Summary
Two new species of Barleria subg. Barleria (Acanthaceae) are described from the Soutpansberg Centre of Plant Endemism in Limpopo Province of South Africa: Barleria soutpansbergensis and B. spinosissima. The habitat requirements, conservation status and taxonomic affinities of each species are discussed. Barleria soutpansbergensis is considered to be closely allied to the widespread and frequently cultivated southern African species B. obtusa, whilst B. spinosissima is morphologically most similar to B. bremekampii which has a restricted distribution in northern South Africa and Zimbabwe. Remarkably, the two new species are postulated to occasionally hybridise within the Soutpansberg. Both species are currently assessed as of Least Concern despite their restricted ranges. Barleria spinosissima is noted to be amongst the most densely spiny species of Barleria and, indeed, is a contender as one of the spiniest species of plant in South Africa.
Similar content being viewed by others
Introduction
Barleria L. is one of the most species-rich genera in the Acanthaceae family and is well represented in southern tropical and subtropical Africa, where several centres of diversity and endemism in the genus are noted (Balkwill & Balkwill 1998; Darbyshire 2015; Darbyshire et al. 2019a, 2019b). As in other parts of its range, many of the southern African species of Barleria are highly range-restricted. Of the 47 species of Barleria currently accepted in South Africa, Eswatini and Lesotho, 18 (38%) are endemic whilst a further 11 species either have the large majority of their ranges within these countries or are narrow cross-border endemics that just extend beyond South Africa (I. Darbyshire, unpubl. data). The genus was last treated in full in southern Africa by Obermeyer (1933), and whilst this was an excellent account, our knowledge of the genus has increased significantly in the proceeding 89 years through large numbers of additional collections and field observations and through a range of taxonomic, floristic and phylogenetic studies (e.g. Balkwill & Balkwill 1997; Darbyshire 2015; Darbyshire et al. 2019a, 2019b, 2021; Comito 2019; Comito et al. 2022). New, highly range-restricted species continue to be discovered in southern Africa. For example, the spectacular Barleria greenii M.Balkwill & K.Balkwill was described from black clays soils on dolerite in uThukela District of KwaZulu-Natal (Balkwill et al. 1990), B. dolomiticola M.Balkwill & K.Balkwill was discovered on dolomitic outcrops in the Capricorn District Municipality of Limpopo Province (Balkwill et al. 1992) and, more recently, B. lebomboensis I.Darbysh., McCleland & Froneman was described from shallow, gravel soils with rhyolite outcrops in the Lebombo Mountains of Eswatini (Darbyshire et al. 2017). Ongoing studies on southern African taxa within the genus are likely to yield further localised novelties in this region. For example, several new taxa have been proposed in a study of the B. bechuanensis-irritans-pungens-rigida complex (Nyirenda 2012; Nyirenda & Balkwill 2018), and preliminary investigations into sect. Prionitis Nees from southern Africa suggest that there may be undescribed taxa present (I. Darbyshire, unpubl. data).
Here, we report on two new species in Barleria subg. Barleria (sensu Darbyshire et al. 2019a) that occur in the Soutpansberg region of Limpopo Province, northeastern South Africa: Barleria soutpansbergensis I.Darbysh. & K.Balkwill allied to B. obtusa Nees, and B. spinosissima I.Darbysh. & K.Balkwill allied to B. bremekampii Oberm. Both of these species were first collected in the 1930s and have been known as taxonomic novelties for some time, but neither has been formally described to date.
The family Acanthaceae is named for the thorny or spiny nature of many of the species. Barleria spinosissima takes this to the limit and qualifies as one of the spiniest species of Barleria, of Acanthaceae in general, and, indeed, as one of the spiniest species of plant in South Africa.
Materials and Methods
The study region
The two species described in this work are concentrated in the Soutpansberg, a range of mountains in Vhembe District of Limpopo Province. This range stretches for about 200 km between the town of Vivo in the west and the Punda Milia Rest Camp in Kruger National Park in the east, and covers an area of approximately 6,700 km2 (Hahn 2017). The highest peaks are between 1700 – 1750 m elevation, with the base of the massif being as low as 200 m in elevation. The geology of this massif comprises basalts overlain by quartzite (Barker et al. 2006; Hahn 2011, 2017).
This mountain range has long been known as a site of importance for plant diversity and endemism and, together with the Blouberg range to the west, it was delimited as the Soutpansberg Centre of Plant Endemism (CPE) by van Wyk & Smith (2001). The Soutpansberg and Blouberg are separated by c. 40 km of semi-arid savanna and each have their own complement of endemic species and differing geomorphology, so are considered by Hahn (2011, 2017) to be better treated as both separate geomorphic and biogeographic units. The Soutpansberg supports a high alpha diversity of vascular plants, comprising 2,443 taxa within 922 genera, 187 families and 64 orders according to the most recently compiled checklist (Hahn 2019). This high diversity is driven in part by the large range of habitats which form a rich and varied mosaic over short distances, varying from moist forest through to semi-desert scrub (Hahn 2017).
Hahn (2017) provided a detailed account of the endemic flora of Soutpansberg, together with the Blouberg and adjacent Makgabeng mountain ranges. A total of 44 endemic taxa in 32 genera and 32 families was recorded for these three ranges, of which 22 taxa in 19 genera and 14 families are endemic to the Soutpansberg alone. Two previous endemic Acanthaceae are noted: Blepharis spinipes Vollesen which occurs on the dry rocky slopes of the western Soutpansberg (Vollesen 2000) and Justicia montis-salinarum A.Meeuse (Immelman 1995), which is a Soutpansberg-Blouberg endemic.
Hahn (2017) concluded that “the Soutpansberg, Blouberg and Makgabeng serve as essential biological refugia and it is critical that they be conserved” (p. 324). All three ranges fall within the vast Vhembe UNESCO-MAB Biosphere Reserve, a protected landscape covering over 30,000 km2 in northeast Limpopo Province. Portions of these massifs are also protected within national or federal nature reserves. For example, there are four such reserves in the western section of the Soutpansberg, namely the Happy Rest, Luvhondo, Roodewal and Studholme Nature Reserves (UNEP-WCMC & IUCN 2021).
