Abstract
Matricellular proteins comprise several families of secreted proteins that function in higher animals at the interface between cells and their surrounding extracellular matrix. Targeted gene disruptions that result in loss of viability in mice have revealed critical roles for several matricellular proteins in murine embryonic development, including two members of the cellular communication network (CCN) gene family. In contrast, mice lacking single or multiple members of the thrombospondin (THBS) gene family remain viable and fertile. The frequency of loss of function mutants, identified using human deep exome sequencing data, provided evidence that some of the essential genes in mice, including Ccn1, are also essential genes in humans. However, a deficit in loss of function mutants in humans indicated that THBS1 is also highly loss-intolerant. In addition to roles in embryonic development or adult reproduction, genes may be loss-intolerant in humans because their function is needed to survive environmental stresses that are encountered between birth and reproduction. Laboratory mice live in a protected environment that lacks the exposures to pathogens and injury that humans routinely face. However, subjecting Thbs1−/− mice to defined stresses has provided valuable insights into functions of thrombospondin-1 that could account for the loss-intolerance of THBS1 in humans.
Graphical Abstract

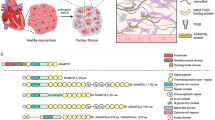
Stress response models using transgenic mice have identified protective functions of thrombospondin-1 in the cardiovascular system (red) and immune defenses (blue) that could account for its intolerance to loss of function mutants in humans
Similar content being viewed by others
Introduction
Several families of secreted proteins have been identified in higher animals that regulate cell behavior by interacting with structural components of the extracellular matrix, secreted cytokines and proteases, and specific cell surface receptors. These are collectively known as matricellular proteins (Bornstein 1995; Murphy-Ullrich and Sage 2014). Matricellular proteins include members of the thrombospondin, tenascin, secreted protein acidic and rich in cysteine (SPARC), small integrin-binding ligand N-linked glycoproteins (SIBLING), and cellular communication network (CCN) gene families (Murphy-Ullrich and Sage 2014; Leask 2020).
Mutations have been identified in matricellular protein genes including CCN6, SPARC, SMOC1, SPOCK1, TNXB, DMP1, DSPP, THBS1, THBS2, and COMP that cause inherited genetic disorders in humans or are linked to increased disease risk (Hurvitz et al. 1999; Topol et al. 2001; Bristow et al. 2005; Stenina et al. 2007; Burke et al. 2009; Abouzeid et al. 2011; Okada et al. 2011; Rainger et al. 2011; Staines et al. 2012; Dhamija et al. 2014; Mendoza-Londono et al. 2015; Posey et al. 2018). Insights into whether any matricellular protein genes are essential in mammals have been gained by studying targeted gene knockouts in mice. These studies demonstrated that CCN1, CCN2, and SMOC1 are essential for viability (Mo et al. 2002; Ivkovic et al. 2003; Mo and Lau 2006; Okada et al. 2011). In contrast, the other homozygous null mice reported to date are viable and fertile (Jones and Jones 2000; Svensson et al. 2002; Hankenson et al. 2005a, 2005b; Kutz et al. 2005; Bradshaw 2009; Midwood and Orend 2009; Canalis et al. 2010; Bouleftour et al. 2016).
Are essential matricellular genes in mice also essential in humans?
The role of specific genes in human viability cannot be directly addressed, but many recessive genes involved in lethal genetic diseases in humans impair viability when disrupted in mice. Conversely, loss of viability in mice may be predictive of an essential function in humans (Dawes et al. 2019). Two of the matricellular protein genes known to be essential for mouse viability are members of the CCN family (Mo et al. 2002; Ivkovic et al. 2003; Mo and Lau 2006). We recently examined the frequency of LoF mutations for CCN family members in The Genome Aggregation Database (gnomAD, v2.1.1), which includes 125,748 deep-sequenced exomes and 15,701 full genome sequences from unrelated individuals with no known severe pediatric genetic disease risk (Karczewski et al. 2020). Over our lifespan, germinal DNA is subject to random mutations caused by background ionizing radiation and oxidative stress. Although epigenetic modifications may partially protect some essential genes from such random mutations (Zhang 2022), the expected number of LoF mutations is proportional to the length of the open reading frame (Lek et al. 2016). Based on the length of the CCN1 open reading frame, 17.2 LoF mutants were expected among the 141,456 individuals in gnomAD, but only 3 LoF mutants were observed. Therefore, CCN1 had a significantly elevated probability of LoF intolerance (pLI = 0.71) (Kaur and Roberts 2021). This is consistent with the essential function of Ccn1 in mouse prenatal development (Mo et al. 2002; Mo and Lau 2006) and establishes that mutation frequency data can be informative for evaluating loss-intolerance for matricellular protein genes in a human population.
Consistent with its essential function in mice (Ivkovic et al. 2003), the 7 observed CCN2 LoF mutants in gnomAD were less than the predicted 12.4 LoF mutants, but this deficit was not sufficient to yield a significant pLI. Because the relatively short coding sequences of CCN genes limits interpretation of LoF mutants, exome data from a larger population will be needed confirm or exclude intolerance to LoF for CCN members other than CCN1. We also examined the frequency of missense mutations in the CCN family and found the largest deficits in CCN1 and CCN2 (Kaur and Roberts 2021), but again data from additional individuals would be required to confirm significance.
