Abstract
A phylogenetic analysis of nuLSU and ITS sequences representing genera previously included in Dacampiaceae indicates that the family is strongly polyphyletic and that the type species of Dacampia is placed in Pleosporales. The genus Munkovalsaria s. str. is placed in Didymosphaeriaceae (Pleosporales). Polycoccum s. str. and two species of Clypeococcum are shown to form a new lineage sister to the Trypetheliaceae in Trypetheliales and described here as Polycoccaceae. Other members of Polycoccum s. lat. are included in the Pleosporales and are closely related to lichenicolous Phoma-like species of the family Phaeosphaeriaceae. The genus Didymocyrtis is resurrected for these species and for lichenicolous species previously assigned to Diederichia, Diederichomyces, Leptosphaeria and Phoma. The genera Diederichia and Diederichomyces are synonymized with Didymocyrtis. The new combinations Didymocyrtis bryonthae, D. cladoniicola, D. foliaceiphila, D. infestans, D. kaernefeltii, D. melanelixiae, D. pseudeverniae, D. ramalinae, D. slaptoniensis and D. xanthomendozae are made, and the new name D. epiphyscia is introduced for Phoma physciicola. Some anamorph-teleomorph relationships are resolved, such as Didymocyrtis ramalinae–Phoma ficuzzae and Didymocyrtis consimilis–Phoma caloplacae, the phylogenetic results being supported by single ascospore cultures that lead to the asexual stage producing pycnidia and conidia in culture. Speciation by host switching is assumed to be important in the genus Didymocyrtis. An identification key to Didymocyrtis species is provided.
Similar content being viewed by others
Introduction
The family Dacampiaceae Körb. includes lichenicolous, lichenized and saprobic fungi forming blackish, perithecioid ascomata, a pseudoparenchymatous exciple, branched-anastomosing pseudoparaphyses, bitunicate asci, and septate to muriform, usually brown ascospores (Hyde et al. 2013). The family was placed in Dothideales in the past (Eriksson and Hawksworth 1986, 1993; Hawksworth and Diederich 1988; Henssen 1995), but is currently considered as ‘Dothideomycetes, family incertae sedis’ (Hyde et al. 2013; Wijayawardene et al. 2014). Dacampiaceae is considered to be a heterogeneous assemblage including about 110 species in 10 genera: Aaosphaeria, Clypeococcum, Dacampia, Eopyrenula, Leptocucurthis, Munkovalsaria, Polycoccum, Pseudonitschkia, Pyrenidium and Weddellomyces (Hyde et al. 2013).
Few sequences of Dacampiaceae are available in GenBank. One sequenced species is Munkovalsaria appendiculata Aptroot, collected on dead culms of Zea mays and assigned to Pleosporales in a phylogenetic analysis by Aptroot (2004); it also was found to cluster with Montagnula opulenta (Didymosphaeriaceae as recently circumscribed by Ariyawansa et al. 2014) in a phylogeny by Wang et al. (2007). Sequences of M. donacina (generic type) were generated by Pitt et al. (2014), but not included in a phylogenetic analysis. A 28S rDNA sequence of Polycoccum vermicularium was also deposited in GenBank (AY961601), but we found it to be of a poor quality and its reliability is questioned here.
Many described species of Dacampiaceae are lichenicolous, but limited sequence data for lichenicolous species means they are not commonly included in cladograms based on molecular data sets (e.g., Lutzoni et al. 2004; Zhang et al. 2009). Despite some recent progress in placing lichenicolous species in Dothideomycetes (Lawrey et al. 2011, 2012; Ruibal et al. 2011; Ertz et al. 2013; Pérez-Ortega et al. 2013; Ertz and Diederich 2015), questions remains about whether the placement and content of Dacampiaceae, including lichenicolous species, are correct.
Polycoccum, the most species-rich genus of Dacampiaceae and one that includes only lichenicolous species, has never been adequately studied using molecular data and is here studied in detail. Polycoccum had been little used for lichenicolous ascomycetes until its reinstallation by Santesson (1960), but in the past two decades it has become one of the most species-rich genera of lichenicolous fungi, with 57 species accepted by Lawrey and Diederich (2015) and new species described almost every year. A first key was given by Vězda (1969, 10 species). The most complete key so far available is that compiled by Hawksworth and Diederich (1988, 23 species worldwide), and there are additional more recently-published regional keys (e.g., Atienza et al. 2003, 13 species from Spain; Calatayud 2004, 5 species from the Sonoran desert region; Ihlen and Wedin 2008, 14 species from Sweden; Gardiennet 2012, 13 species from France) that illustrate the diversity of this genus in various parts of the world.
The relationship of Polycoccum to other members of the Dothideales has never been established with certainty. Crivelli (1983: 193) suggested a close relationship among Dacampia, Polycoccum and Pyrenidium (sub Dacampiosphaeria). This view was further elaborated by Hawksworth and Diederich (1988), when they proposed to treat these three genera together with Byssothecium, Clypeococcum and Weddellomyces in the family Dacampiaceae in the Dothideales. However, until now these hypotheses have not been adequately tested.
This paper aims at (1) testing the monophyly of the family Dacampiaceae, (2) elucidating the phylogenetic position of the genera Clypeococcum, Diederichia and Polycoccum, (3) testing the monophyly of the genus Polycoccum, (4) reinstating the genus Didymocyrtis, and (5) testing some anamorph-teleomorph relationships among lichenicolous members of the genera Polycoccum s. lat. and Phoma s. lat.
Material and methods
Morphological study
Herbarium specimens are deposited in BR, CANL, E, GZU, M, NY, UBC and in the private collections of S. Beeching, F. Berger, P. Diederich, J. Hafellner and J. Etayo. External morphology of herbarium specimens was examined and measured under Leica MZ 7.5 (magnification up to ×50), Wild M3 (magnification up to ×40) and Olympus SZX12 (magnification up to ×90) stereomicroscopes. Macroscopic photographs were done using a Canon 40D camera with a Canon MP-E 65 mm lens or a Nikon BD Plan 10× microscope objective, StackShot (Cognisys) and Helicon Focus (HeliconSoft) for increasing the depth of field; or with a Color View I digital camera connected to an Olympus SZX12 stereomicroscope. Hand-made sections of ascomata and thallus were studied in water, 5 % KOH (K), or 1 % I2 Lugol’s reagent without (I) or with KOH pre-treatment (K/I) using Leica DMLB, Leica DMRE and Olympus BX51 microscopes (magnification up to ×1000). Sectioning was performed by one of us (JH) using a freezing microtome (Leitz, sections of 12–15 μm), but squash preparations were also used, especially for ascus analysis. Conidiogenesis of conidia was studied in water and erythrosin B (ALDRICH 19,826-9) in 10 % ammonia. Measurements of asci, ascospores and conidia all refer to material examined in tap water. Conidial size of a large number of conidia was measured for some specimens, and the average (\( \overline{\mathrm{X}} \)) and standard deviation (SD) calculated. Such measurements are given as \( \overline{\mathrm{X}} \) ± SD, surrounded by the extreme values (between parentheses), followed by the number of measurements (n). For the graphical representation of the conidial size distribution (Fig. 6), each specimen is represented by an ellipse, of which the centre represents the average length and breadth, the large radius represents the SD of conidial length and the small radius the SD of conidial breadth. The graphical representation has been done using the PostScript language. Microscopic photographs were prepared using either a Leica DMLB microscope with differential interference contrast and fitted with a Leica EC3 camera, or an Olympus BX51 microscope with interference contrast and connected to a Color View I digital camera.
Molecular techniques
Cultures were isolated from ascospores (single ascospore cultures for sexual stages of Didymocyrtis ramalinae, for Didymocyrtis aff. consimilis Ertz 17617b, Berger 26876, and for Didymocyrtis slaptoniensis MoraA-B; two-ascospores isolate for Didymocyrtis consimilis 12041; and multi-ascospores isolate for Didymocyrtis melanelixiae and D. slaptoniensis 12009), or from conidia (multiconidia cultures) of freshly collected material on malt-yeast extract medium as described by Yoshimura et al. (2002) (Fig. 9). Thin sections were made through ascomata or pycnidia, and the outer wall was removed with a sterile razor blade to expose ascospores or conidia, which were then spread directly on malt-yeast extract agar. Isolated ascospores were immediately transferred to new petri dishes to attain single-spore cultures. Germination of conidia and ascospores was often observed within a day. The cultures were kept at room temperature in the laboratory of the Botanic Garden Meise and exposed to a natural day-light regime. Cultures maintained at George Mason University were kept at 18 °C in 12 h light-12 h dark cycles. No experiments were done to test whether different light or temperature conditions could improve the growth rate. All the strains were fast-growing and therefore only a few weeks were required to obtain sufficient material for DNA extraction. In some cases, hand-made sections of the hymenium or thallus were used for direct PCR as described in Ertz et al. (2014). The outer wall of ascomata was removed with a sterile razor blade to isolate the hymenium. The material was then added to a tube containing the PCR reaction mixture and amplified directly. Genomic DNA was isolated from cultures using the CTAB extraction protocol (Doyle and Doyle 1990). Amplification reactions were prepared for a 50 μl final volume containing 5 μl 10× DreamTaq Buffer (Fermentas), 1.25 μl of each of the 20 μM primers, 5 μl of 2.5 mg mL−1 bovin serum albumin (Fermentas #B14), 4 μl of 2.5 mM each dNTPs (Fermentas), 1.25 U DreamTaq DNA polymerase (Fermentas), and 1 μl of template genomic DNA or tiny fragments of fungal material. A targeted fragment of about 1.1 kb at the 5′end of the nuLSU rDNA was amplified using primers LIC15R (Miadlikowska et al. 2002) with LR6 (Vilgalys and Hester 1990). A fragment of about 0.6 kb of the nuITS rDNA was amplified using primers ITS1F and ITS4 (White et al. 1990). The yield of the PCRs was verified by running the products on a 1 % agarose gel using ethidium bromide. Both strands were sequenced by Macrogen® using amplification primers. Additional primers were used for the sequencing of nuLSU: LR3, more rarely LR3R and LR5 (Vilgalys and Hester 1990) (Vilgalys’ website, http://www.botany.duke.edu/fungi/mycolab). Sequence fragments were assembled with Sequencher version 4.6 (Gene Codes Corporation, Ann Arbor, Michigan). Sequences were subjected to MEGABLAST searches to verify their closest relatives and to detect potential contaminations.
Taxon selection and phylogenetic analyses
Although not possible for all taxa, we tried to achieve a sample series with at least two specimens of each newly sequenced species, preferably from different localities, to avoid misidentifications caused by contamination or sequencing errors. Our sample series include sequences obtained from the type species of Dacampia, Didymocyrtis and Polycoccum that are important for taxonomic conclusions. For the nuLSU phylogenetic tree, the closest relatives of the new sequences based on BLAST searches were retrieved from GenBank. Additional taxa were selected from Hyde et al. (2013) and Nelsen et al. (2014) to include a wide array of families belonging to Dothideomycetes and an exhaustive list of genera belonging to Trypetheliales. The original nuLSU matrix used in Ertz and Diederich (2015) was used as main template. The resulting nuLSU matrix consisted of 109 sequences, mainly from a wide variety of Dothideomycetes. Some Arthoniomycetes were also included. Three outgroup species were chosen to represent the class Eurotiomycetes (Caliciopsis pinea and Capronia munkii) and the class Leotiomycetes (Lachnum virgineum). Caliciopsis pinea was used as the rooting taxon in the related analyses. The ITS matrix was assembled manually and consisted of 58 sequences, including 52 sequences of Didymocyrtis and 6 sequences chosen from the most closely related genera in Trakunyingcharoen et al. (2014). Phaeosphaeria lycopodina was used as the rooting taxon in these analyses. The alignments were improved manually using MacClade 4.05 (Maddison and Maddison 2002). Terminal ends of sequences, ambiguous aligned regions and introns were delimited manually and excluded from the nuLSU dataset. The nuLSU data set consisted of 1160 unambiguously aligned characters, of which 463 were variable. The ITS data set consisted of 594 unambiguously aligned characters, of which 120 were variable.
The best-fit model of DNA evolution GTR+I+G was chosen for the nuLSU data set and the GTR+I for the ITS data set using the Akaike information criterion (AIC; Akaike 1973) as implemented in Modeltest v. 3.7 (Posada and Crandall 1998). Bayesian analyses were carried out using the Metropolis-coupled Markov chain Monte Carlo method (MCMCMC) in MrBayes v. 3.2.3 (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003) on the CIPRES portal (Miller et al. 2010). Analyses were run under the selected model of nucleotide substitution with six rate categories. Two parallel MCMCMC runs were performed, with each run using four independent chains and 100,000,000 generations for the nuLSU data set and for the ITS data set, and sampling trees every 1000th generation. Convergence diagnostics were estimated using the PSRF (Potential scale reduction factor) where values closer to one indicated convergence between runs (Gelman and Rubin 1992), and using TRACER v. 1.6 by plotting the log-likelihood values of the sample points against generation time (Rambaut and Drummond 2007). Posterior probabilities (PP) of the nuLSU and ITS matrices were determined by calculating a majority-rule consensus tree generated from the 150,002 post-burnin trees of the 200,002 trees sampled by the two MCMCMC runs using the sumt option of MrBayes. In addition, a Maximum Likelihood (ML) analysis was performed on the nuLSU and ITS data sets using GARLI (Zwickl 2006, v. 0.951 for OSX) with default settings, and a single most likely tree was produced (–lnL = 11690.8948 for the nuLSU tree; –lnL = 1941.3522 for the ITS tree). One thousand bootstrap pseudoreplicates were used to calculate a majority rule consensus tree in PAUP* 4.0b10 (Swofford 2002) to assess the Maximum Likelihood bootstrap values (ML-bs). ML-bs ≥ 70 % and PP ≥ 95 % were considered to be significant. Phylogenetic trees were visualized using FigTree v. 1.4.2 (Rambaut 2012).
Results
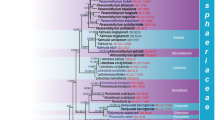
We obtained 54 new sequences (20 nuLSU and 34 ITS) belonging to 15 species from Austria, Belgium, Canada, Canary Islands (Gomera), Madeira, France (including Corsica), Iceland, Luxembourg, Scotland, Switzerland and USA (Table 1). The Bayesian nuLSU tree we recovered did not contradict the ML nuLSU tree topology for the strongly supported branches and hence only the ML tree is shown with the branches having ML-bs ≥ 70 % in bold and with the posterior probabilities of the Bayesian analysis added above the internal branches (Fig. 1). The recovered Bayesian ITS tree did not contradict the ML ITS tree’s topology for the strongly supported branches and hence only the ML tree is shown with the branches having ML-bs ≥ 70 % in bold and with the posterior probabilities of the Bayesian analysis added above the internal branches (Fig. 2).
Phylogenetic relationships among 109 samples within Dothideomyceta (with three outgroup taxa) based on a data set of nuLSU sequences that resulted from a Maximum Likelihood analysis using Garli. Internal branches with Maximum Likelihood bootstrap values ≥ 70 obtained from a Garli analysis are considered strongly supported and represented by thicker lines. Posterior probabilities ≥ 95 resulting from a Bayesian analysis are shown above internal branches. The newly sequenced specimens are in bold. The old and most commonly used generic name is indicated within brackets for the species now placed in Didymocyrtis. Collecting numbers of the authors following the species names act as specimen and sequence identifiers
Phylogenetic relationships among 52 samples of Didymocyrtis based on a data set of ITS sequences that resulted from a Maximum Likelihood analysis using Garli. Internal branches with Maximum Likelihood bootstrap values ≥ 70 obtained from a Garli analysis are considered strongly supported and represented by thicker lines. Posterior probabilities ≥ 95 resulting from a Bayesian analysis are shown above internal branches. The newly sequenced specimens are in bold. Collecting numbers of the authors following the species names act as specimen and sequence identifiers. The geographical origin and the host lichen species of the sequenced specimens of Didymocyrtis are also indicated. The old and most commonly used generic name is indicated within brackets for the species now placed in Didymocyrtis. The length of the branch represented by dashed lines was reduced by 50 % for editing reason
The genera Clypeococcum, Dacampia, Diederichia (here synonymized with Didymocyrtis, see Taxonomy section) and Polycoccum are included in a molecular phylogeny for the first time, all except the first being represented by the type species. The status of Clypeococcum remains unclear until the type, C. cladonema is sequenced. The backbone of our nuLSU phylogenetic tree is poorly resolved. However, main groups such as Arthoniales, Capnodiales, Eremithallales, Hysteriales, Trypetheliales, Tubeufiales and Venturiales are strongly supported by both analyses, even though the relationships among such groups are not supported. Polycoccum is resolved as polyphyletic, with species being placed in two main distantly-related lineages corresponding to Trypetheliales (as newly defined) and Pleosporales. Trypetheliales is strongly supported and divided into two strongly supported main lineages corresponding to Polycoccaceae (newly described here), and to Trypetheliaceae. Polycoccaceae includes two genera, Clypeococcum and Polycoccum, of which the latter is paraphyletic. Pleosporales is only supported by the Bayesian analysis (PP = 96).
Dacampia, the type genus of Dacampiaceae, is strongly supported by both analyses (ML-bs = 92, PP = 100) and placed in Pleosporales. It is sister to Paraleptosphaeria orobanches, but this relationship is not supported. The relationship of Dacampiaceae with other families of Pleosporales cannot be resolved with certainty using only nuLSU sequences and the available representatives of Pleosporales. Didymocyrtis is nested in Pleosporales and includes eleven specimens that form a poorly supported clade with very low resolution. Figure 2 presents a more detailed phylogeny of Didymocyrtis based on ML analysis of ITS sequence data. According to this analysis, the Didymocyrtis clade is strongly supported (ML-bs = 72, PP = 100) and includes species previously placed in Diederichia, Leptosphaeria, Phoma (or the new genus Diederichomyces recently described by Trakunyingcharoen et al. 2014) and Polycoccum. Didymocyrtis cladoniicola does not form a monophyletic group, unlike the other species in the clade, which are strongly supported by both analyses. However, relationships among the species are usually poorly supported. In the Didymocyrtis consimilis clade, samples from Caloplaca hosts cluster together, but this relationship is only supported by the Bayesian analysis (PP = 97).
Most taxa now treated in Didymocyrtis were previously known either as species of Leptosphaeria or Polycoccum (sexual stage) or of Diederichia or Phoma (asexual stage). Co-occurrences of both stages on the same host thallus have rarely been observed and documented until recently. Nevertheless, our phylogenetic analyses clearly show that both stages belong to the same clade, representing the genus Didymocyrtis (Figs. 1 and 2), for which the much younger Diederichia and Diederichomyces are made synonyms.
Interestingly, our ITS phylogenetic tree highlights several potential anamorph-teleomorph relationships, as follows: 1. Phoma ficuzzae is shown to represent the asexual stage of Didymocyrtis (Leptosphaeria) ramalinae; moreover, an asexual Phoma-like stage (with pycnidia and conidia) was obtained in all our cultures of single ascospore isolates of D. ramalinae. 2. ITS sequences of the asexual Phoma-like stage and the sexual stage of Didymocyrtis melanelixiae are identical and both stages clearly represent the same species. 3. ITS sequences of the asexual Phoma caloplacae and the sexual Didymocyrtis consimilis on Caloplaca gr. cerina are identical and both stages clearly represent the same species. 4. An asexual Phoma-like stage (with pycnidia and conidia) was obtained in our cultures of single ascospore isolates of Didymocyrtis aff. consimilis (specimen Ertz 17617b, on Cladonia cf. pocillum, initially identified as Polycoccum laursenii), and the corresponding ITS sequence is identical to the one of a Phoma-like anamorph collected in the same locality and on the same host, C. pocillum (specimen Diederich 17465).
