Abstract
Tomopteridae are transparent, predatory Annelida inhabiting pelagic ocean zones. Despite being well-known for their fast metachronal swimming and species-specific bioluminescence, our knowledge of morphological adaptations in these fascinating holopelagic worms remains extremely limited. In particular, the evolutionary scenarios and adaptive changes related to the transition from putative benthic ancestors to recent free-swimming groups remain poorly investigated and understood. Therefore, we investigated different taxa and developmental stages within the holopelagic Tomopteridae. We used a comparative morphological approach, including a range of microscopic methods, in our investigations focused on the anterior nervous system and prominent sensory structures, such as nuchal organs and tentacular cirri, in early developmental and adult stages of four tomopterid species. Our data show that Tomopteridae undergo heterochronic, lecithotrophic development with early visibility of adult-like features, which is consistent with earlier investigations. Furthermore, our ultrastructural examinations of the tomopterid nuchal organ highlight the conservativism in the fine structure and development of this prominent polychaete chemosensory organ. Nevertheless, our data indicate ultrastructural differences, such as an extraordinary number of supporting cell types and a bipartite olfactory chamber, potentially related to their pelagic lifestyle. In contrast to previous assumptions, it is shown that the supporting structures in the cirrus-like appendages of the first chaetiger contain prominent intracellular skeletal elements rather than annelid chaetae. These findings highlight the need for further investigations to understand Annelida’s immense morphological diversity of organ systems. Furthermore, our data demonstrate the necessity of functional analyses to understand Annelida’s adaptive radiation of sensory and neuronal structures.
Similar content being viewed by others
Introduction
In contrast to the vast majority of marine annelids, Tomopteridae are holoplanktonic organisms inhabiting the pelagic zone of all oceans (Fernández-Álamo, 2022; Pleijel, 2001). Usually adult marine Annelida live in the benthic realm, although larvae that spend a relatively short time in the open water body are quite common in many taxa. Notably, only a few annelid taxa exhibit a holopelagic lifestyle (Halanych et al., 2007; Purschke et al., 2014; Struck & Halanych, 2010). Nevertheless, at least nine different annelid lineages inhabit the pelagic zone and have convergently evolved specific pelagic adaptations such as lack of pigments and transparency, parapodia adapted to permanent swimming, floating appendages, or bioluminescence (Struck & Halanych, 2010). Those holopelagic taxa have been shown to originate from both, pelagic and benthic ancestors, and thus either resemble derived larval or adult forms (Halanych et al., 2007). The larva-like taxa Poeobius meseres Heath, 1930, Flota spp. Hartman, 1967 (Flabelligeridae) and Chaetopterus pugaporcinus Osborn et al., 2007 (Chaetopteridae) are related to lineages with adults exhibiting a more or less sedentary life style (Halanych et al., 2007; Helm et al., 2018a; Struck et al., 2011; Weigert et al., 2014). In contrast, those showing adult-like characteristics are considered to be part of Phyllodocida, a clade of vagile and often predatory annelids possessing well-developed biramous parapodia (Halanych et al., 2007). Within these, most taxa are related to or even nested within Phyllodocidae, such as Alciopidae, Thyphloscolecidae, and Lopadorhynchidae (Halanych et al., 2007; Struck & Halanych, 2010; Tilic et al., 2022). However, the probably best-known and most intensively studied taxon among these fascinating holopelagic annelids is Tomopteridae (Fernández-Álamo, 2022). Their placement in the phylogenetic tree has been uncertain for quite a long time until first multigene as well as phylogenomic analyses placed them as sister to a clade comprising Glyceridae and Goniadidae (Struck et al., 2007; Tilic et al., 2022). This implies a benthic ancestry for Tomopteridae as well.
Tomopteridae are predators, perfectly adapted to the pelagial due to their transparency—a well-known feature of many pelagic organisms (Johnsen & Widder, 1998)—, and their fern leaf-shaped body and paddle-like parapodia that enable them to perform metachronous swimming at high speed (Daniels et al., 2021). Interestingly, tomopterids combine two different propulsion modes and perform metachronal paddling with undulations of the flexible body resulting in very effective swimming (Daniels et al., 2021). Furthermore, Tomopteridae are well-known for producing species-specific bioluminescence with highly specialized parapodial light glands (Gouveneaux & Mallefet, 2013; Gouveneaux et al., 2017). Besides, Tomopteridae can be easily recognized by their very long pair of anterior chaetigerous cirriform appendages called whiskers, which almost reach the posterior end of the body. These appendages represent a characteristic unique to tomopterids (Fernández-Álamo, 2022). In contrast, the relative morphological homogeneity of tomopterid species makes their taxonomy and species determination difficult resulting in an uncertainty in the number of valid species (Fernandez-Alamo, 2022). However, knowledge of the lifestyle, development, and morphological characteristics of Tomopteris is limited to a few investigations, and the data for understanding the evolution of these holopelagic specialists is still scarce (Åkesson, 1962, 1964; Rakusa-Suszczewski, 1968; Franzén, 1982; Fernández-Álamo, 2022). Especially, ultrastructural investigations are rare and have only been carried out on the nephridia (Bartolomaeus, 1997).
To unravel the adaptive morphological changes related to a transition from a benthic to a holopelagic lifestyle, we examined several Tomopteridae species and life stages. Although Tomopteridae are regularly found in plankton samples, they are often not numerous disregarding from rare exceptions reporting high densities (Åkesson, 1962; Pleijel, 2001; Fernandez-Alamo, 2022). Thus, in order to obtain as many different stages as possible, sufficient material for the investigations and to get an idea about morphological diversity within the group, we examined more than only one species. We used a comparative morphological approach, including various microscopic techniques, to highlight changes of morphological characters related to open ocean life. We focus on developmental neuronal changes of the anterior nervous system, functional morphology of the anterior cirriform appendages and the nuchal organs as one of the best-studied sensory structures in Annelida (Purschke, 2016). Our findings will help to understand the general functional adaptations of taxa in the pelagic ocean zone.
Material and methods
Material
The study was performed on various Tomopteris species in plankton samples collected at the following locations: Roscoff, France (Tomopteris septentrionalis Steenstrup, 1849; adults, collected in 2012); Kristineberg, Sweden (Tomopteris helgolandica Greef, 1879; early developmental stages for transmission electron microscopy [TEM], about 6–7 days after fertilization, unknown date of collection), Raunefjord (depth around 150–200 m) close to the Espegrend Marine Research Field Station (Bergen, Norway; T. helgolandica and Tomopteris planktonis Apstein, 1900; adult and early developmental stages, collected in 2016), and open water plankton samples in Florida, USA (Tomopteris pacifica Izuka, 1914; collected in 2016). Embedded T. helgolandica specimens from Kristineberg were provided by the late Bertil Åkesson. T. pacifica larval stages were collected and fixed by Karen Osborn (Smithsonian National Museum of Natural History, Washington, DC, USA). For initial observations and determination of species, some early and adult stages were used immediately for life observations under a dissecting or compound microscope.
Confocal laser–scanning microscopy (CLSM)
Anatomical details were investigated in whole animal preparations using standard immunohistochemical staining protocols. Stainings were always performed using at least five (for adults) or 15 (for early stages) specimens for each stage of each species. While the specificities of the antibodies used have all been established in numerous invertebrates, we cannot entirely exclude the possibility that a given antiserum may bind to related antigens in the investigated specimens. Therefore, we refer to observed labeled profiles as showing antigen-like immunoreactivity (-LIR). Negative controls were obtained by omitting the primary antibody to check for antibody specificity and showed no fluorescence signal.
The individuals were relaxed in a 7% magnesium chloride (MgCl2) seawater (1:1) solution and then fixed in 4% paraformaldehyde (PFA) in 1 × phosphate-buffered saline (PBS) with Tween (PTW; 0.05 M phosphate buffer, 0.3 M sodium chloride [NaCl], 0.1% Tween 20; pH 7.4) for 2 h at room temperature (RT). After fixing, the specimens were stored in PTW containing 0.005% sodium azide (NaN3) at 4 °C.
Experimental specimens were rinsed twice for 5 min in PTW at RT and then transferred into PTW containing 10 μg/mL proteinase K for 10–20 min. After two short rinses in glycine (2 mg glycine/mL in PTW) and three 5-min washes in PTW, specimens were refixed using 4% PFA in PTW containing 0.1% Tween for 20 min at RT. Next, specimens were rinsed twice for 5 min in PTW, twice for 5 min in 0.1 M Tris hydrochloride with 0.1% Tween (THT; pH 8.5), and blocked with 5% goat serum (Sigma-Aldrich Chemie GmbH; Steinheim, Germany; 25 μl goat serum in 500 μl THT) for 2 h. Then, specimens were incubated with primary antibodies against serotonin (5-hydroxytryptamine [5-HT]; ImmunoStar Inc., Hudson, WI, USA; 1 µl in 500 µl of 5% goat serum) or tubulin (acetylated-α-tubulin; clone 6-11B-1; Merck, Darmstadt, Germany; 2 µl in 500 µl of 5% goat serum) for 48–72 h at 4 °C. Finally, specimens were rinsed twice for 10 min in 1 M NaCl and then washed five times for 30 min in THT.
Subsequently, specimens were incubated with the secondary goat anti-mouse 633 (Alexa Fluor 633 goat anti-mouse immunoglobulin G [IgG; H + L]; Thermo Fisher Scientific, Waltham, MA, USA, 1 μl in 500 μl of 5% goat serum) and goat anti-rabbit 488 (Alexa Fluor 488 goat anti-rabbit IgG (H + L), Thermo Fisher Scientific; 1 μl in 500 μl of 5% goat serum) antibodies for 48–72 h at 4 °C.
After staining, specimens were rinsed five times for 30 min in THT and then twice for 5 min in PTW. Next, specimens were incubated with 4′,6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific; 5 μl in PTW) overnight at 4 °C. For staining of f-actin filaments, treated specimens were incubated in a solution containing phalloidin-rhodamine (5 μl methanolic stock solution in 500 μl PTW) (Invitrogen). After incubation, overnight at 4 °C specimens were dehydrated and cleared as described below.
