Abstract
Over the past 70 years, numerous authors studied the platyhelminth fauna near the island of Sylt in the eastern North Sea, each with a specific focus on varying aspects of ecology, morphology or systematics, and most of them dealing with a single habitat type. These data are combined with new data to get a comprehensive view of species richness, the distribution of individual species across tidal levels and sediment types and the resulting communities. So far, 398 platyhelminth species have been recorded around Sylt island, plus a still growing number of unidentified or undescribed species, in particular from subtidal waters. The distribution over habitat types (as defined by sediment types and tidal level) is described for the known species. Neighbouring habitat types overlap in species composition, and faunal composition changes continuously over environmental gradients. The number of species recorded per habitat type mirrors the sampling intensity and varied between some 20 species in poorly studied habitats and 150 in the well-studied intertidal sand flats. Corrected for variations in sampling intensity, platyhelminth species richness showed no significant differences between sediment types and only moderate variation over tidal levels. On a larger spatial scale, three faunal assemblages can be differentiated: the supratidal harbours brackish-water species (mesohaline in the upper and polyhaline in the lower supratidal), the intertidal polyhaline-marine species with a wide tolerance of variations in physical factors and the subtidal marine (± stenohaline) species. With respect to sediment type, mud and sand dwellers are well separated in the supra- and subtidal belt but less in the intertidal. Provided these rules are general, I conclude platyhelminth species richness in a given section of coastline mainly depends on the ranges of environmental factors covered. Nineteen new species encountered during this study are described.
Similar content being viewed by others
Introduction
Systematic studies on the platyhelminth fauna of the island of Sylt date back to 1949 (Ax 1951) and persisted until the 1990s. During that period, dozens of studies resulted in > 100 publications on the Sylt platyhelminth fauna, each with a customised focus on varying aspects of ecology, morphology, ultrastructure or systematics, and most of them dealing with a single habitat type. Information about the ecological demands of single species is thus scattered over many papers. For the rarer species, in particular, informational content per study often is too small to identify the habitat type supporting persistent populations. Nevertheless, every single study holds some bits of information. In order to retrieve this information, I united the previous records in a single data matrix and combined it with new data collected after 2015.
This comprehensive view of localities enables analyses beyond the limits of a classical one-habitat study. As an example, year-round presence of a species in a single locality may be a hint towards a persistent population, but might also result from specimens displaced from a neighbouring population with a far higher abundance. Since the combined data matrix includes almost all habitat types present around Sylt island, it can be used to distinguish between these alternatives and help to recognise the habitat type(s) actually facilitating persistent populations. In addition, we can get information about species-specific habitat requirements with respect to environmental factors such as tidal level and sediment composition that had been routinely included in the past studies. This also yields information on habitat specificity of the species: are the tolerated ranges wide, allowing specimens to occur over many habitat types, or are they narrow, restricting each species to a single well-defined habitat? And is the number of tolerated habitat types all the same over the entire tidal gradient, or do species inhabiting higher tidal levels generally tolerate wider ranges of environmental factors because physical factors such as salinity and temperature are more variable there?
Finally, the combined matrix of all Sylt data also enables analyses on a supra-specific level of organisation: does species richness vary over tidal levels or sediment types, or do all habitat types harbour similar numbers of species? How does community composition change along environmental gradients such as tidal level, continuous, discontinuous or not at all? Thus, can all species basically be found everywhere or can the Sylt platyhelminth fauna by separated into well-defined communities?
Material and methods
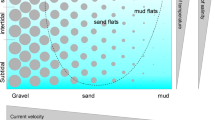
In the eastern North Sea, Sylt belongs to a chain of barrier islands separating the North Sea from the Wadden Sea. According to this position, the island coast includes both wave-exposed sandy beaches facing to the North Sea and sheltered habitats along the Wadden side, including salt marshes and beaches, tidal flats with sediments ranging from mud to coarse sand and a subtidal section ranging from muddy fine sand in the most sheltered sites to very coarse sand in the highly dynamic tidal inlets (Fig. 1). Together with the existence of a marine field station (the ‘Wattenmeerstation Sylt’ of ‘Biologische Anstalt Helgoland’, now part of ‘Alfred-Wegener-Institut, Helmholtz-Zentrum für Polar-und Meeresforschung’), this wide range of sediment types was the reason why Sylt became a centre for meiofaunal research in general, and platyhelminth research in particular.
Until the 1980s, most studies aimed to analyse the species composition and the seasonal dynamics of species; later studies often included field experiments to analyse ecological effects. Accordingly, the data that derived from these studies are heterogeneous in structure, including both qualitative and quantitative data, from un-replicated large sediment cores from the subtidal (with focus on the species spectrum) to highly-replicated but minute sample volumes. The latter in particular came from manipulative field experiments in the intertidal; only abundance data from un-manipulated control sites were used for the current analysis. The methods used to retrieve platyhelminths from the sediment varied both temporarily and spatially; temporarily with the development of new extraction methods, and spatially with the need to adapt extraction methods to the sediment type studied (e.g. Noldt and Wehrenberg 1984; Armonies and Hellwig 1986). This diversity of methods introduced some degree of systematic error, in particular to the comparability of abundance estimates. Therefore, this study is mainly based on presence-absence data with a focus on the main effects of environmental factors while detailed analyses of contrasts between different habitats are out of scope; they demand for adequately planned further studies.
About 100 papers have been evaluated for this analysis (see Online resource 1) which covered different years and seasons. The data presentation in these papers varies as well, from a rough description of localities only, over mean abundances per sampling site to detailed results from every replicate sediment core and depth level. Similarly, some papers include GPS-coordinates of the sampling sites and detailed descriptions of habitat type, sediment composition and tidal level while others only give very rough information. Therefore, the decision whether or not to include the data in this analysis was individual for each paper, based on the quality of available environmental data. Where quantitative abundance data were available, abundances were all transformed to 10 cm−2 and stored in a separate matrix (Online resource 2, Table 2-2) that was then reduced to presence-absence level (Online resource 2, Table 2-3) and united with the original matrix of presence-absence data (Online resource 2, Table 2-1) to give the final matrix (Online resource 2, Table 2-4) which is the base for the following analyses. However, many of the studies dealt with single platyhelminth families only; species outside these families were ignored. Thus, non-existence of a species in a data set does not necessarily mean it did not occur. The current evaluation therefore only deals with positive records of species.
Habitats are classified according to tidal level and sediment composition which are considered key-factors for meiofaunal distribution (Coull 1988; Giere et al. 1988). Tidal level separates the species according to their demand for humidity; those who permanently need fluid water are restricted to the subtidal, those satisfied with humidity may also occupy the higher levels. I used seven classes for tidal level, arranged according to an increasing demand for humidity and decreasing tolerance to temporary desiccation:
-
(1)
The upper supratidal, delimited as the zone higher than 0.5 m above spring high tide level; this zone is only flooded during gales and includes the uppermost part of sandy beaches, sometimes with first pioneer plants, and the plant associations Armerietum maritimae in un-grazed and Juncetum gerardii in grazed salt marshes (all vegetation associations according to Ellenberg 1982).
-
(2)
The lower supratidal, between the upper supratidal and spring high tide level; this zone becomes submerged during moderate storms from a westerly (onshore) direction. Without vegetation in exposed beaches and with the plant association Puccinellietum maritimae in salt marshes.
-
(3)
The belt between spring and neap high tide level; in exposed areas without vegetation while a seasonal Salicornietum may occur in sheltered areas, occasionally replaced by Spartina townsendii.
-
(4)
The intertidal between the neap tide levels; this belt is regularly flooded by the tides except during strong offshore wind, and regularly emerged except during strong onshore wind. In sheltered positions occasionally with seagrasses Zostera marina and Zostera noltii.
-
(5)
The belt between spring and neap low tide level.
-
(6)
The shallow subtidal between neap low tide level and some 5 m below mean low tide level; in the turbid waters of the Wadden Sea, this may be the maximum depth for positive primary production of phytobenthos.
-
(7)
The subtidal deeper than 5 m below mean low tide level. Maximum water depth in the Sylt area is some 35 m in the main tidal channel ‘List deep’; offshore North Sea sediments have hitherto been studied to a depth of some 20 m which is reached approximately 50 km west of the island.
Since the overwhelming majority of platyhelminths are sediment dwellers, the sediment type is another key factor for habitat classification. Sediment composition is influenced by many environmental factors such as exposure, currents, nature and amount of organic matter, and in turn it determines substrate-related physiographic parameters such as porosity, permeability and oxygen supply (Giere et al. 1988; Giere 2008). Most studies used a field classification system to describe sediment type according to the classes mud, very fine sand, fine sand, medium sand, coarse sand and very coarse sand. This is a rather rough classification because it does not distinguish, for example, between pure medium sand and medium sand mixed with a few percent of fine sand or mud—which may be enough to decrease available pore size and thus accessibility to some meiofaunal species. In addition, sediment composition may vary on spatial scales of a few cm, both horizontally (e.g. due to accumulation of fine particles in ripple troughs but not on crests) as well as vertically in laminated sediments as may be brought about by physical factors (such as stronger currents removing fine particles, deposition of fine particles after a storm or by areal import of particles) or by biotic factors (such as selective deposit feeding lugworms (Reise 1985)). Thus, a finer classification of sediment types was desirable. However, during this analysis, I need to adhere to the classification given in the evaluated studies. Contrary to all other sediment size classes, very coarse sand occurred in very few cores only and was assumed to be not adequately represented. Therefore, in the habitat matrix, the few records of very coarse sand were added to the coarse sand unit. Thus, in this study, a ‘habitat type’ is defined as a point in seven tidal-level by five sediment-type matrices, which results in a total of 35 habitat types for the Sylt area.
Questions and statistical methods
Because of the methodological inconsistence of data, this study concentrates on more general questions on the platyhelminth faunal composition in the Sylt area, viz.
-
(1)
Species richness: How are platyhelminth species distributed over habitat types? How does species richness vary over habitat types, and what are possible causes of variability?
-
(2)
Species niches: What are the realised niches of single species and where can we find persistent populations?
-
(3)
Community organisation: How does community composition change over environmental gradients, continuous or discontinuous? In the latter case, what factors may be responsible and what are the joint requirements of the resulting communities?
Study intensity and thus the amount of data strongly varied over habitats. The sandy intertidal was the best studied, in particular because most field experiments used these easy-to-access areas. Subtidal studies were limited by the drought of the vessel used for sampling and by possible sediment disturbance by the ship’s propeller in shallow waters. Accordingly, the shallow subtidal is likely to be under-sampled. The same is true for some other habitat types that are rare and/or difficult to access in the Sylt area. Direct evaluation of the number of localities per species and habitat type may therefore be skewed by study intensity. To compensate for that, I calculated the frequency per habitat type, i.e. the percentage of cores that contained a species. These semi-quantitative frequencies strongly correlate with abundances: irrespective of core size, a high abundance increases the probability to encounter the species, and the reverse, frequent encounters add to a high abundance. A high frequency/abundance is thus expected to indicate environmental conditions suitable for a species. However, whether or not the high abundance habitats harbour optimal physical conditions for a species cannot be decided from these data because the realised physical niche may have been limited by biotic factors such as predation, competition or food availability.
The distributions of study intensity and species richness over habitat types are visualised using 3D-contour plots with distance-weighted least square smoothing (Statistica©); the smoothing procedure (using standard parameters of the statistics package) partially compensates for differences between habitat types in the number of data points and thus produces a ‘normalized’ picture of species richness. Input data for each of the graphs were the class of tidal level for the x-axis, class of median grain size for the y-axis and the corresponding species numbers for the z-axis contour. The effect of variable study intensity over habitat types was tested by ANCOVAs controlling for the co-variant effects of tidal level and sediment type, respectively, to find out which aspect of study intensity (the number of studies, number of studied sediment cores or their total size, respectively) influenced the recorded species richness the most.
To visualise species-specific habitat use, two 3D-contour plots as described above are given for each of the species encountered in the Sylt area. Based on Table 2-4 in Online resource 2, the first plot shows the number of records per habitat type to answer the question ‘where has the species been found?’, and the second shows frequencies, thus ‘where is the species most abundant?’. These graphs summarise the present state of knowledge on the species-specific realised niches which may be used to derive hypotheses on the potential niches and the species-specific limiting factors. This paper only includes a few examples of these results; the full set of graphs is given in Online resource 3.
The similarity of species composition over habitats was estimated by Jaccard’s index which gives the percentage overlap of species composition in two habitats. This was calculated 34 times, once for each habitat type, with the habitat in focus as a fixed point for comparisons (deeper subtidal mud missing because of a lack of data).
The results of this study are described in two chapters: Results (1) deals with the community-related aspects which are subsequently discussed. New species encountered during this study are described in Results (2), with a discussion of its own for each new taxon.
Results (1) community-related aspects
Species richness
Until June 2020, some 465 marine and brackish-water platyhelminth species have been recorded from the island of Sylt (Online resource 1). However, only 398 of them are formally described, the rest are unidentified or undescribed species that are currently only known from mostly unpublished photographs and/or drafts left by previous investigators. These unidentified species are not included in the following analyses.
The number of recorded platyhelminth species per habitat type varied between some 20 and 150, with highest species richness in the sandy intertidal and the sandy deeper subtidal (Fig. 2). These are the most intensely studied habitats; for the sandy intertidal, the number of available records is higher than for any other habitat type (Fig. 3) though the analysed sediment volumes often were rather low. For the deeper subtidal sands, the number of sediment cores was only intermediate but their volume exceptionally large.
Accordingly, ANCOVAs revealed that the number of records is a significant covariant of recorded species richness over sediment classes (Table 1); adjusted for this effect, species richness still tended to be highest in medium sand (Fig. 4) but this was not statistically significant.
