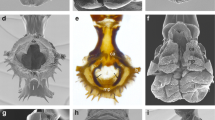
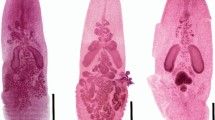
Abstract
Orthonectida is an enigmatic group of parasitic invertebrates with an unclear taxonomic position. Recent molecular studies demonstrated that Orthonectida belongs to Annelida; however, the lack of morphological data does not allow to follow the evolutionary pathway from free-living annelids to parasitic orthonectids. Here, we studied the nervous and the muscular systems in the male and female orthonectid Rhopalura litoralis using confocal laser scanning microscopy and immunohistochemistry. The muscular system is formed by four outer longitudinal muscular bundles and several inner transversal muscles. The nervous system of females is represented by a well-developed cerebral ganglion and a nerve plexus in the body. In males, the cerebral ganglion is significantly smaller, and the body plexus is absent. Instead, a pair of nerves with three pairs of serially organized nerve cells runs posteriorly from the ganglion along the lateral sides of the body. Analyses of the structure of all the orthonectids studied so far suggest that reduction and simplification of the free-living males and females are the dominant mode of evolution in orthonectids.








Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bondarenko, N., Bondarenko, A., Starunov, V., & Slyusarev, G. (2019). Comparative analysis of the mitochondrial genomes of Orthonectida: Insights into the evolution of an invertebrate parasite species. Molecular Genetics and Genomics, 294, 715–727.
Brooks, D., & McLennan, D. A. (1993). Macroevolutionary patterns of morphological diversification among parasitic flatworms (Platyhelminthes: Cercomeria). Evolution, 47(2), 495–509.
Caullery, M. (1961). Classe des Orthonectides (Orthonectida Giard 1877). In: P.P. Grassé (Ed.), Traité de Zoologie, tome IV, fasc. 1 (pp. 695–706). Masson & Cie.
Filippova, A., Purschke, G., Tzetlin, A. B., & Müller, M. C. M. (2010). Musculature in polychaetes: comparison of Myrianida prolifera (Syllidae) and Sphaerodoropsis sp. (Sphaerodoridae). Invertebrate Biology, 129(2), 184–198. https://doi.org/10.1111/j.1744-7410.2010.00191.x
Gąsiorowski, L., Furu, A., & Hejnol, A. (2019). Morphology of the nervous system of monogonont rotifer Epiphanes senta with a focus on sexual dimorphism between feeding females and dwarf males. Frontiers in Zoology 16, 33. https://doi.org/10.1186/s12983-019-0334-9
Giard, A. (1877). Sur les Orthonectida, classe nouvelle d’animaux parasites des Echinodermes et des Turbellariés. Comptes Rendus De L’académie Des Sciences, 85, 812–814.
Hamlet, B., Van Schyndel, D., Adema, C. M., Lewis, L. A., & Locker, E. S. (1996). The phylogenetic position of Rhopalura ophiocomae (Orthonectida) based on 18S ribosomal DNA sequence analysis. Molecular Biology and Evolution, 13, 1187–1191.
Hartmann, D. W. (1925). Mesozoa. In W. Kükenthal & T. Krumbach (Eds.), Handbuch der Zoologie, Bd 1 (pp. 996–1014). Walcer de Gruyter & Co.
Helm, C., & Capa, M. (2015). Comparative analyses of morphological characters in Sphaerodoridae and allies (Annelida) revealed by an integrative microscopical approach. Frontiers in Marine Science, 1, 82.