For anti-synapsin staining, specimens were treated as described in Helm et al. (2014). Preparations were fixed overnight in 1% aqueous formaldehyde containing 18.4 mM zinc chloride, 135 mM NaCl, and 35 mM sucrose. After at least three 15-min rinses in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-buffered saline (10 mM HEPES, 25 mM saccharose, 150 mM NaCl, 5 mM potassium chloride, 5 mM calcium chloride), specimens were incubated in an 80% methanol/20% dimethylsulfoxide (DMSO) solution for 1.5 h at RT. Next, specimens were transferred to 100% methanol for at least 1 h at RT. Then, specimens were rehydrated stepwise and finally incubated for 10 min in Tris-buffered saline (buffer I; 50 mM Tris and 150 mM NaCl; pH 7.4). Next, specimens were transferred into buffer I containing 5% normal goat serum (buffer II; Sigma-Aldrich, St. Louis, MO, USA; 1% DMSO and 0.0005% NaN3) and incubated for 1 h at RT. Then, specimens were incubated with the primary monoclonal mouse anti-synapsin antibody 3C11 (Developmental Studies Hybridoma Bank, Iowa City, IA, USA; 1:30 dilution in buffer II) for 72 h at 4 °C. Next, specimens were rinsed thrice for 2 h in buffer II and incubated with the secondary goat anti-mouse Alexa Fluor 568 antibody (AnaSpec, Fremont, CA, USA; 1:1000 dilution in buffer II) for 48 h at 4 °C. Finally, specimens were washed once in buffer II and twice in buffer I and then dehydrated using an ascending ethanol series.
Subsequently, specimens were dehydrated using an ascending isopropanol series, cleared using Murray’s clear (1:2 benzyl alcohol and benzyl benzoate), and embedded between two coverslips using DPX mounting medium (Merck). Finally, specimens were analyzed with a Leica TCS SP5 confocal laser-scanning microscope (Leica Microsystems, Wetzlar, Germany). Confocal image stacks were processed with the Leica AS AF v2.3.5 (Leica Microsystems) and Imaris 9.3 (Bitplane AG, Zurich, Switzerland) software.
Scanning electron microscopy (SEM)
SEM specimens were fixed in a 2.5% glutaraldehyde solution buffered in 0.05 M phosphate with 0.3 M PBS (pH 7.2) at 4 °C for 1 h and stored in the same buffer. Specimens were post-fixed in 1% osmium tetroxide (OsO4) buffered in 0.05 M phosphate with 0.3 M saline at 4 °C for 30 min and then immediately dehydrated using an ascending ethanol series. The dehydrated specimens in 100% ethanol were transferred to microporous specimen capsules (Electron Microscopy Sciences, Hatfield, PA, USA) for critical point drying. Next, specimens were mounted on conductive carbon adhesive tabs on pin stubs (Electron Microscopy Sciences). Then, specimens were sputter-coated with 40% gold:60% palladium (Polaron SC502 Sputter Coater) and examined under a Zeiss SUPRA 55VP Field Emission SEM. Images were acquired using a secondary electron detector at 3 kV accelerating voltage with a 30-μm aperture. The final images were processed using Adobe Photoshop CC and Illustrator CC (San Jose, CA, USA).
TEM
Electron microscopy specimens of T. septentrionalis and T. helgolandica were relaxed in 8% MgCl2 isotonic with seawater immediately before fixation. The Roscoff specimens (T. septentrionalis) were fixed in a solution of picric acid, PFA, and glutaraldehyde (phosphate-buffered; 0.075 M, pH 7.2) adjusted to the osmolality of seawater with sucrose (SPAFG) (see Ermak & Eakin, 1976). The Kristineberg specimens were fixed in 2.5% glutaraldehyde buffered with cacodylate buffer adjusted to the appropriate salinity with sodium chloride (0.1 M cacodylate and 0.24 M NaCl;.pH 7.4). After initial fixation, the fixative was replaced, and the specimens were incubated for 2 h at 4 °C. Next, specimens were post-fixed in 1% OsO4 (prepared as described above) for 1 h at 4 °C and then washed once with the respective buffer. Then, specimens were dehydrated using an ethanol series with increasing concentrations (30%, 50%, 70%, 80%, 2 × 95%, 2 × 100%; 5 min at 4 °C until 70%, then 10 min at RT). While early developmental stages were left intact after dehydration, adults were dissected into smaller parts (e.g., parapodia and head region) due to their larger size. Next, specimens were washed twice for 5 min in a 1:1 solution of 100% ethanol and intermedium propylene oxide and then washed twice for 10 min in pure propylene oxide. This solution was replaced with intermedium and embedding medium mixtures (propylene oxide: Araldite/Epon [PolyBed 812]). The intermedium was then allowed to evaporate overnight. Specimens were transferred into fresh Araldite/Epon for 5 min at 60 °C before final embedding. After two repetitions, specimens were placed into the embedding molds. Polymerization was performed for 72 h at 60 °C.
Two pre-adult specimens of T. helgolandica were used for a complete series of ultrathin Sects. (70 nm) of the anterior end obtained using diamond knives (Diatome, Biel, Switzerland) and UC6 or UC7 Leica ultra-microtomes (Wetzlar, Germany). Only the chaetigourus cirri were cut from the adult specimens at certain intervals after preparing semi-thin Sects. (1 µm) for orientation. Next, sections were placed on single-slot grids coated with pioloform support films. Then, they were contrasted at 20 °C with 2% uranyl acetate (30 min) and 0.5% lead citrate (20 min) in a Nanofilm Surface Analysis Ultrastainer (Göttingen, Germany). Finally, the sections were examined with Zeiss EM 902A and Libra 120 TEMs (Oberkochen, Germany). Images were acquired using CCD cameras (Image SP, Mohrenweis, Germany). Image panels were prepared using Adobe Photoshop CS and Illustrator CS.
Results
Neurogenesis and the anterior nervous system of Tomopteris pacifica
The post-embryonic development of Tomopteris pacifica is characterized by lecithotrophy, which in this case implies a reduction of larva-like characters such as trochal bands (besides the prototroch) and a prominent apical tuft, as well as a non-feeding development due to yolk deposition in the eggs. The early post-embryonic stages have a prominent prototroch, but the first signs of adult-like features, such as the addition of segments at the posterior end and distinct body appendages, appear early during ontogenesis (Fig. 1A, B). Therefore, early developmental stages already show four pairs of body appendages in addition to the prototroch (Fig. 1B). Notably, while the anterior-most appendage—which later forms the adult tentacular cirrus—is uniramous and cirrus-like, the remaining three body appendage pairs are biramous (Fig. 1B). Nevertheless, anti-α-tubulin staining showed similar innervation of all appendages (Fig. 2B) with prominent neurite bundles branching off from the ventral nerve cord in a comparable manner.
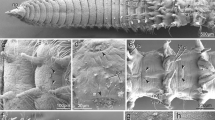
Tomopteris pacifica. Developmental stages. SEM images. A Spherical trochophore ca. 48–72-h post-fertilization. B Elongated embryo at ca. 5 days post-fertilization (dpf) with four segments and rudiments of parapodia, Roman numerals refer to segment numbers. C Dorsal view of larva/juvenile at 6–7 dpf showing parapodia formation; note first cirrus-like appendage. Nuchal organs (no) visible as cilia semicircles in front of the prototroch (pt). D Ventral view of larva/juvenile at ca. 8–10 dpf. E Detailed view of the nuchal organ (no) at 6–7 dpf; note the semicircular cilia arrangement. F Detailed view of parapodium II at 8–10 dpf; the dorsal branch (db) had cilia (ci) and a paddle-like shape, while the ventral branch (vb) appeared cirrus-like. Key: ci, cilia; db, parapodium dorsal branch; ep, episphere; hy, hyposphere; I and II, segment numbers; no, nuchal organ; pa, parapodium; pt, prototroch; vb, ventral parapodium branch
Tomopteris pacifica. Neurogenesis. Confocal maximum projections. A Early developmental stages are characterized by a large amount of yolk and a prominent prototroch (pt). B At 5 days post-fertilization (dpf), the larval stages possess four pairs of well-developed trunk appendages and a distinct prototroch (pt). Note that the anterior-most appendage (I) is uniramous while all other appendages appear biramous. C Slightly older stages show a well-developed ventral nerve cord (vnc) with outgoing parapodial neurite bundles (pn) innervating the body appendages; serotonergic somata form serial clusters along the ventral nerve cord. D A closer examination of larvae at around 6–7 dpf shows the presence of a prominent nuchal nerve (nn) innervating the nuchal organs and originating from the dorsal part of the larval brain (br). The insert shows the innervation of the nuchal organ. For better understanding, the region of the ciliated tuft of the nuchal organ (nt) is highlighted by the dashed pink circle and the course of the innervating nuchal nerves (nn) is marked with a dashed yellow line. E At 8–10 dpf, the developmental stages still possess a distinct prototroch nerve ring, but the remaining body organization is already adult-like. Anterior is at the top of all images. Anti-acetylated-α-tubulin staining is in white, anti-5-HT staining is in red/yellow, and DAPI staining of the cell nuclei is in blue. Key: I and II, appendages with at least one cirrus-like structure; br, brain; cc circumesophageal connective; mo, mouth opening; nn, nuchal nerve; nt, ciliated tuft of the nuchal organ; pn, parapodial nerve; pr, prototroch nerve ring; pt, prototroch; vnc, ventral nerve cord
In older, segmented stages, the anterior-most cirrus-like appendage relocates towards the apical end close to the prominent prototroch (Fig. 1C). Innervation remains unchanged, and distinct serial somata clusters show 5-HT-like immunoreactivity (-LIR; Fig. 2C). In addition, distinct 5-HT positive somata become detectable around the mouth opening and in the prototroch region (Fig. 2C). Moreover, the first signs of the developing nuchal organs appear anteriorly as horseshoe-shaped, densely ciliated pits slightly anterior to the prototroch (Fig. 1C, E). Antibody stainings show a prominent neurite bundle innervating the nuchal organ running from the dorsal brain region toward the horseshoe-shaped organ (Fig. 2D, insert).
In juvenile stages, the body appendages elongate and show a Tomopteris-like paddle-like shape. Simultaneously, additional serially arranged appendages appear along the trunk (Fig. 1D, F). Neuronal innervation along the developing trunk now resembles that observed later in adult specimens. Therefore, four distinct commissures are visible in the brain. The two ventral-most form the ventral roots, and the remaining two form the dorsal roots within Tomopteris (Fig. 2E). The circumesophageal connectives are observable as dense neurite bundles that interconnect the brain and ventral nerve cord. Notably, the neurite bundles innervating appendage I branch off solely from the circumesophageal connective (Figs. 2E and 3C). A distinct prototroch ring nerve is still observable in this stage (Figs. 2E and 3B, C). The ventral nerve cord consists of two main neurite bundles. Anti-α-tubulin staining shows prominent, serially arranged parapodial nerves, and at least two other segmental nerves are observable (Fig. 3C). In addition, the ventral nerve cord has serially arranged 5-HT positive somata in every parapodia-bearing segment (Fig. 3B).