Species richness also co-varied with the number of records over tidal levels (Table 2). Adjustment for this co-variant effect left variations over tidal levels still significant with three maxima in the upper supratidal, mid-intertidal and deeper subtidal, respectively (Fig. 5). Thus, platyhelminth species richness varies over tidal levels but less over sediment types.
Distributional patterns of single species
On average, single species were recorded 33 times in the Sylt area and occupied 5.8 habitat types (as defined by the 5 × 7 sediment types × depth classes matrix). The number of recorded sediment classes, tidal levels and habitat types all strongly correlate with the number of records per species (Fig. 6). Only a few species showed narrow ranges despite a higher number of records (habitat specialists). On the other hand, no species occurred everywhere; all but one were limited to less than half of the habitat types (Fig. 6e).
The distribution over habitat types was charted twice for each species, based on the number of records and on frequency, respectively (Online resource 3). The uneven study intensity over habitat types (Fig. 3) often caused strong deviations between number-of-records and frequency-based patterns. Nematoplana coelogynoporoides, for example, was most often recorded from intertidal medium sand (Fig. 7), whereas the percentage of cores containing the species was highest in coarse sand, from the supratidal to the shallow subtidal (Fig. 8). Thus, for this species, sediment type seems to be more important than tidal level.
Contrary to that, there are two hot-spots for the number of records in Neoschizorhynchus parvorostro (Fig. 9) while frequency-based data indicate it is basically a subtidal species (Fig. 10) where it occupies a wide range of sediment types. Thus, for this species, tidal level seems to be more important than sediment type. Just as in a few other species, its vertical distribution in the sediment varied over tidal levels: in the intertidal it was limited to deeper sediment layers while it also occurred close to the sediment surface in subtidal habitats.
With focus on the habitat holding maximum abundance, three basic distributional types can be distinguished. Neoschizorhynchus parvorostro is a representative of a species group with maximum abundance in the deeper subtidal and decreasing abundance/frequency towards the intertidal (Fig. 10). In the second group, abundance centres in the intertidal such as in Archilopsis arenaria (Fig. 11), and in the third in the supratidal as in Macrostomum tenuicauda (Fig. 12). These three groups are also mirrored in the platyhelminth species richness over depth classes (Fig. 5). The supratidal group combines species known as inhabitants of brackish waters and a few freshwater species known to tolerate brackish conditions (Online resource 1, Table 1-4). Species richness of this group is highest in the upper supratidal and decreases towards the intertidal (Fig. 5). The reversed pattern is found in the subtidal group of species, with maximum abundance in the deeper subtidal that levels out in the intertidal. In Fig. 5, this group forms the peak of species richness at the seaward side. The intermediate peak in Fig. 5 comes from the third species group with a population centre in the intertidal.
With respect to sediment composition, supratidal mud-dwellers like Macrostomum tenuicauda (Fig. 12) are well separated from supratidal sand-dwellers like Proschizorhynchus gullmarensis (Fig. 13) and the same seems to apply for the subtidal (though the mud-side is still understudied in the subtidal). In contrast, species with peak abundance in the intertidal seem to be less specific to sediment composition.
A few species do not fit the sediment classification scheme; they live on seagrasses and algae and rarely, or never, showed up in the sediment underneath. However, this assemblage is still under-sampled in the Sylt area.
Similarity of faunal associations
Since individual species have rather wide environmental niches that cover several sediment types and depth classes, the faunal assemblages of neighbouring habitat types merge into one another, though at different levels (Fig. 14). Associations in the upper supratidal and deeper subtidal have relatively little overlap with their adjoining (lower supratidal and shallow subtidal, respectively) habitats and both of them are well separated from the intertidal ones. Thus, there is a tripartition into supratidal, intertidal and deeper subtidal assemblages again. With respect to sediment composition, the mud and coarse sand associations are well separated in the upper supratidal and the deeper subtidal, but far less in intermediate tidal levels (Fig. 14). Here, some highly motile species may temporarily show up in any sediment type and increase faunal affinities between distant sediment classes.
Discussion
Species-specific ranges and delimitation of communities
Individual species showed relatively wide ranges of tolerance for environmental factors and most species occupied several habitat types. However, the habitat ranges depictured in Online resource 3 are limited by several factors. First, in most species, only sexually mature species can be determined to species level while determination efforts in juveniles usually stop at a generic or higher systematic level. Since these juveniles are not included in the current data matrix, the real number of occupied habitats is likely to be higher than recorded, though there are exceptions. As an example, most authors of the original data did not hesitate to identify juvenile (sexually immature) Proseriata Unguiphora with paired eyes but no statocyst with Nematoplana coelogynoporoides which is currently the only known species of the genus in the Sylt area. Thus, the niche representation of the few species with striking secondary characters may be more detailed than in the usual case where immature individuals were not considered (as long as the species-specificity of the secondary characters holds true, at least). A second limitation is study intensity (Fig. 6) indicating that species currently recorded a few times only may just seem to be limited to a narrow range of habitat types while they may prove to be far wider distributed once more records are available. Only few species showed narrow ranges despite a higher number of records; these species either came from the upper supratidal or the deeper subtidal. Possibly they occupy further habitat types beyond the geographical limits of the studied area, i.e. deeper waters or more limnetic/terrestrial areas, respectively. Finally, we need to remember that this data evaluation can only indicate the realised part of the potential niche which may be truncated by unknown biotic interactions as well as additional physical factors.
Compared to the open sea, variability of physical factors like temperature and salinity is higher in the shallows of the Wadden Sea and still increases in the landward direction. In this boreal zone, supratidal species need to be able to survive wide ranges of temperature and salinity, with extremes like winter frost and summer heat, and salinity variations from freshwater after heavy rainfall to evaporation-driven hyper-salinity, which may all last for weeks or even months. Thus, species able to survive in the supratidal should be able to occupy the entire spectrum of habitats studied—but they clearly do not. Instead, they are either restricted to the supratidal or only marginally enter the intertidal zone. In the German Bight with an average seawater salinity of some 30 psu, the supratidal belt constitutes the transitional zone from marine to limnetic conditions, with an average salinity in the brackish range. With respect to the platyhelminth fauna, it is occupied by ‘true’ brackish water species, i.e. species that do not only tolerate brackish conditions for some time but that need the brackish range of salinity (Ax 2008). Besides the North Sea supratidal, these species also occupy the brackish zone of estuaries and the entire Baltic Sea which has brackish conditions throughout (Armonies 1988a).
The deeper subtidal represents the opposite side of the spectrum of habitats available around Sylt island. In the subtidal, variability of most physical factors is much smaller than at higher tidal levels. In addition, there is no need to endure tidal exposure, i.e. subtidal species always have an aquatic environment. This is different in higher tidal levels where the upper sediment layers are no more saturated with water during low tide and specimens need to survive in damp air, instead. During this study, some platyhelminths from the subtidal were observed to burst after they got in contact with air during sample processing. Thus, besides the ranges of physical factors, the temporary absence of liquid water may be another factor restricting some species to the permanently submerged subtidal. This might explain why some species such as Neoschizorhynchus parvorostro live close to the sediment surface in the subtidal but are restricted to larger sediment depth at their intertidal border of occurrence. In reversed view, limitation of a species to deeper sediment layers in the intertidal may be a hint towards a subtidal population centre.
The assemblage of intertidal species takes an intermediate position. They also need wide ranges of tolerance for variations in physical factors, but because of regular submergence, physical extremes occur for shorter periods than in the supratidal. Against the brackish conditions in the supratidal, intertidal salinity usually remains in the marine to polyhaline range. In part, the wide ranges of occupied habitat types noted for intertidal species may be a consequence of the wide tolerance ranges needed in this tidal level. In addition, the high mobility found for quite a number of intertidal platyhelminth species (Armonies 1989) may contribute to extended habitat ranges. At night, these species may actively leave the sediment and swim in the water column for a while (Armonies 1988b, 1988c, 1988d). While swimming, they get transported with the tidal currents and so return to the sediment some distance remote from their starting point, depending on the period of swimming and the velocity of tidal currents (Armonies 1990). During tidal emergence, these species have no chance to leave the sediment again, even if the last point of return may hold unfavourable conditions—they have to endure until the next period of submergence. Thus, single specimens may be found outside the ranges of environmental factors allowing for a persistent population. The same may occur when superficial sediment layers get eroded during stormy weather, and meiofaunal species get suspended in the water column against their will (e.g. Xylander and Reise 1984); these eroded species may finally also land in an unfavourable habitat type. As a consequence, the typical or preferred habitat type of a species cannot be derived from a single locality. Both active swimming and passive erosion from the sediment have currently only been studied in intertidal habitats and may contribute to the wider environmental ranges observed for intertidal platyhelminth species. Whether or not these types of mobility also occur in the supra- or subtidal has not yet been studied. But since both ways need a water cover, they are limited to storm tides in the supratidal and may not be quantitatively important, there.
Platyhelminth diversity
With some 400 described species, the island of Sylt presumably harbours the most diverse platyhelminth fauna known, so far, for such a small area. For comparison, the Belgian coastline (N-S extension some 40 km, just as the island of Sylt) is known to harbour some 150 platyhelminth species and some 250 including the adjoining localities in Northern France and the Netherlands Delta area (Schockaert et al. 1989). Presumably, the higher Sylt numbers result from the combination of a high study intensity with a high diversity of habitat types occurring around the island. Within habitat types, species richness was significantly affected by the number of studies, far less by the number of sediment cores or by their volume (Tables 1 and 2). Since most studies were independent from each other, each selected their study sites individually and so studied geographically different sites and/or different patches. Thus, patchiness may be the main reason why species numbers within a habitat class strongly increased with the number of studies but less with replication, i.e. the number of cores (de Wolf 1989).
Despite the high study intensity in the Sylt area, the platyhelminth species inventory is far from being complete, as indicated by numerous drafts and photographs of unidentified species left by previous investigators. And in new samples collected during the past years, about every second sample from the Sylt subtidal contained undescribed species (Armonies 2018, 2020) that are not included in this analysis. Subtidal species numbers are therefore under-estimates while the number of new species was rather limited in intertidal sampling (Armonies 2017). This is in accordance with habitat-specific sampling intensity (Fig. 4); conversely, the figure indicates the habitat types that have been insufficiently studied: besides, the hard-to-access shallow subtidal and the rare spots of deeper subtidal mud, easy-to-access supralittoral habitats have also been understudied, so far. With respect to beaches, this is due to the large sampling depth often needed in the supratidal section where platyhelminths, just as other meiofauna, may reach down beyond the ground water table (Schmidt 1968). Some of the coarse-grained beaches thus need to be sampled down to a sediment depth of some 2 m while meiofaunal abundance strongly decreases in the landward direction, at the same time. Consequently, high sampling effort is needed. With respect to salt marshes, only un-grazed muddy and grazed fine-sandy marshes have hitherto been studied. Further, marsh types are likely to harbour further species, in particular un-grazed marshes where decomposing plant litter gives additional habitat for meiofauna (currently included in ‘supratidal mud dwellers’). These supratidal habitats are likely to contribute further brackish-water and brackish-tolerant freshwater species, in particular the species known for the Baltic but not for the North Sea, up to now.
Conclusions
Platyhelminth populations in the shallows of the North Sea tolerate rather wide ranges of salinity and sediment composition; therefore, single species occupy several habitat classes, with decreasing abundance towards the margins. Species composition changes continuously over gradients of tidal level and grain size. Distinctly delimited communities are only visible on a large spatial scale and may be roughly described as brackish-water species in the supratidal, polyhaline-marine species in the intertidal, and stenohaline marine species in the subtidal. With respect to sediment composition, mud and sand dwellers are well separate in the supra- and subtidal but less in the intertidal level. The overall high species richness in the Sylt area is explained by a wide range of habitat types and a high study intensity which may have revealed a higher percentage of the total platyhelminth fauna present than anywhere else. However, due to a lack of areas studied in similar intensity, it is not clear whether or not some 400 species along some 40 km of coastline (N–S extension, total outline of Sylt Island some 100 km) is a usual dimension for platyhelminths, on a worldwide scale.
Results (2) new species
This analysis includes data from new and formerly unpublished localities that contained quite a number of undescribed species. For 19 of them morphological observations are sufficient for a species description (Table 3). Supplementary information on the genus Microstomum and the species Microstomum crildensis, Bradynectes sterreri, Bradynectes robinhoodensis, and Kataplana mesopharynx is given in Online resource 4.
Microstomum spirale sp. nov.
https://zoobank.org/3A191FB4-537E-4F56-8933-1BACB0B793E6(Figs. 15 and 16)
Localities (1) Type locality: List Deep, coarse sand, water depth 23 m (55.0640°N, 8.4340°E; 7 May 2020, 17 individuals). (2) List Deep, medium sand, water depth 6 m (55.0594°N, 8.4113°E; 25 Oct 2019, 1 individual). (3) North Sea, 10 km WNW of Sylt, coarse sand, water depth 14 m (55.0350°N, 8.2219°E; 6 Nov 2019). (4) North Sea, 40 km WNW of Sylt, fine sand, water depth 18 m (55.1304°N, 7.7949°E; 31 Mar 2020). (5) North Sea, 20 km W of Sylt, very fine sand, water depth 16 m (55.0473°N, 008.0818°E, 26 May 2020, 2 individuals).
Material Live observations, photographs; six whole mounts, one designated holotype (AWI Sylt P2020-101), and four paratypes (AWI Sylt P2020-102–P2020-106).
