Abstract
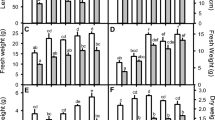
The oxidative stress response was studied in five different clones of Saccharum spontaneum (IMP-564, IS76-216, Pamba, SES-90 and Taiwan) by analyzing the physiological and biochemical parameters and gene expression pattern. The sixty days-old plants were sprayed with different concentrations of 30% H2O2 (300 ppm, 500 ppm and 1000 ppm) for consecutive three days in glass house. Adaptive response was recorded in terms of chlorophyll fluorescence, chlorophyll stability index (CSI), activities of peroxidase (POX) and super oxide (SOD), lipid peroxidation (LPO), protein and proline content at 48 h and 72 h of H2O2 treatment. The IMP -564 clone recorded high SOD and lower lipid peroxidation levels. Based on chlorophyll fluorescence, CSI, peroxidase, SOD and lipid peroxidation values, and the clone SES-90 recorded positive trend for stress tolerance. CSI showed significant difference between genotypes and different concentrations of H2O2. As the genotypes IMP-564 and SES-90 showed positive response to oxidative stress tolerance, the S. spontaneum genotype SES-90 was selected for comparative gene expression studies with Erianthus sp clones IJ76-389 and IK76-91. Different concentrations of H2O2 and different time intervals (control, 500 ppm and 1000 ppm for 48 h and 72 h) were used for gene expression studies. The stress responsive genes viz. Ascorbate peroxidase gene (Apx) Nam, Ataf1 and Cuc2 gene (NAC), Ethylene response factor gene (Erf), Glutathione S-transferases gene (Gst), Myeloblastosis antisense gene (Mybas) and Catalase gene (Cat) showed differential expression among the genotypes of S. spontaneum and Erianthus sp. Among the genotypes, SES 90 and IK76-91 showed better tolerance against oxidative stress and the expression levels of stress responsive genes in the genotype IK76-91 was higher than the genotype SES 90.













Similar content being viewed by others
Change history
07 November 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12355-022-01218-z
Abbreviations
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- POX:
-
Peroxidase
- LPO:
-
Lipid peroxidation
- CF:
-
Chlorophyll fluorescence
- Fv/Fm:
-
Variable fluorescence/Maximum fluorescence
- CSI:
-
Chlorophyll stability index
- TFs:
-
Transcription factors
- qRT-PCR:
-
Quantitative Reverse Transcriptase—Polymerase Chain Reaction
- H2O2 :
-
Hydrogen peroxide
References
Ambawat, S., P. Sharma, N.R. Yadav, and C.Y. Ram. 2013. MYB transcription factor genes as regulators for plant responses: An overview. Physiology and Molecular Biology of Plants 19 (3): 307–321. https://doi.org/10.1007/s12298-013-0179-1.
Arisi, A.M., G. Cornic, L. Jouanin, and C.H. Foyer. 1998. Overexpression of iron superoxide dismutase in transformed poplar modifies the regulation of photosynthesis at low CO2 partial pressures or following exposure to the prooxidantherbicide methyl viologen. Plant Physiology 117: 565–574.
Augustine, S.M., J.A. Narayan, D.P. Syamaladevi, C. Appunu, M. Chakravarthi, and V. Ravichandran. 2015. Erianthus arundinaceus HSP70 (EaHSP70) over expression increases drought and salinity tolerance in sugarcane (Saccharum spp. hybrid). Plant Science 232: 23–34. https://doi.org/10.1016/j.plantsci.2014.12.012.
Boaretto, L.F., G. Carvalho, L. Borgo, S. Creste, M.G. Landell, and P. Mazzafera. 2014. Water stress reveals differential antioxidant responses of tolerant and non-tolerant sugarcane genotypes. Plant Physiology and Biochemistry 74: 165–175. https://doi.org/10.1016/j.plaphy.2013.11.016.
