Abstract
The order Onygenales is classified in the class Eurotiomycetes of the subphylum Pezizomycotina. Families in this order have classically been isolated from soil and dung, and two lineages contain causative agents of superficial, cutaneous and systemic infections in mammals. The ecology and habitat choices of the species are driven mainly by the keratin and cellulose degradation abilities. The present study aimed to investigate whether the ecological trends of the members of Onygenales can be interpreted in an evolutionary sense, linking phylogenetic parameters with habitat preferences, to achieve polyphasic definitions of the main taxonomic groups. Evolutionary processes were estimated by multiple gene genealogies and divergence time analysis. Previously described families, namely, Arthrodermataceae, Ajellomycetaceae, Ascosphaeraceae, Eremascaceae, Gymnoascaceae, Onygenaceae and Spiromastigoidaceae, were accepted in Onygenales, and two new families, Malbrancheaceae and Neogymnomycetaceae, were introduced. A number of species could not be assigned to any of the defined families. Our study provides a revised overview of the main lines of taxonomy of Onygenales, supported by multilocus analyses of ITS, LSU, TUB, TEF1, TEF3, RPB1, RPB2, and ribosomal protein 60S L10 (L1) (RP60S) sequences, combined with available data on ecology, physiology, morphology, and genomics.
Similar content being viewed by others
Introduction
Phylogenetic studies using barcoding genes (Chen et al. 2015), as well as those corroborated by whole-genome sequence data (Wang et al. 2009; Li et al. 2021), have demonstrated that the order Onygenales Cif. ex Benny & Kimbr. is one of the derived clades of the fungal kingdom, experiencing recent diversification. The main driver of evolution is keratin degradation, a unique ability that has shaped large clades, such as Arthrodermataceae, within the order (Currah 1985; Summerbell 2000). Several families show pronounced adaptation to mammalian hosts, which are evolutionarily recent (Sharpton et al. 2009; Muñoz et al. 2018). This has culminated in the emergence of anthropophilic species, i.e., those adapted to Homo sapiens, one of the most recent vertebrates.
The orders Arachnomycetales Gibas, Sigler & Currah, Eurotiales G.W. Martin ex Benny & Kimbr. and Onygenales, together with some unclassified taxa, constitute the subclass Eurotiomycetidae (Schoch et al. 2020; Wijayawardene et al. 2020). Members of the Onygenales show pronounced sexual state morphology, with mostly gymnothecial fruitbodies, frequently elaborate hyphal extensions, and some well-differentiated asexual forms of sporulation (Currah 1985; Hubka et al. 2013; de Hoog et al. 2017; Woodburn et al. 2019). This has enabled the classification of genera and families on the basis of morphology. However, in the absence of sexual states and macroconidia, phylogenetically remote microconidial species are morphologically similar, which has led to highly polyphyletic genera such as Chrysosporium. Such groups require taxonomic revision based on modern criteria. A major problem is the absence of interpretable type specimens for numerous taxa in Onygenales. Therefore, one of the aims of the present paper is to redefine higher taxa and essential species, with the deposition of neotypes or epitypes where necessary.
Several groups within Onygenales have long been recognized for their pronounced ecological preferences, e.g., association with bees in Ascosphaeraceae, systemic infections of mammals in Ajellomycetaceae, and superficial infections of mammals in Arthrodermataceae. In the present article, we will investigate the extent to which ecological parameters can assist in defining groups at the generic or familial levels. Given these ecologies, the revised order might provide a model for a polyphasic approach to a taxonomy that includes parameters that play a role in the course and speed of evolution, in contrast to the present taxonomy that is largely phylogenetic and using anonymous markers. The ultimate aim is to define taxa as biological entities together with their ecological and morphological features rather than solely applying phylogeny.
Materials and methods
Strains
Strains were selected based on the species that have been described in the order Onygenales according to NCBI taxonomy (http://www.ncbi.nlm.nih.gov/taxonomy) and Index Fungorum (www.indexfungorum.org). In total, sequences belonging to 97 genera, 385 species and 553 strains were analysed in this study (Table 1; Supplementary Table 1). Available strains were obtained from the Centraalbureau voor Schimmelcultures reference collection (housed at Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands) and inoculated on Sabouraud’s glucose agar (SGA, BD Difco™). Cultures were incubated at 24 °C for 14‒21 days. Sequences of the remaining strains and for the largest subunit of RNA polymerase II (RPB1), the second largest subunit of RNA polymerase II (RPB2) and translation elongation factor 1-alpha (TEF1) were retrieved from the NCBI nucleotide databank. Whole genome data for 53 strains (Supplementary Table 1) was obtained from NCBI Genome and Joint Genome Institute MycoCosm (https://mycocosm.jgi.doe.gov/mycocosm) databases and added to phylogenetic tree analyses. Ecology and ascomata morphology for the type species were retrieved from the original articles where the species were described for the first time.
DNA extraction, PCR and sequencing
DNA extraction was performed using the cetyltrimethylammonium bromide protocol (Su et al. 2019) and MasterPure™ Yeast DNA Purification Kit (Epicentre, Madison, WI, USA). The quantity and quality of the isolated DNA was evaluated using a NanoDrop ND-1000 spectrophotometer with ND-1000 v3.3.0 software (Coleman Technologies, Wilmington, NC, USA). Five gene regions, the rDNA internal transcribed spacer (ITS), D1-D2 region of large subunit (LSU), partial β-tubulin (TUB), translation elongation factor 3 (TEF3) and ribosomal protein 60S L10 (L1) (RP60S), were amplified using the primers ITS4-ITS5, LR0R-LR5, TUB2Fd-TUB4Fd, EF3-3185F/EF3-3538R and 60S-908R/60S506F, respectively (de Hoog et al. 2017; Dukik et al. 2017). PCRs were carried out as described by Stielow et al. (2015). PCR products were visualized on 1.5% agarose gels and cycle-sequenced using Applied Biosystems BigDye Terminator version 3.1 (Thermo Fisher Scientific) after purification. Bidirectional sequencing was performed using a capillary electrophoresis system (3730 × l DNA analyser; Life Technologies, Carlsbad, CA, USA). The obtained sequences were manually inspected and stored in a BIOLOMICS database.
Phylogenetic analyses
Alignments were generated using PASTA (Mirarab et al. 2015) and MAFFT v7 with default settings and trimmed using ClipKIT with the smart-gap function (Steenwyk et al. 2020). The percent similarity between strains was determined using BIOEDIT v7.2 (Hall 1999). ModelFinder (Kalyaanamoorthy et al. 2017) on IQ-TREE software (Nguyen et al. 2015) was used to find the best-fitting model for each gene according to the Bayesian Information Criterion (BIC). Phylogenetic trees were constructed using the maximum likelihood (ML) methods implemented in IQ-TREE software (Minh et al. 2020b). Branch support values were measured using both ultrafast bootstraps and SH-like approximate likelihood ratio tests (Guindon et al. 2010; Minh et al. 2020a). Additionally, MRBAYES v3.2.7 (Ronquist and Huelsenbeck 2003) with default settings on the CIPRES portal (http://www.phylo.org/) was used for ITS and LSU phylogeny. Aspergillus fumigatus strain Af293 (Aspergillaceae, Eurotiales) was used as an outgroup to define families. Single-locus analyses for ITS and LSU were performed in two groups: Group I contained type species with additional strains from each species, and Group II contained only type species. Strains that had more data for eight loci were selected for each species and used for the multilocus phylogenetic analyses. To determine the phylogeny of the genera, family-based ITS analyses were performed. The characteristics of the trees were analysed using the AMAS (Alignment Manipulation And Summary) tool (Borowiec 2016) with a Python package. Command files were prepared on Alignment Transformation Environment (ALTER) (http://www.sing-group.org/ALTER/) for the maximum likelihood phylogeny analyses and with MESQUITE v2.75 (Maddison and Maddison 2019) for the Bayesian inference analyses. TREEVIEW v1.6.6, MEGA v6 (Tamura et al. 2013) and iTOL v6 (Letunic and Bork 2019) were used to visualize and edit trees. Branches composing the backbone starting with > 1 species with bootstrap values ≥ 80% were collapsed and considered “supported clades”. Branches formed in the supported clades with > 1 species with bootstrap values ≥ 80% were considered “groups”. The ratio of supported/unsupported clades with a low number of branches outside of the clades was taken as a parameter to define tree quality.
To increase the robustness of the ML topologies observed in the concatenated dataset, the gene concordance factor (gCF) and the site concordance factor (sCF) implemented in IQTREE2 software were also shown together with ultrafast bootstrap (UFBoot) and Shimodaira–Hasegawa approximate likelihood ratio test (SH-aLRT) values (Guindon et al. 2010; Hoang et al. 2018; Minh et al. 2020a). For every clade in the multilocus Onygenales tree, gCF is defined as the percentage of conclusive gene trees containing that partition. On the other hand, sCF is stated as the percentage of decisive alignment sites (SNPs) supporting the branch of a family or a clade in the ML tree. The trees were deposited in TREEBASE under accession number 28949.
Relative divergence time estimation
The absolute time of divergence, relative orders of the taxa and spacing of speciation events within Onygenales were estimated based on the RelTime method implemented in MEGA 7 with the General Time Reversible (GTR) model with gamma distributed rates [5 categories (+ G, parameter = 0.6881)] (Tamura et al. 2012; Kumar et al. 2016) using fossils of Paleopyrenomycites devonicus (Pezizomycotina, 410 Mya) for the calibrating point (Samarakoon et al. 2019). The analysis involved 356 nucleotide sequences. There were a total of 7065 positions in the final dataset. In addition to Arachnomyces spp. (Arachnomycetales) and Aspergillus fumigatus (Eurotiales); Arthrobotrys oligospora (Orbiliales, Orbiliomycetes), Ascobolus immersus (Pezizales, Pezizomycetes), Botrytis cinerea (Helotiales, Leotiomycetes), Cladonia grayi (Lecanorales, Lecanoromycetes), Colletotrichum simmondsii (Glomerellales, Sordariomycetes), Dactylellina haptotyla (Orbiliales, Orbiliomycetes), Microthyrium microscopicum (Microthyriales, Dothideomycetes), Piedraia hortae (Capnodiales, Dothideomycetes), Pyronema omphalodes (Pezizales, Pezizomycetes), Sclerophora sanguinea (Coniocybales, Coniocybomycetes) and Usnea florida (Lecanorales, Lecanoromycetes) were used as representatives of the main lineages of Ascomycota. Candida tropicalis and Saccharomyces cerevisiae (Saccharomycetales, Saccharomycetes) were used as outgroups for the analysis.
Results
In the current study, a total of 1667 sequences for LSU (n = 421), ITS (n = 519), TUB (n = 189), RP60S (n = 123), TEF1 (n = 119), TEF3 (n = 144), RPB1 (n = 71) and RPB2 (n = 97) were examined. Among these sequences, 860 [ITS, n = 339; LSU, n = 270; TUB, n = 122; RP60S, n = 73; RPB1, n = 25 and RPB2, n = 46] represented the type strains. A combined tree consisted of eight loci data of 342 strains. This dataset was found to be 7014 bp in length and contained 3706 parsimony-informative sites. The best-fitting model for each gene according to the BIC on IQ-TREE software is shown in Table 2 together with a summary of the alignments for each locus.
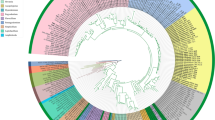
The results of the combined analysis of eight loci using 342 sequences yielded 14 clades with ≥ 90% support for Bayesian probability and ≥ 80% support for maximum likelihood analyses. Families, based on their type genera and type species, were resolved as Ajellomycetaceae, Arthrodermataceae, Ascosphaeraceae, Eremascaceae, Gymnoascaceae, Onygenaceae and Spiromastigoidaceae (Fig. 1). Onygenaceae were found to be polyphyletic, breaking up into three supported clades, as in the LSU analyses. Arthrodermataceae were found in the ultimate position, and Ascosphaeraceae were at the base, with Eremascaceae. Other clades below Arthrodermataceae were placed as follows: Onygenaceae, Gymnoascaceae, Ajellomycetaceae and Spiromastigoidaceae. Strains belonging to Arachnomycetaceae (Arachnomycetales Gibas, Sigler & Currah) were included in the analyses but were found outside the order Onygenales in all phylogenetic trees.
Phylogeny, ecology and key physiological features of the species classified in Onygenales. Phylogenetic tree obtained by combined analysis of eight loci using 342 sequences based on Bayesian analysis and maximum likelihood analysis using the GTR + I + G4 + F model in IQ-TREE-ML. Branch values of ≥ 90% for Bayesian probability and ≥ 80% for maximum likelihood and type species of the genera are shown in bold. The names and neotypes proposed in the present study are shown in red. The species that need nomenclatural revision are written in square brackets. Aspergillus fumigatus was used as an outgroup
The results of the LSU phylogeny of 409 strains (Group I LSU analysis) showed one unsupported and 13 supported clade branches (bootstrap support ≥ 80%). The ultimate clade of Arthrodermataceae was followed by Onygenaceae, Gymnoascaceae, Ascosphaeraceae and Spiromastigoidaceae, with Ajellomycetaceae in the ancestral position (Supplementary Figure 1). In this phylogeny, members formerly classified in Gymnoascaceae were found in two supported clades. Onygenaceae formed three clades. Ajellomycetaceae was subdivided into two clades, with Emmonsiellopsis species in an ancestral position, while members of the Eremascaceae were found within Ajellomycetaceae. Meanwhile, Group II LSU analysis (n = 269) revealed 13 supported and three unsupported clades, and Gymnoascaceae members were subdivided into one unsupported and three supported clades (Supplementary Figure 2).
Phylogenetic analysis using only ITS locus sequences of 488 strains (Group I ITS analysis) revealed 15 clades with bootstrap support values of ≥ 80% (Supplementary Figure 3). The genus Sigleria, a member of Spiromastigoidaceae, was found far from the remaining members of the family, and members of Nannizziopsiaceae (correct name according to the Index Fungorum database: Nannizziopsidaceae; access date 15 March 2022) were placed far apart from other members of Onygenaceae Group C. Species attributed to Onygenaceae were scattered over four supported clades along with species previously described as incertae sedis. The classical family Gymnoascaceae was found to have 72/94% support, with a basal clade formed by Kraurogymnocarpa trochleospora (CBS 591.71) and Diploospora rosea (DAOM 250100). Eremascaceae was found to be a separate supported clade. The tree topology revealed the soilborne and halophilic genus Spiromastigoides to be positioned at the base of the tree, while the family Arthrodermataceae with mammal-associated species was placed in the most derived position. Using the ITS sequences of 323 strains (Group II), 12 supported and three unsupported clades were obtained. In addition, the genus Paranannizziopsis was found to be distant from the members of Nannizziopsis (Supplementary Figure 4) in the ITS Group II phylogeny.
Among the phylogenies of the other loci, Eremascaceae were not represented in the TUB, TEF3 or RP60S analyses. Furthermore, no TEF3, RPB1, RPB2 or RP60S records were found for the members of Neogymnomycetaceae, while Ascosphaeraceae could not be included in the RP60S analysis. The most variable results were obtained for the Onygenaceae family, changing from one to five clades. The highest supported/unsupported ratio was found in the RP60S analysis (11/0) because the family Arthrodermataceae was divided into three supported clades. In addition, Ascosphaeraceae were found in a doubtful position, close to Arthrodermataceae. In contrast, the lowest ratio was found in TEF3 analysis (11/2), with four Onygenaceae and two Arthrodermataceae clades. Both RPB1 and RPB2 phylogenies revealed topologies similar to those of the multilocus phylogeny (Fig. 2).
Phylogenetic analyses of Onygenales species based on a RP60S; b RPB1; c TUB; d TEF3; e RPB2; f TEF1-α; g multilocus sequences; h multilocus sequences without incertae sedis; i LSU Group I; j LSU Group II; k ITS Group II; and l ITS Group I obtained using the maximum likelihood criterion. Aspergillus fumigatus was used as the outgroup. The characteristics of the alignments are listed in Table 2
The highest rate of supported/unsupported clade value among the ITS, LSU and multilocus trees was found in the ITS Group I phylogeny (15/0), followed by multilocus phylogeny. However, members of Onygenaceae and Spiromastigoidaceae were divided into more groups in the ITS phylogeny than in the multilocus analyses. In addition, clades were supported with higher bootstrap values in the multilocus analysis. Therefore, multilocus phylogeny was chosen to demonstrate the combined ecology, physiology and phylogeny data (Fig. 3). A comparison of all the phylogenetic trees together with the substitution models for each tree are summarized in Table 3.