Thbs1, a nonessential gene in mice that is intolerant to LoF in humans
Several matricellular genes that are not essential for viability in mice had significant pLI values in the gnomAD data, including THBS1, THBS2, SPARC, SPOCK1, and TNR (Kaur and Roberts 2021). Thbs1−/− mice are viable, fertile, and appeared healthy except for lung inflammation (Lawler et al. 1998). Because Thbs1 belongs to a family of 5 genes, essential roles for Thbs1 could be masked by compensatory induction of other members in its gene family in Thbs1−/− mice. However, such compensation is unlikely because double and triple knockout combinations with Thbs2, Thbs3, and Comp inactivation were also viable (Agah et al. 2002; Posey et al. 2008), In addition to compensation by paralogous genes, gene essentiality is quantitatively limited by cellular evolvability, wherein adaptive evolution activates compensatory mechanisms to bypass loss of an essential gene function (Liu et al. 2015).
In contrast to the Thbs1−/− mouse data, significant deficits in the rates of missense and predicted LoF nonsense mutations in THBS1 were found in the human gnomAD dataset (v2.1.1). Compared to the 56 expected LoF mutants, only 7 were observed, and the pLI for THBS1 was 1.00, indicating this gene to be highly loss-intolerant (Kaur and Roberts 2021). In addition, none of the 7 individuals with THBS1 LoF mutant alleles were homozygotes. The original report that mice with homozygous LoF mutants in Thbs1 are viable and fertile excluded essential roles for this gene in embryonic development or reproduction (Lawler et al. 1998). Subsequent studies identified a role for Thbs1 in ovarian follicular development and ovulation in mice and primates (McGray et al. 2011; Bender et al. 2019), but the limited effect on fertility is unlikely to account for the high pLI in humans. THBS1 also had a significant deficiency in missense mutations (516 observed versus 721 expected, Z score = 2.72) (Kaur and Roberts 2021). However, the distribution of missense mutations in THBS1 did not clearly identify specific residues or regions of the protein that mediate an essential function, apart from variants previously identified through genome-wide association studies linking a polymorphism in THBS1 with early myocardial infarction and altered calcium binding (Topol et al. 2001; Carlson et al. 2008).
Insights into protective functions of Thbs1 in mice
Aside from critical roles in embryonic development and adult reproduction, selective pressures against gene LoF can arise from any function that decreases the probability that an individual will survive long enough to successfully reproduce. Several relevant functions for matricellular proteins in postnatal survival have been revealed when adult knockout mice were subjected to specific stresses (Roberts et al. 2012; Calabro et al. 2014; Murphy-Ullrich and Sage 2014; Soto-Pantoja et al. 2015; Kim et al. 2018; Stenina-Adognravi and Plow 2019).
The diverse biological functions of thrombospondin-1 in regulation of angiogenesis, vascular homeostasis, connective tissue organization, responses to injury, synaptogenesis, and immune responses are mediated by its interactions with multiple receptors and extracellular ligands (Murphy-Ullrich 2019; Kaur et al. 2021). Thus, studies using knockout mice can also be informative to identify which receptors or ligands mediate specific functions. CD36 and CD47 are cell surface receptors for thrombospondin-1. the resistance of Thbs1−/− mice to injuries caused by ischemia is phenocopied by Cd47−/− but not by Cd36−/− mice (Isenberg et al. 2007b). Correspondingly, LoF mutants in human CD36 are relatively common (Yanai et al. 2000), whereas CD47 was highly intolerant to LoF in the gnomAD data (pLI = 0.92) (Kaur and Roberts 2021), Additional receptors and interaction partners of thrombospondin-1 that share its elevated pLI in humans include stromal interaction molecule (STIM1, pLI = 0.78), loss of which is perinatal lethal in mice (Varga-Szabo et al. 2008), and low density lipoprotein receptor-related protein 1 (LRP1, pLI = 1.0), loss of which is embryonic lethal in mice (Herz et al. 1992). However, interaction partners other than thrombospondin-1 may also contribute to the essential functions of these receptors.
Studies subjecting Thbs1−/− mice and Cd47−/− mice to defined stresses have identified several protective functions of CD47-dependent thrombospondin-1 signaling that could improve postnatal survival. These functions broadly involve enhancing the ability of the cardiovascular system to respond to acute injuries or supporting the immune system in responding to infectious diseases (Fig. 1).
Thrombospondin-1 functions that may select against LoF mutants in humans. Hemostasis and maintenance of central blood pressure are essential for survival of acute injuries. Both processes are regulated by CD47-dependent thrombospondin-1 signaling, which inhibits the biosynthesis of nitric oxide (NO) by endothelial nitric oxide synthase (eNOS), the activation of soluble guanylate cyclase (sGC) by NO, and the downstream signaling mediated by cGMP-dependent protein kinases in vascular smooth muscle cells and platelets. Thrombospondin-1 also enhances platelet function by preventing the proteolytic inactivation of von Willebrand factor by ADAMTS13. Immune defenses against viral, bacterial, and fungal pathogens are also critical for survival to reproductive age. Thrombospondin-1 regulates innate and adaptive immune responses through CD47, interaction with α6β1 integrin that activates superoxide (O2−) production by NADPH oxidase-2 (Nox2), direct activation of immunosuppressive transforming growth factor-β1 (TGFβ1), and incorporation into supramolecular attack particles that mediate sustained delivery of granzyme B and perforin by NK and CD8 T cells to kill infected cells
Because thrombospondin-1 is a major component of platelet α-granules that is rapidly released at sites of injury, a potential role in hemostasis was one of the first hypotheses tested using Thbs1−/− mice (Lawler et al. 1998). The initial examination of platelet function in Thbs1−/− mice using washed platelets found no defect in platelet activation in vitro. However, a subsequent study revealed that a positive function of thrombospondin-1 in promoting platelet activation requires the presence of arginine, which was absent in the washed platelet medium. Arginine is the substrate for biosynthesis of nitric oxide, a potent negative regulator of platelet activation, and activation of Thbs1−/− or Cd47−/− platelets was defective when tested in the presence of physiological levels of nitric oxide or arginine (Isenberg et al. 2008b). Thrombospondin-1 signaling via CD47 redundantly inhibits nitric oxide biosynthesis and cGMP signaling in platelets and other vascular cells (Isenberg et al. 2006, 2008b) (Fig. 1). Thrombospondin-1 released from platelet α-granules thereby mediates positive feedback to overcome the physiological antithrombotic function of nitric oxide at sites of injury.
