Abstract
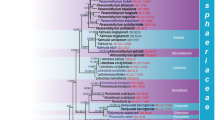
A phylogenetic analysis of nuLSU and ITS sequences representing genera previously included in Dacampiaceae indicates that the family is strongly polyphyletic and that the type species of Dacampia is placed in Pleosporales. The genus Munkovalsaria s. str. is placed in Didymosphaeriaceae (Pleosporales). Polycoccum s. str. and two species of Clypeococcum are shown to form a new lineage sister to the Trypetheliaceae in Trypetheliales and described here as Polycoccaceae. Other members of Polycoccum s. lat. are included in the Pleosporales and are closely related to lichenicolous Phoma-like species of the family Phaeosphaeriaceae. The genus Didymocyrtis is resurrected for these species and for lichenicolous species previously assigned to Diederichia, Diederichomyces, Leptosphaeria and Phoma. The genera Diederichia and Diederichomyces are synonymized with Didymocyrtis. The new combinations Didymocyrtis bryonthae, D. cladoniicola, D. foliaceiphila, D. infestans, D. kaernefeltii, D. melanelixiae, D. pseudeverniae, D. ramalinae, D. slaptoniensis and D. xanthomendozae are made, and the new name D. epiphyscia is introduced for Phoma physciicola. Some anamorph-teleomorph relationships are resolved, such as Didymocyrtis ramalinae–Phoma ficuzzae and Didymocyrtis consimilis–Phoma caloplacae, the phylogenetic results being supported by single ascospore cultures that lead to the asexual stage producing pycnidia and conidia in culture. Speciation by host switching is assumed to be important in the genus Didymocyrtis. An identification key to Didymocyrtis species is provided.










Similar content being viewed by others
References
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F (eds) Second International Symposium on Information Theory. Akademiai Kiado, Budapest
Alstrup V (1981) Notes on some lichens and lichenicolous fungi from Greenland. Nord J Bot 1:120–124
Alstrup V, Cole MS (1998) Lichenicolous fungi of British Columbia. Bryologist 101:221–229
Alstrup V, Hawksworth DL (1990) The lichenicolous fungi of Greenland. Medd Grønl Biosci 31:1–90
Alstrup V, Svane S, Søchting U (2004) Additions to the lichen flora of Denmark VI. Graphis Scr 15:45–50
Alstrup V, Grube M, Motiejūnaitė J, Nordin A, Zhurbenko M (2008) Lichenicolous fungi from the Skibotn area, Troms, Norway. Graphis Scr 20:1–8
Alstrup V, Kocourková J, Kukwa M, Motiejūnaitė J, von Brackel W, Suija A (2009) The lichens and lichenicolous fungi of South Greenland. Folia Cryptogam Est 46:1–24
Aptroot A (1995a) Redisposition of some species excluded from Didymosphaeria (Ascomycotina). Nova Hedwigia 60:325–379
Aptroot A (1995b) A monograph of Didymosphaeria. Stud Mycol 37:1–160
Aptroot A (2004) Two new ascomycetes with long gelatinous appendages collected from monocots in the tropics. Stud Mycol 50:307–311
Aptroot A, Lücking R, Sipman HJM, Umaña L, Chaves JL (2008) Pyrenocarpous lichens with bitunicate asci—a first assessment of the lichen biodiversity inventory in Costa Rica. Bibl Lichenol 97:1–162
Ariyawansa HA, Tanaka K, Thambugala KM, Phookamsak R, Tian Q, Camporesi E, Hongsanan S, Monkai J, Wanasinghe DN, Mapook A, Chukeatirote E, Kang JC, Xu JC, McKenzie EHC, Jones EBG, Hyde KD (2014) A molecular phylogenetic reappraisal of the Didymosphaeriaceae (= Montagnulaceae). Fungal Divers 68:69–104. doi:10.1007/s13225-014-0305-6