Morphological analysis and specimen citation
Herbarium specimens of the two new species housed at GLOW, J, K, NBG and PRE were analysed using standard herbarium practices (herbarium abbreviations follow Thiers, continuously updated). Prior to dissection, flowers were soaked in Aerosol OT 5% solution; all other characters were measured on dry material. All duplicates cited have been seen by at least one of the authors. Herbarium studies were supplemented by field observations and photographs of the species and morphologically similar species where possible, and included a short targeted fieldtrip in May 2021 by two of the authors (K. Balkwill & W. Froneman). All relevant taxonomic literature on Barleria (e.g. Obermeyer 1933; van der Bank et al. 2000; Darbyshire 2010, 2015; Darbyshire et al. 2019b) was consulted when comparing the new species to previously described taxa and JSTOR Global Plants (https://plants.jstor.org/) was consulted to view types. Specimens are cited following the method of Edwards & Leistner (1971). Within quarter degree squares, localities are arranged chronologically for the first collection at each locality, then specimens are arranged by locality and within localities, specimens are arranged chronologically.
Conservation assessments
The species conservation (extinction risk) assessments follow the Categories and Criteria of the IUCN Red List (IUCN 2012) and the guidelines for their use (IUCN Standards and Petitions Committee 2019). Extent of occurrence (EOO) and Area of Occupancy (AOO) were calculated using the GeoCAT tool (Bachman et al. 2011). AOO was calculated using the standard 2 × 2 km grid cell size as recommended by the IUCN Standards and Petitions Committee (2019).
Taxonomic account
Barleria soutpansbergensis I.Darbysh. & K.Balkwill sp. nov. Type: South Africa, Soutpansberg Distr., Waterpoort area, Vancollers Pass, fl. & fr. 25 June 1990, K. Balkwill, M.-J. Balkwill & K. Melville 5885 (holotype K; isotypes B, E, J, LISC, M, MO, PRE).
http://www.ipni.org/urn:lsid:ipni.org:names:77297279-1
Barleria sp. nov. aff. B. obtusa sensu van der Bank et al. (2000: 22 – 27).
Small shrub or much-branched perennial herb, 30 – 100 cm tall, non-spiny, aromatically glandular; stems at first green but soon turning sandy-brown, older stems softly woody; leafy stems densely pubescent with patent or slightly declinate white-buff eglandular hairs 0.4 – 0.8 mm long and interspersed shorter patent glandular hairs, these more numerous on young stems, usually also with interspersed longer ascending pale yellowish-buff bristly hairs 1 – 1.3 mm long. Leaves subsessile or petiole of mature leaves 2 – 4.5 mm long; blade ± elliptic, 9 – 27 × 6.5 – 16.5 mm (length: width ratio 1.1 – 2.2: 1), base rounded or obtuse, margin entire, somewhat revolute when young, apex rounded or obtuse, indumentum as stems, dense when young and giving the leaves a distinct grey-green appearance, glandular hairs most numerous towards margins and on adaxial surface, long bristly hairs restricted to main veins abaxially and along margin; secondary veins 3 – 5 per side, these and the reticulate tertiary veins purplish-brown and conspicuous beneath particularly when young. Inflorescence of opposite axillary cymes towards apex of branches, cymes single-flowered or more rarely with a second flower-bud developing (no examples of two mature flowers on a single cyme seen), sometimes together forming weakly-defined terminal spikes; each cyme sessile or peduncle to 1.5 mm long; bracts foliaceous but often reducing upwards, those towards stem apex often obovate, 4.5 – 7 × 3.5 – 5 mm with base more acute than leaves, indumentum as leaves but glandular hairs often more dense; bracteoles linear, linear-oblanceolate or very narrowly oblong, 4 – 10 × 0.8 – 2 mm, green but later turning scarious, apex acute or obtuse, midrib prominent abaxially, densely pale-pubescent and with ± numerous glandular hairs in the distal half. Calyx at first pale green or purplish-green with darker venation but later turning pale brown-scarious, not markedly accrescent; anterior lobe oblong-oblanceolate, 7 – 13 × 2.3 – 5 mm, apex often biapiculate or shallowly notched for up to 1 (– 1.5) mm, margin subentire or often with 1 – 4 inconspicuous and irregular teeth distally, surface with 4 or 6 subparallel main veins, external surface densely pale-pubescent and with few to often numerous glandular hairs; posterior lobe as anterior lobe but 7.5 – 14.5 mm long, apex rounded to obtuse and apiculate, surface with 5 or 7 main veins; lateral lobes linear-lanceolate, 7 – 11 mm long. Corolla pale blue to mauve with yellow throat, rarely white with yellow throat, 20 – 34 mm long, pubescent externally with mixed eglandular and longer glandular hairs, most numerous on lateral lobes; tube 10.5 – 19 mm long, narrowly campanulate above attachment point of stamens, c. 3.5 – 5 mm wide at mouth; limb in “2+3” configuration; abaxial and lateral lobes broadly obovate or laterals obovate-elliptic, 9.5 – 16.5 × 6.5 – 9.5 mm, apices rounded; adaxial lobes ovate-elliptic, 4.5 – 7.5 × 2.8 – 4 mm, apices acute or obtuse (ratio of adaxial lobe length: lateral lobe length 0.4 – 0.55: 1). Stamens two, inserted 7 – 8.5 mm from base of corolla tube, filaments 15.5 – 25.5 mm long, shortly pubescent at base; anthers long-exserted, 2.6 – 3.3 mm long; lateral staminodes 1.3 – 2.3 mm long, shortly pubescent, antherodes either vestigial or one theca more developed and up to 0.7 mm long; adaxial staminode ± 1.5 mm long, without antherode. Pistil drying black; ovary with few pale straight hairs towards apex; style glabrous; stigma long-exserted, clavate, 0.5 – 0.75 mm long. Capsule drying black or dark brown, fusiform in face view, 4-seeded, 11 – 13.5 mm long, glabrous except for few short hairs towards apex; seeds only seen in immature state, covered in hygroscopic hairs which dry purplish-black. Figs 1, 2.