Summary of the morphology, physiology, ecology and phylogeny of Onygenales. A circular maximum-likelihood tree was constructed with IQ-TREE-ML (Nguyen et al. 2015) using ClipKIT-trimmed alignments (Steenwyk et al. 2020) of eight loci (Supplementary Table 1). The type of ascomata and ascomatal appendages are used as morphological parameters. a peridial hyphae with coiled appendages of the genus Arthroderma (Currah 1985); b ascomata of Ctenomyces surrounded by pectinate appendages (Currah 1985); c ascomata of Shanorella covered with incompositoperidum and spiral appendages (Currah 1985); d reticuloperidium of Auxarthronopsis with spine-like, straight to curved appendages (Currah 1985); e reticuloperidium of Uncinocarpus with uncinate appendages (Currah 1985); f fruiting body of Onygena species (Currah 1985); g cleistothecial ascomata of Aphanoascus and Keratinophyton without appendages (Currah 1985); h reticuloperidium of Malbranchea (Auxarthron) with spine-like, straight to curved appendages (Currah 1985), the difference between the appendage morphology of Malbranchea and Auxarthronopsis being that Malbranchea never produces multiseptate appendages (Sharma et al. 2013); i boat hook-shaped appendages of the genus Gymnoascus (Currah 1985); j mature stalked fruiting body of Narasimhella (Thirumalachar and Mathur 1966); k ascomata of Blastomyces with spiral appendages (Currah 1985); l ascomata of Spiromastigoides with curved appendages (Rizzo et al. 2014); m coiled appendages of Polytolypa (Scott et al. 1993); n spore cyst of Ascosphaera (David Minter, Whitby); o completely naked asci of Eremascus (de Bary 1884). Numbers on the branches represent SH-aLRT support (%) / ultrafast bootstrap support (%)
The ecological preferences of species can be classified as soil/oligotrophic (soil that contains low nutrition, i.e., sandy soil, cave soil, etc.), soil/keratinophilic, dung/agricultural, skin/nail, hair/feather, insect/pollen, osmotic habitats, systemic, plant and other/unknown (Fig. 4). Four main ascomata morphology types were noted: cleistothecium, gymnothecium, spore cyst and naked fruitbody (Fig. 3). Fruitbodies on a stipe structure reported in Onygena corvina, Onygena equina and Narasimhella poonensis were classified as “other”.
Although 21 families were introduced in Onygenales between 1833 and 2014, descriptions of these families were mostly based on their ascomata and peridium morphology; however, most of them are currently obsolete because they were not supported in later studies (Table 1). Judging from the multilocus phylogeny, ecology and reconstruction data, the order Onygenales can be described with the core families Arthrodermataceae, Ajellomycetaceae, Ascosphaeraceae, Eremascaceae, Gymnoascaceae, Onygenaceae and Spiromastigoidaceae. The family Nannizziopsidaceae clustered amidst members of Onygenaceae, and therefore, Nannizziopsidaceae are preliminarily considered a group within Onygenaceae rather than a separate family. Clades supported by high bootstrap values but lacking a type of described family are indicated as incertae sedis. Meanwhile, two clades with type species supported by high bootstrap values were newly introduced as Malbrancheaceae and Neogymnomycetaceae, respectively. Relationships between genera within each family were determined by ITS phylogeny because of the higher variability of this marker, enabling the comparison of more closely related entities for more recent speciation events (Berbee and Taylor 2001; Stielow et al. 2015) and the number of available sequences in public databases being larger than that of other loci. Branches formed by Harorepupu aotearoa, Pectinotrichum llanense and Testudomyces verrucosus were found to be relatively long in multilocus analyses (Fig. 1). The first species was found outside of defined families in all phylogenetic analyses; therefore, it was regarded as incertae sedis. The other two species were found to be related to Onygenaceae (Fig. 1; Supplementary Figures 3 and 4).
The results from phylogenetic analyses (Fig. 1) can be summarized as follows. Clade 1 comprises members of the family Arthrodermataceae, including the keratinophilic genera Arthroderma, Ctenomyces, Epidermophyton, Guarromyces, Lophophyton, Microsporum, Nannizzia, Paraphyton and Trichophyton. Ecology of the species was found mainly invading fur, skin and nails (73/120), followed by soil (37/120). Most geophilic species cluster in basal positions in the family, while anthropophilic species were in derived positions, and zoophiles were located in the middle of the tree (Fig. 1).
Members of Clades 2, 3 and 4 have previously been described in Arthrodermataceae and Onygenaceae or were regarded as incertae sedis (Crous et al. 2017; Wijayawardene et al. 2017). Since the Arthrodermataceae members are keratinophilic and have only gymnothecial ascomata while the morphology and ecology among the Onygenaceae members are variable, Clades 2 and 3 could have been related to Onygenaceae. However, the present study determined the families based on the phylogenetic position of the type species, and Clades 2, 3 and 4 are found remote from the type of Onygenaceae (Fig. 1). Additionally, even though there is still quite a distance between these three clades and Arthrodermataceae, they were found to be closer to Arthrodermataceae than Onygenaceae. Nevertheless, they were not included in the Arthrodermataceae because of the variable ascomata types among the species and the long branch distance to members of Arthrodermataceae. The ecological preferences of members of the clades also showed a large diversity: all categories are represented, except for association with systemic diseases and insects or pollen. Keratinophilic species as well as cellulolytic species isolated from plant debris were included in these clades. Clade 2 included the type species Shanorella (type species S. spirotricha) and Chrysosporium vallenarense; Clade 3 (with 86% Bayesian probability and 68% ML bootstrap support) included the type species Myotisia cremea and Leucothecium emdenii; Clade 4 (100% Bayesian probability and ML bootstrap support) contained the types Arachnotheca (type species A. glomerata), Apinisia (type species A. glomerata), and Myriodontium (type species M. keratinophilum).
Clade 5 contains members of the family Onygenaceae. Five groups were distinguishable in this clade (Fig. 1): Group 5-I was represented by the oligotrophic species Keratinophyton (type species K. terreum); Group 5-II contained species Aphanoascus (type species A. fulvescens). Compared to Group 5-I, isolates of Group 5-II showed an association with nutrient-rich soil, dung and keratinous substrates (Fig. 1; Supplementary Table 1). Group 5-III contained Amauroascus (type species A. niger), Brunneospora (type species B. reticulata), Byssoonygena ceratinophila and Coccidioides (type species C. immitis). Members of the latter genus are thermal dimorphic fungi that cause systemic mycosis in mammals via the inhalation of environmental propagules, leading to endosporulating spherule formation and hematogenous dissemination in the host (Van Dyke et al. 2019). Group 5-IV included Onygena equina, which is the type species of the family and the entire order. In addition, Ascocalvatia alveolata from carnivore dung and another reptile-related fungus, Ophidiomyces ophidiicola, and the soil-borne fungus Pseudoamauroascus (type species P. australiensis) were included in this group. Group 5-V included mainly reptile-associated species of Nannizziopsis (type species N. vriesii) and Paranannizziopsis (type species P. australasiensis), which have been classified in the separate family Nannizziopsidaceae by Stchigel et al. (2013d). Additionally, Aphanoascella (type species A. galapagosensis) and Emydomyces (type species E. testavorans), which were described from infections in turtles (Sutton et al. 2013; Woodburn et al. 2019), and another keratinophilic genus, Pectinotrichum (type species P. llanense), were also found in Onygenaceae.
Members of Clade 6 (100% Bayesian probability and ML bootstrap support) belong to the genus Malbranchea (type species M. pulchella) (Fig. 1). Keratinolytic activity varies among different species. The majority of strains analysed had been isolated from eutrophic soil and dung (Supplementary Table 1; Fig. 4). Although M. ostraviensis (as Auxarthron ostraviense) and M. umbrina (as A. umbrinum) were occasionally reported from onychomycosis cases (Orr et al. 1963a; Hubka et al. 2013), M. zuffiana (as A. zuffianum) was once isolated from the lung of a dog (Emmons 1954), and M. filamentosa (as A. filamentosum) was isolated from the skin of a snake (Sigler et al. 2002a); these species were not proven to be pathogenic (Bowman et al. 1996). Combining the previously described morphological characteristics (Solé et al. 2002a; Sarrocco et al. 2015; Zhang et al. 2021) and ecology of the strains with current phylogenetic results, Clade 6 was considered a separate family. The name Auxarthron was synonymized with Malbranchea by Rodríguez-Andrade et al. (2021), and the previously proposed family name Auxarthraceae is invalid (MycoBank, access date February 11, 2022). Therefore, the name Malbrancheaceae was chosen to accommodate the species in Clade 6.
Clade 7, with 100% Bayesian probability and 98% ML bootstrap support in the multilocus data tree (Fig. 1), included Auxarthronopsis bandhavgarhensis, Canomyces reticulatus, Currahmyces indicus, Neogymnomyces demonbreunii and Renispora flavissima. Except for the type species of Auxarthronopsis from India, all species of this genus were recorded from karst caves in China (Supplementary Table 1). All type strains of the described species were found to be keratinophilic. In consideration of a stable phylogenetic position supported with different loci analyses, as well as a consistent habitat of the species, the new family name Neogymnomycetaceae is proposed for this clade.
Clade 8 was classified as incertae sedis and formed by the type of Amauroascus aureus and xerophilic Diploospora rosea.
Members of Gymnoascaceae are placed in Clade 9 (83% ML bootstrap support) (Fig. 1) with the type species of the genera Arachniotus (type species A. ruber), Gymnascella (type species G. aurantiaca), Gymnoascoideus (type species G. petalosporus), Gymnoascus (type species G. reessii), which is also the type genus of the family, and Narasimhella (type species N. poonensis). The ecological key words of the clade were dominantly oligotrophic and soil (33/79), followed by dung (20/79). The clade also contains halophilic species (Sphaerosporium equinum and Sporendonema casei) isolated from cheese, which is a unique habitat in Onygenales.
Clade 10 contained the family Ajellomycetaceae with 100% support in the multilocus data tree (Fig. 1). Ajellomycetaceae includes the genera Blastomyces (type species B. dermatitidis), Emergomyces (type species E. pasteurianus), Helicocarpus (type species H. griseus), Histoplasma (type species H. capsulatum) and Paracoccidioides (type species P. brasiliensis). The genus Emmonsia has been reduced to synonymy of Blastomyces (Jiang et al. 2018), while the remaining species were transferred to Emergomyces (Jiang et al. 2020). The geophilic genus Emmonsiellopsis (type species E. terrestris) was described in Ajellomycetaceae in an ancestral position based on ML analyses of ITS, LSU, RPB2, TEF3 and TUB2 genes (Jiang et al. 2018).
Clade 11 included the members of the family Spiromastigoidaceae; genera Sigleria (type species S. carmichaelii), Spiromastigoides (type species Sp. warcupii), which is a member of the family, and Pseudospiromastix (type species P. tentaculata) (Fig. 1). Members of this clade show xerophilic characteristics, and keratinolytic and cellulolytic activity is variable among the members (Currah 1994; Hirooka et al. 2016). To date, only two species, Sp. asexualis and Sp. albida were reported as causative agents of deep infections in mammals (Rizzo et al. 2014; Stchigel et al. 2017).
Clade 12 was classified as incertae sedis and formed by the type of Polytolypa (type species P. hystiricis) and Amaurascopsis (type species A. perforata) along with Chrysosporium chiropterorum and C. lobatum (Fig. 1).
Clade 13 comprises Ascosphaeraceae species (100% bootstrap support in all trees) and is represented by Ascosphaera apis, which is the type species of the family. All members of the clade are associated with bee habitats, including nests, pollen and larvae; they can be saprotroph or pathogenic for bees (Wynns 2015).
The last clade, Clade 14, was formed by members of Eremascaceae, including the insect-related fungus Dactylodendron pinicola (formerly known as Arthrographis pinicola) and the osmophilic species Eremascus albus. Both LSU Group I and Group II analysis were not compatible with the other phylogenetic results, such that Eremascaceae were embedded in Ajellomycetaceae (Supplementary Figures 1 and 2).
Simply structured, thallic microconidia are produced throughout the order Onygenales. Species with enteroarthric conidia are generally classified under Malbranchea and species with holoarthric conidiation under Chrysosporium. Both genera were already described in the nineteenth century and have been neotypified with Malbranchea pulchella, neotype strain CBS 202.38 and Chrysosporium merdarium, neotype strain CBS 388.68, respectively (Saccardo 1882; Corda 1833; Carmichael 1962; van Oorschot 1980). Numerous described species are phylogenetically remote from these types, clustering within or outside the order Onygenales and require reclassification. Therefore, in the present study, the Chrysosporium species in the families Arthrodermataceae, Onygenaceae and Spiromastigoidaceae were reclassified when neotypes were available; otherwise, names were maintained according to their first description.
Relative divergence time estimation
The results of RelTime analysis showed that the diversification of species in Onygenales occurred 103 Mya and in two main directions: one with Ajellomycetaceae, Ascosphaeraceae, Eremascaceae and Spiromastigoidaceae and the other with Arthrodermataceae, Malbrancheaceae, Gymnoascaceae, Neogymnomycetaceae and Onygenaceae. The earliest species of the order were found to be related to Gymnoascaceae (79–70 Mya), while the most recent species were found close to Arthrodermataceae (43–15 Mya). The results are summarized in Fig. 5.
Estimated times of molecular divergence in Onygenales by RelTime analysis with multilocus phylogeny of 356 taxa. The tree was scaled to time on the basis of the calibration point of Pezizomycotina given by Samarakoon et al. (2019). Estimated times are given in red, and values in black on the branches represent SH-aLRT, bootstrap, gene concordance factor (gCF) and site concordance factor (CF) (%). Two main extinction events, the Permian–Triassic (251 Mya) and Cretaceous–Tertiary (K-T, 66 Mya), are shown in red circles on the timeline. According to these results, current members of the Onygenales appeared at the end of the Cretaceous period in the Mesozoic era. Therefore, Onygenales can be concluded to be a recently evolved order. The first members of the order Spiromastigoidaceae and Gymnoascaceae were found at the end of the Cretaceous period, when flowering plants became abundant (Silvestro et al. 2021) and bees (Genise et al. 2020) and snakes (Caldwell et al. 2015) evolved. Nevertheless, the results showed that both bee- and reptile-associated members of the order appeared after the K-T extinction 62 Mya and 54 Mya, respectively
Taxonomy
Onygenales Cif. ex Benny & Kimbr.—Mycotaxon 12(1): 8, 1980
Type family: Onygenaceae Burnett—Outl. Bot. (London): 159, 1833; type genus: Onygena Pers.—Observ. Mycol. (Lipsiae) 2: 71, 1800; type species: Onygena equina (Willd.) Pers. Neotype designated here: CBS-H 15271, on cow hoof, The Netherlands, 1970, H.A. van der Aa. Culture derived from the type CBS 947.70 = ATCC 22731 = IFO 31785.
The order Onygenales was first described by Ciferri (nomen nudum, Atti Ist. Bot. Univ. Pavia, Ser. 5, 14: 239. 1957: without Latin diagnosis; Art. 36 ICBN). Later, Benny and Kimbrough (1980) redefined the order and distinguished three families: Dendrosphaeraceae, Gymnoascaceae and Onygenaceae. The order frequently includes keratinophilic species with sessile or stipitate ascocarps, with or without appendages, or sessile, composed of loose, interwoven hyphae (gymnothecium), or naked asci, fruitbodies being absent; asci ovoidal or subclavate, evanescent, containing eight spores; ascospores hyaline or brightly colored; holo- or entero-thallic conidiogenesis, lateral or intercalary; additional septate macroconidia may be present (Currah 1985).
The type species of Onygenales was first described by Willdenow (1787) as Lycoperdon equinum from hooves of horses. Later, Persoon (1800 [1799]) reported that L. equinum is different from other Lycoperdon species, which are now known as a basidiomycetous sac-like fungus (due to their sac-like bodies), and consequently introduced the genus Onygena, with the type species Onygena equina (Persoon, 1800 [1799]), commonly known as the horn stalkball. Type material is not known to be preserved. A culture of the neotype specimen is available as CBS 947.70. This culture stabilizes the nomenclature of the order Onygenales.
Currah (1985) reviewed the order Onygenales and included cellulolytic species in the family Myxotrichaceae. However, later studies questioned its assignment, and at present, the family has been excluded from the order (Currah 1994; Hambleton 1998; Leclerc et al. 1994; Sugiyama et al. 1999). Later, Wang et al. (2006) confirmed that Myxotrichaceae is a family of Leotiomycetes based on phylogenetic analysis of SSU + LSU + 5.8S nuc-rDNA data. Similarly, the taxonomic position of Arachnomycetaceae was doubtful for a long time. The generic type Arachnomyces was first described in Onygenaceae by Malloch and Cain (1971). Abbott et al. (1996) suggested reclassifying the genus within Gymnoascaceae based on ascospore structure and inability to degrade keratin, even though many members of the family have an apparent affinity to keratinous substrates (Ulfig et al. 1998; Scott and Untereiner 2004). Arachnomyces differs morphologically from Gymnoascaceae by its conidial type and by the presence of cleistothecia rather than gymnothecia. Multilocus studies of conserved genes combined with morphological and molecular characteristics (Gibas et al. 2002a; Sun et al. 2019) suggested that Arachnomycetaceae deserve the status of a separate order as Arachnomycetales. The results of the current study also confirmed that members of Arachnomycetaceae cannot be classified in the order Onygenales (Fig. 1; Supplementary Figures 1‒4).