Arnold F (1874) Lichenologische Fragmente XVI. Flora (Regensburg) 57:81–89, 97–110, 137–144, 150–155, 173–175, tab
Atienza V, Calatayud V, Hawksworth DL (2003) Notes on the genus Polycoccum (Ascomycota, Dacampiaceae) in Spain, with a key to the species. Lichenologist 35:125–135
Aveskamp MM, de Gruyter J, Woudenberg JHC, Verkley GJM, Crous PW (2010) Highlights of the Didymellaceae: a polyphasic approach to characterise Phoma and related pleosporalean genera. Stud Mycol 65:1–60
Boerema GH (1997) Contributions towards a monograph of Phoma (coelomycetes)—V. Subdivision of the genus in sections. Mycotaxon 64:321–333
Calatayud V (2004) Polycoccum. In: Nash TH III, Ryan BD, Diederich P, Gries C, Bungartz F (eds) Lichen flora of the Greater Sonoran Desert Region, vol II. Lichens Unlimited, Tempe, pp 684–686
Chaverri P, Samuels GJ (2013) Evolution of habitat preference and nutrition mode in a cosmopolitan fungal genus with evidence of interkingdom host jumps and major shifts in ecology. Evolution 67:2823–2837
Clements FE, Shear CL (1931) The genera of fungi. MW Wilson, New York, p 456
Coppins BJ, Coppins AM (2001) Phoma physciicola. Bull Br Lichen Soc 89:78
Crivelli PG (1983) Ueber die heterogene Ascomycetengattung Pleospora Rabh. Vorschlag für eine Aufteilung. Zürich. Diss. ETH Nr. 7318. pp 1–215
de Gruyter J, Aveskamp MM, Woudenberg JHC, Verkley GJM, Groenewald JZ, Crous PW (2009) Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycol Res 113:508–519
de Gruyter J, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW (2013) Redisposition of Phoma-like anamorphs in Pleosporales. Stud Mycol 75:1–36. doi:10.3114/sim0004
De los Ríos A, Grube M (2000) Host-parasite interfaces of some lichenicolous fungi in the Dacampiaceae (Dothideales, Ascomycota). Mycol Res 104:1348–1353
Del Prado R, Schmitt I, Kautz S, Palice Z, Lücking R, Lumbsch HT (2006) Molecular data place Trypetheliaceae in Dothideomycetes. Mycol Res 110:511–520
Diederich P (1990) New or interesting lichenicolous fungi 1. Species from Luxembourg. Mycotaxon 37:297–330
Diederich P, Kocourková J, Etayo J, Zhurbenko M (2007) The lichenicolous Phoma species (coelomycetes) on Cladonia. Lichenologist 39:153–163
Diederich P, Lawrey JD, Sikaroodi M, van den Boom PPG, Ertz D (2011 online/2012 printed) Briancoppinsia, a new coelomycetous genus of Arthoniaceae (Arthoniales) for the lichenicolous Phoma cytospora, with a key to this and similar taxa. Fungal Divers 52:1–12. doi:10.1007/s13225-011-0105-1
Diederich P, Ertz D, Eichler M, Cezanne R, van den Boom P, Van den Broeck D, Sérusiaux E (2014) New or interesting lichens and lichenicolous fungi from Belgium, Luxembourg and northern France. XV. Bull Soc Nat Luxemb 115:157–165
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Eriksson O, Hawksworth DL (1986) Outline of the ascomycetes—1986. Syst Ascomycetum 5:185–324
Eriksson OE, Hawksworth DL (1993) Outline of the ascomycetes—1993. Syst Ascomycetum 12:51–257
Ertz D, Diederich P (2015) Dismantling Melaspileaceae: a first phylogenetic study of Buelliella, Hemigrapha, Karschia, Labrocarpon and Melaspilea. Fungal Divers 70:141–164. doi:10.1007/s13225-015-0321-1
Ertz D, Diederich P, Brand AM, van den Boom P, Sérusiaux E (2008) New or interesting lichens and lichenicolous fungi from Belgium, Luxembourg and northern France. XI. Bull Soc Nat Luxemb 109:35–51