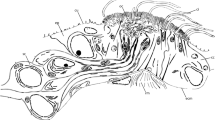
Tomopteris spp. Nervous system. Light microscopic images (A) and confocal maximum projections (B-I). A Adult T. helgolandica show palp-like anterior-most head appendages and a prominent first pair of modified parapodia (II) and distinct eye spots (ey); note that the cirrus-like anterior-most head appendage of the larval stages (I in Fig. 2) is missing in adults of this species. B Anti-5-HT staining in late T. helgolandica larvae shows an adult-like arrangement of neuronal structures; only the prototroch nerve ring (pr) represents a typical larval character. C All body appendages in late developmental stages of T. helgolandica are innervated by neurite bundles originating in the ventral nerve cord (vnc). D A dorsal view of adult T. planktonis shows the ring-like structure of the prominent nuchal organs (no) and the neurites innervating the palp-like appendages (pl). A detailed view of the nuchal organ (insert) shows that the ring of motile cilia (mci) surrounding the olfactory chamber resembles a dense neurite meshwork (arrows) and the nuchal nerve (nn). E Muscular staining in the same species shows two muscle bundles running into the palp-like appendages that represent a continuation of the body-wall longitudinal musculature; the bases of the second pair of appendages have dense musculature arranged in a cone-like pattern. F Anterior margin of the palp-like appendages in T. planktonis are characterized by numerous neurites densely traversing the entire structure, each terminating anteriorly in a receptive process. G Neurite bundles innervating the palp-like appendages in T. helgolandica (arrows) originate from anterior brain regions and form a dense scaffold of parallel neurite bundles branching out into the appendage. H A ventral view of T. planktonis shows numerous neurite bundles running into the palp-like appendage and originating from the epidermal peripheral nervous system. I Anti-5-HT and anti-synapsin staining (insert) resemble the bilobed character of the brain area and neuropile in Tomopteris. Anterior is at the top of all images. Anti-5-HT staining is red (B) or orange (I). Anti-f-actin staining is orange (E). Anti-tubulin staining is shown in (C–D) and (F, G, H). The insert in (I) shows anti-synapsin staining in yellow. Key: br, brain; ey, eye; mci, motile cilia; mo, mouth opening; nb, neurite bundle; no, nuchal organ; nn, nuchal nerve; pa, parapodium; pl, palp-like appendage; pn, parapodia nerve; pr, prototroch nerve ring; vnc, ventral nerve cord
The adult nervous system (based on the observations in T. helgolandica and T. planktonis) is characterized by a dense scaffold of neurite bundles covering the entire anterior end (Fig. 3D). Anti-α-tubulin staining clearly shows the prominent innervation of the adult palp-like appendages and nuchal organs (Fig. 3D, F, G). The palp-like head appendages are characterized by a dense meshwork of sensory nerves mainly innervating the anterior margin of the organ. These sensory structures are innervated by numerous parallel neurite bundles running towards the brain (Fig. 3F, G). Interestingly, the latter parallel bundles do not branch off from distinct brain areas but originate from the entire anterior end of the adult brain (Fig. 3G, H). Staining of the musculature shows two distinct muscle bundles, representing a continuation of the longitudinal body muscles that branch out into the palp-like appendages (Fig. 3E). Anti-α-tubulin staining shows that the nuchal organs are characterized by ciliated rings with a dense meshwork of tubulinergic neurite bundles (Fig. 3G). Anti-5HT and anti-synapsin stainings show the bilobed arrangement of the brain somata and synaptic areas (Fig. 3I).
Tentacular cirri
In the juveniles of all species investigated, the tentacular cirri or cirriform appendages of the first segment are the only sensory appendages present at this stage (labeled I; Fig. 1C, D). The so-called tentacles or palp-like appendages that are the most anterior appendages have not yet developed, and the second parapodium is similar to following parapodia and unmodified (see also Åkesson, 1962). Later in development, this parapodium forms the second pair of very long tentacular cirri or cirriform appendages (whiskers). Each appendage of the first pair is unbranched, cirrus-like, and tapering distally. They arise ventrolaterally from the trunk and lie beside the brain (Figs. 1C and 4A). The cirrus comprises epidermal cells, receptor cell neurites, two long slender cells with intracellular skeletal elements, and a muscle fiber bundle (Fig. 4B–D). Chaetae are absent. Basally, a few gland cell processes are present, which open to the exterior at the cirrus’ base (Figs. 4D and 5D, E). A conspicuous feature is the absence of nuclei in the entire cirrus; all nuclei of cells contributing to the cirrus proper are located in a basal area bulging into the trunk (Fig. 4A, C, D).
Tomopteris helgolandica. Tentacular (first) cirrus of juvenile. TEM. A Half of a cross-section showing the base of a tentacular cirrus emerging lateroventrally; brain somata (so) adjacent to somata contributing to cirrus are circled. B Cross-section of cirrus with two skeletal elements (se), two monociliary receptor cell processes (rc), and muscle fiber bundle (mu). C Enlargement of (A) showing intracellular skeletal elements (arrowheads denote cells with skeletal rods, and arrows denote extracellular matrix [ECM] bulging into the body); note the absence of nuclei in the cirrus proper. D Base of the cirrus with skeletal elements (arrowheads) and spherically arranged somata of cells contributing to the cirrus (arrows denote ECM surrounding somata); nuclei are (n) distributed around the nucleus-free central and distal regions. Specimens shown are at the same stage as in Fig. 1C, D. Key: cc, circumoesophageal connective; ci, cilium; cu, cuticle; ec, epidermal cell; ecm, extracellular matrix; gc, gland cell; mo, mouth cavity; mu, muscle fiber; n, nucleus; ne, neurite; pe, pigmented eye; rc, receptor cell; se, skeletal element; so, somata; tc, tentacular cirrus
Epidermal supportive cells constitute most of the epithelium. They cover comparatively large but flat apical areas (about 0.8–0.9 µm thick), and only in certain places they form flat extensions (about 3 µm thick and 0.9 µm wide) extending deeper into the cirrus between the neurites and other cells (Fig. 4D). Epidermal cells on the prostomium adjacent to the cirri are about 7 µm thick in regions without nuclei. Basally, they form multiple layers (Fig. 4C). The epidermal cells have short, low-density microvilli traversing the thin cuticle (Figs. 4B and 5A, E). The microvilli surmount the cuticle for a short distance and terminate in apical swellings. The cuticle is only 350–600-nm thick and comprises three layers: a basal layer almost devoid of material (210–450 nm) followed by a fibrillary layer of medium electron density (80 nm) and an undulating outer layer of high electron density (35–60 nm). This cuticle is identical to cuticles observed elsewhere on the body. Apically, a single layer of ovoid vesicles (0.6–0.9 µm in the longest direction) is present in the epidermal cells (Figs. 4C, D; 5E, F), which are electron-lucent and contain very little fibrillar material. Typical apical junctional complexes comprise a zonula adherens followed by a septate junction interconnecting the epithelial cells.
The most conspicuous epidermal cells in these appendages are two cells each bearing one long intracellular supporting structure (Figs. 4A–D and 5A–C, F). These cells are deeply anchored in the basal region inside the body (Fig. 4C, D), and their apical regions almost reach the tip of the cirrus. The skeletal elements are electron-dense and oval in cross-section (1.3 by 0.7 µm). Their longitudinal axis shows a characteristic striation pattern with a periodicity of 57.3 nm: a 24.5-nm wide denser band is followed by a lighter band 32.7 nm wide; the latter is subdivided by a 10-nm-wide dark band after 10 nm (Fig. 5A, B). While these rods are very close to the cell membrane in certain places where they converge to just a few nanometers (Fig. 5A), the distance is about the same as the rod’s thickness in other places. Desmosome-like or other attachment structures were not observed. These cells are epithelial, since apically they are part of the outer epidermal cell layer and covered by the cuticle (Fig. 5A, C).
Tomopteris helgolandica. Tentacular cirrus of juvenile. TEM. A Longitudinal section of the intracellular skeletal element (se) with regular cross striation pattern. B Skeletal element, periodicity, and substructure of the striation pattern. C Process of rod-bearing cells reaching the epithelial surface of the epidermis (arrow). D Gland cell opening at the base of cirrus with a circle of microvilli (arrow). E Group of distal gland cell processes close to opening at the base of cirrus. F Central part of the cirrus formed by numerous neurites covered by supportive epidermal cells (ec); note densely arranged microtubules (arrowheads) in specific processes. G, H, I More detailed images of receptor cell apices, each with a single short cilium (ci) resting on the basal body (bb) without rootlet; arrowheads denote microtubules. Key: bb, basal body; ci, cilium; cu, cuticle; ecm, extracellular matrix; ec, epidermal cell; gc, gland cell; m, mitochondrion; mu, muscle fiber; mv, microvillus; ne, neurite; se, skeletal element; ve, vesicle
The neurites are concentrated in the inner part of the cirrus, and occasionally, one turns towards the surface and terminates between the supporting cells (Figs. 4B and 5G–I). These terminals each have a single short cilium (up to 1.5 µm long). No such apical dendrite is visible in most sections, two of which were rarely encountered (Fig. 4B). Apically, these dendrites are about 0.8 µm wide and become somewhat larger just below the junctional complexes. The cilia possess a 9 + 2 × 2 axoneme and arise from a basal body without rootlets (Fig. 5G–I). Many cilia bear a lateral swelling containing some vesicle-like inclusions (Fig. 5G). The neurites contain numerous microtubules following their longitudinal axis, almost reaching the apical regions (Fig. 5F, G, I). In addition, a few mitochondria and dense-core and light vesicles are present in the neurites (Fig. 5F). Up to 75 neurites were counted in cross sections through the basal cirri region. Neuron somata were not encountered inside the appendage.
The musculature only comprises a bundle of about ten flat longitudinal muscle fibers separated from the other cells by a 30–40-nm-thick extracellular matrix (ECM) surrounding the bundle (Fig. 4B, D). Cross-sections show the muscle fibers measure about 3.5 by 0.5 µm and contain a myofilament-free part facing the interior (Fig. 4B). All myofilaments belong to a single layer comprising a single set of obliquely arranged myomeres. Z-elements are represented as rods best visible on longitudinal sections (not shown). Occasionally, mitochondria and tubules of the sarcoplasmic reticulum are interspersed between the myofilaments. The sarcoplasmic reticulum forms dyads with the cell membrane at certain places. The myofilament-free part contains a few mitochondria and granular material, presumably representing glycogen. While neurites directly border the ECM surrounding the muscle fibers, neuromuscular junctions with these neurites were not observed.
For comparison, the second pair of tentacular appendages was investigated in an adult T. septentrionalis specimen (Fig. 6A–D). In addition to the so-called tentacles, these represent the only pair of such appendages in this species’ adult stage. Their most conspicuous structure is an extremely long, strong, rigid chaeta serving as an internal skeletal element (Fig. 6A–C). Intracellular skeletal elements as present in the larval cirri are absent in the adult cirri. A single layer of epidermal cells forming a multicellular follicle composed of numerous cells surrounds the chaeta (Fig. 6A–D). Proximally, this follicle extends deep into the body cavity. In turn, the coelom reaches into the basal part of the appendage (Fig. 6A). The appendage is formed by the epidermis in this region, which covers a sheath of muscle fibers, a large coelomic cavity, and the epidermal follicle with the chaeta in the center. The coelomic cavity is subdivided into four spaces by muscular septa. The appendage gradually tapers distally, and the size of the coelomic spaces and associated tissues decreases. More distally, the appendage is formed only of epidermal cells; musculature and coelom are absent, and the chaeta is positioned eccentrically, enclosed by a thinner sheath of epidermal cells (Fig. 6C). This structure gives the appendage a flattened oval appearance in cross Sects. (110 by 47 µm; Fig. 6C), while the chaeta remains 42 µm in diameter.
