Abstract
Introduction
The purpose of this study was to estimate the cost-effectiveness of atezolizumab plus chemotherapy in patients with metastatic non-squamous non-small cell lung cancer (NSCLC) from the United States (US) payers’ perspective in the first-line treatment.
Methods
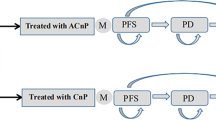
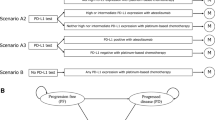
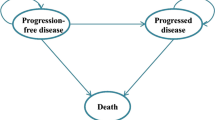
A mathematical Markov model was developed to estimate cost and effectiveness of atezolizumab combination therapy versus carboplatin plus nab-paclitaxel alone in the first-line therapy of metastatic non-squamous NSCLC from the data of IMpower130. Costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs) were analyzed, and model robustness was assessed by sensitivity analysis. Additional subgroup analyses were performed as well.
Results
Compared to chemotherapy, treatment with atezolizumab plus chemotherapy yields an increase of 0.16 QALYs with an increase in cost of $109,809.13, resulting in an ICER of $670,309.66 per QALY. The most influential factor in this model was the cost of atezolizumab. Probabilistic sensitivity analysis showed that there was 0% probability that atezolizumab plus chemotherapy was cost-effective at willingness-to-pay (WTP) values of $150,000 per QALY. The results of subgroup analyses showed that the ICER remained greater than $150,000/QALY across the all patient subgroups.
Conclusion
First-line treatment with atezolizumab in combination with carboplatin plus nab-paclitaxel is not a cost-effective option in patients with metastatic non-squamous NSCLC.


Similar content being viewed by others
References
Fitzmaurice C, Akinyemiju TF, Al Lami FH, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990–2016: a systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553–68.
Bonotto M, Gerratana L, Iacono D, et al. Treatment of metastatic breast cancer in a real-world scenario: is progression-free survival with first line predictive of benefit from second and later lines? Oncologist. 2015;20:719–24.
Herbst RS, Redman MW, Kim ES, et al. Cetuximab plus carboplatin and paclitaxel with or without bevacizumab versus carboplatin and paclitaxel with or without bevacizumab in advanced NSCLC (SWOG S0819): a randomised, phase 3 study. Lancet Oncol. 2018;19:101.
Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2019;69(2019):363–85.
Qiao F, Li N, Li W. Integrative bioinformatics analysis reveals potential long non-coding RNA biomarkers and analysis of function in non-smoking females with lung cancer. Med Sci Monit. 2018;24:5771–8.
Zhou D, Xie M, He B, Gao Y, Chen Q. Microarray data re-annotation reveals specific lncRNAs and their potential functions in non-small cell lung cancer subtypes. Mol Med Rep. 2017;16:5129–36.
Zou T, Yin J, Zheng W, et al. Rho GTPases: RAC1 polymorphisms affected platinum-based chemotherapy toxicity in lung cancer patients. Cancer Chemother Pharmacol. 2016;78:249–58.
Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:192–237.
National Comprehensive Cancer Network (NCCN) (2020) NCCN clinical practice guidelines in oncology (NCCN guidelines®): non-small cell lung cancer. V1. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed 24 Nov 2019.
Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65.
Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–301.
US Food and Drug Administration. FDA approves atezolizumab with chemotherapy and bevacizumab for first-line treatment of metastatic non-squamous NSCLC. FDA. 2019. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm627874.htm. Accessed 8 May 2019.
West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–37.
Goldstein DA, Chen Q, Ayer T, et al. First- and second-line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: a United States-based cost-effectiveness analysis. J Clin Oncol. 2015;33:1112–8.
Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol. 2011;11:139.
Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13:e195–203.
Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84.
Handorf EA, McElligott S, Vachani A, et al. Cost effectiveness of personalized therapy for first-line treatment of stage IV and recurrent incurable adenocarcinoma of the lung. J Oncol Pract. 2012;8:267–74.
XiaoMin W, Zhang Y, Tan CQ, Zeng X, Peng L. First-line nivolumab plus ipilimumab vs sunitinib for metastatic renal cell carcinoma: a cost-effectiveness analysis. JAMA Oncol. 2019;5:491–6.
ASP Drug Pricing Files. Centers for Medicare and Medicaid Services. 2019. https://www.cms.gov/Medicare/Medicare-Fee-forService-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2019ASPFiles.html. Accessed October 2019.
Drugs.com. Erlotinib prices, coupons and patient assistance programs. https://www.drugs.com/price-guide/erlotinib. Accessed October 2019.