The phylogenetic, morphological and ecological characteristics of recognized families in the order Onygenales are summarized below.
Clade 1
Arthrodermataceae Locq. ex Currah
Type genus: Arthroderma Curr.—Outl. Brit. Fung. (London): 357, 1860; type species: Arthroderma curreyi Berkeley. Epitype CBS 353.66, from dune soil, UK, A.E. Apinis, 1966 (de Hoog et al. 2017).
Ancestral members of this family generally have white, pale yellow or yellowish-brown, globose ascomata with appendages; thin-walled, transparent, subglobose to globose asci; hyaline to pale yellow, smooth, minute, oblate to oblate-discoid or oblate-convex ascospores (Currah 1985). They often produce large, multiseptate macroconidia in addition to one-celled, sessile microconidia of the chrysosporium-like (except genus Ctenomyces) (Currah 1985). In the derived, mostly human-associated species (i.e., Trichophyton rubrum), sexuality tends to be lost (Persinoti et al. 2018), and as in the case of T. tonsurans and T. equinum, species might evolve as separate clones with a specific mating-type (Gräser et al. 2006; Kano et al. 2014; Kandemir et al. 2020). The family comprises the genera Arthroderma, Ctenomyces, Epidermophyton, Guarromyces, Keratinophyton, Lophophyton, Microsporum, Nannizzia, Paraphyton and Trichophyton (de Hoog et al. 2017) (Fig. 6). Phylogenetic results showed that type strains of several described Chrysosporium species and that of Pectinotrichum chinense (ex-type culture LC5811) clustered in the family among species of Arthroderma (Figs. 1 and 6). Additionally, Shanorella spirotricha (ex-type culture CBS 305.56) and Leucothecium emdenii (ex-type culture CBS 576.73) were found to be close to Arthrodermataceae (Fig. 1). Available molecular and morphological data for the last two species were combined, and they are judged as incertae sedis, as discussed in the related sections below.
Phylogenetic tree of Arthrodermataceae based on ITS sequences obtained with Bayesian analysis and maximum likelihood analysis using the GTR + G4 + I + F model in IQ-TREE-ML. Branch values of ≥ 90% for Bayesian probability and ≥ 80% for maximum likelihood and type species of the genera are shown in bold. The species that need nomenclatural revision are enclosed in square brackets. Neotypes and new combinations are indicated in red. Onygena corvina and Onygena equina were used as the outgroup species
Members of Arthrodermataceae are characteristically mammal-associated fungi with keratinolytic abilities. Otčenášek and Dvořák (1975) classified them as geophilic, zoophilic and anthropophilic, depending on the part of the life cycle that is environmental or closer to the mammalian or human host. De Hoog et al. (2018) used transmissibility as a criterion to distinguish environmental pathogens from zoophilic pathogens, with infection taking place either via environmental propagules or by direct host-to-host transmission. However, determination of the natural habitat of a species may not always be easy. Geophilic, zoophilic and anthropophilic occurrences are statistical trends rather than diagnostic parameters. These ecological trends are also approximately reflected in sexuality, clonality and loss of specialized reproduction (de Hoog et al. 2017; Persinoti et al. 2018). Anthropophilic species are necessarily at the top of phylogenetic trees, given that Homo sapiens is the most recent host (Gräser et al. 2000; Fig. 1). Transmission occurs by skin flakes loaded with amorphous fungal cells, associated with significant morphological reduction compared to the elaborate environmental sexual forms commonly observed in geophiles (Li et al. 2010). Zoophilic species lie between these two edges by mostly sporulating and showing sexual reproduction upon mating (Metin and Heitman 2017). Changes in habitat and host preference at the species level are usually accompanied by slight differences in phenotypic and physiological characteristics (Kandemir et al. 2020; Su et al. 2019).
Since dermatophytes are among the well-studied groups in the order Onygenales in terms of laboratory identification, antifungal resistance and taxonomy (de Hoog et al. 2017; Dukik et al. 2017; Gupta et al. 2017; L’Ollivier and Ranque 2017; Martinez-Rossi et al. 2018; Khurana et al. 2019; Monod 2019; Su et al. 2019; Baert et al. 2020; Čmoková et al. 2020), we summarize currently available data for the remaining family members below.
During phylogenetic analyses, several GenBank records were encountered with other generic names than the ones above but clustered in Arthrodermataceae. Two strains were deposited as Onygena corvina CBS 225.60 and CBS 281.48 (LSU MH869510 and FJ358287, respectively) and were misidentified. Both strains showed 100% LSU identity with Arthroderma crocatum, type strain IHEM 5251, KT155108 (Hainsworth et al. 2021). This was confirmed by CBS 225.60 having 99.6% ITS identity with A. crocatum CBS 130.70 (AJ877223) in the present study.
Corda (1833) introduced the genus Chrysosporium, with Chrysosporium corii as a type species. The original leather material, dating back to 1833 from herbarium in Prague (PR), Czech Republic, is preserved at the Canadian National Mycological Herbarium with a slide of the type DAOM 55541 (personal communication with Jennifer Wilkinson). To stabilize the current nomenclature, van Oorschot (1980) proposed C. merdarium as a type species of the genus, using slide DAOM 43321 as a reference. However, this material is not interpretable either, and the status of the species remains uncertain. Numerous species have been described in Chrysosporium based on morphology, which according to modern criteria, are nondiagnostic. The described Chrysosporium species appear highly polyphyletic, i.e., C. longispora (current name Paranannizziopsis longispora) in Onygenales (Sigler et al. 2013), C. pilosum (current name Arachnomyces pilosus) in Arachnomycetales (Sun et al. 2019), C. verrucosum (current name Pseudogymnoascus pannorum is in Thelebolales (Minnis and Lindler 2013), C. xerophilum (current name Xerochrysium xerophilum) is in Eurotiales (Pitt et al. 2013) and C. thermophilum (current name Thermothelomyces thermophilusis) in Sordariales (Marin-Felix et al. 2015). Given this polyphyly and the fact that no interpretable type material exists, we regard Chrysosporium as nomen confusum.
The ex-type strain of Chrysosporium kuzurovianum, CBS 667.78, has been recorded as a synonym (van Oorschot 1980) of C. keratinophilum (ex-type culture CBS 104.62). However, it differs by its colony color, larger conidia, the presence of multiple conidial shapes and the absence of arthroconidia (Scharapov 1974; van Oorschot 1980). This is confirmed by phylogeny (Figs. 1 and 6) showing affinity to Arthroderma multifidum (99.7% LSU to KT155237, 97% ITS to KT155892). Therefore, the following new combination is proposed:
Arthroderma kuzurovianum (Scharapov) Kandemir & de Hoog, comb. nov. – MycoBank MB842352.
Basionym: Chrysosporium kuzurovianum Scharapov—Nov. Sist. Niz. Rast. 11: 266, 1974; ex-type culture: CBS 667.78 = UAMH 4322 = VKM F-2119, from black soil, Russia.
Van Oorschot (1980) examined CBS 277.77 and proposed a new combination Chrysosporium pannicola (Corda) v. Oorschot & Stalpers. Information about the status of the herbarium specimen described on cloth and deposited in National Museum, Prague, Czech Republic (PRM), was requested from PRM; however, we have not received any response. Even though the herbarium specimen was indicated in previous reports (van Oorschot 1980), ex-type culture from the holotype has not been designated. Therefore, the following epitype strain is designated here: CBS 277.77, from an unknown source, isolated by G.F. Orr in 1961. Current results show that this strain clusters in Arthrodermataceae. Note that Labuda et al. (2021) reclassified the species in Keratinophyton, but this was based on supposed synonymy with the type strain CBS 116.63 of Keratinophyton evolceanui above. The following combination is required for the strain CBS 277.77:
Arthroderma pannicola (Corda) Kandemir & de Hoog, comb. nov. – MycoBank MB842353.
Basionym: Capillaria pannicola Corda—Icon. Fung. 1: 10. 1837 ≡ Sporotrichum pannicola (Corda) Rabenhorst—Deutschl. KryptFl. 1: 78, 1844 ≡ Chrysosporium pannicola (Corda) van Oorschot & Stalpers—Stud. Mycol. 20: 43, 1980 ≡ Keratinophyton pannicola (Corda) Labuda et al.—IMA Fungus 12(1):17, 2021. ex-type culture: CBS 277.77 = UAMH 1125 = Orr O-736 from an unknown source, isolated by G.F. Orr, in 1961.
Ctenomyces Eidam—Beitr. Biol. Pfl. 3: 274, 1880; type species: Ctenomyces serratus Eidam. ex-neotype culture: CBS 187.61 = ATCC 15504 = IMI 86199 = NRRL A-11176, from soil, Australia. Herb. No: CBS H-14818 (Orr and Kuehn 1963a).
Ctenomyces serratus was originally thought to be a resting or sclerotial phase of Arthroderma curreyi bearing comb-like appendages. Since the original material was lost, Benjamin (1956) described C. serratus as a separate species, and Orr and Kuehn (1963) indicated a neotype, IMI 86199. The genus Ctenomyces was described as having orange-brown cleistothecia, a peridium composed of a thin layer of densely interwoven hyphae, and ctenoid appendages consisting of thick-walled, asperulate cells. Conidia are verrucose, thick-walled, and lightly pigmented, commonly with ampulliform swelling (Benjamin 1956; Zhang et al. 2019). The habitat of Ctenomyces species is soil enriched with bird feathers (Currah 1985; Zhang et al. 2019); no animal pathology is known. Isolation of known Ctenomyces species involves hair baiting techniques using chicken feathers and human hair, and hence species are probably keratinophilic like other members of the family. Gymnothecia of Ctenomyces species bear characteristic comb-like appendages on the peridia, which Currah (1985) suggested to enhance the adaptation of the fungus to be transported via avian feathers. In addition, ascospores in Ctenomyces are large (mostly > 8 μm in length; Currah 1985; Zhang et al. 2019). Currently, C. albus, C. indicus, C. serratus, C. peltricolor, C. obovatus and C. vellereus have been included in the genus. Zhang et al. (2019) referred to the identification key for Ctenomyces species.
Van Oorschot (1980) introduced Ctenomyces as a sexual morph of Myceliophthora, which was later confirmed by phylogenetic analysis of M. vellerea (van den Brink et al. 2012). In the same study, M. vellerea was found phylogenetically (ITS, TEF1 and RPB2) remote from the type species of the genus M. lutea and was renamed Ctenomyces.
Ctenomyces vellereus (Sacc. & Speg.) Kirk—Index Fungorum 120: 1, 2014 ≡ Sporotrichum vellereum Sacc. & Speg.—Michelia 2 (7): 287, 1881 ≡ Myceliophthora vellerea (Sacc. & Speg.) van Oorschot—Stud. Mycol. 20: 47, 1980. Neotype designed here: CBS 479.76, from soil, Egypt, isolated by H.M. Yusef.
Ctenomyces bossae Milochevitch (1935) (GenBank MH855761, ITS and GenBank MH867274, LSU) was described with the type strain CBS 176.36. However, phylogenetic results showed that this species is remote from the type species of the genus C. serratus (Zhang et al. 2019), clustering with Trichophyton tonsurans and T. equinum (Fig. 6), which is in agreement with human skin as a source of its isolation.
Vanbreuseghem (1952) introduced the genus Keratinomyces, typified by K. ajelloi, to include dermatophytes with fusiform, smooth-walled macroconidia and lacking microconidia. Molecular studies have shown that K. ajelloi is a synonym of Arthroderma uncinatum (de Hoog et al. 2017), while a second species, K. longifusus, has been reclassified as Nannizzia fulva (de Hoog et al. 2017). Keratinomyces ceretanicus was the only species left in the genus, and as it was phylogenetically remote from A. uncinatum, the genus Guarromyces was erected (de Hoog et al. 2017). The type strain FFBA 328 was described from forest soil in Catalonia (Punsola and Guarro 1984), but the only available record is CBS 269.89 = FMR 3063 = UAMH 6412, an isolate from soil in Chile deposited by J. Guarro in 1988; this isolate was used as a neotype (de Hoog et al. 2017).
The genus Thallomicrosporon was introduced with the type species Thallomicrosporon kuehnii (ex-type culture CBS 119.64; Benedek 1964). Benedek (1969) suggested that this species may have derived from another dermatophyte as a stable mutant. Molecular analyses in the current study showed that the type strain of T. kuehnii is identical to CBS 258.61, the type strain of Nannizzia gypsea (99.7% LSU identity to NG064029 and 100% ITS identity to NR131271) and is therefore regarded as a synonym.
Zhang et al. (2017c) isolated a dermatophyte strain from soil and based on the ITS data and the dermatophyte taxonomy at the time, they identified the strain as Microsporum guizhouense. According to current approaches in dermatophyte taxonomy (de Hoog et al. 2017; Dukik et al. 2020), this species should be classified in the genus Nannizzia. In the present study, ex-type culture GZUIFR-EB2001M was found in the same cluster with Nannizzia species based on the ITS data analysis (Fig. 6). Therefore, the following combination was proposed:
Nannizzia guizhouensis (Zhang, Zou, Han & Liang) Kandemir & de Hoog, comb. nov. – MycoBank MB843402.
Basionym: Microsporum guizhouense Zhang, Zou, Han & Liang—Mycosystema 36(5): 537, 2017. Holotype: EB2001M; ex-type culture: GZUIFR-EB2001M isolated from tobacco farmland soil by YR Wang in 2012, China.
Clade 2‒4 Incertae sedis
The genus Shanorella contains a single species, S. spirotricha, with characteristic peridial hyphae with coiled appendages (Benjamin 1956), and it can be differentiated from Ctenomyces by its spiral rather than ctenoid appendages on the peridium (Benjamin 1956). The habitat choice of bird feathers is similar in both genera (Benjamin 1956; Currah 1985; Zhang et al. 2019), and the specialized peridial hyphae probably enhance attachment to feathers. In the current study, S. spirotricha clustered in Clade 2 with the type of Chrysosporium vallenarense (CBS 627.83) in both ITS (with 93% similarity) and LSU analyses (with 97% similarity). Based on phylogenetic results, they formed a separate clade basal to the Arthrodermataceae (Fig. 1; Supplementary Figures 1‒4). In the current study, Shanorella was maintained as incertae sedis; Chrysosporium vallenarense requires nomenclatural revision.
Clades 3 and 4 contain several generic species, i.e., Leucothecium emdenii (ex-type culture CBS 576.73), Myotisia cremea (ex-type culture CBS 141864 = CCF 5407), Arachnotheca glomerata (ex-type culture CBS 348.71 = ATCC 22733 = UAMH 3551), Arthropsis hispanica (ex-type culture CBS 351.92 = FMR 4058), Apinisa graminicola (ex-type culture CBS 721.68 = ATCC 18745 = IMI 126422 = Orr O-1125), Kuehniella racovitzae (ex-type culture CBS 156.77 = ATCC 28557 = NRRL 6154) and Myriodontium keratinophilum (ex-type culture CBS 947.73) (Fig. 1).
The genus Myotisia was introduced with the type species Myotisia cremea (ex-type culture CBS 141864) from bat droppings (Crous et al. 2017) and classified in Onygenaceae based on morphology (gymnothecium with globose, smooth-walled and hyaline ascospores, malbranchea-like conidial morph; Crous et al. 2017). The genus Arthrographis Cochet ex Sigler & Carmich. (1976), represented by the type species Arthrographis kalrae (ex-type culture CBS 693.77), is phylogenetically found to belong to Eremomycetaceae in Dothideomycetes (Giraldo et al. 2014). However, ITS, LSU and multilocus phylogeny data showed a cluster containing Arthrographis alba (ex-type culture CBS 370.92, from marine sediment, Spain) and Leucothecium emdenii, the generic type species (ex-type culture CBS 576.73, from agricultural soil, The Netherlands), in a basal position to Arthrodermataceae with different levels of support (Fig. 1; Supplementary Figures 1‒4). Giraldo et al. (2014) applied sequences of ITS and D1/D2 domains of the 28S rRNA and underlined that A. alba is the asexual morph of L. emdenii. The species was published to be weakly keratinolytic and does not grow at 40 °C (Gené et al. 1996).
Arthropsis hispanica and Arachnotheca glomerata grouped together, and ITS and LSU similarities between the two species were found to be 88% and 97%, respectively. In addition, in both species, conidia are formed by fragmentation of hyphal branches (von Arx 1971; Ulfig et al. 1995). Since the type species of the genus Arthropsis (type species A. truncata; ex-type culture UAMH 4430) is classified in Sordariomycetes (Giraldo et al. 2014), a nomenclatural revision is required for A. hispanica.