Additional studies of thrombospondin-1/CD47 signaling in vascular smooth muscle cells demonstrated that thrombospondin-1 enhances vasoconstriction and reduces local blood flow by blocking the function of nitric oxide to relax vascular smooth muscle (Isenberg et al. 2007a) (Fig. 1). Thus, signaling mediated by CD47 at an acute injury site where thrombospondin-1 is released by platelets can limit bleeding by simultaneously enhancing platelet hemostasis and vasoconstriction. These functions could cause individuals with LoF in THBS1 or CD47 to experience increased mortality due to blood loss when acutely injured.
Apart from these CD47-dependent signaling functions, thrombospondin-1 enhances hemostasis by its ability to directly inhibit ADAMTS13 (Bonnefoy et al. 2006) (Fig. 1). ADAMTS13 is a protease that cleaves von Willebrand factor, which inhibits the critical function of von Willebrand factor to mediate platelet activation and thrombus formation of when exposed to collagen at a site of vascular injury (Bonnefoy et al. 2006). ADAMTS13 was not significantly intolerant to LoF in humans (observed/expected LoF mutants = 0.52 (90% range 0.39–0.68), pLI = 0.0), but ADAMTS13 deficiency in mice can shorten their lifespan (Cassis et al. 2018). Another study identified a direct role of thrombospondin-1 to promote platelet adhesion and thrombus formation on exposed collagen by engaging its receptor CD36 (Kuijpers et al. 2014), which is not loss-intolerant in humans (Kaur and Roberts 2021). Thus, thrombospondin-1 released by platelets has multiple CD47-dependent and CD47-independent functions that limit bleeding at a site of injury.
Apart from its functions in hemostasis, regulation of nitric oxide signaling by thrombospondin-1/CD47 can improve survival of cardiovascular stresses that cause acute loss of blood pressure (Fig. 1). Cd47−/− mice were more susceptible to death when subjected to isoflurane anesthesia using conditions that were well-tolerated by wild type mice (Isenberg et al. 2009). Death was caused by loss of blood pressure, and autonomic blockade similarly led to accelerated vascular collapse and death of Thbs1−/− mice compared to wild type mice (Isenberg et al. 2009). The decreased stability of blood pressure regulation in the absence of thrombospondin-1 or CD47 could be another significant selective pressure that prevents accumulation of THBS1 and CD47 LoF mutants. Increased mortality could result from traumatic injuries or from blood loss as a complication of childbirth.
Unlike humans, laboratory mice live in an environment that minimizes their exposure to pathogens. Prior to the relatively recent development of antibiotics and vaccines, infectious disease was a major factor that limited survival of children to adulthood (Mackenbach and Looman 1988; DiLiberti and Jackson 1999). Thus, genes for which LoF significantly increases the risk of death from common infectious diseases may be loss-intolerant in humans without impairing the viability of laboratory mice. The functions of thrombospondin-1 in immune regulation are complex and are mediated by several thrombospondin-1 receptors (Forslow et al. 2007; Martin-Manso et al. 2008; Sarfati et al. 2008; Stein et al. 2016; Kaur et al. 2021). Correspondingly, loss of Thbs1 in mice has been demonstrated to either increase or decrease their survival following exposure to different bacterial, viral, or fungal pathogens (Lawler et al. 1998; Martin-Manso et al. 2012a; Qu et al. 2018; Binsker et al. 2019; Arun et al. 2020).
Studies have identified several mechanisms by which thrombospondin-1 protects mice from specific pathogens. Initially, Thbs1−/− mice were reported to have a chronic lung inflammatory phenotype (Lawler et al. 1998), which was subsequently attributed to loss of the ability of thrombospondin-1 to activate latent TGFβ1 (Crawford et al. 1998). The increased immunosuppressive activity of TGFβ1 in wild type mice would then prevent lung inflammation. This could also occur independent of infection, which is consistent with a report that thrombospondin-1 limits injury and collagen and CCN2 expression in lungs treated with bleomycin (Ezzie et al. 2011). However, the originally reported spontaneous lung inflammation in Thbs1−/− mice may relate to exposure to a specific pathogen in the vivarium because the lung phenotype was lost when the mice were rederived in a different vivarium (Isenberg et al. 2008a). In another study, thrombospondin-1 protected mice from lung injury caused by Pseudomonas aeruginosa by inhibiting pathogen and host proteolytic activities (Qu et al. 2018).
