Abstract
Purpose
3β-hydroxysteroid dehydrogenase type 2 deficiency (3βHSD2D) is a very rare variant of congenital adrenal hyperplasia (CAH) causing less than 0.5% of all CAH. The aim was to review the literature.
Methods
PubMed was searched for relevant articles.
Results
3βHSD2D is caused by HSD3B2 gene mutations and characterized by impaired steroid synthesis in the gonads and the adrenal glands and subsequent increased dehydroepiandrosterone (DHEA) concentrations. The main hormonal changes observed in patients with 3βHSD2D are elevated ratios of the Δ5-steroids over Δ4-steroids but molecular genetic testing is recommended to confirm the diagnosis. Several deleterious mutations in the HSD3B2 gene have been associated with salt-wasting (SW) crisis in the neonatal period, while missense mutations have been associated with a non-SW phenotype. Boys may have ambiguous genitalia, whereas girls present with mild or no virilization at birth. The existence of non-classic 3βHSD2D is controversial. In an acute SW crisis, the treatment includes prompt rehydration, correction of hypoglycemia, and parenteral hydrocortisone. Similar to other forms of CAH, glucocorticoid and mineralocorticoid replacement is needed for long-term management. In addition, sex hormone replacement therapy may be required if normal progress through puberty is failing. Little is known regarding possible negative long-term consequences of 3βHSD2D and its treatments, e.g., fertility, final height, osteoporosis and fractures, adrenal and testicular tumor risk, and mortality.
Conclusion
Knowledge is mainly based on case reports but many long-term outcomes could be presumed to be similar to other types of CAH, mainly 21-hydroxylase deficiency, although in 3βHSD2D it seems to be more difficult to suppress the androgens.
Similar content being viewed by others
Introduction
Congenital adrenal hyperplasia (CAH) is a group of disorders caused by deficiency of one of five enzymes that are responsible for making cortisol from cholesterol in the adrenal glands [1,2,3]. 21-hydroxylase deficiency (21OHD) is the most common disorder causing CAH (95-99%) followed by 11-beta-hydroxylase deficiency (11OHD) [2, 4,5,6,7].
3β-hydroxysteroid dehydrogenase type 2 deficiency (3βHSD2D) [8, 9], is a very rare type of CAH affecting <0.5% of all CAH [4, 5], and with <1/1,000,000 estimated prevalence at birth [10]. This disorder is caused by HSD3B2 gene mutations and characterized by impairment of steroid synthesis in the gonads and the adrenal glands [11]. This leads to decreased cortisol, aldosterone, and androstenedione concentrations, however, renin, ACTH, and dehydroepiandrosterone (DHEA) concentrations are increased with DHEA being converted to testosterone by extra-adrenal 3βHSD1 [2]. The first cases of 3βHSD2D were reported by Bongiovanni in USA 1962 [12]. The clinical presentation varies according to the type (severity) of the genetic mutation and may include salt-wasting (SW) in both sexes, incomplete masculinization in males, and virilization in females. Elevated Δ5-17-hydroxypregnenolone is the best single biological marker or indicator of 3βHSD2D [13], but molecular genetic testing is recommended to confirm the diagnosis [14]. Glucorticoid and mineralocorticoid replacement therapy constitutes the main treatment [15]. In addition, sex hormones may be required for some patients who fail to progress through puberty [16].
The aim of this review is to provide a summary of currently available knowledge of CAH due to 3βHSD2D.
Physiology
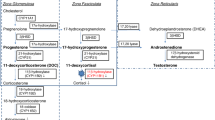
The adrenal glands are vital organs where steroidogenesis (in adrenal cortex) and catecholamine production (adrenal medulla) take place. The adrenal cortex has three compartments: zona glomerulosa, zona fasciculata, and zona reticularis [17] (Fig. 1). In the first step of the steroidogenesis StAR transports the cholesterol across the membrane, and then cholesterol is converted pregnenolone by the P450 side chain cleavage enzyme [18]. Within the zona glomerulosa, HSD3B2 converts pregnenolone to progesterone, which eventually is converted into aldosterone by a series of enzymatic processes involving CYP21A2 and aldosterone synthase [17]. In the zona fasciculata, CYP17A1 hydroxylates pregnenolone to form 17-hydroxyprogesterone (17OHP) which is then converted via several enzymes, including CYP11B1 and HSD3B2, to form cortisol. In the zona reticularis, 17-hydroxypregnenolone is converted to DHEA by CYP17A1. Then, HSD3B2 converts DHEA to androstenedione, which is a precursor of sex hormones [18]. The conversion of the Δ5-3β-hydroxysteroids (pregnenolone, 17-hydroxypregnenolone, and DHEA) to a Δ4-3-ketosteroids (progesterone, 17OHP, and androstenedione) by HSD3B2 involves dehydrogenation followed by an isomerization reaction [19]. Similarly, within the Leydig cells in the testis, cholesterol is converted to pregnenolone, 17-hydroxypregnenolone, DHEA and androstenedione. HSD17B3/AKR1C3 converts androstenedione or androstenediol to testosterone [20]. DHEA is converted by SULT2A1 to the more stable sulfated form (DHEAS). DHEAS has longer half-life (<10 h) and only 20% diurnal variation (DHEA ~30 min and 300%, respectively) [21] and is therefore measured more often than DHEA, for practical reasons. DHEAS can then be reconverted to DHEA by steroid sulfatase (STS) (~70%) but also to a certain degree by SULT2A1 located in the liver and kidney [22, 23].
a Normal steroidogenesis in the adrenal cortex. The pathways of aldosterone, cortisol, and androgen synthesis and the enzymatic steps from the precursor cholesterol are shown. b Adrenal hormonal synthesis and enzyme expression pattern. ZG zona glomerulosa, ZF zona fasciculata, ZR zona reticularis, CYP11B2 aldosterone synthase, CYP17A1 17α-hydroxylase/17,20-lyase, CYP11B1 11β-hydroxylase, CYB5A, cytochrome b5, SULTA1 steroid sulfotransferase type 2A1
Pathophysiology
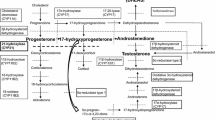
21OHD and 11OHD impair steroidogenesis in the adrenal glands only [1,2,3, 6, 17]. In contrast, severe form of 3βHSD2D causes defects of steroidogenesis in both adrenal glands and gonads [2, 3, 15]. Figure 2 illustrates the pathophysiology of 3βHSD2D.
HSD3B2 catalyzes reactions responsible for synthesis of a 3-keto-Δ4 A-ring, which is an essential part of endogenous mineralocorticoids, glucocorticoids, progestins, and androgens [3, 12, 24]. As a result, 3βHSD2D impairs the synthesis of progesterone, the precursor hormone of aldosterone, 17OHP, the precursor for cortisol, androstenedione, testosterone, and estrogen in the adrenal glands and gonads [13, 24]. Reduced levels of cortisol decrease the negative feedback on the pituitary gland causing excess ACTH production. Subsequently, ACTH drives the accumulation of β-hydroxy-Δ5-steroids pregnenolone, 17-hydroxypregnenolone, and DHEA, and their sulfates [25]. These precursor steroids cannot compensate for the cortisol and aldosterone deficiencies resulting in electrolyte disturbances and SW in most patients [12]. In the peripheral tissues, the intact isoenzyme HSD3B1 enzyme converts circulating DHEA to testosterone [16].
Elevated level of androstenedione leads to relatively high level of testosterone in females, however, it fails to achieve full compensation for absence of testosterone synthesis in males. In 46,XY neonates testosterone deficiency causes genital ambiguity. On the other hand, in 46,XX neonates, the relatively high level of testosterone may cause clitoromegaly and partial labioscrotal fusion. In addition, undiagnosed females may present with precocious pubarche, acne, hirsutism, and menstrual disturbances [26].
The human type I isoenzyme 3βHSD is the isoenzyme, encoded by HSD3B1 gene and is expressed in peripheral tissue including skin, mammary glands, and placenta [9, 11, 14]. It has 372 amino acids and shares more than 90% homology with the type II isoenzyme [27, 28]. The human type I isoenzyme 3βHSD catalyzes transformation of DHEA into sex steroids including testosterone and estradiol [9].
Clinical presentation
The phenotype of 3βHSD2D varies according to the genetic defect from severe SW form in neonates to mild menstrual disorders in older females [13, 15, 20].
Incomplete masculinization in males
In normal 46,XY fetuses, androgens are required for penile development including the urethra and fusion of the labial-scrotal folds that normally takes place before 12 weeks of gestation [29]. Severe form of 3βHSD2D is associated with varying manifestations of incomplete masculinization including severe hypospadia, micropenis, bifid scrotum, and undescended testis [16, 20, 29].
Female virilization
Depending on the genetic mutations, 46,XX infants can show enlarged clitoris, incomplete labial fusion and genital hyperpigmentation [30]. In contrast, some girls can have normal external genitalia which may delay diagnosis and they can subsequently present with adrenal crisis [31]. Older girls and women with genetically confirmed non-SW 3βHSD2D can present with androgen symptoms of hirsutism, premature pubarche or menstrual disorders including oligomenorrhea and primary amenorrhea [16, 32].