Bowler, C., W. Van Camp, M. Van Montagu, and D. Inzé. 1994. Superoxide dismutases in plants. Critical Reviews in Plant Science 13: 199–218.
Cattivelli, L., F. Rizza, F.W. Badeck, E. Mazzucotelli, A.M. Mastrangelo, E. Francia, C. Mare, A. Tondelli, and A.M. Stanca. 2008. Drought tolerance improvement in crop plants: an integrated view from breeding to genomics. Field Crops Research 105: 1–14.
Cavalcanti, F.R., J.T.A. Oliveira, A.S. Martins-Miranda, R.A. Viégas, and J.A.G. Silveira. 2004. Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. The New Phytologist 163 (3): 563–571.
Chagas, R.M., J.A.G. Silveira, R.V. Ribeiro, V.A. Vitorello, and H. Carrer. 2008. Photochemical damage and comparative performance of superoxide dismutase and ascorbate peroxidase in sugarcane leaves exposed to paraquat-induced oxidative stress. Pesticide Biochemistry and Physiology 90: 181–188.
Chakraborty, A., and S. Bhattacharjee. 2015. Differential competence of redox-regulatory mechanism under extremes of temperature determines growth performances and cross tolerance in two indica rice cultivars. Journal of plant physiology 176: 65–77. https://doi.org/10.1016/j.jplph.2014.10.016.
Chen, J.H., H.W. Jiang, E.J. Hsieh, H.Y. Chen, C.T. Chien, H.L. Hsieh, et al. 2012. Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiology 158: 340–351. https://doi.org/10.1104/pp.111.181875.
Cui, M., M. Haider, P. Chai, J. Guo, P. Du, H. Li, W. Dong, B. Huang, Z. Zheng, L. Shi, X. Zhang, and S. Han. 2021. Genome-wide identification and expression analysis of AP2/ERF transcription factor related to drought stress in cultivated peanut (Arachis hypogaea L.). Frontiers in Genetics 12: 750–761. https://doi.org/10.3389/fgene.2021.750761.
Deng, X.P., Y.J. Cheng, X.B. Wu, S.S. Kwak, W. Chen, and A.E. Eneji. 2012. Exogenous hydrogen peroxide positively influences root growth and metabolism in leaves of sweet potato seedlings. Australian Journal of Crop Science. 6: 1572–1578.
Ding, N., A. Wang, X. Zhang, Y. Wu, R. Wang, H. Cui, et al. 2017. Identification and analysis of glutathione S-transferase gene family in sweet potato reveal divergent GST-mediated networks in aboveground and underground tissues in response to abiotic stresses. BMC Plant Biology 17: e225. https://doi.org/10.1186/s12870-017-1179-z.
Exposito-Rodriguez, M., P.P. Laissue, G. Yvon-Durocher, N. Smirnoff, and P.M. Mullineaux. 2017. Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nature Communications 8 (1): 49. https://doi.org/10.1038/s41467-017-00074-w.
Fang, Y., K. Liao, H. Du, Y. Xu, H. Song, and X. Li. 2015. A stress- responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. Journal of Experimental Botany 66: 6803. https://doi.org/10.1093/jxb/erv386.
Ferreira, T., M.S. Tsunada, D. Bassi, P. Araújo, L. Mattiello, and G.V. Guidelli. 2017. Sugarcane Water Stress Tolerance Mechanisms and Its Implications on Developing Biotechnology Solutions. Frontiers in Plant Science 8: 1077. https://doi.org/10.3389/fpls.2017.01077.
Gill, S.S., and N. Tuteja. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48: 909–930. https://doi.org/10.1016/j.plaphy.2010.08.016.
Hamim, Violita, and Triadiati and Miftahudin. 2017. Oxidative stress and photosynthesis reduction of cultivated (Glycine max L.) and wild soybean (G.tomentella L.) exposed to drought and paraquat. Asian Journal of Plant Science 16: 65–77.