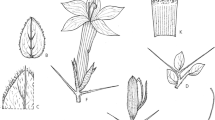
Barleria soutpansbergensis. A habit, flowering stems; B leaves, from distal portion of the stem (left) and proximal portion of the stem (right); C detail of glandular and eglandular hairs on leaves; D calyx and pair of bracteoles, from left to right: bracteole, posterior calyx lobe, anterior and lateral calyx lobes, second bracteole; E corolla, face view; F androecium; G style and stigma; H capsule. All from K. & M-J. Balkwill 9326 (J), except H from K. Balkwill et al. 9108a. drawn by sandie burrows.
RECOGNITION. Barleria soutpansbergensis is morphologically similar to B. obtusa but is easily separated by the more strongly zygomorphic corolla in which the adaxial lobes are less than half as long as the lateral and abaxial lobes (vs only slightly smaller in B. obtusa), in the corolla throat being yellow and lacking white stripes (vs blue with white stripes), and in the dense eglandular indumentum on the young foliage and inflorescences giving the plants a grey-green appearance (vs indumentum less dense, plants usually appearing green even when young).
DISTRIBUTION. This species is largely restricted to the slopes of the Soutpansberg in Limpopo Province of South Africa. There is one collection from Glen Ferness Dam, on the northern slopes of the Blouberg, where it may be more widespread. It is therefore a further Soutpansberg-Blouberg endemic, restricted to the Soutpansberg CPE sensu van Wyk & Smith (2001). See Map 1A.
SPECIMENS EXAMINED. SOUTH AFRICA. Limpopo Prov.: 2229 (Waterpoort): Zoutpansberg [Soutpansberg], lower northern slopes, Farm Zoutpan 193 (–CD), fr. Nov. 1932, A. Obermeyer et al. 162 (PRE); Farm Dorpsrivier of SV Fourie No. M5696, just S of Waterpoort, N slope of Soutpansberg (–DC), fl. 20 April 1976, E. J. van Jaarsveld 1252 (K, PRE); cultivated in Lowveld Bot. Garden, ex Farm Dorpsrivier of SV Fourie, M5696, fl. 29 March 1978, E. Buitendag 1150 (PRE); Farm Crimea 747 MS near Waterpoort (–DC), fl. 4 July 1982, F. Venter 8837 (PRE); Soutpansberg Distr., Waterpoort area, Vancollers Pass (–DC), fl. & fr. 25 June 1990, K. Balkwill et al. 5885 (holotype K; isotypes B, E, J, LISC, M, MO, PRE); Zoutpansberg [Soutpansberg], Franz Hoek farm (–DD), fl. & fr. 19 July 1935, E. E. Galpin 14924 (K, PRE); Soutpansberg Distr., on the farm Prince 758MS (currently Medike) (–DD), fl. 10 April 1993, K. & M-J. Balkwill 8263 (E, J); Zoutpansberg Distr., 3.5 miles S of P. O. Wylliespoort (–DD), fl. & fr. 1 May 1948, L. E. Codd 4167 (PRE); Wylliespoort (–DD), fl. March 1990, W. Froneman 180 (J); Louis Trichardt [Makhado], on farm Davenham 740 MS on the N-facing slopes of the Soutpansberg (–DD), fl. 11 May 1983, K. Balkwill & J. Manning 713 (K, PRE); Davenham 740 MS, above two dams (–DD), fl. 8 May 1991, K. & M-J. Balkwill 6322 (B, E, J, K, MO, RSA); Davenham 740 MS, stream running towards neighbouring farm (–DD), fl. 29 April 1995, K. & M-J. Balkwill 9305 (B, E, J, K, M, MO, RSA); Davenham 740 MS, NW corner of farm (–DD), fl. 30 April 1995, K. & M-J. Balkwill 9326 (B, E, J, K, MO); Aintree 782 MS (–DD), fl. 16 March 1985, P. Raal 393 (PRE); Soutpansberg, Lokovhela 793 farm, path going down to waterfall from farmhouse (–DD), fl. 11 May 1991, H. F. Glen 2689 (PRE); Soutpansberg Distr., on the farm Zwarthoek 796 MS, walk to the waterfall (–DD), fl. 11 May 1991, K. & M-J. Balkwill 6401 (E, J, K, RSA); On the farm Zwarthoek (–DD) 796 MS, fl. 13 May 1994, K. Balkwill et al. 9108a (J, K); Louis Trichardt [Makhado], Farm Little Leigh, ± 10 km N of town (–DD), fl. & fr. 28 May 2006, J. J. Meyer 4814 (PRE); Makhado, Soutpansberg on the Mashovela Lodge Property (–DD), fl. 21 Feb. 2019, W. Froneman 1106 (GLOW). 2328 (Baltimore): Bochum distr., NW slopes of the Blouberg, on the farm Glenferness 1 LS, near Glenferness Dam (–BB), fr. 4 Dec. 1990, K. Balkwill et al. 6002 (B, CM, J, OSH). 2330 (Tzaneen): Entabene (–AB), fl. & fr. 30 July 1935, L. E. Taylor 731 (PRE).
HABITAT & ECOLOGY. This species occurs in dry open bushland or woodland, growing amongst rock crevices, on rocky slopes or along ravines and gulleys (kloofs) with thin sandy soils, at 900 – 1325 m elevation. It typically occurs in hot, dry, exposed sites in Soutpansberg Mountain Bushveld (Mucina & Rutherford 2006).
