Abstract
Purpose of Review
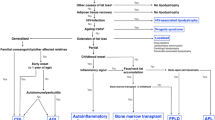
Genetic or acquired lipodystrophies are characterized by selective loss of body fat along with predisposition towards metabolic complications of insulin resistance, such as diabetes mellitus, hypertriglyceridemia, hepatic steatosis, polycystic ovarian syndrome, and acanthosis nigricans. In this review, we discuss the various subtypes and when to suspect and how to diagnose lipodystrophy.
Recent Findings
The four major subtypes are autosomal recessive, congenital generalized lipodystrophy (CGL); acquired generalized lipodystrophy (AGL), mostly an autoimmune disorder; autosomal dominant or recessive familial partial lipodystrophy (FPLD); and acquired partial lipodystrophy (APL), an autoimmune disorder. Diagnosis of lipodystrophy is mainly based upon physical examination findings of loss of body fat and can be supported by body composition analysis by skinfold measurements, dual-energy x-ray absorptiometry, and whole-body magnetic resonance imaging. Confirmatory genetic testing is helpful in the proband and at-risk family members with suspected genetic lipodystrophies. The treatment is directed towards the specific comorbidities and metabolic complications, and there is no treatment to reverse body fat loss. Metreleptin should be considered as the first-line therapy for metabolic complications in patients with generalized lipodystrophy and for prevention of comorbidities in children. Metformin and insulin therapy are the best options for treating hyperglycemia and fibrates and/or fish oil for hypertriglyceridemia.
Summary
Lipodystrophy should be suspected in lean and muscular subjects presenting with diabetes mellitus, hypertriglyceridemia, non-alcoholic fatty liver disease, polycystic ovarian syndrome, or amenorrhea. Diabetologists should be aware of lipodystrophies and consider genetic varieties as an important subtype of monogenic diabetes.

Similar content being viewed by others
References
Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350(12):1220–34.
Chiquette E, et al. Estimating the prevalence of generalized and partial lipodystrophy: findings and challenges. Diabetes Metab Syndr Obes. 2017;10:375–83.
Andre P, et al. Metabolic and cardiac phenotype characterization in 37 atypical Dunnigan patients with nonfarnesylated mutated prelamin A. Am Heart J. 2015;169(4):587–93.
Gonzaga-Jauregui C, et al. Clinical and Molecular prevalence of lipodystrophy in an unascertained large clinical care cohort. Diabetes. 2020;69(2):249–58.
Udler MS, et al. Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: A soft clustering analysis. PLoS Med. 2018;15(9):e1002654.
Patni N, Garg A. Congenital generalized lipodystrophies--new insights into metabolic dysfunction. Nat Rev Endocrinol. 2015;11(9):522–34.
Misra A, Garg A. Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine (Baltimore). 2003;82(2):129–46.
Dunnigan MG, et al. Familial lipoatrophic diabetes with dominant transmission. A new syndrome Q J Med. 1974;43(169):33–48.
Misra A, Peethambaram A, Garg A. Clinical features and metabolic and autoimmune derangements in acquired partial lipodystrophy: report of 35 cases and review of the literature. Medicine (Baltimore). 2004;83(1):18–34.
Agarwal AK, et al. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet. 2002;31(1):21–3.
Magre J, et al. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet. 2001;28(4):365–70.
Kim CA, et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab. 2008;93(4):1129–34.
Hayashi YK, et al. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest. 2009;119(9):2623–33.
Payne F, et al. Mutations disrupting the Kennedy phosphatidylcholine pathway in humans with congenital lipodystrophy and fatty liver disease. Proc Natl Acad Sci U S A. 2014;111(24):8901–6.
Rubio-Cabezas O, et al. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med. 2009;1(5):280–7.
Albert JS, et al. Null mutation in hormone-sensitive lipase gene and risk of type 2 diabetes. N Engl J Med. 2014;370(24):2307–15.
Farhan SM, et al. A novel LIPE nonsense mutation found using exome sequencing in siblings with late-onset familial partial lipodystrophy. Can J Cardiol. 2014;30(12):1649–54.
Donadille B, et al. Partial lipodystrophy with severe insulin resistance and adult progeria Werner syndrome. Orphanet J Rare Dis. 2013;8:106.
Herbst KL, et al. Kobberling type of familial partial lipodystrophy: an underrecognized syndrome. Diabetes Care. 2003;26(6):1819–24.