Kerbl, A., Bekkouche, N., Sterrer, W., & Worsaae, K. (2015). Detailed reconstruction of the nervous and muscular system of Lobatocerebridae with an evaluation of its annelid affinity. BMC Evolutionary Biology, 15, 277. https://doi.org/10.1186/s12862-015-0531-x
Kerbl, A., Fofanova, E. G., Mayorova, T. D., Voronezhskaya, E. E., & Worsaae, K. (2016). Comparison of neuromuscular development in two dinophilid species (Annelida) suggests progenetic origin of Dinophilus gyrociliatus. Frontiers in Zoology, 13(1), 49. https://doi.org/10.1186/s12983-016-0181-x
Kerbl, A., Conzelmann, M., Jékely, G., & Worsaae, K. (2017). High diversity in neuropeptide immunoreactivity patterns among three closely related species of Dinophilidae (Annelida). Journal of Comparative Neurology, 525(17), 3596–3635. https://doi.org/10.1002/cne.24289
Kuper, M., & Purschke, G. (2001). The excretory organs in Sphaerodorum flavum (Phyllodocida, Sphaerodoridae): A rare case of co-occurrence of protonephridia, coelom and blood vascular system in Annelida. Zoomorphology, 120(4), 191–203. https://doi.org/10.1007/s004350000035
Kozloff, E. N. (1969). Morphology of the orthonectid Rhopalura ophiocomae. Journal of Parasitoogy, 55, 171–195.
Kozloff, E. N. (1971). Morphology of the orthonectid Ciliocincta sabellariae. Journal of Parasitoogy, 57, 377–406.
Leasi, F., Fontaneto, D., & Melone, G. (2010). Phylogenetic constraints in the muscular system of rotifer males: Investigation on the musculature of males versus females of Brachionus manjavacas and Epiphanes senta (Rotifera, Monogononta). Journal of Zoology, 282(2), 109–119. https://doi.org/10.1111/j.1469-7998.2010.00721.x
Lindvall, O., & Björklund, A. (1974). The glyoxylic acid fluorescence histochemical method: A detailed account of the methodology for the visualization of central catecholamine neurons. Histochemistry, 39, 97–127.
Mikhailov, K. V., Slyusarev, G. S., Nikitin, M., Logacheva, M. D., Penin, A. A., Aleoshin, V. V., & Panchin, Y. V. (2016). The genome of Intoshia linei affirms orthonectids as highly simplified spiralians. Current Biology, 26, 1768–1774.
Poulin, R. (2011). Evolutionary ecology of parasites. 2nd Ed. Princeton University Press.
Pawlowski, J., Montoya-Burgos, J. -I., Fahrni, J. F., Wuest, J., & Zaninetti, L. (1996). Origin of the Mesozoa inferred from 18S rRNA gene sequences. Molecular Biology and Evolution, 13, 1128–1132.
Scherholz, M., Redl, E., Wollesen, T., Todt, C., & Wanninger, A. (2015). From complex to simple: Myogenesis in an aplacophoran mollusk reveals key traits in aculiferan evolution. BMC Evolutionary Biology, 15, 201. https://doi.org/10.1186/s12862-015-0467-1
Schiffer, P. H., Robertson, H. E., & Telford, M. J. (2018). Orthonectids are highly degenerate annelid worms. Current Biology, 28, 1970–1974. https://doi.org/10.1016/j.cub.2018.04.088
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J. -Y., White, D. J., Hartenstein, V., Eliceiri, K., Tomancak, P., & Cardona, A. (2012). Fiji: An open-source platform for biological-image analysis. Nature Methods, 9, 676–682. https://doi.org/10.1038/nmeth.2019
Slyusarev, G. S. (1994). The fine structure of the female Intoshia variabili (Alexandrov & Sljusarev) (Mesozoa: Orthonectida). Acta Zoologica, 75, 311–321.
Slyusarev, G. S. (2018). 2. Orthonectida. In A. Schmidt-Rhaesa (Ed.), Handbook of Zoology. Miscellaneous Invertebrates (pp. 11–40). De Gruyter. https://doi.org/10.1515/9783110489279-002
Slyusarev, G. S., & Starunov, V. V. (2016). The structure of the muscular and nervous systems of the female Intoshia linei (Orthonectida). Organisms Diversity and Evolution, 16, 65–71.