Carlson JJ, Canestaro W, Ravelo A, Wong W. The cost-effectiveness of alectinib in anaplastic lymphoma kinase-positive (ALK+) advanced NSCLC previously treated with crizotinib. J Med Econ. 2017;20:671–7.
Mistry R, May JR, Suri G, et al. Cost-effectiveness of ribociclib plus letrozole versus palbociclib plus letrozole and letrozole monotherapy in the first-line treatment of postmenopausal women with HR+/HER2− advanced or metastatic breast cancer: a US payer perspective. J Manag Care Spec Pharm. 2018;24:514–23.
Huang M, Lou YY, Pellissier J, et al. Cost effectiveness of pembrolizumab vs. standard-of-care chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in the United States. Pharmacoeconomics. 2017;35:831–44.
Ting J, Tien-Ho P, Xiang P, et al. Cost-effectiveness and value of information of erlotinib, afatinib, and cisplatin-pemetrexed for first-line treatment of advanced EGFR mutation-positive non-small-cell lung cancer in the United States. Value Health. 2015;18:774–82.
Cho SK, Hay JW, Barzi A. Cost-effectiveness analysis of regorafenib and TAS-102 in refractory metastatic colorectal cancer in the United States. Clin Colorectal Cancer. 2018;17:e751–61.
Goldstein DA, Ahmad BB, Chen Q, et al. Cost-effectiveness analysis of regorafenib for metastatic colorectal cancer. J Clin Oncol. 2015;33:3727–32.
Zhang Y, Baik SH, Fendrick AM, Baicker K. Comparing local and regional variation in health care spending. N Engl J Med. 2012;367:1724–31.
Wu B, Zhang Q, Sun J. Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced renal-cell carcinoma. J Immunother Cancer. 2018;6:124.
Hoyle M, Green C, Thompson-Coon J, et al. Cost-effectiveness of temsirolimus for first line treatment of advanced renal cell carcinoma. Value Health. 2010;13:61–8.
Redman JM, Gibney GT, Atkins MB. Advances in immunotherapy for melanoma. BMC Med. 2016;14:20.
Emens LA, Ascierto PA, Darcy PK, et al. Cancer immunotherapy: opportunities and challenges in the rapidly evolving clinical landscape. Eur J Cancer. 2017;81:116–29.
Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32.
Long GV, Atkinson V, Cebon JS, et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial. Lancet Oncol. 2017;18:1202–10.
Criss SD, Mooradian MJ, Watson TR, et al. Cost-effectiveness of atezolizumab combination therapy for first-line treatment of metastatic nonsquamous non-small cell lung cancer in the United States. JAMA Netw Open. 2019;2:e1911952.
Wan X, Luo X, Tan C, et al. First-line atezolizumab in addition to bevacizumab plus chemotherapy for metastatic, nonsquamous non-small cell lung cancer: a United States-based cost-effectiveness analysis. Cancer. 2019;125:3526–34.
Georgieva M, Lima JPDN, Aguiar P, Lopes GD, Haaland B. Cost-effectiveness of pembrolizumab as first-line therapy for advanced non-small cell lung cancer. Lung Cancer. 2018;124:248–54.
She Longjiang, Huabin Hu, Liao Mengting, et al. Cost-effectiveness analysis of pembrolizumab versus chemotherapy as first-line treatment in locally advanced or metastatic non-small cell lung cancer with PD-L1 tumor proportion score 1% or greater. Lung cancer. 2019;138:88–94.
Insinga RP, Vanness DJ, Feliciano JL, et al. Cost-effectiveness of pembrolizumab in combination with chemotherapy in the 1st line treatment of non-squamous NSCLC in the US. J Med Econ. 2018;21:1191–205.
Acknowledgements
Funding
This manuscript, including the journal’s Rapid Service Fee, was supported by grants from the Hunan Natural Science Foundation of China (No. 2018JJ3852). All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Dong Ding, Huabin Hu, Mengting Liao, Yin Shi, Longjiang She, Linli Yao, Youwen Zhu, Shan Zeng, and Jin Huang have nothing to disclose.
Compliance with Ethics Guidelines
The model structure and data were based on results of IMpower130, the US publicly available databases, and published literature. As such, this article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors and did not require the approval of an independent ethics committee.
Data Availability
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.11955858.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ding, D., Hu, H., Liao, M. et al. Cost-Effectiveness Analysis of Atezolizumab Plus Chemotherapy in the First-Line Treatment of Metastatic Non-Squamous Non-Small Cell Lung Cancer. Adv Ther 37, 2116–2126 (2020). https://doi.org/10.1007/s12325-020-01292-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01292-3