CONSERVATION STATUS. This species has a small range, with an Extent of Occurrence of 951 km2 and an Area of Occupancy of 60 km2, which would qualify the species as Endangered under criteria B1 and B2 of the IUCN Red List, if there were known threats. However, some collectors have noted that the species is locally common within its restricted range: “common” (van Jaarsveld 1252; Venter 8837); “very local but common where it occurs” (Glen 2689). Some of the localities are in private or provincial nature reserves and the wider area is part of a biosphere reserve (Vhembe Biosphere Reserve 2021) and so are afforded some protection. Furthermore, the habitat in which the species occurs is unsuitable for cultivation and although there are proposals to mine coal just north of the Soutpansberg, this is outside of the area in which Barleria soutpansbergensis occurs. We thus propose a provisional threat status of Least Concern (LC).
NOTES. This species was originally identified as a form of Barleria obtusa in herbaria. Barleria obtusa is a widespread and frequent species in eastern South Africa, and is commonly cultivated under the name “bush violet” or “blue bush violet”. The Soutpansberg species is morphologically similar to B. obtusa in terms of growth habit, foliage, lack of spines and, in particular, calyx morphology. However, there are clear and consistent differences, notably in indumentum and corolla morphology and colour, that warrant recognition of two distinct species as noted in the Recognition section (and compare Figs 1 & 3). In addition, B. soutpansbergensis differs from most populations of B. obtusa in having usually single-flowered and subsessile cymes, whereas B. obtusa usually has lax 2 – 7-flowered cymes with slender peduncles that are widely divergent from the stems (Fig. 2B). However, some populations of B. obtusa are noted to have more contracted, few- or single-flowered cymes, including some of the cultivated forms (e.g. H. J. Schlieben 7934 [K]; see discussion in Darbyshire 2015). In such cases, the differences in corolla form and colour and in vegetative indumentum separate the two species reliably. Based on current knowledge, B. obtusa does not occur in the Soutpansberg and so the two species are allopatric (van der Bank et al. 2000).
Although they are not considered to be closely related, Barleria soutpansbergensis has been found to putatively hybridise naturally with B. heterotricha Lindau where the two species are sympatric (van der Bank et al. 2000). Van Wyk 5538 from Vancollers Pass is an example of a possible hybrid between these species. On initial inspection, it appears very close to B. soutpansbergensis, and it lacks any stellate hairs which are characteristic of B. heterotricha Lindau. However, it has a longer corolla tube c. 25.5 mm long with the stamens inserted in the distal half of the tube, which is similar to B. heterotricha and unlike the other collections of B. soutpansbergensis where the corolla tube is only up to 19 mm long and with the stamens inserted below the midpoint. This collection also has ovate leaves and more acute leaf, bract and calyx lobe apices than in B. soutpansbergensis, all of which could point towards an affinity with B. heterotricha. This collection is therefore omitted from the description of B. soutpansbergensis presented here. True B. soutpansbergensis is also known to occur at Vancollers Pass (K. Balkwill et al. 5885).
Plants with spiny bracteoles that are otherwise morphologically close to Barleria soutpansbergensis and occur alongside that species are considered to be potential hybrids with B. spinosissima I.Darbysh. & K.Balkwill which is newly described below (Fig. 4), although these spiny forms have not, so far, been found growing together with both of the putative parents. Such plants correlate with the putative hybrid with B. bremekampii (sensu lato) discussed by van der Bank et al. (2000). Barleria spinosissima is very different morphologically to B. soutpansbergensis in, for example, being a harshly spiny shrub. However, they do share a similar corolla morphology and colour in which the adaxial pair of corolla lobes is much reduced in comparison to the lateral and abaxial lobes, and the corolla throat is yellow (Figs 1 and 5C – E).
The existence of these putative hybrids led van der Bank et al. (2000) to consider the possibility that Barleria soutpansbergensis is the result of hybridisation. They conducted an alloenzyme electrophoretic analysis to attempt to establish if B. soutpansbergensis represents a hybrid swarm and, if so, what the most likely parentage is, and whether it has diverged sufficiently from its putative parents in order to be recognised as a distinct species. The results of that study supported the recognition of a new species by demonstrating sufficiently high levels of genetic differentiation between B. soutpansbergensis and the other species included in the study (B. heterotricha, B. obtusa and “B. bremekampii”, the latter actually being B. spinosissima), indicating there are effective barriers in place to gene flow between the populations studied. However, the genetic distance between B. soutpansbergensis, B. obtusa and B. spinosissima was found to be towards the lower end of the range for congeneric species, based on previous comparable studies (Nei 1978). This could potentially lend support to the hypothesis that B. soutpansbergensis was derived from a past hybridisation event and, if so, that B. obtusa and B. spinosissima are more likely to be involved in the parentage than B. heterotricha. However, this hypothesis requires further testing with cytological data and modern molecular phylogenetic techniques.
Samples of some of these species were included in a recent RADseq molecular phylogenetic study of Barleria (Comito 2019; Comito et al. 2022). In that study, Barleria soutpansbergensis was resolved in a clade of subg. Barleria together with B. obtusa, B. bremekampii and the Zimbabwean Great Dyke endemic B. molensis Wild. Within this clade, B. soutpansbergensis was resolved with high support as sister to B. bremekampii. In the same phylogeny, B. heterotricha was resolved in a clade together with other members of subg. Barleria with a stellate indumentum, and this clade is not closely related to the B. obtusa-B. bremekampii clade (Comito et al. 2022).
Putative hybrids with Barleria heterotricha :
SOUTH AFRICA. 2229 (Waterpoort): Waterpoort, Vancollers Pass (–DC), fl. & fr. 20 May 1982, van Wyk 5538 (PRE); Soutpansberg distr., Davenham 740 MS, above two dams (–DD), fl. 8 May 1991, K. & M-J. Balkwill 6324 ( J); Davenham 740 MS, valley running past house (–DD), fl. 8 May 1991, K. & M-J. Balkwill 6348 (B, CM, E, J, M, RSA); Davenham 740 MS, about 200 m from main gate along road from main house (–DD), fl. 9 May 1991, K. & M-J. Balkwill 6349 (B, CM, E, J, LISC, M, MO, RSA).