Etymology The species name refers to the shape of the stylet
Diagnosis Microstomum without eyes, with a long preoral intestine, rhabdite bundles scattered over entire body and adhesive papillae all around body except the anterior end; stylet spiral-shaped
Description Unpigmented animals without eyes spots, anterior end conical in the first zooid of a chain (Fig. 15a) but broadly rounded in recently separated zooids and solitary specimens (Fig. 15c); caudal end rounded (Fig. 15a–c). Body length up to 1 mm in two-zooid chains, up to 1.5 mm for four-zooids, and up to 1.4 mm in sexual solitaries. With adhesive papillae over entire body length except the anterior tip, most numerous at caudal end. Rhabdites 8 to 10 μm long, in bundles of 5–12 (mostly 8–10) scattered over entire body, best seen in un-squeezed specimens (Fig. 16b). However, the few specimens obtained from muddy sediment had less rhabdite bundles with less rods per bundle. Preoral intestine long, extending anterior to brain, nearly reaching the anterior end in specimens recently isolated from the zooid chain. Ciliary pits cup-shaped in relaxed specimens but just a slight depression in stretched organisms. A few individuals with nematocysts, most abundant in the caudal half of the body.
With a single median ovary starting directly behind the pharynx. Testis single, lateral in the second body half; copulatory bulb ovoid (about 50 × 80 μm) with a twisted stylet. The stylet develops from the distal end (tube diameter 4 μm, distal end cut-off obliquely with an opening of 10 μm; Fig. 16a); proximally the tube diameter increases steadily. The maximum measured stylet length was 158 μm with a proximal opening of 12 μm.
Discussion So far, four described species of the genus share the combination of the characters eyes absent, preoral intestine long and rhabdites and adhesive papillae present both anteriorly and posteriorly. Among these species, sexual organs are only known for Microstomum papillosum Graff, 1882 which has a stylet distinctly differing from M. spirale sp. nov. Microstomum breviceps Marcus, 1951 from Brazil is characterised by a spatulate tail plate resembling parotoplanids (see Fig. 9 in Marcus 1951). In Microstomum weberi Atherton and Jondelius 2019, both rhabdite bundles and adhesive papillae are restricted to the body ends while they occur all over the body in M. spirale sp. nov. Finally, in Microstomum afzelii Atherton and Jondelius 2019, adhesive papillae are restricted to the very anterior and caudal body ends while they occur all over the body except the anterior end in M. spirale sp. nov. Thus, the character combination in M. spirale sp. nov. fits none of the species with unknown reproductive organs.
Microstomum semicirculare sp. nov.
https://zoobank.org/C32F4A74-1B29-49E2-9E0C-E33569EAF101(Fig. 17)
Localities (1) North Sea, coarse sand 10 km W of List, water depth 14 m (55.0350°N, 008.2219°E, 6 Nov 2019, 8 individuals). (2) Type locality: List Deep, coarse sand, repeatedly in water depths 10 to 26 m (Jan to May 2020, always 1-2 individuals). (3) North Sea, very fine sand 20 km W of List (55.0473°N, 008.0818°E, 26 May 2020, 1 individual)
Material Live observations, photographs; 2 whole mounts, one designated holotype (AWI Sylt P2020-108), the other paratype (P2020-109).
Etymology The species name refers to the shape of the stylet
Diagnosis Microstomum with a slightly conical anterior and a rounded caudal end, with a slight constriction between brain and pharynx, U-shaped ciliary pits, without eyes, with a long preoral intestine, few faint rhabdite bundles near the mouth opening and adhesive papillae all around body; stylet semicircular
Description Animals with a broadly oval body shape, no eyespots, unpigmented but most with brownish intestinal contents, four-zooid chain up to 1.5 mm long, solitaries up to 1.2 mm. Anterior end slightly conical with a rounded tip and a slight constriction between brain and pharynx. Ciliary pits located in the constriction, U-shaped in relaxed specimens; otherwise, inconspicuous and hard to see at all. Adhesive papillae abundant at the broadly rounded caudal end and in a lower density along the entire body; anterior adhesive papillae only seen in the first zooid of a chain but not in zooids freshly isolated from a chain (as visible by the intestine still reaching to the anterior end). In specimens with the anterior end closed by an epidermal layer, the preoral intestine reached to half distance between anterior end and the brain. Rhabdites faint, two bundles of about 10 slender rods was seen close to the mouth opening in one individual but none detected in others, possibly because of their faint nature. Nematocysts spread over entire body, numerous in some individuals but scarce or absent in others, presumably depending on the previous diet.
Ovary unpaired, median, from mid-body to the beginning of the last quarter; testis and copulatory organ behind. Copulatory bulb (about 50 × 30 μm) weakly muscular. The stylet is a delicate thin-walled tube of 100 μm length that forms a semi-circle 70 μm in diameter. Proximal opening of the tube 10 μm, continuously tapering to a distal diameter of 3.5 μm with a distal opening obliquely cut-off. In most individuals, the stylets were very delicate thin-walled and easily bent or crumpled during preparation. This delicate nature seems to be a transitional state during stylet formation: studying further species day-by-day, the stylet walls became continuously more robust.
Discussion Four of the currently known marine Microstomum species share the characters absence of eye pigmentation, a pre-pharyngeal intestine extending anterior to the brain and presence of adhesive papillae in the anterior and posterior body (Atherton and Jondelius 2019). Among these, M. breviceps Marcus, 1951 is characterised by its unique spatulate posterior end while M. afzelii and M. weberi (both Atherton and Jondelius 2019) bear adhesive papillae at the anterior and caudal body ends but none along the intermediate margins. Thus, M. papillosum Graff, 1882 is most similar to M. semicirculare sp. nov. In both species the stylet is a curved tube continuously tapering towards the distal end. While the tube forms a half circle in M. semicirculare sp. nov., the proximal and distal ends of the tube are bent outwards in M. papillosum. Both species bear rhabdites at the anterior end, faint ones in M. semicirculare sp. nov., but striking and more abundant in M. papillosum. Finally, in M. papillosum, the anterior end is rounded and the caudal end slightly tapered while this is reversed in M. semicirculare sp. nov.
Austromacrostomum pedistylum sp. nov.
https://zoobank.org/958D20FD-A30A-49D7-8694-5AB7EABB0844(Figs. 18 and 19)
Type locality Eastern North Sea, 10 km WNW off the island of Sylt (55.0355°N, 008.2134°E), fine sand in 14 m water depth (9 Apr 2018, 4 individuals).
Material Live observations including photographs; one whole mount designated holotype (AWI Sylt P2020-312).
Etymology The species name refers to the foot-like (Latin: pes) shape of the stylet tip
Diagnosis Austromacrostomum with a penis stylet ending in a broad foot-shaped tip distally and lacking a lateral appendage, bursal apparatus with a long mouth-piece and a semicircular mid-piece.
Description Unpigmented organisms of 0.9 to 1.1 mm body length, with a pair of closely-spaced reddish-brown eyes in front of the brain and rhabdites of 4–5 μm length abundant all over the body; caudal end broadly rounded (Fig. 18a). Ovaries paired, in the beginning of the caudal fifth of the body; gut simple, caudally limited by the ovaries. Testis large, unpaired, right-sided in the end of the anterior body half. Common genital atrium and genital pore in the caudal tenth of the body (Fig. 18a, b).
The bursal organ consists of a mouth-piece and a mid-piece; a sperm tube was not observed. The total size (proximal opening of mid-piece to distal opening of mouth-piece) is 32 μm. The mouth-piece is a slender tube 1 μm in diameter and 11 μm in length with two distal swellings, the proximal one stronger (diameter 4 μm) than the distal one (2.5 μm). The mid-piece is a semi-circular tube proximally starting with a small opening of some 1 μm and then rapidly widening to a diameter of 5–6 μm; the distal half of the mid-piece is strongly the sclerotic while the proximal half is rather faint (Figs. 18c and 19a, b).
A seminal vesicle was only seen in alive organisms; it is a slight widening of the spermatic duct without an obvious muscular cover. The granular vesicle, in contrast, is strongly muscular. It is oval in shape, some 50 μm long and 25 μm wide and filled with granular secretions except a narrow central sperm duct. It connects to the penis stylet by a rather wide duct of 12 μm length.
The penis stylet is a winded tube starting with a funnel 6.5 μm in diameter that rapidly narrows to 2.5/3 μm inner/outer diameter and the surrounds the accessory stylet by three quarters of a whorl (Fig. 19). Then it forms a wide spiral, passing its own proximal funnel and the accessory stylet and finally ends in a foot-shaped structure. Total length of the penis stylet along its winded course is some 135 μm and the distance funnel to tip 62 μm (single measurement from holotype). In the distal third, the stylet walls are markedly strengthened. The stylet tip resembles a human foot with a prominent heel, a slightly depressed sole and rather flat toes; distance heel to tip of toes is 13 μm. Sometimes, a fringe of unknown nature was seen to derive from the foot sole.
The accessory stylet is weakly sigmoid in shape. It starts as a tube of 12 μm diameter that continuously narrows to 2/2.5 μm inner/outer diameter close to the tip where it enlarges again to form a funnel with an opening diameter of 4.5 μm. While its proximal end is only slightly turned, the distal third forms a quarter of a circle. Measured along its winded course the length of the accessory stylet is 72 μm and the distance proximal to distal funnel is 61 μm. The glandular organ attached to the accessory stylet was an elongate tube of some 100 μm length and 20 μm width; however, just as the seminal vesicle, it was weakly developed only. Probably, the studied specimens had to yet reach full maturity.
Discussion The combination of paired ovaries and a single testis, a typical penis stylet and a long and tube-shaped accessory stylet, eyes present and rhabdites scattered all over the body fits two dolichomacrostomid genera, viz. Austromacrostomum and Cylindromacrostomum (both Rieger, 1971b). A major difference between these genera is the shape of the sperm tubes which are spiral-shaped in Austromacrostomum but long and whip-shaped in Cylindromacrostomum (Janssen et al. 2015). Unfortunately, sperm tubes were not yet developed in the studied specimens. Thus, the classification with Austromacrostomum is preliminary, mainly based on the shape of the stylet tip which is also rather broad and plate-like in Austromacrostomum arumoidicornum Janssen et al., 2015 while the stylets of the Cylindromacrostomum species all have a tube-shaped tip. Independent from the generic classification, the new species is characterised by the shape of the penis stylet with a foot-shaped distal end and the lack of a lateral appendage as is usually found in Dolichomacrostomida.
Currently, only four individuals of this species have been found (see localities) and there are no DNA samples available. An attempt to re-sample the type locality only yielded juveniles that could not unequivocally be identified with the new species.
Cirrifera paucispina sp. nov.
https://zoobank.org/E037F252-FD7A-466B-8B6B-A1161D1A5A9B(Figs. 20 and 21)
Type locality Eastern North Sea, 7.5 km WNW off the island of Sylt (54.9294°N, 008.1868°E), fine sand in 15 m water depth (26 Nov 2019, 2 individuals).
Material Live observations including photographs; two whole mounts, one designated holotype (AWI Sylt P2020-205), and one paratype (P2020-206).
Etymology The species name refers to the low number of cirrus spines, from Latin pauci = few.
Diagnosis Cirrifera with an unpaired seminal vesicle and a mushroom-shaped male copulatory organ including a low number of spines.
Description Very slender organisms, stretched 5–7 mm long and about 200 μm in diameter. Body whitish with numerous but rather small (4 to 6 μm) slightly yellowish epidermal glands. Brain encapsulated and far anteriorly, statocyst anterior of the brain, and with an intestinal diverticulum extending beyond the statocyst. The pharynx is spherical to ovoid and positioned unusually far caudally in the last fifth of the body.
Vitellary vesicles arranged in lateral rows from the second fifth of the body backwards to the end of the last forth, followed by the paired germaries; no more vitellary follicles were seen behind the germaries or the pharynx. Testis follicles numerous, arranged in a median row. Genital pore half-way between the pharynx and the caudal end, i.e. in the beginning of the last tenth of the body. The male copulatory organ lies anteriorly of the genital pore and the unpaired seminal vesicle behind. Genital atrium inconspicuous, with lateral junctions of the germo-vitelloducts and voluminous shell glands.
The seminal vesicle is rather small, drop-shaped in the smaller individual observed but more elongate in the larger one. It is connected to the copulatory organ by a single and rather wide seminal duct that passes the genital atrium dorsally. In its distal part, the duct is enlarged and surrounded by prostatic glands; though I found no prostatic secretions inside, this section of the spermatic duct may functionally replace the prostatic vesicle. Most distally, the spermatic duct ends in a cirrus consisting of relatively few (about 40) slightly curved spines of 10 to 12 μm without a basal plate. The distal part of the copulatory organ including the cirrus is enclosed in a hemispheric muscular bag; together with the distal part of the spermatic duct (functional prostatic gland) this gives the copulatory organ a mushroom-shaped appearance.
Discussion Currently, the genus Cirrifera Sopott, 1972 includes eight species. C. paucispina sp. nov. differs from all of them in the position of the pharynx (very far caudal) and the small cirrus consisting of comparatively few spines (some 40 against one to several hundreds). Within the genus, it joins C. boletiformae Sopott, 1972, C. dumosa Sopott, 1972 and C. genitoductus Jouk, Martens & Schockaert, 2007 which all have a single seminal vesicle. The closest relative of C. paucispina sp. nov. may be C. boletiformae; both species lack a prostate vesicle, instead prostatic glands open directly into the seminal duct close to the copulatory bulb.
Parotoplanina trigintaspina sp. nov.
https://zoobank.org/22EAA752-4811-45E3-99F5-91CF01CC7F87(Fig. 22)
Localities List Deep, the main tidal inlet to the Wadden Sea north of Sylt: (1) Coarse sand from 27 m water depth (55.0652°N, 8.4412°E; 14 Apr 2020, 6 individuals). (2) Medium sand, 26 m water depth (55.0623°N, 8.4557°E; 3 Mar 2020, 1 individual). (3) Type locality: coarse sand off the eastern tip of Ellenbogen, 10.5 m water depth (55.0465°N, 008.4657°E; 12 Mar 2018, 4 individuals). Previously observed at the same locality by Noldt (3 Aug 1984, unpublished record). (4) Coarse sand, 23 m water depth (55.0640°N, 8.4340°E; 7 May 2020, 3 individuals). (5) Medium sand, 6 m water depth (55.0699°N, 8.4327°E; 7 May 2020, 1 individual).