Loss of Thbs1 or Cd47 in mice may also increase death from infectious diseases by impairing T cell and natural killer cell immunity (Fig. 1). CD47 is required for optimal defense against some bacterial, viral and Candida albicans fungal infections (Lindberg et al. 1996; Navarathna et al. 2015; Nath et al. 2018). Gene expression profiling indicated protective effects of thrombospondin-1 and CD47 on innate and adaptive immune cells (Navarathna et al. 2015; Nath et al. 2018). Thrombospondin-1 also enhances superoxide production via NADPH oxidase-2 in macrophages and neutrophils in an integrin α6β1-dependent manner (Martin-Manso et al. 2008; Roberts et al. 2017). The catalytic subunit of NADPH oxidase-2 encoded by CYBB is highly loss intolerant (observed/expected LoF mutants = 0.04 (0.01–0.19), pLI = 1.00) (Karczewski et al. 2020).
Recently, thrombospondin-1 was identified as an essential component of supramolecular attack particles produced by CD8 T cells and natural killer cells (Ambrose et al. 2020; Balint et al. 2020). Supramolecular attack particles are coated with a fragment of thrombospondin-1 and deliver the cytotoxic agents granzyme B and perforin to target cells, and the sustained delivery mediated by these particles increases that killing of refractory targets (Chang et al. 2022).
Conclusions
Studies have identified beneficial as well as detrimental effects of Thbs1 gene disruption on the ability of mice to survive exposure to specific pathogens or respond to a variety of physiological stresses (McMaken et al. 2011; Martin-Manso et al. 2012b; Soto-Pantoja et al. 2015; Zhao et al. 2015; Qu et al. 2018; Arun et al. 2020). Although each of the mechanisms outlined in this review have the potential to contribute to the selective pressure preventing loss of function in THBS1 in humans, it is premature to rank their relevance. To date, the evidence is most compelling for protective functions of thrombospondin-1 in hemostasis to account for its loss-intolerance. Future genome wide association studies may identify additional missense mutations in THBS1 that contribute to essential functions in humans and provide clues to the specific thrombospondin-1 interaction partners involved. The insights gained from stress models that revealed protective functions of Thbs1 and Cd47 in mice may also be applicable for identifying molecular mechanisms underlying the deficits in LoF and missense mutants observed for other thrombospondins and members of other matricellular gene families in humans.
Data availability
All data is contained in the manuscript or the indicated public databases.
Abbreviations
- CCN:
-
Cellular communication network
- gnomAD:
-
Genome aggregation database
- LoF:
-
Loss of function
- pLI:
-
Probability of loss-of-function intolerance
- THBS:
-
Thrombospondin
References
Abouzeid H, Boisset G, Favez T, Youssef M, Marzouk I, Shakankiry N, Bayoumi N, Descombes P, Agosti C, Munier FL, Schorderet DF (2011) Mutations in the SPARC-related modular calcium-binding protein 1 gene, SMOC1, cause waardenburg anophthalmia syndrome. Am J Hum Genet 88:92–98. https://doi.org/10.1016/j.ajhg.2010.12.002
Agah A, Kyriakides TR, Lawler J, Bornstein P (2002) The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol 161:831–839. https://doi.org/10.1016/S0002-9440(10)64243-5
Ambrose AR, Hazime KS, Worboys JD, Niembro-Vivanco O, Davis DM (2020) Synaptic secretion from human natural killer cells is diverse and includes supramolecular attack particles. Proc Natl Acad Sci U S A 117:23717–23720. https://doi.org/10.1073/pnas.2010274117
Arun A, Rayford KJ, Cooley A, Rachakonda G, Villalta F, Pratap S, Lima MF, Sheibani N, Nde PN (2020) Thrombospondin-1 Plays an essential role in yes-associated protein nuclear translocation during the early phase of trypanosoma cruzi infection in heart endothelial cells. Int J Mol Sci. https://doi.org/10.3390/ijms21144912
Balint S, Muller S, Fischer R, Kessler BM, Harkiolaki M, Valitutti S, Dustin ML (2020) Supramolecular attack particles are autonomous killing entities released from cytotoxic T cells. Science 368:897–901. https://doi.org/10.1126/science.aay9207
Bender HR, Campbell GE, Aytoda P, Mathiesen AH, Duffy DM (2019) Thrombospondin 1 (THBS1) promotes follicular angiogenesis, luteinization, and ovulation in primates. Front Endocrinol (lausanne) 10:727. https://doi.org/10.3389/fendo.2019.00727
Binsker U, Kohler TP, Hammerschmidt S (2019) Contribution of human thrombospondin-1 to the pathogenesis of gram-positive bacteria. J Innate Immun 11:303–315. https://doi.org/10.1159/000496033