Myriodontium keratinophilum was described from keratinous substrates in Italy (Samson and Polonelli 1978) and as a cause of infections in humans in two reports (Maran et al. 1985; Kochhar et al. 2018). The identification of the fungi in these reports was based on the colony and microscopic morphology only. Cano et al. (1997) described M. keratinophilum as an asexual morph of Neoarachnotheca keratinophila (ex-type culture: FMR 4016, from marine sediments, Spain; Herb. IMI 351982 is stored in the Kew Herbarium, UK). The other strains, Arachniotus albicans (holotype IMI 100875; ex-type cultures CBS 151.65 = ATCC 22478 = IHEM 4423), Chrysosporium carmichaelii (ex-type culture CBS 643.79), C. georgiae CBS 625.79, C. pallidum (holotype HMAS 247992; ex-type culture CGMCC: 3.19575) and C. undulatum (holotype IMI 375884; ex-type cultures CBS 964.97 = FMR 6101), need revision since they are remote from the type species of their genera based on both LSU and ITS analyses.
Clade 5 Onygenaceae Berk.
Type genus: Onygena Pers.—Observ. Mycol. (Lipsiae) 2: 71, 1800; type species: Onygena equina (Willd.) Pers. ≡ Lycoperdon equinum Willd.—Fl. Berol. Prodr.: 412, 1787. Herb. No: CBS H-15271, from cow hoof, Germany, H.A. van der Aa, 1970. ex-neotype culture: CBS 947.70 = ATCC 22731 = IFO 31785.
The family Onygenaceae is one of the largest groups containing a large number of species in the order Onygenales (Figs. 1 and 7). Genera are morphologically and ecologically diverse, and the family includes dimorphic pathogens next to environmental saprobes (Fig. 1). The majority of the species are found on keratinous substrates and in soil (Fig. 4). Sexual states are described with pseudoparenchymatous cleistothecia or elaborate gymnothecia, and asexual morphs are chrysosporium-like or malbranchea-like conidia (Currah 1985).
Phylogenetic tree of Onygenaceae based on ITS sequences obtained with Bayesian analysis and maximum likelihood analysis using the GTR + G4 + I + F model in IQ-TREE-ML. Branch values of ≥ 90% for Bayesian probability and ≥ 80% for maximum likelihood and type species of the genera are shown in bold. Neotypes and new combinations are indicated in red. The species that need nomenclatural revision are enclosed in square brackets. Arthroderma redellii and Arthroderma quadrifidum were used as the outgroup species
In multilocus data analyses, Clade 5 was shown to be the main clade of Onygenaceae (with 100% bootstrap support). Six groups were individualized (Fig. 1). Additionally, sequences of the type of Chrysosporium europae (ex-type culture CBS 321.86 = UAMH 4587, from soil, Spain) and Pectinotrichum llanense (ex-type culture CBS 882.71 = ATCC 18921 = IHEM 4440 = IMI 155644, from Savannah soil, Venezuela) also clustered in Clade 5 as separate unresolved branches (Fig. 1). Uncinocarpus reesii (ex-type culture CBS 121.77 = ATCC 34,533 = IMI 211,204 = UAMH 3880, from feather, Australia) was also found to be an unresolved branch in the clade according to the LSU phylogeny (Supplementary Figures 1 and 2). This soilborne species has often been regarded to be closely related to the environmental pathogens in Coccidioides, which was confirmed by genome comparisons (Sharpton et al. 2009), but at a relatively large distance and with other taxa in between (Untereiner et al. 2002), similar to our data.
Group 5-I Keratinophyton Randhawa & Sandhu
Type species: Keratinophyton terreum Randhawa & Sandhu—Sabouraudia 3: 253, 1964; ex-type culture: CBS 342.64 = IFO 31781 = IMI 108689 = ATCC 16413 = UAMH 4066, from lawn soil, India.
Members of Keratinophyton and Aphanoascus share cleistothecia with a pseudoparenchymatic peridium, and they can be found on keratinous substrates and dung (Cano et al. 2002a; Sutton et al., 2013) (Supplementary Table 1). The two groups are distinguished by their ascospore morphology and differences in the ITS locus (Sutton et al. 2013; Crous et al. 2017) (Fig. 7). Both genera have been shown to be separate according to ITS sequences in previous studies (Sutton et al. 2013; Crous et al. 2017; Labuda et al. 2021). In the current study, differences in the LSU locus also confirmed the differentiation of Aphanoascus and Keratinophyton species (Supplementary Figures 1 and 2). The Keratinophyton group includes several species described in Chrysosporium, an ancient genus that has been regarded as having a doubtful identity. Labuda et al. (2021) reclassified the following recently described species in Keratinophyton: C. clavisporum (ex-type culture: G801), C. echinulatum (ex-type culture: CBS 141178), C. evolceanui (ex-type culture: CBS 116.63), C. fluviale (ex-type culture: CBS 100809), C. qinghaiense (ex-type culture: GZUIFR-11), C. hubeiense (ex-type culture: EM66601), C. linfenense (ex-type culture: GZAC-H31), C. minutisporum (ex-type culture: CBS 101577), C. submersum (ex-type culture: CBS 101575), and C. siglerae (ex-type culture: UAMH 6541). The results of all phylogenetic analyses in the current study confirmed these changes with the following additional combinations:
Keratinophyton alvearium (Liu & Cai) Kandemir & de Hoog, comb. nov. – MycoBank MB842355. Basionym: Chrysosporium alvearium Liu & Cai—Mycosphere 9(6): 1096, 2018; holotype HMAS 247780, ex-holotype culture CGMCC 3.18783 = LC 11684 = LF1882 isolated from hive-stored pollen collected in Italian honey bee colonies in the flowering season of Brassica campestris by Y.Z. Zhao, 2016.
Keratinophyton indicum (Garg) Kandemir & de Hoog, comb. nov. – MycoBank MB842356. Basionym: Trichophyton indicum Randhawa & Sandhu—Mycopath. Mycol. Appl. 20: 227, 1963 ≡ Chrysosporium indicum (Randhawa & Sandhu) Garg—Sabouraudia 4(4): 262, 1966; ex-type culture: CBS 117.63 = ATCC 22401 = UAMH 1274, Herb. IMI 147544, isolated from soil, India, by H.S. Randhawa, 1960. The ITS similarity between K. indicum and the closest species, K. linfenense, was 98% in 574 bp.
Trichophyton evolceanui was described with the type CBS 116.63 by Randhawa and Sandhu (1963) from Indian soil. However, since the morphological structures were originally described as macroconidia that appeared to be lost in subcultures, with only hyaline, aseptate echinulate thalloconidia remaining, it was renamed Chrysoporium evolceanui by Garg (1966). In the present study, the type strain of C. evolceanui (CBS 116.63) was found in Clade 5 within the Keratinophyton Group (Fig. 7), and the following new combination is proposed:
Keratinophyton evolceanui (Randhawa & Sandhu) Kandemir & de Hoog, comb. nov. – MycoBank MB842357. Basionym: Trichophyton evolceanui Randhawa & Sandhu—Mycopath. Mycol. Appl. 20: 232, 1963 ≡ Chrysosporium evolceanui (Randhawa & Sandhu) Garg—Sabouraudia 4(4): 262, 1966; ex-type culture: CBS 116.63 (van Oorschot 1980), Herb. IMI 147545, isolated by R.S. Sandhu from soil, India, 1961.
Group 5-II Aphanoascus (Cooke) Apinis
Type: Aphanoascus fulvescens (Cooke) Apinis—Mycopath. Mycol. Appl. 35: 99, 1968. Basionym: Badhamia fulvescens Cooke—Grevillea 4(30): 69, 1875 ≡ Eurotium fulvescens (Cooke) Cooke—Grevillea 8 (45): 11, 1879 ≡ Pyrobolus fulvescens (Cooke) Kuntze—Revis. Gen. Pl. (Leipzig) 2: 868, 1891 ≡ Anixiopsis fulvescens (Cooke) de Vries—Mykosen 12: 120, 1969. Cooke described the species on an old sacking from the UK, but this material is not known to be preserved. Neotype designated here: UAMH 4114 = IMI 74750, from soil, UK, P.M. Stockdale, 1958.
= Castanedomyces australiensis Cano, Pitarch & Guarro—Stud. Mycol. 47: 167, 2002; ex-type culture: IMI 370017 = FMR 5484, from soil, Australia.
The deposition of a neotype stabilizes the current concept of Aphanoascus as described recently by Labuda et al. (2021). However, the nomenclatural history of this genus is confusing. In 1875, Cooke published what he thought was a myxomycete and named it Badhamia fulvescens. One year later, Hansen (1877) introduced Eurotium stercorarium, isolated from old fox manure. Based on Hansen’s description, Cooke (1879) re-examined B. fulvescens and renamed it Eurotium fulvescens. Zukal (1890) recognized that E. stercorarium Hansen differs from other Eurotium species, and he described Aphanoascus cinnabarinus isolated from crocodile dung from his collection in Vienna. After this description, Hansen re-examined the type specimen of E. stercorarium and proposed a new genus, Anixiopsis, with the type species Anixiopsis stercoraria. De Vries (1964) accepted Aphanoascus cinnabarinus Zukal as a synonym of Anixiopsis stercoraria Hansen. To resolve this confusion, Apinis (1968) examined the type specimens, except the type specimen for Zukal’s A. cinnabarinus, which was not available, and found that the strains from Hansen and Cook’s specimens were identical. Apinis (1968) combined the generic name priority (Zukal’s, in 1890 against Hansen’s in 1897) with specific epithet priority (Cooke’s in 1875 against Zukal’s, in 1890) and proposed a new combination Aphanoascus fulvescens, instead of Aphanoascus cinnabarinus, which should be a type species. Meanwhile, since the original material of A. cinnabarinus Zukal was lost, Udagawa and Takada (1973) designated a neotype ATCC 26215 (= CBS 267.72) from pepper soil in Japan in 1973. However, this neotype was later shown to have a deviating conidial state, named Paecilomyces cinnabarinus (Jong and Davis 1975), and decades after the introduction of the neotype, A. cinnabarinus was moved to the genus Talaromyces (Yilmaz et al. 2014). Aphanoascus was synonymized with Anixiopsis Hansen (1897), even though they were considered two different genera by several researchers based on their cleistothecia differences in color and size, late or early closure of their peridial walls and ascospore morphology (de Vries 1969; Guého and de Vroey 1986). In the current study, Aphanoascus fulvescens was chosen as a conserved name. The neotype was chosen as Aphanoascus fulvescens UAMH 4114 for several reasons. First, the generic name Aphanoascus was described prior to Anixiopsis. Second, the material for UAMH 4114 was described in the UK similar to Cooke’s original material (Cooke 1875).
Another keratinophilic fungus, Castanedomyces australiensis (Cano et al. 2002b; ex-type culture FMR 5484), was described with the same morphological characteristics as A. fulvescens (i.e., ascomata with membranous peridium and lenticular ascospores with an equatorial crest and polar thickenings), but the two species were distinguished by a difference of 15 nucleotides in the 18S region (Cano et al. 2002b). According to the results of the ITS analysis in the current study (Fig. 7), C. australiensis can be considered a synonym of A. fulvescens (99.8% ITS identity to KT155718).
Chrysosporium tropicum (Carmichael 1962; ex-type culture CBS 171.62, from woolen overcoat, the Solomon Islands) showed a 100% identical ITS and LSU profile with the keratinophilic species Aphanoascus verrucosus (Cano and Guarro 1990; ex-type culture CBS 468.88 from arable soil, Spain). Therefore, C. tropicum is considered a synonym of A. verrucosus. Similarly, the type of C. shanxiense (ex-type culture GZAC EB1601 M.1) was synonymized with A. verrucosus (99% ITS identity to NR_131309.1), and C. articulatum was synonymized with A. reticulisporus (100% ITS identity to MH859002). Another Chrysosporium species, C. jingzhouense (Zhang et al. 2017a; holotype GZUIFR-EB1303M), was found in the same cluster as the types of A. reticulispora and A. keratinophilus (with 97% and 94% ITS identity, respectively). Similarly, C. crassitunicatum (Kushwaha and Agrawal 1977; ex-type culture: CBS 167.78) was found to be close to the type of A. pinarensis (ex-type culture: CBS 113874) with 96% ITS identity. Therefore, the following combinations were proposed for these two species:
Aphanoascus jingzhouensis (Zhang, Han & Liang) Kandemir & de Hoog, comb. nov. – MycoBank MB842358. Basionym: Chrysosporium jingzhouense Zhang, Han & Liang—Phytotaxa 303(2): 175, 2017; holotype GZUIFR-EB1303M, from farmland soil, China.
Aphanoascus crassitunicatus (Kushwaha & Agarwal) Kandemir & de Hoog, comb. nov. – MycoBank MB842359. Basionym: Chrysosporium crassitunicatum Kushwaha & Agarwal—Trans. Br. Mycol. Soc. 68(3): 464, 1977; ex-type culture CBS 167.78, Herb. IMI 185320, from buried human hair, India.
CBS 783.70 is the type strain of Xynophila mephitalis (Malloch & Cain 1971), defining this genus. As shown earlier by Cano and Guarro (1990), this genus is not significantly different from Aphanoascus and should be regarded as a synonym. Similarly, the type species of Neoxenophila, N. foetida (Apinis & Clark 1974; ex-type culture: CBS 453.75), is found amidst Aphanoascus species; the genus is considered a synonym, confirming Cano and Guarro (1990). Chrysosporium lucknowense (Garg 1966; ex-type culture: CBS 143.77) and C. mephiticum (Sigler et al. 1986; ex-type culture: CBS 320.86) clustered with Aphanoascus foetidus (Cano et al. 2002a; ex-type culture: CBS 453.75) in all trees. Cano et al. (2002a) reported C. lucknowense to be an asexual morph and C. mephiticum a synonym of A. foetidus. In the current study, the ITS similarity of C. lucknowense, C. mephiticum and A. foetidus was found to be above 98% (NR_145206.1, NR_145207.1 and KT55907, respectively), and the LSU similarity was above 99% (NG_063936.1, MH873650 and AB040693, respectively). Additionally, C. guizhouiense (ex-type culture: EM14.2002) also placed in this cluster with 98% ITS identity to the type of A. foetidus (NR_158324.1 vs. KT55907). For precise species resolution, multilocus studies of a larger number of strains are needed.
Group 5-III Amauroascus Schröt.
Type: Amauroascus niger Schröt.—Krypt.-Fl. Schlesien (Breslau) 3.2(1–2): 211, 1893 ≡ Arachniotus niger (Schröt.) Kuehn, Orr & Varsavsky—Mycopath. Mycol. Appl. 25: 106, 1965; ex-type culture: CBS 114.61 = ATCC 22339 = IFO 32599 = NRRL A-10697, from soil, USA, G.F. Orr.
In addition to the type species of Amauroascus, the group included the type species of Brunneospora (B. reticulata), Byssoonygena (B. ceratinophila), Coccidioides (C. immitis) and Uncinocarpus (U. reesii) (Fig. 7).
The genus Amauroascus was found to be polyphyletic (Fig. 1; Supplementary Figures 1‒4). Nomenclatural confusion about this genus goes back to 1893, when Schroeter described two new genera, Amauroascus and Arachniotus, at the same time (Schroeter 1893). In Amauroascus, he included Eidam’s Gymnoascus verrucosus (Eidam 1886; from moldy leather boots) and his own new species Amauroascus niger (Schroeter 1893; from old badger dung). The only criterion to differentiate between these two genera was the color of the ascospores. Since this is unfit for generic distinction, some researchers considered these two genera congeneric (Benjamin 1956; Kuehn 1958; Orr et al. 1965), whereas von Arx (1971) recognized Amauroascus as a separate genus and indicated A. verrucosus as a type species, albeit without an explanation. However, A. niger was already cited as the lectotype of the genus earlier (Clements and Shear 1954; Benjamin 1956), and it was suggested to follow this choice according to article 8.1 (ICBN) (Currah 1985).
Rammeloo (1982) compared the type specimens of Onygena mutata Quélet (1875; from the hoofs of an ox) and A. verrucosus (Eidam) Schroeter (1893; from moldy leather boots) and reported that the two species were identical, confirming the conclusions of Orr et al. (1965). However, he chose to change the name O. mutata to Amauroascus mutatus for the combined species. Since A. verrucosus was described earlier, it had priority over A. mutatus. Therefore, the following epitype is designated here for Amauroascus verrucosus: CBS 181.70, from soil, USA, G.F. Orr. This strain has an alternative collection number ATCC 22395.