Material Live observations, photographs; eight whole mounts, one designated holotype (AWI Sylt P2020-301), and two paratypes (P20203-302 and -303). Unpublished photographs and drawings by Uwe Noldt
Etymology The species name refers to the high number of needles in the copulatory organ, from Latin triginta = thirty.
Diagnosis Parotoplanina with a copulatory organ including a circle of some 30 small spines
Description Mature animals up to 5.3 mm long and 0.5 mm in diameter. Pharynx in mid-body, strongly muscular, 350 to 400 μm in diameter, oriented along the dorsoventral axis. Anterior end knob-shaped offset, with longer tactile cilia; ventral row of adhesive papilla present and with a pair of large anterior glands. Brain encapsulated with distinct lateral nerve cords. The tail end forms a slightly triangular plate densely covered by adhesive papillae; further adhesive papillae laterally over entire body length.
Testis follicles numerous, in pre-pharyngeal lateral rows; germaries positioned at base of pharynx, followed by lateral rows of vitelline follicles (a few of them pre-pharyngeal and lateral of the pharynx but the major part post-pharyngeal) reaching back to the beginning of the seminal vesicle. Copulatory organ in the caudal fifth of the body, followed by a long seminal vesicle. Directly in front of the copulatory organ, one specimen was just forming an egg-capsule some 330 μm in diameter.
The copulatory organ is an almost spherical muscular bulb of some 110 μm enclosing some 30–34 short needles a cylindrical arrangement. The needles are very slightly bent with a minute triangular projection about 1 μm from the tip. Since their outline tends to fade away in the proximal part, length measurements in different specimens varied between 26 and 32 μm but they were all the same length in a single individual; thus, length differences between specimens may reflect the developmental state. A small amount of prostatic secretions was seen inside the circle of spines but no prostatic vesicle. Instead, the seminal vesicle directly joins the copulatory organ. The seminal vesicle is a long (350–450 μm) but narrow (diameter 25–35 μm) tube; the paired seminal ducts enter laterally just behind the copulatory organ. Except its very caudal end, all of the seminal vesicle is covered by strong circular muscles that form a tube continuing to the base of the needles in the copulatory organ. The diameter of the seminal vesicle varies according to the state of contraction of these circular muscles, while in the copulatory organ, contraction causes the needle tips to spread outward while their orientation is cone-shaped in the relaxed condition.
Vesicular tissue without well-defined outlines was seen dorso-laterally of the seminal vesicle next to the entrance of the seminal ducts. This is interpreted as the seminal bursa, according to position of the primary type. However, its connection to the genital atrium could not be seen in alive individuals.
Discussion In the current system of Otoplanidae, genera are characterised with special emphasis on the type of the seminal bursa and the arrangement of the prostatic vesicle (Ax 1956a). The combination of a primary-type seminal bursa and a prostatic vesicle widely fused with the copulatory organ in the new species matches two genera, viz. Parotoplanina Ax, 1956a and Praebursoplana Ax, 1956a (Online resource 5). Species of the latter genus deviate from all other parotoplanids in pharynx position (unusually far caudally) and the restriction of vitelline follicles to the pre-pharyngeal region. Thus, the new species was classified with Parotoplanina. However, the number and origin of the efferent bursal ducts (‘Bursastiele’ in Ax 1956a) could not be verified from live observations; hence, the classification is provisional. With a copulatory organ including a high number of uniform small needles P. trigintaspina sp. nov. deviates from all parotoplanids described so far which encouraged me to describe it as a new species.
When Ax shaped the current system of Otoplanidae in 1956, the characters he selected were sufficient to define clearly delimited genera. However, many additional species have been described afterwards, many of them with character combinations that no more fit the system. Thus, the entire taxon Otoplanidae is in urgent need for a revision.
Postbursoplana syltensis sp. nov.
https://zoobank.org/CE7A2E70-843F-4FCB-9CD3-5F10B833E03C(Figs. 23 and 24)
Type locality Lister Ley, medium sand, water depth 5 m (55.0426°N 008.4799E, 9 July 2015, 1 individual; 30 May 1984, 1 individual and 11 July 1984, 2 individuals, leg. Uwe Noldt).
Material Own live observations and photographs, unpublished drawings and photographs from earlier findings by Uwe Noldt. Holotype is a series of photographs deposited at Pangaea (https://doi.pangaea.de/10.1594/PANGAEA.936533).
Etymology The species name refers to the type locality.
Diagnosis Small sized Postbursoplana with a copulatory organ including paired lateral needles and eight central needles, four large and four small ones.
Description Very small individuals with a body length of only 0.6 to 0.8 mm alive. Pharynx in mid-body, almost spherical, about 70 × 80 μm. Anterior end knob-shaped offset, with longer tactile cilia and paired anterior glands; brain encapsulated. Tail end conical with adhesive papillae, further adhesive papillae laterally over entire body length.
Testis follicles numerous, in pre-pharyngeal lateral rows; germaries positioned besides pharynx, followed by lateral rows of vitelline follicles reaching backwards to the genital pore. Copulatory organ and longish seminal vesicle (100 × 50 μm) in the caudal fifth of the body. The copulatory organ encloses 12 very slender needles of 3 different types. The central group consists of four longer and four shorter needles, all with a small triangular projection 3 μm from the tip in the small and 5 μm from the tip in the large needles. Within an individual, the longer and shorter needles, respectively, were all the same length but between individuals their size varied between 62 to 70 μm for the longer and 44 to 51 μm for the shorter needles. The lateral group is formed by two pairs of slightly curved needles 55 to 58 μm in length, also with a triangular projection some 6–7 μm from the tip. In weakly squeezed specimens, the shafts of these lateral needles are convergent giving a bifurcated appearance, but stronger coverslip pressure indicates that they are not fused. Along their entire length, the lateral needles are accompanied by glands holding fine granular secretions, presumably the prostatic glands. Proximally, the copulatory organ directly joins the seminal vesicle, an intermediate prostatic vesicle was not seen.
Discussion The genus Postbursoplana Ax, 1956a currently comprises 10 species. In all of them, the copulatory organ encloses a central group and lateral needles; the latter are paired in all species except P. minima (Table 4). All species differ in the number, size and shape of the needles. The combination of paired lateral needles and eight needles in the central bundle only occurs in P. noldti and P. syltensis sp. nov. (Table 4). While the central group consists of six smaller hooks and two larger spines that support a delicate central funnel with a bulgy stem in P. noldti, there are four longer and four shorter needles in the central group in P. syltensis sp. nov.
Orostylis biforaminis sp. nov.
https://zoobank.org/A178462B-4FEF-485E-8981-7A75CF96F000(Fig. 25)
Type locality South-eastern North Sea, 10 km west of the Island of Sylt (55.0359°N, 8.2216°E; 6 Nov 2019): very fine sand, 13.5 m water depth.
Material Live observations and photographs. Three whole mounts, one designated holotype (AWI Sylt P2020-218), and two paratypes (P2020-219 and -220).
Etymology: The name refers to the stylet with two separate openings for sperm and prostatic secretions, respectively. From Latin bi = two and foramen = opening.
Diagnosis: Species of Orostylis characterised by a small (31 μm) stylet with a secondary funnel and a wide median tube that is only slightly curved. With eyes, pharynx rim with a circle of small papillae and six tentacles.
Description. Free swimming about 0.8 mm long, very slender, rather transparent, with small paired eyes. Cylindrical pharynx in the anterior third of the body, total length about 1/5 of the body length; its anterior end with a collar separated by a slight constriction from the rest of the pharynx. The collar rim bears a circle of small (2–3 μm long) papillae and six tentacles (16–18 μm long) that are anchored in the collar for half their length while the rest projects anteriorly into a thin-walled oral tube that connects the pharynx to the sub-terminal mouth opening.
Testes paired, on either side of the body behind the pharynx; copulatory organ alongside the pharynx in contracted or behind the pharynx in stretched specimens. It is weakly muscular with a long and slender seminal and a small prostate vesicle that enter the sclerotized stylet separately.
The stylet is a slightly curved tube obliquely cut-off distally and with two funnels proximally. The first funnel is a direct extension of the stylet tube; it receives the prostatic secretions. The second funnel is laterally attached to the first one with its opening turned at an angle of about 120°; this funnel receives the sperm. The second funnel seems to be less sclerotized and therefore appears to be a lateral flap, at first view. Both funnels are similar in size (proximal diameter some 8 μm) and enter the tube-part of the stylet side-by-side. This median tube-part is 24 μm long and slightly curved; its diameter reduces from 3.4 μm behind the funnels to 2.4 μm at the beginning of the opening where the tube is cut-off obliquely. This cut produces an oval opening 1.5 μm wide and 5.4 μm long; most distally, the tube ends in a twisted tip. The total length of the stylet (funnel to tip) is 31 μm. The male genital canal is rather long and weakly muscular. It could be followed to close to the mouth opening; presumably, here it enters the pre-pharyngeal cavity.
Discussion. With a sclerotized stylet in the anterior part of the body and a male atrial opening in the pre-pharyngeal cavity, Orostylis biforaminis sp. nov. shares the main characters of the genus Orostylis Gobert et al., 2022. In addition, a pharynx with a distal rim bearing tentacles or papillae is commonly found in this genus. According to stylet morphology, O. asinaraensis Gobert, Jouk, Revis & Artois, 2022 and O. gallicus Gobert, Monnens & Artois, 2022 may be closest related to O. biforaminis sp. nov. These three species have a stylet with an asymmetric, lateral proximal aperture described as a long, triangular ‘handle’ in O. asinaraensis and as a broad flap in O. gallicus, respectively (Gobert et al. 2022). The second funnel in O. biforaminis sp. nov. resembles the broad flap in O. gallicus, so these species may be closely related. So far, the combination of a wide proximal funnel (with a lateral secondary funnel) and a rather wide and only slightly curved median stylet tube is only found in O. biforaminis sp. nov.
Mediovortex gen. nov.
https://zoobank.org/B910A2A5-4A3B-48C9-8D7A-2F809AF330F0
Diagnosis Provorticidae with the genital opening in the middle of the body; paired testes and the copulatory organ anterior and paired vitellaries and germaries caudal of the genital opening. Prostate united, i.e. seminal vesicle and vesicle granulorum next to one another in the copulatory organ.
Etymology The genus name refers to the median (Latin: medio) position of the genital opening
Description and discussion Neodalyellida with mouth anterior, male and female gonads paired, copulatory organ with a tubular stylet, with bursa and seminal receptacle present, are united in the family Provorticidae Beklemischev, 1927. In this family, the usual position of the genital opening is close to the caudal end while it is dislocated towards the middle of the body (or slightly behind in the very beginning of the caudal body half, depending on the organism’s degree of stretching) in Mediovortex sp. nov. This causes an exchange in the relative positions of the germaries and vitellaries: the germaries are situated anterior of the vitellaries. In addition, the dislocation of the genital pore towards mid-body results in a reversed orientation of the germaries, with the most maturate egg cells pointing towards the anterior.
Within Provorticidae, a copulatory organ with seminal and prostatic vesicles next to one another points to the sub-family Provorticinae Luther, 1962. Here, Mediovortex gen. nov. combines characters of the genus Provortex Graff, 1882 (germaries separate from vitellaries) with those of Vejdovskya Graff, 1905 (absence of eyes, longish body shape, pharynx relatively weak) while the central position of the genital pore separates Mediovortex gen. nov. from both.
Mediovortex inversa sp. nov.
https://zoobank.org/E2D046FF-AD54-4586-96F5-162D17C6D335(Figs. 26 and 27)
Localities Sandbank in tidal inlet ‘Lister Ley’. (1) Type locality: well sorted fine sand, 5.1 m water depth (55.0349°N, 008.4720°E, 25 Feb 2019, 2 individuals); (2) moderately well sorted fine sand, 3.7 m water depth (55.0357°N, 008.4747°E, 11 Mar 2019, 1 individual).
Material Live observations and photographs; two whole mounts, one designated holotype (AWI Sylt P2020-313) and one paratype (AWI Sylt P2020-314).
Etymology The species name refers to the inverse orientation of the germaries with most maturate egg cells pointing anterior.
Diagnosis Currently as genus; the stylet is a curved funnel of 32 μm length with a proximal opening of 21 μm and a needle-shaped appendage of 6 μm at its distal end.
Description Slender organisms of 1.0 to 1.2 mm body length, both ends gently rounded, unpigmented. Pharynx doliiformis weakly muscular as in species of Vejdovskya, brain just in front of pharynx, no eye pigmentations. Mouth opening subanterior, with voluminous glands that reach backwards to the middle of the pharynx. Prominent tactile hairs were not seen. Genital opening with a small genital atrium, positioned in the very beginning of the caudal body half.
Testes paired but close together, in the end of the anterior body half, connected to the spherical to ovoid seminal vesicle by short but rather wide deferent ducts. Distally, the seminal vesicle directly joins the copulatory organ, which is longish with a weak muscular cover; prostatic glands enter the copulatory organ side-by-side with the sperm. The stylet is a curved funnel of 32 μm length with a proximal opening of 21 μm and a needle-shaped appendage of 6 μm at its distal end. The sperm are extremely long threads.
Germaries and vitellaries are separated from each other and connected to the genital atrium by a short but wide female genital duct. Besides this duct, a slightly drop-shaped organ enters the genital atrium; though it contained no sperm it is assumed to be a seminal receptacle. In the germaries, the most maturate eggs are oriented towards the genital pore, i.e. anteriorly. The vitellaries stretch between the anterior side of the germaries to almost the caudal end of the body.
Discussion See Mediovortex gen. nov.; Mediovortex inversa sp. nov. is the type species for the new genus.
Proceropharynx spiculatus sp. nov.
https://zoobank.org/E382904C-9E1F-4735-AE30-F884AFB5715D(Figs. 28 and 29)
Localities Type locality: Lister Ley, medium to fine sand of a sandbank, 5 m water depth (55.0347°N, 008.4723°E, 28 Feb 2018, 1 individual)
Material Live observations including drawings and photographs. Holotype is a series of photographs deposited at Pangaea (https://doi.pangaea.de/10.1594/PANGAEA.936533).