Tomopteris septentrionalis. First parapodium of adult (second larval parapodium) of Tomopteris septentrionalis. TEM. A A low-power micrograph of a cross-section near the base with an enlarged cutout from C (inset) showing three monociliary receptor cells (arrows). Parapodium is formed by cuticle, epidermis, and muscular sheath. Chaeta (ch) is located centrally inside the epidermal follicle (ef), followed by a coelomic cavity (coe); muscular septa (sep) subdivide the coelom into four separate cavities. The epidermis (ec) covers a strong muscular sheath mainly comprising longitudinal fibers (mu). Note neurite bundles in the epidermis (arrows). Rectangles labeled 7A, 7B, and 7D mark the positions of enlargements shown in Fig. 7A, B, D, respectively. B Enlargement of a chaetal follicle showing nuclei (n) of follicle cells with little heterochromatin and large nucleoli. C The same parapodium cut more distally; parapodium is only formed by epidermal cells; chaeta is located eccentrically, surrounded by thin follicle cells; beside the chaeta is a strong neurite bundle that follows the longitudinal axis. D Enlargement of a chaeta showing typical honeycomb-like substructure of annelid chaetae. Key: ch, chaeta; coe, coelomic cavity; cu, cuticle; ec, epidermal cell; ecm, extracellular matrix; ef, epidermal follicle; mu, muscle fiber; n, nucleus; ne, neurite; sep, muscle septum
Tomopteris septentrionalis. First parapodium of adult. TEM, A Cuticle (cu), epidermis (ec) with part of a neurite bundle (ne) and muscular sheath (mu). B Follicle cell (fc) from basal region of a chaetal follicle; note nucleus (n) with little heterochromatin and large nucleolus (nu). C Coelomic lining of chaetal follicle comprises an extracellular matrix (ecm), muscle fibers (mu), and coelothelial cover (ct); the coelothel cells are connected by zonula adherens (za) and covered by prominent glycocalyx (gx). D Coelomic lining of the parapodial body wall; muscle fibers (mu) are covered by a thin lining of coelothelial cells (ct). Details are as specified in Fig. 6A. Key: ch, chaeta; coe, coelom; ct, coelothelial cell; cu, cuticle; ec, epidermal cell; ecm, extracellular matrix; fc, follicle cell; gx, glycocalyx; m, mitochondrion; mu, muscle fiber; n, nucleus; ne, neurite; nu, nucleolus; rer, rough endoplasmic reticulum; tf, tonofilaments; za, zonula adherens
This chaeta tapers into a fine tip towards its distal end; proximally, close to its base, it becomes 45–50 µm thick. Cross-sections through the cirrus at different levels show that the chaeta has a honeycomb structure throughout its length, consisting of numerous tube-like substructures (Fig. 6D). The chaeta stains are comparatively electron-lucent. However, the individual tubes are easily distinguishable by more or less distinct, thin, somewhat more electron-dense lines. An electron-dense rim marks each tube’s central lumen, which is relatively narrow compared to the width of the entire tubes (0.04 × 0.08 µm vs. 1.3 µm). There is little structural variation throughout the entire length and perimeter of the chaeta, except for a decrease in the diameter of the individual tubes towards the chaeta’s periphery, where the outermost chitinous tubes are only 0.6 µm thick (Fig. 6D).
The epidermis of the appendage comprises mainly supportive cells, the structure of which changes according to the position along the cirrus. Basally, the epidermis is 2.5–7.3 µm thick and covered by a 1.6-µm-thick cuticle. In regions where basiepithelial neurite bundles are present, the entire epidermis may measure up to 24 µm (Fig. 6A). More distally, the thickness of the epidermis cells surrounding the chaeta decreases and can be only 400 nm thick (Fig. 6C). In regions with nuclei, the epithelial sheath around the chaeta is up to 4 µm thick. The same applies to the cuticle, which is only 0.3 µm thick in this area. The epidermal cells have a low microvilli density (2.8/µm2) traversing the cuticle (Fig. 6A). The microvilli surmount the cuticle for a short distance and terminate in apical swellings. Thin bundles of tonofilaments enter the microvilli, the former terminate in hemidesmosomes after a short distance. The fine structure of the cuticle corresponds to that described above for the larval appendages except for the thickness of the basal part containing little material. In addition, there is a thin transitional layer (200 nm), followed by the medium electron density fibrillary layer (80–110 nm). The epithelial cells are highly interdigitated, and only a few nuclei were observed in each section. Occasionally, a few gland cells are present. Apically, typical junctional complexes comprising zonula adherens followed by a septate junction interconnect the epithelial cells. The cells rest on a prominent electron-dense ECM (0.3 µm thick on average).
The chaetal follicle comprises numerous cells, which form a cylinder about 20 µm (12–24 µm) thick in the basal part. The cells are comparatively electron-dense and characterized by a well-developed endomembrane system and numerous basal–apical oriented tonofilament bundles. They terminate in flat, plasma-membrane-associated electron-dense plaques (60 nm thick). They are basally connected with the underlying muscular sheath via the 200-µm-thick ECM and apically with the chaeta. Apically, the membrane forms an undulating surface precisely following the shape of the chaeta so that the “waves” have a periodicity of about 700 nm and a height of 200 nm. The plaques are always situated in wave troughs (Fig. 6D). The nuclei contain little heterochromatin and a large nucleolus (Fig. 6B). Distally, the follicle cells have a similar undulating surface but lack the electron-dense plaques. Here, the nuclei are flattened and contain more heterochromatin.
In addition, the appendage comprises numerous neurites in a basiepithelial position following its longitudinal axis (Fig. 6A, C). Basally, the neurites form several small bundles concentrated on one side of the cirrus (Fig. 6A). Neurites counts on entire cross-sections indicate their total number to be about 1400–1500. Basally, they are organized into six more or less distinct bundles, the largest of which comprises more than 400 neurites (Fig. 6A). More distally, where mesodermal tissues are absent, these neurites form a single bundle running along the center of the appendage parallel to the chaeta. Their number has only slightly decreased up to this area. Unlike the chaeta, the neurite bundle is accompanied by a few somata, presumably belonging to neurons; the nuclei of the supportive epidermal cells are flattened and contain more heterochromatin. Receptor cells were found distally but not proximally in the few sections investigated. These receptor cells showed a similar morphology to those described above; their dendritic processes are monociliary, each bearing a short cilium without a rootlet. Occasionally, a few processes were united to form small groups or tufts of receptor cells.
The epidermis is underlain by a strong continuous muscular sheath about 24–30 µm thick, comprised mainly of densely arranged longitudinal fibers (Fig. 6A). Occasionally, a few circular fibers are present between the epidermis and longitudinal fibers. The muscle fibers are flattened and oriented with their narrow sides towards the epithelium and coelomic cavity. They are obliquely striated and equipped with a central sarcoplasm core devoid of myofilaments containing mitochondria and sarcoplasmic reticulum tubules. Nuclei were rarely observed but are also situated in the center. The muscular layer is covered by thin and flat cell processes, presumably forming a complete coelothelial cover, often only about 40 nm thick. The coelothelial cells are connected by adhering junctions and are covered by a fuzzy glycocalyx-like coat (200–350 nm thick). The muscular septa subdividing the coelomic cavity and connecting the chaeta to the outer sheath of musculature are comprised of a single muscle fiber layer covered by coelothelial cells. These continue towards the follicle, which is enveloped by a similar arrangement of muscle and coelothelial cells.
Nuchal organ
In the juveniles of T. helgolandica investigated by TEM, the paired nuchal organs remain represented by a paired horseshoe-shaped ciliary patch anterior to the prototroch, the opening of which points anteriorly (Fig. 1C–E). Later in development, the nuchal organs become enlarged, and their ciliary bands form a closed ovoid structure situated laterally on the head region, also called nuchal epaulets (Fig. 3D). This latter stage was not investigated by TEM.
The organs comprise three types of supportive cells and primary sensory cells (Fig. 8A–C; Suppl. Fig. 1A–C). The sensory dendrites terminate in a depression in the center of the ciliary band, forming an olfactory chamber covered by supportive cell processes and the cuticle (Figs. 8A and 9A; Suppl. Fig. 1A, B). These elements are continuous with the smooth anterior end surface, making the olfactory chamber externally invisible (Fig. 1C–E). While the olfactory chamber is connected anteriorly to the subcuticular space (Suppl. Fig. 1A), more posteriorly, the chamber appears completely separate and internal (Fig. 9A; Suppl. Fig. 1B). Therefore, the olfactory chamber forms a tube-like invagination subdivided into anterior and posterior parts by a plug of electron-dense cuticular material (Fig. 8A, C; Suppl. Fig. 1B). The remaining olfactory chamber part is densely filled with sensory receptor cell processes (Figs. 8A and 9B). Such a dense innervation of the olfactory chamber is also visible by antibody staining (Fig. 3D, insert). The posteriormost part of the olfactory chamber is solely formed by receptor cells, which then continue as the nuchal nerve and run toward the brain (Fig. 3D), which at this stage is still situated behind the nuchal organ (Suppl. Fig. 1C). In the stages investigated, the nuchal nerve comprises about 50 receptor cell dendrites.