Han, C., Q. Liu, and Y. Yang. 2009. Short-term effects of experimental warming and enhanced ultraviolet-B radiation on photosynthesis and antioxidant defense of Picea asperata seedlings. Plant Growth Regulation 58: 153–162. https://doi.org/10.1007/s10725-009-9363-2.
Harb, A., D. Awad, and N. Samarah. 2016. Gene expression and activity of antioxidant enzymes in barley (Hordeum vulgare L.) under controlled severe drought. Journal of Plant Interactions. 10: 109–116. https://doi.org/10.1080/17429145.2015.1033023.
Hirayama, T., and K. Shinozaki. 2010. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant Journal 61: 1041–1052. https://doi.org/10.1111/j.1365-313X.2010.04124.x.
Hong, Y., H. Zhang, L. Huang, L. Dayong, and F. Song. 2016. Over expression of a stress-Responsive NAC transcription Factor Gene ONAC022 improves Drought and salt Tolerance in Rice. Frontiers in Plant Science 10: 3389.
Hu, W.H., X.S. Song, and K. Shi. 2008. Changes in electron transport, superoxide dismutase and ascorbate peroxidase isoenzymes in chloroplasts and mitochondria of cucumber leaves as influenced by chilling. Photosynthetica 46: 581. https://doi.org/10.1007/s11099-008-0098-5.
Ito, Y., K. Koji, M. Kyonoshin, T. Teruaki, K. Masatomo, S. Motoaki, S. Kazuo, and Y.S. Kazuko. 2006. Functional Analysis of Rice DREB1/CBF-type Transcription Factors Involved in Cold-responsive Gene Expression in Transgenic Rice. Plant and Cell Physiology 47: 141–153.
Jia, B., M. Sun, X. Sun, R. Li, Z. Wang, J. Wu, et al. 2016. Overexpression of GsGSTU13 and SCMRP in Medicago sativa confers increased salt–alkaline tolerance and methionine content. Physiologia Plantarum 156: 176–189. https://doi.org/10.1111/ppl.12350.
Kate, M., and N.J. Giles. 2000. Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany 51: 659–668. https://doi.org/10.1093/jexbot/51.345.659.
Kohila, S., and R. Gomathi. 2018. Adaptive physiological and biochemical response of sugarcane genotypes to high-temperature stress. Indian Journal of Plant Physiology 23 (2): 245–260.
Kumari, A., P. Das, A.K. Parida, and P.K. Agarwal. 2015. Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Frontiers in Plant Science 6: 537. https://doi.org/10.3389/fpls.2015.00537.
Levine, A., R. Tenhaken, R. Dixon, and C. Lamb. 1994. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593. https://doi.org/10.1016/0092-8674(94)90544-4.
Li, J., C. Chen, J. Wei, Y. Pan, C. Su, and X. Zhang. 2019. SpPKE1, a multiple stress-responsive gene confers salt tolerance in tomato and tobacco. International Journal of Molecular Sciences 20 (10): 2478. https://doi.org/10.3390/ijms20102478.
Liang, X., L.U. Zhang, S.K. Natarajan, and D.F. Becker. 2013. Proline mechanism of stress survival. Antioxidants and Redox Signaling 19: 998–1011. https://doi.org/10.1089/ars.2012.5074.
Livak, K.J., and T.D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2DDC(T) method. Methods 25: 402–408.
Maheshwari, R., and R.S. Dubey. 2009. Nickel-induced oxidative stress and the role of antioxidant defence in rice seedlings. Plant Growth Regulation 59: 37–49. https://doi.org/10.1007/s10725-009-9386-8.
Manimekalai, R., N. Jini, M. Gokul, A. Selvi, R. Gomathi, and R. Arun kumar. 2018a. Biochemical and physiological response to oxidative stress in cultivated sugarcane and wild genera. Indian Journal of Plant Physiology 23: 1–10. https://doi.org/10.1007/s40502-018-0368-6.