In several other species both sexual and asexual stages sometimes occur on the same host thallus or apothecia, a phenomenon that leads to the following hypotheses: 1. Phoma denigricans is the asexual stage of D. bryonthae (both confined to Lecanora epibryon). 2. An unnamed Phoma-like fungus occasionally accompanying perithecia of D. slaptoniensis on Xanthoria parietina may be the asexual stage of that species. 3. Finally, a sexual stage typical of Didymocyrtis has been found growing with Phoma xanthomendozae, wherein the perithecia are intimately intermixed with pycnidia and macroscopically indistinguishable from them, and is considered to represent the same species.
Taxonomy
Pleosporales Luttr. ex M. E. Barr
Prodromus to class Loculoascomycetes (Amherst) 67 (1987). Type: Pleospora Rabenh. ex Ces. & De Not. MycoBank: MB 90563.
Note. Pleosporales is the largest order of Dothideomycetes with 41 families and includes saprobic fungi on dead plant material, pathogens on living plants, hyperparasites on fungi or insects, and lichenized species (Zhang et al. 2011; Hyde et al. 2013).
Dacampiaceae Körb.
(as ‘Dacampieae’), Syst. Lich. Germ.: 322 (1855). Type: Dacampia A. Massal. MycoBank: MB 80680.
Notes. 1. Dacampiaceae was considered as a heterogeneous family in the past, with ten genera accepted by Hyde et al. (2013). Our molecular data resolved the family as polyphyletic. The genus Polycoccum and members of Clypeococcum are accommodated in the new family Polycoccaceae, while the genus Munkovalsaria is placed in Didymosphaeriaceae (Fig. 1). No molecular data are available for the other genera currently placed in Dacampiaceae, i.e. Aaosphaeria, Eopyrenula, Leptocucurthis, Pseudonitschkia, Pyrenidium and Weddellomyces.
2. The genus Dacampia is related to members of Leptosphaeriaceae in our nuLSU tree (e.g., Paraleptosphaeria orobanches, Fig. 1). The relationship of Dacampiaceae with other families of Pleosporales will require more detailed study. Leptosphaeriaceae will become a synonym of Dacampiaceae if Dacampia is shown to be nested within it.
Dacampia A. Massal. (Fig. 3)
Sulla Lec. Hook. Schaer.: 7 (1853). Type: Dacampia hookeri (Borrer) A. Massal. MycoBank: MB 1401.
Syn.: Xenosphaeria Trevis., Conspect. Verruc.: 18 (1860). Type: Xenosphaeria hookeri (Schaer.) Trev., lectotypified by Clements and Shear (1931). MycoBank: MB 5823.
Lichenicolous or lichenized with Coccomyxa and external cephalodia with Nostoc. Ascomata perithecioid, with a central ostiole, lacking setae, black, immersed to semi-immersed, with a pseudoparenchymatic ascomatal wall, in longitudinal section seen as a textura angularis with thick-walled, reddish to dark brown cells. Hamathecium of interascal filaments (paraphysoids) with ramifications and anastomoses. Asci bitunicate, subcylindrical, apically thickened when mature, with a small, sometimes indistinct, ocular chamber, 8-spored, ascal wall and hymenial gel I–, more rarely K/I+ bluish. Ascospores 1–2-seriate, pale brown to dark brown, pigmented from an early stage of development, muriform, ellipsoid to fusiform, often constricted at the septa, smooth, without visible gelatinous sheath. Asexual stage unknown.
Note. The genus Dacampia includes 15 species, most of them being lichenicolous. The type species, Dacampia hookeri, is lichenized (except juvenile stages that might facultatively transform the thallus of Solorina bispora), while the lichenicolous Dacampia engeliana modifies its host lichen to form a thallus structure similar to that found in D. hookeri (Henssen 1995; Hyde et al. 2013; de los Ríos and Grube 2000). The genus might be heterogeneous, as some lichenicolous Dacampia species that do not form such thallus structures might be more distantly related, but molecular data are currently missing for them.
Phaeosphaeriaceae M. E. Barr
Mycologia 71: 948 (1979). Type: Phaeosphaeria I. Miyake. MycoBank: MB 81637.
Note. Phaeosphaeriaceae is a large and important family in the Pleosporales (Hyde et al. 2013, Phookamsak et al. 2014). Lawrey et al. (2012) placed all lichenicolous Phoma species in Phaeosphaeriaceae, while de Gruyter et al. (2009, 2013) stated that Phoma s. str. should be restricted only to Didymellaceae. Eventually, Trakunyingcharoen et al. (2014) described the new genus Diederichomyces to accommodate most of the lichenicolous Phoma. But as shown here, several older generic names are available for the lichenicolous Phoma species, of which Didymocyrtis is the oldest.
Didymocyrtis Vain.
Acta Soc. Fauna Flora Fenn. 49(2): 221, 263 (1921). Type: Didymocyrtis consimilis Vain., lectotype, selected here. MycoBank: MB 1554.
Syn. nov.: Diederichia D. Hawksw., Lichenologist 35: 206 (2003). Type: Diederichia pseudeverniae (Etayo & Diederich) D. Hawksw. MycoBank: MB 28744.
Syn. nov.: Diederichomyces Crous & Trakunyingcharoen in Trakunyingcharoen et al., IMA Fungus 5: 393 (2014). Type: Diederichomyces xanthomendozae (Diederich & Freebury) Crous & Trakunyingcharoen. MycoBank: MB 810828.
Ascomata (unknown in some species) perithecioid (pseudothecia); ascomatal wall dark brown, pseudoparenchymatous, in longitudinal section consisting of polyhedral, tangentially flattened cells forming a textura angularis, with the pigment deposited in the hyphal cell walls. Hamathecial filaments paraphysoids, persistent, septate, with some branches and anastomoses. Asci fissitunicate, narrowly cylindrical, endoascus laterally thickened when young, apically thickened when mature, with a small ocular chamber, ascal wall I– and K/I– except dextrinoid reaction of ascal periplasma, hymenial ‘gel’ I– and and K/I–, 8-spored. Ascospores ± uniseriate to half-overlapping, pale brown to brown, usually pigmented from an early stage of development (mature ones pale brown only in some species), relatively thin-walled (compared to those of true Polycoccum-species), transversally 1–3-septate, upper half-spore slightly broader than lower one, with rounded ends, most species with a distinct sculpture in light microscopy, a distinct perispore visible in young spores of some species in K.
Conidiomata (unknown in some species) Phoma-like, with unilocular, ostiolate pycnidia; pycnidia usually undistinguishable from perithecia, except sometimes by the smaller size; pycnidial wall similar to perithecial wall. Conidiophores lacking. Conidiogenous cells attached to the conidiomal wall and lining the cavity, hyaline; conidiogenesis phialidic, not proliferating. Conidia hyaline, simple, smooth-walled, not embedded in a gelatinous matrix, usually with rounded ends.
Notes. 1. Boerema (1997) subdivided the genus Phoma into nine sections with sexual stages in the genera Didymella, Leptosphaeria, Mycosphaerella and Pleospora. de Gruyter et al. (2009) confirmed the polyphyletic character of Phoma in the Pleosporineae by the application of molecular methods (see also Zhang et al. 2009). The generic type, Phoma herbarum, grouped in the Didymellaceae, and therefore, Phoma s. str. is considered to be restricted to the Didymellaceae. Aveskamp et al. (2010) confirmed that Phoma species appear not only in the Didymellaceae, but also in the related clades Leptosphaeriaceae, Phaeophaeriaceae and Pleosporaceae. In a recent phylogenetic study focusing on species of Sphaerellopsis, Trakunyingcharoen et al. (2014) described the new genus Diederichomyces as including most of the lichenicolous Phoma species that had been assigned to the Phaeosphaeriaceae by Lawrey et al. (2012). As shown here, the genus Diederichia also belongs to this clade. However, the generic name Didymocyrtis Vain., neglected practically from its publication (Vainio 1921), is also available and is here used for this group (see Taxonomy section). As a consequence Diederichia and Diederichomyces are synonymized with Didymocyrtis.
2. Vainio (1921) described both the genus Didymocyrtis and the species D. consimilis in detail, and mentioned D. physciicola (Nyl.) Vain. (based on Mycoporum physciicola Nyl., a later heterotypic synonym of Sphaerellothecium parietinarium (Linds.) Hafellner & V. John) and Mycoporum epistygium Nyl. (Vainio 1921: 222) (an ascomycete of unclear relationship on Melanelia stygia). Mycoporum epistygium was combined into Didymocyrtis epistygia (Nyl. ex Vain.) Vain. later in the same publication (Vainio 1921: 263) and, therefore, Didymocyrtis consimilis cannot be treated as the holotype and is here selected as lectotype.
3. Didymocyrtis differs from Polycoccum s. str. mainly by narrowly cylindrical asci, ± monostichously arranged ascospores, thin paraphysoids, thin-walled, medium (reddish)-brown ascospores and a Phoma-like anamorphic stage. As well, Polycoccum s. str. often induces galls formation on the host lichens, whereas Didymocyrtis does not.
4. Many other lichenicolous Phoma-like species have been described in the past (Lawrey and Diederich 2015), and some of these will certainly be combined later in Didymocyrtis, as soon as sequences of them become available. However, these species should not be combined in this genus, based on morphological characters alone. E.g., sequences of Phoma puncteliae Diederich & Lawrey placed this species ouside of Didymocyrtis (Lawrey et al. 2012) and Trakunyingcharoen et al. (2014) introduced the new genus Xenophoma to accommodate the species. Similarly, Phoma cytospora (Vouaux) D. Hawksw. has been shown to belong to Arthoniales, and the new genus Briancoppinsia has subsequently been described for it (Diederich et al. 2011).
5. Didymocyrtis is also a generic name used for protists in the family Coccodiscidae (e.g. Gilg et al. 2010).
Key to species of Didymocyrtis
-
1 Fructifications are perithecioid ascomata.......................2
-
1 Fructifications are pycnidial conidiomata....................9
-
2 Ascospores predominantly 2- or 3-septate....................3
-
2 Ascospores predominantly 1-septate............................4
-
3 Ascospores predominantly 2-septate, some 1- and 3-septate intermixed, up to 15 μm long; on Parmeliaceae.......................................D. melanelixiae
-
3 Ascospores predominantly 3-septate, some 1- or 2-septate ascospores intermixed, usually longer than 15 μm; on Ramalina [specimens on Protousnea, named Leptosphaeria protousneae, will also key out here]..........................................................D. ramalinae
-
4 Ascospores 16–20 × 8–10 μm; on Teloschistes ......................................................................D. kaernefeltii
-
4 Ascospores up to 15 × 7 μm long......................................5
-
5 Many asci longer than 90 μm; ascospores 13–15 × 6–7 μm.............................................................................6
-
5 Most asci up to 90 μm long.........................................7
-
6 Ascospores 13–15 × 6–7 μm; on Xanthoria parietina..............................................D. slaptoniensis
-
6 Ascospores 11–13 × 6–7 μm; on Xanthomendoza..........................D. xanthomendozae
-
7 All ascospores 1-septate, 10–13 × 4.5–6 μm; on Teloschistes................................................D. infestans
-
7 Some 2-septate ascospores intermixed; on apothecia of crustose lichens.............................................................8
-
8 Verruculose sculpture of ascospores distinct; ascospores 11.5–14 × 4–5 μm; on Lecanora epibryon [conidia, if present, ± ellipsoid, 7–8 × 3–4 μm].......................................................D. bryonthae
-
8 Sculpture of ascospores very minute and often indistinct; ascospores 12–15 × 5–6 μm; on Caloplaca [conidia, if present, broadly ellipsoid, 4.5–6.5 × 2.5–4.5 μm, to subglobose, 5–6 μm diam.]...........................D. consimilis
-
9 Conidia mostly 16–22 × 6–9 μm, multiguttulate; on Pseudevernia furfuracea...................D. pseudeverniae
-
9 Conidia smaller, less than 8 × 6 μm, usually 1–2-guttulate.....................................................................10
-
10 Conidia broadly ellipsoid, l/b ratio < 1.6, mostly 1-guttulate....................................................................11
-
10 Conidia narrowly ellipsoid, l/b ratio > 1.4, mostly 2-guttulate....................................................................13
-
11 Conidia mostly 3.8–5.1 × 3.2–3.8 μm, with one large guttule; on Parmeliaceae [some specimens of D. consimilis s. lat. and of D. ramalinae may also key out here] .......................................D. melanelixiae
-
11 Conidia at least 4.5 × 3.5 μm....................................12
-
12 Pycnidia (50–)100–150 μm diam.; conidia broadly ellipsoid; on Physcia aipolia........D. epiphyscia s. str.
-
12 Pycnidia 70–100(–130) μm diam.; conidia broadly ellipsoid to subspherical; on Caloplaca (gr. cerina or gr. tiroliensis) [D. consimilis s. lat. also on Cladonia pocillum, Heterodermia and Melanohalea exasperatula]..........................................D. consimilis
-
13 Conidia very narrowly ellipsoid, l/b ratio mainly 2.4–3; on Cladonia and Parmelia ..............D. foliaceiphila
-
13 Conidia narrowly ellipsoid, l/b ratio mainly 1.4–2.3............................................................................14
-
14 Conidial breadth mainly < 3 μm...............................15
-
14 Conidial breadth mainly > 3 μm...............................17
-
15 Conidia 6–8 × 2.5–3.5 μm; on Xanthoria parietina..................................................D. slaptoniensis
-
15 Conidia shorter, mainly less than 6.5 × 3 μm [two genetically distinct species that can hardly be distinguished morphologically].........................................16
-
16 Pycnidia (50–)100–150 μm diam.; on Physcia adscendens, P. tenella and Xanthoria parietina .....................................................D. epiphyscia s. lat.
-
16 Pycnidia (40–)50–100(–140) μm; on Cladonia, Ramalina, Squamarina and Parmeliaceae ...........................................................D. cladoniicola
-
17 Conidial l/b ratio > 2..................................................18
-
17 Conidial l/b ratio < 2 [two genetically distinct species of which the asexual stages are morphologically very similar]......................................................................19
-
18 Conidia mainly 7–8 × 3–4 μm; on Lecanora epibryon (hymenium) [ascospores, if present, mainly 11.5–14 × 4–5 μm]..................................................D. bryonthae
-
18 Conidia mainly 6–8 × 2.5–3.5 μm; on Xanthoria parietina [ascospores, if present, mainly 13–15 × 6–7 μm]..................................................D. slaptoniensis
-
19 Pycnidia 105–135 μm diam.; on Ramalina [ascospores, if present, 3-septate].....D. ramalinae
-
19 Pycnidia 140–160 μm; on Xanthomendoza [ascospores, if present, 1-septate]..................................D. xanthomendozae
Didymocyrtis bryonthae (Arnold) Hafellner comb. nov.
Endococcus bryonthae Arnold, Flora (Regensburg) 57: 141 ([21. März] 1874); Didymosphaeria bryonthae (Arnold) G. Winter, Rabenh. Krypt.-Fl., 2. Aufl., 1(2): 430 (1885); Microthelia bryonthae (Arnold) Kuntze, Revisio generum plantarum 3: 498 (1898); Mycoporum bryonthae (Arnold) Jatta, Syll. Lich.: 494 (1900); Tichothecium bryonthae (Arnold) Jatta, Fl. Ital. Crypt. Lich.: 841 (1911); Sphaeria bryonthae (Arnold) H. Olivier, Bull. Int. Acad. Géogr. Bot. 17: 170 (1907); Polycoccum bryonthae (Arnold) Vězda, Ceská Mycol. 23: 109 (1969). Type: [Austria, Nordtirol, Serlos-Gruppe], Kalkboden links ober der Ochsenalm, Matreier Grube, Waldrast in Tirol, 6000′ [Wiener Fuß = c. 1900 m alt.], on Lecanora epibryon (as Lecanora subfusca var. bryontha), Aug. 1873, F. Arnold (M – holotype!). MycoBank: MB 814022
Syn.?: Phoma denigricans Hafellner, Herzogia 10: 18 (1994). Type: Italy: Trentino, Dolomiten, Pordoi-Joch, N-Fuß des Sass Beccle, 46°29′05″ N, 11°48′40″ E, c. 2300 m, Hänge mit niedrigen Dolomitschrofen, on Lecanora epibryon (apoth.), 25 Oct. 1984, J. Hafellner 11989 (GZU – holotype!). MycoBank: MB 447502
Ascomata in dense groups on the apothecia of the host, almost completely immersed in the apothecial hymenium that becomes black and might finally bleach, more protruding when the host hymenium has collapsed, subspherical, 150–200(–250) μm diam.; wall dark brown, often somewhat paler towards the base, laterally c. 12–15 μm diam., of about 4–6 layers of cells. Paraphysoids 1–1.5 μm diam. Asci 60–80 × 7–10 μm. Ascospores ± uniseriate, pale brown, 1-septate, some (less than 5 %) with an additional septum in the upper cell, (10–)11.5–14(–16) × 4–5 μm, often slightly constricted at the septum; septum with externally protruding torus; distinct verruculose sculpture visible in light microscopy.
Conidiomata of supposed asexual stage immersed in the hymenium of host apothecia, several per apothecium, blackening the apothecial surface, in longitudinal section broadly oval to subspherical, c. 80–100 × 100–130 μm diam.; wall dark brown above, paler brown towards the base, of c. 3–5 layers of cells; conidiogenous cells broadly pear-shaped, 7–9 × 4–5 μm. Conidia ellipsoid to somewhat tapering towards the lower end to oblong, many biguttulate, (6–)7–8 × 3–4 μm, l/b ratio 2–2.3.
For further descriptions and illustrations of the teleomorph see Arnold (1874), and of the supposed anamorph see Hafellner (1994).
Distribution and hosts. Taking into consideration published records, and adding the data for the supposed anamorph (Phoma denigricans) and of the specimens cited below, the known distribution can be summarized as follows: Europe (Austria, Germany, Italy, Poland, Svalbard, Sweden), Asia (Mongolia, Russia), and North America (Canada, Greenland, USA); in the hymenium of Lecanora epibryon (e.g., Arnold 1874; Hafellner 1994; Kukwa and Flakus 2009; Santesson 1993; Triebel and Scholz 2001; Zhurbenko 1996, 2009b).
Notes. 1. The collection Arnold, Lich. exs. 615 sub Endococcus bryonthae given as type by various authors (e.g., Aptroot 1995b) is in fact a topotype; that specimen was collected on 4 Sept. 1874 and therefore after the appearance of the protologue (March 1874).
2. Hawksworth and Diederich (1988: 297) and Aptroot (1995b: 59) erroneously described the host of Arnold, Lich. exs. 615 (sec. Arnold duplicates of a later collection from the locus classicus) as Pertusaria bryontha; however, according to Arnold and the specimens seen by us, it is Lecanora epibryon.
3. Sometimes ascomata of Didymocyrtis bryonthae and pycnidia of Phoma denigricans can be observed on the same specimen (e.g., Hafellner 38111, 76036, including on one host apothecium). Comparable observations have also been reported from Svalbard and Siberia by Zhurbenko (2009b: 147, 148). Such co-occurrences are one of the arguments for our opinion that the two taxa represent the sexual and asexual stages of the same fungal species.
4. For differences between Didymocyrtis bryonthae and D. consimilis, see notes below under D. consimilis.
5. The record on Lecanora bryopsora (Hafellner 2008) could not be confirmed. The specimen, on which this record was based, shows infested L. epibryon growing intermingled with an undetermined sorediate crust which evidently is not L. bryopsora.