Salt-wasting
Several deleterious mutations in the HSD3B2 gene have been described that can cause SW during the first few weeks of life and may be fatal if not treated adequately [31, 32]. Biochemical findings include hyponatremia, hyperkalemia, metabolic acidosis and hypoglycemia [15, 33]. On the other hand, missense mutations in the coding region of HSD3B2 gene is associated with non-SW form due to the presence of some residual enzymatic activity, about 10%, is sufficient to prevent aldosterone deficiency [16, 24, 32, 34, 35].
Hypoglycemia
Recurrent episodes of hypoglycemia were reported to be a presenting feature in a suspected case of 3βHSD2D but the genotype was not performed to confirm the diagnosis [36]. Another patient presented during second day of life with hypoglycemia, later on, the molecular genetics confirmed 3βHSD2D [31].
Diagnosis
In case of SW phenotype, 3βHSD2D is usually diagnosed within the first few weeks of life. In case of non-SW phenotype, patients may be diagnosed at any time before puberty [37]. However, the diagnosis has rarely been further delayed and patients can present with gender role related concerns during adulthood [38]. Overall, the patients tend to be diagnosed at a younger age in 46,XY children due to a higher rate of genital ambiguity compared to females [34, 39]. Also, there seems to be an underrepresentation of 46,XX patients, which might be explained by lack of diagnosis in milder form of 3βHSD2D in females. Also, females with severe form may die undiagnosed in a neonatal adrenal crisis more often than males [15].
When adrenal insufficiency is suspected in the setting of an adrenal crisis (i.e., an acute hemodynamic disturbance with hyponatremia, hyperkalemia and often hypoglycemia), blood should be drawn for steroid hormone measurements [15], but without delaying the lifesaving acute treatment with intravenous (or intramuscular) hydrocortisone [40, 41]. Low cortisol with high ACTH is consistent with primary adrenal insufficiency [42].
As 3βHSD2D catalyzes the conversion of Δ5-steroids (pregnenolone, 17-hydroxypregnenolone, DHEA, androstenediol) to Δ4-steroids (progesteron, 17OHP, androstenedione, testosterone), the main hormonal changes observed in patients with 3βHSD2D are high ratios of the Δ5- over Δ4-steroids [24, 43]. This includes raised 17-hydroxypregnenolone to 17OHP and DHEA(S) to androstenedione ratios in serum, and pregnanetriol to pregnanediol ratio in urine [15, 44, 45].
ACTH stimulation test and hormonal profiles
Morning administration of 250 μg of synthetic ACTH followed by measurements of plasma Δ5-17-hydroxypregnenolone (5–17P), cortisol, Δ4-17-hydroxyprogesterone (17OHP), DHEA(S), and androstenedione can be used to improve the diagnostic process of 3βHSD2D [3, 13]. Hormonal criteria for the diagnosis of 3βHSD2D have been developed from a previous study [13], where hormonal profiles of patients with homozygous/compound heterogeneous HSD3B2 mutations and people with normal HSD3B2 genes were compared. ACTH stimulation test shows, apart from diminished cortisol, an exaggerated response and high level of Δ5-17-hydroxypregnenolone in patients with homozygous/compound heterozygous HSD3B2 mutations and varies according to patient age (Table 1) [13, 46].
In general, Δ5-17-hydroxypregnenolone above 100 nmol/L, either basal or after ACTH stimulation, is the best single biological criterion of 3βHSD2D [16, 31, 37]. The hormonal profile cannot distinguish heterozygous carriers from normal people [3, 47].
Other biochemical findings are elevated renin, relatively high level of testosterone in girls, elevated 17OHP (via peripheral conversion, see below), elevated DHEA(S), elevated urinary Δ5-OHP, and DHA metabolites [16].
Molecular analysis and genetic studies
There are two isoenzymes of human 3βHSD which are encoded by different genes located on the p13.1 region of chromosome 1 [14, 15]. Both genes are included within a DNA fragment of around 7.8 kB and consist of four exons and three introns [19, 24, 34]. The HSD3B2 gene encodes the human type II 3βHSD isoenzyme and is expressed in the adrenal cortex and in the gonads. The isoenzyme is essential for the adrenal synthesis of glucocorticoids, mineralocorticoids, and sex steroids [9, 10, 34, 36]. More than 40 mutations have been found in the HSD3B2 gene causing 3βHSD2D and a few of them have been identified in isolated populations (Table 2) [10, 15, 16, 20, 24, 29,30,31, 35, 37, 48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64].
In general, frameshift mutations, in-frame deletions, and nonsense mutations introducing a premature termination codon are associated with severe form of 3βHSD2D resulting in SW phenotype [14, 34, 65]. The locations of these mutations suggest that at least the first 318 amino acids out of 371 are required for HSD3B2 activity [14]. In contrast, missense mutations are associated with some residual enzymatic activity and non-SW phenotype [14]. There have been no reported mutations of the HSD3B1 gene in human so far [32, 44].
Neonatal screening
Newborns with atypical external genitalia should undergo hormonal profile analysis prior to hospital discharge to avoid presentation with SW crisis [66, 67]. Neonatal screening for 21OHD by detecting elevated level of 17OHP has been implemented in most developed countries [68]. 3βHSD2D can result in an increase in the level of circulating 17OHP due to peripheral conversion of high levels of accumulated Δ5-steroids by the isoenzyme 3βHSD type 1. There have been previous case reports of false positive, for 21OHD, neonatal screen for infants with 3βHSD2D [31, 65]. Accordingly, neonates with elevated 17OHP should undergo molecular genetic confirmation to confirm the type of enzymatic deficiency [14, 31, 68].
Non-classic form of 3βHSD2D
Prior to the implementation of molecular genetic studies, it was thought that many children with premature pubarche, and females with hirsutism and menstrual irregularities might have a mild, late-onset (non-classic) form of 3βHSD2D [35, 37, 45]. This was supported by controversial hormonal criteria based on exaggerated Δ5-steroid production after ACTH stimulation test and elevated 17OHP to cortisol ratios [32]. However, genetic studies failed to detect any mutations in the HSD3B2 gene in this group of patients [24, 29, 35, 37], and treatment with glucocorticoids and mineralocorticoids did not improve signs of androgen excess [29, 32]. A previous report has shown normalization of the hormonal profile after treatment with GnRH agonist for two patients diagnosed with polycystic ovarian syndrome (PCOS) associated with 3βHSD2D [69]. The exact mechanism of exaggerated Δ5-steroid production after ACTH stimulation is not clear and it might be related to a form of PCOS or other unidentified mechanism causing alteration in intra-adrenal 3βHSD activity [32]. A not uncommon presentation among adult women with mild hyperandrogenism is that they are found to have elevated serum DHEAS and/or reported to have “partial 3βHSD2D”, based on urine steroid profiling but with no HSD3B2 gene mutations identified. The diagnosis usually ends up being PCOS. Thus, non-classic 3βHSD2D, if it exists, is extremely rare [2], in contrast to non-classic 21OHD [70, 71].
Pubertal status
Few patients have been evaluated after puberty [15, 20, 33, 72,73,74,75]. With good compliance with glucocortiocid and mineralocorticoid replacement therapy [15], most 46,XX patients have shown progressive feminization at appropriate age with menstruations [15, 33, 74]. In contrast, one female with severe HSD3B2 mutations had minimal breast development at age 14.7 years, required gonadotropin injections and estrogen treatment to develop full feminization. However, with cessation of estrogen and progesterone replacement treatment, her menstrual cycle ceased and she developed ovarian cysts [16, 76].
The pubertal development has been reported in some males with HSD3B2 mutations. Most of these patients entered puberty spontaneously without need for testosterone supplementation [15, 20, 33, 72, 74, 75, 77]. This could be explained by peripheral conversion of DHEAS to testosterone by HSD17B5 activity [10, 20].
Gynecomastia
In adult males with 3βHSD2D, HSD3B1 converts the high amount of androgen precursors (DHEA and androstenediol) in peripheral tissues to androstenedione or testosterone [20]. Then HSD17B1, HSD17B5, and CYP19A1 enzymes catalyze the conversion of androstenedione and testosterone to estrogens [20]. High level of estrogens is associated with gynecomastia in males [10, 20, 72]. Testosterone replacement therapy was found to reduce gynecomastia by suppressing gonadotrophin synthesis via negative feedback [20].
Final height
Final height has been reported in a few patients and the adult height seemed to be within the target range when control of the hyperandrogenism during the growth period had been good [15], but otherwise the final height was reduced [75].
Fertility
3βHSD is required for biosynthesis of not only mineralocorticoids and glucocorticoids, but also sex hormones. Accordingly, males with 3βHSD2D may suffer from decreased spermatogenesis and infertility. Also, females may have menstrual irregularity and infertility [20]. However, there is very limited information about fertility, semen analysis and testicular histology in patients with 3βHSD2D [15, 20, 73, 75]. In case reports of 46,XY patients, semen analyses have shown azoospermia [15, 75]. Moreover, testicular histology in adult males with 3βHSD2D showed spermatogenic arrest at the level of spermatogonia [20, 78]. In contrast, a patient with severe HSD3B2 mutations, with annual follow-ups from birth until the age of 23 years old, demonstrated normal sperm production probably attributed to his good compliance with treatment [10]. This might suggest that fertility is possible even with severe mutations. One case of an adult male fathering two children has been reported, however, there was no genetic testing to confirm his paternity [10]. In 21OHD, fertility has been shown to be impaired in both females and males [4, 79,80,81,82,83,84,85,86], however, the fertility may be normal if the male has been diagnosed and treated early on since the neonatal period. If this is also true in 3βHSD2D is unknown.