Ertz D, Lawrey JD, Common RS, Diederich P (2013 online/2014 printed) Molecular data resolve a new order of Arthoniomycetes sister to the primarily lichenized Arthoniales and composed of black yeasts, lichenicolous and rock-inhabiting species. Fungal Divers 66:113–137. doi:10.1007/s13225-013-0250-9
Ertz D, Tehler A, Irestedt M, Frisch A, Thor G, van den Boom P (2014 online/2015 printed) A large-scale phylogenetic revision of Roccellaceae (Arthoniales) reveals eight new genera. Fungal Divers 70:31–53. doi:10.1007/s13225-014-0286-5
Etayo J (1996a) Aportación a la flora liquénica de las Islas Canarias. I. Hongos liquenícolas de Gomera. Bull Soc Linn Provence 47:93–110
Etayo J (1996b) Contribución al conocimiento de los líquenes y hongos liquenícolas de Mallorca (Islas Baleares, España). Bull Soc Linn Provence 47:111–121
Etayo J (2004) Líquenes y hongos liquenícolas de los Pirineos occidentales y norte de la Península Ibérica. Naturzale Cuad Cienc Nat 18:143–167
Etayo J (2010a) Líquenes y hongos liquenícolas de Aragón. Guineana 16:1–501
Etayo J (2010b) Líquenes y hongos liquenícolas del País Vasco. Catálogo del año 2010. Ihobe Flora (Bilbao) 6:1–87
Etayo J, Diederich P (1996) Lichenicolous fungi from the western Pyrenees, France and Spain. II. More deuteromycetes. Mycotaxon 60:415–428
Etayo J, Osorio HS (2004) Algunos hongos liquenícolas de Sudamérica, especialmente del Uruguay. Comun Bot Mus Nacionales Hist Nat Antropol 6(129):1–19
Etayo J, Sancho LG (2008) Hongos liquenícolas del Sur de Sudamérica, especialmente de Isla Navarino (Chile). Bibl Lichonol 98:1–302
Gardiennet A (2012) Découverte de Polycoccum slaptoniense D. Hawksw. en France. Bull Info Assoc Fr Lichénol 37:107–111
Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Stat Sci 7:457–511
Gilg IC, Amaral-Zettler LA, Countway PD, Moorthi S, Schnetzer A, Caron DA (2010) Phylogenetic affiliations of Mesopelagic Acantharia and Acantharian-like environmental 18S rRNA genes off the Southern Californian Coast. Protist 161:197–211
Hafellner J (1994) Beiträge zu einem Prodromus der lichenicolen Pilze Österreichs und angrenzender Gebiete I. Einige neue oder seltene Arten. Herzogia 10:1–28
Hafellner J (1996) Bemerkenswerte Funde von Flechten und lichenicole Pilze auf makaronesischen Inseln IV. Einige bisher übersehene lichenicole Arten der Kanarischen Inseln. Cryptog Bryol Lich 17:1–14
Hafellner J (2008) Zur Diversität lichenisierter und lichenicoler Pilze im Gebiet der Koralpe (Österreich: Kärnten und Steiermark, Slowenien). Mitt Naturwiss Ver Steiermark 138:29–112
Halıcı MG, Hawksworth DL (2008) Two new species of Dacampia (Ascomycota, Dacampiaceae), with a key to and synopsis of the known species of the genus. Fungal Divers 28:49–54
Halıcı MG, Candan M, Calatayud V (2009) Dacampia rubra sp. nov. (Ascomycota, Dacampiaceae), a lichenicolous fungus on vagrant Aspicilia species. Mycotaxon 108:235–240
Halıcı MG, Candan M, Güllü M, Özcan A (2014) Phoma recepii sp. nov. from the Caloplaca cerina group in Turkey. Mycotaxon 129:163–168
Hawksworth DL (1981) The lichenicolous Coelomycetes. Bull Br Mus Nat Hist Bot ser 9(1):1–98
Hawksworth DL (1994) Notes on British lichenicolous fungi: VII. Lichenologist 26:337–347
Hawksworth DL, Diederich P (1988) A synopsis of the genus Polycoccum (Dothideales), with a key to accepted species. Trans Br Mycol Soc 90:293–312
Henssen A (1995) Studies on the biology and structure of Dacampia (Dothideales), a genus with lichenized and lichenicolous species. Cryptog Bot 5:149–158