Spiny form, putative hybrids with Barleria spinosissima :
SOUTH AFRICA. 2229 (Waterpoort): Soutpansberg Distr., on the farm Zwarthoek 796 MS (–DD), fl. 13 May 1994, K. Balkwill et al. 9108 (J, K); Davenham 740 MS, above house (–DD), fl. 29 April 1995, K. & M-J. Balkwill 9311 (B, E, J, K). Fig. 3.
Barleria spinosissima. A growth habit and typical habitat; B & C growth habit showing spininess; D flower and young fruits; E lateral view of inflorescences. Barleria bremekampii. F – H inflorescences and flowers, for comparison. photos: a & e k. balkwill, b & d w. froneman, c m.-j. cadman, all from Vancollers Pass, Soutpansberg; f & g w. froneman, Thabazimbi; h m.-j. cadman, Bela-Bela [Warmbaths].
Barleria spinosissima. A habit, flowering stem; B leaf; C bract; D detail of glandular and eglandular hairs on inflorescence; E calyx, posterior lobe to the left, anterior and lateral lobes to the right; F corolla lobes, face view; G dissected corolla with androecium and style / stigma; H capsule. All from W. Froneman 157 (J), except F & G from from K. Balkwill et al. 9310 (J). drawn by sandie burrows.
Barleria spinosissima I.Darbysh. & K.Balkwill sp. nov. Type: South Africa, Soutpansberg Distr., Farm Waterpoort 712MS, Vancollers Pass, just as the road goes over the crest of the cliffs, 22°54'50"S, 29°36'30"E, 830m, fl. 6 May 2021, K. Balkwill & W. Froneman 14246 (holotype J; isotypes B, E, K, MO, PRE).
http://www.ipni.org/urn:lsid:ipni.org:names:77297280-1
Barleria bremekampii sensu van der Bank et al. (2000: 22 – 27), pro parte.
Harshly spiny shrub or shrublet 30 – 100 cm tall; stems sandy-brown or green-brown, older stems woody and gnarled; leafy stems with few to numerous ascending to appressed bristly hairs 0.4 – 0.9 mm long, numerous short spreading eglandular hairs 0.1 – 0.2 mm long, and few to numerous interspersed spreading glandular hairs ± 0.2 mm long. Axillary spines derived from bracteoles of old or aborted (sterile) inflorescences, stalked, paired or in a group of four, stalk 4 – 14 mm long, spines 8 – 15 mm long, sandy-brown. Leaves subsessile or petiole to 1.7 mm long; blade broadly elliptic to ovate-elliptic or obovate-elliptic, 3.7 – 11.5 × 2.3 – 7.5 mm (length: width ratio 1.3 – 2.1: 1), base acute or obtuse, margin entire, apex acute to rounded and with a conspicuous mucro 0.4 – 2 mm long, adaxial surface, margin and veins beneath with appressed or ascending bristly hairs, with finer and shorter spreading hairs beneath and with scattered short glandular hairs, these becoming more numerous on the leaves in the distal portion of the branches; secondary veins 3 – 5 per side. Inflorescences axillary in distal portions of branches, cymes unilateral, 2 – 6-flowered, 17 – 33 mm long, often rather lax, sometimes compound, with lateral inflorescences issuing from the axils of some bracteoles; peduncle 3.3 – 8.5 mm long, slender, this and rachis glandular-pubescent and eglandular-puberulous, with interspersed bristly ascending hairs; bracts foliaceous; bracteoles spinose, pairs unequal in length, longer bracteole of each pair 10.5 – 17.5 mm long, at first purplish but soon turning reddish-brown, blade absent, triangular in cross-section, margin entire, glandular-pubescent and with few short ascending bristly hairs. Calyx at first green or purplish but later turning brown-scarious, not markedly accrescent; anterior lobe lanceolate or oblong-lanceolate, 9.7 – 14.5 × 2 – 3.7 mm, apex attenuate into a short spine, rarely with 2 apical spines, margin subentire or usually with 1 – 3 spinulose teeth distally, surface with subparallel main veins but only midrib prominent in flower, external surface with few to more numerous short ascending bristly hairs and numerous short patent glandular hairs, not obscuring the calyx surface; posterior lobe as anterior lobe but 10.2 – 17.5 × 2 – 3.5 mm; lateral lobes lanceolate and often markedly attenuate, 6.7 – 12 × 1.5 – 2.5 mm. Corolla bright magenta-mauve to blue with yellow throat and with darker magenta streaks towards the mouth, 26 – 33.5 mm long, pubescent externally with eglandular hairs and with longer glandular hairs mainly on lateral lobes; tube 17.5 – 23 mm long, cylindrical, somewhat campanulate above attachment point of stamens, c. 4 – 5 mm wide at mouth; limb in “2+3” configuration; abaxial lobe broadly obovate, 6.5 – 11.5 × 6.5 – 8 mm, apex rounded; lateral lobes as abaxial lobe but 7.5 – 12 × 6.2 – 8.7 mm; adaxial lobes elliptic, 3.2 – 5.5 × 2.4 – 4 mm, apices obtuse (ratio of adaxial lobe length: lateral lobe length 0.4 – 0.6: 1). Stamens two, inserted slightly above the midpoint of the corolla tube, filaments 17 – 20.5 mm long, shortly pubescent at base; anthers exserted, 3.5 – 4.3 mm long; lateral staminodes 0.6 – 1 mm long, pubescent, antherodes vestigial, up to 0.35 mm long, no pollen observed; adaxial staminode 0.4 – 1.2 mm long, without antherode. Pistil drying black; ovary with few to more numerous pale straight hairs towards apex; style glabrous above base; stigma clavate, 0.5 – 0.8 mm long. Capsule drying black, fusiform in face view, 4-seeded, (10.5 –) 12.5 – 15 mm long, with sparse straight pale hairs towards apex; seeds ± 4.5 mm in diameter, covered in hygroscopic hairs which dry shiny-brown. Figs 5A & B, 6.