Bonnefoy A, Daenens K, Feys HB, De Vos R, Vandervoort P, Vermylen J, Lawler J, Hoylaerts MF (2006) Thrombospondin-1 controls vascular platelet recruitment and thrombus adherence in mice by protecting (sub)endothelial VWF from cleavage by ADAMTS13. Blood 107:955–964.https://doi.org/10.1182/blood-2004-12-4856
Bornstein P (1995) Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol 130:503–506. https://doi.org/10.1083/jcb.130.3.503
Bouleftour W, Juignet L, Bouet G, Granito RN, Vanden-Bossche A, Laroche N, Aubin JE, Lafage-Proust MH, Vico L, Malaval L (2016) The role of the SIBLING, bone sialoprotein in skeletal biology-contribution of mouse experimental genetics. Matrix Biol 52–54:60–77. https://doi.org/10.1016/j.matbio.2015.12.011
Bradshaw AD (2009) The role of SPARC in extracellular matrix assembly. J Cell Commun Signal 3:239–246. https://doi.org/10.1007/s12079-009-0062-6
Bristow J, Carey W, Egging D, Schalkwijk J (2005) Tenascin-X, collagen, elastin, and the Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet 139C:24–30. https://doi.org/10.1002/ajmg.c.30071
Burke A, Creighton W, Tavora F, Li L, Fowler D (2009) Decreased frequency of the 3’UTR T>G single nucleotide polymorphism of thrombospondin-2 gene in sudden death due to plaque erosion. Cardiovasc Pathol. https://doi.org/10.1016/j.carpath.2008.12.013
Calabro NE, Kristofik NJ, Kyriakides TR (2014) Thrombospondin-2 and extracellular matrix assembly. Biochim Biophys Acta 1840:2396–2402. https://doi.org/10.1016/j.bbagen.2014.01.013
Canalis E, Smerdel-Ramoya A, Durant D, Economides AN, Beamer WG, Zanotti S (2010) Nephroblastoma overexpressed (nov) inactivation sensitizes osteoblasts to bone morphogenetic protein-2, but nov is dispensable for skeletal homeostasis. Endocrinology 151:221–233. https://doi.org/10.1210/en.2009-0574
Carlson CB, Liu Y, Keck JL, Mosher DF (2008) Influences of the N700S thrombospondin-1 polymorphism on protein structure and stability. J Biol Chem 283:20069–20076. https://doi.org/10.1074/jbc.M800223200
Cassis P, Cerullo D, Zanchi C, Corna D, Lionetti V, Giordano F, Novelli R, Conti S, Casieri V, Matteucci M, Locatelli M, Taraboletti G, Villa S, Gastoldi S, Remuzzi G, Benigni A, Zoja C (2018) ADAMTS13 deficiency shortens the life span of mice with experimental diabetes. Diabetes 67:2069–2083. https://doi.org/10.2337/db17-1508
Chang HF, Schirra C, Ninov M, Hahn U, Ravichandran K, Krause E, Becherer U, Balint S, Harkiolaki M, Urlaub H, Valitutti S, Baldari CT, Dustin ML, Jahn R, Rettig J (2022) Identification of distinct cytotoxic granules as the origin of supramolecular attack particles in T lymphocytes. Nat Commun 13:1029. https://doi.org/10.1038/s41467-022-28596-y
Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SMF, Lawler J, Hynes RO, Boivin GP, Bouck N (1998) Thrombospondin-1 is a major activator of TGF-b1 in vivo. Cell 93:1159–1170. https://doi.org/10.1016/s0092-8674(00)81460-9
Dawes R, Lek M, Cooper ST (2019) Gene discovery informatics toolkit defines candidate genes for unexplained infertility and prenatal or infantile mortality. NPJ Genom Med 4:8. https://doi.org/10.1038/s41525-019-0081-z
Dhamija R, Graham JM Jr, Smaoui N, Thorland E, Kirmani S (2014) Novel de novo SPOCK1 mutation in a proband with developmental delay, microcephaly and agenesis of corpus callosum. Eur J Med Genet 57:181–184. https://doi.org/10.1016/j.ejmg.2014.02.009
DiLiberti JH, Jackson CR (1999) Long-term trends in childhood infectious disease mortality rates. Am J Public Health 89:1883–1885. https://doi.org/10.2105/ajph.89.12.1883
Ezzie ME, Piper MG, Montague C, Newland CA, Opalek JM, Baran C, Ali N, Brigstock D, Lawler J, Marsh CB (2011) Thrombospondin-1-deficient mice are not protected from bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 44:556–561. https://doi.org/10.1165/rcmb.2009-0019OC
Forslow A, Liu Z, Sundqvist KG (2007) Receptor communication within the lymphocyte plasma membrane: a role for the thrombospondin family of matricellular proteins. Cell Mol Life Sci 64:66–76. https://doi.org/10.1007/s00018-006-6255-8
Hankenson KD, Hormuzdi SG, Meganck JA, Bornstein P (2005a) Mice with a disruption of the thrombospondin 3 gene differ in geometric and biomechanical properties of bone and have accelerated development of the femoral head. Mol Cell Biol 25:5599–5606. https://doi.org/10.1128/MCB.25.13.5599-5606.2005
Hankenson KD, James IE, Apone S, Stroup GB, Blake SM, Liang X, Lark MW, Bornstein P (2005b) Increased osteoblastogenesis and decreased bone resorption protect against ovariectomy-induced bone loss in thrombospondin-2-null mice. Matrix Biol 24:362–370. https://doi.org/10.1016/j.matbio.2005.05.008
Herz J, Clouthier DE, Hammer RE (1992) LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell 71:411–421. https://doi.org/10.1016/0092-8674(92)90511-a