Selected specimens examined (all on Lecanora epibryon): Sexual stage: Austria: Salzburg, Nationalpark Hohe Tauern, Glockner Gruppe, NW-Grat des Großen Margrötzen Kopfs W ober dem Hochtor, 47°05′10″ N, 12°50′10″ E, 2620 m, GF 8943/1, 1996, Hafellner 38111 & Wittmann (hb. Hafellner) (with asexual stage). Steiermark, Nordalpen (Nördliche Kalkalpen), Totes Gebirge, Hochangern-Massiv N von Liezen, Nazogl, knapp NE vom Gipfel auf dem Rücken gegen den Angerkogel, 47°36′45″ N, 14°13′50″ E, 2050 m, GF 8351/3, 2010, Hafellner 76036 & Muggia (GZU) (with asexual stage). Greenland: W-Grönland, Gem. Umanak, Hänge über Marmorilik, 50–300 m, 1983, Poelt & Ullrich (GZU). Italy: Lombardia, prov. Brescia, Eastern Alps, Central Alps, Ortler-group (Stelvio-group), Cima di Cadì N above Passo del Tonale, N below the summit, 46°16′35″ N, 10°34′15″ E, 2590 m, 2006, Hafellner 75406 & Muggia (GZU). Mongolia: Chubsugul Chimak, Tomim-Brigade, 1983, Huneck MVR 83-43 (GZU). Russia: Siberia, Yakutiya (Sakha-Yakutiya Republic), Lena River delta, right bank of Olenek channel/river branch, near fishermans’ house Novyi Chai-Tumus, 45 WNW Krest-Tumsa cape and Sokol polar station, 72°22′N, 125°40′E, 10–40 m, 1998, Zhurbenko 9896 (M).
Asexual stage: Austria: Steiermark, Zentralalpen, Niedere Tauern, Schladminger Tauern, SE-Abhänge der Steirischen Kalkspitze, W der Giglachseehütte zwischen Preuneggsattel und Akarscharte, 47°16′50″–17′00″ N, 13°37′50″–38′10″ E, 1970–2080 m, GF 8747/2, 2001, Obermayer 9166 (GZU). Germany: Bayern, Landkreis Berchtesgadener Land, Steinernes Meer, Viehkogel, auf dem Gipfel, 2150 m, GF 8543, 1985, Wunder 4030 & Türk (M). Greenland: W-Grönland, Gem. Umanak, Marmorilik, N-seitige Hänge S über dem Fjord Qaumarujuk, 5–200 m, 1983, Poelt & Ullrich (GZU).
Didymocyrtis cladoniicola (Diederich, Kocourk. & Etayo) Ertz & Diederich comb. nov.
Phoma cladoniicola Diederich, Kocourk. & Etayo, Lichenologist 39: 157 (2007); Diederichomyces cladoniicola (Diederich, Kocourk. & Etayo) Crous & Trakunyingcharoen in Trakunyingcharoen et al., IMA Fungus 5: 401 (2014). Type: USA, Minnesota, Cottonwood County, 2 miles W of US 71, 14.5 miles N of Windom, 44°06′40″ N, 95°04′30″ W, 400 m, seasonally dry, flat, Sioux quartzite outcrop on N side of road, on Cladonia pyxidata, 28 Sept. 1991, W. R. Buck 20859A (NY – holotypus!; hb. Diederich – isotypus!). MycoBank: MB 814023.
Ascomata unknown.
Conidiomata immersed in pale necrotic areas of the thallus or partially erumpent, black, subspherical to pyriform, (40–)50–100(–140) μm diam.; pycnidial wall brown, 10–12 μm thick, composed of several layers of cells, outer cells dark brown, 5–8(–10) μm diam., inner cells hyaline. Conidiogenous cells short-ampulliform, 2.5–4.5 μm high, 2.5–4 μm wide. Conidia ellipsoid, biguttulate, with a small guttule near each apex, (3.8–)4.7–5.9(–7.3) × (2.0–)2.4–3.0(–3.5) μm, l/b ratio (1.4–)1.7–2.2(–2.8) (n = 472) [from Diederich et al. 2007].
For further descriptions and illustrations, see Diederich et al. (2007), Lawrey et al. (2012) and Trakunyingcharoen et al. (2014).
Distribution and hosts (*confirmed by sequences). Europe (Belgium, Czech Republic, France incl. Corsica, Germany, Spain, Ukraine), Africa (Canary Islands), and North America (USA: Minnesota; Greenland); on Cladonia cervicornis, C. convoluta, C. fimbriata, C. foliacea*, C. furcata, C. phyllophora, C. pocillum, C. pyxidata, C. ramulosa, C. rangiformis*, C. symphycarpia*, Flavoparmelia caperata, Parmelina tiliacea*, Ramalina pollinaria*, R. polymorpha* and Squamarina cartilaginea* (Alstrup et al. 2009; Diederich et al. 2007; Etayo 2010a; Lawrey et al. 2012).
Note. This species was originally described from Cladonia species. ITS sequences, however, showed that specimens on Parmelina, Ramalina and Squamarina are almost surely conspecific. The four sequences from mainland France originate from the same calcareous outcrop, where the species parasitized Cladonia foliacea, C. rangiformis, C. symphycarpia and Ramalina pollinaria (all sequenced) and Squamarina cartilaginea (not sequenced). This unexpected low host specificity may be explained by the rather aggressive parasitic way of life of this species that eventually kills the host thallus.
Additional specimens examined: Canary Islands: Gomera, NW of Imada, Raso Grande, volcanic outcrop, 28°05′36″ N, 17°14′52″ W, 1288 m, summit of a volcanic outcrop, on Ramalina polymorpha, 2011, Ertz 16296 (BR). France: Corsica, Saint-Florent, au nord de la route entre Tettola et Santa Maria, 42°41′39″ N, 9°19′4″ E, pelouse calcaire xérique, sur Cladonia foliacea, 2011, Ertz 16464 (BR).
Didymocyrtis consimilis Vain. (Figs. 4a–h and 9a–b)
Didymocyrtis consimilis s. lat. on Caloplaca (a–h), on Melanohalea exasperatula (i–l) and on Heterodermia (M). a on Caloplaca tirolensis (Hafellner 83640); b on C. cerina (Türk 44065a-7); c on C. stillicidiorum (Hafellner 83640); d section through conidioma (left) and ascoma (right) (in water; Gardiennet 12008); e section through overmature ascoma with brown ascospores (in water; Hafellner 41836); f ascomatal wall in surface view (in KOH; Hafellner 41836); g ascospores (in KOH; Hafellner 41836); h section through conidioma, showing subspherical conidia (in water; Türk 44065a-7). i black immersed conidiomata on Melanohalea exasperatula (Berger 27218); j asci with ascospores in water; k asci with ascospores becoming blue in Lugol’s reagent (j–k Berger 26876); l ellipsoid conidia (Berger 27218). m on Heterodermia, conidia (in water; Berger 27251). Scale bars: a–c, i = 200 μm; d = 50 μm; e–f, h = 10 μm; g, j–m = 5 μm
Acta Soc. Fauna Flora Fenn. 49(2): 221, 263 (1921). Type: [Finland], ‘in Selkäsaari in par. Simo in Ostrobotnia bor., on bark of Sorbus’, on Caloplaca cerina (hymenium), Räsänen (TUR-VAIN 32734 – holotype!). MycoBank: MB 431576.
Syn. nov.: Phoma caloplacae D. Hawksw., Bull. Br. Mus. nat. Hist. (Bot.) 9: 50 (1981); Diederichomyces caloplacae (D. Hawksw.) Crous & Trakunyingcharoen in Trakunyingcharoen et al., IMA Fungus 5(2): 401 (2014). Type: [Russia, Krasnoyarsk Krai], Yeniseysk, Stolba, 60°20′ N, on Caloplaca cerina (apothecia), 1 July 1876, Brenner 1027b p.p. (UPS – holotype!). MycoBank: MB 112547.
Ascomata in dense groups on the apothecia of the host, almost completely immersed in the apothecial hymenium that becomes mis-coloured. Ascoma in longitudinal section subspherical, (70–)100–150 μm diam.; wall medium to dark brown, laterally c. 10–15 μm diam., consisting of c. 3–4 layers of cells. Paraphysoids 1–1.5 μm diam. Asci 50–70 × 7–10 μm. Ascospores ± uniseriate or half-overlapping, pale brown, 1-septate, some (less than 5 %) with an additional septum in the upper cell, 12–15 × (4.5–)5–6(–6.5) μm, hardly constricted at the septum, externally protruding torus at the septum indistinct, verruculose sculpture hardly visible in light microscopy.
Conidiomata several per hymenium, scattered, completely immersed at first, later protruding over the collapsed hymenium, dark brown, somewhat darker as the discoloured hymenial surface of the infested apothecium, in longitudinal section subglobose, 70–100(–130) μm diam.; wall of conidiomata dark brown in upper part, pale brown to subhyaline towards the base, c. 10–15 μm diam., of c. 3–5 layers of tangentially flattened cells. Conidiogenous cells subglobose to broadly obpyriform, 5–6 μm diam. Conidia 1–2-guttulate, rather variable in form and size, broadly ellipsoid, 4.5–6.5 × 2.5–4.5 μm, to subglobose, (4–)5–6(–7) μm diam., l/b ratio < 1.5.
For further descriptions and illustrations of the asexual stage, see Hawksworth (1981), Ihlen and Wedin (2008), Lawrey et al. (2012) and Trakunyingcharoen et al. (2014).
Distribution and hosts. Up to now the sexual stage of the species has not been distinguished from that of Didymocyrtis bryonthae, a fungus confined to the Lecanora subfusca group (L. epibryon). Therefore all reports of ‘Polycoccum bryonthae’ can be taken as referring to Didymocyrtis consimilis as long as a Caloplaca of the C. cerina or C. tiroliensis group is given as host for a record. Taking this into consideration and adding data for the asexual stage (Phoma caloplacae) and specimens cited below, the known distribution can be summarized as follows: Europe (Austria, Finland, France, Germany, Italy, Poland, Slovakia, Slovenia, Spain, Sweden), Asia (Iran, Israel, Russia, Turkey), and North America (Canada, Greenland); in the hymenium of Caloplaca cerina, C. cerina var. muscorum, C. stillicidiorum, C. tiroliensis (e.g., Alstrup and Hawksworth 1990; Halıcı et al. 2014; Hawksworth 1981; Hawksworth and Diederich 1988; Kondratyuk et al. 2005; Kukwa and Flakus 2009; Lawrey et al. 2012; Magnusson 1952 sub Phoma physciicola p. p.; Santesson et al. 2004; Seaward et al. 2008; Wittmann and Türk 1990; Zhurbenko 2009a).
Notes. 1. Two specimens labeled Didymocyrtis consimilis are preserved in TUR-VAIN: no. 32734 with locality data as in protologue, hence the type specimen, but with a host different from that given in the protologue, identified as Placodium gilvum, 17 June 1916, V. Räsänen; and no. 32733 with locality data differing from that given in the protologue (Finland, Ostrobotnia borealis, Simo, Lammasletto, Pihlajalla), and host as in the protologue, identified as Placodium gilvum var. cyanolepra, without date, V. Räsänen.
2. In the type specimen, this species is growing on a corticolous Caloplaca cerina. Records of this fungus on corticolous Caloplaca species are relatively rare but do occur also outside Scandinavia (see below). At least in Central Europe the species is much more common on Caloplaca species growing over bryophytes and decaying vascular plants in alpine environments. These populations were usually identified as Polycoccum bryonthae, a fungus described from Lecanora epibryon and which is treated here as separate species. Both species infest the hymenium of their hosts in which either ascomata or conidiomata, or both develop. Sometimes ascomata (Didymocyrtis consimilis) and pycnidia (Phoma caloplacae) can be observed on the same specimen (e.g., specimens Wilfling 1847, Hafellner 20068) or even in one host apothecium (e.g., specimens Hafellner 78736, 75304).
3. Didymocyrtis consimilis differs from the evidently closely related D. bryonthae by smaller ascomata, somewhat broader ascospores, a less conspicuous ascospore sculpture, distinctly broader conidia, and by its host selection.
4. Didymocyrtis consimilis has always been considered to be host specific on the Caloplaca cerina group, but our nuITS analyses (Fig. 2) revealed the existence of populations on other hosts (Cladonia pocillum, Heterodermia, Melanohalea exasperatula) that have very similar ITS sequences and are morphologically similar (Figs. 4i–m and 9c–d). Some morphological differences were observed, as follows: Conidia on the unusual hosts tend to be more elongate ellipsoid, vs. broadly ellipsoid in D. consimilis s. str. on Caloplaca gr. cerina: specimen Berger 27251 on Heterodermia has conidia of 4–5 × 3–3.5 μm with one large oil drop (Fig. 4m); specimen Berger 27218 on Melanohalea exasperatula has conidia with one large or two small oil drops, 4–6 × 3–4(–5) μm (Fig. 4i); specimen Diederich 17465 on Cladonia pocillum has conidia (3.5–)4–4.5 × 2.5–3 μm. The sexual stage on Melanohalea exasperatula (Berger 26876) has ascospores with a faint and homogeneous amyloid reaction in Lugol (Fig. 4k), (9–)10.5–13(–14) × (4–)4.5–5.5(–6) μm; moreover the two isolates on Melanohalea produce long yellowish acicular crystals in culture with the agar medium turning dark reddish-brown (Fig. 9d), but as the cultures of D. consimilis s. lat. were done in different laboratories and in different conditions, it is unclear if this might be used as a diagnostic character. The sexual stage on Cladonia cf. pocillum (Ertz 17617b) has smaller ascospores, 8.5–10.5 × 3.5–4 μm, and was thus first identified as Polycoccum laursenii Zhurb. (Zhurbenko and Alstrup 2004), a species described from Alaska on Cladonia pocillum (but only four ascospores were measured, the specimen being very tiny). Phoma heterodermiae S. Y. Kondr., L. Lökös & J.-S. Hur, described from South Korea on Heterodermia hypoleuca, with conidia measuring 3.5–5 × 3–4(–4.5) μm, might be conspecific with the material studied by us on Heterodermia. More studies using a larger taxon sampling and a multigene approach will be needed to investigate this group in detail.
Selected specimens examined: Sexual stage: Austria: Kärnten, Nationalpark Hohe Tauern, [Glockner-Gruppe], Sattelalpe W von Heiligenblut, 1 km SE der Bricciuskapelle, 47°03′01″ N, 12°48′10″ E, 1600 m, GF 8942/2, on Caloplaca stillicidiorum, 1987, Hafellner 20068 & Walther (GZU) (with asexual stage). Salzburg, Pongau, Niedere Tauern, Radstädter Tauern, Kleinarltal, beim Jägersee c. 5 km S von Kleinarl, entlang der Straße am Ostufer des Sees, 47°14′15″ N, 13°20′02″ E, 1100 m, GF 8746/3, on C. cerina, 2006, Hafellner 41836 (GZU). Steiermark, Nordalpen, Mürztaler Alpen, Thalerkogel NE von Trofaiach, kurz N vom Gipfel, 47°28′40″ N, 15°03′15″ E, 1640 m, GF 8556/1, on C. stillicidiorum, 2011, Hafellner 78736 (GZU) (with asexual stage). Steiermark, Nordalpen, Mürzsteger Alpen, Rauschkogel NE von Turnau, 47°36′40″ N, 15°22′15″ E, 1660 m, GF 8358/3, on C. tirolensis and C. stillicidiorum, 2005, Hafellner 83640 (GZU). Tirol, Nordalpen, Karwendel, Hinterautal, Umgebung vom Kotwaldsee, 47°22′41″ N, 11°21′26″ E, 1070 m, GF 8634/1, on C. cerina, 2008, Türk 44065a-7 & Pfleger (GZU). Finland: Ostrobotnia borealis, Simo, Lammasletto, Pihlajalla, on C. cerina (as “Placodium gilvum var. cyanolepra”) (apoth.), without date, Räsänen (TUR-VAIN 32733). France: Côte-d’Or, L’Etang-Vergy, combe Ragon, on C. cerina, 2012, Gardiennet 12041 (hb. Gardiennet). Italy: Trentino-Alto Adige, prov. Bolzano (Südtirol), Southern Alps, Dolomiti, M. Seceda (Geisler Spitzen) NE of Ortisei (St. Ulrich), on the ridge just W above Forc Pana (Pana Scharte), 46°36′05″ N, 11°44′05″ E, 2500 m, on C. tiroliensis, 2002, Hafellner 75196 (GZU).
Asexual stage: Austria: Steiermark, Niedere Tauern, Wölzer Tauern, Gumpeneck SE von Gröbming, Gipfelpyramide, NW-seitig, 47°23′50″ N, 14°00′50″ E, 2180 m, GF 8650/1, on C. stillicidiorum/C. tiroliensis, 1993, Hafellner & Wilfling 1847 (GZU) (with sexual stage). Canada: Saskatchewan, RM of Val Marie No. 17, Grasslands National Park, West Block, Frenchman River Valley, Belza’s Ranch, 49°9.1′ N, 107°31.0′ W, 800 m, on bones, on C. stillicidiorum, 2010, Freebury 1357 (CANL). Germany: Bayern, Nordalpen, Chiemgauer Alpen, Hochgern, 16 km SW von Traunstein, oberste N-Hänge knapp unter dem Gipfel, 47°45′04″ N, 12°30′53″ E, 1700 m, on C. stillicidiorum, 2009, Hafellner 75177 (GZU). Italy: Lombardia, prov. Brescia, Eastern Alps, Central Alps, Ortler-group (Stelvio-group), Cima di Cadì N above Passo del Tonale, N below the summit, 46°16′35″ N, 10°34′15″ E, 2590 m, on C. cerina var. muscorum, 2006, Hafellner 75407 & Muggia (GZU). Slovakia: Carpates, Tatra Magna, pars orientalis, Belanské Tatry, in monte Bujačí, 1900 m, on C. tiroliensis, 1985, Farkas, Kyselová & Vězda (GZU). Slovenia: Southern Alps, Julian Alps, massif of Mangart NE of Bovec, NE slopes of Mali vrh S opposite to Mangartska koča (Mangart refuge), 46°26′00″ N, 13°38′35″ E, 1960 m, on C. cerina var. muscorum, 2003, Hafellner 75304 (GZU). Spain: Prov. Barcelona, Sierra de Montseny, Hänge NE der Paßhöhe des Coll Formich, 1200 m, on C. cerina, 1983, Hafellner 17354 (GZU). Sweden: Torne Lappmark, Umgebung von Abisko, über Kieselkalk auf dem Kliff Paddos S von Abisko, 620–630 m, on C. tiroliensis, 1967, Poelt (GZU, under the name of the host).
Selected specimens examined on unusual hosts and therefore referred here as D. aff. consimilis: Sexual stage: Austria: Oberösterreich, Sauwald, Kopfing, 540 m, MTB 7547, auf Melanohalea exasperatula, 2012, Berger 26876 (hb. Berger). Luxembourg: S of Obercorn, Kiemerchen, 49°30′00″ N, 5°53′53″ E, 385 m, bois clairs et friches dans une ancienne carrière, sur squamules de Cladonia cf. pocillum au sol, 2012, Ertz 17617b (BR).