RECOGNITION. Barleria spinosissima is morphologically similar to B. bremekampii but is separated by the inflorescence having shorter, non-silky glandular hairs and sparser eglandular hairs, these not obscuring the calyx surfaces (vs inflorescence densely silky-hairy with mixed long glandular and eglandular hairs, these together obscuring the calyx surfaces in herbarium specimens); smaller leaves 3.7 – 11.5 × 2.3 – 7.5 mm (vs 12 – 30 × 8 – 22 mm); and a clavate stigma 0.5 – 0.8 mm long (vs stigma linear, 0.8 – 1.3 mm long). In addition, the spininess of B. spinosissima is striking — indeed, many plants look like a cushion of spines with the leaves borne within the spines and only the limb of the corolla protruding. In B. spinosissima, the inflorescence is borne on a peduncle approximately 5.3 – 9.3 mm long and subsequent internodes of the peduncle are 3.3 – 8.5 mm long, the inflorescence can be compound, with lateral inflorescences issuing from the axils of some bracteoles, the bracteoles are straight (not curved), the distal bracteoles are similar in length to the proximal pair and there may be up to 6 or more flowers (and thus pairs of bracteoles) in each inflorescence. In B. bremekampii, the inflorescence is usually born on a short peduncle 0.7 – 3.3 mm long (although occasionally 9 mm long), subsequent internodes of the inflorescence are 0 – 1.4 mm long, the inflorescence is simple, the bracteoles are slightly curved, the second and subsequent pairs of bracteoles are ± much smaller than the proximal pair in sterile inflorescences and there are usually only up to 4 flowers in the inflorescence. Thus, although the proximal bracteoles in B. spinosissima are shorter (10.5 – 17.5 mm vs 17 – 31 mm long) and less stout, the fewer distal bracteoles in fertile inflorescences and the smaller and fewer distal bracteoles in the sterile inflorescences make the overall appearance of B. bremekampii much less spiny. The bracteoles in B. spinosissima soon turn reddish-brown, whereas those of B. bremekampii soon turn straw-coloured. See Fig. 5.
DISTRIBUTION. The known distribution of this species is restricted to the slopes of the Soutpansberg in Limpopo Province of South Africa, with one doubtful record from the Modimolle region. A population has recently been discovered on Tshipise Koppie which is an outlier sandstone inselberg of the Karoo Supergroup formation (see Hahn 2011) just north of Soutpansberg. The extreme spininess of this species is likely to deter collection, so it may well be more widespread in the Soutpansberg than the records suggest and possibly present in the Blouberg. See Map 1B.
SPECIMENS EXAMINED. SOUTH AFRICA. Limpopo Prov.: 2229 (Waterpoort): Zoutpansberg [Soutpansberg], Farm Zoutpan 193, in kloof behind homestead (–CD), in bud, 22 Nov. 1932, A. Obermeyer et al. 124 (PRE); Zoutpansberg [Soutpansberg], Crewe Farm (–DC), fl. & fr. 23 Aug. 1930, J. Hutchinson & J. B. Gillett 4437 (K, SRGH); Farm George 749 MS (–DC), fl. 4 July 1982, S. Venter 8845 (LYD, PRE); Waterpoort, Vancollers Pass (–DC), fl. & fr. 20 May 1982, A. E. van Wyk 5543 (PRE, PRU); Vancollers Pass (–DC), fl. 25 June 1990, K. Balkwill et al. 5891 (B, E, J, MO); Vancollers Pass (–DC), st. 26 April 1991, W. Froneman 157 (GLOW, J); Farm Waterpoort 712MS, Vancollers Pass, just as the road goes over the crest of the cliffs, fl. 6 May 2021, K. Balkwill & W. Froneman 14246 (holotype J; isotypes B, E, K, MO, PRE); Soutpansberg, Farm Parkfield 725 MS, on the eastern slope of Wylliespoort (–DD), fl. 1979, S. P. Fourie 153 (PRE); Wylliespoort (–DD), fl. March 1990, W. Froneman 141 (GLOW, J); Wylliespoort, fl. 31 May 1950, H. Hall 4 (NBG); Venda, Fripp [= Mudimeli], langs Bobbejaankop (–DD), fl. 10 April 1979, E. Netshiungani 839 (J, PRE); Soutpansberg Distr., N of the Soutpansberg, on the farm Davenham 740 MS (–DD), fl. 29 April 1995, K. Balkwill et al. 9310 (B, E, J, RSA). 2329 (Pietersburg [= Polokwane]): Soutpansberg Distr., ± 1 mile from Dandy Farm [possibly Dundee 216 LS] on road to this farm branching off to the N at Sandrivier bridge on Louis Trichardt [Makhado] – Mara Vivo road (–BA), fl. 3 April 1957, A. D. J. Meeuse 10219 (PRE). 2230 (Messina [Musina]): Tshipise, Farm Honnet 137MT, Tshipise Forever Resort, Tshipise Koppie, just below upper steep slopes, 616 m, fl. 6 May 2021, K. Balkwill & W. Froneman 14247 (J, K, MO, PRE).
Imprecise and unlikely locality: Nylstroom region, fl. July 1949, F. van der Merwe s.n. [in PRE 58906] (PRE).
HABITAT & ECOLOGY. This species occurs among rock crevices on large outcrops, along dry gulleys and in woodland on stony ground and sandy soil including on steep slopes of the Soutpansberg Mountain Bushveld (Mucina & Rutherford 2006); it is recorded at elevations of 625 – 1340 m.