Apinisia queenslandica (ex-type culture: CBS 280.77 = IMI 121675 = IFM 47370) was described from bird feathers in Australia by Apinis and Rees (1976). The species was subsequently placed in Uncinocarpus based on microscopic morphology (Sigler et al. 1998) and then moved to Amauroascus based on a higher ITS sequence similarity compared to Uncinocarpus reesii (Solè et al. 2002a). Solè et al. (2002a) synonymized Brunneospora reticulata (Guarro et al. 1987b; ex-type culture: FMR 784, from arable soil, Spain) and Orromyces spiralis (Ghosh and Sur, 1985; ex-type culture: GS-191, from soil, India) with U. queenslandica. These species are all keratinophilic and possess gymnothecia, helical peridial appendages, pigmented ascospores and chrysosporium-like anamorphs. The only difference mentioned in the description of the species was in terms of ascospore shape, i.e., globose in Apinisia, ellipsoidal in Brunneospora, oval in Orromyces and ovoid to globose in Uncinocarpus (Apinis and Rees 1976; Ghosh and Sur 1985; Guarro et al. 1987b; Solè et al. 2002a). In the current study, Amauroascus verrucosus and Amauroascus niger grouped with U. reesii, whereas A. queenslandica and B. reticulata formed a separate, supported branch in this group (Figs. 1 and 7). The similarity between A. queenslandica (ex-type culture: CBS 280.77) and B. reticulata (ex-type culture: UAMH 5704) was found to be 98% (in 546 bp). On the other hand, the similarity of Apinisia queenslandica (AJ390394) with U. reesii (NR_111092.1) and with A. niger (NR_159639.1) was 81%. Although the strain CBS 280.77 was first described as Apinisia, it was found to be placed far from the generic type, Apinisia graminicola (La Touche 1968; ex-type culture: CBS 721.68 on rotting Poaceae, UK). For Orromyces, ex-type (GS-191) and isotype cultures (GS-141) of O. spiralis were reportedly deposited in the fungal herbarium of the Botany Laboratory, Department of Sciences, Regional College of Education, Bhubaneswar, Orissa (Ghosh and Sur 1985). However, we did not receive any response from the herbarium regarding the presence of this species, and no related information is available from other collections. Therefore, Orromyces spiralis is considered doubtful and has been abandoned. The following combination is proposed for A. queenslandica:
Brunneospora queenslandica (Apinis & Rees) Kandemir & de Hoog, comb. nov. – MycoBank MB842360. Basionym: Apinisia queenslandica Apinis & Rees—Trans. Br. Mycol. Soc. 67(3): 524, 1976 = Uncinocarpus queenslandicus (Apinis & Rees) Sigler—Can. J. Bot. 76(9): 1632, 1999; ex-type culture: CBS 280.77 = IMI 121675 from feathers of domestic fowl, Australia, Apinis and Rees, 1965.
Byssoonygena ceratinophila (ex-type culture: ATCC 64724 = FMR 785 = IMI 316057 = UAMH 5669) was also found in Group III and clustered with Brunneospora queenslandica (ex-type culture: CBS 280.77; Fig. 7), which are keratinophilic, possess gymnothecia and brown ascospores, whereas Byssoonygena lacks peridial appendages and has a malbranchea-like anamorph (Guarro et al. 1987a).
The genus Coccidioides Stiles (Rixford and Gilchrist 1896) is represented by two species: the generic type Coccidioides immitis and Coccidioides posadasii (Fisher et al. 2002). Coccidioides is a unique genus in Onygenaceae, having an environmental asexual life cycle and a pathogenic phase with spherules and endospores in mammalian hosts (Lewis et al. 2015). The genus combines keratinophily and thermal dimorphism with xerotolerance, being able to grow in soils with low pH levels and temperatures between –6.5 °C and 60.6 °C and tolerating up to 8% NaCl (del Rocío Reyes-Montes et al. 2016; de Hoog et al. 2005; Barker 2018). Previously, some arthroconidial fungi isolated from soil were described as the Malbranchea state of C. immitis and named Malbranchea dendritica and Malbranchea gypsea (Sigler and Carmichael 1976); however, despite their morphological resemblance with C. immitis, they were not shown to form endosporulating spherules in tissue (Orr 1972). Malbranchea gypsea was found to be cellulolytic (Sigler and Carmichael 1976). The phylogeny also supported that Malbranchea and Coccidioides are two different genera (Fig. 1). Consequently, M. gypsea was transferred to Spiromastigoides, and M. dendritica was kept in Malbranchea.
Group 5-IV Onygena Pers.
Type species: See above.
The genus Onygena contains 12 species, excluding the varieties (Index Fungorum, access date 9 February 2021). Among these species, O. lycoperdon was recorded as nomen nudum (MycoBank.org; access date 15 March 2022), O. mougeotii was recorded as a synonym of O. equina and Onygena mutata was renamed as Amauroascus mutatus (Rammeloo 1982) (see section Group 5-III, Amauroascus). There are no known type specimens or isolates for O. apus (Berkeley and Broome 1851; from decaying bones in UK), O. bommerae (Saccardo 1913; on feathers and bones of birds), O. caprina (Fuckel 1870; from a sheep horn), O. hypsipus (Ditmar 1813; on marten excretion mixed with mouse bones), O. piligena (Fries 1829; from sheep wool and murine fur) and O. ungulina (Rostrup 1894; on horse hooves). Therefore, they have been considered doubtful. Only the specimens that were identified as O. equina, which is the type species of the family Onygenaceae determining the order Onygenales, and O. corvina are represented by herbarium specimens in collections. Since the original type materials are not known to be preserved for both species, neotypes are indicated in the present study.
Onygena corvina Alb. & Schwein.—Consp. Fung. (Leipzig): 113, 1805. Neotype designed herewith: Herb. No: CBS H-15266, on rotting bird remains, The Netherlands, H.A. van der Aa, 1972. Culture derived from type: CBS 152.73.
The additional species Aphanoascella galapagosensis (ex-type culture: CBS 132345, isolated from a Galápagos tortoise Chelonoidis nigra; Sutton et al. 2013) and another reptile-associated member, Emydomyces testavorans (ex-type culture: ATCC TSD-145) (Woodburn et al. 2019), also cluster in Onygenaceae (Figs. 1 and 7). Even though they formed a monophyletic group based on multilocus data analyses, mutual distances and distances to other reptile-related members of the clade were considerable in ITS and LSU analyses (Supplementary Figures 1, 2 and 4).
The genus Pseudomalbranchea (type species P. gemmata) was recently described by Rodríguez-Andrade et al. (2021) from a human bronchial washing specimen, and the ex-type culture CBS 146933 clustered in Onygenaceae Group 5-IV together with keratinophilic and soil-borne Pectinotrichum. The ITS similarity between the two genera was 69% (NR_119467.1 vs. LR701761). The soil-borne genus Pectinotrichum (type species P. llanense, ex-type culture: CBS 882.71) is morphologically similar to Arthroderma in having gymnothecia with characteristically arched hyphae with short and long appendages that are usually slightly curved or bent at the apices (Varsavsky and Orr 1971). The GenBank accession number AJ390391 for the ITS locus of the type species was found to be different from that of MH860394, NR_119467.1 and LR136983. Phylogenetic results with the first one showed P. llanense in Arthrodermataceae together with Pectinotrichum chinense, while the results with the other records placed the species in Onygenaceae. Molecular analyses based on small subunit (SSU) and LSU showed that generic type species P. llanense clusters in the family Onygenaceae (Sugiyama et al. 1999; Sugiyama and Mikawa 2001), while P. chinense is amidst species of Arthroderma (Fig. 6). Consequently, the following combination is proposed:
Arthroderma chinense (Zhang & Cai) Kandemir & de Hoog, comb. nov. – MycoBank MB842354. Basionym: Pectinotrichum chinense Zhang & Cai—Persoonia 39: 21, 2017; ex-type culture: LC5811, from soil from a karst cave, China, isolated by Z.F. Zhang & S.Y. Cai.
Group 5-V Nannizziopsis Currah.
Type species: Nannizziopsis vriesii (Apinis) Currah—Mycotaxon 24: 164, 1985 ≡ Rollandina vriesii Apinis—Trans. Br. Mycol. Soc. 55(3): 501, 1970; ex-type culture: CBS 407.71 = CMI 149994, from Ameiva skin and lung, The Netherlands, collected by A.E. Apinis, isolated by G.A. de Vries.
Rollandina vriesii (Apinis 1970) was described based on the description of the type species of the genus, namely, Rollandina capitata (Patouillard 1905; type specimen from green waste, Vietnam, in Herb. FH). However, re-examination of the type specimen of R. capitata yielded different descriptions by different researchers (Benjamin 1956; Apinis 1970). Von Arx (1971) considered the genus Rollandina to be doubtful because the type species is not known in pure culture. Currah (1985) followed von Arx, indicating Rollandina as a nomen confusum and proposed a new genus, Nannizziopsis, which was classified in Onygenaceae to accommodate R. vriesii.
Stchigel et al. (2013) combined the physiology, morphology and multilocus phylogeny of Nannizziopsis and several chrysosporium-like strains, mostly isolated from reptile habitats, and proposed a new family, Nannizziopsidaceae. The predominant reptile-associated ecology, combined with discrete, spherical ascomata with a peridium of loosely interwoven, hyaline hyphae; eight-spored, spherical asci; and thick-walled, smooth, hyaline ascospores (Stchigel et al. 2013), was suggestive of an independent evolutionary course. In the larger phylogeny of Onygenales, this family does not clearly form an individual entity separate from Onygenaceae (Fig. 7). The reptile-associated fungus Ophidiomyces ophiodiicola (ex-type culture: CBS 122913) (Sigler 2013), isolated from a granuloma of a black rat snake, was found to be remote from Nannizziopsis and Paranannizziopsis in Onygenaceae (Figs. 1 and 7).
Clade 6 Malbrancheaceae Kandemir & de Hoog, fam. nov. MycoBank 843,372.
Type genus: Malbranchea Sacc.—Michelia 2(no. 8): 639, 1882; type species: Malbranchea pulchella Sacc. & Penz.; on wet cardboard, France; a slide of the type: ex-DAOM 41374.
= Malbranchea bolognesii-chiurcoi Vuill., Pollacci & Nann.—Archivi di Biologia 1: 255, 1925; ex-type culture: CBS 202.38 = MUCL 8435 = IFM 41308, from thoracic ulcer of a man, Italy = Malbranchea kambayashii Kambay.—Archiv für Dermatologie und Syphilis 170: 106, 1934; ex-type culture: CBS 203.38 = MUCL 8436 = UAMH 3794 from a facial skin lesion, China = Auxarthron californiense Orr & Kuehn—Can. J. Bot. 41: 1439, 1963; ex-type culture: CBS 129.62 = ATCC 15600 = IHEM 4422 = NRRL A-1122, from dung of pack rat, the USA.
The members of the genera Auxarthron and Malbranchea were placed in Clade 6 with 100% support in all phylogenetic analyses but formed an unsupported clade (40% bootstrap support) in ITS Group II analysis (Supplementary Figure 4). The genus Auxarthron has been described with mesh-like gymnothecia, well-differentiated thick-walled peridial hyphae with knuckle-joint septal enlargements (usually in 1‒3 septa) (except A. concentricum, which lacks both knuckle joints and peridial appendages), punctate ascospores and an asexual morph in Malbranchea (Orr et al. 1963a; Currah 1985; Sigler et al. 2002a; Sarrocco et al. 2015). The genus was classified as a monophyletic group in Onygenaceae (Currah 1985; Sarrocco et al. 2015; Rodríguez-Andrade et al. 2021). However, in the present study, the group was considered a separate family because this monophyletic group was found to be distant from the type species of Onygenaceae (Figs. 1 and 8). Members of the family can be found in eutrophic environments enriched by dung, such as in cave soil, or on animal dung. During the present study, Rodríguez-Andrade et al. (2021) synonymized Auxarthron with Malbranchea based on the phylogenetic analysis of the concatenated ITS-LSU sequences, and the same authors provided an identification key for the species in the genus. In the present study, the following combinations were proposed accordingly:
Phylogenetic tree of Malbrancheaceae based on ITS sequences obtained with Bayesian analysis and maximum likelihood analysis using the SYM + G4 + I model in IQ-TREE-ML. Branch values of ≥ 90% for Bayesian probability and ≥ 80% for maximum likelihood and type species of the genera are shown in bold. New combinations are indicated in red. Arthroderma curreyi was used as the outgroup
Malbranchea reticulata (Zukal) Kandemir & de Hoog, comb. nov. – MycoBank MB842883. Basionym: Gymnoascus reticulatus Zukal—Verh. Zool.-Bot. Ges. Wien 37: 40, 1887 ≡ Auxarthron reticulatum (Zukal) Orr & Plunkett—Can. J. Bot. 41(10): 1443, 1963; neotype culture CBS 201.64 = ATCC 18426 = O-1020 = NRRL A-10,748 designated by Orr et al. (1963a), isolated from wood slat from a greenhouse flat, USA by G. F. Orr.
Malbranchea pseudoreticulata (Currah) Kandemir & de Hoog, comb. nov. – MycoBank MB842884. Basionym: Amauroascus pseudoreticulatus Currah—Mycotaxon 24: 134, 1985 ≡ Auxarthron pseudoreticulatum (Currah) Solé, Cano & Guarro—Mycol. Res. 106(4): 393, 2002; holotype UAMH 3117, isolated from lizard dung, Mexico by R.K. Benjamin.
Malbranchea thaxteri (Kuehn) Kandemir & de Hoog, comb. nov. – MycoBank MB842885. Basionym: Myxotrichum thaxteri Kuehn—Mycologia 47(6): 878, 1956 ≡ Gymnoascus umbrinus var. thaxteri (Kuehn) Apinis—Mycol. Pap. 96: 14, 1964 ≡ Auxarthron thaxteri (Kuehn) Orr & Kuehn—Mycologia 63(2): 200, 1971; type: CBS 248.58 = NRRL 1714 isolated from opossum dung, Haiti by R. Thaxter.
Clade 7 Neogymnomycetaceae Kandemir & de Hoog, fam. nov. MycoBank MB842886
Type genus: Neogymnomyces Orr—Can. J. Bot. 48(6): 1065, 1970; type species: Neogymnomyces demonbreunii (Ajello & Cheng) Orr—Can. J. Bot. 48(6): 1065, 1970 ≡ Gymnoascus demonbreunii Ajello & Cheng—Mycologia 59(4): 692, 1967; ex-type culture: CBS 427.70 = ATCC 18394 = O-3200 = M19, from soil, USA, J. Brandsberg. Herb. No: CBS H-7197, provided by G.F. Orr, 1970.
The clade was formed by species mainly isolated from dung and soil (Fig. 4) and supported with a 98% bootstrap value in multilocus data analysis (Fig. 1). In addition to Neogymnomyces, the generic types Auxarthronopsis bandhavgarhensis (ex-type culture: CBS 134524, from soil inside hollow tree, India), Canomyces reticulatus (ex-type culture: MCC 1486, from soil under a tree, India), Currahmyces indicus (ex-type culture: MCC 1548, from hen-populated soil, India) and Renispora flavissima (ex-type culture: CBS 708.79 from soil in barn housing Myotis velifer, USA) were placed in the clade. Based on the generic types of the genera, Clade 7 can be divided into two main groups: Group Neogymnomyces and Group Auxarthronopsis. Canomyces reticulatus, Auxarthronopsis stercicola (ex-type culture: CGMCC3.19639, from animal feces in karst Cave, China) and Auxarthronopsis pulverea (ex-type culture: CGMCC3.19312, from plant debris in karst Cave, China) clustered in the same group based on LSU and multilocus data analyses (Fig. 1; Supplementary Figures 1 and 2); however, Canomyces was in a different group in the ITS tree (Fig. 9; Supplementary Figures 3 and 4). The similarity of Auxarthronopsis pulverea and Canomyces reticulatus was 84% for ITS and 98% for LSU loci. Similarly, ITS identity for Auxarthronopsis stercicola and Canomyces reticulatus was found to be 85%, while the LSU identity was 98%. Morphological differences between these species were recorded in previous studies (Zhang et al. 2021; Sharma and Shouche 2021). The ITS similarity of Auxarthronopsis stercicola and A. pulverea was 97%, while the LSU similarity between these two species and the type species A. bandhavgarhensis was 85% and 86%, respectively. These results support that A. stercicola and A. pulverea belong to the same genus, but they should be excluded from Auxarthronopsis. Additionally, Amauroascus purpureus (ex-type culture: UAMH 8294, from cultivated soil, Japan), Amauroascus volatilis-patellus (ex-type culture: CBS 249.72, from clay soil, USA) and Nannizziopsis mirabilis (ex-type culture: UAMH 7712, from forest soil, USA) in Clade 7 also need nomenclatural revision since they are remote from their generic types Amauroascus niger (ex-type culture: CBS 114.61, from soil, USA) and Nannizziopsis vriesii (ex-type culture: CBS 407.71, from lizard skin and lung, The Netherlands).