Testicular adrenal rest tumors
During abdominal surgery, the presence of ectopic adrenocortical tissue is a common incidental finding in otherwise healthy individuals without clinical significance [87]. In patients with CAH and during period of suboptimal treatment, high levels of ACTH and angiotensin II can stimulate adrenal-like cells causing development of testicular adrenal rest tumors (TARTs) and rarely ovarian adrenal rest tumors [75, 87]. The prevalence of TARTs varies between 34 and 94% according to different reports in males with CAH due to 21OHD [82, 85, 88, 89]. TARTs have been reported in some patients with 3βHSD2D [15, 63, 75], but it is difficult to estimate the prevalence. Also, it has been demonstrated that presence of TARTs has a negative impact on fertility in males with 21OHD [82, 88, 90]. Similarly, in previous case reports, males with 3βHSD2D and TARTs have been found to have severely impaired spermatogenesis [63, 75, 82]. High dose of corticosteroids might reduce the size of TART [63]. It has been recommended that all patients with CAH should undergo regular testicular examination with ultrasonography [1, 7, 90]. Even though these recommendations were primarily written for 21OHD it can be assumed that males with 3βHSD2D have equal benefits.
Bone mineral density and fractures
Supraphysiological glucocorticoid replacement has harmful effects on bone mineral density (BMD) via multiple mechanisms [91, 92]. Only one case of 3βHSD2D and BMD measurements has been reported, and has showed osteoporosis [75]. In general, studies in adults with CAH have demonstrated impaired BMD [4, 93,94,95,96,97,98,99,100], even though there are exceptions with normal BMD [101, 102], and better than other DSD conditions [103]. Prednisolone may be associated with worse BMD than hydrocortisone [95, 97, 104, 105]. Fractures have not been reported in 3βHSD2D so far but may be increased in CAH in general [93, 95, 97, 99, 100, 103].
Obesity, diabetes, and cardiovascular disease
Obesity, including severe, has been reported in patients with 3βHSD2D [63, 75], probably due to iatrogenic Cushing syndrome. It could be assumed that the prevalence of obesity, diabetes and cardiovascular disease in 3βHSD2D is similar to most other forms of CAH, most commonly 21OHD, and mainly due to glucocorticoid excess but androgen excess and/or deficiency may also contribute. The majority of studies including adults and children with CAH have reported an increased body fat mass assessed by DXA [96, 101, 102, 106, 107], which enables separation between lean mass (which may be increased due to hyperandrogenism) from fat mass. Elevated cardiometabolic risk, including insulin resistance [4, 94, 108,109,110,111,112,113,114,115,116,117], has been reported in a large number of studies on CAH, with a few reporting increased rate of established cardiovascular disease [103, 118], and diabetes (including gestational diabetes) [81, 109, 118]. Very few individuals with CAH above 50 years of age have been included in studies, and thus it could be expected that the rate will increase since cardiovascular disease and diabetes usually develop later in life [1].
Psychiatric diseases
Psychiatric disorders have so far not been reported in studies with exclusively 3βHSD2D recruited [119]. In studies of CAH psychiatric diseases have only occasionally been investigated and these have shown an increased rate [103, 120, 121], especially of depression [122], alcohol misuse [120, 121], and suicidality [103, 120].
Adrenal tumors
Chronic elevation of ACTH will lead to hyperplasia of the adrenal cortex and sometimes subsequent tumor formation [123,124,125]. Adrenal tumors have so far not been reported in 3βHSD2D but are known to affect 11–82% of patients with other CAH variants [124, 126, 127]. Adrenal incidentalomas, i.e., adrenal tumors found serendipitously by imaging for other reasons than suspected adrenal tumor or disease [128], have sometimes been the initial presentation of CAH, including classic CAH, both in case reports and adrenal incidentaloma cohorts [125, 129,130,131,132,133,134].
Mortality
Very little is known about the mortality in individuals with 3βHSD2D. The introduction of glucocorticoid replacement and increased awareness have increased the survival of classic 21OHD [5], and this is most probably also the case in 3βHSD2D. In population studies, patients with CAH had generally an increased mortality rate (hazard ratio 3–5) and died 6.5–18 years earlier, compared to controls [122, 135]. Adrenal crisis was the main cause of death [135], iterating the importance of stress dosing during acute illness [40, 41]. Mortality studies in pure 3βHSD2D will probably never be performed due to its rareness.
Treatment
Glucocorticoid and mineralocorticoid replacement is similar to other forms of CAH. In SW crisis, treatment includes prompt rehydration, correction of hypoglycemia, and parenteral hydrocortisone (intravenous or intramuscular) [15, 40, 41]. For follow-up children are treated with hydrocortisone in a dose of 10–15 mg/m2/day. Long-acting glucocorticoids such as dexamethasone and prednisolone, known to suppress growth in children, can be used during adulthood [7, 33, 67]. Compared to 21OHD it seems to be more difficult to suppress the androgens in 3βHSD2D, which could be speculated be due to the DHEAS as a constant source of DHEA, testosterone and DHT. This may result in a need for slightly higher doses of glucocorticoids in 3βHSD2D with subsequently more long-term negative outcomes. Mineralocorticoid replacement can be achieved with fludrocortisone 0.1 mg/day [33] with regular monitoring of plasma renin activity [1, 7, 67]. Sex hormone replacement therapy should be considered for patients who show delayed progression through puberty [16]. In addition, testosterone replacement therapy might be considered for male patients with testosterone responsive microphallus to augment penile growth [33]. Hormonal replacement therapy should be combined with regular clinical and biochemical evaluation of these patients [15]. Surgical intervention might be indicated in some circumstances including undescended testis [63], hypospadias repair [20], and severe genital virilization [136,137,138]. Bilateral adrenalectomy has occasionally been used in selective cases with 21OHD or 11OHD to better control hyperandrogenism and/or to be able to lower the glucocorticoid doses with similar control of the hyperandrogenism [139]. Its utility in 3βHSD2D is currently unclear.
Conclusion
3βHSD2D is a very rare form of CAH and the phenotype varies according to the severity of the HSD3B2 mutations. In severe forms, the neonate can present with SW crisis but the diagnosis can be delayed in mild forms until adolescence. Hormonal criteria for the diagnosis of 3βHSD2D have been developed and it was proposed that Δ5-17-hydroxypregnenolone above 100 nmol/L, either basal or after ACTH stimulation, is the best single biological criterion of 3βHSD2D. However, molecular genetic testing is recommended to confirm the diagnosis. Glucocorticoid and mineralocorticoid replacement are the main treatments. Sex hormone replacement and surgical corrective procedures may be indicated in some patients. On the basis of case reports, 3βHSD2D may be associated with infertility, obesity, osteoporosis, TARTs, and reduced final height. However, very little is known about mortality, cardiovascular health, mental health, and adrenal tumor risk due to the rareness of 3βHSD2D but can be presumed to be elevated, and similar to 21OHD. Although in 3βHSD2D it seems to be more difficult to suppress the androgens, subsequently leading to slightly higher glucocorticoid doses. This may result in more long-term negative outcomes.