CONSERVATION STATUS. This species has a highly restricted range, with an Extent of Occurrence of 1,248 km2 and an Area of Occupancy of 44 km2, which would qualify the species as Endangered under IUCN Red List criteria B1 and B2, if there were known threats. However, because of its spininess, the species is likely to occur more widely than is suggested by the number of herbarium specimens. One collector has indicated that the species is locally common (Netshiungani 839). Some of the localities are in private or provincial nature reserves and there are efforts in place for wider and more formalised conservation for more of the Soutpansberg range. Furthermore, the habitat in which the species occurs is unsuitable for cultivation. There are extensive coal reserves north of the Soutpansberg (Vhembe Municipality 2021) and one proposal for a mine will possibly eliminate the Bobbejaankop locality (Coal of Africa Limited 2013). We propose a provisional threat status of Least Concern (LC), but suggest that this should be re-evaluated on a regular basis, particularly if the plans for coal mining in the region are taken forward.
NOTES. Since its first discovery in the 1930s, this species has been included within the concept of Barleria bremekampii. That species is otherwise centred on the Waterberg Range in the vicinity of Thabazimbi and Modimolle some 250 km or more to the southwest of the Soutpansberg, although it has also been collected from Messina in northern Limpopo (Rogers 20737), and the type specimen is apparently from Lupane in Matabeleland, western Zimbabwe from where it has never been recollected (Obermeyer 1933; Darbyshire 2015). The two share a similar growth habit, both being markedly spiny shrubs with 2 – several-flowered unilateral cymes and blue to mauve corollas with a yellow throat and with the adaxial pair of lobes significantly smaller than the lateral and abaxial lobes. However, they differ in a number of characters as noted in the Recognition section, most notably in the length and colour of the spinose bracteoles, the inflorescence indumentum, and the size of the leaves, which together give the two plants a conspicuously different gestalt (Fig. 5). Given these morphological differences, it is considered most appropriate that the Soutpansberg populations be treated as a distinct species. In addition, B. bremekampii typically has proportionately broader anterior and posterior calyx lobes up to 5 mm wide, but there is some overlap in this character. The lower end of the size ranges for the bracteole and calyx measurements for B. bremekampii given in Darbyshire (2015) are from Germishuizen 368 (K), which appears to be an aberrantly small specimen. In that specimen, the bracteoles are only 10 – 13 mm long, but in all other material seen, the proximal pair of bracteoles in each cyme is always ≥ 17 mm long.
Although Barleria bremekampii and B. spinosissima are apparently allopatric, one specimen of the latter — van der Merwe s.n. (PRE) — is recorded as from “Regio Nylstroom”, with no further locality information given. Given that Nylstroom [= Modimolle] is a considerable distance to the southwest of the other collections of this species and is within the core range of true B. bremekampii and that the collector did not give the specimen a number in the field, the provenance of this collection must be in doubt; it is possible that there has been some confusion over the labelling of this specimen.
A further species that is somewhat similar to Barleria spinosissima is the recently described Barleria hydeana I.Darbysh. from the Great Dyke of Zimbabwe (Darbyshire 2015). This species shares with B. spinosissima and B. bremekampii a similar growth habit and a similar corolla in which the adaxial pair of lobes is much reduced relative to the lateral and abaxial lobes (see Hyde et al. 2021 for images of this species). The inflorescence indumentum is also similar to B. spinosissima. However, B. hydeana is easily separated from both by having single-flowered cymes with the flowers being conspicuously pedicellate, and in having proportionately narrower, (oblong-) elliptic leaves with the length: width ratio 2.4 – 3: 1 (vs 1.3 – 2.1: 1 in B. spinosissima and B. bremekampii). As noted in Darbyshire (2015), B. hydeana could be confused with another Great Dyke endemic, B. molensis, when in fruit although the flowers are very different from that species. Given that both B. bremekampii and B. molensis are resolved in the same clade in the recent RADseq phylogenomic analysis of Barleria (Comito 2019; Comito et al. 2022) — the B. obtusa-B. bremekampii clade as discussed under B. soutpansbergensis above — there is little doubt that both B. hydeana and B. spinosissima will be further members of this clade.
References
Bachman, S., Moat, J., Hill, A. W., de la Torre, J. & Scott, B. (2011). Supporting red list threat assessments with GeoCAT: Geospatial conservation assessment tool. ZooKeys 150: 117 – 126.
Balkwill, M.-J. & Balkwill, K. (1997). Delimitation and infra-generic classification of Barleria (Acanthaceae). Kew Bull. 52: 535 – 573.
____ & ____ (1998). A preliminary analysis of distribution patterns in a large, pantropical genus, Barleria L. (Acanthaceae). J. Biogeogr. 25: 95 – 110.
____, ____ & Vincent, P. L. D. (1990). Systematic studies in the Acanthaceae: A new species of Barleria from Natal. S. Afr. J. Bot. 56: 571 – 576.
____, Stalmans, M. & Balkwill, K. (1992). Systematic studies in the Acanthaceae: a new species of Barleria from the northeastern Transvaal, with some notes on its ecology. S. Afr. J. Bot. 58: 286 – 291.
Barker, O. B., Brandl, G., Callaghan, C. C., Ericsson, P. G. & Van der Neut, M. (2006). The Soutpansberg and Waterberg Groups and the Blouberg Formation. pp. 301 – 318 in: M. R. Johnson, C. R. Anhaeusser & R. J. Thomas (eds), The Geology of South Africa. Geological Society of South Africa and Council for Geoscience, Pretoria.
Coal of Africa Limited (2013). Greater Soutpansberg Mopane Project: Environmental Impact Assessment and Environmental Management Programme. Available at: https://sahris.sahra.org.za/sites/default/files/additionaldocs/001%20Mopane%20EMP%20Section%201%20FINAL%2008-11-2013.pdf [Accessed 12 Feb. 2021]
Comito, R. (2019). A RADseq phylogeny of Barleria (Acanthaceae) resolves fine-scale relationships. Unpubl. M.Sc. Thesis, California State University, Long Beach.
____, Darbyshire, I., Kiel, C., McDade, L. & Fisher, A. E. (2022). A RADseq phylogeny of Barleria (Acanthaceae) resolves fine-scale relationships. Molec. Phylogenet. Evol. 169: 107428.