Phylogenetic tree of Neogymnomycetaceae based on ITS sequences obtained with Bayesian analysis and maximum likelihood analysis using the TNe + G4 + F model in IQ-TREE-ML. Branch values of ≥ 90% for Bayesian probability and ≥ 80% for maximum likelihood and type species of the genera are shown in bold. A new combination is indicated in red. The species that need nomenclatural revision are enclosed in square brackets. Nannizziopsis arthrosporioides and Nannizziopsis vriesii were used as outgroup species
Another keratinophilic fungus, Chlamydosauromyces punctatus (ex-type culture: UAMH 9990, from skin of lizard, USA), is also placed in Clade 7 along with Neogymnomyces species based on ITS phylogeny (Fig. 9; Supplementary Figures 3 and 4). With the original description of C. punctatus, Neogymnomyces species were not included in the analyses since the molecular data were not available at that time (Sigler et al. 2002b). In the present study, ITS similarity between C. punctatus and N. demonbreunii was found to be 94%. Both genera are keratinophilic and have gymnothecial ascomata; however, their ascospore and culture morphologies are different (Sigler et al. 2002b; Doveri et al. 2012). The following recombination is proposed for C. punctatus:
Neogymnomyces punctatus (Sigler, Hambleton & Paré) Kandemir & de Hoog, comb. nov. – MycoBank MB842361. Basionym: Chlamydosauromyces punctatus Sigler, Hambleton & Paré—Stud. Mycol. 47: 127, 2002; ex-type culture: UAMH 9990 from shed skin of lizard (Chlamydosaurus kingii), USA, isolated by J. Paré, 2001.
Clade 8 Incertae sedis
Two original materials of the xerophilic species Diploospora rosea (epitype: DAOM 250100, from dust, Micronesia) and Amauroascus aureus (ex-type culture: CBS 593.71, from decaying wood in cavity of stump, Japan) formed a clade in multilocus data analysis (Fig. 1). ITS Group I analysis revealed different results from multilocus and LSU data analyses, such that A. aureus was found in a basal position to Clade 7 Neogymnomycetaceae and D. rosea was found close to Gymnoascaceae (Supplementary Figure 3). The ex-type strain CBS 593.71 (ATCC 18654 = NRRL 12184 = Orr O-2512 = IMI 151274 from decayed wood ex tree stump cavity, Japan) of A. aureus was first described as a neotype for Arachniotus aureus (Kuehn et al. 1964b) based on the descriptions provided by Eidam (1886) for Gymnoascus aureus. Currently, it is known as the type of Amauroascus aureus (von Arx 1971). In all phylogenetic analyses, this strain was found remote from the type species of these genera (Fig. 1; Supplementary Figures 1‒4), therefore considered incertae sedis.
The genus Diploospora was described with the type species Diploospora rosea by Grove (1916). Nearly 100 years after its first discovery, another strain (DAOM 250,100) was reported from dust in a residence in Micronesia (Tanney et al. 2015). It was described with dull pink colonies and oblong, apiculate conidia borne in long chains showing mixed acropetal and basipetal extension. Conidiogenesis was also described as a mixture of blastic and thallic (Tanney et al. 2015). Considering the source of the reported strains, D. rosea seems to be xerophilic and adapted to the indoor environment. In addition, mite feces seen on the holotype were consistent with the dung affinity of Gymnoascaceae members (Tanney et al. 2015) (Fig. 10). Further molecular analyses are required to define this species’ position in Onygenales.
Phylogenetic tree of Gymnoascaceae based on ITS sequences obtained with Bayesian analysis and maximum likelihood analysis using the TIM2 + I + G4 + F model in IQ-TREE-ML. Branch values of ≥ 90% for Bayesian probability and ≥ 80% for maximum likelihood and type species of the genera are shown in bold. The species that need nomenclatural revision are enclosed in square brackets. Arthroderma flavescens and Arthroderma vespertilii were used as the outgroup species
Clade 9 Gymnoascaceae Baranetzky
Type genus: Gymnoascus Baranetzky—Bot. Ztg. 30: 158, 1872; type species: Gymnoascus reessii Baran., ex-type culture: CBS 392.64, from soil, USA, G.F. Orr. Herb. No: CBS H-13156.
Ascocarps in this family are mostly yellowish, greenish, reddish or brownish shades, with a gymnoperidium composed of a loose network of hyaline or pigmented hyphae. Asci globose to ovoid; ascospores are hyaline, yellowish or greenish, spherical, ovoidal or oblate, smooth- and thick-walled, sometimes with equatorial grooves and ridges and/or polar thickenings (Currah 1985; Doveri et al. 2012).
Based on the number of described species, Gymnoascaceae is one of the largest families of Onygenales (Solé et al. 2002b). Members of the family are eutrophic and can be found in soil rich in organic matter, various types of dung, and decaying vegetation (Fig. 4). Species (e.g., Gymnoascus reessii) have also been isolated from the lungs of wood rats and prairie dogs (Emmons 1954) without any lesions in the lungs, and they have been found to survive mouse passage without causing disease. This survival ability is similar to that of the dimorphic fungus Emmonsia crescens (now known as Emergomyces crescens), which is commonly carried asymptomatically by terrestrial rodents (Jiang et al. 2020). Survival mechanisms in the lung for such environmental pathogens are mostly related to in vitro and in vivo conidial changes (Jiang et al. 2018, 2020), and for Gymnoascaceae members, these mechanisms are not known to exist. Skin and nail scrapings and animal hair are the main sources of dermatophytes because of their high keratin content (Currah 1985; de Hoog et al. 2017). Members of Gymnoascaceae are frequently found colonizing similar keratinized material shed from humans and other mammals (Currah 1985). Baiting techniques with horse and/or human hair or pieces of hooves have successfully been used for the selective isolation of keratinolytic Gymnoascaceae and the examination of their gymnothecial structures (von Arx 1986; Sharma and Singh 2013).
Osmotolerance might be another trait that enhances the wide distribution of Gymnoascaceae. Sporendonema casei and Sphaerosporium equinum have repeatedly been isolated from cheese, which seems to be their preferred habitat (Ropars et al. 2012; Hermet et al. 2014). According to available records, almost all strains have only been isolated from the rinds of hard cheeses. This habitat lacks moisture, and the relatively high free fatty acid content makes this part highly acidic compared to the interior of the cheese. In addition, the rind has higher levels of crude protein and salt (calcium and phosphorus) (Davies 1938). The ripening process takes place at low temperatures (4‒18 °C), and given the high acid and salt concentrations, these species can be considered xerophilic and halophilic. However, Scaramuzza et al. (2015) showed that S. casei can also be isolated from the air of cheese processing rooms.
Other members of the family Gymnoascaceae show other types of halotolerance, e.g., Gymnoascus halophilus, from lake sediments and can tolerate NaCl concentrations of up to 22.5% (Zhou et al. 2016). Species found under conditions of extreme dryness, such as Gymnoascus desertorum, may possess similar survival factors. Desert soil colonization can also be enhanced by oligotrophism. Possibly due to the wide range of habitat choices of the members of Gymnoascaceae, physiological trends are apparent in this family, suggesting alternative lifestyles, such as eutrophism on animal dung. Temperature relationships seem equally variable: Gymnoascus stercorarius, found in compost, can grow at temperatures up to 50 °C, while Arachniotus ruber, from coyote dung, requires a low temperature of 5 °C for its isolation (Zhou et al. 2016; Currah 1985).
After the description of the family and the genus Gymnoascus in 1872, 20 genera were included in Gymnoascaceae (Table 1). Species of the genera were reclassified in the family several times by different researchers, mostly using peridium and ascospore morphology as taxonomic criteria (e.g., von Arx 1986; Currah 1985; Apinis 1964). Gymnascella was introduced by Peck (1884) for the single species Gymnascella aurantiaca from old bones in a damp place. The fungus lacked an ascigerous fruitbody, and asci formed clusters between undifferentiated hyphae. Arachniotus Schroeter (1893) was described as having three species that were originally classified in Gymnoascus: G. candidus Eidam, G. aureus Eidam and G. ruber van Tieghem. Arachniotus candidus (Eidam) Schroeter was considered the generic type species. The genus had snow-white or yellowish gymnothecia and thin-walled peridial elements covering the asci in a net-like form, similar to a fragile membrane (Orr et al. 1977a). Pseudoarachniotus Kuehn (1957) was described to separate some Arachniotus species that lacked discrete ascocarps or peridial elements, formation of croziers and lack of conidia on phialides. Type species was indicated as Pseudoarachniotus roseus. However, in describing this genus, Gymnascella was not taken into account. Later, Pseudoarachniotus was used to accommodate species that could not be classified as either Arachniotus or Gymnascella (Orr et al. 1977a). Petalosporus Ghosh, Orr & Kuehn, with the type species Petalosporus nodulosus, was described as having discrete ascocarps, characteristic enlargements at the nodes of smooth hyphae, which comprise a rudimentary peridium and petaloid arrangement of ascospores in asci (Ghosh et al. 1963). Narasimhella Thirum. & Mathur (1966) (type species, N. poonensis) was described for species with well-developed stipitate fruitbodies without peridium; the stipe is composed of upwards growing hyphal bundles, while the irregularly globular, fertile head portion contains groups of asci. Crozier formation was similar to that of Pseudoarachniotus. The genus Plunkettomyces Orr, represented by the type species Plunkettomyces littoralis, has peridial elements that are smooth or coarsely tuberculate, radiating outward from the centrum or the base of the asci (Orr 1977a). Ascospores were lenticular, with a yellow-orange to orange-brown longitudinal band (Orr 1977a). Plunkettomyces littoralis was thought to represent an intermediate between Gymnascella and Pseudoarachniotus. Disarticulatus Orr, with the type species Disarticulatus devroeyi, had yellow-orange to orange gymnothecia, which tended to disarticulate into components with variously shaped, thick-walled cells (Orr 1977a). Ascospores were described as spherical or ellipsoidal, with a slight lateral bulge (Orr 1977a). Disarticulatus was found to be similar to Petalosporus, except for the absence of the petaloid arrangement of ascospores in the asci. Gymnoascoideus was introduced by Orr et al. (1977b) to describe species lacking gymnothecia but having typical elements with branching at right angles, unsharpened at the apices and covered by asexual spores in and around the ascal clusters. Gymnoascoideus shows discrete gymnothecia lacking appendages, and similar to Petalosporus, its ascospores show a petaloid pattern in the asci, but in contrast, they lack lateral bulges. Gymnoascoideus petalosporus was assigned as the type species. Kraurogymnocarpa Udagawa & Uchiyama, represented by Kraurogymnocarpa lenticulospora, was described with yellowish-green ascomata with a disarticulating reticuloperidium, bivalvate ascospores, and weak cellulolytic activity (Udagawa and Uchiyama 1999a). The discrete ascomata have short spines and straight or slightly curved appendages. Mallochia Arx & Samson was introduced to classify Pseudoarachniotus echinulatus into a separate genus (Arx and Samson 1986). In Mallochia echinulata, similar to Narasimhella, obovate asci develop from croziers. However, ascospores in the latter species are bivalvate, with an equatorial furrow surrounded by two rims and covered with blunt spines (von Arx and Samson 1986). The generic names introduced in Gymnoascaceae are shown in Fig. 11.
Summary of the genera described in Gymnoascaceae. Gray boxes indicate the genera to which the synonyms of the type species belong. Type species for each genus given in the brown boxes are Arachniotus ruber, Diploospora rosea, Gymnascella aurantiaca, Gymnoascoideus petalosporus, Gymnoascus reessii, Kraurogymnocarpa lenticulispora, Mallochia echinulata, Narasimhella poonensis, Petalosporus nodulosus, and Sporendonema casei. Sphaerosporium is described with the type species S. lignatile Schwein. (1832), which is not classified in Gymnoascaceae; therefore, only S. equinum is shown in the figure
From this summary of genera attributed to Gymnoascaceae on morphological grounds, it appears that a limited set of characters is used in different combinations to define the genera. Many of these fungi have impressive morphologies, which taxonomists acknowledge. However, re-examination of deposited strains or type materials revealed that the morphology may vary within species. For example, de Vroey (1966) examined several strains of Pseudoarachniotus roseus and noted that the presence or absence of discrete ascocarpic elements was variable, and he consequently regarded P. roseus as a synonym of Arachniotus dankaliensis. Similarly, in describing Arachniotus flavoluteus, Kuehn and Orr (1959) observed some ascal clusters that were devoid of peridial elements, as in Pseudoarachniotus. Phylogenetic studies also indicated that species with slight morphological differences may share the same molecular characteristics (Solé et al. 2002b), and vice versa. A Bayesian inference tree of the ITS locus of Gymnoascaceae (Fig. 10) showed that members of several of the genera mentioned above are not clearly separated into monophyletic groups; no generic substructure can be recognized that matches the current concepts. In contrast, Sporendonema and Sphaerosporium, which have not been associated with Gymnoascaceae, clustered within this amalgamate (Figs. 1 and 10).
Phylogenetic results in the current study revealed five groups in the family: Group I Gymnascella, Group II Arachniotus, Group III Gymnoascus, Group IV Narasimhella and Group V Gymnoascoideus. Group names were determined by choosing the well-supported branches with high bootstrap values. Type species for each genus in the group were detected. In the case of more than one type species for different genera in the same group, the oldest type species is maintained to name the group.
Group 9-I Gymnascella Peck
Type species: Gymnascella aurantiaca Peck—Ann. Rep. N.Y. St. Mus. Nat. Hist. 35: 143, 1882. Neotype designated here: UAMH 3138, from lizard dung, USA, R.K. Benjamin, 1969. Alternative strain number: RSA 1483.
= Arachniotus verruculosus Orr & Kuehn—Mycologia 64(1): 62, 1972. ex-type culture: CBS 655.71 = ATCC 18897 = IMI 155648 = NRRL 3716 = QM 9290 = DPG 138 from clay soil, USA.
Orr and Kuehn (1972) described A. verruculosus based on the presence of discrete ascocarps, smooth, thick-walled, ovoid to oblate ascospores and characteristic, coarsely verrucose, loose peridial elements around the asci. Currah (1985) synonymized A. verruculosus with Gymnascella aurantiaca Peck, and the results of the present study confirmed this, with 99% ITS similarity between the type species (Supplementary Table 2).
Habitats of the species in this group mostly tended to be dung and soil, especially highly nutritional clay soil (Fig. 4; Supplementary Table 1). The group contains halotolerant and thermotolerant species such as G. thermotolerans and G. stercorarius. Similarly, Petalosporus anodosus has been reported to grow more rapidly at 37 °C than at 30 °C (Kuehn et al. 1964a). Plunkettomyces littoralis, which is known to occur only in marine habitats, clustered in the same group. These findings might be a clue for a common halophilic ancestor of Group 9-I Gymnascella and Group 9-II Arachniotus, which contains species predominantly isolated from environments with high salt content.
The genus Pseudoarachniotus was introduced to include species without discrete ascomata and with a loose peridium, ascocarp initials and croziers, evanescent asci and ascospores in variable shapes (globose, ovoid, elliptical or lenticular) and colors (hyaline, red or shades of yellow) (Kuehn 1957). Although Kuehn did not consider Gymnascella with his description of Pseudoarachniotus, Currah (1985) placed several species previously described in Arachniotus and Pseudoarachniotus with oblate ascospores in Gymnascella. Phylogenetic analyses showed that these species can be united under the name Gymnascella based on ≥ 90% ITS similarity with the neotype of G. aurantiaca (Supplementary Table 2).
Gymnascella dankaliensis (Castell.) Currah—Mycotaxon 24: 77, 1985; ex-type culture: CBS 117.38 = UAMH 3552, from camel skin, Africa ≡ Arachniotus dankaliensis (Castell.) v. Beyma—Antonie van Leeuwenhoek 8: 105, 1942 ≡ Gymnoascus dankaliensis (Castell.) v. Arx—Persoonia 13(2): 177, 1986 ≡ Trichophyton dankaliense Castell.—J. Trop. Med. Hyg. 40: 315, 1937.
= Pseudoarachniotus terrestris Thirum. & Mathur—Mycopath. Mycol. Appl. 40(2): 102, 1970 fide von Arx loc. cit.; ex-type culture: CBS 339.65 from soil, India.
= Pseudoarachniotus flavoluteus (Kuehn & Orr) Orr, Ghosh & Roy—Mycologia 69(1): 154, 1977 ≡ Arachniotus flavoluteus Kuehn & Orr—Mycologia 51(6): 864, 1961; ex-type culture: CBS 627.71 from soil, USA.
= Pseudoarachniotus thirumalacharii Mathur—Mycopath. Mycol. Appl. 40(2): 101, 1970 fide v. Arx loc. cit.; ex-type culture: CBS 294.66 from soil, India.