Cao H, Hegele RA. Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet. 2000;9(1):109–12.
Shackleton S, et al. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet. 2000;24(2):153–6.
Agarwal AK, Garg A. A novel heterozygous mutation in peroxisome proliferator-activated receptor gamma gene in a patient with familial partial lipodystrophy. J Clin Endocrinol Metab. 2002;87(1):408–11.
George S, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304(5675):1325–8.
Gandotra S, et al. Perilipin deficiency and autosomal dominant partial lipodystrophy. N Engl J Med. 2011;364(8):740–8.
Dyment DA, et al. Biallelic mutations at PPARG cause a congenital, generalized lipodystrophy similar to the Berardinelli-Seip syndrome. Eur J Med Genet. 2014;57(9):524–6.
Novelli G, et al. Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am J Hum Genet. 2002;71(2):426–31.
Agarwal AK, et al. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum Mol Genet. 2003;12(16):1995–2001.
Lessel D, et al. Mutations in SPRTN cause early onset hepatocellular carcinoma, genomic instability and progeroid features. Nat Genet. 2014;46(11):1239–44.
Cabanillas R, et al. Nestor-Guillermo progeria syndrome: a novel premature aging condition with early onset and chronic development caused by BANF1 mutations. Am J Med Genet A. 2011;155A(11):2617–25.
Eriksson M, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423(6937):293–8.
De Sandre-Giovannoli A, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300(5628):2055.
Graul-Neumann LM, et al. Marfan syndrome with neonatal progeroid syndrome-like lipodystrophy associated with a novel frameshift mutation at the 3' terminus of the FBN1-gene. Am J Med Genet A. 2010;152A(11):2749–55.
Garg A, et al. Whole exome sequencing identifies de novo heterozygous CAV1 mutations associated with a novel neonatal onset lipodystrophy syndrome. Am J Med Genet A. 2015;167A(8):1796–806.
Weedon MN, et al. An in-frame deletion at the polymerase active site of POLD1 causes a multisystem disorder with lipodystrophy. Nat Genet. 2013;45(8):947–50.
Masotti A, et al. Keppen-Lubinsky syndrome is caused by mutations in the inwardly rectifying K+ channel encoded by KCNJ6. Am J Hum Genet. 2015;96(2):295–300.
Thauvin-Robinet C, et al. PIK3R1 mutations cause syndromic insulin resistance with lipoatrophy. Am J Hum Genet. 2013;93(1):141–9.
Agarwal AK, et al. PSMB8 encoding the beta5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am J Hum Genet. 2010;87(6):866–72.
Corvillo F, et al. Autoantibodies Against Perilipin 1 as a Cause of Acquired Generalized Lipodystrophy. Front Immunol. 2018;9:2142.
Garg A. Lipodystrophies. Am J Med. 2000;108(2):143–52.
Garg A. Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96(11):3313–25.
Nolis T. Exploring the pathophysiology behind the more common genetic and acquired lipodystrophies. J Hum Genet. 2014;59(1):16–23.
Hussain I, Patni N, Garg A. Lipodystrophies, dyslipidaemias and atherosclerotic cardiovascular disease. Pathology. 2019;51(2):202–12.
Pardini VC, et al. Leptin levels, beta-cell function, and insulin sensitivity in families with congenital and acquired generalized lipoatrophic diabetes. J Clin Endocrinol Metab. 1998;83:503–8.
Seip M, Trygstad O. Generalized lipodystrophy, congenital and acquired (lipoatrophy). Acta Paediatr Suppl. 1996;413:2–28.
Westvik J. Radiological features in generalized lipodystrophy. Acta Paediatr Suppl. 1996;413:44–51.
Brunzell JD, Shankle SW, Bethune JE. Congenital generalized lipodystrophy accompanied by cystic angiomatosis. Ann Intern Med. 1968;69(3):501–16.
Fleckenstein JL, et al. The skeleton in congenital, generalized lipodystrophy: evaluation using whole-body radiographic surveys, magnetic resonance imaging and technetium-99m bone scintigraphy. Skelet Radiol. 1992;21(6):381–6.
Chandalia M, et al. Postmortem findings in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 1995;80(10):3077–81.
Anonymous. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 1-1975. N Engl J Med. 1975;292(1):35–41.