Asexual stage: Austria: Bezirk Rohrbach, Neustift, Rannariedl, 300 m N Schloss, 48°29′02″ N, 13°46′18″ E, 485 m, MTB7548, auf Juglans regia, auf Melanohalea exasperatula, 2013, Berger 27218 (hb. Berger). Luxembourg: SWW de Belvaux, Kiemreech (M8.51), terricole, dans une ancienne exploitation minière, sur Cladonia pocillum, 2012, Diederich 17465 (hb. Diederich). Madeira: Achada da Madeira, Laurisilva entlang der Levada Rib da Urzal, 32°46′37″ N, 16°58′55″ W, 655 m, auf Heterodermia, 2013, Berger 27251 (hb. Berger).
Didymocyrtis epiphyscia Ertz & Diederich nom. nov. (Figs. 5a–c and 6)
Didymocyrtis epiphyscia (a–i) and D. slaptoniensis (j–l). a–c D. epiphyscia s. str. conidiomata on Physcia aipolia apothecia (a Freebury 1409; b–c Diederich 17482): habitus, section through conidioma, conidia in water. d–f D. epiphyscia s. lat. conidiomata on P. tenella thallus (Diederich 17478): habitus, section through conidioma, conidia in water. g–i D. epiphyscia s. lat. conidiomata on Xanthoria parietina apothecium (Diederich 17479): habitus, section through conidioma, conidia in water. j–l D. slaptoniensis on X. parietina thallus (Obermayer 13130): conidiomata, ascomata, conidia in water. Scale bars: a, d, g, j–k = 200 μm; b, e, h = 10 μm; c, f, i, l = 5 μm
Conidial size distribution (length versus breadth, in μm) of selected Didymocyrtis specimens occurring on Physcia and Xanthoria (each specimen is represented by an ellipse, based on the measurement of at least 20 conidia from one conidioma; large radius of ellipse = standard deviation (SD) of conidial length; small radius = SD of conidial breadth). Blue = Didymocyrtis epiphyscia s. str. on Physcia aipolia. Grey = D. epiphyscia s. lat. on P. adscendens and P. tenella. Red = D. epiphyscia s. lat. on Xanthoria parietina. Green = D. slaptoniensis on X. parietina
Phoma physciicola Keissler, Hedwigia 50: 294 (1911). Type: Austria, Steiermark, Gams bei Hieflau, on Physcia aipolia, June 1910, K. von Keissler (W 1910/609 – holotype, non vid., examined by Hawksworth 1981). MycoBank: MB 814024
Ascomata unknown.
Conidiomata immersed in necrotic areas of the host hymenium, more rarely of the thallus, black, subspherical, (50–)100–150 μm diam.; pycnidial wall brown, inner cells paler, darker near the ostiole. Conidiogenous cells ampulliform, 4–7 μm diam. Conidia ellipsoid, with one or two small guttules, (4.0–)4.6–6.1(–7.8) × (3.2–)3.5–4.2(–5.0) μm, l/b ratio (1.0–)1.2–1.6(–2.0) (n = 150).
For a description and further illustrations, see Hawksworth (1981).
Distribution and hosts. Europe (Austria, France, Great Britain: Scotland and Luxembourg) and North America (Canada), on Physcia aipolia (hymenium, rarely thallus). The species has also been reported on Physcia stellaris (Coppins and Coppins 2001), Physconia distorta (Hawksworth 1981) and Phaeophyscia orbicularis (von Brackel 2011: 82), but we did not examine any material on those last two host genera. The material on Physcia adscendens and P. tenella with narrower conidia is treated below as D. epiphyscia s. lat. Finally, reports from other host genera, e.g. on Parmelia sulcata (von Brackel 2007, 2009; Zhurbenko et al. 2012), almost surely refer to other species.
Notes. 1. This species was originally described as parasitizing the hymenium of Physcia aipolia. Although we did not re-examine the holotype, which was carefully studied, described and illustrated by Hawksworth (1981), there is no doubt that our recent specimens in the hymenium of P. aipolia belong to the same species. In the description of Hawksworth (1981), conidia were described as slightly narrower [4–5.5(–6) × 2.5–3.5(–4) μm], but this author regarded the species in a broader sense, also including specimens on Physconia. In the original description, conidia were given as ‘ca. 6 × 4 μm’, which perfectly agrees with our observations.
2. Phoma physciicola Keissler cannot be combined in Didymocyrtis, because of the earlier combination Didymocyrtis physciicola (Nyl.) Vain., a synonym of Sphaerellothecium parietinarium (see Note 2 under Didymocyrtis), and a new name for Phoma physciicola must therefore be introduced in Didymocyrtis. On the other hand, the older name Phoma epiphyscia Vouaux is of unknown application and might threaten the current nomenclature of Didymocyrtis (see Note under Didymocyrtis slaptoniensis) if sequence data become available from a specimen collected close to the neotype locality in Greenland and on the same host Phaeophyscia sciastra. To avoid such unwanted future nomenclatural changes, we choose the same epithet ‘epiphyscia’ as a nomen novum for Phoma physciicola.
3. Rather unexpectedly, Phoma specimens parasitizing the thallus of Physcia adscendens and P. tenella, but also the thallus and hymenium of Xanthoria parietina, are genetically very close to the specimens on Physcia aipolia, suggesting that they might belong to the same species, Phoma epiphyscia. However, as conidia on those hosts are constantly much narrower, we treat them below as D. epiphyscia s. lat.
Specimens examined (all on Physcia aipolia): Canada: Quebec, Les Collines-de-l’Outaouais Regional County, Gatineau Park, near Intersection Highway 105 & 366, 45°37′56″ N, 75°56′26″ W, edge of forest near La Pêche River, 2010, Freebury 1119A (CANL); ibid., 2011, Freebury 1409 (CANL). France: Côte d’Or, Val-Suzon, En Neudry, 2014, Gardiennet 14079 (hb. Gardiennet). Meuse, SE of Montmédy, Marville, cimetière de St Hilaire, 2013, Diederich 17724 (hb. Diederich). Great Britain: Scotland, Argyll Main, VC 98, Inverary, Glen Shira, along boundary wall near Allt Buidhe, S of Kilbraan, 1996, Coppins 16745 (E). Luxembourg: W of Steinfort, old sandstone quarries, 2012, Diederich 17482 (hb. Diederich); ibid., 2015, Diederich 17766 (hb. Diederich).
Didymocyrtis epiphyscia Ertz & Diederich s. lat. (Figs. 5d–i, 6 and 9g–h)
Ascomata unknown.
Conidiomata and conidiogenous cells as in Didymocyrtis epiphyscia, but conidia much narrower, biguttulate or rarely multiguttulate, (3.7–)4.6–6.4(–8.0) × (2.0–)2.5–3.1(–3.5) μm, l/b ratio (1.2–)1.6–2.3(–3.5) (n = 290).
Distribution and hosts. We have seen material from Europe (Belgium, France and Luxembourg), on Physcia adscendens, P. tenella (thallus) and Xanthoria parietina (thallus and apothecia). Specimens likely to be the same species have also been reported from Germany (von Brackel 2007, on P. tenella, sub Phoma physciicola; von Brackel 2009, on P. tenella, sub Phoma physciicola; von Brackel 2010, on P. adscendens, sub Phoma epiphyscia) and Italy (von Brackel 2011, on P. tenella, sub Phoma epiphyscia). Literature reports on Xanthoria parietina are more problematic, as they might as well belong to the putative anamorph of Didymocyrtis slaptoniensis.
Notes. 1. As specimens on Xanthoria parietina from Belgium, northern France and Luxembourg are genetically and morphologically almost identical to the specimens on Physcia adscendens and P. tenella, we consider them as belonging to the same species. Our phylogenetic analyses, using ITS sequences, place these specimens together with Didymocyrtis epiphyscia s. str., which differs, however, by constantly much broader conidia.
2. Several asexual specimens from Austria on Xanthoria parietina were growing close to Didymocyrtis slaptoniense, and we tentatively consider them as representing the asexual stage of that species. Morphologically, conidia from the putative D. slaptoniense asexual stage are larger, (5–)6–8(–9) × 2.5–3.5 μm.
3. The name Phoma epiphyscia Vouaux was introduced for a species on Phaeophyscia orbicularis and Xanthoria parietina, collected in northern France (Vouaux 1914). As the type is presumed to be lost (Hawksworth 1981), Alstrup and Hawksworth (1990) neotypified the name on a Greenland specimen on Phaeophyscia sciastra. These authors don’t describe the neotype, but state that ‘The only difference we noted is that the conidia are up to 7 × 3 μm, and not only to 6 × 3 μm as given by Vouaux’. In the identification key (p. 14), they describe the material on P. sciastra as ‘Conidia 4–6 × 2–3 μm, rounded at the ends; conidiomata immersed’. Following the conidial dimensions, Phoma epiphyscia could represent the species we are studying here on Physcia and Xanthoria. However, sequences from a similar specimen on P. sciastra are needed before proposing any conclusions.
4. The material on Physcia adscendens, P. tenella and Xanthoria is morphologically hardly distinguishable from Didymocyrtis cladoniicola, of which conidia were given by Diederich et al. (2007) as mainly 4.7–5.9 × 2.4–3.0, l/b ratio 1.7–2.2 (n = 472). The pycnidia were said to be (40–)50–100(–140) μm in D. cladoniicola, slightly smaller than those on Physcia and Xanthoria. Both species are, however, genetically distinct (see Fig. 2).
Specimens examined: Belgium: Rochefort, Ave-et-Auffe, colline schisto-calcaire au centre du village d’Ave, 220 m, on P. adscendens, 2012, Ertz 17411 (BR); ibid., on X. parietina, Ertz 17413, 17414 (BR). Wibrin, lieu-dit ‘Au Bec du Fèyi’, rive gauche du ruisseau du Fond de Minée, 440 m, on X. parietina, 2012, Ertz 17422 (BR). France: Finistère, Camaret-sur-Mer, Pointe de Pen Hir, 48°15′31″ N, 4°37′11″ W, 35 m, on P. adscendens, 2012, Ertz 17461 (BR). Meuse, SE of Montmédy, Marville, cimetière de St Hilaire, on X. parietina, 2013, Diederich 17726 (hb. Diederich). Côte d’Or, Saint-Seine-sur-Vingeanne, rive de la Vingeanne au niveau de la D960, sur X. parietina sur Populus, 2011, Gardiennet 12008 (hb. Gardiennet). Luxembourg: S of Obercorn, Kiemerchen, 49°30′00″ N, 5°53′53″ E, 385 m, on P. tenella, 2012, Ertz 17615 (BR). SWW of Belvaux, Kiemreech, on P. tenella, 2012, Diederich 17478 (hb. Diederich); ibid., on X. parietina, 2012, Diederich 17479 (hb. Diederich). Strassen, Tossebierg, old sandstone quarry, on X. parietina, 2014, Diederich 17759 (hb. Diederich).
Didymocyrtis foliaceiphila (Diederich, Kocourk. & Etayo) Ertz & Diederich comb. nov.
Phoma foliaceiphila Diederich, Kocourk. & Etayo, Lichenologist 39: 159 (2007); Diederichomyces foliaceiphila (Diederich, Kocourk. & Etayo) Crous & Trakunyingcharoen in Trakunyingcharoen et al., IMA Fungus 5: 401 (2014). Type: Czech Republic, Central Bohemia, distr. Rakovník, Křivoklátsko protected landscape area, between Nezabudice and Roztoky villages, Nezabudické skály nature reserve, 50°01′ N, 13°51′ E, 255 m, in Quercus petraea forest below steep slope of rocky outcrops, on Cladonia foliacea, 2002, J. Kocourková (PRM 896164 – holotypus, non vid.). MycoBank: MB 814025.
Ascomata unknown.
Conidiomata immersed in pale necrotic areas of the thallus or partially erumpent, black, subspherical to pyriform, 50–100 μm diam.; pycnidial wall brown, 10–12 μm thick, composed of several layers of cells, outer cells dark brown, 5–8(–10) μm diam., inner cells hyaline. Conidiogenous cells short-ampulliform, 4–5.5 × 2.5–5 μm. Conidia ellipsoid, biguttulate, with a small guttule near each apex, (5.0–)5.8–7.1(–7.5) × (2.0–)2.2–2.7(–3.0) μm, l/b ratio (2.0–)2.4–3.0(–3.5) (n = 100) [from Diederich et al. 2007].
For further descriptions and illustrations, see Diederich et al. (2007), Lawrey et al. (2012) and Trakunyingcharoen et al. (2014).
Distribution and hosts (*confirmed by sequences). Europe (Belgium, Czech Republic, France, Germany, Lithuania, Luxembourg, Netherlands, Spain), on Cladonia fimbriata, C. foliacea, C. furcata, C. rangiformis, C. squamosa* and Parmelia sulcata* (von Brackel 2010; Diederich et al. 2007; Etayo 2010a; Lawrey et al. 2012; Motiejūnaitė et al. 2011).
Notes. 1. This species was originally described from Cladonia species. ITS sequences, however, showed that specimens on Parmelia sulcata are almost surely conspecific. The five sequences used in our analysis originate from the same siliceous outcrop in the Belgian Ardennes and obviously represent a genetically uniform population parasitizing both Cladonia spp. and Parmelia sulcata.
2. Trakunyingcharoen et al. (2014) described the new Paraphaeosphaeria parmeliae Crous & Trakunyingcharoen, said to be lichenicolous on Parmelia sulcata, and based on a culture (CBS 131728) that ‘was originally identified as Phoma foliaceiphila’. However, this culture was isolated by us from specimen Ertz 15257, which represents typical P. foliaceiphila. Furthermore, our original culture produced pycnidia with typical P. foliaceiphila conidia. As a conclusion, it is highly likely that the culture used by Trakunyingcharoen et al. (2014) was contaminated, which would mean that the new Paraphaeosphaeria parmeliae is a contaminant obtained in laboratory and therefore of uncertain origin and ecology.
Didymocyrtis infestans (Speg.) Hafellner comb. nov.
Didymosphaeria infestans Speg., Anal. Soc. Cient. Argentina 12 (4): 176 (1881). Didymosphaerella infestans (Speg.) Cooke, Grevillea 18 (86): 29 (1889). Microthelia infestans (Speg.) Kuntze, Revisio Generum Plantarum 3: 498 (1898). Endococcus infestans (Speg.) Speg., Anales del Museo Nacional de Buenos Aires 19: 369 (1909). Discothecium infestans (Speg.) Vouaux, Bull. Soc. Mycol. France 29: 56 (1913). Polycoccum infestans (Speg.) Etayo, Bull. Soc. Linn. Provence 47: 105 (1996). Type: Argentina, Buenos Aires, Las Conchas, on apothecia of Teloschistes chrysophthalmus, 1 May 1881, C. Spegazzini 5854 (LPS – holotype, non vid., fide Etayo 1996a). MycoBank: MB 814026.
Ascomata developing in the hymenium and/or thallus branches of the host, immersed, finally somewhat protruding. Ascoma in longitudinal section subspherical to pyriform, 250–350 μm diam.; wall brown, laterally c. 10–20 μm diam., consisting of about 3–4 layers of cells. Paraphysoids 1–1.5 μm diam. Asci 70–95 × 7–9(–10) μm. Ascospores ± uniseriate, medium brown, 1-septate (practically no tendency to develop a second septum), 10–13(–14) × 4.5–6 μm, not or somewhat constricted at the septum, with distinct verruculose sculpture visible in light microscopy.
Conidiomata unknown.
For further descriptions and illustrations of the sexual stage see Spegazzini (1881) and Etayo (1996a).
Distribution and hosts. Africa (Canary Islands) and South America (Argentina); Teloschistes chrysophthalmus, T. flavicans. The record on Ramalina sp. (Etayo 1996a) needs to be revised.
Notes. 1. The host of the type specimen was originally identified as Teloschistes flavicans. However, Etayo (1996a: 106) revised it as Teloschistes chrysophthalmus.
2. The proposed transfer of the species to Didymocyrtis is based on morpho-anatomical characters; molecular data are still lacking.
3. D. infestans differs from D. kaernefeltii, also occurring upon Teloschistes, by its smaller, medium brown ascospores and smaller asci. Didymocyrtis slaptoniensis has somewhat longer asci, somewhat larger ascospores with a pale brown spore wall and a more distinct septal torus.
Specimen examined: Canary Islands: Gomera, Hermigua, mirador de El Bailadero, bosque de tejos (Erica scoparia) en la cresta, 1010 m, on Teloschistes flavicans, 1994, Etayo 13142 (hb. Etayo).
Didymocyrtis kaernefeltii (S. Y. Kondr.) Hafellner comb. nov.
Polycoccum kaernefeltii S. Y. Kondr., Ukr. Bot. Zhurn. 65: 566 (2008). Type: Australia, Victoria, 20 km SW of on the road to Patchewollah, 35°10.52′ S, 142°12.32′ E, c. 100 m, on Acacia and other shrubs along the road, on Teloschistes chrysophtalmus, 15 Jan. 1999, I. Kärnefelt 992401 (LD – holotypus, non vid., fide Kondratyuk 2008). MycoBank: MB 814034.
Ascomata dispersed, developing in the hymenium of the host apothecia, entirely immersed, only the ostiolar region visible. Ascoma in longitudinal section subspherical, 250–350 μm diam.; wall brown, laterally c. 15–25 μm diam., consisting of about 3–4 layers of cells. Paraphysoids 1.5–2 μm diam. Asci 90–115 × 10–13(–15) μm. Ascospores ± uniseriate, pale brown, mostly 1-septate, 2-septate intermixed (>10 %), 16–20(–23) × 8–10 μm, distinctly constricted at the primary septum, with a distinct verruculose sculpture visible in light microscopy.
Conidiomata unknown.
For a description and further illustrations of the sexual stage see Kondratyuk (2008).
Distribution and hosts. Australia (South Australia, Victoria, Queensland); on Teloschistes chrysophtalmus (Kondratyuk 2008).
Notes. 1. The proposed transfer of the species to Didymocyrtis is based on morpho-anatomical characters. Molecular data are still lacking.
2. To date, Didymocyrtis kaernefeltii is the most variable known species regarding ascospore septation in the genus. Two-septate spores are quite common (> 10 %) in a squash preparation. The additional septum develops more frequently in the upper half of the spore but occasionally also in the lower half. Aseptate and 3-septate spores are rare (< 1 %).
3. According to our data, ascus width measurements given in the protologue are too low, and the indicated average of ascospore length is somewhat too high.
4. Didymocyrtis kaernefeltii has also been reported growing on the apothecia of Caloplaca cerina from the Caucasus in Russia (Zhurbenko and Kobzeva 2014), but that record needs to be confirmed. We saw another species growing on various Caloplaca species (specimens from Afghanistan, Armenia and Cyprus) for which the description provided by Zhurbenko and Kobzeva (2014) fits perfectly. Although the ascospores of this species are surprisingly similar (with some differences in shape and pigmentation), we think that it is neither Didymocyrtis kaernefeltii nor D. consimilis. With our concept it is not even a Didymocyrtis, because the asci are broadly cylindrical to subventricose.
Selected specimens examined (all on Teloschistes chrysophtalmus): Australia: South Australia, Nairne to Mount Barker road, 2 km S of Nairne, 35°03′ S, 138°55′ E, 1981, Mayrhofer 2724a (GZU). Queensland, Sellin’s Road 1 km E of Mt. Mee State Forest station, 27°05′ S, 152°45′ E, 500 m, 1986, Hafellner 41806 & Stevens (hb. Hafellner).