Hitch C (1997) New, rare and interesting British lichen and lichenicolous fungus records. Br Lichen Soc Bull 80:46–58
Hitch C (ed) (2011) New, rare and interesting lichens. Br Lichen Soc Bull 109:75–90
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17:754–755
Hyde KD, Gareth Jones EB, Liu J-K et al (2013) Families of Dothideomycetes. Fungal Divers 63:1–313. doi:10.1007/s13225-013-0263-4
Ihlen PG, Wedin M (2008) An annotated key to the lichenicolous Ascomycota (including mitosporic morphs) of Sweden. Nova Hedwigia 86:275–365
Jaklitsch WM, Fournier J, Dai DQ, Hyde KD, Voglmayr H (2015) Valsaria and the Valsariales. Fungal Divers. doi:10.1007/s13225-015-0330-0
Kocourková J, von Brackel W (2005) Einige für Bayern neue flechtenbewohnende Pilze – Beitrag zu einer Checkliste I. Ber Bayer Bot Ges 75:3–10
Kondratyuk S (2008) Polycoccum kaernefeltii sp. nova (Dothideales), a new lichenicolous fungus on Teloschistes chrysophthalmus (L.) Th. Fr. Ukr Bot Zhurn 65:565–571
Kondratyuk S, Nevo E, Wasser S (2005) New and rare lichen-forming and lichenicolous fungi from the Carmel Mountains, Israel. Ukr Bot Zhurn 62(1):100–110
Körber GW (1865) Parerga lichenologica. Breslau 385-501, (i)-xvi pp
Kukwa M, Flakus A (2009) New or interesting records of lichenicolous fungi from Poland VII. Species mainly from Tatra Mountains. Herzogia 22:191–211
Kukwa M, Szymczyk R, Kowalewska A (2013) New or interesting records of lichenicolous fungi from Poland IX. Herzogia 26:159–168
Lawrey JD, Diederich P (2003) Lichenicolous fungi: interactions, evolution, and biodiversity. Bryologist 106:80–120
Lawrey JD, Diederich P (2015) Lichenicolous fungi – worldwide checklist, including isolated cultures and sequences available. URL: http://www.lichenicolous.net [02/22/2015]
Lawrey JD, Diederich P, Nelsen MP, Sikaroodi M, Gillevet PM, Brand AM, van den Boom P (2011) The obligately lichenicolous genus Lichenoconium represents a novel lineage in the Dothideomycetes. Fungal Biol 115:176–187
Lawrey JD, Diederich P, Nelsen MP, Freebury C, Van den Broeck D, Sikaroodi M, Ertz D (2012) Phylogenetic placement of lichenicolous Phoma species in the Phaeosphaeriaceae (Pleosporales, Dothideomycetes). Fungal Divers 55:195–213
Lutzoni F, Kauff F, Cox CJ, McLaughlin D, Celio G, Dentinger B, Padamsee M, Hibbett D, James TY, Baloch E, Grube M, Reeb V, Hofstetter V, Schoch C, Arnold AE, Miadlikowska J, Spatafora J, Johnson D, Hambleton S, Crockett M, Shoemaker R, Sung G-H, Lücking R, Lumbsch T, O’Donnell K, Binder M, Diederich P, Ertz D, Gueidan C, Hansen K, Harris RC, Hosaka K, Lim Y-W, Matheny B, Nishida H, Pfister D, Rogers J, Rossman A, Schmitt I, Sipman H, Stone J, Sugiyama J, Yahr R, Vilgalys R (2004) Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. Am J Bot 91:1446–1480
Maddison D, Maddison W (2002) MacClade Version 4.03PPC: analysis of phylogeny and character evolution. Sinauer Associates, Sunderland
Magnusson AH (1952) Lichens from Torne Lappmark. Ark Bot Ser 2 2(2):45–249
Merinero S, Bidussi M, Gauslaa Y (2015) Do lichen secondary compounds play a role in highly specific fungal parasitism? Fungal Ecol 14:125–129
Miadlikowska J, McCune B, Lutzoni F (2002) Pseudocyphellaria perpetua, a new lichen from Western North America. Bryologist 105:1–10