Darbyshire, I. (2010). Barleria, pp. 325 – 442. In: H. J. Beentje (ed.), Flora of Tropical East Africa. Royal Botanic Gardens, Kew.
____ (2015). Barleria. In: J. R. Timberlake & E. S. Martins (eds), Flora Zambesiaca Vol. 8 (6): 37 – 122. Royal Botanic Gardens, Kew.
____, Fisher, A., Kiel, C. A. & McDade, L. A. (2019a). Phylogenetic relationships among species of Barleria (Acanthaceae, Lamiales): molecular data reveal complex patterns of morphological evolution and support a revised classification. Taxon 68: 92 – 111.
____, McCleland, W. & Froneman, W. (2017). Barleria lebomboensis (Acanthaceae), an endangered new species from the Lebombo Mountains of Swaziland. Phytotaxa 323: 173 – 181.
____, Tripp, E. A. & Chase, F. M. (2019b). A taxonomic revision of Acanthaceae tribe Barlerieae in Angola and Namibia. Part 1. Kew Bull. 74–5: 1 – 85.
____, ____ & ____ (2021). A taxonomic revision of Acanthaceae tribe Barlerieae in Angola and Namibia. Part 2. Kew Bull. 76: 127 – 190.
Edwards, D. & Leistner, O. (1971). A degree reference system for citing biological records in Southern Africa. Mitt. Bot. Staatssaml. München 10: 501 – 509.
Hahn, N. (2011). Refinement of the Soutpansberg Geomorphic Province, Limpopo, South Africa. Trans. R. Soc. South Africa 66: 32 – 40.
____ (2017). Endemic flora of the Soutpansberg, Blouberg and Makgabeng. S. Afr. J. Bot. 113: 324 – 336.
____ (2019). Indigenous vascular plants of the Soutpansberg, South Africa. Bothalia 49: a2402 [5 pages].
Hyde, M. A., Wursten, B. T., Ballings, P. & Coates Palgrave, M. (2021). Flora of Zimbabwe: Species information: Barleria hydeana. Available at: https://www.zimbabweflora.co.zw/speciesdata/species.php?species_id=203180 [Accessed 20 Jan. 2021]
Immelman, K. L. (1995). Justicia. In: O. A. Leistner (ed.), Flora of southern Africa 30 (3): 18 – 46.
IUCN (2012). IUCN Red List Categories and Criteria. Version 3.1. Second Edition. IUCN Species Survival Commission, Gland & Cambridge.
IUCN Standards and Petitions Committee (2019). Guidelines for Using the IUCN Red List Categories and Criteria. Version 14. Prepared by the Standards and Petitions Committee. Available at: http://www.iucnredlist.org/documents/RedListGuidelines.pdf [Accessed 15 Dec. 2020]
Mucina, L. & Rutherford, M. C. (2006). The vegetation of South Africa, Lesotho and Swaziland. Strelitzia 19, pp. 807. SANBI, Pretoria.
Nei, M. (1978). Molecular evolutionary genetics. Columbia University Press, New York.
Nyirenda, F. C. (2012). Morphological variation in five related species of Barleria (Acanthaceae). Unpubl. M.Sc. dissertation, University of the Witwatersrand, Johannesburg.
____ & Balkwill, K. (2018). Significance of variation and evaluation of potential characters in three morphologically similar species of Barleria sect. Barleria (Acanthaceae) in southern Africa. Pl. Syst. Evol. 304: 77 – 92.
Obermeyer, A. A. (1933). A revision of the South African species of Barleria. Ann. Transvaal Mus. 15: 123 – 180.
Thiers, B. [continuously updated]. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium. Available at: http://sweetgum.nybg.org/science/ih/ [Accessed 22 Jan. 2021]
UNEP-WCMC & IUCN (2021). Protected Planet. Discover the world’s protected areas. Available at: https://www.protectedplanet.net/en [Accessed 22 Jan. 2021]
Van der Bank, F. H., van der Bank, M., Balkwill, M.-J. & Balkwill, K. (2000). Electrophoretic evidence for an undescribed species of Barleria L. S. Afr. J. Bot. 66: 22 – 27.
Van Wyk, A. E. & Smith, G. F. (2001). Regions of Floristic Endemism in Southern Africa. A review with emphasis on succulents. Umdaus Press, Hatfield.
Vhembe Biosphere Reserve (2021). Vhembe Biosphere Reserve. Available at: https://www.vhembebiosphere.org/ [Accessed 13 Feb. 2021]
Vhembe Municipality (2021). Mining and Agriculture. Available at: http://www.vhembe.gov.za/led/mining-agriculture [Accessed 13 Feb. 2021]
Vollesen, K. (2000). Blepharis: A taxonomic revision. Royal Botanic Gardens, Kew.
Acknowledgements
We are grateful to Grant Otto and Mandy-Jane Cadman for their initial work on these species, which has informed our understanding of the species and their possible origins. We thank Norbert Hahn for assistance in the field, and Tshipise Forever Resort and Tertius Steyn of ZZ2 for permission to access field sites in 2021. We are grateful to the herbaria which have facilitated access to their collections through loans, visits and images. We thank colleagues at the C. E. Moss Herbarium (J) for their diverse and unstinting support. Kallie Franz is thanked for providing accommodation and permission to work on Davenham. We are highly grateful to Sandie Burrows for producing the excellent illustrations of the new species. Financial assistance from the University of the Witwatersrand (Research Incentive Scheme) and the National Research Foundation (Incentive Funding for Rated Researchers UID 119295) to K. Balkwill is acknowledged and appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Darbyshire, I., Balkwill, K. & Froneman, W. Two new species of Barleria (Acanthaceae) from the Soutpansberg of Limpopo Province, South Africa. Kew Bull 77, 475–489 (2022). https://doi.org/10.1007/s12225-022-10018-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12225-022-10018-3