Didymocyrtis melanelixiae (Brackel) Diederich, Harris & Etayo comb. nov. (Fig. 7a–e)
Didymocyrtis melanelixiae (a–e) and D. pseudeverniae (f–h). a ascomata (right) and conidiomata (left) on Punctelia rudecta (Harris 57475); b conidiomata on Cetrelia (Diederich 9177); c–d asci with ascospores (c in water; d in KOH; Etayo 20035); e conidia (in water; Beeching 15964). f D. pseudeverniae conidiomata on Pseudevernia furfuracea (Etayo 12600); g section through conidioma; h conidia (g–h: in water; Diederich 16755). Scale bars: a–b = 500 μm; f = 200 μm; c–d, g–h = 10 μm; e = 5 μm
Phoma melanelixiae Brackel, Herzogia 24: 81 (2011). Type: Italy, Basilicata, Prov. di Potenza, Monte Vulture, near “Femmina Morta”, 40°57′24″ N, 15°37′17″ E, 1145 m, in mixed forest of oaks, elders and maples, on the bark of Acer pseudoplatanus, on Melanelixia glabra, 22 Aug. 2010, W. & G. v. Brackel 5658 (M – holotypus, non vid.). MycoBank: MB 814027.
Ascomata dispersed, developing in necrotic areas of the host thallus, visible as black dots often provided with paler apical dots indicating the ostioles. Ascoma in longitudinal section subspherical or flattened with protruding ostiolar region, 200–250 μm diam.; wall brown, laterally c. 15–20 μm diam., consisting of about 3–4 layers of cells, cells 5–10 μm diam. Paraphysoids 1–2.5 μm diam. Asci 70–100 × 7.5–10.5 μm, 8-spored (but often with fewer spores reaching maturity). Ascospores ± uniseriate to half-overlapping, medium to dark brown, (1–)2(–3)-septate, 11.5–15 × 4–5.5 μm, the middle cell slightly wider than the end cells, slightly constricted at the septa, torus indistinct, with a distinct verruculose sculpture visible in light microscopy.
Conidiomata immersed in pale necrotic areas of the thallus surrounded by a black line, black, subspherical to ellipsoid, 50–100 μm diam.; pycnidial wall brown, dark brown around the ostiole, 9–15 μm thick, composed of 3–5 layers of cells 3–9 μm diam., inner cells hyaline. Conidiogenous cells ampulliform, mainly 5–9 × 3–4.5 μm. Conidia broadly ellipsoid to subspherical, with one large guttule or rarely two smaller guttules, (3.5–)3.8–5.1(–6.2) × (2.8–)3.2–3.8(–4.3) μm, l/b ratio (1.0–)1.1–1.5(–1.8) (n = 140).
Distribution and hosts. Europe (Belgium, France, Italy, Spain, Switzerland, United Kingdom: Scotland), North America (Canada: British Columbia and New Brunswick, USA: Georgia, Maine and North Carolina), South America (Ecuador) and Indian Ocean (La Reunion); on the thallus of Parmeliaceae, including Canoparmelia, Cetrelia, Hypotrachyna, Melanelixia, Parmelia s. str., Parmotrema, Platismatia, Pseudevernia, Punctelia and Usnea.
Notes. 1. An unknown Didymocyrtis sexual stage had been collected twice on Punctelia in the USA, accompanied by a Phoma-like asexual stage. A similar Phoma-like fungus had been collected on Cetrelia in the same locality as one of the two sexual specimens. DNA sequences have shown that both the sexual and asexual stages, collected on Punctelia and Cetrelia, belong to the same, previously unsequenced species. Subsequently, two further sexual specimens on Melanelixia and Punctelia became available from Spain and Ecuador. Examination of unidentified Phoma-like specimens on diverse parmelioid hosts eventually resulted in a larger number of specimens. Amongst the hitherto described lichenicolous Phoma species, Phoma melanelixiae has been described on a parmelioid host, and the description perfectly fits the Phoma-like asexual stage studied by us. Although neither the type of P. melanelixiae, nor any other material from the same host genus, Melanelixia, has been sequenced, we believe that there is little doubt that all the material studied by us belongs to P. melanelixiae, especially after the recent discovery of the sexual stage on Melanelixia glabra in Spain, and the new combination in Didymocyrtis is consequently proposed here. A further specimen from Italy on Parmotrema crinitum, published by von Brackel (2015)) as Phoma cf. melanelixiae, with a detailed description and illustrations, clearly fits our new species concept of D. melanelixiae.
2. The sexual stage of the new species is distinguished from Didymocyrtis ramalinae by the smaller and mostly 2-septate ascospores. The asexual stage is distinguished from most lichenicolous Phoma-like species by the relatively broad conidia having a single relatively large guttule, often filling more than half of the cell content. Measurements of numerous conidia from all specimens have shown that while the conidial length is rather variable, the breadth is more constant, so that the shape ranges from subspherical to broadly ellipsoid.
3. An undescribed species of Didymocyrtis, here treated as D. aff. consimilis, has been collected by one of us on Melanohalea exasperatula (sexual stage: Berger 26876; asexual stage: Berger 27218). That species is genetically only distantly related to D. melanelixiae, and the sexual stage is easily distinguished by the 1-septate ascospores. The asexual stage, however, is rather similar to that of D. melanelixiae, and further study is needed to better circumscribe the species on Melanohalea and to identify if any of the asexual specimens now included in D. melanelixiae are, in fact, the undescribed species.
4. Xenophoma puncteliae (Diederich & Lawrey) Crous & Trakunyingchaeroen (= Phoma puncteliae Diederich & Lawrey), described from Punctelia rudecta in the USA, differs by smaller pycnidia, 40–60 μm diam., shorter conidiogenous cells, 2.5–5 × 3.5–4.5 μm, and much smaller conidia, 2.5–3 × 2–2.4 μm (Lawrey et al. 2012). The species is known only in the asexual stage.
5. Following the original description of Phoma fistulata Etayo and Osorio (2004), this species resembles Didymocyrtis melanelixiae. However, re-examination of an isotype (hb. Etayo) showed that the pycnidial wall is very different (composed of well-delimited cells with an irregularly formed margin, similar to pieces of a jigsaw puzzle); the conidiogenesis is difficult to study, but certainly different (no ampulliform or ellipsoid conidiogenous cells were observed); conidia are embedded in a gelatinous matrix (they are free-swimming in microscopical preparations of Didymocyrtis species); and the typical guttulation of Didymocyrtis conidia could not be observed. As the current specimen is too old to be cultured and sequenced, we can not take a decision on the taxonomic position of this species.
Additional specimens examined: Sexual stage: Ecuador: Prov. Tungurahua, Baños de Agua Santa, sendero de la cascada del Bascún, 1760 m, on saxicolous Punctelia rudecta, 1999, Etayo 20035 & Palice (QCA, hb. Etayo). Spain: Navarra, Carretera de Zabalza a Adoain, N-2103, despoblados de Urraúl, viejo camino de Aspurz, on Melanelixia glabra, 2015, Etayo 29169 (hb. Etayo). USA: Georgia, Murray Co., Chattahoochee National Forest, Cohutta Wilderness, Tearbritches Trail from Forest Serv. Rd. 68 to Bald Mountain, 34°52′ N, 84°38′ W, 1100–1200 m, on Punctelia appalachensis, 1992, Buck 21688 (NY). Maine, Hancock Co., Township of T28 MD, Lead Mountain (Humpback), along trail from parking area to water catchment area, 44°51′28″ N, 68°05′52″ W, on P. rudecta, 2012, Harris 57475 (NY, hb. Diederich).
Asexual stage (sequenced specimens): USA: Maine, Hancock Co., Township of T28 MD, Lead Mountain (Humpback), along trail from parking area to water catchment area, 44°51′28″ N, 68°05′52″ W, on P. rudecta, 2012, Harris 57475-B, 57476 (NY); ibid., on Cetrelia olivetorum, Harris 57465 (NY).
Asexual stage (non-sequenced specimens, provisionally included in this species; specimens Harris 58925 and Buck 63062 examined by R.C. Harris): Belgium: Etalle, SE of Buzenol, vallée de la Rouge-Eau, en aval du passage de la route Etalle – St-Léger, on Platismatia glauca, 1984, Diederich 5550 (hb. Diederich). Canada: British Columbia, Wells Gray Provincial Park, Clearwater valley, 26 km N of Clearwater, near Kingfishers Wood Cottages, 51°51′23″ N, 120°00′52″ W, 730 m, on corticolous Parmelia sulcata, 2008, Diederich 17272 & Ertz (hb. Diederich). New Brunswick, Albert County, Fundy National Park, Maple Grove Trail, 45°35′23″ N, 64°59′01″ W, 225 m, on P. rudecta, 2013, Harris 58925 (NY). France: Pyrénées-Atlantiques, SW of Larrau, near road to Spain, on C. olivetorum, 1990, Diederich 9177 (hb. Diederich). Pyrénées-Atlantiques, à 1 km du Col d’Ibardin, La Redoute des Emigrés, on Parmotrema reticulatum, 1995, Etayo 12734 (hb. Etayo). La Reunion: Cilaos, Forêt du Grand Matarum, sentier vers le Piton des Neiges, 21°07′13″ S, 55°29′03″ E, 1550 m, on Usnea, 2012, Ertz 18043 (BR). Switzerland: Valais, SE of Les Haudères, forêt de Tauge, on Pseudevernia furfuracea, 2012, Diederich 17328 (hb. Diederich). United Kingdom: Isle of Skye, Broadford, wood near the coast N of Isle Ornsay (NG 70 14), on Hypotrachyna taylorensis, 1987, Diederich 8793 (hb. Diederich). USA: Georgia, Dekalb Co., Arabia Mt. Park, Vaughter’s Farm, on Canoparmelia texana, 2015, Beeching 15964 (hb. Beeching). North Carolina, Camden Co., North River Game Land, E end of Indian Island, N of Indian Island Rd./Sassafras Lane, 3 mi E of Sandy Hook Rd., 36°17′08″ N, 75°59′27″ W, 1 m, on P. rudecta, 2012, Harris 57318-A (NY). North Carolina, Gates Co., Chowan Samp Game Land, E shore of the Chowan River, Buck Rd. 0.9 mi NW of Sandbanks Rd. (CR 1200), 7 mi N of US 13 in Storys, 36°31′15″ N, 76°54′09″ W, 2 m, on Quercus, on Parmotrema submarginale, 2012, Harris 57985 (NY). North Carolina, Dare Co., Alligator River National Wildlife Refuge, W of Whipping Creek Road, 0.5 mi N of jct w/ Chip Road, 35°40′31″ N, 75°57′45″ W, 1 m, on P. rudecta, 2014, Buck 63062 (NY).
Didymocyrtis pseudeverniae (Etayo & Diederich) Ertz & Diederich comb. nov. (Figs. 7f–h and 9e–f)
Macrophomina pseudeverniae Etayo & Diederich, Mycotaxon 60: 419 (1996); Diederichia pseudeverniae (Etayo & Diederich) D. Hawksw., Lichenologist 35: 206 (2003). Type: Spain, Huesca, road Sabiñánigo to Boltaña, Perto del Serrablo, Sierra del Gabardón, km 39 near Laguarta, 1200 m, on Pseudevernia furfuracea, 5 July 1993, J. Etayo 12597 (MA-Lich – holotype; hb. Etayo – isotype, non vid.). MycoBank: MB 814028
Ascomata unknown.
Conidiomata immersed in pinkish necrotic areas of the host thallus, dark brown to black, subspherical, 130–170 μm diam.; pycnidial wall brown, inner cells paler, darker near the ostiole. Conidiogenous cells ampulliform, 7–10 × 6–7 μm. Conidia subcylindrical, ellipsoid or irregular in form, with numerous small guttules, (14–)16–22(–26) × 6–9 μm [from Etayo and Diederich 1996].
For further illustrations, see Etayo and Diederich (1996).
Distribution and hosts. Europe (Great Britain, Italy, Latvia, Lithuania, Spain, Switzerland), on Pseudevernia furfuracea (von Brackel 2013; Etayo 2004; Etayo and Diederich 1996; Hitch 1997; Motiejūnaitė and Grochowski 2014; Motiejūnaitė et al. 2011).
Note. This species is distinguished by the particularly large conidia that are multiguttulate, contrary to most other Didymocyrtis species, which typically have 1–2-guttulate conidia.
Specimens examined (all on Pseudevernia furfuracea): Spain: Aragón, Huesca, Araguës del Puerto, 1991, Etayo 770 (hb. Diederich). Sierra de Illón, monte Belbún, Navascués, 2 km from Castillonuevo, 1994, Etayo 12600 (hb. Diederich). Switzerland: Valais, Evolène, Les Haudères, Forêt de Tauge, 2008, Diederich 16755 (hb. Diederich). Valais, 3 km S of Haute-Nendaz, road to Siviez, between main road and Planchouet, 2012, Diederich 17327, 17338 (hb. Diederich).
Didymocyrtis ramalinae (Roberge ex Desm.) Ertz, Diederich & Hafellner comb. nov. (Figs. 8a–d and 9i)
Didymocyrtis ramalinae (a–d) and Leptosphaeria protousneae (e–j). a Ascomata on Ramalina fastigiata (Santesson Fungi Lich. Exs. 318); b conidiomata on R. fastigiata (Van den Broeck 2900); c asci and ascospores (in water; Paul 27i2013); d conidia (in water; Van den Broeck 2900). e–j Leptosphaeria protousneae on Protousnea (isotype, hb. Etayo); e immersed ascomata; f ascomatal view in surface view showing isodiametric cells (in KOH); g id., with elongate cells; h hamathecium (in KOH); i–j asci (in KOH). Scale bars: a–b, e = 200 μm; f–j = 10 μm; c–d = 5 μm
Cultures of Didymocyrtis species. a D. consimilis (Freebury 1357); b D. consimilis conidia (Freebury 1357); c D. aff. consimilis on Cladonia pocillum (Ertz 17617b); d D. aff. consimilis on Melanohalea exasperatula (Berger 26876); e D. pseudeverniae (Diederich 17327); f D. pseudeverniae conidia (Diederich 17338); g D. epiphyscia s. lat. on Physcia tenella (Ertz 17615); h D. epiphyscia s. lat. on Xanthoria parietina (Gardiennet 12008); i D. ramalinae (Van den Broeck 2900). Scale bars: c = 1 cm; g = 5 mm; e, h = 2 mm; a, d = 1 mm; i = 500 μm; b, f = 10 μm
Sphaeria ramalinae Roberge ex Desm., Ann. Sci. Nat., Bot., 3. sér., 11: 354 (1849) ; Leptosphaeria ramalinae (Desm.) Sacc., Syll. Fung. 2: 84 (1883); Heptameria ramalinae (Desm.) Cooke, Grevillea 18 (86): 33 (1889); Phaeospora ramalinae (Desm.) Vouaux, Bull. Soc. Mycol. France 29: 74 (1913). Type: France, without locality and date, on Ramalina fastigiata (thallus), Roberge (?PC – holotypus, non vid.) MycoBank: MB 814029.
Syn. nov.: Phoma ficuzzae Brackel, Sauteria 15: 109 (2008); Diederichomyces ficuzzae (Brackel) Crous & Trakunyingcharoen, IMA Fungus 5(2): 401 (2014). Type: Italy, Sicily, Prov. Palermo, Bosco della Ficuzza, road from Ficuzza to S, 37°52′00.6″ N, 13°23′17.3″ E, 910 m, in grazed coppice forest mainly of oaks and ashes, on the bark of Pyrus amygdaliformis, on Ramalina fraxinea, 9 Aug. 2006, Brackel (hb. IVL 3983 – holotype; M-0044890 – isotype, non vid.). MycoBank: MB 519643.
? Syn.: Pyrenidium ucrainicum S. Y. Kondr., L. Lőkös et J.-S. Hur, Acta Bot. Hung. 56: 364 (2014). Type: Ukraine, Crimean AR, Sudak town, “Novy Svit” Botanical Reserve in the vicinity of Novy Svit settlement, SSW slopes of Mt Sykht-Lar, on bark of Juniperus excelsa, in thalli of Ramalina sp., 44°49′19″N, 34°55′05″E, ca 153 m alt., 17 Nov. 2013, Kondratyuk 21324 & Virchenko (KW-L 70284 – holotype; non vid. [no answer from KW]).
Ascomata dispersed, immersed in the apothecial margins, the hymenium and the gradually bleaching branches of the host thallus, visible as black dots, often with paler apical dots indicating the ostioles, more rarely on the apothecial disk and then almost sessile. Ascoma in longitudinal section subspherical with protruding ostiolar region, c. 200–300 μm diam.; wall brown, laterally c. 10–20 μm diam., consisting of c. 3–4 layers of cells. Paraphysoids 1–1.5 μm diam. Asci 90–110 × 9–12 μm. Ascospores ± uniseriate to half-overlapping, dark brown, 3-septate, some only with 1 or 2 septa (<1 %), (13–)14–20 × 5–6.5(–7) μm, the upper half spore (namely second cell) slightly wider than the lower one, not or slightly constricted at the septum, torus indistinct, with distinct verruculose sculpture visible in light microscopy.
Conidiomata immersed in pale necrotic areas of the thallus or apothecial margin surrounded by a black line, black, subspherical, 105–135 μm diam.; wall brown, dark brown around the ostiole, 7–10 μm thick, composed of 2–4(–5) layers of cells 8–10 × 3–4.5 μm, inner cells hyaline. Conidiogenous cells short-ampulliform to subglobose, mainly 6–7.5 × 4–5.5 μm. Conidia ellipsoid, biguttulate, with a small guttule near each apex, 5–7 × 3–4 μm, l/b ratio 1.5–2 [from von Brackel 2008a and our own observations].
For further descriptions and illustrations of the asexual stage, see von Brackel (2008a) and Lawrey et al. (2012); for the sexual stage, see Vouaux (1913).
Distribution and hosts. Europe (e.g. Denmark, France, Great Britain, Italy, Lithuania, Luxembourg, Poland, Portugal, Spain), Africa (Canary Islands, South Africa), and Australasia (Australia). The sexual stage has been recorded on Ramalina calicaris, R. celastri, R. exiguella, R. farinacea, R. fastigiata, R. lacera, R. pollinaria, R. subgeniculata and Ramalina sp., and the asexual stage on R. fastigiata and R. fraxinea (e.g., Alstrup et al. 2004; van den Boom and Giralt 2012; von Brackel 2008a; Diederich 1990; Etayo 1996a, b, 2010b; Hafellner 1996; Hitch 2011; Kukwa et al. 2013; Lawrey et al. 2012; Motiejūnaitė et al. 2011, 2012; Vouaux 1913).
Notes. 1. The conidial size is rather variable in this species. In the original description (von Brackel 2008a), conidia were given as (5.5–)5.9–6.8(–7.6) × (3.1–)3.5–3.9(–4.2) μm, l/b ratio (1.4–)1.6–1.9(–2.1) (n = 40). In specimen Van den Broeck 2900 from France, conidia were smaller, (4.8–)4.9–5.4(–5.8) × (3.2–)3.2–3.6(–4.0) μm, l/b ratio (1.3–)1.4–1.6(–1.8). In a culture obtained from Van den Broeck 2900, conidia were (5.0–)5.6–6.4(–6.7) × (2.8–)2.9–3.5(–3.8) μm, l/b ratio (1.3–)1.7–2.1(–2.2). Similarly, the pycnidial diameter is variable, 105–135 μm in von Brackel (2008a)) and 40–70 μm in Van den Broeck 2900, but similar to Brackel’s measurements in a culture obtained from Van den Broeck 2900. Lawrey et al. (2012) concluded that the species is probably much more variable than the few specimens examined suggest.