Tomopteris helgolandica. Nuchal organ, juvenile. TEM. A Nuchal organ comprises three types of supportive cells (suc-1, -2, and -3), a spacious olfactory chamber (oc) extends deep into the body lined by numerous sensory dendrites (sd). The olfactory chamber is filled with sensory processes (spr) originating in sensory dendrites; arrows indicate connections between the olfactory chamber and the external subcuticular space. The section is ~ 350 nm behind Suppl. Fig. 1A. B A type-1 supportive cell (suc-1) with microvilli (mv) and motile cilia (mci) traversing the cuticle (cu). C A type-2 supportive cell (suc-2) is characterized by dense vesicles (dve) and long electron-dense microvilli (mv) covered by an electron-dense cuticular plug (cu) covering the olfactory chamber’s inner part. Key: bb, basal body; cr, ciliary rootlet; cu, cuticle; dv, dense vesicle; m, mitochondrion; mci, motile cilium; mv, microvillus; oc, olfactory chamber; sci, sensory cilium; sd, sensory dendrite; spr, sensory process; suc-1/2/3, supportive cell types 1, 2, and 3; ve, vesicle; za, zonula adherens
Tomopteris helgolandica. Nuchal organ, details of receptor cells, juvenile, TEM. A Outer part of the olfactory chamber (oc), showing that the epithelium is primarily formed by monociliary sensory dendrites (sd), some with basal bodies (arrows). The olfactory chamber has numerous sensory processes (spr), and vesicle-like structures appearing empty (asterisks). The apical region of dendrites often contains dense cored (arrowheads) and other vesicles. B Sensory dendrite (sd) with very short sensory cilium and shaft branches into microvillus-like structures (arrowhead). C Sensory dendrite sending out a microvillus (arrow). D Sensory dendrite (sd) with three basal bodies (arrowheads), long cilia (sci), and a microvillus (smv). E Same cell as in (D) in a different section; note microtubules inside sensory cilia (arrowheads). F. Biciliated sensory dendrite (sd) with long cilia; arrowheads denote cilia with various microtubule patterns. Key: bb, basal body; cu, cuticle; m, mitochondrion; oc, olfactory chamber; sci, sensory cilium; sd, sensory dendrite; sj, septate junction; smv, sensory microvillus; spr, sensory process; suc-3, type-3 supportive cell; ve, vesicle; za, zonula adherens
The type-1 supportive cells are multiciliated and have a cuticle almost identical to that of the remaining parts of the body (Fig. 8A, B; Suppl. Fig. 1A–C). The cilia penetrate the cuticle and are the only sign of this organ when viewed externally. Each cell has approximately 15 cilia and numerous microvilli, but most microvilli terminate below the cuticular surface (Fig. 8A, B). The cilia have a 9 × 2 + 2 axoneme resting on typical basal bodies and anchored in the cytoplasm by short rootlets (Fig. 8B). The supportive cells show a flattened rectangular outline with their longest axis perpendicular to the animal’s anterior–posterior axis. They are arranged in a row. Basally behind the olfactory chamber, the supportive cells envelop a dendrite bundle, forming the nuchal nerve (Suppl. Fig. 1C). The cuticle is similar in structure and thickness as described above for the appendages. However, above the ciliated supportive cells, the basal part is wider and finally merges with the olfactory chamber (Fig. 8A; Suppl. Fig. 1A–C). This cuticle part contains only little material. The middle layer has a medium electron density and appears somewhat thicker than elsewhere on the body. It contains irregularly shaped dense plaques (Fig. 8A). The supportive cells are further characterized by endocytic activity signs (e.g., coated pits, vesicles, and endosomes; Fig. 8B). All supportive cells and sensory dendrites are interconnected by typical junctional complexes comprising a zonula adherens followed by a septate junction (Figs. 8B and 9A, B, F).
The other supportive cells are unciliated. The type-2 supportive cells form the border between the olfactory chamber’s anterior and posterior parts and are characterized by evenly distributed small electron-dense vesicles (220–250 nm in diameter). Apically, they have comparatively long microvilli extending into the olfactory chamber (Fig. 8A, C). The space between these microvilli is completely occupied by the electron-dense cuticular material mentioned above, which separates the chamber’s anterior and posterior parts. The type-3 supportive cells are also unciliated and structurally similar to normal epidermal cells. They partly line the olfactory chamber and, as supportive epidermal cells, they contain numerous electron-lucent vesicles (Fig. 8A; Suppl. Fig. 1A–C).
The dendritic processes of the primary receptor cells terminate in the anterior or posterior olfactory chamber (Figs. 8A and 9A; Suppl. Fig. 1A, B), where they form two groups of adjacent cells surrounded by supportive cells. Apically, these dendritic processes measure 1–1.5 µm in diameter. They can be distinguished from the supportive cells by their more electron-lucent cytoplasm, well-developed microtubules, and smooth endoplasmic reticulum cisternae. Additionally, the cell processes contain small dense cored vesicles and electron-lucent vesicles, and a few small mitochondria (Fig. 9A–D). Most dendrites give rise to a single cilium comprising a basal body and a short shaft with a 9 × 2 + 0 axoneme. Shortly above the basal body (about 200 nm), the shaft splits into microvillus-like structures (Fig. 9B). Rootlets are missing. In addition, microvilli emerge from the processes that intermingle with ciliary branches, proceed in various directions, and finally occupy the olfactory chamber (Fig. 9A, C). The second type of sensory dendrite occurs in the olfactory chamber’s posterior part (Fig. 9D–F). These are slightly larger in apical diameter and have three cilia emerging close together with a few accompanying microvilli. Unlike the cilia of other dendrites, these are unbranched, possess longer shafts, and continuously decrease in diameter (Fig. 9F). While they contain microtubules throughout their entire length, their pattern in the axoneme changes along its longitudinal axis. The axoneme microtubules start with a 9 × 2 + 0 pattern and then progressively decrease in number so that doublets are only present basally; more distally, various singlet numbers were observed.
Discussion
Development and anterior nervous system
Our observations on Tomopteridae development are consistent with those of Åkesson (1962). Accordingly, Tomopteris shows “typical” lecithotrophic development and adult-like features early in ontogeny. A distinct apical organ, characteristic for annelid trochophora larvae (e.g., Purschke, 2016) is missing. Notably, other larval features, such as a well-developed prototroch and the related prototroch nerve ring, are observable even in older individuals. Similar heterochrony and related intermixing of larval and adult features during development can be observed in several errant Annelida and appears highly comparable between closely related groups.
Therefore, developmental investigations of Platynereis dumerilii (Audouin & Milne Edwards, 1833), Platynereis massiliensis (Moquin-Tandon, 1869), and Neanthes arenaceodentata (Moore, 1903) uncovered comparable characteristics in early ontogeny and neurogenesis (Fischer et al., 2010; Winchel et al., 2010; Helm et al., 2014; Starunov et al., 2017). Hence, a comparable transition of the anterior parapodia-bearing segments during development and their involvement in head formation (cephalization) is also described for Platynereis sp., Neanthes arenaceodentata, but is also well-known for sedentary species such as Sabellaria alveolata (Linnaeus, 1767) (Faroni-Perez et al., 2016). Furthermore, a pre-adult development of nuchal organs is also confirmed for the latter taxa. For the sedentary Sabellaridae and Spionidae, a highly comparable neural innervation of those larval nuchal organs—similar to the adult conditions—is described as well (Helm et al., 2018b). Therefore, the larval nuchal organs of Tomopteridae, Sabellariidae, Spionidae, as well as Amphinomidae (own unpubl. observation) are all innervated by neurite bundles originating from the dorsal brain in post-embryonic developmental stages. Additionally, in all these cases, the associated ciliated nuchal pits or tufts are exhibited anterior of the prototroch (as described herein and known for several Sabellariidae and Amphinomidae) or within the non-ciliated dorsal gap of the latter (as known for several Sabellariidae and Spionidae). Furthermore, in all of these cases, a shift of the position and/or size of the nuchal organ has been observed.
Nevertheless, comparable datasets for other—especially errant—taxa and putatively closely related groups such as Glyceridae or Sphaerodoridae are missing but are needed for reconstructing general developmental features of Tomopteridae and within Errantia given their proposed phylogenetic position (Tilic et al., 2022).
The situation is much better concerning a comparative perspective on the adult anterior nervous system. Numerous detailed investigations of putatively related errant taxa such as Syllidae (Orrhage, 1996; Schmidbaur et al., 2020), Phyllodocidae (Orrhage & Eibye-Jacobsen, 1998), Sigalionidae (Beckers et al., 2022), Dorvilleidae (Müller & Henning, 2004), and Eunicidae (Zanol, 2010) are available. The adult (anterior) nervous system shares numerous characteristics with other Errantia, including several commissures in the brain and the structure of the innervation of prominent sensory organs such as nuchal organs. Therefore, our data highlight the general conservativism of the annelid nervous system. Even transitioning from a benthic to a pelagic environment does not change the general patterns and developmental constraints of the neuronal scaffold.
Sensory structures
General aspects
Annelids possess highly diverse sensory structures, either in the form of more or less complex sensory organs or as receptor cells occurring in groups or as isolated cells (Purschke, 2005, 2016; Storch & Schlötzer-Schrehardt, 1988; Verger-Bocquet, 1992). Among the sensory organs described to date for annelids, Tomopteridae are known to possess palp-like appendages, also called frontal antennae, tentacular cirri, eyes, and nuchal organs (Fernández-Álamo, 2022). Because the palp-like appendages occur later in development than the stages investigated, they could not be studied with TEM here. The CLSM observations show that they represent important sensory elements, especially since their frontal part bears numerous receptive structures. To a lesser extent, this also applies to the parapodia and the entire body surface.
The only receptor cells studied by TEM in T. helgolandica were present on the tentacular appendages or in the nuchal organs; the latter will be discussed separately below. Several morphologically distinguishable receptor cells are usually present in a given species, irrespective of their occurrence on specific body parts (Purschke, 2005, 2016; Storch & Schlötzer-Schrehardt, 1988; Verger-Bocquet, 1992). More than one type of receptor cell has been described in the few species studied to date, especially on the sensory appendages (palps, antennae, tentacular, and parapodial cirri; Boilly-Marer, 1972; Dorsett & Hyde, 1969; Purschke et al., 2017). The greatest diversity observed occurs on the cirri in Eurythoe complanata (Pallas, 1776), equipped with four morphologically distinct ciliary receptor cells and a photoreceptor-like receptor (Purschke et al., 2017). However, as in T. helgolandica juveniles and adults, only one morphological distinct receptor cell type has been described to occur on the cirri of Harmothoe imbricata (Linnaeus, 1767) by Lawry (1967), both of which are monociliary. While not possessing parapodial appendages, a similar situation has been described for Polygordius appendiculatus Fraipont, 1887 (Wilkens & Purschke, 2009). Only one receptor cell type was found on the prostomium and the palps, which are the only sensory appendages in Polygordius species. These are typical collar receptors frequently present in aquatic invertebrates (Purschke, 2005; Purschke et al., 2017; Schmidt-Rhaesa, 2007). Unique characteristics of these monociliary receptor cells in T. helgolandica are the absence of a circle of specialized microvilli, their short length (only slightly rising above the cuticle), and the occurrence of lateral swellings containing vesicle-like inclusions. The absence of collar receptors is not unique for T. helgolandica, having also been reported for Sabellaria alveolata (Linnaeus, 1767) by Meyer et al. (2021). Unfortunately, the functions of most of these receptor cells are more or less unknown and speculative based on their morphology (Meyer et al., 2021; Purschke, 2005).