References
H. Falhammar, M. Thoren, Clinical outcomes in the management of congenital adrenal hyperplasia. Endocrine 41(3), 355–373 (2012). https://doi.org/10.1007/s12020-011-9591-x
D. El-Maouche, W. Arlt, D.P. Merke, Congenital adrenal hyperplasia. Lancet 390(10108), 2194–2210 (2017). https://doi.org/10.1016/S0140-6736(17)31431-9
W.L. Miller, Mechanisms in endocrinology: rare defects in adrenal steroidogenesis. Eur. J. Endocrinol. 179(3), R125–R141 (2018). https://doi.org/10.1530/EJE-18-0279
W. Arlt, D.S. Willis, S.H. Wild, N. Krone, E.J. Doherty, S. Hahner, T.S. Han, P.V. Carroll, G.S. Conway, D.A. Rees, R.H. Stimson, B.R. Walker, J.M. Connell, R.J. Ross, United Kingdom Congenital Adrenal Hyperplasia Adult Study, Executive: Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J. Clin. Endocrinol. Metab. 95(11), 5110–5121 (2010). https://doi.org/10.1210/jc.2010-0917
S. Gidlof, H. Falhammar, A. Thilen, U. von Dobeln, M. Ritzen, A. Wedell, A. Nordenstrom, One hundred years of congenital adrenal hyperplasia in Sweden: a retrospective, population-based cohort study. Lancet Diabetes Endocrinol. 1(1), 35–42 (2013). https://doi.org/10.1016/S2213-8587(13)70007-X
K. Bulsari, H. Falhammar, Clinical perspectives in congenital adrenal hyperplasia due to 11beta-hydroxylase deficiency. Endocrine 55(1), 19–36 (2017). https://doi.org/10.1007/s12020-016-1189-x
P.W. Speiser, W. Arlt, R.J. Auchus, L.S. Baskin, G.S. Conway, D.P. Merke, H.F.L. Meyer-Bahlburg, W.L. Miller, M.H. Murad, S.E. Oberfield, P.C. White, Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 103(11), 4043–4088 (2018). https://doi.org/10.1210/jc.2018-01865
N.A. Al-Jurayyan, Ambiguous genitalia: two decades of experience. Ann. Saudi Med. 31(3), 284–288 (2011). https://doi.org/10.4103/0256-4947.81544
A.R. Benkert, M. Young, D. Robinson, C. Hendrickson, P.A. Lee, K.A. Strauss, Severe salt-losing 3beta-hydroxysteroid dehydrogenase deficiency: treatment and outcomes of HSD3B2 c.35G>A homozygotes. J. Clin. Endocrinol. Metab. 100(8), E1105–E1115 (2015). https://doi.org/10.1210/jc.2015-2098
B. Donadille, M. Houang, I. Netchine, J.P. Siffroi, S. Christin-Maitre, Human 3beta-hydroxysteroid dehydrogenase deficiency associated with normal spermatic numeration despite a severe enzyme deficit. Endocr. Connect 7(3), 395–402 (2018). https://doi.org/10.1530/EC-17-0306
E. Rheaume, J. Simard, Y. Morel, F. Mebarki, M. Zachmann, M.G. Forest, M.I. New, F. Labrie, Congenital adrenal hyperplasia due to point mutations in the type II 3 beta-hydroxysteroid dehydrogenase gene. Nat. Genet 1(4), 239–245 (1992). https://doi.org/10.1038/ng0792-239
A.M. Bongiovanni, The adrenogenital syndrome with deficiency of 3 beta-hydroxysteroid dehydrogenase. J. Clin. Invest. 41, 2086–2092 (1962). https://doi.org/10.1172/JCI104666
C. Lutfallah, W. Wang, J.I. Mason, Y.T. Chang, A. Haider, B. Rich, M. Castro-Magana, K.C. Copeland, R. David, S. Pang, Newly proposed hormonal criteria via genotypic proof for type II 3beta-hydroxysteroid dehydrogenase deficiency. J. Clin. Endocrinol. Metab. 87(6), 2611–2622 (2002). https://doi.org/10.1210/jcem.87.6.8615
Y. Morel, F. Mebarki, E. Rheaume, R. Sanchez, M.G. Forest, J. Simard, Structure-function relationships of 3 beta-hydroxysteroid dehydrogenase: contribution made by the molecular genetics of 3 beta-hydroxysteroid dehydrogenase deficiency. Steroids 62(1), 176–184 (1997)
N. Alos, A.M. Moisan, L. Ward, M. Desrochers, L. Legault, G. Leboeuf, G. Van Vliet, J. Simard, A novel A10E homozygous mutation in the HSD3B2 gene causing severe salt-wasting 3beta-hydroxysteroid dehydrogenase deficiency in 46,XX and 46,XY French-Canadians: evaluation of gonadal function after puberty. J. Clin. Endocrinol. Metab. 85(5), 1968–1974 (2000). https://doi.org/10.1210/jcem.85.5.6581
A.M. Moisan, M.L. Ricketts, V. Tardy, M. Desrochers, F. Mebarki, J.L. Chaussain, S. Cabrol, M.C. Raux-Demay, M.G. Forest, W.G. Sippell, M. Peter, Y. Morel, J. Simard, New insight into the molecular basis of 3beta-hydroxysteroid dehydrogenase deficiency: identification of eight mutations in the HSD3B2 gene eleven patients from seven new families and comparison of the functional properties of twenty-five mutant enzymes. J. Clin. Endocrinol. Metab. 84(12), 4410–4425 (1999). https://doi.org/10.1210/jcem.84.12.6288
A.F. Turcu, R.J. Auchus, Adrenal steroidogenesis and congenital adrenal hyperplasia. Endocrinol. Metab. Clin. North Am. 44(2), 275–296 (2015). https://doi.org/10.1016/j.ecl.2015.02.002
S.F. Witchel, Congenital adrenal hyperplasia. J. Pediatr. Adolesc. Gynecol. 30(5), 520–534 (2017). https://doi.org/10.1016/j.jpag.2017.04.001
A.H. Payne, D.B. Hales, Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 25(6), 947–970 (2004). https://doi.org/10.1210/er.2003-0030
M.A. Burckhardt, S.S. Udhane, N. Marti, I. Schnyder, C. Tapia, J.E. Nielsen, P.E. Mullis, E. Rajpert-De Meyts, C.E. Fluck, Human 3beta-hydroxysteroid dehydrogenase deficiency seems to affect fertility but may not harbor a tumor risk: lesson from an experiment of nature. Eur. J. Endocrinol. 173(5), K1–K12 (2015). https://doi.org/10.1530/EJE-15-0599
L. Starka, M. Duskova, M. Hill, Dehydroepiandrosterone: a neuroactive steroid. J. Steroid Biochem Mol. Biol. 145, 254–260 (2015). https://doi.org/10.1016/j.jsbmb.2014.03.008
S. Sharp, E.V. Barker, M.W. Coughtrie, P.R. Lowenstein, R. Hume, Immunochemical characterisation of a dehydroepiandrosterone sulfotransferase in rats and humans. Eur. J. Biochem 211(3), 539–548 (1993)
C. Longcope, Dehydroepiandrosterone metabolism. J. Endocrinol. 150(Suppl), S125–127 (1996)
J. Simard, E. Rheaume, R. Sanchez, N. Laflamme, Y. de Launoit, V. Luu-The, A.P. van Seters, R.D. Gordon, M. Bettendorf, U. Heinrich et al.. Molecular basis of congenital adrenal hyperplasia due to 3 beta-hydroxysteroid dehydrogenase deficiency. Mol. Endocrinol. 7(5), 716–728 (1993). https://doi.org/10.1210/mend.7.5.8316254
S. Pang, Congenital adrenal hyperplasia owing to 3 beta-hydroxysteroid dehydrogenase deficiency. Endocrinol. Metab. Clin. North Am. 30(1), 81–99 (2001).
A.M. Bongiovanni, A.W. Root, The adrenogenital syndrome. N. Engl. J. Med 268, 1283–1289 (1963). https://doi.org/10.1056/NEJM196306062682308.
M. Doi, Y. Takahashi, R. Komatsu, F. Yamazaki, H. Yamada, S. Haraguchi, N. Emoto, Y. Okuno, G. Tsujimoto, A. Kanematsu, O. Ogawa, T. Todo, K. Tsutsui, G.T. van der Horst, H. Okamura, Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat. Med. 16(1), 67–74 (2010). https://doi.org/10.1038/nm.2061
M.M. Alswailem, O.S. Alzahrani, D.S. Alhomaidah, R. Alasmari, E. Qasem, A.K. Murugan, A. Alsagheir, I. Brema, B.B. Abbas, M. Almehthel, A. Almeqbali, A.S. Alzahrani, Mutational analysis of rare subtypes of congenital adrenal hyperplasia in a highly inbred population. Mol. Cell. Endocrinol. 461, 105–111 (2018). https://doi.org/10.1016/j.mce.2017.08.022
J. Simard, E. Rheaume, F. Mebarki, R. Sanchez, M.I. New, Y. Morel, F. Labrie, Molecular basis of human 3 beta-hydroxysteroid dehydrogenase deficiency. J. Steroid Biochem Mol. Biol. 53(1-6), 127–138 (1995)
L. Zhang, H. Sakkal-Alkaddour, Y.T. Chang, X. Yang, S. Pang, A new compound heterozygous frameshift mutation in the type II 3 beta-hydroxysteroid dehydrogenase (3 beta-HSD) gene causes salt-wasting 3 beta-HSD deficiency congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 81(1), 291–295 (1996). https://doi.org/10.1210/jcem.81.1.8550766