2. Leptosphaeria protousneae Etayo (Fig. 8e–j) was described from Chile and is known only from the type locality, where it was found growing as a parasite on the lacinia of the lichen genus Protousnea (Etayo and Sancho 2008; known only from sexual stage). L. protousneae closely resembles Didymocyrtis ramalinae, apart from the perithecial wall cells which were described as elongate in L. protousneae and isodiametric in D. ramalinae. We, however, found that this character is variable in all Didymocyrtis species. The asci and ascospores were also reported to be smaller in D. ramalinae, but we have found that the spore dimensions are rather variable within our sequenced specimens of this species, sometimes within a single specimen. The perithecia in L. protousneae are breaking through fissures in the cortex while in D. ramalinae, the perithecia usually remain immersed in the thallus, but this might be the results of the thin laciniae of the Protousnea host thallus. Although we were unable to find any substantial morphological differences between the taxa, due to the remote geographical distribution of L. protousneae and the different host genus, we prefer to wait for molecular data and more material from South America before deciding on a possible synonymy with Didymocyrtis ramalinae.
Selected specimens examined (all on Ramalina): Sexual stage: Australia: Queensland, Tandora about 25 km ENE of Maryborough, 25°27′ S, 152°52′ E, sea level, on R. exiguella, 1986, Hafellner 18174 & Rogers (GZU). Canary Islands: Tenerife, Macizo de Anaga, Monte de las Mercedes, NE ober Las Mercedes bei der Abzweigung nach Llano de los Viejos, 28°31′30″ N, 16°17′ W, 760 m, auf Ramalina sp., 1989, Hafellner 35107 (hb. Hafellner). France: Corsica, N d’Ajaccio, côte sud du Golfe de Sagone, route à l’est du village de Pevani, Capu di u Monte, 355 m, 42°01′11″ N, 8°43′13″ E, rocher siliceux exposé, sur Ramalina sp., 2011, Ertz 16399 (BR). Great Britain: Scotland, VC 95, Moray, Brodie, SE of Plyon Cott, on R. fastigiata on Crataegus in hedgerow, 18 m, Grid NH973.577, 13 i 2013, Paul s.n. (E); ibid., Forres, Beech Road, on R. fastigiata on Acer pseudoplatanus, 20 m, Grid NJ04020.57892, 27 i 2013, Paul s.n. (E). Italy: Basilicata, Prov. Potenza, NW-Abhänge der Coppola di Paola SE von Rotonda, 1400 m, on R. fastigiata, 1979, Hafellner 41837 (hb. Hafellner). Luxembourg: Hoscheid, along road, on R. fastigiata, 1967, Van Wersch (hb. Diederich). South Africa: Cape Province, south coast, about 48 km E of Pletttenberg Bay, Tsitsikamma Coastal National Park, along the ‘Loerie Trail’ N of the camp, 34°30′ S, 23°53′ E, on Ramalina sp., 1987, Wetschnig & Wetschnig (GZU). Spain: Castello Prov., Morella, Els Campbello, 40°37′ N, 0°06′ W, 1000 m, on R. fastigiata, 1994, Calatayud, Santesson Fungi Lich. Exs. 318 (hb. Diederich). Sweden: Bohuslän, Lysekil commune, Skaftö par., Islandsberg, 1 km S of Grundsund, on trail to Islandsbergs Huvud, c. 50 m, on R. fastigiata, 1992, Hafellner 30485 (hb. Hafellner).
Asexual stage (all on Ramalina): France: Pas-de-Calais, Equihen-Plage, dunes d’Escault, 50°39′55″ N, 1°34′37″ E, on Acer, on R. fastigiata, 2009, Van den Broeck 2900 (BR, hb. Diederich) [culture JL334-09, CBS 128019]. Great Britain: Scotland, VC 95, Moray, Earlsmill, by Muckle Burn, on R. fastigiata, 34 m, Grid NH972.562, 10 i 2013, Paul s.n. (E).
Didymocyrtis slaptoniensis (D. Hawksw.) Hafellner & Ertz comb. nov. (Figs. 5j–l and 6)
Polycoccum slaptoniense D. Hawksw., Lichenologist 26(4): 342 (1994). Type: Great Britain, England, South Devon, Slapton, Slapton Ley National Nature Reserve, east end of Duck Marsh near The Causeway, on Sambucus nigra, on Xanthoria parietina, 2 Oct. 1993, D. L. Hawksworth (IMI 359711 – holotype, non vid., fide Hawksworth 1994). MycoBank: MB 814030.
Ascomata usually scattered, sometimes in small groups, developing in the hymenium, the apothecial margin and the thallus (when on the thallus, ascomata protruding and surrounded by a thallus collar; when immersed in the hymenium they protrude very little and lack a collar; the thalline collar and the nearest parts of the host thallus may turn purple-red making the infection spot rather conspicuous, with only the ostiolar region becoming exposed and visible as black, somewhat protruding dots). Ascoma in longitudinal section globose to broadly oval, (150–)200–300 μm diam.; wall dark red-bown, laterally c. 15–20 μm diam., up to 40 μm towards the apex, consisting of c. 4–5 layers of cells. Paraphysoids 1.5–2 μm diam. Asci (80–)90–110 × 7–10 μm. Ascospores ± uniseriate, pale brown, 1-septate, (11–)13–15 × (5.5–)6–7 μm, with verruculose sculpture and with torus protruding on the outside in semimature spores (torus of mature spores not protruding any further).
Conidiomata of supposed asexual stage on the thallus and occasionally in the hymenium, immersed when young, later protruding. Pycnidia developing on the thallus remain obtected by a thin layer of host plectenchyma forming a more or less distinct collar, very similar to teleomorphic pseudothecia but smaller. Pycnidia in longitudinal section more or less globose, 80–120(–150) μm diam.; wall brown throughout and c. 10 μm diam. Conidiogenous cells ellipsoid to flask-shaped, hyaline, 8–10 (–12) × 5–6 μm. Conidia ellipsoid to oblong, many somewhat bent, (5–)6–8(–9) × 2.5–3.5 μm, with two small guttules near the ends, l/b ratio c. 2.
For further descriptions and illustrations of the sexual stage, see Hawksworth (1994).
Distribution and hosts. Europe (Austria, Belgium, England, France, Germany, Italy, Liechtenstein, Luxembourg, Portugal, Switzerland), on the thallus and apothecia of Xanthoria parietina (van den Boom and Giralt 2012; von Brackel 2008b, 2009; Diederich et al. 2014; Ertz et al. 2008; Gardiennet 2012; Hawksworth 1994; Kocourková and von Brackel 2005; Roux et coll. 2014).
Note. Sometimes ascomata of Didymocyrtis slaptoniensis and Phoma-like pycnidia can be observed on the same specimen (e.g., Obermayer 13130). This supposed Phoma-like anamorph (Fig. 5j, l) fits the description of Phoma epiphyscia Vouaux, described from northern France. Vouaux (1914) mentioned two host species in the protologue, namely Xanthoria parietina and Phaeophyscia orbicularis. As the type is not among the remnants of the Vouaux herbarium (Rondon 1969) and is apparently lost, Alstrup and Hawksworth (1990) designated a neotype originating from Greenland and infesting Phaeophyscia sciastra. As the conspecificity of the fungi growing on Phaeophyscia and that upon Xanthoria parietina is only speculative (no comparable teleomorph is known on Phaeophyscia), we hesitate to use Vouaux’s name for the fungus growing on Xanthoria.
Selected specimens examined (all on Xanthoria parietina): Sexual stage: Austria: Steiermark, Oststeirisches Riedelland, 5.2 km east of the centre of Graz, Ragnitz, 50 m S of the brook Ragnitzbach (Grazbach), 47°04′24″ N, 15°30′26″ E, 405 m, GF 8958/2, 2014, Obermayer 13130 (GZU) (with asexual stage). Belgium: Hermeton-sur-Meuse, section de 1.5 km de la vallée de l’Hermeton en amont de la confluence avec la Meuse, 100 m, 2005, Ertz 9057 & Duvivier (BR). France: Côte d’Or, Véronnes, Combe du Chatelet, vers la chapelle Sainte-Anne, 2012, Gardiennet 12009 (hb. Gardiennet). Ardennes, Fumay, rive droite de la Meuse, entre la carrière du Pont de Fumay et l’ancienne ardoisière de Follemprise, Mora (BR). Rhône-Alpes, Haute-Savoie, Western Alps, Bornes Massif (Le Massif des Bornes), Burzier NW above Sallanches, SE below the parking area at Route de Doran, 45°57′22″ N, 6°36′42″ E, 935 m, 2011, Hafellner 82564, 82565 (GZU) (with asexual stage). Liechtenstein: Eastern Alps, Rätikon, W below the village Triesenberg by the road to Vaduz, 47°06′45″ N, 09°32′30″ E, 750 m, 2008, Hafellner 83057 (GZU). Luxembourg: NW von Ahn, Verbuschung am Wegrand westlich vom Kräizuet, 2014, Cezanne & Eichler 9590 (hb. Diederich) Switzerland: canton Bern, municipality of Wengi, 6 km E of Lyss, N of Äschwald (0.6 km NE of the pond Golihuebweiher), 47°05′00″ N, 07°22′53″ E, 488 m, 2014, Berger & Zimmermann (GZU).
Asexual stage: Austria: Burgenland, Südburgenland, Güssing, Schlossberg, Schlossplatz, 47°03′29″ N, 16°19′27″ E, 310 m, 2012, Berger 26196 (hb. Berger). Steiermark, Nordalpen, Nördliche Kalkalpen, Hochschwab-Gruppe, Seetal W von Seewiesen, 10 km NE von Aflenz, 47°37′15″ N, 15°15′20″ E, 930 m, GF 8357/4, 2011, Hafellner 78042, 78044 (GZU).
Didymocyrtis xanthomendozae (Diederich & Freebury) Diederich & Freebury comb. nov.
Phoma xanthomendozae Diederich & Freebury, in Lawrey et al., Fungal Diversity 55: 208 (2012). Type: Canada, Quebec, Les Collines-de-l’Outaouais RCM, Gatineau Park, near Wakefield, grassy ditch beside Route 05, 45°37.8′ N, 75°56.4′ W, on fallen Salix, on Xanthomendoza hasseana, 3 May 2010, C. Freebury 1122 (CANL – holotypus; hb. Diederich – isotypus!). Ex-type culture: JL451-10, CBS 129666. MycoBank: MB814031.
Ascomata rare, macroscopically undistinguishable from pycnidia (see description below); globose to broadly oval, 180–250 μm diam.; wall dark red-brown, 20–35 μm diam., consisting of c. 4–5 layers of cells. Paraphysoids 1.5–2.5 μm diam. Asci c. 75–110 × 8–9.5 μm. Ascospores ± uniseriate, pale brown, 1-septate, (10.5–)11–13(–14) × (5.5–)6–7(–7.5) μm (n = 20), with verruculose sculpture and with torus protruding on the outside in semimature spores (not protruding further in mature spores).
Conidiomata immersed in the host apothecia, more rarely in the host thallus, black, subspherical, c. 140–160 μm diam.; pycnidial wall brown, 14–17 μm thick, composed of 3–5 layers of cells, outer cells dark brown, 5–7.5 μm diam., inner cells pale brown. Conidiogenous cells elongate ampulliform to subcylindrical, 5–10 × 2.5–3.5 μm. Conidia ellipsoid, (1–)2-guttulate, (4.5–)5.6–7.1(–8.6) × (2.9–)3.3–4.3(–4.6) μm, l/b ratio (1.2–)1.4–2.0(–2.5) (n = 60) [from Lawrey et al. 2012].
For further descriptions and illustrations of the asexual stage and of cultures, see Lawrey et al. (2012).
Distribution and hosts. The species is known from Canada (Quebec and Saskatchewan); it inhabits apothecia and more rarely the thallus of corticolous Xanthomendoza hasseana and X. montana, and eventually kills the host (Lawrey et al. 2012).
Notes. 1. This species was initially described in Phoma, as only pycnidia had been observed. However, a few perithecia have since been observed in one of the specimens, and these are typical for Didymocyrtis.
2. Phylogenetically, this species is very close to Didymocyrtis slaptoniensis, which also grows on a related host, Xanthoria parietina, and therefore we wondered if they were conspecific. However, we decided to treat them as distinct, for several reasons. (1) In Europe, D. slaptoniensis appears to be strongly host specific, inhabiting a single species of the genus Xanthoria, whilst D. xanthomendozae is known only from North America on Xanthomendoza species; (2) ascospores of D. slaptoniensis (mainly 13–15 × 5–7 μm) are longer than those of D. xanthomendozae; (3) pycnidia of D. slaptoniensis are much smaller in diam. than those of D. xanthomendozae; (4) conidia in D. slaptoniensis (mainly 6–8 × 2.5–3.5 μm) are slightly longer and distinctly narrower than those of D. xanthomendozae; (5) conidiogenous cells in D. slaptoniensis (ellipsoid, flask-shaped, mainly 8–10 × 5–6 μm) are much broader than those of D. xanthomendozae, which are mainly subcylindrical.
Sexual stage specimen examined: Canada: Quebec, Les Collines-de-l’Outaouais RCM, Gatineau Park, 45°37′56″ N, 75°56′26″ W, on Xanthomendoza hasseana, 2011, Freebury 1413, 1415 (CANL).
Trypetheliales Lücking, Aptroot & Sipman
in Aptroot et al., Bibl. Lichenol. 97: 13 (2008). Type: Trypethelium Spreng. MycoBank: MB 533049.
Notes. 1. The order Trypetheliales was described to accommodate the single family Trypetheliaceae, a group of mainly lichenized ascomycetes placed in Dothideomycetes in a molecular phylogeny by Del Prado et al. (2006). Species in this order are characterized by perithecioid ascomata appearing solitary or aggregated in pseudostromata, which have either separate ostioles or with ascomatal chambers laterally fused to share a common ostiole, a dark brown to carbonized perithecial wall, branched and anastomosing paraphysoids embedded in a distinct gelatinous matrix, bitunicate asci, and hyaline or rarely brown ascospores with variously developed endospore thickenings that give the lumina a diamond-shaped or more rarely a round outline (Aptroot et al. 2008; Hyde et al. 2013; Nelsen et al. 2014). The ascospores may be muriform or transversely septate.
2. In the present study, we introduce the new family Polycoccaceae for the genera Polycoccum and Clypeococcum. We provisionally place it in the order Trypetheliales based on molecular and morphological data. Polycoccaceae species differ from those of Trypetheliaceae by their non-lichenized, lichenicolous habit, and 1-septate, brown, usually smaller ascospores; but they are otherwise similar in having perithecioid ascomata, branched and anastomosing paraphysoids in a hymenial gel matrix, and bitunicate asci.
Polycoccaceae Ertz, Hafellner & Diederich, fam. nov.
Type: Polycoccum Saut. ex Körb., Parerga Lich.: 470 (1865). MycoBank: MB 814032
Lichenicolous fungi, belonging to the Trypetheliales. Ascomata arising singly, often becoming grouped, sometimes united by a clypeus or enclosed in galls, immersed with only the ostiole visible to erumpent and the upper half exposed when mature, perithecioid, subglobose to obpyriform, dark brown to black, ostiolate, neck not extended and scarcely distinguishable from the ascomatal wall; wall even in thickness or somewhat broader near the ostiole, composed of 3–6 layers of polyhedral pseudoparenchymatous cells, radially compressed in vertical section and roughly isodiametric in surface view, forming a textura angularis; outer layers of cells with ± evenly thickened walls, brown to dark brown and continuing below the centrum; inner layers less intensely pigmented to hyaline with thin-walled cells. Hamathecium composed of a branched and anastomosing net of thin and regularly septate to remotely septate narrow hyphal filaments, probably trabeculate pseudoparaphyses (paraphysoids); periphyses absent; hymenial gel I+ blue to violet or unchanged. Asci broadly cylindrical to subclavate, shortly stalked, bitunicate, fissitunicate, wall thicker in the upper part of mature asci, with a small internal apical beak, 2–8-spored. Ascospores irregularly distichously arranged in the asci, ellipsoid, 1-septate (euseptate); cells ± equal in size or the upper cell larger and broader, apices generally rounded or rarely attenuated, somewhat constricted at the septum, brown to dark brown when mature, smooth or delicately verruculose, sometimes with a gelatinous sheath.
Note. The family currently includes the genera Clypeococcum and Polycoccum. It is the sister group to Trypetheliaceae in our molecular phylogeny using nuLSU sequences (Fig. 1).
Polycoccum Saut. ex Körb. (Fig. 10)
Morphological features of the genus Polycoccum. a–d, Polycoccum trypethelioides (a galls on Stereocaulon with immersed ascomata; b section through ascoma; c asci with ascospores; d ascospores; in water; a Goward 95-282; b–d Diederich 17508). e–g P. vermicularium (e section through ascomata in gall; f ascus; g ascospores; in water; Diederich 17545). h–m P. pulvinatum (h galls with immersed ascomata on Physcia caesia; i–j section through ascomata; k ascus; l–m ascospores; i–l in water; m in KOH; Diederich 17389). Scale bars: h = 1 mm; a = 200 μm; b, e, i–j = 50 μm; c–d, f–g, k–m = 5 μm
Parerga Lich.: 470 (1865). Type: Polycoccum sauteri Körb. [= Polycoccum trypethelioides (Th. Fr.) R. Sant., fide Santesson 1960]. MycoBank: MB 4309.
Syn.: Lophothelium Stirt., Scott. Naturalist 9: 37 (1887). Type: Lophothelium acervatum Stirt. [= Polycoccum trypethelioides (Th. Fr.) R. Sant., fide Hawksworth and Diederich 1988]. MycoBank: MB 2945.
Note. The core group of Polycoccum is characterized by thick-walled, often distinctly ornamented ascospores. Further characters of Polycoccum s. str. are the formation of ascomata on the host thallus, a tendency to induce the formation of galls, and certain features of the ascomatal wall (cells ± isodiametric in longitudinal section), the hamathecium (consisting of relatively thick paraphysoids), the ascus (broadly cylindrical to subclavate, generally with irregularly distichously arranged ascospores). Variations exist; for example, the ascospores of the generic type, Polycoccum trypethelioides, are often irregularly uniseriate.
Polycoccum trypethelioides (Th. Fr.) R. Sant. (Fig. 10a–d)
Svensk Bot. Tidskr. 54: 505 (1960). For synonyms see Hawksworth and Diederich (1988). Type: Norway, Troms Prov., Tromsö, on Stereocaulon alpinum, 1857, Fries (UPS, holotype, non vid.) (fide database of UPS herbarium). MycoBank: MB 119595.
Ascomata immersed in subglobose galls on the thallus of the host, 150–250 μm diam. Paraphysoids 1.5–2.5 μm wide. Asci elongate-clavate, (75–)80–100 × (14–)16–19 μm, 8-spored. Ascospores overlapping uni- to biseriate, broadly ellipsoid to soleiform (the upper cell much larger in size, the lower only about 1/3 the length of the spore), brown to dark brown, smooth (according to Hawksworth and Diederich 1988) or verruculose (according to Orange 1990), thick-walled, 14–22 × 8–10 μm.
Conidiomata described by Zhurbenko (2010) as ‘pycnidial, black, subglobose, ostiolate, 50–100 μm diam., immersed to erumpent, crowded and intermixed with the ascomata. Conidiogenous cells holoblastic, arising from the pycnidial wall, subcylindrical to elongate-ampulliform and strongly tapered towards the apex, with 1–2 annelations, c. 14–15 × 2.5–3.5 μm; conidia simple, (narrowly) oblong to elliptic, with rounded apex and truncated base, hyaline, smooth-walled, (3.5–)4–5(–7) × 1.5–2 μm, l/b = (1.8–)2.3–3.4(–4.7) (n = 47).
For further descriptions, illustrations and synonymy, see Hawksworth and Diederich (1988) and Zhurbenko (2010).