Millanes AM, Truong C, Westberg M, Diederich P, Wedin M (2014) Host switching promotes diversity in host-specialized mycoparasitic fungi: uncoupled evolution in the Biatoropsis-Usnea system. Evolution 68:1576–1593
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES science gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 November 2010, New Orleans, pp 1–8
Motiejūnaitė J, Grochowski P (2014) Miscellaneous new records of lichens and lichenicolous fungi. Herzogia 27:193–198
Motiejūnaitė J, von Brackel W, Stončius D, Preikša Ž (2011) Contribution to the Lithuanian flora of lichens and allied fungi. III. Bot Lith 17:39–47
Motiejūnaitė J, Berglund T, Czarnota P, Himelbrant D, Högnabba F, Konoreva LA, Korchikov ES, Kubiak D, Kukwa M, Kuznetsova E, Leppik E, Lõhmus P, Prigodina Lukošienė I, Pykälä J, Stončius D, Stepanchikova I, Suija A, Thell A, Tsurykau A, Westberg M (2012) Lichens, lichenicolous and allied fungi found in Asveja Regional Park (Lithuania). Bot Lith 18:85–100
Muggia L, Gueidan C, Knudsen K, Perlmutter G, Grube M (2012 online/2013 printed) The lichen connections of black fungi. Mycopathologia 175:523–535. doi:10.1007/s11046-012-9598-8
Muggia L, Kopun T, Ertz D (2015) Phylogenetic placement of the lichenicolous, anamorphic genus Lichenodiplis and its connection to Muellerella-like teleomorphs. Fungal Biol (in press)
Nelsen MP, Lücking R, Aptroot A, Andrew CJ, Cáceres M, Rivas Plata E, Gueidan C, da Silva Canêz L, Knight A, Ludwig LR, Mercado-Díaz JA, Parnmen S, Lumbsch HT (2014) Elucidating phylogenetic relationships and genus-level classification within the fungal family Trypetheliaceae (Ascomycota: Dothideomycetes). Taxon 63:974–992
Orange A (1990) New or interesting lichens and lichenicolous fungi from Iceland. Acta Bot Isl 10:37–44
Pérez-Ortega S, Suija A, de los Ríos A (2011) The connection between Abrothallus and its anamorph state Vouauxiomyces established by Denaturing Gradient Gel Electrophoresis (DGGE). Lichenologist 43:277–279
Pérez-Ortega S, Suija A, Crespo A, de los Ríos A (2013 online/2014 printed) Lichenicolous fungi of the genus Abrothallus (Dothideomycetes: Abrothallales ordo nov.) are sister to the predominantly aquatic Janhulales. Fungal Divers 64:295–304. doi:10.1007/s13225-013-0269-y
Phookamsak R, Liu JK, McKenzie EHC, Manamgoda DS, Ariyawansa H, Thambugala KM, Dai DQ, Camporesi E, Chukeatirote E, Wijayawardene NN, Bahkali AH, Mortimer PE, Xu JC, Hyde KD (2014) Revision of Phaeosphaeriaceae. Fungal Divers 68:159–238. doi:10.1007/s13225-014-0308-3
Pitt WM, Urbez-Torres JR, Trouillas FP (2014) Munkovalsaria donacina from grapevines and Desert Ash in Australia. Mycosphere 5:656–661. doi:10.5943/mycosphere/5/5/6
Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817–818
Rambaut A (2012) FigTree v1.4.2, available from: http://tree.bio.ed.ac.uk/software/figtree/
Rambaut A, Drummond AJ (2007) Tracer v1.6, Available from http://beast.bio.ed.ac.uk/
Rondon Y (1969) L’herbier des champignons parasites des lichens de l’Abbe L. Vouaux. Rev Bryol Lichenol 36:737–745
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Roux et coll. (2014) Catalogue des lichens et des champignons lichénicoles de France métropolitaine. Édit. Henry des Abbayes, Fougères, 1525 p
Ruibal C, Millanes AM, Hawksworth DL (2011) Molecular phylogenetic studies on the lichenicolous Xanthoriicola physciae reveal Antarctic rock-inhabiting fungi and Piedraia species among closest relatives in the Teratosphaeriaceae. IMA Fungus 2:97–103