A. Nordenstrom, M.G. Forest, A. Wedell, A case of 3beta-hydroxysteroid dehydrogenase type II (HSD3B2) deficiency picked up by neonatal screening for 21-hydroxylase deficiency: difficulties and delay in etiologic diagnosis. Horm. Res. 68(4), 204–208 (2007). https://doi.org/10.1159/000102593
S. Pang, The molecular and clinical spectrum of 3beta-hydroxysteroid dehydrogenase deficiency disorder. Trends Endocrinol. Metab. 9(2), 82–86 (1998)
B.S. Bin-Abbas, N.A. Sakati, A.A. Al-Ashwal, Congenital adrenal hyperplasia due to 3 beta-hydroxysteroid dehydrogenase type II deficiency in 4 Saudi children. Long term follow up. Saudi Med J. 25(9), 1293–1295 (2004)
J. Simard, R. Sanchez, F. Durocher, E. Rheaume, C. Turgeon, Y. Labrie, V. Luu-The, F. Mebarki, Y. Morel, Y. de Launoit et al.. Structure-function relationships and molecular genetics of the 3 beta-hydroxysteroid dehydrogenase gene family. J. Steroid Biochem Mol. Biol. 55(5-6), 489–505 (1995)
S. Marui, A.J. Russell, F.J. Paula, I. Dick-de-Paula, J.A. Marcondes, B.B. Mendonca, Genotyping of the type II 3beta-hydroxysteroid dehydrogenase gene (HSD3B2) in women with hirsutism and elevated ACTH-stimulated delta(5)-steroids. Fertil. Steril. 74(3), 553–557 (2000)
M.C. Konar, S. Goswami, B.G. Babu, A.K. Mallick, Hypoglycemia due to 3beta-hydroxysteroid dehydrogenase type II deficiency in a newborn. Indian Pediatr. 52(11), 981–983 (2015)
T.H. Johannsen, D. Mallet, H. Dige-Petersen, J. Muller, K.M. Main, Y. Morel, M.G. Forest, Delayed diagnosis of congenital adrenal hyperplasia with salt wasting due to type II 3beta-hydroxysteroid dehydrogenase deficiency. J. Clin. Endocrinol. Metab. 90(4), 2076–2080 (2005). https://doi.org/10.1210/jc.2004-1374
B.B. Mendonca, W. Bloise, I.J. Arnhold, M.C. Batista, S.P. Toledo, M.C. Drummond, W. Nicolau, E. Mattar, Male pseudohermaphroditism due to nonsalt-losing 3 beta-hydroxysteroid dehydrogenase deficiency: gender role change and absence of gynecomastia at puberty. J. Steroid Biochem. 28(6), 669–675 (1987)
B.B. Mendonca, M.C. Batista, I.J. Arnhold, W. Nicolau, G. Madureira, V.S. Lando, M.B. Kohek, D.G. Carvalho, W. Bloise, Male pseudohermaphroditism due to 5 alpha reductase deficiency associated with gynecomastia. Rev. Hosp. Clin. Fac. Med. 42(2), 66–68 (1987)
R.L. Rushworth, D.J. Torpy, H. Falhammar, Adrenal crises: perspectives and research directions. Endocrine 55(2), 336–345 (2017). https://doi.org/10.1007/s12020-016-1204-2
R.L. Rushworth, D.J. Torpy, C.A. Stratakis, H. Falhammar, Adrenal crises in children: perspectives and research directions. Horm. Res. Paediatr. 89(5), 341–351 (2018). https://doi.org/10.1159/000481660
S.R. Bornstein, B. Allolio, W. Arlt, A. Barthel, A. Don-Wauchope, G.D. Hammer, E.S. Husebye, D.P. Merke, M.H. Murad, C.A. Stratakis, D.J. Torpy, Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 101(2), 364–389 (2016). https://doi.org/10.1210/jc.2015-1710
A. Kulle, N. Krone, P.M. Holterhus, G. Schuler, R.F. Greaves, A. Juul, Y.B. de Rijke, M.F. Hartmann, A. Saba, O. Hiort, S.A. Wudy, E.C. Action, Steroid hormone analysis in diagnosis and treatment of DSD: position paper of EU COST Action BM 1303 ‘DSDnet'. Eur. J. Endocrinol. 176(5), P1–P9 (2017). https://doi.org/10.1530/EJE-16-0953
C.E. Fluck, A.V. Pandey, Steroidogenesis of the testis—new genes and pathways. Ann. Endocrinol. 75(2), 40–47 (2014). https://doi.org/10.1016/j.ando.2014.03.002
M. Gortakowski, R. Conroy, L. Aguiar, H. Allen, Premature pubarche in a child with abnormal 3beta-hydroxysteroid dehydrogenase function and Klinefelter syndrome: the intriguing relationship between androgen deficiency and excess. Clin. Case Rep. 5(1), 57–60 (2017). https://doi.org/10.1002/ccr3.742
L.M. Mermejo, L.L. Elias, S. Marui, A.C. Moreira, B.B. Mendonca, M. de Castro, Refining hormonal diagnosis of type II 3beta-hydroxysteroid dehydrogenase deficiency in patients with premature pubarche and hirsutism based on HSD3B2 genotyping. J. Clin. Endocrinol. Metab. 90(3), 1287–1293 (2005). https://doi.org/10.1210/jc.2004-1552
S. Pang, G. Carbunaru, A. Haider, K.C. Copeland, Y.T. Chang, C. Lutfallah, J.I. Mason, Carriers for type II 3beta-hydroxysteroid dehydrogenase (HSD3B2) deficiency can only be identified by HSD3B2 genotype study and not by hormone test. Clin. Endocrinol. 58(3), 323–331 (2003)
J. Simard, E. Rheaume, J.F. Leblanc, S.C. Wallis, G.F. Joplin, S. Gilbey, J. Allanson, G. Mettler, M. Bettendorf, U. Heinrich et al.. Congenital adrenal hyperplasia caused by a novel homozygous frameshift mutation 273 delta AA in type II 3 beta-hydroxysteroid dehydrogenase gene (HSD3B2) in three male patients of Afghan/Pakistani origin. Human Mol. Genet. 3(2), 327–330 (1994)
E. Rheaume, R. Sanchez, F. Mebarki, E. Gagnon, J.C. Carel, J.L. Chaussain, Y. Morel, F. Labrie, J. Simard, Identification and characterization of the G15D mutation found in a male patient with 3 beta-hydroxysteroid dehydrogenase (3 beta-HSD) deficiency: alteration of the putative NAD-binding domain of type II 3 beta-HSD. Biochemistry 34(9), 2893–2900 (1995)
B.B. Mendonca, A.J. Russell, M. Vasconcelos-Leite, I.J. Arnhold, W. Bloise, B.L. Wajchenberg, W. Nicolau, R.G. Sutcliffe, A.M. Wallace, Mutation in 3 beta-hydroxysteroid dehydrogenase type II associated with pseudohermaphroditism in males and premature pubarche or cryptic expression in females. J. Mol. Endocrinol. 12(1), 119–122 (1994)
E. Rheaume, R. Sanchez, J. Simard, Y.T. Chang, J. Wang, S. Pang, F. Labrie, Molecular basis of congenital adrenal hyperplasia in two siblings with classical nonsalt-losing 3 beta-hydroxysteroid dehydrogenase deficiency. J. Clin. Endocrinol. Metab. 79(4), 1012–1018 (1994). https://doi.org/10.1210/jcem.79.4.7962268
A.J. Russell, A.M. Wallace, M.G. Forest, M.D. Donaldson, C.R. Edwards, R.G. Sutcliffe, Mutation in the human gene for 3 beta-hydroxysteroid dehydrogenase type II leading to male pseudohermaphroditism without salt loss. J. Mol. Endocrinol. 12(2), 225–237 (1994)
R. Sanchez, F. Mebarki, E. Rheaume, N. Laflamme, M.G. Forest, F. Bey-Omard, M. David, Y. Morel, F. Labrie, J. Simard, Functional characterization of the novel L108W and P186L mutations detected in the type II 3 beta-hydroxysteroid dehydrogenase gene of a male pseudohermaphrodite with congenital adrenal hyperplasia. Human Mol. Genet. 3(9), 1639–1645 (1994)
R. Sanchez, E. Rheaume, N. Laflamme, R.L. Rosenfield, F. Labrie, J. Simard, Detection and functional characterization of the novel missense mutation Y254D in type II 3 beta-hydroxysteroid dehydrogenase (3 beta HSD) gene of a female patient with nonsalt-losing 3 beta HSD deficiency. J. Clin. Endocrinol. Metab. 78(3), 561–567 (1994). https://doi.org/10.1210/jcem.78.3.8126127
F. Mebarki, R. Sanchez, E. Rheaume, N. Laflamme, J. Simard, M.G. Forest, F. Bey-Omar, M. David, F. Labrie, Y. Morel, Nonsalt-losing male pseudohermaphroditism due to the novel homozygous N100S mutation in the type II 3 beta-hydroxysteroid dehydrogenase gene. J. Clin. Endocrinol. Metab. 80(7), 2127–2134 (1995). https://doi.org/10.1210/jcem.80.7.7608265