Tentacular cirri
The modified and cirriform parapodia of the first two segments are, at least in part, sensory appendages, given their innervation and receptor cells. The presence of muscle fibers in both appendages suggests they are also highly motile locomotory organs to aid swimming behavior primarily as balancing and floating devices (Åkesson, 1962; Fernández-Álamo, 2022). These appendages are reported to contain supporting chaetae, usually interpreted as aciculae (Åkesson, 1962; Fernández-Álamo, 2022). The first pair of appendages may only be present in larvae or juveniles and become reduced or detached in adults in certain species, as is the case for the species investigated here (Åkesson, 1962; Fernández-Álamo, 2022; Meyer, 1926). The predominant neurite modality present in each appendage is generally considered to represent sensory (afferent) fibers, with only a small portion considered to be efferent, such as innervating muscle fibers and gland cells (Bullock & Horridge, 1969; Horridge, 1963; Lawry, 1967; Purschke, 2016). Neurite counts in appendages are rare in polychaetes (Horridge, 1963; Lawry, 1967; Purschke et al., 2017). While neurite numbers in the first pair of (larval) appendages are comparatively low, those of the second pair (the whiskers) are similar to the parapodial cirri of the other examples. However, given their extraordinary length, their number appears somewhat lower. Since no muscle fibers were present in the most distal parts shown, an afferent orientation appears highly conceivable and supports the hypothesis that sensory processes underlie the vast majority of neurites in annelid appendages.
The presence of two supporting structures in each of the first pair of tentacular cirri in T. helgolandica can be confirmed (Åkesson, 1962). However, as shown by our data, these supporting structures are not represented by typical annelid chaetae, as suggested by previous histological observations (Åkesson, 1962; see Hausen, 2005 for chaetae ultrastructure). They are neither extracellular nor sheathed by a cellular sheath or cellular follicle, contrasting with true intracellular structures; each is formed by a single cell. This fact likely underlies structural differences from normal or typical annelid chaetae reported by previous light microscopy investigations (Åkesson, 1962; Hachfeld, 1926). Speculations about a completely different development mode of these chaetae, such as chaetoblast absence and formation by follicle cells (Åkesson, 1962), can now be resolved by the proof of a completely different structure.
The intracellular elements show a characteristic striation pattern typical of collagen-like proteins, keratin-like elements, and ciliary rootlets (Purschke, 1985; Westheide, 1979). Such intracellular elements are rare in annelids and have seldom been reported. Comparable skeletal elements have also rarely been described outside annelids. For example, they have been described as forming the hard structure in the stylets of some nemertean species (Stricker, 1984) and copulatory stylets of platyhelminths (Ehlers & Ehlers, 1980). Similar skeletal structures have been described in annelids from the copulatory organs in Microphthalmus cf. similis and the so-called tongue-like organ present in the ventral pharynx of Nerillidae, Protodrilidae, and Saccocirridae (Jouin, 1978; Purschke, 1985, 1988; Purschke & Jouin, 1988; Tzetlin et al., 1992; Westheide, 1979). Each supportive cell contains only one skeletal rod in these species, consistent with the Tomopteris appendage.
From unpublished observations, similar supporting elements are also known to occur in Microphthalmidae cirri (Westheide unpublished in Purschke, 1985). Their presence in the sensory appendages of the anterior end (i.e., palps, antennae, and tentacular cirri) of Microphthalmidae (formerly Hesionidae, see Salazar-Vallejo et al., 2019) has been confirmed from our TEM results (Fig. 10A–D). In Microphthalmus similis Bobretzky, 1870, and Hesionides arenaria Friedrich, 1937, intracellular skeletal elements are present in some supportive cells (Fig. 10A–D). As in the other examples described above, each supportive cell contains only one skeletal rod. Their number in the different appendages varied between the species investigated. In addition, the skeletal elements are thinner, probably weaker, and more flexible than those in T. helgolandica. These appendages also differ in the absence of muscle fibers. Therefore, they are only passively moveable and represent solely sensory appendages in Microphthalmidae.
TEM images of Polychaete appendages with intracellular supporting structures. A, B Hesionides arenaria. A Slightly oblique section of the median antenna with four larger and three smaller intracellular skeletal rods (se). B Cross-section through apical part of the tentacular cirrus comprising two skeletal rods (se). C, D Microphthalmus similis. C Tentacular cirrus with about ten skeletal elements (se) situated somewhat eccentrically; the central part of cirrus comprises numerous neurites (ne). D Intracellular skeletal elements (se) with striation patterns surrounded by numerous neurites (ne). Key: cu, cuticle; ec, epidermal cell; g, Golgi apparatus; ne, neurite; rc, receptor cell; sci, sensory cilium; se, skeletal elements
In all examples mentioned, the intracellular elements are always formed by a single cell, but the periodicity reported differs somewhat between them (Jouin, 1978; Purschke, 1985; Tzetlin et al., 1992; Westheide, 1979). For example, from 65 nm in Protodrilus to 67 nm in Trochonerilla, Nerillidium, and Nerilla compared to 57 nm in T. helgolandica. As in T. helgolandica, these elements end apically free in the cytoplasm of the respective cells, explaining the lack of a pointed tip usually present in chaetae as already noted by Åkesson (1962). A brief mention of isolated ciliary rootlets in the antennae of Oxydromus flexuosus (Delle Chiaje, 1827) (as Ophiodromus flexuosus) by Storch (1971) indicates the possibility of a more widespread occurrence of similar skeletal elements in annelid cirri. Interestingly, all known examples belong to the clade Errantia with no reports from its sister clade Sedentaria or more basal branching taxa such as Amphinomida. However, our knowledge remains too incomplete to enable a more conclusive interpretation of their composition and evolutionary history.
Nuchal organs
Nuchal organs belong to, or even represent, the most prominent sensory organ in polychaete annelids which are present in almost every lineage (Beckers & Tilic, 2021; Purschke, 1997, 2016; Verger-Bocquet, 1992). Therefore, they have been thought to represent an important morphological autapomorphy of either polychaetes or Annelida (Rouse & Pleijel, 2001; Struck et al., 2011). Since they are absent in the lineages branching off first in the annelid phylogenetic tree, Palaeoannelida and Chaetopteriformia (see Weigert et al., 2014; Helm et al., 2018a), this is now believed to represent a primary absence rather than a loss (Beckers et al., 2019a, b). Consequently, this led to the current alternate hypothesis that nuchal organs were not present in the annelid stem species and evolved later in annelid evolution within the stem lineage of Amphinomida/Sipuncula and Pleistoannelida (Beckers & Tilic, 2021; Beckers et al., 2019b).
Usually situated at the interface between prostomium and peristomium, nuchal organs are externally visible as densely ciliated areas or patches of various shapes, showing considerable morphological variability (Purschke, 1997; Storch & Schlötzer-Schrehardt, 1988; Verger-Bocquet, 1992). Especially in burrowing or tube-dwelling forms, they are situated in more or less deep pits or grooves and can be withdrawn into the body, probably for protection during burrowing activities. Besides a few loss and reduction cases, several taxa possess extremely large and well-developed nuchal organs, which extend posteriorly over several trunk segments. Examples usually mentioned are Amphinomidae and certain Spionidae, Syllidae, and Phyllodocidae members (Buhre & Purschke, 2021; Purschke, 1997, 2005). While known to possess enlarged nuchal organs (Fernández-Álamo, 2022; Pleijel, 2001), Tomopteridae have mostly been neglected in this respect.
A common morphological feature of all these enlarged nuchal organs is their elongated bands of cilia forming semicircles, circles, ovals, or even long parallel bands on either side of the body (Beckers & Tilic, 2021; Jelsing, 2003; Jelsing & Jacobsen, 2010; Söderström, 1930). These large nuchal organs are typically called caruncles when situated on a bulging ridge and nuchal epaulets on flat structures (Beckers & Tilic, 2021; Purschke, 1997, 2005). In adult Tomopteris spp., the nuchal organs form epaulets. Our results confirm those of Åkesson (1962), who showed that they start as a pair of small ciliary patches during ontogenetic development. Somewhat later, they become semicircular, and a closed oval cilia ring is present in the adult stage. There is some interspecific size variability, but they generally extend beyond the pigmented eyes in adults (Fernández-Álamo, 2022; Pleijel, 2001).
In contrast to the external variability of nuchal organs, their anatomical structure is almost identical, comprising only a few but highly similar cell types throughout all taxa investigated (Buhre & Purschke, 2021; Purschke, 1997, 2005; Verger-Bocquet, 1992). These organs comprise ciliated supporting cells, bipolar primary receptor cells, a retractor muscle (if they can be retracted), and direct innervation from the brain’s posterior region (Buhre & Purschke, 2021; Helm et al., 2018b; Jelsing & Jacobsen, 2010; Purschke, 1997, 2005, 2016; Rullier, 1951; Schmidtberg & Dorresteijn, 2010; Verger-Bocquet, 1992). The supportive and receptor cells form an olfactory chamber separated from the exterior that houses the sensory processes. Therefore, visible cilia usually belong to supportive cells. These cilia are motile, non-sensory, and most likely responsible for a quick exchange of sensory stimuli. Occasionally, other cell types may be present, such as additional supportive cells that can be structurally distinguished from normal epidermal cells (West, 1978; Rhode, 1990; Purschke, 1997, 2000). Generally, diverging nuchal organ descriptions are based on incomplete, preliminary, or in some cases, erroneous interpretations (e.g., Storch & Welsch, 1969).
In T. helgolandica, an extraordinary number of three supportive cell types have been found. The type-1 supportive cell corresponds to the ciliated supportive cells described above. In contrast, a unique feature of T. helgolandica is the so-called type-2 supportive cell, which does not possess cilia and lines part of the deeper olfactory chamber. Its structure suggests it most likely secretes the dense cuticular material separating the olfactory chamber into anterior and posterior parts. The type-3 supportive cells line one side of the olfactory chamber and can be regarded as belonging to the nuchal organ despite being structurally similar to normal epidermal cells.
In nuchal organs not situated in more or less deep pits, the olfactory chamber is often separated from the outside by a modified cover formed of microvilli or cuticles of the supportive cells (Buhre & Purschke, 2021; Purschke, 1997). However, nuchal organs without such covers have been found in certain errant polychaetes. Those reported to date are Nephtys caeca (Fabricius, 1780), Eulalia viridis (Linnaeus, 1767), Eteone longa (Fabricius, 1780), Phyllodoce mucosa Örsted, 1843 (as Anaitides mucosa), Glycera unicornis Lamarck, 1818 (as Glycera rouxii), Hediste diversicolor (Müller, 1776) (as Nereis diversicolor), Platynereis dumerilii (Audouin & Milne Edwards, 1833), and Microphthalmus similis Bobretzky, 1870 (Purschke, 1997, 2005; Rhode, 1990; Schmidtberg & Dorresteijn, 2010; Whittle & Zahid, 1974). Interestingly, all of these belong to an unnamed Phyllodocida subclade comprising Nephtyiformia, Nereidiformia (including Microphthalmidae), and Glyceriformia (Tilic et al., 2022). Errant species outside this group, such as those of the large Syllidae taxon, possess corresponding covering structures (Lewbart & Riser, 1996; Purschke, 1997). However, data on the nuchal organs in Aphroditiformia, the scale worms suggested as the sister group of Syllidae, are still missing. Available data on Eunicida, the sister group of Phyllodocida, also report the presence of such covering structures (Purschke, 1997; Rhode, 1989). Therefore, the absence of specialized covers in this clade may represent an apomorphy of this unnamed subclade.