Garg A, et al. Peculiar distribution of adipose tissue in patients with congenital generalized lipodystrophy. J Clin Endocrinol Metab. 1992;75(2):358–61.
Haque WA, et al. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab. 2002;87(5):2395–8.
Antuna-Puente B, et al. Higher adiponectin levels in patients with Berardinelli-Seip congenital lipodystrophy due to seipin as compared with 1-acylglycerol-3-phosphate-o-acyltransferase-2 deficiency. J Clin Endocrinol Metab. 2010;95(3):1463–8.
Van Maldergem L, et al. Genotype-phenotype relationships in Berardinelli-Seip congenital lipodystrophy. J Med Genet. 2002;39(10):722–33.
Agarwal AK, et al. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 2003;88(10):4840–7.
Upreti V, et al. An unusual cause of delayed puberty: Berardinelli- Seip syndrome. J Pediatr Endocrinol Metab. 2012;25(11-12):1157–60.
Maguire M, et al. Pregnancy in a woman with congenital generalized lipodystrophy: leptin's vital role in reproduction. Obstet Gynecol. 2012;119(2 Pt 2):452–5.
Jiang M, et al. Lack of testicular seipin causes teratozoospermia syndrome in men. Proc Natl Acad Sci U S A. 2014;111(19):7054–9.
Ebihara C, et al. Seipin is necessary for normal brain development and spermatogenesis in addition to adipogenesis. Hum Mol Genet. 2015;24(15):4238–49.
Karhan AN, et al. Biallelic CAV1 null variants induce congenital generalized lipodystrophy with achalasia. Eur J Endocrinol. 2021;185(6):841–54.
Cao H, et al. Heterozygous CAV1 frameshift mutations (MIM 601047) in patients with atypical partial lipodystrophy and hypertriglyceridemia. Lipids Health Dis. 2008;7:3.
Patni N, Hegele RA, Garg A. Caveolar dysfunction and lipodystrophies. Eur J Endocrinol. 2022;186(3):C1–4.
Garg A. Gender differences in the prevalence of metabolic complications in familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab. 2000;85(5):1776–82.
Patni N, et al. Regional body fat changes and metabolic complications in children with Dunnigan lipodystrophy-causing LMNA Variants. J Clin Endocrinol Metab. 2018;104(4):1099–108.
Garg A, Peshock RM, Fleckenstein JL. Adipose tissue distribution pattern in patients with familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab. 1999;84(1):170–4.
Haque WA, et al. Risk factors for diabetes in familial partial lipodystrophy, Dunnigan variety. Diabetes Care. 2003;26(5):1350–5.
Hegele RA. Premature atherosclerosis associated with monogenic insulin resistance. Circulation. 2001;103(18):2225–9.
Patni N, et al. A novel syndrome of generalized lipodystrophy associated with pilocytic astrocytoma. J Clin Endocrinol Metab. 2015;100(10):3603–6.
Haddad N, et al. Acquired generalized lipodystrophy under immune checkpoint inhibition. Br J Dermatol. 2020;182(2):477–80.
Jehl A, et al. Acquired generalized lipodystrophy: a new cause of anti-PD-1 immune-related diabetes. Diabetes Care. 2019;42(10):2008–10.
Savage DB, et al. Complement abnormalities in acquired lipodystrophy revisited. J Clin Endocrinol Metab. 2009;94(1):10–6.
Park JY, et al. Type 1 diabetes associated with acquired generalized lipodystrophy and insulin resistance: the effect of long-term leptin therapy. J Clin Endocrinol Metab. 2008;93(1):26–31.
Kumar R, et al. Acquired generalised lipodystrophy and type 1 diabetes mellitus in a child: a rare and implacable association. BMJ Case Rep. 2018;2018:bcr-2018.
Srinivasan S, et al. A polygenic lipodystrophy genetic risk score characterizes risk independent of BMI in the diabetes prevention program. J Endocr Soc. 2019;3(9):1663–77.
Handelsman Y, et al. The clinical approach to the detection of lipodystrophy - an AACE consensus statement. Endocr Pract. 2013;19(1):107–16.
Brown RJ, et al. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab. 2016;101(12):4500–11.
Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40(3):497–504.
Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med Sci Sports Exerc. 1980;12(3):175–81.
Dezenberg CV, et al. Predicting body composition from anthropometry in pre-adolescent children. Int J Obes Relat Metab Disord. 1999;23(3):253–9.