T. Tajima, K. Fujieda, J. Nakae, N. Shinohara, M. Yoshimoto, T. Baba, E. Kinoshita, Y. Igarashi, T. Oomura, Molecular analysis of type II 3 beta-hydroxysteroid dehydrogenase gene in Japanese patients with classical 3 beta-hydroxysteroid dehydrogenase deficiency. Hum. Mol. Genet 4(5), 969–971 (1995)
S. Nayak, P.A. Lee, S.F. Witchel, Variants of the type II 3beta-hydroxysteroid dehydrogenase gene in children with premature pubic hair and hyperandrogenic adolescents. Mol. Genet Metab. 64(3), 184–192 (1998). https://doi.org/10.1006/mgme.1998.2715
S. Pang, W. Wang, B. Rich, R. David, Y.T. Chang, G. Carbunaru, S.E. Myers, A.F. Howie, K.J. Smillie, J.I. Mason, A novel nonstop mutation in the stop codon and a novel missense mutation in the type II 3beta-hydroxysteroid dehydrogenase (3beta-HSD) gene causing, respectively, nonclassic and classic 3beta-HSD deficiency congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 87(6), 2556–2563 (2002). https://doi.org/10.1210/jcem.87.6.8559
M. Welzel, N. Wustemann, G. Simic-Schleicher, H.G. Dorr, E. Schulze, G. Shaikh, P. Clayton, J. Grotzinger, P.M. Holterhus, F.G. Riepe, Carboxyl-terminal mutations in 3beta-hydroxysteroid dehydrogenase type II cause severe salt-wasting congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 93(4), 1418–1425 (2008). https://doi.org/10.1210/jc.2007-1874
B. Rabbani, N. Mahdieh, M.T. Haghi Ashtiani, A. Setoodeh, A. Rabbani, In silico structural, functional and pathogenicity evaluation of a novel mutation: an overview of HSD3B2 gene mutations. Gene 503(2), 215–221 (2012). https://doi.org/10.1016/j.gene.2012.04.080
Y. Huang, J. Zheng, T. Xie, Q. Xiao, S. Lu, X. Li, J. Cheng, L. Chen, L. Liu, [A novel homozygous mutation p.E25X in the HSD3B2 gene causing salt wasting 3beta-hydroxysteroid dehydrogenases deficiency in a Chinese pubertal girl: a delayed diagnosis until recurrent ovary cysts]. Zhonghua Er Ke Za Zhi 52(12), 948–951 (2014)
M.S. Baquedano, M. Ciaccio, R. Marino, N. Perez Garrido, P. Ramirez, M. Maceiras, A. Turjanski, L.A. Defelipe, M.A. Rivarola, A. Belgorosky, A novel missense mutation in the HSD3B2 gene, underlying nonsalt-wasting congenital adrenal hyperplasia. new insight into the structure-function relationships of 3beta-hydroxysteroid dehidrogenase type II. J. Clin. Endocrinol. Metab. 100(1), E191–E196 (2015). https://doi.org/10.1210/jc.2014-2676
A. Guven, S. Polat, Testicular Adrenal rest tumor in two brothers with a novel mutation in the 3-beta-hydroxysteroid dehydrogenase-2 gene. J. Clin. Res Pediatr. Endocrinol. 9(1), 85–90 (2017). https://doi.org/10.4274/jcrpe.3306
S.L. Teasdale, A. Morton, Adrenarche unmasks compound heterozygous 3beta-hydroxysteroid dehydrogenase deficiency: c.244G>A (p.Ala82Thr) and the novel 931C>T (p.Gln311*) variant in a non-salt wasting, severely undervirilised 46XY. J. Pediatr. Endocrinol. Metab. 30(3), 355–360 (2017). https://doi.org/10.1515/jpem-2016-0348
V. G. Araújo, R. S. Oliveira, K. P. Gameleira, C. B. Cruz, A. Lofrano-Porto, 3β-hydroxysteroid dehydrogenase type II deficiency on newborn screening test. Arq. Bras. Endocrinol. Metab. 58(6), 650–655 (2014). https://doi.org/10.1590/0004-2730000003098
C. Bizzarri, N. Olivini, S. Pedicelli, R. Marini, G. Giannone, P. Cambiaso, M. Cappa, Congenital primary adrenal insufficiency and selective aldosterone defects presenting as salt-wasting in infancy: a single center 10-year experience. Ital. J. Pediatr. 42(1), 73 (2016). https://doi.org/10.1186/s13052-016-0282-3
R. Podgorski, D. Aebisher, M. Stompor, D. Podgorska, A. Mazur, Congenital adrenal hyperplasia: clinical symptoms and diagnostic methods. Acta Biochim. Pol. 65(1), 25–33 (2018). https://doi.org/10.18388/abp.2017_2343
H. Falhammar, A. Wedell, A. Nordenstrom, Biochemical and genetic diagnosis of 21-hydroxylase deficiency. Endocrine 50(2), 306–314 (2015). https://doi.org/10.1007/s12020-015-0731-6
F. Gonzalez, D.A. Hatala, L. Speroff, Adrenal and ovarian steroid hormone responses to gonadotropin-releasing hormone agonist treatment in polycystic ovary syndrome. Am. J. Obstet. Gynecol. 165(3), 535–545 (1991)
H. Falhammar, A. Nordenstrom, Nonclassic congenital adrenal hyperplasia due to 21-hydroxylase deficiency: clinical presentation, diagnosis, treatment, and outcome. Endocrine 50(1), 32–50 (2015). https://doi.org/10.1007/s12020-015-0656-0
F. Hannah-Shmouni, R. Morissette, N. Sinaii, M. Elman, T.R. Prezant, W. Chen, A. Pulver, D.P. Merke, Revisiting the prevalence of nonclassic congenital adrenal hyperplasia in US Ashkenazi Jews and Caucasians. Genet Med 19(11), 1276–1279 (2017). https://doi.org/10.1038/gim.2017.46
P.A. Parks, G.A. Bermudez, C.S. Anast, A.M. Bongiovanni, M.I. New, Pubertal boy with the 3β-hydroxy steroid dehydrogenase defect. J. Clin. Endocrinol. Metab. 33(2), 269–278 (1971). https://doi.org/10.1210/jcem-33-2-269
O. Janne, J. Perheentupa, L. Viinikka, R. Vihko, Testicular endocrine function in a pubertal boy with 3beta-hydroxysteroid dehydrogenase deficiency. J. Clin. Endocrinol. Metab. 39(1), 206–209 (1974). https://doi.org/10.1210/jcem-39-1-206
Y.T. Chang, H.E. Kulin, L. Garibaldi, M.J. Suriano, K. Bracki, S. Pang, Hypothalamic-pituitary-gonadal axis function in pubertal male and female siblings with glucocorticoid-treated nonsalt-wasting 3 beta-hydroxysteroid dehydrogenase deficiency congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 77(5), 1251–1257 (1993). https://doi.org/10.1210/jcem.77.5.8077318
E. Lolis, C.C. Juhlin, A. Nordenstrom, H. Falhammar, Extensive bilateral adrenal rest testicular tumors in a patient with 3beta-hydroxysteroid dehydrogenase type 2 deficiency. J. Endocr. Soc. 2(6), 513–517 (2018). https://doi.org/10.1210/js.2018-00082
M. Zachmann, M.G. Forest, E. De Peretti, 3 beta-hydroxysteroid dehydrogenase deficiency follow—study a girl pubertal bone age. Horm. Res. 11(6), 292–302 (1979). https://doi.org/10.1159/000179067
M. Yoshimoto, T. Kawaguchi, R. Mori, E. Kinoshita, T. Baba, T. Tajima, K. Fujieda, T. Suzuki, H. Sasano, Pubertal changes in testicular 3 beta-hydroxysteroid dehydrogenase activity in a male with classical 3 beta-hydroxysteroid dehydrogenase deficiency showing spontaneous secondary sexual maturation. Horm. Res. 48(2), 83–87 (1997). https://doi.org/10.1159/000185492
G. Schneider, M. Genel, A.M. Bongiovanni, A.S. Goldman, R.L. Rosenfield, Persistent testicular delta5-isomerase-3beta-hydroxysteroid dehydrogenase (delta5-3beta-HSD) deficiency in the delta5-3beta-HSD form of congenital adrenal hyperplasia. J. Clin. Invest. 55(4), 681–690 (1975). https://doi.org/10.1172/JCI107977
J. Jaaskelainen, M. Hippelainen, O. Kiekara, R. Voutilainen, Child rate, pregnancy outcome and ovarian function in females with classical 21-hydroxylase deficiency. Acta Obstet. Gynecol. Scand. 79(8), 687–692 (2000)
F. Gastaud, C. Bouvattier, L. Duranteau, R. Brauner, E. Thibaud, F. Kutten, P. Bougneres, Impaired sexual and reproductive outcomes in women with classical forms of congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 92(4), 1391–1396 (2007). https://doi.org/10.1210/jc.2006-1757
K. Hagenfeldt, P.O. Janson, G. Holmdahl, H. Falhammar, H. Filipsson, L. Frisen, M. Thoren, A. Nordenskjold, Fertility and pregnancy outcome in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hum. Reprod. 23(7), 1607–1613 (2008). https://doi.org/10.1093/humrep/den118.