As is typical in polychaete nuchal organs, the sensory cells of T. helgolandica are bipolar receptor cells terminating in clusters at the bottom of the olfactory chamber (Buhre & Purschke, 2021; Purschke, 1997, 2005; Verger-Bocquet, 1992). Most receptor cells correspond to those generally described as having only one branched cilium with a modified axoneme and additional microvilli. As is typical, rootlets are either absent or weakly developed. However, the other receptor cell type with three cilia may represent a specific feature of T. helgolandica, although more than one cilium has also been reported in a few other species. Examples within Errantia are Nephtys caeca (Fabricius, 1870), Hediste diversicolor (Müller, 1776) (as Nereis diversicolor), Glycera unicornis Lamarck, 1818 (as Glycera rouxii), Eulalia viridis (Linnaeus, 1776), Eteone longa (Fabricius, 1870), and Phyllodoce mucosa Örsted, 1843 (as Anaitides mucosa) (see Whittle & Zahid, 1974; Rhode, 1990). Receptor cells without cilia have rarely been reported (Purschke, 1997). In addition, a bipartite olfactory chamber is also uncommon in annelids. While the stages investigated by TEM are early juveniles, their nuchal organs appear to be fully functional, indicating the great importance of this sensory system in animals living in all ocean depths, from the surface to abyssal waters (Fernández-Álamo, 2022). In general, chemosensory systems are central to animal behavior and are important sensory systems in aquatic annelids (Chartier et al., 2018; Lindsay, 2009), particularly holopelagic forms living partly or wholly in an environment without sunlight. To date, chemosensory activity has been shown to occur in nuchal organs rather than on various body parts (Chartier et al., 2018).
Conclusions
Our comparative investigations of different Tomopteris species provide detailed insights into neuronal and sensory structure development in this fascinating taxon of holopelagic annelids. Our integrative methodological approach has shown Tomopteridae to have a heterochronic, lecithotrophic development with early visibility of adult-like features and a complex adult anterior nervous system. The supporting structures in the cirrus-like appendages of the first chaetiger contain prominent intracellular skeletal elements rather than annelid chaetae, as had been previously assumed. Among annelids, such skeletal elements are so far only known from Phyllodocida. Furthermore, our ultrastructural examinations of sensory structures, such as the nuchal organ, have shown conservativism in the fine structure and development of this prominent polychaete characteristic complex. Nevertheless, the here found divergent receptor and supportive cell types and specific olfactory chamber also illustrate the structural flexibility of highly conserved organs such as the nuchal organ to adapt to a pelagic lifestyle.
Therefore, our findings highlight the need for further comparative but detailed investigations to understand the immense morphological diversity of organ systems in Annelida. Furthermore, our data demonstrate the necessity of functional analyses to understand the adaptive radiation of sensory polychaete structures.
Data availability
TEM materials (embedded blocks, semi-thin sections, and ultrathin sections) are stored at the Department of Zoology and Developmental Biology at the University of Osnabrueck. All TEM images taken are stored in the Omero database hosted at the University of Osnabrueck. These images are available from the corresponding authors upon reasonable request.
References
Åkesson, B. (1962). The embryology of Tomopteris helgolandica (Polychaeta). Acta Zoologia (Stockholm), 43, 135–199.
Åkesson, B. (1964). On the eyes of Tomopteris helgolandica (Tomopteridae, Polychaeta). Acta Zoologica, 45(3), 179–189.
Bartolomaeus, T. (1997). Structure and development of the nephridia of Tomopteris helgolandica (Annelida). Zoomorphology, 117, 1–11.
Beckers, P., & Tilic, E. (2021). Fine structure of the brain in Amphinomida (Annelida). Acta Zoologia (Stockholm), 102, 483–495.
Beckers, P., Helm, C., Purschke, G., Worsaae, K., Hutchings, P., & Bartolomaeus, T. (2019a). The central nervous system of Oweniidae (Annelida) and its implications for the structure of the ancestral annelid brain. Frontiers in Zoology, 16, 6. https://doi.org/10.1186/s12983-019-0305-1
Beckers, P., Helm, C., & Bartolomaeus, T. (2019b). The anatomy and development of the nervous system in Magelonidae (Annelida) – Insights into the evolution of the annelid brain. BMC Evolutionary Biology, 19, 173. https://doi.org/10.1186/s12862-019-1498-9
Beckers, P., Pein, C., & Bartolomaeus, T. (2022). Fine structure of mushroom bodies and the brain in Sthenelais boa (Phyllodocida, Annelida). Zoomorphology, 141(1), 19–36.
Boilly-Marer, Y. (1972). Étude ultrastructurale des cirres parapodiaux des neridens atoques (Annélides, Polychètes). Zeitschrift für Zellforschung und Mikroskopische Anatomie, 131, 309–327.
Bullock, T. H. & Horridge, G. A. (1969). Structure and function of the nervous system in invertebrates. Freeman & Co.
Buhre, J. S., & Purschke, G. (2021). Ultrastructure and functional morphology of the dorsal organs in Scoloplos armiger (Annelida, Sedentaria, Orbiniida). Zoomorphology, 140, 437–452.
Chartier, T. F., Deschamps, J., Dürichen, W., Jékely, G., & Arendt, D. (2018). Whole-head recording of chemosensory activity in the marine annelid Playtnereis dumerilii. Open Biology, 8, 180139.
Daniels, J., Aoki, N., Havassy, J., Katija, K., & Osborn, K. J. (2021). Metachronal swimming with flexible legs: A kinematics analysis of the midwater polychaete Tomopteris. Integrative and Comparative Biology, 61(5), 1658–1673.
Dorsett, D. A., & Hyde, R. (1969). The fine structure of the compound sense organs in the cirri of Nereis diversicolor. Zeitschrift Für Zellforschung Und Mikroskopische Anatomie, 97, 512–527.
Ehlers, B., & Ehlers, U. (1980). Struktur und Differenzierung penialer Hartgebilde von Carenscoilia bidentata Sopott (Turbellaria, Proseriata). Zoomorphologie, 95, 159–167.
Ermak, T. H., & Eakin, R. M. (1976). Fine structure of the cerebral and pygidial ocelli in Chone ecaudata (Polychaeta: Sabellidae). Journal of Ultrastructure Research, 54, 243–260.
Faroni-Perez, L., Helm, C., Burghardt, I., Hutchings, P., & Capa, M. (2016). Anterior sensory organs in Sabellariidae (Annelida). Invertebrate Biology, 135(4), 423–447.
Fischer, A. H., Henrich, T., & Arendt, D. (2010). The normal development of Platynereis dumerilii (Nereididae, Annelida). Frontiers in Zoology, 7(1), 1–39.
Fernández-Álamo, M. A. (2022). Tomopteridae Grube, 1850. In G. Purschke, W. Westheide & M. Böggemann (Eds.), Handbook of zoology. Annelida. Vol. 4 Pleistoannelida, Errantia II. (pp. 400–412) Berlin: De Gruyter.
Franzén, Å. (1982). Ultrastructure of the biflagellate spermatozoon of Tomopteris helgolandica Greef, 1879 (Annelida, Polychaeta). Gamete Research, 6(1), 29–37.
Gouveneaux, A., Flood, P. R., Erichsen, E. S., Olsson, C., Lindström, J., & Mallefet, J. (2017). Morphology and fluorescence of the parapodial light glands in Tomopteris helgolandica and allies (Phyllodocida: Tomopteridae). Zoologischer Anzeiger, 268, 112–125.
Gouveneaux, A., & Mallefet, J. (2013). Physiological control of bioluminescence in a deep-sea planktonic worm, Tomopteris helgolandica. Journal of Experimental Biology, 216(22), 4285–4289.
Hachfeld, G. (1926). Beiträge zur Kenntnis der Tomopteris catharina Gosse. Zeitschrift für Wissenschaftliche Zoologe, 128, 133–181.
Halanych, K. M., Cox, L. N., & Struck, T. H. (2007). A brief review of holopelagic annelids. Integrative and Comparative Biology, 47(6), 872–879.
Hausen, H. (2005). Chaetae and chaetogenesis in polychaetes (Annelida). Hydrobiologia, 535/536, 37–52.
Helm, C., Adamo, H., Hourdez, S., & Bleidorn, C. (2014). An immunocytochemical window into the development of Platynereis massiliensis (Annelida, Nereididae). International Journal of Developmental Biology, 58(6–8), 613–622.
Helm, C., Beckers, P., Bartolomaeus, T., Drukewitz, S. H., Kourtesis, I., Weigert, A., Purschke, G., Worsaae, K., Struck, T. H., & Bleidorn, C. (2018a). Convergent evolution of the ladder-like ventral nerve cord in Annelida. Frontiers in Zoology, 15, 36. https://doi.org/10.1186/s12983-018-0280-y
Helm, C., Bok, M. J., Hutchings, P., Kupriyanova, E., & Capa, M. (2018b). Developmental studies provide new insights into the evolution of sense organs in Sabellariidae (Annelida). BMC Evolutionary Biology, 18, 149. https://doi.org/10.1186/s12862-018-1263-5
Horridge, G. A. (1963). Propioreceptors, bristle receptors, efferent sensory impulses, neurofibrils and number of axons in the parapodial nerve of the polychaete Harmothoe. Proceedings of the Royal Society of London B Biological Sciences, 157, 199–222.
Jelsing, J. (2003). Ultrastructural studies of dorsal ciliated organs in Spionidae (Annelida: Polychaeta). Hydrobiologia, 496, 241–251.
Jelsing, J., & Jacobsen, D. (2010). Ultrastructure of the extensively developed nuchal organs in Laonice bahusienis (Annelida: Canalipalpata: Spionidae). Journal of Morphology, 271, 376–382.
Johnsen, S., & Widder, E. A. (1998). Transparency and visibility of gelatinous zooplankton from the Northwestern Atlantic and Gulf of Mexico. The Biological Bulletin, 195(3), 337–348.
Jouin, C. (1978). Anatomical and ultrastructural study of the pharyngeal bulb in Protodrilus (Polychaeta, Archiannelida). II. The stomodeal epithelium and its cuticle. Tissue and Cell, 10, 289–301.
Lawry, J. V. Jr. (1967). Structure and function of the parapodial cirri of the polynoid polychaete, Harmothoë. Zeitschrift für Zellforschung und Mikroskopische Anatomie, 82, 345–361.
Lewbart, G. A., & Riser, N. W. (1996). Nuchal organs in the polychaete Parapionosyllis manca (Syllidae). Invertebrate Biology, 115, 286–298.
Lindsay, S. M. (2009). Ecology and biology of chemoreception in polychaetes. Zoosymposia, 2, 339–367.