Distribution and hosts. Known from Europe (Austria, British Isles, Canada, Finland, Germany, Iceland, Norway, Russia, Sweden) and North America (Canada, Greenland, USA) (Alstrup 1981; Alstrup and Cole 1998; Alstrup et al. 2008; von Keissler 1930; Hawksworth and Diederich 1988; Körber 1865 [Polycoccum sauteri, type of Polycoccum, type from Austria]; Orange 1990; Zhurbenko 2007, 2010; Zhurbenko and Santesson 1996) on diverse Stereocaulon species.
Selected specimens examined: Austria: Tirol, Samnaun-Gruppe, Furgler W ober Serfaus, am Grat zwischen dem Furgler Joch und dem Gipfel, 47°02′40″ N, 10°30′50″ E, 2800–2900 m, GF 8929, on Stereocaulon, 1991, Hafellner 30184 (hb. Hafellner). Canada: British Columbia, 25 km N of Kispiox, near Skeena River, on S. tomentosum, 1995, Goward 95-282 (UBC, hb. Diederich). Iceland: SE of Höfn, between road 1 and Jökulsárlón, 1 km W of bridge, 64.04251° N, 16.20525° W, 5 m, terricolous, on Stereocaulon, 2013, Diederich 17508 (hb Diederich).
Discussion
Polyphyly of Dacampiaceae. The phylogenetic position of the family Dacampiaceae is shown here for the first time by the placement of the genus Dacampia in Pleosporales. Notably, while most Dacampiaceae species are lichenicolous or saprobic, the type species of Dacampia, D. hookeri, is lichenized (Henssen 1995; Hyde et al. 2013) (Fig. 3a). Dacampia hookeri is also one of the rare lichenized fungi now confirmed in Pleosporales, an order that otherwise includes mainly saprobic fungi on dead plant material and pathogens on living plants (Hyde et al. 2013). This species clusters with the lichenicolous Dacampia engeliana in our phylogenetic tree (Fig. 1). Atypically for a lichenicolous fungus, D. engeliana modifies its host lichen (Solorina) to form a thallus structure similar to that found in D. hookeri (Henssen 1995; de los Ríos and Grube 2000) (Fig. 3b), a feature supporting their close phylogenetic relationship. Other lichenicolous Dacampia species that do not form such thallus structures might therefore be expected to be more distantly related. Dacampia is closely related to members of Leptosphaeriaceae (e.g., Paraleptosphaeria orobanches, Fig. 1); however, based on the current dataset, the relationship of Dacampiaceae with other families of Pleosporales is unclear, and resolving this will require more detailed study. Surprisingly, Dacampiaceae is strongly polyphyletic since other genera originally assigned to this family, such as Clypeococcum, Polycoccum and Munkovalsaria, are part of other lineages (Fig. 1). Wang et al. (2007) and Hyde et al. (2013) suggested that the family was heterogeneous because it was not strongly characterized morphologically, the only distinguishing characters being multilayered and rather soft ascomatal walls, and relatively wide ostioles.
Placement of genera previously included in Dacampiaceae. One of the most striking results of our study is the placement of Polycoccum s. str. and two species of Clypeococcum as the sister group to the Trypetheliaceae (Trypetheliales), a family of mainly lichenized tropical fungi. This sister relationship being well-supported, we suggest including Polycoccum in Trypetheliales, while the position of Clypeococcum will need to be confirmed by the sequencing of its type species. We recognize this new, well-supported lineage of lichenicolous fungi as the new family Polycoccaceae (see Taxonomy section). This relationship is somewhat similar to Arthoniomycetes, where the non-lichenized Lichenostigmatales, including black yeasts, lichenicolous and rock-inhabiting taxa, are sister to the mainly lichenized Arthoniales (Ertz et al. 2013), and it suggests that the lichenicolous life habit might have played a key role in the transition from non-lichenized to lichenized life habits that led to the evolution of some of the main groups of lichenized fungi. The long, isolated branch formed by Trypetheliaceae within Dothideomycetes (e.g., Muggia et al. 2012; Hyde et al. 2013) is indeed broken by the new lineage of Polycoccaceae. The genus Clypeococcum, here represented by C. placopsiiphilum and C. psoromatis, is nested within Polycoccum and renders this genus paraphyletic. According to Hawksworth and Diederich (1988), Clypeococcum differs from Polycoccum by the hyphal rather than pseudoparenchymatous ascomatal walls, which are massively thickened around the ostiole, and by the ascomata occurring in groups united by a common clypeus. It is unclear from the data included in our phylogenetic tree whether Clypeococcum species need to be included in Polycoccum or if Polycoccaceae has to be split into several genera. The long branches within Polycoccaceae suggest a fast-evolving nuLSU gene contrasting with low morphological variability, which results in few phenotypic characters useful for the recognition of different genera.
Interestingly, several members previously assigned to the genus Polycoccum are placed in the Pleosporales and are thus distantly related to the Trypetheliales/Polycoccaceae. Phenotypic characters such as the ascus type clearly support the placement of these ‘Polycoccum’ species in the Pleosporales (see Taxonomy section). These ‘Polycoccum’ species are closely related to ‘Phoma-like’ lichenicolous fungi assigned to the family Phaeosphaeriaceae (Lawrey et al. 2012), and all these taxa are treated below as the ‘Didymocyrtis clade’.
Another genus originally assigned to Dacampiaceae and included in our phylogenetic tree (Fig. 1) is Munkovalsaria [generic type: M. donacina (Niessl) Aptroot]. Aptroot (1995a) established this genus to include fungi with a black stroma that forms a clypeus around the ostiole; branched-anastomosing pseudoparaphyses; cylindrical bitunicate asci with eight uniseriate ascospores (K/I–); and reddish to deep brown, euseptate ascospores that are surrounded by a thin gelatinous sheath. Three nuLSU sequences of Munkovalsaria were available on GenBank. Munkovalsaria appendiculata was already placed in the Pleosporales as sister species to Montagnula opulenta (Didymosphaeriaceae) in a phylogenetic study by Wang et al. (2007). In our nuLSU tree, Munkovalsaria appendiculata and M. donacina cluster together in Pleosporales. Our phylogenetic study shows M. appendiculata and M. donacina clustered with Montagnula opulenta and Bimuria novae-zelandiae, which suggests that Munkovalsaria does not belong to Dacampiaceae as originally classified but to Didymosphaeriaceae as recently circumscribed by Ariyawansa et al. (2014). A third species, Munkovalsaria rubra Aptroot, van der Aa & Petrini, was found to cluster with Valsaria insitiva in a multilocus phylogeny (Zhang et al. 2009), but it was placed outside the order Pleosporales. Subsequently, M. rubra was accommodated in the new family Valsariaceae (Valsariales) and combined in the genus Myrmaecium (Jaklitsch et al. 2015).
Anamorph-teleomorph relationships in Didymocyrtis. Several potential anamorph-teleomorph relationships were highlighted in our ITS phylogenetic tree (e.g. Phoma ficuzzae/Didymocyrtis ramalinae, Phoma caloplacae/Didymocyrtis consimilis; see Results section). Molecular and phylogenetic analyses already suggested anamorph-teleomorph relationships among lichenicolous fungi belonging to other taxonomic groups: in the Abrothallales (Dothideomycetes), the connection between Abrothallus and its anamorph stage Vouauxiomyces was established by Denaturing Gradient Gel Electrophoresis (Pérez-Ortega et al. 2011); in the Lichenostigmatales (Arthoniomycetes) Phaeosporobolus usneae was shown to be the anamorphic stage of Lichenostigma maureri (Ertz et al. 2013) and in the Chaetothyriales (Eurotiomycetes), the species Lichenodiplis lecanorae and Muellerella atricola were suggested to represent, respectively, the anamorph and teleomorph stages of the same fungus (Muggia et al. 2015). The co-occurrence of ascomata and conidiomata on the same lichen thallus often suggests evidence of such relationships. Halıcı and Hawksworth (2008) and Halıcı et al. (2009) previously suggested the existence of anamorph-teleomorph relationships within the genus Dacampia, given the observation of Phoma-like conidiomata in intimate association with ascomata of Dacampia rubra and D. muralicola. The generic position of these two species remains to be confirmed by molecular data, but in the meantime the co-occurrence of Phoma-like asexual stages should be considered in support of their placement within the Pleosporales, where such anamorphs are frequent.
Species boundaries and host specificity in Didymocyrtis. Most lichenicolous fungi are assumed to be highly host specific, confined to a single lichen species or genus. The causes for this are not known, but may involve resistance to lichen secondary metabolites (Merinero et al. 2015) and/or nutritional or mechanical requirements that can be met only by specific lichen hosts (Lawrey and Diederich 2003). Assumptions about host specificity depend heavily on the prevailing species concepts applied to both lichen parasites and their hosts. Since for lichenicolous fungi, species concepts have sometimes been based largely on host ecology, presumed species boundaries and host specificities should be considered as hypotheses to be tested using a combination of morphological, genetic, distributional and ecological information.
In most members of Didymocyrtis, species boundaries are relatively well-established and supported by a combination of characters. Some easily distinguished Didymocyrtis species can be viewed as narrow specialists, restricted to a single host lichen genus or species. For example, D. ramalinae apparently grows only on species of Ramalina, while D. pseudeverniae is found only on Pseudevernia furfuracea. Similarly, D. slaptoniensis and D. xanthomendozae appear to be closely related sister species that colonize separate hosts and may have arisen as a result of recent host-switching. Didymocyrtis slaptoniensis, well-known primarily from the sexual stage, is found only on Xanthoria parietina, and D. xanthomendozae, known from the sexual and asexual stages, is found only on Xanthomendoza spp.
Other morphologically and genetically distinguishable Didymocyrtis species appear to have much broader host ecologies, being found on a variety of sometimes unrelated lichen hosts. This situation was first described by Lawrey et al. (2012), who found that D. cladoniicola and D. foliaceiphila, previously assumed to be restricted to Cladonia hosts (Diederich et al. 2007), were also able to infect lichens belonging to other non-related hosts. Didymocyrtis cladoniicola is now known from Cladonia, Parmelina, Ramalina, Squamarina, and D. foliaceiphila is now found on Cladonia and Parmelia. To these we can also add D. melanelixiae, which occurs on many members of Parmeliaceae, including Canoparmelia, Cetrelia, Hypotrachyna, Melanelixia, Parmelia s. str., Parmotrema, Platismatia, Pseudevernia and Punctelia.
For other Didymocyrtis species, conflicting information blurs species boundaries and makes the assessment of host specificity difficult. For example, D. consimilis is well-known mainly from the asexual stage (as syn. Phoma caloplacae) and has always been considered to be host specific on the Caloplaca cerina group. Our ITS analyses (Fig. 2), however, revealed the existence of populations on other hosts (Cladonia pocillum, Heterodermia, Melanohalea exasperatula) that are genetically almost identical (only one different position in the ITS alignment supports the separation of both groups, D. consimilis s. str. and D. aff. consimilis in Fig. 2). They are also morphologically similar, although conidia tend to be more elongate ellipsoid on hosts other than Caloplaca gr. cerina, and in culture some isolates look different from the others. It is impossible to tell if these specimens represent a single parasite species that colonizes a variety of host lichens or several host-specific parasites. Similarly, our ITS tree (Fig. 2) indicates that specimens identified as Didymocyrtis epiphyscia are nearly identical genetically but are found on Physcia aipolia (host of the type specimen), P. adscendens, P. tenella and Xanthoria parietina. In this species, however, there appear to be two morphologically distinct clusters, with specimens from P. aipolia having broad conidia and those from P. adscendens, P. tenella and X. parietina having narrow conidia. In this case, morphology would lead us to recognize two species on different lichen hosts, but the ITS sequencing results suggest that speciation may not yet have taken place.
Although we do not know with certainty the evolutionary processes responsible for the remarkable variation in Didymocyrtis host ecology, we assume that speciation by host switching is an important factor. If this is the case, our results appear to include examples of this process at various stages of development. Host switching is known to lead to speciation in mycoparasites (Chaverri and Samuels 2013), including lichenicolous species (Millanes et al. 2014) and is assumed to result from host-specific parasites occasionally exploiting alternative hosts in the habitat. Over time, it is speculated that this could have led to specialization on hosts different from the ancestral species and to the eventual isolation of distinct host-specific species.
Our results include specimens that might represent the earliest stages of this speciation process, most notably Didymocyrtis epiphyscia and D. consimilis, in which specimens from different hosts can sometimes be distinguished morphologically but with little or no genetic differentiation in ITS. We also recognize species that appear to have resulted from recent speciation initiated by a host switch. These include D. slaptoniensis (on Xanthoria parietina) and D. xanthomendozae (on Xanthomendoza), each of which is sufficiently distinct both morphologically and genetically to be recognized as species. Finally, Didymocyrtis includes clearly delimited species that are narrowly host-specific (D. ramalinae and D. pseudeverniae), and other that are host-generalized (D. cladoniicola, D. foliaceiphila and D. melanelixiae). This wide variety of host-parasite interactions in Didymocyrtis species makes them especially attractive for investigations of speciation mechanisms and the causes of high or low host specialization in mycoparasites. Hypotheses based on our findings should be developed and tested with a higher number of specimens and using markers that are more informative than ITS.
References
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F (eds) Second International Symposium on Information Theory. Akademiai Kiado, Budapest
Alstrup V (1981) Notes on some lichens and lichenicolous fungi from Greenland. Nord J Bot 1:120–124
Alstrup V, Cole MS (1998) Lichenicolous fungi of British Columbia. Bryologist 101:221–229
Alstrup V, Hawksworth DL (1990) The lichenicolous fungi of Greenland. Medd Grønl Biosci 31:1–90
Alstrup V, Svane S, Søchting U (2004) Additions to the lichen flora of Denmark VI. Graphis Scr 15:45–50
Alstrup V, Grube M, Motiejūnaitė J, Nordin A, Zhurbenko M (2008) Lichenicolous fungi from the Skibotn area, Troms, Norway. Graphis Scr 20:1–8
Alstrup V, Kocourková J, Kukwa M, Motiejūnaitė J, von Brackel W, Suija A (2009) The lichens and lichenicolous fungi of South Greenland. Folia Cryptogam Est 46:1–24
Aptroot A (1995a) Redisposition of some species excluded from Didymosphaeria (Ascomycotina). Nova Hedwigia 60:325–379
Aptroot A (1995b) A monograph of Didymosphaeria. Stud Mycol 37:1–160
Aptroot A (2004) Two new ascomycetes with long gelatinous appendages collected from monocots in the tropics. Stud Mycol 50:307–311
Aptroot A, Lücking R, Sipman HJM, Umaña L, Chaves JL (2008) Pyrenocarpous lichens with bitunicate asci—a first assessment of the lichen biodiversity inventory in Costa Rica. Bibl Lichenol 97:1–162
Ariyawansa HA, Tanaka K, Thambugala KM, Phookamsak R, Tian Q, Camporesi E, Hongsanan S, Monkai J, Wanasinghe DN, Mapook A, Chukeatirote E, Kang JC, Xu JC, McKenzie EHC, Jones EBG, Hyde KD (2014) A molecular phylogenetic reappraisal of the Didymosphaeriaceae (= Montagnulaceae). Fungal Divers 68:69–104. doi:10.1007/s13225-014-0305-6
Arnold F (1874) Lichenologische Fragmente XVI. Flora (Regensburg) 57:81–89, 97–110, 137–144, 150–155, 173–175, tab
Atienza V, Calatayud V, Hawksworth DL (2003) Notes on the genus Polycoccum (Ascomycota, Dacampiaceae) in Spain, with a key to the species. Lichenologist 35:125–135
Aveskamp MM, de Gruyter J, Woudenberg JHC, Verkley GJM, Crous PW (2010) Highlights of the Didymellaceae: a polyphasic approach to characterise Phoma and related pleosporalean genera. Stud Mycol 65:1–60
Boerema GH (1997) Contributions towards a monograph of Phoma (coelomycetes)—V. Subdivision of the genus in sections. Mycotaxon 64:321–333
Calatayud V (2004) Polycoccum. In: Nash TH III, Ryan BD, Diederich P, Gries C, Bungartz F (eds) Lichen flora of the Greater Sonoran Desert Region, vol II. Lichens Unlimited, Tempe, pp 684–686
Chaverri P, Samuels GJ (2013) Evolution of habitat preference and nutrition mode in a cosmopolitan fungal genus with evidence of interkingdom host jumps and major shifts in ecology. Evolution 67:2823–2837
Clements FE, Shear CL (1931) The genera of fungi. MW Wilson, New York, p 456
Coppins BJ, Coppins AM (2001) Phoma physciicola. Bull Br Lichen Soc 89:78
Crivelli PG (1983) Ueber die heterogene Ascomycetengattung Pleospora Rabh. Vorschlag für eine Aufteilung. Zürich. Diss. ETH Nr. 7318. pp 1–215
de Gruyter J, Aveskamp MM, Woudenberg JHC, Verkley GJM, Groenewald JZ, Crous PW (2009) Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycol Res 113:508–519
de Gruyter J, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW (2013) Redisposition of Phoma-like anamorphs in Pleosporales. Stud Mycol 75:1–36. doi:10.3114/sim0004
De los Ríos A, Grube M (2000) Host-parasite interfaces of some lichenicolous fungi in the Dacampiaceae (Dothideales, Ascomycota). Mycol Res 104:1348–1353
Del Prado R, Schmitt I, Kautz S, Palice Z, Lücking R, Lumbsch HT (2006) Molecular data place Trypetheliaceae in Dothideomycetes. Mycol Res 110:511–520
Diederich P (1990) New or interesting lichenicolous fungi 1. Species from Luxembourg. Mycotaxon 37:297–330