Manimekalai, R., N. Jini, M. Gokul, A. Selvi, K. Pradheep, and R. Gomathi. 2018b. Hydrogen peroxide induced oxidative stress in sugarcane and response expression pattern of stress responsive genes through quantitative RT-PCR. Sugar Tech 20: 681–691. https://doi.org/10.1007/s12355-018-0604-4.
Manimekalai, R., N. Jini, M. Gokul, A. Selvi, A. Meena, R. Gomathi, and B. Ram. 2016. Genome wide analysis of NAC gene family ‘sequences’ in sugarcane and its comparative phylogenetic relationship with rice, sorghum, maize and Arabidopsis for prediction of stress associated NAC genes. Agri Gene 3: 1–11. https://doi.org/10.1016/j.aggene.2016.10.003.
Matysik, J., B. Bhalu. Alia, and P. Mohanty. 2002. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Current Science 82: 525–532.
Mhamdi, A., G. Queval, S. Chaouch, S. Vanderauwera, B.F. Van, and G. Noctor. 2010. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. Journal of Experimental Botany 61 (15): 4197–4220. https://doi.org/10.1093/jxb/erq282.
Mishra, S., A.B. Jha, and R.S. Dubey. 2011. Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma 248 (3): 565–577. https://doi.org/10.1007/s00709-010-0210-0.
Mittler, R., S. Vanderauwera, N. Suzuki, G. Miller, V.B. Tognetti, and K. Vandepoele. 2011. ROS signaling: The new wave? Trends in Plant Science 16: 300–309. https://doi.org/10.1016/j.tplants.2011.03.007.
Mizoi, J., K. Shinozaki, and K. Yamaguchi-Shinozaki. 2012. AP2/ERF family transcription factors in plant abiotic stress responses. Biochimica et biophysica acta 1819 (2): 86–96. https://doi.org/10.1016/j.bbagrm.2011.08.004.
Mohan, M.M., S.L. Narayana, and S.M. Ibrahim. 2000. Chlorophyll Stability Index (CSI): Its impact on salt tolerance in rice. International Rice Research Notes 25: 38–39.
Nakashima, K., and K. Yamaguchi-Shinozaki. 2006. Regulons in osmotic stress responsive and cold stress-responsive gene expression in plants. Physiologia Plantarum 126: 62–71.
Noctor, G., and C. Foyer. 1998. Ascorbate and glutathione: Keeping active oxygen under control. Annu Reviwe of Plant Physiology 49: 249–279. https://doi.org/10.1146/annurev.arplant.49.1.249.
Paramjeet, K., N. Sharma, M. Kumar and S. Kaur. 2014. Changes in growth and antioxidative enzyme activities in Vicia faba L. seedlings under chromium stress. Indian Journal of Plant Physiology 19(2), 101–106
Pujari, D.S., and S.V. Chanda. 2002. Effect of salinity stress on growth, peroxidase and IAA oxidase activities in vigna seedlings. Acta Physiologia Plantarum 24 (4): 435–439.
Roy, S. 2015. Function of MYB domain transcription factors in abiotic stress and epigenetic control of stress response in plant genome. Plant Signalling and Behaviour 10: 1559–2324. https://doi.org/10.1080/15592324.2015.1117723.
Sanghera, G.S., and A. Kumar. 2018. Recent perspectives towards enhancing drought tolerance in sugarcane. Journal of Plant Science Researh 34 (1): 23–34.
Sharma, P., and R.S. Dubey. 2007. Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Reports 26 (11): 2027–2038. https://doi.org/10.1007/s00299-007-0416-6.
Shinozaki, K., K. Yamaguchi-Shinozaki, and M. Seki. 2003. Regulatory network of gene expression in the drought and cold stress responses. Current Opinion in Plant Biology 6: 410–417. https://doi.org/10.1016/s1369-5266(03)00092-x
Song, X., Y. Wang, and X. Lv. 2015. Responses of plant biomass, photosynthesis and lipidperoxidation to warming and precipitation change in two dominant species (Stipa grandis and Leymus chinensis) from North China Grasslands. Ecology and Evolution 6 (6): 1871–1882. https://doi.org/10.1002/ece3.1982.