Meyer, A. (1926). Die Segmentalorgane von Tomopteris catharina (Gosse) nebst Bemerkungen über das Nervensystem, die rosetten-förmigen Organe und die Coelombewimperung. Zeitschrift für Wissenschaftliche Zoologe, 127, 297–402.
Meyer, C., André, T., & Purschke, G. (2021). Ultrastructure and functional morphology of the appendages in the reef-building polychaete Sabellaria alveolata (Annelida, Sedentaria, Sabellida). BMC Zoology, 6, 5. https://doi.org/10.1186/s40850-021-000068-8
Müller, M. C. & Henning, L. (2004). Ground plan of the polychaete brain—I. Patterns of nerve development during regeneration in Dorvillea bermudensis (Dorvilleidae). Journal of Comparative Neurology, 471:1, 49–58.
Orrhage, L. (1996). On the microanatomy of the brain and the innervation and homologues of the cephalic appendages of Hesionidae and Syllidae (Polychaeta). Acta Zoologica, 77, 137–151.
Orrhage, L., & Eibye-Jacobsen, D. (1998). On the anatomy of the central nervous system of Phyllodocidae (Polychaeta) and the phylogeny of phyllodocid genera: A new alternative. Acta Zoologica, 79(3), 215–234.
Purschke, G. (1985). Anatomy and ultrastructure of ventral pharyngeal organs and their phylogenetic importance in Polychaeta (Annelida). II. The pharynx of the Nerillidae. Mikrofauna Marina, 2, 23–60.
Purschke, G. (1988). Pharynx. In W. Westheide & C.O. Hermans (Eds.), The ultrastructure of polychaeta. Microfauna Marina, 4, 177–197.
Purschke, G. (1997). Ultrastructure of nuchal organs in polychaetes (Annelida) - New results and review. Acta Zoologia (Stockholm), 78, 123–143.
Purschke, G. (2005). Sense organs in polychaetes (Annelida). Hydrobiologia, 535(536), 53–78.
Purschke, G. (2016). Annelida: Basal groups and Pleistoannelida. In A. Schmidt-Rhaesa, S. Harzsch, & G. Purschke (Eds.), Structure and evolution of invertebrate nervous systems (pp. 254–312). Oxford.
Purschke, G., Bleidorn, C., & Struck, T. (2014). Systematics, evolution and phylogeny of Annelida–A morphological perspective. Memoirs of Museum Victoria, 71, 247–269.
Purschke, G., & Jouin, C. (1988). Anatomy and ultrastructure of the ventral pharyngeal organs of Saccocirrus (Saccocirridae) and Protodriloides (Protodriloidae fam. n.) with remarks on the phylogenetic relationships within the Protodrilida (Annelida, Polychaeta). Journal of Zoology, 215, 405–432.
Purschke, G., Hugenschütt, M., Ohlmeyer, L., Meyer, H., & Weihrauch, D. (2017). Structural analysis of the branchiae and dorsal cirri in Eurythoe complanata (Annelida, Amphinomida). Zoomorphology, 136, 1–18.
Pleijel, F. (2001). Tomopteridae. In G. W. Rouse & F. Pleijel (Eds.), Polychaetes (pp. 139–140). Oxford.
Rakusa-Suszczewski, S. (1968). Predation of Chaetognatha by Tomopteris helgolandica Greff. ICES Journal of Marine Science, 32(2), 226–231.
Rhode, B. (1989). Ultrastructural investigations on the nuchal organ of the protandric polychaete, Ophryotrocha puerilis (Polychaeta, Dorvilleidae). Zoomorphology, 108, 315–322.
Rhode, B. (1990). Ultrastructure of nuchal organs in some marine polychaetes. Journal of Morphology, 206, 95–107.
Rouse, G. W., & Pleijel, F. (2001). Polychaete systematics. In G. W. Rouse & F. Pleijel (Eds.), Polychaetes (pp. 8–17). Oxford.
Rullier, F. (1951). Étude morphologique, histologique et physiologique de l’organe nuchal chez les annélides polychètes sédentaires. Annales de l’Institut Oceanographique de Monaco, 25, 207–341.
Salazar-Vallejo, S. I., de León-González, J. A., & Carrera-Parra, L. F. (2019). Phylogeny of Microphthalminae Hartmann-Schröder, 1971, and revision of Hesionella Hartman, 1939, and Struwela Hartmann-Schröder, 1959 (Annelida, Errantia). PeerJ, 7, e7723.
Schmidt-Rhaesa, A. (2007). The evolution of organ systems. Oxford University.
Schmidtberg, H., & Dorresteijn, A. (2010). Ultrastructure of the nuchal organs in the polychaete Platynereis dumerilii (Annelida, Nerididae). Invertebrate Biology, 129, 252–265.
Schmidbaur, H., Schwaha, T., Franzkoch, R., Purschke, G., & Steiner, G. (2020). Within-family plasticity of nervous system architecture in Syllidae (Annelida, Errantia). Frontiers in Zoology, 17, 1. https://doi.org/10.1186/s12983-020-00359-9
Söderström, A. (1930). Über segmental wiederholte “Nuchalorgane” bei Polychäten. Zoologiska Bidrag från Uppsala, 12, 1–18.
Starunov, V. V., Voronezhskaya, E. E., & Nezlin, L. P. (2017). Development of the nervous system in Platynereis dumerilii (Nereididae, Annelida). Frontiers in Zoology, 14(1), 1–20. https://doi.org/10.1186/s12983-017-0211-3
Storch, V. (1971). Elektronenmikroskopische Untersuchungen an Rezeptoren von Anneliden (Polychaeta, Oligochaeta). Zeitschrift Für Mikroskopisch-Anatomische Forschung, 85, 55–84.
Storch, V. & Schlötzer-Schrehardt, U. (1988). Sensory structures. In W. Westheide & C.O. Hermans (Eds.), The Ultrastructure of Polychaeta. Microfauna Marina, 4, 121–133.
Storch, V., & Welsch, U. (1969). Zur Feinstruktur des Nuchalorgans von Eurythoe complanata (Pallas) (Amphinomidae, Polychaeta). Zeitschrift Für Zellforschung, 100, 411–420.
Stricker, S. A. (1984). Styletogenesis in nemertean worms: The ultrastructure of organelles involved in intracellular calcification. Journal of Morphology, 179, 119–134.
Struck, T. H., Schult, N., Kusen, T., Hickman, E., Bleidorn, C., McHugh, D., & Halanych, K. M. (2007). Annelid phylogeny and the status of Sipuncula and Echiura. BMC Evolutionary Biology, 7, 57. https://doi.org/10.1186/1471-2148-7-57
Struck, T. H., Paul, C., Hill, N., Hartmann, S., Hoesel, C., Kube, M., Lieb, B., Meyer, A., Tiedemann, R., Purschke, G., & Bleidorn, C. (2011). Phylogenomic analyses unravel annelid evolution. Nature, 471, 95–98.
Struck, T. H., & Halanych, K. M. (2010). Origins of holopelagic Typhloscolecidae and Lopadorhynchidae within Phyllodocidae (Phyllodocida, Annelida). Zoologica Scripta, 39(3), 269–275.
Tilic, E., Stiller, J., Campos, E., Pleijel, F., & Rouse, G. W. (2022). Phylogenomics resolves ambiguous relationships within Aciculata (Errantia, Annelida). Molecular Phylogenetics and Evolution, 166, 107339.
Tzetlin, A. B., Purschke, G., Westheide, W., & Saphonov, M. V. (1992). Ultrastructure of enteronephridia and general description of the alimentary canal in Trochonerilla mobilis and Nerillidium troglochaetoides (Polychaeta, Nerillidae). Acta Zoologica (Stockholm), 73, 163–176.
Verger-Bocquet, M. (1992). Polychaeta: Sensory structures. In F.W. Harrison & S.L. Gardiner (Eds.), Microscopic Anatomy of Invertebrates. Vol. 7. Annelida (pp. 181–196). Wiley-Liss.
Weigert, A., Helm, C., Meyer, M., Nickel, B., Arendt, D., Hausdorf, B., Santos, S. R., Halanych, K. M., Purschke, G., Bleidorn, C., & Struck, T. H. (2014). Illuminating the base of the annelid tree using transcriptomics. Molecular Biology and Evolution, 31, 1391–1401.
Westheide, W. (1979). Ultrastruktur der Genitalorgane interstitieller Polychaeten. II. Männliche Kopulationsorgane mit intrazellulären Stilettstäben in einer Microphthalmus-Art. Zoologica Scripta, 8, 111–118.
Whittle, A. C., & Zahid, Z. R. (1974). Fine structure of nuchal organs in some errant polychaetous annelids. Journal of Morphology, 144, 167–184.
Wilkens, V., & Purschke, G. (2009). Central nervous system and sense organs, with special reference to photoreceptor elements, in Polygordius appendiculatus (Annelida), an interstitial polychaete with uncertain phylogenetic affinities. Invertebrate Biology, 128, 46–64.
Winchell, C. J., Valencia, J. E., & Jacobs, D. K. (2010). Confocal analysis of nervous system architecture in direct-developing juveniles of Neanthes arenaceodentata (Annelida, Nereididae). Frontiers in Zoology, 7, 1. https://doi.org/10.1186/1742-9994-7-17
Zanol, J. (2010). Homology of prostomial and pharyngeal structures in Eunicida (Annelida) based on innervation and morphological similarities. Journal of Morphology, 271(9), 1023–1043.
Acknowledgements
We are grateful to the head of the Department of Zoology and Developmental Biology at the University of Osnabrueck (Germany), Prof Dr. A. Paululat, for support. We thank S. Dykstra (University of Osnabrueck) for preparing the ultrathin sections. We also thank K. Etzold and W. Mangerich (University of Osnabrueck) for their technical assistance. Furthermore, we thank Karen Osborn (Smithsonian National Museum of Natural History, Washington, DC, USA) for help with the collection of larval stages and Nataliya Budaeva (University Museum of Bergen, Bergen, Norway) for help with the SEM observations.
Funding
Open Access funding enabled and organized by Projekt DEAL. During sample collection, CH was funded by a Personal Research Fellowship from the German Science Foundation DFG (HE 7224/1–1).
Author information
Authors and Affiliations
Contributions
CH and GP conceived the study. GP provided and focused on the TEM data. CH provided and focused on the SEM and CLSM data. Both authors analyzed the data and contributed to writing and discussing the manuscript. Both authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
We neither used endangered species nor were the investigated animals collected in protected areas. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Consent for publication
All authors have read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Purschke, G., Helm, C. Development and structure of the anterior nervous system and sense organs in the holopelagic annelid Tomopteris spp. (Phyllodocida, Errantia). Org Divers Evol 23, 505–527 (2023). https://doi.org/10.1007/s13127-023-00603-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-023-00603-0