H. Falhammar, H.F. Nystrom, U. Ekstrom, S. Granberg, A. Wedell, M. Thoren, Fertility, sexuality and testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia. Eur. J. Endocrinol. 166(3), 441–449 (2012). https://doi.org/10.1530/EJE-11-0828
D.E. Reichman, P.C. White, M.I. New, Z. Rosenwaks, Fertility in patients with congenital adrenal hyperplasia. Fertil. Steril. 101(2), 301–309 (2014). https://doi.org/10.1016/j.fertnstert.2013.11.002
A. Strandqvist, H. Falhammar, P. Lichtenstein, A.L. Hirschberg, A. Wedell, C. Norrby, A. Nordenskjold, L. Frisen, A. Nordenstrom, Suboptimal psychosocial outcomes in patients with congenital adrenal hyperplasia: epidemiological studies in a nonbiased national cohort in Sweden. J. Clin. Endocrinol. Metab. 99(4), 1425–1432 (2014). https://doi.org/10.1210/jc.2013-3326
C. Bouvattier, L. Esterle, P. Renoult-Pierre, A.B. de la Perriere, F. Illouz, V. Kerlan, V. Pascal-Vigneron, D. Drui, S. Christin-Maitre, F. Galland, T. Brue, Y. Reznik, F. Schillo, D. Pinsard, X. Piguel, G. Chabrier, B. Decoudier, P. Emy, I. Tauveron, M.L. Raffin-Sanson, J. Bertherat, J.M. Kuhn, P. Caron, M. Cartigny, O. Chabre, D. Dewailly, Y. Morel, P. Touraine, V. Tardy-Guidollet, J. Young, Clinical outcome, hormonal status, gonadotrope axis, and testicular function in 219 adult men born with classic 21-hydroxylase deficiency. A French National Survey. J. Clin. Endocrinol. Metab. 100(6), 2303–2313 (2015). https://doi.org/10.1210/jc.2014-4124
H. Falhammar, L. Frisen, C. Norrby, C. Almqvist, A.L. Hirschberg, A. Nordenskjold, A. Nordenstrom, Reduced frequency of biological and increased frequency of adopted children in males with 21-hydroxylase deficiency: a Swedish Population-Based National Cohort Study. J. Clin. Endocrinol. Metab. 102(11), 4191–4199 (2017). https://doi.org/10.1210/jc.2017-01139
H.L. Claahsen-van der Grinten, K. Duthoi, B.J. Otten, F.C. d'Ancona, C.A. Hulsbergen-vd Kaa, A.R. Hermus, An adrenal rest tumour in the perirenal region in a patient with congenital adrenal hyperplasia due to congenital 3beta-hydroxysteroid dehydrogenase deficiency. Eur. J. Endocrinol. 159(4), 489–491 (2008). https://doi.org/10.1530/EJE-08-0311
N.M. Stikkelbroeck, B.J. Otten, A. Pasic, G.J. Jager, C.G. Sweep, K. Noordam, A.R. Hermus, High prevalence of testicular adrenal rest tumors, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 86(12), 5721–5728 (2001)
M. Engels, K. Gehrmann, H. Falhammar, E.A. Webb, A. Nordenstrom, F.C. Sweep, P.N. Span, A.E. van Herwaarden, J. Rohayem, A. Richter-Unruh, C. Bouvattier, B. Kohler, B.B. Kortmann, W. Arlt, N. Roeleveld, N. Reisch, N. Stikkelbroeck, H.L. Claahsen-van der Grinten, Lg dsd, Gonadal function in adult male patients with congenital adrenal hyperplasia. Eur. J. Endocrinol. 178(3), 285–294 (2018). https://doi.org/10.1530/EJE-17-0862
H.L. Claahsen-van der Grinten, B.J. Otten, M.M. Stikkelbroeck, F.C. Sweep, A.R. Hermus, Testicular adrenal rest tumours in congenital adrenal hyperplasia. Best. Pract. Res. Clin. Endocrinol. Metab. 23(2), 209–220 (2009). https://doi.org/10.1016/j.beem.2008.09.007.
G. Mazziotti, A.M. Formenti, S. Frara, E. Roca, P. Mortini, A. Berruti, A. Giustina, MANAGEMENT OF ENDOCRINE DISEASE: Risk of overtreatment in patients with adrenal insufficiency: current and emerging aspects. Eur. J. Endocrinol. 177(5), R231–R248 (2017). https://doi.org/10.1530/EJE-17-0154
H. Falhammar, Skeletal fragility induced by overtreatment of adrenal insufficiency. Endocrine 59(2), 239–241 (2018). https://doi.org/10.1007/s12020-017-1501-4
H. Falhammar, H. Filipsson, G. Holmdahl, P.O. Janson, A. Nordenskjold, K. Hagenfeldt, M. Thoren, Fractures and bone mineral density in adult women with 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 92(12), 4643–4649 (2007). https://doi.org/10.1210/jc.2007-0744.
G.P. Finkielstain, M.S. Kim, N. Sinaii, M. Nishitani, C. Van Ryzin, S.C. Hill, J.C. Reynolds, R.M. Hanna, D.P. Merke, Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 97(12), 4429–4438 (2012). https://doi.org/10.1210/jc.2012-2102
K.R. Koetz, M. Ventz, S. Diederich, M. Quinkler, Bone mineral density is not significantly reduced in adult patients on low-dose glucocorticoid replacement therapy. J. Clin. Endocrinol. Metab. 97(1), 85–92 (2012). https://doi.org/10.1210/jc.2011-2036.
I. Nermoen, I. Bronstad, K.J. Fougner, J. Svartberg, M. Oksnes, E.S. Husebye, K. Lovas, Genetic, anthropometric and metabolic features of adult Norwegian patients with 21-hydroxylase deficiency. Eur. J. Endocrinol. / Eur. Fed. Endocr. Soc. 167(4), 507–516 (2012). https://doi.org/10.1530/EJE-12-0196.
H. Falhammar, H. Filipsson Nystrom, A. Wedell, K. Brismar, M. Thoren, Bone mineral density, bone markers, and fractures in adult males with congenital adrenal hyperplasia. Eur. J. Endocrinol. 168(3), 331–341 (2013). https://doi.org/10.1530/EJE-12-0865
A. Bachelot, J.L. Golmard, J. Dulon, N. Dahmoune, M. Leban, C. Bouvattier, S. Cabrol, J. Leger, M. Polak, P. Touraine, Determining clinical and biological indicators for health outcomes in adult patients with childhood onset of congenital adrenal hyperplasia. Eur. J. Endocrinol. 173(2), 175–184 (2015). https://doi.org/10.1530/EJE-14-0978
D. El-Maouche, S. Collier, M. Prasad, J.C. Reynolds, D.P. Merke, Cortical bone mineral density in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin. Endocrinol. 82(3), 330–337 (2015). https://doi.org/10.1111/cen.12507
F. Ceccato, M. Barbot, N. Albiger, M. Zilio, P. De Toni, G. Luisetto, M. Zaninotto, N.A. Greggio, M. Boscaro, C. Scaroni, V. Camozzi, Long-term glucocorticoid effect on bone mineral density in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur. J. Endocrinol. 175(2), 101–106 (2016). https://doi.org/10.1530/EJE-16-0104
N.M. Stikkelbroeck, W.J. Oyen, G.J. van der Wilt, A.R. Hermus, B.J. Otten, Normal bone mineral density and lean body mass, but increased fat mass, in young adult patients with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 88(3), 1036–1042 (2003)
P. Christiansen, C. Molgaard, J. Muller, Normal bone mineral content in young adults with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm. Res. 61(3), 133–136 (2004). https://doi.org/10.1159/000075588.
H. Falhammar, H. Claahsen-van der Grinten, N. Reisch, J. Slowikowska-Hilczer, A. Nordenstrom, R. Roehle, C. Bouvattier, B.P.C. Kreukels, B. Kohler, Lg dsd, Health status in 1040 adults with disorders of sex development (DSD): a European multicenter study. Endocr. Connect. 7(3), 466–478 (2018). https://doi.org/10.1530/EC-18-0031
J. Jaaskelainen, R. Voutilainen, Bone mineral density in relation to glucocorticoid substitution therapy in adult patients with 21-hydroxylase deficiency. Clin. Endocrinol. 45(6), 707–713 (1996)
K.R. Frey, T. Kienitz, J. Schulz, M. Ventz, K. Zopf, M. Quinkler, Prednisolone is associated with a worse bone mineral density in primary adrenal insufficiency. Endocr. Connect. 7(6), 811–818 (2018). https://doi.org/10.1530/EC-18-0160
K. Hagenfeldt, E. Martin Ritzen, H. Ringertz, J. Helleday, K. Carlstrom, Bone mass and body composition of adult women with congenital virilizing 21-hydroxylase deficiency after glucocorticoid treatment since infancy. Eur. J. Endocrinol. 143(5), 667–671 (2000). 1430667 [pii]
A. Halper, B. Sanchez, J.S. Hodges, A.S. Kelly, D. Dengel, B.M. Nathan, A. Petryk, K. Sarafoglou, Bone mineral density and body composition in children with congenital adrenal hyperplasia. Clin. Endocrinol. 88(6), 813–819 (2018). https://doi.org/10.1111/cen.13580
H. Falhammar, H. Filipsson Nystrom, A. Wedell, M. Thoren, Cardiovascular risk, metabolic profile, and body composition in adult males with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur. J. Endocrinol. 164(2), 285–293 (2011). https://doi.org/10.1530/EJE-10-0877.
H. Falhammar, H. Filipsson, G. Holmdahl, P.O. Janson, A. Nordenskjold, K. Hagenfeldt, M. Thoren, Metabolic profile and body composition in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 92(1), 110–116 (2007). https://doi.org/10.1210/jc.2006-1350.
H. Falhammar, H. Filipsson, G. Holmdahl, P.O. Janson, A. Nordenskjold, K. Hagenfeldt, M. Thoren, Increased liver enzymes in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr. J. 56(4), 601–608 (2009).