Srivastava, S., and R.S. Dubey. 2011. Manganese-excess induces oxidative stress, lowers the pool of antioxidants and elevates activities of key antioxidative enzymes in rice seedlings. Plant Growth Regulation 64: 1–16. https://doi.org/10.1007/s10725-010-9526-1.
Tanou, G., A. Molassiotis, and G. Diamantidis. 2009. Induction of reactive oxygen species and necrotic death-like destruction in strawberry leaves by salinity. Environmental and Experimental Botany 65 (2–3): 270–281. https://doi.org/10.1016/j.envexpbot.2008.09.005.
Upadhyaya, H., M.H. Khan, S.K. Panda, and S.K. Dhyani. 2007. Hydrogen peroxide induces oxidative stress in detached leaves of Oryza sativa L. Plant Physiology 33 (1–2): 83–95.
Van Toai, T.T., and C.S. Bolles. 1991. Post-anoxic injury in soybean (Glycine max) seedlings. Plant Physiology 9: 588–592.
Vijayalakshmi, D., S. Srividhya, S. Muthulakshmi, and R. Satishraj. 2014. Induction of Oxidative Stress by Hydrogen Peroxide Treatment in Rice Genotypes to Study the Osmolyte Accumulation Pattern and Antioxidant Capacity. Journal of Stress Physiology and Biochemistry 10 (3): 37–46.
Wang, X., H. Han, J. Yan, F. Chen, and W. Wei. 2015. A new AP2/ERF transcription factor from the oil plant Jatropha curcas confers salt and drought tolerance to transgenic tobacco. Applied Biochemistry and Biotechnology 176 (2): 582–597. https://doi.org/10.1007/s12010-015-1597-z.
Xu, W., K. Cui, A. Xu, L. Nie, J. Huang, and S. Peng. 2015. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiologiae Plantarum 37: 9. https://doi.org/10.1007/s11738-014-1760-0.
Xu, Z., and G.S. Zhou. 2006. Combined effects of waterstress and high temperature on photosynthesis, nitrogenmetabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta 224: 1080–1090.
Xu, Z., G.S. Zhou, and H. Shimizu. 2009. Effects of soildrought with nocturnal warming on leaf stomatal traits andmesophyll cell ultrastructure of a perennial grass. Crop Science 49: 1843–1851.
Xu, Z., G.S. Zhou, G. Han, and Y. Li. 2011. Photosynthetic potential and its association with lipid peroxidation inresponse to high temperature at different leaf ages in maize. Journal of Plant Growth Regulation 30: 41–50.
Yamaguchi-Shinozaki, K., and K. Shinozaki. 2006. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annual review of plant biology 57: 781–803. https://doi.org/10.1146/annurev.arplant.57.032905.105444.
You, J., and Z. Chang. 2015. ROS Regulation during abiotic stress response in crop plants. Frontiers in Plant Science. 6: 1092. https://doi.org/10.3389/fpls.2015.01092.
Zheng, X., B. Chen, G. Lu, and B. Han. 2008. Over expression of a NAC transcription factor enhances rice drought and salt tolerance. Biochemical and Biophysical Research Communication 379: 985–989.
Zonglie, H., K. Lakkineni, Z. Zhang, and D.S. Verma. 2000. Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiology 122: 1129–1136. https://doi.org/10.1104/pp.122.4.1129.
Author information
Authors and Affiliations
Contributions
RM planned and executed the work; JN conducted the experiments and prepared the MS; AS edited the MS, and AK analyzed the data.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Narayanan, J., Manimekalai, R., Selvi, A. et al. Physiological, Biochemical and Molecular Responses to Oxidative Stress in Saccharum spontaneum. Sugar Tech 25, 282–293 (2023). https://doi.org/10.1007/s12355-022-01189-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-022-01189-1