Santesson R (1960) Lichenicolous fungi from northern Spain. Svensk Bot Tidskr 54:499–522
Santesson R (1993) The lichens and lichenicolous fungi of Sweden and Norway. SBT-förlaget, Lund, pp 240
Santesson R, Moberg R, Nordin A, Tønsberg T, Vitikainen O (2004) Lichen-forming and lichenicolous fungi of Fennoscandia. Museum of Evolution, Uppsala University, Uppsala, pp 359
Seaward MRD, Sipman HJM, Sohrabi M (2008) A revised checklist of lichenized, lichenicolous and allied fungi for Iran. In: Facetten der Flechtenforschung. Festschrift zu Ehren von Volkmar Wirth. Sauteria 15:459–520
Spegazzini C (1881) Fungi argentini additis nonnullis brasiliensibus montevideensibusque. Pugillus quartus. Anal Soc Cient Argentina 12:13–30, 63–82, 97–117, 174–189, 208–227, 241–258
Swofford DL (2002) PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland
Trakunyingcharoen T, Lombard L, Groenewald JZ, Cheewangkoon R, Toanun C, Alfenas AC, Crous PW (2014) Mycoparasitic species of Sphaerellopsis, and allied lichenicolous and other genera. IMA Fungus 5:391–414
Triebel D, Scholz P (2001) Lichenicolous fungi from Bavaria as represented in the Botanische Staatssammlung München. Sendtnera 7:211–231
Vainio EA (1921) Lichenographia Fennica I. Pyrenolichenes iisque proximi Pyrenomycetes et Lichenes imperfecti. Acta Soc Fauna Flora Fenn 49:1–274
van den Boom PPG, Giralt M (2012) Checklist and three new species of lichens and lichenicolous fungi of the Algarve (Portugal). Sydowia 64:149–208
Vězda A (1969) Beiträge zur Kenntnis der flechtenbewohnenden Pilze in der Tschechoslowakei. II. – Zwei neue Arten: Opegrapha rinodinae sp. nov. und Polycoccum galligenum sp. nov. Ceská Mykol 23:104–109
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246
von Brackel W (2007) Weitere Funde von flechtenbewohnenden Pilzen in Bayern. Beitrag zu einer Checkliste III. Ber Bayer Bot Ges 77:5–26
von Brackel W (2008a) Phoma ficuzzae sp. nov. and some other lichenicolous fungi from Sicily, Italy. In: Facetten der Flechtenforschung. Festschrift zu Ehren von Volkmar Wirth. Sauteria 15:103–120
von Brackel W (2008b) Some lichenicolous fungi collected during the 20th meeting of the Società Lichenologica Italiana in Siena. Notiz Soc Lichenol Ital 21:63–66
von Brackel W (2009) Weitere Funde von flechtenbewohnenden Pilzen in Bayern – Beitrage zu einer Checkliste IV. Ber Bayer Bot Ges 79:5–55
von Brackel W (2010) Weitere Funde von flechtenbewohnenden Pilzen in Bayern – Beitrage zu einer Checklist V. Ber Bayer Bot Ges 80:5–32
von Brackel W (2011) Lichenicolous fungi and lichens from Puglia and Basilicata (southern Italy). Herzogia 24:65–101
von Brackel W (2013) Miscellaneous records of lichenicolous fungi from the Italian Alps. Herzogia 26:141–157
von Brackel W (2015) Lichenicolous fungi from central Italy with note on some remarkable hepaticolous, algicolous and lichenized fungi. Herzogia 28:212–281
von Keissler K (1930) Die Flechtenparasiten. Dr L. Rabenhorst’s Kryptogamen-Flora von Deutschland, Österreich und der Schweiz 8:1–712
Vouaux L (1912–14) Synopsis des champignons parasites des lichens. Bull Soc mycol Fr 28:177–256; 29:33–128, 399–494; 30:135–198, 281–329
Wang HK, Aptroot A, Crous PW, Hyde KD, Jeewon R (2007) The polyphyletic nature of Pleosporales: an example from Massariosphaeria based on rDNA and RBP2 gene phylogenies. Mycol Res 111:1268–1276