Slyusarev, G. S., Nesterenko, M. A., & Starunov, V. V. (2019). The structure of the muscular and nervous systems of the male Intoshia linei (Orthonectida). Acta Zoologica, 100(4), 451–458. https://doi.org/10.1111/azo.12279
Slyusarev, G. S., Starunov, V. V., Bondarenko, A. S., Zorina, N. A., Natalya, I., & Bondarenko, N. I. (2020). Extreme genome and nervous system streamlining in the invertebrate parasite Intoshia variabili. Current Biology, 30, 1–7. https://doi.org/10.1016/j.cub.2020.01.061
Struck, T. H. (2006). Progenetic species in polychaetes (Annelida) and problems assessing their phylogenetic affiliation. Integrative and Comparative Biology, 46(4), 558–568. https://doi.org/10.1093/icb/icj055
Struck, T. H., Golombek, A., Weigert, A., Franke, F. A., Westheide, W., Purschke, G., Bleidorn, K., & Halanych, K. M. (2015). The evolution of annelids reveals two adaptive routes to the interstitial realm. Current Biology, 25, 1993–1999. https://doi.org/10.1016/j.cub.2015.06.007
Stunkard, H. W. (1954). The life-history and systematic relations of the Mesozoa. Quarterly Review of Biology, 29, 230–244.
Tekle, Y. I., Raikova, O. I., Ahmadzadeh, A., & Jondelius, U. (2005). Revision of the Childiidae (Acoela), a total evidence approach in reconstructing the phylogeny of acoels with reversed muscle layers. Journal of Zoological Systematics and Evolutionary Research, 43, 72–90.
Vortsepneva, E., Tzetlin, A., & Tsitrin, E. (2009). Nervous system of the dwarf ectoparasitic male of Scolelepis laonicola (Polychaeta, Spionidae). Zoosymposia, 2, 437–445.
Wanninger, A., & Haszprunar, G. (2002). Chiton myogenesis: Perspectives for the development and evolution of larval and adult muscle systems in molluscs. Journal of Morphology, 251, 103–113. https://doi.org/10.1002/jmor.1077
Westheide, W. (1987). Progenesis as a principle in meiofauna evolution. Journal of Natural History, 21, 843–854.
Windoffer, R., & Westheide, W. (1988). The nervous system of the male Dinophilus gyrociliatus (polychaeta, dinophilidae): II. Electron microscopical reconstruction of nervous anatomy and effector cells. Journal of Comparative Neurology, 272, 475–488. https://doi.org/10.1002/cne.902720403
Worsaae, K., & Kristensen, R. M. (2005). Evolution of interstitial Polychaeta (Annelida). Hydrobiologia, 535(536), 319–340. https://doi.org/10.1007/s10750-004-4417-y
Worsaae, K., & Rouse, G. W. (2008). Is Diurodrilus an annelid? Journal of Morphology, 269, 1426–1455. https://doi.org/10.1002/jmor.10686
Zverkov, O. A., Mikhailov, K. V., Isaev, S. V., Rusin, L. Y., Popova, O. V., Logacheva, M. D., Penin, A. A., Moroz, L. L., Panchin, Y. V., Lyubetsky, V. A., & Aleoshin, V. V. (2019). Dicyemida and Orthonectida: Two stories of body plan simplification. Frontiers in Genetics, 10, 443.
Acknowledgements
We are thankful to Dr. M. Makarov for providing accommodation and facilities for field work at the Barents Sea and to the staff of the Marine Biological Station of the Saint Petersburg State University for the assistance in collecting the material. The study was performed at the Core Facility Centers for Microscopy and Microanalysis, Center for Molecular and Cell Technologies, center “CHROMAS,” and center “Culture Collection of Microorganisms” of St. Petersburg State University. The research was partially supported by the budget program AAAA-A19-119020690076-7 to VVS. The main financial support for this study was provided by the Russian Foundation for Basic Research (RFBR) grant № 19-04-00218 and Russian Science Foundation grant 19-74-10013 to N.I.B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. We used neither endangered species nor investigated animals collected in the protected areas.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13127_2021_519_MOESM1_ESM.png
Supplementary file1 (PNG 505 KB). Partial z-projection of a lateral side of a female Rhopalura littoralis. The arrowheads label places where the striation of the muscular cells is clearly seen.
Rights and permissions
About this article
Cite this article
Slyusarev, G.S., Bondarenko, N.I., Skalon, E.K. et al. The structure of the muscular and nervous systems of the orthonectid Rhopalura litoralis (Orthonectida) or what parasitism can do to an annelid. Org Divers Evol 22, 35–45 (2022). https://doi.org/10.1007/s13127-021-00519-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-021-00519-7