Etymology The species name refers to the spines present in the distal part of the ejaculatory duct, from Latin spiculum = spine.
Diagnosis Species of Proceropharynx with a cirrus of fine ridges proximally and a group of strong spines distally; copulatory bursa with an irregular pattern of fine hardened ridges.
Description Slender unpigmented specimens, anterior end broadly rounded, caudal end triangular in shape; free-swimming 0.8 mm long. Without eye pigmentation but with anterior glands that form a striking package before the brain. Pharynx with voluminous pre-pharyngeal glands positioned in the second half of the body, mouth opening at 80% and genital opening at 85% of body length.
Arrangement of genital organs as usual in the genus: paired testes are situated laterally directly behind the brain and are partly fused in the median line; paired vitellaries stretch laterally from the testes to the mouth opening; the single ovary and all atrial organs are situated in the last quarter of the body. In alive specimens, the deferent ducts could not be traced until they were swollen to external seminal vesicles. The copulatory organ is inversely pear-shaped (about 70 μm long and 30 μm wide). In its proximal half, the central ejaculatory duct is enlarged to an internal seminal vesicle and surrounded by prostatic glands (Fig. 29a, b). In this proximal part, the outer muscle layer of the copulatory organ contains strong circular muscles (Fig. 28b); by contraction, these circular muscles may act as a pump. However, since circular muscles are weak in the most proximal tenth of the copulatory organ, parts of the prostatic glands appear to be outside the copulatory organ once the circular muscles are constricted (Fig. 29c). In the distal part of the copulatory organ, the ejaculatory duct is surrounded by parenchyma and bears the fine hardened ridges typically for the genus. These ridges end in the most distal part and are replaced by a bundle of (apparently five) strong spines.
The common genital opening leads into the common genital atrium. The male copulatory organ enters the common atrium from the anterior side and the oviduct laterally from the left. The seminal bursa consists of a smaller (about 12 μm) roundish vesicle containing intact sperm and a larger caudal part that contained packages of sperm in different stages of digestion. Presumably, the small vesicle acts as a seminal receptacle storing partner sperm for future fecundation while the larger part is a resorptive vesicle digesting excess sperm. The duct connecting the small vesicle to the caudal part of the genital atrium was well-defined in alive specimens, but its connection to the ovary was not clearly defined. The uterus (terminology used by Ehlers 1972) is a pear-shaped sac of about 20 × 35 μm that enters the common atrium from the right. Finally, the copulatory bursa opens to the dorsal side; in live observations, it is visible only as an area enclosed by fine hardened ridges in an irregular pattern, like a crumpled paper bag.
Discussion The genus Proceropharynx Ehlers, 1972 is characterised by a cirrus of fine ridges. Currently, the genus contains 3 species, P. anophthalmus (Meixner, 1929) Ehlers, 1972, P. litoralis Ehlers, 1972, and P. profundum Willems, Sandberg & Jondelius, 2007. These and the new species differ in the presence/absence of spines in addition to the cirrus ridges (no spines in P. anophthalmus and P. litoralis) and in the size of these spines (many small ones in P. profundum and few large ones in P. spiculatus sp. nov.). According to the present localities, all four species seem to be restricted to the ecoprovince ‘Northern European Seas’ (Spalding et al. 2007).
Coronhelmis lamellatus sp. nov.
https://zoobank.org/4F091F23-4918-4EBD-AC3C-8476C06DE989(Fig. 30)
Type locality North Sea, pure fine sand 48 km WNW of Sylt island, water depth 20.5 m (55.1506°N, 007.6582°E; 2 July 2020, 3 individuals)
Material Live observations and photographs. Two whole mounts, one designated holotype (AWI Sylt P2020-216), and one paratype (AWI Sylt P2020-217).
Etymology The species name refers to tip of the stylet which bears a half-circle of lamella-shaped spines.
Diagnosis Coronhelmis with a stylet of 40 μm equipped with a high number of leaf-shaped lamellae distally.
Description Slender specimens up to 1.6 mm long, anterior end with dense rows of rhabdites, no eye pigmentations. Pharynx 100 μm in diameter, positioned in the posterior half of the body. Paired vitellaries laterally from the brain to almost the rear end. Germaries in the last sixth of the body, weakly developed in the studied specimens. A seminal receptacle could not be seen.
Male system with paired testes anterior the pharynx and paired seminal vesicles behind. Muscular copulatory organ spherical (diameter 80 μm) in un-squeezed and piriform (60 × 100 μm) in squeezed specimens. The stylet (total length 40 μm) consists of a U-shaped proximal part (called ‘Manschette’ in Luther 1962) and a distal part with spines. The proximal part is 27 μm long and weakly sclerotized with a diameter of 27 μm proximally and 15 μm distally. Stronger magnification reveals it bears about eight vertical folds or ridges. The distal spines appear as delicate leaf-shaped lamellae of 4–6 μm oriented vertically along the central axis at one side and horizontal ridges at the other. Since the fine structure of these lamellae is beyond the resolution capacity of light microscopy it is not clear whether lamellae and ridges are different structures or identical structures differing in orientation only. Accordingly, the number of lamellae and ridges can only be estimated to some 30 and some 15, respectively.
Discussion Currently, the genus Coronhelmis Luther, 1948 comprises 12 valid species (WoRMS 2020; excluding C. urna Ax, 1954 which is regarded as a junior synonym of C. lutheri Ax, 1951, see Luther 1962, Willems et al. 2005). All of these species differ in stylet morphology and size (Willems et al. 2005). The combination of a large stylet (un-squeezed length 40 μm) with a high number of very small spines is only found in C. lamellatus sp. nov. Very small spines also occur in C. subtilis Ax, 2008 but in this species the proximal stylet part has no vertical ridges in and the distal part no horizontal ones. In C. mimosa Van Steenkiste, Volonterio, Schockaert & Artois, 2008, the distal spines are also very small but total stylet length is much shorter and there are no distal horizontal ridges. Finally, C. lamellatus sp. nov. is the first species of the genus found in a marine subtidal environment.
Promesostoma furcatum sp. nov.
https://zoobank.org/CC2D4F6A-AE4E-47BF-A9CC-422A3B6B9713(Fig. 31)
Localities Tidal inlet ‘Lister Ley’, (1) Coarse sand, 22 m water depth (55.0515°N, 008.4699°E, 7 Jan 2020, 2 individuals; (2) Coarse sand of a sandbank, 10 m water depth (55.0523°N, 008.4756°E, 7 Jan 2020, 1 individual). Previously found in the same area by Wehrenberg (1983). (3) Type locality: coarse sand, 11 m water depth (55.0541°N, 8.4590°E; 3 Mar 2020, 2 individuals).
Material Live observations including drawings and photographs. Four whole mounts, one designated holotype (AWI Sylt P2020-201), and three paratypes (AWI Sylt P2020-202 to -204).
Etymology The species name refers to the lateral appendage of the stylet, from Latin furca = a fork with two branches.
Diagnosis Promesostoma with a rather straight stylet of 109 μm length bearing a two-branched lateral appendage in its distal third.
Description Slender unpigmented specimens with small paired eyes, the pharynx in mid-body, the genital opening half-way between the pharynx and the rounded caudal end, and with rhabdites scattered all over the body. All specimens were in beginning male maturity and 0.5 to 0.6 mm long (free-swimming); fully mature specimens will probably be larger. With paired testes laterally and the male copulatory organ central in front of the pharynx; the latter is elongate and weakly muscular, as is the adjoining deferent duct to the stylet. Female organs not observed.
The stylet is a slender tube of 108 to 111 μm length (mean 109.5 μm, n = 3). Proximally, it is slightly s-shaped with a weakly developed funnel surrounded by a sphincter. The adjoining tube is nearly straight, slightly tapering from proximally 5.0/4.5 μm (outer/inner diameter) to 3.0/2.5 μm near the tip where the tube is cut-off obliquely. The distal third of the stylet bears a lateral appendage of 33 to 35 μm total length composed of an unbranched proximal half and a furcate distal half. One of the distal branches always paralleled the stylet tube over a short distance while the other was considerably strutted apart. The male genital canal is rather wide; an ovoid seminal receptacle branches from its most proximal part. Distally, the genital canal merges with the genital atrium which was rather wide and equipped with a bundle of accessory glands.
Discussion Currently, the genus Promesostoma includes 35 valid species (WoRMS 2020) which mainly differ in details of the male genital tract. A relatively short (around 100 μm) and branched stylet only occurs in seven species of Promesostoma, i.e. P. bipartitum Ax, 1956b, P. balticum Luther, 1918, P. cochleare Karling, 1935, P. paracochlearis Ax, 1952, P. wehrenbergi Armonies, 2018, as well as P. fibulatum Ax & Armonies, 1987 and P. digitosum Ax, 1995. The stylets of the last two species are sharply bent proximally behind the sphincter, with a trident pinnacle projecting from the bent—a character so far only observed in N. American species. In P. bipartitum and P. balticum, the branch occurs in the middle of the stylets and in P. cochleare and P. paracochlearis already after the proximal third. Thus, from the position of the branch in the distal third of the stylets, P. wehrenbergi is most similar to P. furcatum sp. nov. while both species clearly differ in the shape of the stylet tips and the lateral branches. These species may be closely related and they occupy ecologically neighbouring habitats in the SE North Sea, P. wehrenbergi fine to medium sand and P. furcatum sp. nov. coarse sand, both in the shallow subtidal.
Subulagera obscurohamata sp. nov.
https://zoobank.org/2816D7EB-1E89-49FC-8404-D01ECE70E484(Figs. 32, 33 and 34)
Localities (1) Type locality: North Sea, some 8 km W of Sylt, very fine gravelly medium sand, 14.4 m water depth (54.9330°N, 008.1850°E; 26 Nov 2019, 1 individual); (2) Tidal inlet ‘List Deep’, muddy medium sand, 3.8 m water depth (55.0511°N, 008.4431°E, 21 Aug 2018, 1 individual; (3) List Deep, very fine gravelly medium sand, 2.3 m water depth (55.0529°N, 008.4407°E, 7 Aug 2019, 1 individual).
Material Live observations including drawings and photographs. Three whole mounts, one designated holotype (AWI Sylt P2020-309), and two paratypes (AWI Sylt P2020-310 and -311).
Etymology The species name refers to the two hooks (Latin: hamus) included in the stylet, one of which is hidden (Latin: obscurus) in the proximal part.
Diagnosis Unpigmented species of Subulagera with a complex stylet including two large curved hooks, one in the distal ending and one hidden in its proximal part.
Description Slender unpigmented specimens of 1.2 to 1.3 mm body length, with prominent anterior glands, without eye pigmentation; pharynx in the beginning of the caudal third of the body, genital opening half-way between the pharynx and the tapered caudal end.
With paired testes laterally behind the brain and in anterior to the gut, and paired vitellaries beginning in level with the gut and stretching backwards to the genital opening. Male copulatory organ between the pharynx and the genital opening, ovoid (90 × 120 μm), weakly muscular; with voluminous inner and smaller outer parts of the prostatic glands and a central deferent duct. Seminal vesicles rather small and weakly muscular. Paired germaries caudal of the genital opening, with the most-developed egg cells oriented towards the caudal end of the body. Copulatory bursa about the same size as the stylet, positioned close to the genital opening. Accessory glands containing granular secretions close to the genital opening, small in two but strongly developed in the third specimen studied alive. Seminal receptacles were not positively identified in alive specimens.
The stylet looks drop-shaped in low magnification (Fig. 33), with an ovoid (30 × 50 μm) proximal part and a distal curved hook of 40 μm which is partly inserted in the ovoid part. Stronger magnification reveals a complex structure of ridges and folds in the ovoid proximal part, including a second strongly curved hook which becomes more obvious with increasing coverslip pressure (Fig. 34).
Discussion see below.
Subulagera triangularis sp. nov.
https://zoobank.org/8EF96C41-B9B6-4724-9AF9-B8E78D1F5BB0(Figs. 35 and 36)
Type locality: North Sea, 6 km WNW of Sylt, very well sorted fine sand, 5.7 m water depth (55.0865°N, 008.3280°E; 6 Dec 2018, 1 individual). The specimen co-occurred with further three red-coloured species (Pseudoschizorhynchus ruber, Diascorhynchus rubrus and Subulagera rubra) and uncoloured Paromalostomum fusculum, Bradynectes sterreri, Proxenetes cimbricus, Gnathorhynchus conocaudatus and Schizochilus choriurus (only abundant species listed).
Material Live observations on a single individual, including a series of 17 photographs. Since the specimen could not be successfully mounted, the series of photographs is designated holotype and deposited in Pangaea (https://doi.pangaea.de/10.1594/PANGAEA.936533).
Etymology The species name refers to the stylet which is triangular in outline and includes two triangular plates distally.
Diagnosis Subulagera with red-pigmented mesenchyme and a stylet triangular in outline that ends in two lateral triangular plates.
Description A slender unpigmented specimen of 1.3 mm body length. Mesenchyme reddish by dissolved pigment which facilitates recognition of internal organisation. Thus, the anterior glands are easily visible though rather weakly developed. No eye pigmentations; pharynx in the beginning of the caudal third of the body, genital opening half-way between the pharynx and the tapered caudal end.
With paired testes laterally between the brain and the gut, paired vitellaries from the gut to the genital opening. Male copulatory organ between the pharynx and the genital opening, with a very weak muscular cover; it thus varied in outline during live inspection; with voluminous inner and smaller outer parts of the prostatic glands and a central deferent duct. Seminal vesicles rather small and weakly muscular. Paired germaries caudal of the genital opening, with the most developed egg cells oriented towards the caudal end of the body. Copulatory bursa about the same size as the stylet, positioned close to the genital opening. Accessory glands containing granular secretions close to the genital opening.