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols—a guide to methods and applications. Academic, San Diego, pp 315–322
Wijayawardene NN, Crous PW, Kirk PM et al (2014) Naming and outline of Dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Divers 69:1–55. doi:10.1007/s13225-014-0309-2
Wittmann H, Türk R (1990) Die Flechten im Nationalpark Nockberge (Kärnten, Österreich). Kärntner Nationalparkschriften 4:1–112
Yoshimura I, Yamamoto Y, Nakano T, Finnie J (2002) Isolation and culture of lichen photobionts and mycobionts. In: Kranner I, Beckett RP, Varma AK (eds) Protocols in lichenology—culturing, biochemistry, physiology and use in biomonitoring. Springer, Berlin, pp 3–33
Zhang Y, Schoch CL, Fournier J, Crous PW, de Gruyter J, Woudenberg JHC, Hirayama K, Tanaka K, Pointing SB, Spatafora JW, Hyde KD (2009) Multi-locus phylogeny of Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Stud Mycol 64:85–102
Zhang Y, Crous PW, Schoch CL, Hyde KD (2011 online/2012 printed) Pleosporales. Fungal Divers 53:1–221. doi:10.1007/s13225-011-0117-x
Zhurbenko M (1996) Lichens and lichenicolous fungi of the northern Krasnoyarsk territory, central Siberia. Mycotaxon 58:185–232
Zhurbenko MP (2007) The lichenicolous fungi of Russia: geographical overview and a first checklist. Mycol Balc 4:105–124
Zhurbenko MP (2009a) Lichenicolous fungi and some lichens from the Holarctic. Opusc Philolichenum 6:87–120
Zhurbenko M (2009b) Lichenicolous fungi and lichens from the Holarctic. Part II. Opusc Philolichenum 7:121–186
Zhurbenko MP (2010) Lichenicolous fungi and lichens growing on Stereocaulon from the Holarctic, with a key to the known species. Opusc Philolichenum 8:9–39
Zhurbenko MP, Alstrup V (2004) Lichenicolous fungi on Cladonia mainly from the Arctic. Symb Bot Ups 34:477–499
Zhurbenko MP, Kobzeva AA (2014) Lichenicolous fungi from Northwest Caucasus, Russia. Herzogia 27:377–396
Zhurbenko MP, Santesson R (1996) Lichenicolous fungi from the Russian Arctic. Herzogia 12:147–161
Zhurbenko MP, Hermansson J, Pystina TN (2012) Endococcus incrassatus new to Eurasia and some other lichenicolous fungi from the Komi Republic of Russia. Graphis Scr 24:36–39
Zwickl DJ (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. Ph. D. dissertation, The University of Texas at Austin
Acknowledgments
We would like to thank the curators of herbaria and the owners of private collections cited in Materials and Methods for the loan of specimens. Javier Etayo and Richard Harris are acknowledged for their help concerning Didymocyrtis melanelixiae; J.E. also for Leptosphaeria protousneae; and Alain Mora, Bernadette Mora and Jean-Pierre Duvivier for Didymocyrtis slaptoniensis. Ann Bogaerts, Cyrille Gerstmans, Myriam Dehaan and Wim Baert are thanked for technical assistance. Finally, the first author acknowledges financial support from the Fonds National de la Recherche Scientifique (FNRS) from Belgium (F.R.F.C. 2.4567.08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ertz, D., Diederich, P., Lawrey, J.D. et al. Phylogenetic insights resolve Dacampiaceae (Pleosporales) as polyphyletic: Didymocyrtis (Pleosporales, Phaeosphaeriaceae) with Phoma-like anamorphs resurrected and segregated from Polycoccum (Trypetheliales, Polycoccaceae fam. nov.). Fungal Diversity 74, 53–89 (2015). https://doi.org/10.1007/s13225-015-0345-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-015-0345-6