Hurvitz JR, Suwairi WM, Van HW, El-Shanti H, Superti-Furga A, Roudier J, Holderbaum D, Pauli RM, Herd JK, Van HEV, Rezai-Delui H, Legius E, Le MM, Al-Alami J, Bahabri SA, Warman ML (1999) Mutations in the CCN gene family member WISP3 cause progressive pseudorheumatoid dysplasia. Nat Genet 23:94–98. https://doi.org/10.1038/12699
Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD (2006) CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem 281:26069–26080. https://doi.org/10.1074/jbc.M605040200
Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, Kuppusamy P, Wink DA, Krishna MC, Roberts DD (2007a) Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood 109:1945–1952. https://doi.org/10.1158/1078-0432.CCR-06-1758
Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD (2007b) Increasing survival of ischemic tissue by targeting CD47. Circ Res 100:712–720. https://doi.org/10.1161/01.RES.0000259579.35787.4e
Isenberg JS, Maxhimer JB, Hyodo F, Pendrak ML, Ridnour LA, DeGraff WG, Tsokos M, Wink DADRD (2008a) Thrombospondin-1 and CD47 limit cell and tissue survival of radiation injury. Am J Pathol 173:1100–1112. https://doi.org/10.2353/ajpath.2008.080237
Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, Rick ME, Wink DA, Frazier WA, Roberts DD (2008b) Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood 111:613–623. https://doi.org/10.1182/blood-2007-06-098392
Isenberg JS, Qin Y, Maxhimer JB, Sipes JM, Despres D, Schnermann J, Frazier WA, Roberts DD (2009) Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol 28:110–119. https://doi.org/10.1016/j.matbio.2009.01.002
Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM (2003) Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 130:2779–2791. https://doi.org/10.1242/dev.00505
Jones FS, Jones PL (2000) The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn 218:235–259. https://doi.org/10.1002/(SICI)1097-0177(200006)218:23.0.CO;2-G
Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alfoldi J, Wang Q, Collins RL, Laricchia KM, Ganna A, Birnbaum DP, Gauthier LD, Brand H, Solomonson M, Watts NA, Rhodes D, Singer-Berk M, England EM, Seaby EG, Kosmicki JA, Walters RK, Tashman K, Farjoun Y, Banks E, Poterba T, Wang A, Seed C, Whiffin N, Chong JX, Samocha KE, Pierce-Hoffman E, Zappala Z, O’Donnell-Luria AH, Minikel EV, Weisburd B, Lek M, Ware JS, Vittal C, Armean IM, Bergelson L, Cibulskis K, Connolly KM, Covarrubias M, Donnelly S, Ferriera S, Gabriel S, Gentry J, Gupta N, Jeandet T, Kaplan D, Llanwarne C, Munshi R, Novod S, Petrillo N, Roazen D, Ruano-Rubio V, Saltzman A, Schleicher M, Soto J, Tibbetts K, Tolonen C, Wade G, Talkowski ME, Database GA, C., Neale, B. M., Daly, M. J., MacArthur, D. G. (2020) The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581:434–443. https://doi.org/10.1038/s41586-020-2308-7
Kaur S, Bronson SM, Pal-Nath D, Miller TW, Soto-Pantoja DR, Roberts DD (2021) Functions of thrombospondin-1 in the tumor microenvironment. Int J Mol Sci. https://doi.org/10.3390/ijms22094570
Kaur S, Roberts DD (2021) Differential intolerance to loss of function and missense mutations in genes that encode human matricellular proteins. J Cell Commun Signal 15:93–105. https://doi.org/10.1007/s12079-020-00598-9
Kim KH, Won JH, Cheng N, Lau LF (2018) The matricellular protein CCN1 in tissue injury repair. J Cell Commun Signal 12:273–279. https://doi.org/10.1007/s12079-018-0450-x
Kuijpers MJ, de Witt S, Nergiz-Unal R, van Kruchten R, Korporaal SJ, Verhamme P, Febbraio M, Tjwa M, Voshol PJ, Hoylaerts MF, Cosemans JM, Heemskerk JW (2014) Supporting roles of platelet thrombospondin-1 and CD36 in thrombus formation on collagen. Arterioscler Thromb Vasc Biol 34:1187–1192. https://doi.org/10.1161/ATVBAHA.113.302917
Kutz WE, Gong Y, Warman ML (2005) WISP3, the gene responsible for the human skeletal disease progressive pseudorheumatoid dysplasia, is not essential for skeletal function in mice. Mol Cell Biol 25:414–421. https://doi.org/10.1128/MCB.25.1.414-421.2005
Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO (1998) Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest 101:982–992. https://doi.org/10.1172/JCI1684
Leask A (2020) Conjunction junction, what’s the function? CCN proteins as targets in fibrosis and cancers. Am J Physiol Cell Physiol 318:C1046–C1054. https://doi.org/10.1152/ajpcell.00028.2020
Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won HH, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG, Exome Aggregation C (2016) Analysis of protein-coding genetic variation in 60,706 humans. Nature 536:285–291. https://doi.org/10.1038/nature19057
Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ (1996) Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science 274:795–798. https://doi.org/10.1126/science.274.5288.795
Liu G, Yong MY, Yurieva M, Srinivasan KG, Liu J, Lim JS, Poidinger M, Wright GD, Zolezzi F, Choi H, Pavelka N, Rancati G (2015) Gene essentiality is a quantitative property linked to cellular evolvability. Cell 163:1388–1399. https://doi.org/10.1016/j.cell.2015.10.069
Mackenbach JP, Looman CW (1988) Secular trends of infectious disease mortality in The Netherlands, 1911–1978: quantitative estimates of changes coinciding with the introduction of antibiotics. Int J Epidemiol 17:618–624. https://doi.org/10.1093/ije/17.3.618
Martin-Manso G, Galli S, Ridnour LA, Tsokos M, Wink DA, Roberts DD (2008) Thrombospondin-1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity by differentiated U937 cells. Cancer Res 68:7090–7099. https://doi.org/10.1158/0008-5472.CAN-08-0643
Martin-Manso G, Navarathna DH, Galli S, Soto-Pantoja DR, Kuznetsova SA, Tsokos M, Roberts DD (2012a) Endogenous thrombospondin-1 regulates leukocyte recruitment and activation and accelerates death from systemic candidiasis. PLoS ONE 7:e48775. https://doi.org/10.1371/journal.pone.0048775
Martin-Manso G, Navarathna DHMLP, Galli S, Soto-Pantoja DR, Kuznetsova SA, Tsokos M, Roberts DD (2012b) Endogenous thrombospondin-1 regulates leukocyte recruitment and activation and accelerates death from systemic candidiasis. PLoS ONE. https://doi.org/10.1371/journal.pone.0048775
McGray AJ, Gingerich T, Petrik JJ, LaMarre J (2011) Rapid insulin-like growth factor-1-induced changes in granulosa cell thrombospondin-1 expression in vitro. J Reprod Dev 57:76–83. https://doi.org/10.1262/jrd.10-045h
McMaken S, Exline MC, Mehta P, Piper M, Wang Y, Fischer SN, Newland CA, Schrader CA, Balser SR, Sarkar A, Baran CP, Marsh CB, Cook CH, Phillips GS, Ali NA (2011) Thrombospondin-1 contributes to mortality in murine sepsis through effects on innate immunity. PLoS ONE 6:e19654. https://doi.org/10.1371/journal.pone.0019654
Mendoza-Londono R, Fahiminiya S, Majewski J, Tétreault M, Nadaf J, Kannu P, Sochett E, Howard A, Stimec J, Dupuis L, Roschger P (2015) Recessive osteogenesis imperfecta caused by missense mutations in SPARC. Am J Human Genet 96(6):979–985. https://doi.org/10.1016/j.ajhg.2015.04.021
Midwood KS, Orend G (2009) The role of tenascin-C in tissue injury and tumorigenesis. J Cell Commun Signal. https://doi.org/10.1007/s12079-009-0075-1
Mo FE, Lau LF (2006) The matricellular protein CCN1 is essential for cardiac development. Circ Res 99:961–969. https://doi.org/10.1161/01.RES.0000248426.35019.89
Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF (2002) CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol 22:8709–8720. https://doi.org/10.1128/MCB.22.24.8709-8720.2002
Murphy-Ullrich JE (2019) Thrombospondin 1 and its diverse roles as a regulator of extracellular matrix in fibrotic disease. J Histochem Cytochem 67:683–699. https://doi.org/10.1369/0022155419851103
Murphy-Ullrich JE, Sage EH (2014) Revisiting the matricellular concept. Matrix Biol 37:1–14. https://doi.org/10.1016/j.matbio.2014.07.005
Nath PR, Gangaplara A, Pal-Nath D, Mandal A, Maric D, Sipes JM, Cam M, Shevach EM, Roberts DD (2018) CD47 expression in natural killer cells regulates homeostasis and modulates immune response to lymphocytic choriomeningitis virus. Front Immunol 9:2985. https://doi.org/10.3389/fimmu.2018.02985
Navarathna DH, Stein EV, Lessey-Morillon EC, Nayak D, Martin-Manso G, Roberts DD (2015) CD47 promotes protective innate and adaptive immunity in a mouse model of disseminated candidiasis. PLoS ONE 10:e0128220. https://doi.org/10.1371/journal.pone.0128220
Okada I, Hamanoue H, Terada K, Tohma T, Megarbane A, Chouery E, Abou-Ghoch J, Jalkh N, Cogulu O, Ozkinay F, Horie K, Takeda J, Furuichi T, Ikegawa S, Nishiyama K, Miyatake S, Nishimura A, Mizuguchi T, Niikawa N, Hirahara F, Kaname T, Yoshiura K, Tsurusaki Y, Doi H, Miyake N, Furukawa T, Matsumoto N, Saitsu H (2011) SMOC1 is essential for ocular and limb development in humans and mice. Am J Hum Genet 88:30–41. https://doi.org/10.1016/j.ajhg.2010.11.012
Posey KL, Hankenson K, Veerisetty AC, Bornstein P, Lawler J, Hecht JT (2008) Skeletal abnormalities in mice lacking extracellular matrix proteins, thrombospondin-1, thrombospondin-3, thrombospondin-5, and type IX collagen. Am J Pathol 172:1664–1674. https://doi.org/10.2353/ajpath.2008.071094
Posey KL, Coustry F, Hecht JT (2018) Cartilage oligomeric matrix protein: COMPopathies and beyond. Matrix Biol 71–72:161–173. https://doi.org/10.1016/j.matbio.2018.02.023