The stylet is triangular in outline with a proximal diameter of some 27 μm and a total length of 36 μm; proximal part with a complex structure of paralleling lines, distal tip formed by a pair of V-shaped plates. The lateral part of these plates is strongly sclerotic but sclerotization fades away proximally. It is thus not clear whether or not the plates fuse proximally.
Discussion of the species of Subulagera All organisational characters observed conform well with the other species of the genus (i.e. Subulagera mucronata Ehlers, 1974 and Subulagera rubra Martens & Schockaert, 1981); only seminal receptacles were not detected in the studied specimens of S. obscurohamata sp. nov. S. triangularis sp. nov. and S. rubra are both reddish from pigments dissolved in the mesenchyme while S. mucronata and S. obscurohamata sp. nov. are unpigmented. All four species show a complicated stylet structure with many folds or ridges but they clearly differ in the shapes of the strongly sclerotic distal parts of their stylets. S. mucronata has an r-shaped stylet with few folds and ridges against a high number in the other three species. In S. rubra, the stylet shape resembles the letter y, with a very wide proximal and mid-section and a tube-shaped end accompanied by a shorter elongate v-shaped thorn. The stylet of S. obscurohamata sp. nov. is drop-shaped with a very well-defined distal hook that resembles a bird’s bill and a second hook in the proximal section of the stylet. Finally, the stylet of S. triangularis sp. nov. is broadly v-shaped with a distal tip formed by two v-shaped lateral plates.
Ethmorhynchus styliferus sp. nov.
Ethmorhynchus styliferus sp. nov., copulatory organ; a, b schematic; c from whole mount; d from alive organism. Arrows in (a) indicate the constriction between the seminal vesicle and the seminal duct in intermediate state of development; in younger organisms this constriction is far narrower and longer, in older individuals it is no more visible
https://zoobank.org/E0848A76-B585-4D2A-8DF2-68FAAB6BB3D1(Figs. 37 and 38)
Type locality Eastern North Sea, 7.7 km WNW off the island of Sylt (54.9330°N, 008.1850°E), medium to coarse sand in 15 m water depth (26 Nov 2019, 6 individuals).
Material Live observations, photographs; four whole mounts, one designated holotype (AWI Sylt P2020-207), and three paratypes (P2020-208 to -210).
Etymology In this species the cirrus of the copulatory organ includes a stylet, as opposed to the other species of the genus.
Diagnosis Ethmorhynchus with four pairs of proboscis-ampullae and a cirrus including a conical stylet.
Description Slender organisms, free swimming 1.2 to 1.5 mm long and 100 to 120 μm in diameter. Body whitish and rather opaque which is brought about by small drops of epidermal secretions (3 to 4 μm in diameter) densely covering the entire body (Fig. 37e); eyes absent. The pharynx is spherical (diameter 60 μm) in un-squeezed specimens and ovoid (50 × 70 μm) in squeezed ones and positioned in the end of the first third of the body; genital opening sub-terminally in the caudal end.
The proboscis (relaxed 55 μm long and 32 μm in diameter) bears 4 pairs of ampullae filled with granular secretions. These ampullae are cylindrical with a rounded end caudally and stretched to a narrow duct in their anterior half. The base of the proboscis sheath is built by a ring of swollen cells that terminally also contain granular secretions (called ‘Rüsselscheidendrüsen’ in Meixner 1938, Fig. 34). It is not clear, however, whether these swollen cells are supplied with secretions from the proboscis ampullae or whether the ampullae open separately at the proboscis cone.
The specimens are distinctly proterandric, only a single organism had both male and female organs in a functional state of development. The copulatory organ is a cylindrical tube 40 to 50 μm in diameter and 80 to 100 μm in length equipped with a thin layer of outer longitudinal muscles and strong inner circular muscles. Distally, it is followed by a penis papilla without visible musculature which encloses the cirrus with a stylet. The cirrus consists of some 150 needles in a cylindrical arrangement; single needles are composed of a hemispherical basal plate carrying a slightly curved spine. The stylet is conical in shape, with a total length of 27 μm and a proximal opening 7 μm in diameter; distally, it is cut-off obliquely.
About half of the inner volume of the copulatory organ was filled with sperm from the seminal vesicle that joins the copulatory organ proximally without a clear delimitation. In smaller organisms, the seminal vesicle was hemispherical in shape and rather small, barely surpassing the diameter of the copulatory organ; proximally, it merged to a long (some 500 μm) and slender (10 to 15 μm) seminal duct just separated by a short zone with a smaller diameter of the vessel. In maximum male maturity, however, the seminal vesicle and spermatic duct seem to be completely fused and swollen to a winding tube of about half body length and up to twice the diameter of the copulatory organ. Testes could no longer be seen in this developmental stage while paired testes were seen laterally in the beginning of the second body half in younger organisms.
Adjacent to the seminal vesicle, prostatic glands enter the copulatory organ proximally, slightly forced aside by the seminal vesicle. Inside the copulatory organ, a narrow duct without visible content was seen from the region of the prostate secretions entrance down to the penis papilla. In older specimens, this duct could no longer be seen and the prostatic secretions apparently passed the copulatory organ directly besides (but not mixed with) the sperm.
Only a single specimen was in female maturity, with well-developed vitellaries (paired, laterally from the pharynx backwards to a short distance before the copulatory organ) and paired ovaries adjoining the vitellaries distally; testes could no longer be seen in this stage and the seminal vesicle was collapsed to a slender tube again, with few sperm left. Instead, a diffuse tissue took the place of the (largely degenerated) seminal vesicle, presumably the bursa, but sclerotized bursal mouth-pieces were not detected. A wide though apparently empty duct connected the bursa to the genital atrium. Finally, a large bundle of glands (shell glands?) enter the genital atrium from the caudal side.
Discussion The most striking character of this species is the presence of epidermal secretions covering the entire body surface in drops 3–4 μm in diameter. Similar but larger (about 10 μm) drops of epidermal secretions also occur in E. anophthalmus Meixner, 1938 (Karling 1956, p. 265) but not in the freshwater-species E. youngi Kolasa, 1977 which, however, was only provisionally classified within Ethmorhynchus (Kolasa 1977). Further, differential characters of E. styliferus sp. nov. are the presence of secretory proboscis ampullae as typical for Cicerinidae and a cirrus including a stylet; but characters are absent in E. anophthalmus.
Neopolycystis schilkei sp. nov.
https://zoobank.org/337A9487-125B-460A-B774-714F0A5B7780(Figs. 39 and 40)
Localities Wadden Sea near Sylt, intertidal medium to coarse sands. Type locality is ‘Hausstrand’, a semi-exposed beach next to the List ferry landing (55.0154°N, 008.4379°E) where the species occurs in the upper intertidal zone (0.6 to 0.2 m below mhtl) but always in sediment depths > 20 cm below the surface. Previously recorded as Neopolycystis tridentata in the same area by Schilke (1970), Hoxhold (1974), Wellner and Reise (1989), and Armonies (2017). Further records from Sylt island: intertidal coarse sand (Xylander and Reise 1984), sheltered sandy beaches (Hellwig 1987), and sandy shores of salt marsh creeks (Hellwig-Armonies and Armonies 1987). Presumably, the records of N. tridentata from Belgian beaches (Jouk and Schockaert 2002) also refer to this species. However, previous records from the Sylt subtidal (Wellner and Reise 1989; Noldt 1989) all refer to individuals without trident crystals and their identity with Neopolycystis tridentata is documented by figures 34-35 in Noldt 1989.
Material Live observations and photographs. Five whole mounts, one designated holotype (AWI Sylt P2020-210), and 4 paratypes (AWI Sylt P2020-211 to 215).
Etymology The species was already recorded by Schilke in the late 1960s but erroneously identified as Neopolycystis tridentata.
Diagnosis Species of Neopolycystis with a slender stylet starting with a funnel 7–8 μm in diameter and rapidly narrowing to 3 μm; with trident parenchymal inclusions.
Description Small specimens of 0.5 to 0.8 mm body length, occasionally up to 1 mm; body shape, colour, type of movement and general organisation all as in Neopolycystis tridentata. The proboscis is relatively large (up to 200 μm long and 50–60 μm in diameter), the pharynx is slightly ovoid in shape (60–70 μm in diameter and 70–80 μm long) and positioned in the middle of the body or slightly before.
The single ovary lies dorsally in the middle of the posterior body half, directly adjoining to the vitellary which has a horseshoe-shape and lies entirely behind the pharynx; in larger specimens the left side of the vitellary was more voluminous than the right side. The small seminal bursa was half-way between the ovary and the genital atrium. The longish uterus was only seen in two larger specimens; it was an inconspicuous tube some 10 μm in diameter and 120 μm long.
The testes are paired though slightly different in size, with the right-sided testis larger than the left sided one. In larger specimens, they take a lateral position in the rear body half, in smaller specimens they appear more anteriorly besides or even slightly before the pharynx. The deferent ducts towards the seminal vesicle could not be seen in alive specimens. The seminal vesicle is relatively large and ovoid in shape, sometimes even larger than the pharynx. A short, very narrow duct connects it to the conjuncta-type (Karling 1956) copulatory organ with a type IV (terminology according to Artois and Schockaert 2003) prostate vesicle; it is a longish bag in stretched organisms but more ovoid in contracted animals. Most of its volume seems to be taken by hyaline prostate cells that only leave a central deferent duct. A large bundle of glands with granular secretions occurred half-way between the prostatic vesicle and the germary.
The stylet proximally starts with a narrow funnel that rapidly reduces diameter to some 3 μm giving the stylet the shape of a slender, curved tube. The basal part of the stylet (‘Basalstück’ in Karling (1955) is V-shaped. Strong muscle fibres connect the base part of the stylet to the sigmoid accessory stylet which has a total length of 38 to 41 μm and proximally protrudes the stylet by 8 to 10 μm. The small accessory vesicle lies next to the base part of the stylet, and though it has a muscle cover of its own, it seems to be embedded in the muscle package connecting the accessory stylet and the base part of the stylet (Fig. 39b). The accessory vesicle seems to be fully embedded in the muscle cover of the accessory stylet.
The parenchyma contains a variable number of inclusions of unknown function; Schilke (1970) described them as three-spined flat but possibly the 3-D structure is a tetrapod with its four arms arranged according to the axes of a triangular pyramid (Fig. 39c). During embedment in an acid fixative, the inclusions rapidly dissolve.
Discussion Around Sylt island, Neopolycystis tridentata Karling, 1955 was reported from medium to coarse sand of both intertidal and subtidal habitats (references in localities). The subtidal specimens fully match the original description given by Karling (1955) and own data. Intertidal specimens, however, deviate in stylet morphology and dimensions (Fig. 40, Table 5); the most striking difference is the shape of the accessory stylet which is a narrow duct in N. schilkei sp. nov. but a wide rim in N. tridentata. The parenchymal inclusions shaped like trident crystals (described in Schilke 1970) were always found in specimens from the intertidal but are absent in subtidal specimens (Noldt 1989; own observations). Because of the presence of these crystals, most of the former records of N. tridentata from intertidal habitats in fact refer to N. schilkei sp. nov. Other differences between both species such as the shape of the vitellaries, the testes size and the distinctness of the seminal bulb may depend on species maturity and therefore are of low diagnostic value.
Proschizorhynchus angusticirrus sp. nov.
https://zoobank.org/7E36C4BD-FB69-4369-A7E1-AC5A1F4604E5(Figs. 41 and 45a)
Localities (1) Type locality: North Sea some 50 km WNW of Sylt island, pure fine sand from 20 m water depth (55.1506°N, 007.6582°E; 2 July 2020, 3 individuals). (2) Wadden Sea near Sylt, tidal inlet ‘Lister Ley’, medium sand from 10 m water depth (55.0216°N, 008.4580°E; 27 Nov 2017, 1 individual). (3) Tidal inlet ‘List Deep’, fine sand from 7 m water depth (55.0692°N, 008.4420°E; 14 Apr 2020, 1 individual).
Material Live observations, photographs; two whole mounts but one lost, the remaining designated holotype (AWI Sylt P2020-110).
Etymology The species name refers to the shape of the cirrus-sheath which is narrow (Latin: angustus) compared to other species of the genus.
Diagnosis Proschizorhynchus with a narrow cirrus-sheath without distal spines and a long single-curved stylet protruding from the cirrus sheath by half its length.
Description Whitish, medium sized Proschizorhynchus with small paired eye pigmentations and usual arrangement of organs. Copulatory organ pear-shaped, 150 μm long and 90 μm maximum diameter. The distal penis papilla is cylindrical, 70 μm in length, and turned by some 45° from the longitudinal axis of the copulatory organ. It contains a ridged cirrus-sheath of some 60 μm length and 32 μm diameter with a smooth distal opening without spines. The stylet within is single-curved with a total length (plate-shaped proximal part to tip) of 87 μm; it protrudes from the distal cirrus opening by about half of its length. Vagina externa with a hardened mouth piece shaped like a bottle with a curved neck (Fig. 42d); in everted state, the bottle is some 60 μm high with a diameter of 30 μm at the bottom and 9 μm in the neck opening.
Discussion Joint discussion for all new species of Proschizorhynchus see below.
Proschizorhynchus serpentistylus sp. nov.
https://zoobank.org/56224DE2-B7DB-437E-B670-208F7E6C570C(Figs. 42 and 45b)
Type locality North Sea some 50 km WNW of Sylt island, pure fine sand from 20 m water depth (55.1506°N, 007.6582°E; 2 July 2020, 6 individuals). The species occurred together with P. angusticirrus sp. nov., P. frisius sp. nov. and P. triductibus Schilke, 1970
Material Live observations, photographs; four whole mounts, one designated holotype (AWI Sylt P2020-111) and three paratypes (AWI Sylt P2020-112 to P2020-114).