= Pseudoarachniotus roseus Kuehn—Mycologia 49(5): 695, 1957; ex-type culture: CBS 323.58, from ulcerated chicken gizzard, USA.
Gymnascella nodulosa (Ghosh, Orr & Kuehn) Currah—Mycotaxon 24: 88, 1985 ≡ Petalosporus nodulosus Ghosh, Orr & Kuehn—Mycopath. Mycol. Appl. 21: 37, 1963 ≡ Gymnoascus nodulosus (Ghosh, Orr & Kuehn) v. Arx—Persoonia 13(2): 179, 1986; ex-type culture: CBS 577.63 = ATCC 15,318 = IFO 31,789 = IMI 168,760 = NRRL 2882 = QM 8527, from dung of Guinea pig, India.
Ghosh et al. (1963) described the genus Petalosporus primarily based on the floral-shaped arrangement of ascospores. However, this can also be seen in other members of Gymnoascaceae, e.g., Gymnoascus boliviensis and G. petalosporus. In the present study, the type of Petalosporus nodulosus was phylogenetically similar to that of P. afilamentosus. These two species have thick-walled ascospores; P. nodulosus forms a discrete and often fragmentary ascocarp, and its ascospores lack lateral bulges.
Gymnascella afilamentosa (Orr & Kuehn) Currah—Mycotaxon 24: 69, 1985 ≡ Petalosporus afilamentosus Orr & Kuehn—Mycologia 64(1): 62, 1972 ≡ Gymnoascus nodulosus (Ghosh, Orr & Kuehn) Arx—Persoonia 13: 179, 1986; ex-type culture: CBS 658.71 = ATCC 18,901 = IFO 31,787 = NRRL 3717 = DPG 115 = O-2601 = UAMH 3427, from clay soil, USA.
Orr and Kuehn (1972) described the type species of Petalosporus afilamentosus (DPG 115) as lacking discrete ascocarps and having smooth, thick-walled and oblate ascospores with a faint lateral bulge. Our study revealed that P. afilamentosus clusters with G. nodulosus, both of which are closely related to G. dankaliensis and can be found in nutrient-rich environments.
Gymnascella udagawae (Arx) Currah—Syst. Ascom. 7(1): 5, 1988 ≡ Gymnoascus udagawae v. Arx—Persoonia 13(2): 181, 1986; ex-type culture: CBS 950.69 = ATCC 24,072 = IFO 8921 = NHL 2364 = NRRL 5963 = UAMH 5726, from soil, Japan.
Gymnoascus udagawae is found phylogenetically in Group 9-I Gymnascella, with Plunkettomyces littoralis (98% ITS to MH860738) (Fig. 10). Reportedly (von Arx 1986), colonies of two species are bright lemon yellow. However, G. udagawae colonies expand more rapidly (daily rate 3‒4 mm at 25 °C on hay infusion agar), ascospores lack equatorial ridges, and the species has abundant conidia (von Arx 1971, 1986).
Gymnascella littoralis (Orr) Currah—Mycotaxon 24: 87, 1985 ≡ Plunkettomyces littoralis Orr—Mycotaxon 6(1): 34, 1977 ≡ Arachniotus littoralis (Orr) v. Arx—Persoonia 9(3): 397, 1977 ≡ Gymnoascus littoralis (Orr) v. Arx—Persoonia 13(2): 179, 1986; ex-type culture CBS 454.73 = NRRL A-16025 = Orr 3053 = UAMH 3885, from conch shell collected on the beach, Canada.
In the present study, the type strain of Plunkettomyces littoralis CBS 454.73 was found in Group 9-I (Gymnascella), along with G. citrinus and G. udagawae. In the GenBank database, Sano et al. (2010) submitted several ITS sequences (AB566297, AB566285 and AB566287) for several Arachniotus littoralis strains (IFM 58,442, IFM 58,265 and IFM 58,266), which were obtained from skin scrapings of rats. However, these strains did not cluster with the type strain, and the identification of these strains requires more data. In the ITS tree, P. littoralis was found to be close to G. udagawae (98% ITS identity to MH859492) and G. citrinus (98% ITS identity to AJ315836). Morphologically, P. littoralis can be differentiated from G. udagawae by its ascospores having longitudinal rims and from G. citrinus by having an asexual state, which is not known in G. citrinus. In addition, colonies of G. citrinus have a distinct bright lemon-yellow color, and ascospores are oblate with thickened poles (Currah 1985; Orr et al. 1977a). Considering this habitat choice, P. littoralis can be recognized as a halophilic species.
Gymnascella citrina (Massee & Salmon) Orr et al.—Mycologia 69: 134, 1977 ≡ Pseudoarachniotus citrinus (Massee & Salmon) Kuehn—Mycologia 49: 699, 1957 ≡ Arachniotus citrinus Massee & Salmon—Ann. Bot., Lond. 16: 62, 1902 ≡ Gymnoascus citrinus (Massee & Salmon) v. Arx—Persoonia 13: 177, 1986; ex-type culture: 0–195 = CBS 638.72 = NRRL 5873, from soil, USA.
Von Arx (1977) designated CBS 638.72 as the neotype of Gymnascella citrina because according to his observations, this strain possesses the characteristics described by Massee and Salmon (1902) for A. citrinus. In the present study, the ITS sequence obtained from GenBank for CBS 863.72 (G. citrinus, from rat dung, India) was used. It clustered in the Gymnascella group and was found close to the type of G. udagawae (99% ITS identity to MH859492); therefore, CBS 863.72 is considered a synonym of G. udagawae. Previous studies revealed differences between these two species, in that the ascospores of G. udagawae lacked equatorial thickenings and the colony growth rates of G. udagawae and G. citrinus differed (von Arx 1986). Moreover, another strain, NRRL 5970, was also identified as G. citrina; according to multilocus phylogeny analysis, this strain formed a basal branch to Group II-Arachniotus. More data series are needed to determine the phylogeny of G. citrina.
Gymnascella thermotolerans (Zhou & Cai) Kandemir & de Hoog, comb. nov. – MycoBank MB842362. Basionym: Gymnoascus thermotolerans Zhou & Cai, Mycologia 108(1): 188, 2016. Holotype: HMAS 246111. ex-holotype culture: CGMCC 3.17580 = LC 3877 from corn-field soil, China.
The nearest neighbor was P. anodosus (97% ITS identity to MH859182). The ascospores of G. thermotolerans show distinct equatorial and polar thickenings, which are absent from P. anodosus. In addition, the arrangement of ascospores inside the asci shows reticular formation in G. thermotolerans, while the petaloid form is seen in P. anodosus (Kuehn et al. 1964a). Halo- and thermo-tolerant G. stercorarius was also found close to G. thermotolerans, and two species differ from each other by a 4% difference in ITS sequences. Morphologically, G. thermotolerans has yellowish ascomata, whereas G. stercorarius is only known from its conidial state.
Gymnascella anodosa (Kuehn, Orr & Ghosh) Kandemir & de Hoog, comb. nov. – MycoBank MB842363. Basionym: Petalosporus anodosus Kuehn, Orr & Ghosh—Mycopath. Mycol. Appl. 23: 29, 1964; ex-type culture: CBS 518.68 = ATCC 18490 = IFO 31788 = IMI 168759 = NRRL A-10221 = QM 8526, from dung of rabbit, USA.
The type specimen of P. anodosus, NRRL A-10221, was indicated by Kuehn et al. (1964a). The species was described as usually having a discrete ascocarp, absence of a distinct peridium, and a characteristic, petaloid arrangement of ascospores in the asci. In the present study, the type strain was found to be related to G. thermotolerans (94% ITS identity to KP278212) and G. stercorarius (90% ITS identity to KP278214). These two species are known to be thermophilic, although the original description of P. anodosus lacks this information. The isolates grow more rapidly at 37 °C than at 30 °C (Zhou et al. 2016; Kuehn et al. 1964a). Currah (1985) accepted P. anodosus as a synonym of P. nodulosus; however, two species show morphological differences (Kuehn et al. 1964a), which is supported by a 6% ITS difference.
Gymnascella stercoraria (Zhou & Cai) Kandemir & de Hoog, comb. nov. – MycoBank MB842364. Basionym: Gymnoascus stercorarius Zhou & Cai, Mycologia 108(1): 188, 2016. Holotype: HMAS 246109. ex-holotype culture: CGMCC 3.17574 = LC4076 from artificial compost (made from sheep droppings and plant residues), China.
Gymnoascus stercorarius has been reported as a halo- and thermo-tolerant species (maximum near 50 °C, optimum 35 °C; growth up to 22.5% NaCl; Zhou et al. 2016). The species is sister to G. thermotolerans (96% ITS identity to KP278212) and G. anodosa (94% ITS identity to MH859182) in Group 9-I (Gymnascella).
Gymnascella devroeyi (Orr) Currah—Mycotaxon 24: 79, 1985 ≡ Disarticulatus devroeyi Orr—Mycotaxon 6(1): 37, 1977 ≡ Arachniotus devroeyi (Orr) v. Arx—Persoonia 9(3): 398, 1977 ≡ Gymnoascus devroeyi (Orr) v. Arx—Persoonia 13(2): 178, 1986; ex-type culture: ATCC 34425 = O3485 = NRRL 5543 = RV 19682, from soil, Belgium.
Orr (1977a) described D. devroeyi with the type strain ATCC 34425 and examined the secondary strain CBS 546.72 = RV 19653 = Orr 4390 in the same sample set. Both strains were proven to be identical (von Arx 1977). In the current study, only LSU sequences were available for CBS 546.72; based on this material, the species is placed in Group 9-I Gymnascella. Therefore, the genus Disarticulatus was abandoned.
Group 9-II Arachniotus Schröt. 1893
Type species: Arachniotus ruber (v. Tiegh.) Schröt.—Krypt. Fl. Schlesien (Breslau) 3.2(1–2): 210, 1893 ≡ Gymnoascus ruber v. Tiegh.—Bull. Soc. Bot. Fr. 24: 159, 1877 ≡ Pseudoarachniotus ruber (v. Tiegh.) Orr, Ghosh & Roy—Mycologia 69(1): 153, 1977; ex-neotype culture CBS 352.90 = Cox A 157 = MUCL 39748 = IMI 92796 = ATCC 15315 = NRRL 5951 = QM 9289, from soil, UK (Kuehn and Orr 1964).
Gymnoascus ruber was described by van Tieghem (1877) as the type species of the genus. However, the type material has never been located (Kuehn and Orr 1964). Kuehn and Orr (1964) examined four dried herbarium specimens according to the descriptions that were provided by van Tieghem (1877) and Schroeter (1893), and they indicated CBS 352.90 as a neotype for this species. This strain clustered in Group 9-II with A. desertorum, Gymnoascus confluens, Pseudoarachniotus aurantiacus, Sphaerosporium equinum and Sporendonema casei. Arachniotus ruber is frequently isolated from alkaline or neutral soils and from dog and sheep dung. Alkaline soils have a high pH (> 8.0) and contain salts (Qin et al. 2012). This is consistent with the habitat choices of other members of the group. Even though the optimal growth temperature was not noted for A. ruber, it was unable to grow at 37 °C (Kuehn and Orr 1964).
Sporendonema is an ancient genus first described 45 years before the establishment of the family Gymnoascaceae by Desmazières (1827) to accommodate the orange-red cheese fungus S. casei. Hammer and Gillman (1944) and Sigler and Carmichael (1976) conducted extensive studies on the morphology and physiology of this slow-growing fungus in vitro. In contrast to other members of the family, it produces broad, subhyaline to pale pink hyphae that disarticulate into enteroarthric conidia, propagules being produced within the outer walls of thick-walled hyphae (Sigler and Carmichael 1976; Hammer and Gilman 1944). This type of conidiogenesis is also typical for the soil-associated and systemic pathogen Coccidioides (Pan et al. 1994), which is also known to tolerate high salt concentrations and to grow in sandy alkaline soils that are rich in organic matter and salts (del Rocío Reyes-Montes et al. 2016). Optimal growth occurs between 15 °C and 20 °C, with good sporulation at 8 °C and no growth at 30 °C. Hammer and Gillman (1944) reported enhanced growth on cheese agar containing cheese and additional salts. Ropars et al. (2012) used a proprietary medium with a confidential composition provided by starter producers for S. casei, which enhanced growth and sporulation. The authors also compared TUB, TEF1, LSU and ITS sequences. They obtained the best matches for ITS and LSU loci with Arachniotus ruber (97% ITS homology and 99.4% LSU homology) using BLAST. According to the ITS + LSU phylogeny, A. ruber grouped with S. casei strains, whereas in TUB/TEF1 concatenated analyses, A. ruber took a separate, basal position (Ropars et al. 2012). Another isolate of S. casei (HDN16-802) was reported by Ge et al. (2019) from a sediment sample from Zhangzi Island, China, which was selected because of its orange color and remarkable metabolic profile. To our knowledge, this is the first report of S. casei from a substrate other than cheese. In the present study, strains HDN16-802 and CBS 543.75 showed a difference of four bases in ITS sequences (511 bp), and all available S. casei strains were found in the same group containing the type of A. ruber (Fig. 10). Based on the ITS sequences, similarity between A. ruber (MH862216) and S. casei cheese isolates (CBS 543.75, CBS 207.27, strain RS3, MUCL 38539 and MUCL 40630) was found to be 99% and 98% for the sediment isolate of S. casei. Typification and nomenclatural revision for the Sporendonema casei strains will be discussed after further analyses together with Sphaerosporium equinum strains.
Several of the isolates from cheese were reported as Sphaerosporium (Ropars et al. 2012) and were located in Group 9-II. The genus Sphaerosporium includes S. argentinense (Spegazzini 1911), S. equinum (Crane and Schoknecht 1986) and S. lignatile (type of the genus; von Schweinitz 1832). The status of the first species is reported to be uncertain, since this type is not interpretable (Partridge and Morgan-Jones 2002). There is no known type material for S. equinum, and the first description of the species was based on a specimen H.G. 1510 (PC) from old and humid horse hoofs (Crane and Schoknecht 1986). The type material of Sphaerosporium lignatile (#3036, PH on dead wood, USA) was examined morphologically by Partridge and Morgan-Jones (2002) and reported to have larger conidia and more loosely structured sporodochia than those in S. equinum, while both species show similar thallic conidiogenesis. Our understanding of the molecular characteristics of S. equinum and S. lignatile is limited owing to inadequate data, taken from two studies: the first one reported on MUCL S. equinum strains isolated from cheese (Ropars et al. 2012), while the second reported on isolates of S. lignatile from decayed hardwood from the personal collection of Alden C. Dirks (Song et al. 2019). Wanderley Costa et al. (2012) also reported S. equinum isolates from the leaves of mangrove plants in Brazil; however, the researchers did not provide any information about their identification method. It is possible that the strains were misidentified. On the other hand, an isolate from a skin swab of a living bat (Myotis lucifugus) was deposited in the UAMH collection (UAMH 11516), and this strain was reported to be 99% identical to S. equinum MUCL 49171 from Pyrenean cheese from Belgium. Another strain, MUCL 54024, was isolated from an insect pupa in Belgium by C. Decock (personal communication). It was found as a separate branch in the same cluster containing cheese isolates, and it showed 99% ITS and 99.5% LSU identity to MUCL 49171 in the present study (Fig. 10). Similar to previously described habitats for Group 9-II, both bat wings and insect pupae have an osmophilic nature, which supports the growth of S. equinum. Nomenclatural revision of this species will be discussed after further analyses.
Arachniotus aurantiacus (Kamyschko) Arx—Persoonia 6(3): 373, 1971 ≡ Pseudoarachniotus aurantiacus Kamyschko—Nov. Sist. Niz. Rast. 1967(4): 224, 1967 ≡ Gymnascella kamyschkoi Orr, Ghosh & Roy—Mycologia 69(1): 136, 1977 (name change); ex-type culture: CBS 603.67 = ATCC 22394 = NRRL A-18287 = UAMH 3529 = VKM F-1140, from soil, USSR.
The type strain CBS 603.67 of P. aurantiacus has gymnothecia but lacks a peridium (Kamyschko 1967; Orr et al. 1977a). It differs from other Pseudoarachniotus species by its ascospore morphology and from Arachniotus species by its orange or yellow-orange gymnothecium (Orr et al. 1977a). The species phylogenetically is found in Group 9-II clustering with osmophilic S. equinum (98% ITS identity to JQ434576).
Arachniotus confluens (Sartory & Bainier) Apinis—Mycol. Pap. 96: 37, 1964 ≡ Gymnoascus confluens Sartory & Bainier—Bull. Soc. Mycol. Fr. 29: 261, 1913 ≡ Gymnascella confluens (Sartory & Bainier) Currah—Mycotaxon 24: 75, 1985; ex-type culture: CBS 352.66 = BDUN 375 = ATCC 22220 = IMI 100873 = NRRL 5979, from dung, UK.