Qu Y, Olonisakin T, Bain W, Zupetic J, Brown R, Hulver M, Xiong Z, Tejero J, Shanks RM, Bomberger JM, Cooper VS, Zegans ME, Ryu H, Han J, Pilewski J, Ray A, Cheng Z, Ray P, Lee JS (2018) Thrombospondin-1 protects against pathogen-induced lung injury by limiting extracellular matrix proteolysis. JCI Insight. https://doi.org/10.1172/jci.insight.96914
Rainger J, van Beusekom E, Ramsay JK, McKie L, Al-Gazali L, Pallotta R, Saponari A, Branney P, Fisher M, Morrison H, Bicknell L, Gautier P, Perry P, Sokhi K, Sexton D, Bardakjian TM, Schneider AS, Elcioglu N, Ozkinay F, Koenig R, Megarbane A, Semerci CN, Khan A, Zafar S, Hennekam R, Sousa SB, Ramos L, Garavelli L, Furga AS, Wischmeijer A, Jackson IJ, Gillessen-Kaesbach G, Brunner HG, Wieczorek D, van Bokhoven H, Fitzpatrick DR (2011) Loss of the BMP antagonist, SMOC-1, causes Ophthalmo-acromelic (Waardenburg Anophthalmia) syndrome in humans and mice. PLoS Genet 7:e1002114. https://doi.org/10.1371/journal.pgen.1002114
Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS (2012) The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress. Matrix Biol 31:162–169. https://doi.org/10.1016/j.matbio.2012.01.005
Roberts DD, Kaur S, Isenberg JS (2017) Regulation of cellular redox signaling by matricellular proteins in vascular biology, immunology, and cancer. Antioxid Redox Signal 27:874–911. https://doi.org/10.1089/ars.2017.7140
Sarfati M, Fortin G, Raymond M, Susin S (2008) CD47 in the immune response: role of thrombospondin and SIRP-alpha reverse signaling. Curr Drug Targets 9:842–850. https://doi.org/10.2174/138945008785909310
Soto-Pantoja DR, Kaur S, Roberts DD (2015) CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit Rev Biochem Mol Biol 50:212–230. https://doi.org/10.3109/10409238.2015.1014024
Staines KA, MacRae VE, Farquharson C (2012) The importance of the SIBLING family of proteins on skeletal mineralisation and bone remodelling. J Endocrinol 214:241–255. https://doi.org/10.1530/JOE-12-0143
Stein EV, Miller TW, Ivins-O’Keefe K, Kaur S, Roberts DD (2016) Secreted Thrombospondin-1 Regulates Macrophage Interleukin-1beta Production and Activation through CD47. Sci Rep 6:19684. https://doi.org/10.1038/srep19684
Stenina OI, Topol EJ, Plow EF (2007) Thrombospondins, their polymorphisms, and cardiovascular disease. Arterioscler Thromb Vasc Biol 27:1886–1894. https://doi.org/10.1161/ATVBAHA.107.141713
Stenina-Adognravi O, Plow EF (2019) Thrombospondin-4 in tissue remodeling. Matrix Biol 75–76:300–313. https://doi.org/10.1016/j.matbio.2017.11.006
Svensson L, Aszodi A, Heinegard D, Hunziker EB, Reinholt FP, Fassler R, Oldberg A (2002) Cartilage oligomeric matrix protein-deficient mice have normal skeletal development. Mol Cell Biol 22:4366–4371. https://doi.org/10.1128/MCB.22.12.4366-4371.2002
Topol EJ, McCarthy J, Gabriel S, Moliterno DJ, Rogers WJ, Newby LK, Freedman M, Metivier J, Cannata R, O’Donnell CJ, Kottke-Marchant K, Murugesan G, Plow EF, Stenina O, Daley GQ (2001) Single nucleotide polymorphisms in multiple novel thrombospondin genes may be associated with familial premature myocardial infarction. Circulation 104:2641–2644. https://doi.org/10.1161/hc4701.100910
Varga-Szabo D, Braun A, Kleinschnitz C, Bender M, Pleines I, Pham M, Renne T, Stoll G, Nieswandt B (2008) The calcium sensor STIM1 is an essential mediator of arterial thrombosis and ischemic brain infarction. J Exp Med 205:1583–1591. https://doi.org/10.1084/jem.20080302
Yanai H, Chiba H, Morimoto M, Abe K, Fujiwara H, Fuda H, Hui SP, Takahashi Y, Akita H, Jamieson GA, Kobayashi K, Matsuno K (2000) Human CD36 deficiency is associated with elevation in low-density lipoprotein-cholesterol. Am J Med Genet 93:299–304. https://doi.org/10.1002/1096-8628(20000814)93:4%3c299::aid-ajmg9%3e3.0.co;2-7
Zhang J (2022) Important genomic regions mutate less often than do other regions. Nature 602:38–39. https://doi.org/10.1038/d41586-022-00017-6
Zhao Y, Olonisakin TF, Xiong Z, Hulver M, Sayeed S, Yu MT, Gregory AD, Kochman EJ, Chen BB, Mallampalli RK, Sun M, Silverstein RL, Stolz DB, Shapiro SD, Ray A, Ray P, Lee JS (2015) Thrombospondin-1 restrains neutrophil granule serine protease function and regulates the innate immune response during Klebsiella pneumoniae infection. Mucosal Immunol 8:896–905. https://doi.org/10.1038/mi.2014.120
Funding
This work was supported by the Intramural Research Program of the NIH/NCI (ZIA SC009172).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
NA
Consent to participate
NA
Consent for publication
NA
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaur, S., Roberts, D.D. Why do humans need thrombospondin-1?. J. Cell Commun. Signal. 17, 485–493 (2023). https://doi.org/10.1007/s12079-023-00722-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-023-00722-5