Etymology The species name refers to the shape of the stylet which is long and s-shaped (Latin serpens = winded like a snake) as opposed to the c-shape usually found in the genus.
Diagnosis Proschizorhynchus with a long s-shaped stylet protruding from the cirrus sheath by half its length and accompanied by a bunch of distal spines reaching from the cirrus-sheath to the tip of the stylet.
Description Whitish, body length up to 2.5 mm, very slender, no eye pigmentations. Proboscis lips are each accompanied by a row of spherical refractive secretions some 4 μm in diameter; pharynx in the end of the first quarter of the body. Arrangement of reproductive organs as usual in the genus. Copulatory organ 90 μm long and 40 μm in diameter. The penis papilla is some 30 μm long and turned by 90° from the longitudinal axis of the copulatory organ. The ridged cirrus-sheath is nearly as large as the entire papilla; half of its distal opening bears no, the other half a bunch of very long but delicate spines that accompany the stylet to its tip. These spines seem to be tied among each other and appear as a veil rather than spines under weak magnification. The stylet is S-shaped with a total length (plate-shaped proximal part to tip) of 55 μm (natural s-shape) or 70 μm (imaginary stretched); it protrudes from the distal cirrus opening by about half of its length. The bursal mouth piece is a very narrow tube 19 μm in length and 1 μm in diameter with a bottle-shaped widening and a slightly funnel-shaped opening at one end (Fig. 43e, f). However, this was observed in a single specimen only, and since the entire outline was rather faint this might not be the finale state of development.
Proschizorhynchus frisius sp. nov.
https://zoobank.org/90F71FB4-603F-436E-ABDC-88D90FEF86BE(Figs. 43 and 45d)
Type locality North Sea some 50 km WNW of Sylt island, 20 m water depth (55.1506°N, 007.6582°E; 2 July 2020, 5 individuals). The sediment was ‘pure fine sand’ in field classification while lab analyses classified it ‘moderately well sorted fine sand’ with a median diameter of 213 μm and a mud content of 0.1%. The species occurred together with P. angusticirrus sp. nov., P. serpentistylus sp. nov. and P. triductibus.
Material Live observations, photographs; six whole mounts, one designated holotype (AWI Sylt P2020-115) and five paratypes (AWI Sylt P2020-116 to P2020-120).
Etymology The species name refers to the type locality off the coast of northern Friesland.
Diagnosis Proschizorhynchus with eyes, three adhesive girdles, and a stylet protruding from the cirrus sheath by a third of its length; bursal mouth piece with a wide funnel, without a bulbous enlargement.
Description Resting specimens are 1.5 to 2 mm long but may stretch to 3 mm when crawling; whitish except reddish brown parenchyma lateral of the proboscis. With large eye pigmentations and three girdles of adhesive glands, an anterior one positioned directly behind the pharynx, a terminal one in the very body end, and one between. General arrangement of reproductive organs as usual in the genus. Copulatory organ with a distal penis papilla of some 30 μm containing the ridged cirrus-sheath with the stylet. The distal opening of the cirrus-sheath is cut-off obliquely and turned by some 45° from the longitudinal axis of the copulatory organ. The curved stylet is 27 μm long and protrudes from the cirrus-sheath by a quarter to a third of its length. The rim of the cirrus-sheath bears a bunch of spines approximately as long as the protruding part of the stylet.
The bursal mouth piece and the hardened knob of the spermatic duct were only observed in a single specimen; the mouth piece was a curved tube 17 μm in length with a weakly sclerotic funnel with an opening diameter of 6 μm without a bulbous enlargement (Fig. 43c, d); the tube and funnel are separated by strong circular muscles. The spermatic duct just appeared as a ring some 2.5 μm in outer diameter. Whether or not this is the finale state of development remains unclear.
Proschizorhynchus inusitatus sp. nov.
https://zoobank.org/9BD81064-C3A7-46D2-A58A-42B092AE188C(Figs. 44, 45c)
Type locality Wave-exposed western beach of the Island of Sylt, mid-intertidal medium to coarse sand in 0.5 m sediment depth (55.0368°N, 008.3835°E; Aug 1986, 1 individual).
Material This species had already been found in August 1986 and preserved as a whole mount now designated holotype (AWI Sylt P2020-107).
Etymology The species name refers to the highly unusual (Latin: inusitatus) shape of the bursal appendage.
Diagnosis Proschizorhynchus with eyes, a stylet protruding from the cirrus sheath by a third of its length and accompanied by strikingly long sheath-spines, and a bursal appendage starting as a wide tube that breaks into a bunch of lamellae at half length.
Description The preserved specimen is 1.5 mm long with a mid-body diameter of 200 μm in moderately squeezed condition. General organisation as usual in the genus, with medium-sized reddish eyes, and pharynx (diameter 180 μm) in the end of the anterior third of the body. Copulatory organ (90 × 50 μm) with a distal penis papilla of some 45 μm containing the ridged cirrus-sheath with the stylet. The distal opening of the cirrus-sheath is cut-off obliquely and turned by some 90° from the longitudinal axis of the copulatory organ. The curved stylet is 62 μm long and protrudes from the cirrus-sheath by 17 μm, i.e. roughly a quarter of its length. A bunch of spines protrudes from the convex side of the cirrus-sheath; at the rim of the cirrus-sheath these spines originate side-by-side but then seem to fuse and end in a flagelliform curved tip. Total length of these spines is 45 μm which means they are considerably longer than the protruding stylet tip. The bursal appendage (total length 38 μm) is highly unusual in shape (Fig. 44). It starts as a wide (6.5 μm diameter) tube that widens to some 10 μm and then splits into a bunch of lamellae.
Discussion for the new species of Proschizorhynchus Species of Proschizorhynchus are relatively uniform in general morphology and mainly differ in the shape and size of the penis papilla with the stylet (Noldt 1985; Gobert et al. 2020). In addition, the shape of the sclerotic bursal mouthpiece provides valuable diagnostic characters (Fig. 45). Therefore, the discussion will concentrate on these characters.
P. angusticirrus sp. nov. bears no spines along the distal rim of the penis papilla while such spines are present in most species described so far. It thus joins a small group of spineless Proschizorhynchus-species (P. anophthalmus L’Hardy, 1965, P. lunatus Brunet, 1970, P. arnautsae Gobert et al., 2020). Among these, P. lunatus and P. arnautsae have stylets shorter than P. angusticirrus sp . nov. (35 μm or less against 87 μm) while P. anophthalmus has a longer stylet (105–110 μm). Pigmented eyes are only present in P. angusticirrus sp. nov. Other diagnostic characters of P. angusticirrus sp. nov. are the relatively narrow opening of the penis papilla and the relatively small angle this opening is turned from the longitudinal axis of the copulatory organ (some 45° against some 90° commonly found). Finally, the shape and size of the mouth-piece of the vagina may be species-specific though this part of the female tract is not known for all of the species described so far.
Proschizorhynchus serpentistylus sp. nov. bears a stylet curved outside after half of its length thus attaining an s-shape. This is unique for P. serpentistylus sp. nov. because all Proschizorhynchus-species described so far have a stylet curved in a single direction, reaching from comma-shaped in species with a very short stylet such as P. triductibus Schilke, 1970 to nearly a full circle in long-stylet species such as P. algarvensis Gobert et al., 2020. Spines accompanying the stylet from the cirrus-sheath distally are rather common in the genus. A special character of P. serpentistylus sp. nov. is their extension towards the tip of the stylet.
Proschizorhynchus frisius sp. nov. resembles P. tricingulatus with respect to body size, the presence of three adhesive girdles, paired eyes, and the shape of the stylet while the sizes of the penis papilla, the cirrus-sheath, and the stylet are all smaller in P. frisius sp. nov. than in P. tricingulatus. In addition, the bursal mouth piece opens with a wide funnel and lacks a bulbous enlargement. Finally, P. frisius sp. nov. comes from a subtidal (20 m water depth) marine habitat while P. tricingulatus was described from shallow (1 m) brackish waters of the Black Sea (Ax 1959).
Proschizorhynchus inusitatus sp. nov. deviates from all other species of the genus in the shape of the bursal appendage. Generally, the bursal mouth piece is shaped as a narrow tube with a ± distinct funnel while it is a wide tube that splits into a bunch of lamellae in P. inusitatus sp. nov. Thus, this species currently takes an isolated position in the genus.
List of abbreviations in figures
ast | accessory stylet | ga | genital atrium | pv | prostatic vesicle |
b | bursa | ger | germary | rhb | rhabdites |
bp | basal part of the stylet | gl | glands | sb | seminal bulb |
br | brain | go | genital opening | sd | seminal duct |
cb | copulatory bursa | gvd | germovitelloduct | sg | shell glands |
ci | cirrus | lm | longitudinal muscles | sr | seminal receptacle |
cm | circular muscles | m | muscle fibres | sta | statocyst |
cop | copulatory organ | mb | muscular bag | st | stylet |
e | eyes | mo | mouth opening | sv | seminal vesicle |
fd | female duct | pg | prostatic glands | te | testes |
fg | anterior glands | ph | pharynx | u | uterus |
fgc | female genital canal | pr | proboscis | vit | vitellary |
References
Armonies W (1988a) Common pattern of Plathelminth distribution in North Sea salt marshes and in the Baltic Sea. Arch Hydrobiol 111:625–636
Armonies W (1988b) Active emergence of meiofauna from intertidal sediment. Mar Ecol Prog Ser 43:151–159 https://www.jstor.org/stable/24825763
Armonies W (1988c) Hydrodynamic factors affecting behaviour of intertidal meiobenthos. Ophelia 28:183–193. https://doi.org/10.1080/00785326.1988.10430812
Armonies W (1988d) Physical factors influencing active emergence of meiofauna from boreal intertidal sediment. Mar Ecol Prog Ser 49:277–286 https://www.jstor.org/stable/24827638
Armonies W (1989) Semiplanktonic Plathelminthes in the Wadden Sea. Mar Biol 101:521–527. https://doi.org/10.1007/BF00541654
Armonies W (1990) Short-term changes of meiofaunal abundance in intertidal sediments. Helgol Meeresunters 44:375–386. https://doi.org/10.1007/BF02365474
Armonies W (2017) Long-term change of meiofaunal species composition in a sandy beach, with description of 7 new species of Platyhelminthes. Helgol Mar Res. https://doi.org/10.1186/s10152-017-0492-0
Armonies W (2018) Uncharted biodiversity in the marine benthos: the void of the smallish with description of ten new Platyhelminth taxa from the well-studied North Sea. Helgol Mar Res 72:18. https://doi.org/10.1186/s10152-018-0520-8
Armonies W (2020) Analysing long-term change in small benthos: geomorphological dynamics affects method selection. Helgol Mar Res 74:1. https://doi.org/10.1186/s10152-019-0533-y
Armonies W, Hellwig M (1986) Quantitative extraction of living meiofauna from marine and brackish muddy sediments. Mar Ecol Prog Ser 29:37–43 https://www.jstor.org/stable/24817533
Artois TJ, Schockaert ER (2003) Primary homology assessment in the male atrial system of the Polycystididae (Platyhelminthes: Eukalyptorhynchia). Zool Anz 242:179–190. https://doi.org/10.1078/0044-5231-00095
Atherton S, Jondelius U (2019) A taxonomic review and revisions of Microstomidae (Platyhelminthes: Macrostomorpha). PLoS ONE 14(4): e0212073. https://doi.org/10.1371/journal.pone.0212073
Ax P (1951) Die Turbellarien des Eulitorals der Kieler Bucht. Zool Jb, Abt Syst Okol Tiere 80:277–378
Ax P (1952) Turbellarien der Gattung Promesostoma von den deutschen Küsten. Kieler Meeresforsch 8:218–226
Ax P (1954) Die Turbellarienfauna des Küstengrundwassers am Finnischen Meerbusen. Acta Zool Fenn 81:1–54
Ax P (1956a) Monographie der Otoplanidae (Turbellaria). Morphologie und Systematik, vol 13. Akademie der Wissenschaften und der Literatur Abhandlungen der Mathematisch-Naturwissenschaftlichen, pp 1–298
Ax P (1956b) Turbellarien der Gattung Promesostoma von der französischen Atlantikküste. Kieler Meeresforsch 12:110–113
Ax P (1959) Zur Systematik, Ökologie und Tiergeographie der Turbellarienfauna in den ponto-kaspischen Brackwassergebieten. Zool Jahrb Syst 87:43–184
Ax P (1995) New Promesostoma species (Rhabdocoela, Plathelminthes) from the North Inlet salt marsh of Hobcaw Barony, South Carolina, USA. Microfauna Marina 10:313–318
Ax P, Armonies W (1987) Amphiatlantic identities in the composition of the boreal brackish water community of Plathelminthes. A comparison between the Canadian and European Atlantic coast. Microfauna Marina 3:7–80