M.F. Mnif, M. Kamoun, F. Mnif, N. Charfi, N. Kallel, B. Ben Naceur, N. Rekik, Z. Mnif, M.H. Sfar, M.T. Sfar, M. Hachicha, L.A. Keskes, M. Abid, Long-term outcome of patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Am. J. Med. Sci. 344(5), 363–373 (2012). https://doi.org/10.1097/MAJ.0b013e31824369e4
W. Bonfig, F.W. Roehl, S. Riedl, H.G. Dorr, M. Bettendorf, J. Bramswig, E. Schonau, F. Riepe, B. Hauffa, R.W. Holl, K. Mohnike, A.C.S. Group, Blood pressure in a large cohort of children and adolescents with classic adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency. Am. J. Hypertens. 29(2), 266–272 (2016). https://doi.org/10.1093/ajh/hpv087
C.F. Mooij, A.E. van Herwaarden, F. Sweep, N. Roeleveld, C.L. de Korte, L. Kapusta, H.L. Claahsen-van der Grinten, Cardiovascular and metabolic risk in pediatric patients with congenital adrenal hyperplasia due to 21 hydroxylase deficiency. J. Pediatr. Endocrinol. Metab. 30(9), 957–966 (2017). https://doi.org/10.1515/jpem-2017-0068
R. Ozdemir, H.A. Korkmaz, M. Kucuk, C. Karadeniz, T. Mese, B. Ozkan, Assessment of early atherosclerosis and left ventricular dysfunction in children with 21-hydroxylase deficiency. Clin. Endocrinol. 86(4), 473–479 (2017). https://doi.org/10.1111/cen.13275
C.F. Mooij, M.S. Pourier, G. Weijers, C.L. de Korte, Z. Fejzic, H.L. Claahsen-van der Grinten, L. Kapusta, Cardiac function in paediatric patients with congenital adrenal hyperplasia due to 21 hydroxylase deficiency. Clin. Endocrinol. 88(3), 364–371 (2018). https://doi.org/10.1111/cen.13529
D. Rosenbaum, A. Gallo, G. Lethielleux, E. Bruckert, B.I. Levy, M. L. Tanguy, J. Dulon, N. Dahmoune, J.E. Salem, R. Bittar, M. Leban, X. Girerd, P. Touraine, A. Bachelot, CARDIOHCS study group, Early central blood pressure elevation in adult patients with 21-hydroxylase deficiency. J. Hypertens. (2018). https://doi.org/10.1097/HJH.0000000000001850
S. Tamhane, R. Rodriguez-Gutierrez, A.M. Iqbal, L.J. Prokop, I. Bancos, P.W. Speiser, M.H. Murad, cardiovascular and metabolic outcomes in congenital adrenal hyperplasia: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 103(11), 4097–4103 (2018). https://doi.org/10.1210/jc.2018-01862
H. Falhammar, L. Frisen, A.L. Hirschberg, C. Norrby, C. Almqvist, A. Nordenskjold, A. Nordenstrom, Increased cardiovascular and metabolic morbidity in patients with 21-hydroxylase deficiency: a Swedish Population-Based National Cohort Study. J. Clin. Endocrinol. Metab. 100(9), 3520–3528 (2015). https://doi.org/10.1210/JC.2015-2093
E. Daae, K.B. Feragen, I. Nermoen, H. Falhammar, Psychological adjustment, quality of life, and self-perceptions of reproductive health in males with congenital adrenal hyperplasia: a systematic review. Endocrine 62(1), 3–13 (2018). https://doi.org/10.1007/s12020-018-1723-0
H. Falhammar, A. Butwicka, M. Landen, P. Lichtenstein, A. Nordenskjold, A. Nordenstrom, L. Frisen, Increased psychiatric morbidity in men with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 99(3), E554–E560 (2014). https://doi.org/10.1210/jc.2013-3707
H. Engberg, A. Butwicka, A. Nordenstrom, A.L. Hirschberg, H. Falhammar, P. Lichtenstein, A. Nordenskjold, L. Frisen, M. Landen, Congenital adrenal hyperplasia and risk for psychiatric disorders in girls and women born between 1915 and 2010: a total population study. Psychoneuroendocrinology 60, 195–205 (2015). https://doi.org/10.1016/j.psyneuen.2015.06.017
S. Jenkins-Jones, L. Parviainen, J. Porter, M. Withe, M.J. Whitaker, S.E. Holden, C.L. Morgan, C.J. Currie, R.J.M. Ross, Poor compliance and increased mortality, depression and healthcare costs in patients with congenital adrenal hyperplasia. Eur. J. Endocrinol. 178(4), 309–320 (2018). https://doi.org/10.1530/EJE-17-0895
H. Selye, H. Stone, Hormonally induced transformation of adrenal into myeloid tissue. Am. J. Pathol. 26(2), 22 (1950)
S. Jaresch, E. Kornely, H.K. Kley, R. Schlaghecke, Adrenal incidentaloma and patients with homozygous or heterozygous congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 74(3), 685–689 (1992). https://doi.org/10.1210/jcem.74.3.1311000
H. Falhammar, Non-functioning adrenal incidentalomas caused by 21-hydroxylase deficiency or carrier status? Endocrine 47(1), 308–314 (2014). https://doi.org/10.1007/s12020-013-0162-1
N. Reisch, M. Scherr, L. Flade, M. Bidlingmaier, H.P. Schwarz, U. Muller-Lisse, M. Reincke, M. Quinkler, F. Beuschlein, Total adrenal volume but not testicular adrenal rest tumor volume is associated with hormonal control in patients with 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 95(5), 2065–2072 (2010). https://doi.org/10.1210/jc.2009-1929
I. Nermoen, J. Rorvik, S.H. Holmedal, D.L. Hykkerud, K.J. Fougner, J. Svartberg, E.S. Husebye, K. Lovas, High frequency of adrenal myelolipomas and testicular adrenal rest tumours in adult Norwegian patients with classical congenital adrenal hyperplasia because of 21-hydroxylase deficiency. Clin. Endocrinol. 75(6), 753–759 (2011). https://doi.org/10.1111/j.1365-2265.2011.04151.x
J. Patrova, M. Kjellman, H. Wahrenberg, H. Falhammar, Increased mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: a 13-year retrospective study from one center. Endocrine 58(2), 267–275 (2017). https://doi.org/10.1007/s12020-017-1400-8
L. Barzon, C. Scaroni, N. Sonino, F. Fallo, M. Gregianin, C. Macri, M. Boscaro, Incidentally discovered adrenal tumors: endocrine and scintigraphic correlates. J. Clin. Endocrinol. Metab. 83(1), 55–62 (1998)
A. Patocs, M. Toth, C. Barta, M. Sasvari-Szekely, I. Varga, N. Szucs, C. Jakab, E. Glaz, K. Racz, Hormonal evaluation and mutation screening for steroid 21-hydroxylase deficiency in patients with unilateral and bilateral adrenal incidentalomas. Eur. J. Endocrinol. 147(3), 349–355 (2002).
H. Falhammar, M. Thoren, An 88-year-old woman diagnosed with adrenal tumor and congenital adrenal hyperplasia: connection or coincidence? J. Endocrinol. Investig. 28(5), 449–453 (2005).
J. Patrova, I. Jarocka, H. Wahrenberg, H. Falhammar, Clinical outcomes in adrenal incidentaloma: experience from one center. Endocr. Pract. 21(8), 870–877 (2015). https://doi.org/10.4158/EP15618.OR
H. Falhammar, D.J. Torpy, A 42-year-old man presented with adrenal incidentaloma due to non-classic congenital adrenal hyperplasia with a novel CYP21A2 mutation. Intern. Med. J. 46(9), 1115–1116 (2016). https://doi.org/10.1111/imj.13177
H. Falhammar, D.J. Torpy, Congenital adrenal hyperplasia due to 21-hydroxylase deficiency presenting as adrenal incidentaloma: a systematic review and meta-analysis. Endocr. Pract. 22(6), 736–752 (2016). https://doi.org/10.4158/EP151085.RA
H. Falhammar, L. Frisen, C. Norrby, A.L. Hirschberg, C. Almqvist, A. Nordenskjold, A. Nordenstrom, Increased mortality in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 99(12), E2715–E2721 (2014). https://doi.org/10.1210/jc.2014-2957
A. Nordenskjold, G. Holmdahl, L. Frisen, H. Falhammar, H. Filipsson, M. Thoren, P.O. Janson, K. Hagenfeldt, Type of mutation and surgical procedure affect long-term quality of life for women with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 93(2), 380–386 (2008). https://doi.org/10.1210/jc.2007-0556.
J. Almasri, F. Zaiem, R. Rodriguez-Gutierrez, S.U. Tamhane, A.M. Iqbal, L.J. Prokop, P.W. Speiser, L.S. Baskin, I. Bancos, M.H. Murad, Genital reconstructive surgery in females with congenital adrenal hyperplasia: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 103(11), 4089–4096 (2018). https://doi.org/10.1210/jc.2018-01863
A.R. Fernandez-Aristi, A.A. Taco-Masias, L. Montesinos-Baca, Case report: Clitoromegaly as a consequence of Congenital Adrenal Hyperplasia. An accurate medical and surgical approach. Urol. Case Rep. 18, 57–59 (2018). https://doi.org/10.1016/j.eucr.2018.02.011
D. MacKay, A. Nordenstrom, H. Falhammar, Bilateral adrenalectomy in congenital adrenal hyperplasia: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. (2018). https://doi.org/10.1210/jc.2018-00217
Funding
This study was funded by Magnus Bergvall Foundation (Grant Number 2017-02138).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Al Alawi, A.M., Nordenström, A. & Falhammar, H. Clinical perspectives in congenital adrenal hyperplasia due to 3β-hydroxysteroid dehydrogenase type 2 deficiency. Endocrine 63, 407–421 (2019). https://doi.org/10.1007/s12020-018-01835-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-01835-3