Vasandani C, et al. Diagnostic value of anthropometric measurements for familial partial lipodystrophy, Dunnigan Variety. J Clin Endocrinol Metab. 2020;105(7)2132-2141.
Javor ED, et al. Proteinuric nephropathy in acquired and congenital generalized lipodystrophy: baseline characteristics and course during recombinant leptin therapy. J Clin Endocrinol Metab. 2004;89(7):3199–207.
Akinci B, et al. Renal complications of lipodystrophy: a closer look at the natural history of kidney disease. Clin Endocrinol. 2018;89(1):65–75.
Musso C, et al. Spectrum of renal diseases associated with extreme forms of insulin resistance. Clin J Am Soc Nephrol. 2006;1(4):616–22.
Oral EA, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570–8.
Beltrand J, et al. Metabolic correction induced by leptin replacement treatment in young children with Berardinelli-Seip congenital lipoatrophy. Pediatrics. 2007;120(2):e291–6.
Simha V, et al. Comparison of efficacy and safety of leptin replacement therapy in moderately and severely hypoleptinemic patients with familial partial lipodystrophy of the Dunnigan variety. J Clin Endocrinol Metab. 2012;97(3):785–92.
Diker-Cohen T, et al. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab. 2015;100(5):1802–10.
Luedtke A, et al. Thiazolidinedione response in familial lipodystrophy patients with LMNA mutations: a case series. Horm Metab Res. 2012;44(4):306–11.
Simha V, Rao S, Garg A. Prolonged thiazolidinedione therapy does not reverse fat loss in patients with familial partial lipodystrophy, Dunnigan variety. Diabetes Obes Metab. 2008;10(12):1275–6.
Banning F, et al. Insulin secretory defect in familial partial lipodystrophy Type 2 and successful long-term treatment with a glucagon-like peptide 1 receptor agonist. Diabet Med. 2017;34(12):1792–4.
Oliveira J, et al. Glucagon-like peptide-1 analogues - an efficient therapeutic option for the severe insulin resistance of lipodystrophic syndromes: two case reports. J Med Case Rep. 2017;11(1):12.
Melvin A, et al. Roux-en-Y gastric bypass surgery in the management of familial partial lipodystrophy type 1. J Clin Endocrinol Metab. 2017;102(10):3616–20.
Grundfest-Broniatowski S, et al. Successful treatment of an unusual case of FPLD2: The role of Roux-en-Y gastric bypass-case report and literature review. J Gastrointest Surg. 2017;21(4):739–43.
Kozusko K, et al. Clinical and molecular characterization of a novel PLIN1 frameshift mutation identified in patients with familial partial lipodystrophy. Diabetes. 2015;64(1):299–310.
Ciudin A, et al. Successful treatment for the Dunnigan-type familial partial lipodystrophy with Roux-en-Y gastric bypass. Clin Endocrinol. 2011;75(3):403–4.
McGrath NM, Krishna G. Gastric bypass for insulin resistance due to lipodystrophy. Obes Surg. 2006;16(11):1542–4.
Utzschneider KM, Trence DL. Effectiveness of gastric bypass surgery in a patient with familial partial lipodystrophy. Diabetes Care. 2006;29(6):1380–2.
Mandel-Brehm C, et al. Autoantibodies to perilipin-1 define a subset of acquired generalized lipodystrophy. Diabetes. 2022; db211172 (online ahead of print).
Acknowledgments
We thank Tea Huseinbegovic, B.S., for help with the illustration.
Funding
A.G. and N.P are supported by the National Institutes of Health grant R01-DK105448 and A.G. is also supported by the Southwestern Medical Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
N.P. have no financial or non-financial interests that are directly or indirectly related to the work submitted for publication. A.G. consults for Amryt Pharma PLC and Regeneron and has received grant support from Amryt Pharma PLC, Regeneron, Quintiles, Akcea Pharmaceuticals, and Intercept Pharmaceuticals. A.G. is coholder of a patent for “use of leptin for treating human lipoatrophy and a method of determining predisposition to said treatment” but receives no financial compensation.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pathogenesis of Type 2 Diabetes and Insulin Resistance
Rights and permissions
About this article
Cite this article
Patni, N., Garg, A. Lipodystrophy for the Diabetologist—What to Look For. Curr Diab Rep 22, 461–470 (2022). https://doi.org/10.1007/s11892-022-01485-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-022-01485-w