Ax P (2008) Plathelminthes aus Brackgewässern der Nordhalbkugel. Steiner, Stuttgart
Beklemischev WN (1927) Uber die Turbellarienfauna des Aralsees. Zugleich ein Beitrag zur Morphologie und zum System der Dalyelliida. Zool Jb Syst Ökol Geogr Tiere 54:87–138
Brunet M (1970) Turbellariés Schizorhynchidae de sables infralittoraux de la côte marseillaise. Cah Biol Mar 11:279–306
Coull BC (1988) Ecology of the marine meiofauna. In: Higgins RP, Thiel H (eds) Introduction to the study of meiofauna. Smithsonian, Washington, pp 18–38
de Wolf P (1989) The price of patchiness. Helgol Meeresunters 43:263–273. https://doi.org/10.1007/BF02365888
Doe DA (1986) Ultrastructure of the copulatory organ of Haplopharynx quadristimulus and its phylogenetic significance (Plathelminthes, Haplopharyngida). Zoomorphology 106:163–173
Ehlers U (1972) Systematisch–phylogenetische Untersuchungen an der Familie Solenopharyngidae (Turbellaria, Neorhabdocoela). Mikrofauna des Meeresbodens 11:1–78
Ehlers U (1974) Interstitielle Typhloplanoida (Turbellaria) aus dem Litoral der Nordseeinsel Sylt. Mikrofauna des Meeresbodens. 49:1–102
Ellenberg H (1982) Vegetation Mitteleuropas mit den Alpen in ökologischer Sicht. Ulmer, Stuttgart
Faubel A (1984) Experimentelle Untersuchungen zur Wirkung von Rohöl und Rohöl/Tensid-Gemischen im Ökosystem Wattenmeer. X. Turbellaria. Senckenbergiana marit 16:153–170
Giere O (2008) Meiobenthology, 2nd edn. Springer, Berlin
Giere O, Eleftheriou A, Murison DJ (1988) Abiotic factors. In: Higgins RP, Thiel H (eds) Introduction to the study of meiofauna. Smithsonian, Washington, pp 61–78
Gobert S, Monnens M, Eerdekens L, Schockaert E, Reygel P, Artois T (2020) Schizorhynchia Meixner, 1928 (Platyhelminthes, Rhabdocoela) of the Iberian Peninsula, with a description of four new species from Portugal. Eur J Taxonomy 595:1–17. https://doi.org/10.5852/ejt.2020.595
Gobert S, Armonies W, Diez YL, Jouk P, Monnens M, Revis N, Reygel P, Smith J, Van Steenkiste N, Artois T (2022) Orostylis gen. nov., a new genus of Dalytyphloplanida with seven new species (Platyhelminthes: Rhabdocoela). Zootaxa 5115: 29-46. https://doi.org/10.11646/zootaxa.5115.1.2
Graff L v (1882) Monographie der Turbellarien 1. Rhabdocoelida. Engelmann, Leipzig, pp 1–442
Graff L v (1905) Turbellaria II. Rhabdocoelida. In: Schulze FE (ed) Das Tierreich, vol 35. Koenigl Preuss Akad Wissensch, Berlin, pp 1–484
Hallez P (1892) Classification des Triclades. Bull Soc Zool France 17:106–109
Hellwig M (1987) Ökologie freilebender Plathelminthen im Grenzraum Watt-Salzwiese lenitischer Gezeitenküsten. Mikrofauna Marina 3:157–248
Hellwig-Armonies M, Armonies W (1987) Meiobenthic gradients with special reference to Plathelminthes and Polychaeta in an estuarine salt marsh creek—a small-scale model for boreal tidal coasts? Helgol Meeresunters 41:201–216. https://doi.org/10.1007/BF02364700
Hoxhold S (1974) Populationsstruktur und Abundanzdynamik interstitieller Kalyptorhynchia (Turbellaria, Neorhabdocoela). Mikrofauna des Meeresbodens 41:1–134
Janssen T, Vizoso DB, Schulte G, Littlewood DTJ, Waeschenbach A, Schärer L (2015) The first multi-gene phylogeny of the Macrostomorpha sheds light on the evolution of sexual and asexual reproduction in basal Platyhelminthes. Mol Phylogenet Evol 92:82–107. https://doi.org/10.1016/j.ympev.2015.06.004
Jouk P, Schockaert E (2002) Species composition and diversity of free-living Plathelminthes (Turbellaria) from sandy beaches at the Belgian coast. Bulletin van het Koninklijk Belgisch Instituut voor Natuurwetenschappen. Biologie = Bulletin de l'Institut Royal des Sciences Naturelles de Belgique. Biologie, (Suppl.) 72:35-41
Jouk PEH, Martens EE, Schockaert ER (2007) Cirrifera genitoductus sp.n. (Platyhelminthes, Proseriata, Coelogynoporidae) from the Belgian coast, with observations on ist ultrastructure and ist significance for the taxonomy of the Coelogynoporidae. Belg J Zool 137:223–230
Karling TG (1935) Mittelungen über Turbellarien aus dem Finnischen Meerbusen. 2. Promesostoma cochlearis n. sp. Memoranda Societatis pro Fauna et Flora Fennica 10:391–395
Karling TG (1955) Studien ueber Kalyptorhynchien (Turbellaria) V. Der Verwandtschaftskreis von Gyratrix Ehrenberg. Acta Zool Fenn 88:1–39
Karling TG (1956) Morphologisch-histologische Untersuchungen an den männlichen Atrialorganen der Kalyptorhynchia (Turbellaria). Arkiv för Zoologi Ser 2(9):187–279
Karling TG (1966) Marine Turbellaria from the Pacific Coast of North America. IV. Coelogynoporidae and Monocelididae. Ark Zool (Ser. 2) 18:493–528
Kolasa J (1977) Uncinorhynchus karlingi sp. nov. and Ethmorhynchus youngi sp. nov. Microturbellaria. Bulletin Acad Polon Sciences, Sér sci biol Cl II. 25(3):167–171
Lanfranchi A (1969) Nuovi otoplanidi (Turbellaria Proseriata) delle coste della Liguria e della Toscana. Bollettino Zool 36:167–188
Lanfranchi A, Melai M, Meini G (2010) Postbursoplana parafibulata sp nov.: A new flatworm from the Ligurian Sea (Plathelminthes, Rhabditophora, Otoplanidae). Ital J Zool 77:168–171
L'Hardy J-P (1965) Turbellariés Schizorhynchidae des sables de Roscoff II. - Le genre Proschizorhynchus. Cah Biol Mar 6:135–161
Luther A (1907) Über die systematische Stellung der Rhabdocoelen-Familie Catenulidae s. str. (=Stenostomidae Vejd.). Zool Anz 31:718–723
Luther A (1918) Vorläufiges Verzeichnis der rhabdocölen und alloeocölen Turbellarien Finnlands. Medd Soc Fauna Flora Fenn 44:47–52
Luther A (1948) Untersuchungen an rhabdocoelen Turbellarien. VII. Über einige marine Dalyellioida. VIII. Beiträge zur Kenntnis der Typhloplanoida. Acta Zool Fenn 55:1–122
Luther A (1962) Die Turbellarien Ostfennoskandiens III. Neorhabdocoela 1. Dalyellioida, Typhloplanoida: Byrsophlebidae und Trigonostomidae. Fauna Fennica 12:1–71
Marcus E (1951) Turbellaria Brasileiros (9). Bol Fac Fil Ci Letr Univ Sao Paulo Zool 16:5–216
Martens PM, Schockaert ER (1981) Sand dwelling Turbellaria from the Netherlands Delta area. Hydrobiologia 84:113–127
Meini G (2015) Two new Otoplanid species (Platyhelminthes: Rhabditophora: Proseriata) of the genera Orthoplana Steinböck, 1932 and Postbursoplana Ax, 1956 from the Tuscan coast (Italy). Zootaxa 3947(3):425–439
Meixner J (1928) Aberrante Kalyptorhynchia (Turbellaria: Rhabdocoela) aus dem Sande der Kieler Bucht. Zool Anz 77:229–253
Meixner J (1938) Turbellaria (Strudelwürmer). I. Allgemeiner Teil. In: Grimpe G, Wagler E (eds) Tierwelt der Nord- und Ostsee IV.b:1-146. Akademische Verlagsgesellschaft mbH Leipzig
Noldt U (1985) Typhlorhynchus syltensis n. sp. (Schizorhynchia, Plathelminthes) and the Adelphotaxa-relationship of Typhlorhynchus and Proschizorhynchus. Mikrofauna Marina 2:347–370
Noldt U (1989) Kalyptorhynchia (Plathelminthes) from sublittoral coastal areas near the Island of Sylt (North Sea). II. Eukalyptorhynchia. Microfauna Marina 5:295–329
Noldt U, Wehrenberg C (1984) Quantitative extraction of living Plathelminthes from marine sands. Mar Ecol Prog Ser 20:193–201 https://www.int-res.com/articles/meps/20/m020p193.pdf
Reise K (1985) Tidal flat ecology. Springer, Berlin
Rieger RM (1971a) Bradynectes sterreri gen. nov., spec. nov., eine neue psammobionte Macrostomide (Turbellaria). Zool Jb Syst 98:205–235
Rieger RM (1971b) Die Turbellarienfamilie Dolichomacrostomidae Rieger II. Teil. Dolichomacrostominae 1. Zool Jb Syst 98:569–703
Schilke K (1970) Kalyptorhynchia (Turbellaria) aus dem Eulitoral der deutschen Nordseeküste. Helgol wiss Meeresunters 21:143–265. https://doi.org/10.1007/BF01630522
Schmidt P (1968) Die quantitative Verteilung und Populationsdynamik des Mesopsammon am Gezeiten-Sandstrand der Nordseeinsel Sylt. I. Faktorengefüge und biologische Gliederung des Lebensraumes. Int Revue ges Hydrobiol 53: 723-779. https://doi.org/10.1002/iroh.19680530504
Schockaert ER, Jouk PEH, Martens PM (1989) Free-living Plathelminthes from the Belgian coast and adjacent areas. In: Wouters K, Baert L (eds) Verhandelingen van het symposium "Invertebraten van België". KBIN, Brussel, pp 19–25
Sopott B (1972) Systematik und Oekologie von Proseriaten (Turbellaria) der deutschen Nordseekueste. Mikrofauna Meeresboden 13:1–72
Spalding MD, Fox HE, Allen GR, Davidson N, Ferdana ZA, Finlayson M, Halpern BS, Jorge MA, Lombana A, Lourie SA, Martin KD, McManus E, Molnar J, Recchia CA, Robertson J (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 57:573–583. https://doi.org/10.1641/B570707
Van Steenkiste N, Volonterio O, Schockaert E, Artois T (2008) Marine Rhabdocoela (Platyhelminthes, Rhabditophora) from Uruguay, with the description of eight new species and two new genera. Zootaxa 1914:1–33
Wehrenberg C (1983) Besiedlungsstruktur der Turbellaria in sublitoralen Sänden der Nordsee bei Sylt. Diploma-thesis, University Göttingen
Wellner G, Reise K (1989) Plathelminth assemblages from an exposed and a sheltered sandy beach of the Island of Sylt in the North Sea. Microfauna Mar 5:277–294
Willems WR, Artois TJ, Backeljau T, Schockaert ER (2005) Typhloplanoida (Platyhelminthes, Rhabdocoela) from New Caledonia and eastern Australia, with the description of six new taxa. N Z J Zool 32:79–98. https://doi.org/10.1080/03014223.2005.9518400
Willems WR, Wallberg A, Jondelius U, Littlewood DTJ, Backeljau T, Schockaert ER, Artois TJ (2006) Filling a gap in the phylogeny of flatworms: relationships within the Rhabdocoela (Platyhelminthes), inferred from 18S ribosomal DNA sequences. Zool Scr 35:1–17
Willems WR, Sandberg MI, Jondelius U (2007) First report on Rhabdocoela (Rhabditophora) from deep parts of Skagerrak, with the description of four new species. Zootaxa 1616:1–21
WoRMS Editorial Board (2020) World Register of Marine Species. http://www.marinespecies.org at VLIZ. Accessed 17 Jan 2020
Xylander WER, Reise K (1984) Free-living Plathelminthes (Turbellaria) of a rippled sand bar and a sheltered beach: a quantitative comparison at the Island of Sylt (North Sea). Microfauna Marina 1:257–277
Acknowledgements
Thanks to the reviewers for their improvements to this manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the author’s home institute (Alfred Wegener Institut Helmholtz Zentrum für Polar- und Meeresforschung, Wattenmeerstation Sylt, Hafenstr. 43, 25992 List, Germany) and did not involve third-party funding. The institute did not influence the design of the study and collection, analysis, interpretation of data, or writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for animal testing, animal care and use of animals were followed by the author.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements, if applicable. The study is compliant with CBD and Nagoya protocols.
Data availability
The datasets supporting the conclusions of this article are included within the article and the online resources.
Author contribution
Not applicable—single author.
Additional information
Communicated by M. Curini-Galletti
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is registered in ZooBank under: https://zoobank.org/BB104A11-EC05-437C-9569-3640D7CD1B50
This article is a contribution to the Topical Collection Biodiversity and Ecology of the Wadden Sea under changing environments
Supplementary Information
ESM 1
(XLSX 245 kb) Online resource 1 Species list.xlsx contains a taxonomic list of the Sylt platyhelminth species (Table 1-1), references for their localities (Table 1-2), references for the data sources (Table 1-3) and the calculated populations centres with respect to tidal level and sediment type (Table 1-4).
ESM 2
(XLSX 6930 kb) Online resource 2 Data matrix.xlsx contains the quantitative (Table 2-1) and qualitative (Table 2-2) data, the results of reduction of quantitative to presence-absence data (Table 2-3) and the combined presence-absence data (Table 2-4), the resulting sampling intensity per habitat type (Table 2-5) and a documentation of data retrieval from the older ‘Hausstrand’- studies (Table 2-6).
ESM 3
(XLSX 18147 kb) Online resource 3 Platyhelminth distribution over habitat types Sylt.xlsx contains the graphical representations of the distribution of localities and frequency, respectively, for the described species around Sylt island; access via Table 3-1 ‘Species list’.
ESM 4
(DOCX 2313 kb) Online resource 4 Morphological remarks.docx contains supplementary information on the genus Microstomum Schmidt 1848 and the species Microstomum crildensis Faubel 1984, Bradynectes sterreri Rieger 1971a, Bradynectes robinhoodensis Janssen et al. 2015 and Kataplana mesopharynx Ax 1956.
ESM 5
(XLSX 15 kb) Online resource 5 Characters Parotoplaninae.xlsx is the compilation of morphological characters in parotoplanid genera used to classify the new species.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Armonies, W. Platyhelminth fauna of the Island of Sylt: a meta-analysis of distributional patterns and description of 19 new species. Mar. Biodivers. 53, 17 (2023). https://doi.org/10.1007/s12526-022-01309-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-022-01309-w