Diederich P, Kocourková J, Etayo J, Zhurbenko M (2007) The lichenicolous Phoma species (coelomycetes) on Cladonia. Lichenologist 39:153–163
Diederich P, Lawrey JD, Sikaroodi M, van den Boom PPG, Ertz D (2011 online/2012 printed) Briancoppinsia, a new coelomycetous genus of Arthoniaceae (Arthoniales) for the lichenicolous Phoma cytospora, with a key to this and similar taxa. Fungal Divers 52:1–12. doi:10.1007/s13225-011-0105-1
Diederich P, Ertz D, Eichler M, Cezanne R, van den Boom P, Van den Broeck D, Sérusiaux E (2014) New or interesting lichens and lichenicolous fungi from Belgium, Luxembourg and northern France. XV. Bull Soc Nat Luxemb 115:157–165
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Eriksson O, Hawksworth DL (1986) Outline of the ascomycetes—1986. Syst Ascomycetum 5:185–324
Eriksson OE, Hawksworth DL (1993) Outline of the ascomycetes—1993. Syst Ascomycetum 12:51–257
Ertz D, Diederich P (2015) Dismantling Melaspileaceae: a first phylogenetic study of Buelliella, Hemigrapha, Karschia, Labrocarpon and Melaspilea. Fungal Divers 70:141–164. doi:10.1007/s13225-015-0321-1
Ertz D, Diederich P, Brand AM, van den Boom P, Sérusiaux E (2008) New or interesting lichens and lichenicolous fungi from Belgium, Luxembourg and northern France. XI. Bull Soc Nat Luxemb 109:35–51
Ertz D, Lawrey JD, Common RS, Diederich P (2013 online/2014 printed) Molecular data resolve a new order of Arthoniomycetes sister to the primarily lichenized Arthoniales and composed of black yeasts, lichenicolous and rock-inhabiting species. Fungal Divers 66:113–137. doi:10.1007/s13225-013-0250-9
Ertz D, Tehler A, Irestedt M, Frisch A, Thor G, van den Boom P (2014 online/2015 printed) A large-scale phylogenetic revision of Roccellaceae (Arthoniales) reveals eight new genera. Fungal Divers 70:31–53. doi:10.1007/s13225-014-0286-5
Etayo J (1996a) Aportación a la flora liquénica de las Islas Canarias. I. Hongos liquenícolas de Gomera. Bull Soc Linn Provence 47:93–110
Etayo J (1996b) Contribución al conocimiento de los líquenes y hongos liquenícolas de Mallorca (Islas Baleares, España). Bull Soc Linn Provence 47:111–121
Etayo J (2004) Líquenes y hongos liquenícolas de los Pirineos occidentales y norte de la Península Ibérica. Naturzale Cuad Cienc Nat 18:143–167
Etayo J (2010a) Líquenes y hongos liquenícolas de Aragón. Guineana 16:1–501
Etayo J (2010b) Líquenes y hongos liquenícolas del País Vasco. Catálogo del año 2010. Ihobe Flora (Bilbao) 6:1–87
Etayo J, Diederich P (1996) Lichenicolous fungi from the western Pyrenees, France and Spain. II. More deuteromycetes. Mycotaxon 60:415–428
Etayo J, Osorio HS (2004) Algunos hongos liquenícolas de Sudamérica, especialmente del Uruguay. Comun Bot Mus Nacionales Hist Nat Antropol 6(129):1–19
Etayo J, Sancho LG (2008) Hongos liquenícolas del Sur de Sudamérica, especialmente de Isla Navarino (Chile). Bibl Lichonol 98:1–302
Gardiennet A (2012) Découverte de Polycoccum slaptoniense D. Hawksw. en France. Bull Info Assoc Fr Lichénol 37:107–111
Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Stat Sci 7:457–511
Gilg IC, Amaral-Zettler LA, Countway PD, Moorthi S, Schnetzer A, Caron DA (2010) Phylogenetic affiliations of Mesopelagic Acantharia and Acantharian-like environmental 18S rRNA genes off the Southern Californian Coast. Protist 161:197–211
Hafellner J (1994) Beiträge zu einem Prodromus der lichenicolen Pilze Österreichs und angrenzender Gebiete I. Einige neue oder seltene Arten. Herzogia 10:1–28
Hafellner J (1996) Bemerkenswerte Funde von Flechten und lichenicole Pilze auf makaronesischen Inseln IV. Einige bisher übersehene lichenicole Arten der Kanarischen Inseln. Cryptog Bryol Lich 17:1–14
Hafellner J (2008) Zur Diversität lichenisierter und lichenicoler Pilze im Gebiet der Koralpe (Österreich: Kärnten und Steiermark, Slowenien). Mitt Naturwiss Ver Steiermark 138:29–112
Halıcı MG, Hawksworth DL (2008) Two new species of Dacampia (Ascomycota, Dacampiaceae), with a key to and synopsis of the known species of the genus. Fungal Divers 28:49–54
Halıcı MG, Candan M, Calatayud V (2009) Dacampia rubra sp. nov. (Ascomycota, Dacampiaceae), a lichenicolous fungus on vagrant Aspicilia species. Mycotaxon 108:235–240
Halıcı MG, Candan M, Güllü M, Özcan A (2014) Phoma recepii sp. nov. from the Caloplaca cerina group in Turkey. Mycotaxon 129:163–168
Hawksworth DL (1981) The lichenicolous Coelomycetes. Bull Br Mus Nat Hist Bot ser 9(1):1–98
Hawksworth DL (1994) Notes on British lichenicolous fungi: VII. Lichenologist 26:337–347
Hawksworth DL, Diederich P (1988) A synopsis of the genus Polycoccum (Dothideales), with a key to accepted species. Trans Br Mycol Soc 90:293–312
Henssen A (1995) Studies on the biology and structure of Dacampia (Dothideales), a genus with lichenized and lichenicolous species. Cryptog Bot 5:149–158
Hitch C (1997) New, rare and interesting British lichen and lichenicolous fungus records. Br Lichen Soc Bull 80:46–58
Hitch C (ed) (2011) New, rare and interesting lichens. Br Lichen Soc Bull 109:75–90
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17:754–755
Hyde KD, Gareth Jones EB, Liu J-K et al (2013) Families of Dothideomycetes. Fungal Divers 63:1–313. doi:10.1007/s13225-013-0263-4
Ihlen PG, Wedin M (2008) An annotated key to the lichenicolous Ascomycota (including mitosporic morphs) of Sweden. Nova Hedwigia 86:275–365
Jaklitsch WM, Fournier J, Dai DQ, Hyde KD, Voglmayr H (2015) Valsaria and the Valsariales. Fungal Divers. doi:10.1007/s13225-015-0330-0
Kocourková J, von Brackel W (2005) Einige für Bayern neue flechtenbewohnende Pilze – Beitrag zu einer Checkliste I. Ber Bayer Bot Ges 75:3–10
Kondratyuk S (2008) Polycoccum kaernefeltii sp. nova (Dothideales), a new lichenicolous fungus on Teloschistes chrysophthalmus (L.) Th. Fr. Ukr Bot Zhurn 65:565–571
Kondratyuk S, Nevo E, Wasser S (2005) New and rare lichen-forming and lichenicolous fungi from the Carmel Mountains, Israel. Ukr Bot Zhurn 62(1):100–110
Körber GW (1865) Parerga lichenologica. Breslau 385-501, (i)-xvi pp
Kukwa M, Flakus A (2009) New or interesting records of lichenicolous fungi from Poland VII. Species mainly from Tatra Mountains. Herzogia 22:191–211
Kukwa M, Szymczyk R, Kowalewska A (2013) New or interesting records of lichenicolous fungi from Poland IX. Herzogia 26:159–168
Lawrey JD, Diederich P (2003) Lichenicolous fungi: interactions, evolution, and biodiversity. Bryologist 106:80–120
Lawrey JD, Diederich P (2015) Lichenicolous fungi – worldwide checklist, including isolated cultures and sequences available. URL: http://www.lichenicolous.net [02/22/2015]
Lawrey JD, Diederich P, Nelsen MP, Sikaroodi M, Gillevet PM, Brand AM, van den Boom P (2011) The obligately lichenicolous genus Lichenoconium represents a novel lineage in the Dothideomycetes. Fungal Biol 115:176–187
Lawrey JD, Diederich P, Nelsen MP, Freebury C, Van den Broeck D, Sikaroodi M, Ertz D (2012) Phylogenetic placement of lichenicolous Phoma species in the Phaeosphaeriaceae (Pleosporales, Dothideomycetes). Fungal Divers 55:195–213
Lutzoni F, Kauff F, Cox CJ, McLaughlin D, Celio G, Dentinger B, Padamsee M, Hibbett D, James TY, Baloch E, Grube M, Reeb V, Hofstetter V, Schoch C, Arnold AE, Miadlikowska J, Spatafora J, Johnson D, Hambleton S, Crockett M, Shoemaker R, Sung G-H, Lücking R, Lumbsch T, O’Donnell K, Binder M, Diederich P, Ertz D, Gueidan C, Hansen K, Harris RC, Hosaka K, Lim Y-W, Matheny B, Nishida H, Pfister D, Rogers J, Rossman A, Schmitt I, Sipman H, Stone J, Sugiyama J, Yahr R, Vilgalys R (2004) Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. Am J Bot 91:1446–1480
Maddison D, Maddison W (2002) MacClade Version 4.03PPC: analysis of phylogeny and character evolution. Sinauer Associates, Sunderland
Magnusson AH (1952) Lichens from Torne Lappmark. Ark Bot Ser 2 2(2):45–249
Merinero S, Bidussi M, Gauslaa Y (2015) Do lichen secondary compounds play a role in highly specific fungal parasitism? Fungal Ecol 14:125–129
Miadlikowska J, McCune B, Lutzoni F (2002) Pseudocyphellaria perpetua, a new lichen from Western North America. Bryologist 105:1–10
Millanes AM, Truong C, Westberg M, Diederich P, Wedin M (2014) Host switching promotes diversity in host-specialized mycoparasitic fungi: uncoupled evolution in the Biatoropsis-Usnea system. Evolution 68:1576–1593
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES science gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 November 2010, New Orleans, pp 1–8
Motiejūnaitė J, Grochowski P (2014) Miscellaneous new records of lichens and lichenicolous fungi. Herzogia 27:193–198
Motiejūnaitė J, von Brackel W, Stončius D, Preikša Ž (2011) Contribution to the Lithuanian flora of lichens and allied fungi. III. Bot Lith 17:39–47
Motiejūnaitė J, Berglund T, Czarnota P, Himelbrant D, Högnabba F, Konoreva LA, Korchikov ES, Kubiak D, Kukwa M, Kuznetsova E, Leppik E, Lõhmus P, Prigodina Lukošienė I, Pykälä J, Stončius D, Stepanchikova I, Suija A, Thell A, Tsurykau A, Westberg M (2012) Lichens, lichenicolous and allied fungi found in Asveja Regional Park (Lithuania). Bot Lith 18:85–100
Muggia L, Gueidan C, Knudsen K, Perlmutter G, Grube M (2012 online/2013 printed) The lichen connections of black fungi. Mycopathologia 175:523–535. doi:10.1007/s11046-012-9598-8
Muggia L, Kopun T, Ertz D (2015) Phylogenetic placement of the lichenicolous, anamorphic genus Lichenodiplis and its connection to Muellerella-like teleomorphs. Fungal Biol (in press)
Nelsen MP, Lücking R, Aptroot A, Andrew CJ, Cáceres M, Rivas Plata E, Gueidan C, da Silva Canêz L, Knight A, Ludwig LR, Mercado-Díaz JA, Parnmen S, Lumbsch HT (2014) Elucidating phylogenetic relationships and genus-level classification within the fungal family Trypetheliaceae (Ascomycota: Dothideomycetes). Taxon 63:974–992
Orange A (1990) New or interesting lichens and lichenicolous fungi from Iceland. Acta Bot Isl 10:37–44
Pérez-Ortega S, Suija A, de los Ríos A (2011) The connection between Abrothallus and its anamorph state Vouauxiomyces established by Denaturing Gradient Gel Electrophoresis (DGGE). Lichenologist 43:277–279
Pérez-Ortega S, Suija A, Crespo A, de los Ríos A (2013 online/2014 printed) Lichenicolous fungi of the genus Abrothallus (Dothideomycetes: Abrothallales ordo nov.) are sister to the predominantly aquatic Janhulales. Fungal Divers 64:295–304. doi:10.1007/s13225-013-0269-y
Phookamsak R, Liu JK, McKenzie EHC, Manamgoda DS, Ariyawansa H, Thambugala KM, Dai DQ, Camporesi E, Chukeatirote E, Wijayawardene NN, Bahkali AH, Mortimer PE, Xu JC, Hyde KD (2014) Revision of Phaeosphaeriaceae. Fungal Divers 68:159–238. doi:10.1007/s13225-014-0308-3
Pitt WM, Urbez-Torres JR, Trouillas FP (2014) Munkovalsaria donacina from grapevines and Desert Ash in Australia. Mycosphere 5:656–661. doi:10.5943/mycosphere/5/5/6
Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817–818
Rambaut A (2012) FigTree v1.4.2, available from: http://tree.bio.ed.ac.uk/software/figtree/
Rambaut A, Drummond AJ (2007) Tracer v1.6, Available from http://beast.bio.ed.ac.uk/
Rondon Y (1969) L’herbier des champignons parasites des lichens de l’Abbe L. Vouaux. Rev Bryol Lichenol 36:737–745
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Roux et coll. (2014) Catalogue des lichens et des champignons lichénicoles de France métropolitaine. Édit. Henry des Abbayes, Fougères, 1525 p
Ruibal C, Millanes AM, Hawksworth DL (2011) Molecular phylogenetic studies on the lichenicolous Xanthoriicola physciae reveal Antarctic rock-inhabiting fungi and Piedraia species among closest relatives in the Teratosphaeriaceae. IMA Fungus 2:97–103
Santesson R (1960) Lichenicolous fungi from northern Spain. Svensk Bot Tidskr 54:499–522
Santesson R (1993) The lichens and lichenicolous fungi of Sweden and Norway. SBT-förlaget, Lund, pp 240
Santesson R, Moberg R, Nordin A, Tønsberg T, Vitikainen O (2004) Lichen-forming and lichenicolous fungi of Fennoscandia. Museum of Evolution, Uppsala University, Uppsala, pp 359
Seaward MRD, Sipman HJM, Sohrabi M (2008) A revised checklist of lichenized, lichenicolous and allied fungi for Iran. In: Facetten der Flechtenforschung. Festschrift zu Ehren von Volkmar Wirth. Sauteria 15:459–520
Spegazzini C (1881) Fungi argentini additis nonnullis brasiliensibus montevideensibusque. Pugillus quartus. Anal Soc Cient Argentina 12:13–30, 63–82, 97–117, 174–189, 208–227, 241–258
Swofford DL (2002) PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland
Trakunyingcharoen T, Lombard L, Groenewald JZ, Cheewangkoon R, Toanun C, Alfenas AC, Crous PW (2014) Mycoparasitic species of Sphaerellopsis, and allied lichenicolous and other genera. IMA Fungus 5:391–414
Triebel D, Scholz P (2001) Lichenicolous fungi from Bavaria as represented in the Botanische Staatssammlung München. Sendtnera 7:211–231
Vainio EA (1921) Lichenographia Fennica I. Pyrenolichenes iisque proximi Pyrenomycetes et Lichenes imperfecti. Acta Soc Fauna Flora Fenn 49:1–274
van den Boom PPG, Giralt M (2012) Checklist and three new species of lichens and lichenicolous fungi of the Algarve (Portugal). Sydowia 64:149–208
Vězda A (1969) Beiträge zur Kenntnis der flechtenbewohnenden Pilze in der Tschechoslowakei. II. – Zwei neue Arten: Opegrapha rinodinae sp. nov. und Polycoccum galligenum sp. nov. Ceská Mykol 23:104–109
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246
von Brackel W (2007) Weitere Funde von flechtenbewohnenden Pilzen in Bayern. Beitrag zu einer Checkliste III. Ber Bayer Bot Ges 77:5–26
von Brackel W (2008a) Phoma ficuzzae sp. nov. and some other lichenicolous fungi from Sicily, Italy. In: Facetten der Flechtenforschung. Festschrift zu Ehren von Volkmar Wirth. Sauteria 15:103–120
von Brackel W (2008b) Some lichenicolous fungi collected during the 20th meeting of the Società Lichenologica Italiana in Siena. Notiz Soc Lichenol Ital 21:63–66
von Brackel W (2009) Weitere Funde von flechtenbewohnenden Pilzen in Bayern – Beitrage zu einer Checkliste IV. Ber Bayer Bot Ges 79:5–55
von Brackel W (2010) Weitere Funde von flechtenbewohnenden Pilzen in Bayern – Beitrage zu einer Checklist V. Ber Bayer Bot Ges 80:5–32
von Brackel W (2011) Lichenicolous fungi and lichens from Puglia and Basilicata (southern Italy). Herzogia 24:65–101
von Brackel W (2013) Miscellaneous records of lichenicolous fungi from the Italian Alps. Herzogia 26:141–157
von Brackel W (2015) Lichenicolous fungi from central Italy with note on some remarkable hepaticolous, algicolous and lichenized fungi. Herzogia 28:212–281
von Keissler K (1930) Die Flechtenparasiten. Dr L. Rabenhorst’s Kryptogamen-Flora von Deutschland, Österreich und der Schweiz 8:1–712
Vouaux L (1912–14) Synopsis des champignons parasites des lichens. Bull Soc mycol Fr 28:177–256; 29:33–128, 399–494; 30:135–198, 281–329
Wang HK, Aptroot A, Crous PW, Hyde KD, Jeewon R (2007) The polyphyletic nature of Pleosporales: an example from Massariosphaeria based on rDNA and RBP2 gene phylogenies. Mycol Res 111:1268–1276
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols—a guide to methods and applications. Academic, San Diego, pp 315–322
Wijayawardene NN, Crous PW, Kirk PM et al (2014) Naming and outline of Dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Divers 69:1–55. doi:10.1007/s13225-014-0309-2
Wittmann H, Türk R (1990) Die Flechten im Nationalpark Nockberge (Kärnten, Österreich). Kärntner Nationalparkschriften 4:1–112
Yoshimura I, Yamamoto Y, Nakano T, Finnie J (2002) Isolation and culture of lichen photobionts and mycobionts. In: Kranner I, Beckett RP, Varma AK (eds) Protocols in lichenology—culturing, biochemistry, physiology and use in biomonitoring. Springer, Berlin, pp 3–33
Zhang Y, Schoch CL, Fournier J, Crous PW, de Gruyter J, Woudenberg JHC, Hirayama K, Tanaka K, Pointing SB, Spatafora JW, Hyde KD (2009) Multi-locus phylogeny of Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Stud Mycol 64:85–102
Zhang Y, Crous PW, Schoch CL, Hyde KD (2011 online/2012 printed) Pleosporales. Fungal Divers 53:1–221. doi:10.1007/s13225-011-0117-x
Zhurbenko M (1996) Lichens and lichenicolous fungi of the northern Krasnoyarsk territory, central Siberia. Mycotaxon 58:185–232
Zhurbenko MP (2007) The lichenicolous fungi of Russia: geographical overview and a first checklist. Mycol Balc 4:105–124
Zhurbenko MP (2009a) Lichenicolous fungi and some lichens from the Holarctic. Opusc Philolichenum 6:87–120
Zhurbenko M (2009b) Lichenicolous fungi and lichens from the Holarctic. Part II. Opusc Philolichenum 7:121–186
Zhurbenko MP (2010) Lichenicolous fungi and lichens growing on Stereocaulon from the Holarctic, with a key to the known species. Opusc Philolichenum 8:9–39
Zhurbenko MP, Alstrup V (2004) Lichenicolous fungi on Cladonia mainly from the Arctic. Symb Bot Ups 34:477–499
Zhurbenko MP, Kobzeva AA (2014) Lichenicolous fungi from Northwest Caucasus, Russia. Herzogia 27:377–396
Zhurbenko MP, Santesson R (1996) Lichenicolous fungi from the Russian Arctic. Herzogia 12:147–161
Zhurbenko MP, Hermansson J, Pystina TN (2012) Endococcus incrassatus new to Eurasia and some other lichenicolous fungi from the Komi Republic of Russia. Graphis Scr 24:36–39
Zwickl DJ (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. Ph. D. dissertation, The University of Texas at Austin
Acknowledgments
We would like to thank the curators of herbaria and the owners of private collections cited in Materials and Methods for the loan of specimens. Javier Etayo and Richard Harris are acknowledged for their help concerning Didymocyrtis melanelixiae; J.E. also for Leptosphaeria protousneae; and Alain Mora, Bernadette Mora and Jean-Pierre Duvivier for Didymocyrtis slaptoniensis. Ann Bogaerts, Cyrille Gerstmans, Myriam Dehaan and Wim Baert are thanked for technical assistance. Finally, the first author acknowledges financial support from the Fonds National de la Recherche Scientifique (FNRS) from Belgium (F.R.F.C. 2.4567.08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ertz, D., Diederich, P., Lawrey, J.D. et al. Phylogenetic insights resolve Dacampiaceae (Pleosporales) as polyphyletic: Didymocyrtis (Pleosporales, Phaeosphaeriaceae) with Phoma-like anamorphs resurrected and segregated from Polycoccum (Trypetheliales, Polycoccaceae fam. nov.). Fungal Diversity 74, 53–89 (2015). https://doi.org/10.1007/s13225-015-0345-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-015-0345-6