= Arachniotus desertorum Moustafa—Trans. Br. Mycol. Soc. 61(2): 392, 1973 ≡ Pseudoarachniotus desertorum (Moustafa) Orr, Ghosh & Roy—Mycologia 69(1): 158, 1977 ≡ Gymnoascus desertorum (Moustafa) v. Arx—Persoonia 13(2): 178, 1986; ex-type culture: CBS 634.72 = ATCC 26464 = IMI 163480, from halomorphic soil, Kuwait.
Currah (1985) synonymized Arachniotus desertorum and Gymnascella confluens because the differences between the two species (darker colonies and slightly larger ascospores for A. desertorum) did not merit maintaining them as separate species. The ITS analysis provided by Solé et al. (2000a) and the present study support this approach. Both species are placed in Group 9-II and have been isolated from carnivore dung and halomorphic soil samples (Currah 1985). The name Arachniotus confluens was used for both species in the present study because the isolates clustered with the type species Arachniotus and A. confluens have priority over A. desertorum. Members of Arachniotus are considered halotolerant because they are highly adapted to environments with high salt content, such as cheese, marine water sediments and saline soil.
Group 9-III Gymnoascus Baran.
Type species: Gymnoascus reessii Baran.—Bot. Ztg. 30: 158, 1872. Neotype designated here: Herb. No: CBS H-13156, dried culture from soil, USA, isolated by G.F. Orr, 1963. Living strain derived from type CBS 392.64.
= Gymnoascus corniculatus Orr & Plunkett—Mycopath. Mycol. Appl. 21: 1, 1963; ex-type culture: CBS 410.72 = NRRL A-10099 = O-263 = UAMH 3159, from soil, USA.
Variability has been noted in ascocarp morphology and pigmentation among G. reessii isolates. The presence of peridial hyphae may be extensive or rather limited, and they may be smooth or roughened; furthermore, the peridial apices may be simple or elaborate (Orr et al. 1963b). The main criterion for separating Gymnoascus corniculatus from G. reessii was the number of appendages with trifurcate or chandelieroid apical branching (almost 50% in G. corniculatus, versus infrequent in G. reessii), in addition to ascocarp size. In the current ITS sequence data, CBS 410.72 was found in the same cluster as G. reessii at 99% identity. Given the morphological variability of G. reessii, these data led to the conclusion that G. corniculatus can be regarded as a younger synonym.
Gymnoascus longitrichus Orr & Kuehn—Mycopath. Mycol. Appl. 21(1): 9, 1963; ex-type culture: CBS 366.64 = ATCC 18433 = IFO 8167 = IHEM 4416 = NRRL A-10096 = RV 20203, from soil, USA.
Gymnoascus longitrichus was treated as a synonym of G. reessii by Currah (1985). In the present study, G. longitrichus was found to be separate from G. reessii, being more closely related to G. dugwayensis (98% ITS identity to LC146737). Morphologically, G. longitrichus differs from that species by having much longer appendages (160‒500 µm vs. 36‒164 µm; Orr and Kuehn 1972). Both species might share the same ecology since G. dugwayensis was reported from sandy soil and G. longitrichus was isolated from composite soil close to a hot spring area.
Gymnoascus dugwayensis Orr & Kuehn—Mycologia 64(1): 65, 1972; ex-type culture: ATCC = 18,899 = O-3138 = DPG-132 = IFO 31716 = NRRL 3714, from sandy soil, USA.
Unlike the other Gymnoascus species, which have typical “boat hooks” on their short appendages, G. dugwayensis has hooks that are confined to the longer appendages. Appendages in G. dugwayensis are shorter (36‒164 µm; Orr and Kuehn 1972) than those of its nearest neighbor, G. longitrichus (160‒500 µm; Orr et al. 1963b).
Gymnoascus uncinatus Eidam – Cohn’s Beitr. Biol. Pfl. 3: 292, 1880 ≡ Myxotrichum uncinatum (Eidam) Schröt., Krypt.—Fl. Schlesien (Breslau) 3.2(1–2): 212, 1893 ≡ Uncinocarpus uncinatus (Eidam) Currah—Mycotaxon 24: 186, 1985; ex-type culture: CBS 408.72 = NRRL 3610 = O-3215 = RSA 56 = UAMH 3913, from dung, USA, R.K. Benjamin, 1949.
Currah (1985) proposed RSA 56 as a neotype of U. uncinatum. The morphological characteristics of the strain were discussed again by Udagawa and Uchiyama (2000), and the species was included in Gymnoascus based on the presence of lattice-like peridium, coiled ascomatal initials, smooth-walled ascospores and nonthermotolerant behaviour. Phylogenetically, it was confirmed to be a member of Group 9-III, clustering with G. exasperatus (95% ITS identity to KU746682) in the present study.
Gymnoascus exasperatus Liu & Cai—Persoonia 39: 14, 2017; holotype: HMAS 246925; ex-type culture: CGMCC 3.17923 = LC5640, from bat guano, China.
This species is only known from its asexual morph. Sequence data showed that it is related to G. uncinatus (95% ITS identity to KT155648).
Gymnoascus flavus Zhang & Cai—Fungal Divers. 106: 29, 2021; holotype: HMAS 248010; ex-type culture: CGMCC3.19574 = LC12500, from karst cave soil, China.
Zhang et al. (2021) reported that the species was unable to grow on oat meal agar, while growth was observed on synthetic nutrient agar at 23‒25 °C, underlining its oligotrophic character. Similar to the ITS and LSU locus data provided by Zhang et al. (2021), G. flavus formed a basal cluster with G. dugwayensis, G. exasperatus, G. longitrichus, G. reessii, and G. uncinatus in the present study (Fig. 10). Unlike G. reessii and G. uncinatus, there is no sexual morph known for G. flavus. The species can be distinguished from G. exasperatus by abundant intercalaries in addition to terminal and lateral conidia.
Gymnoascus intermedius Orr—Mycotaxon 5(2): 470, 1977; ex-type culture: ATCC 28555 = O-313 = NRRL 6272, from soil, USA.
Gymnoascus intermedius has been isolated from the soil and lung of a pocket mouse. The original material is not known to be preserved. In the present study, two secondary isolates were examined, i.e., IFM 47418 (= UAMH 3833 = NRRL A16935 = O-3139 from soil, Italy) and UAMH 11472 (from skin swab of a living bat, Canada). The first strain clustered within Malbranchea species based on LSU data (Supplementary Figure 1), while the latter clustered in Gymnoascaceae, Group 9-III, along with G. longitrichus and G. dugwayensis, with 99% ITS similarity to both species. In the original description of G. intermedius, Orr (1977b) noted that the ascocarp lacks appendages as well as the “boat-hook” type branches, which can be observed in the former two species. Additionally, the ascospore morphology was noted to be slightly different for these three species as oval to elliptical for G. intermedius, oblate for G. dugwayensis and oval to oblate for G. longitrichus (Orr et al. 1963b; Orr and Kuehn 1972; Orr 1977b). Further analyses are required to determine the status of G. intermedius, and strains IFM 47418 and UAMH 11472 need a nomenclatural revision.
Members of Group 9-III are associated with various types of mammal dung and soil. Gymnoascus longitrichus was isolated from soil close to an alkaline hot spring (Orr et al. 1963b). Hot spring soils have high temperatures, extreme pH values, sparse vegetation, and limited organic carbon (Redman et al. 1999; Kumar et al. 2004). The habitat of Gymnoascus dugwayensis was found in sandy, acidic soil with poor nutrient content. Both species are phylogenetically closely related to G. reessii (Figs. 1 and 10). Gymnoascus reessii can be found in habitats with high nutritional content, such as dung of various types of mammals and soil. All species of Gymnoascus have long gymnothecial appendages. Gymnoascus flavus and G. exasperatus were found in karst caves, which constitute an oligotrophic environment (Jiang et al. 2017). However, G. exasperatus was found on bat guano in one of these caves, providing local patches of substrate rich in carbon, nitrogen, sulphate, phosphorus and chitin (Liu et al. 2013).
Group 9-IV Narasimhella Thirumalachar & Mathur.
Type species: Narasimhella poonensis Thirum. & Mathur—Sydowia 19(1–6): 185, 1966; ex-type culture: CBS 393.71 = ATCC 16197 = HACC 171 = IHEM 3406 = IMI 113696, from soil, India.
= Pseudoarachniotus marginisporus Kuehn & Orr—Mycopath. Mycol. Appl. 19: 257, 1963 ≡ Arachniotus marginisporus (Kuehn & Orr) Udagawa—Trans. Mycol. Soc. Japan 10(3): 103, 1970 ≡ Gymnascella marginispora (Kuehn & Orr) Currah—Mycotaxon 24: 87, 1985 ≡ Narasimhella marginispora (Kuehn & Orr) v. Arx—Persoonia 13(2): 179, 1986 ≡ Gymnoascus marginisporus (Kuehn & Orr) Solé, Cano & Guarro—Stud. Mycol. 47: 145, 2002; ex-type culture: CBS 115.54 = NBRC 9194 = ATCC 15314 = UAMH 3533 = NRRL 2850 = RSA 1553, from buried cable, USA.
The generic type species Narasimhella poonensis was described as having well-developed, stipitate fruitbodies. The sterile stipe is composed of upwards-growing hyphae and an irregularly globular, fertile head portion with groups of asci (Thirumalachar and Mathur 1966). It lacks a peridium and shows crozier formation similar to that of Pseudoarachniotus species. The optimum growth temperature is 28 °C (Thirumalachar and Mathur 1966). NRRL 2850 was listed as the type of P. marginisporus by Kuehn and Orr (1963). Several taxonomic rearrangements were followed. Narasimhella has a peculiar ecology. All isolates of this species have been obtained from buried substrates, such as cables and fabrics buried in dung-enriched soil. Nutrient-rich soil is a common habitat for many Gymnoascaceae members, but none of them have been found to grow on hard underground substrates. The ITS similarity between Narasimhella poonensis (MH857260) and N. marginispora (MH860180) was found to be 99%, and the species were considered to be synonyms, confirming Thirumalachar and Mathur (1966), with N. marginispora as the oldest name. The fungus is described with its habitat choice, thin- and smooth-walled ascospores with minute rims, and ochraceous to very pale-yellow colonies (Kuehn and Orr 1963).
Narasimhella echinulata (Dutta & Ghosh) v. Arx—Gen. Fungi Sporul. Cult., Edn 2 (Vaduz): 94, 1974 ≡ Pseudoarachniotus echinulatus Dutta & Ghosh—Mycologia 55(6): 775, 1963 ≡ Amauroascus echinulatus (Dutta & Ghosh) v. Arx—Persoonia 6(3): 375, 1971 ≡ Mallochia echinulata (Dutta & Ghosh) v. Arx & Samson—Persoonia 13(2): 185, 1986; ex-type culture: CBS 278.64 = ATCC 15317 = UAMH 3561 = NRRL A-12062 = IFO 9192, from rice-field soil, India, G.F. Orr.
The species was first included in Pseudoarachniotus because of the absence of discrete ascomata. It was found to be morphologically different from other Pseudoarachniotus species, primarily by globose and echinulate ascospores (Dutta et al. 1963). Von Arx and Samson (1986) introduced the genus Mallochia to classify Pseudoarachniotus echinulatus. Udagawa and Uchiyama (2002) examined the type specimen and accepted the genus in Amauroascaceae. In the present study, N. echinulata (ex-type culture: IFO 9192) was found to be close to N. punctata (98% ITS identity to AJ315825), N. marginispora (97% ITS identity to MH857260) and N. hyalinospora (98% ITS identity to NR_130659.1). Although morphologically the nearest neighbor was noted to be P. reticulatus (Dutta et al. 1963), the two species were found phylogenetically remote from each other (88% ITS identity to NR_153508.1; 94% LSU identity to NG_056930.1). Morphological differences with N. echinulata include the absence of peripheral rims or extensions and red-capped hyphae, changes in ascospore colors from purple-brown to red-brown with age, and relatively large echinulate ascospores. The ability to grow at 37 °C confirmed this separation.
Narasimhella punctata (Dutta & Ghosh) Kandemir & de Hoog, comb. nov. – MycoBank MB842365. Basionym: Pseudoarachniotus punctatus Dutta & Ghosh—Mycologia 56(2):153, 1964 ≡ Arachniotus punctatus (Dutta & Ghosh) v. Arx—Persoonia 6(3): 373, 1971 ≡ Gymnascella punctata (Dutta & Ghosh) Currah—Mycotaxon 24: 93, 1985 ≡ Gymnoascus punctatus (Dutta & Ghosh) v. Arx—Persoonia 13(2): 180, 1986; ex-type culture: CBS 279.64 = ATCC 22851 = NRRL A-12061 from rice-field soil, India by B.G. Dutta. Herb. No: CBS H-6711.
Strain NRRL A-12061 was described as having punctate ascospores with a broad rim. Discrete ascocarps were absent, and asexual spores could not be observed. The strain was located in Group 9-IV and was found to be similar to N. echinulata (98% ITS similarity to AJ271562), differing by punctate ascospores with a rim, yellow in mass, while those of N. echinulata were echinulate without a rim and purple-brown in mass. Both species were isolated from paddy soil. In addition to high humidity, the major characteristic of paddy soil is a higher available silica content compared to highlands (Takahashi 1968). Although silicon is not a nutrient, it promotes the growth of fungi under oligotrophic conditions (Wainwright et al. 1997).
Narasimhella hyalinospora (Kuehn, Orr & Ghosh) v. Arx—Persoonia 6: 374, 1971 ≡ Pseudoarachniotus hyalinosporus Kuehn, Orr & Ghosh—Mycopath. Mycol. Appl. 14: 217, 1961 ≡ Arachniotus hyalinosporus (Kuehn, Orr & Ghosh) Apinis—Mycol. Pap. 96: 41, 1964 ≡ Rollandina hyalinospora (Kuehn, Orr & Ghosh) Roy, Orr & Ghosh—Taxonomy of Fungi (Proc. Int. Symp. Madras, 1973) 1: 221, 1978 ≡ Gymnascella hyalinospora (Kuehn, Orr & Ghosh) Currah—Mycotaxon 24: 84, 1985 ≡ Gymnoascus hyalinosporus (Kuehn, Orr & Ghosh) Solé, Cano & Guarro—Stud. Mycol. 47: 145, 2002; ex-type culture: CBS 548.72 = NRRL 2881 = BCCM/IHEM 3416 = IMI 99725 = RSA 1529 = UAMH 3155, from dung of Guinea pig, India, G.R. Ghosh, 1959.
Kuehn et al. (1961) described the strain NRRL 2881 = CBS 548.72 as an ex-type culture for Pseudoarachniotus hyalinosporus. In the present study, it clustered with N. punctata, N. echinulata and N. marginispora. These species are all morphologically similar by the absence of discrete ascocarps. Narasimhella punctata can easily be distinguished by its punctate ascospores; N. echinulata has large, echinulate ascospores (4.0–5.5 × 5–6 µm vs. 2.2–2.5 × 2.5–3.3 µm for N. hyalinospora). Ascospores of N. marginospora deviate by having a minute rim. Habitat choice of the members of this group is mostly nutrient-rich environments, such as animal dung and rhizosphere soil.
Narasimhella armeniaca (Solé, Cano & Guarro) Kandemir & de Hoog, comb. nov. – MycoBank MB842366. Basionym: Gymnoascus armeniacus Solé, Cano & Guarro—Stud. Mycol. 47: 145, 2002; ex-type culture: CBS 125.78 = IMI 386570, from dung of kangaroo, India, J.A. von Arx. Herb. No: CBS H-14981.
Gymnoascus armeaniacus was found in Group 9-IV close to P. hyalinosporus (95% ITS similarity in 500 bp). Both P. hyalinosporus and G. armeniacus prefer nutritionally rich habitats, and they were reported to be morphologically similar (Solé et al. 2002b). However, the latter lacks discrete ascomata (Kuehn et al. 1961; Currah 1985). Growth for G. armeniacus was reported at 37 °C but not at 45 °C (Solé et al. 2002b).
Group 9-V Gymnoascoideus Orr, Roy & Ghosh.
Type species: Gymnoascoideus petalosporus Orr, Roy & Ghosh—Mycotaxon 5(2): 460, 1977 ≡ Gymnoascus petalosporus (Orr, Roy & Ghosh) v. Arx—Persoonia 9(3): 397, 1977; ex-type culture: UAMH 1665 = ATCC 34351 = NRRL 6001 = O-3325, from nodular cyst of human eye.
Orr et al. (1977b) described Gymnoascoideus petalosporus with thick- and smooth-walled ascospores in a petaloid arrangement in the asci. Correctly identified strains suggest a preference for keratinous substrates such as human skin (CBS 630.72), horse skin (UAMH 3592), horn (UAMH 3525) and feathers (O-1299), in addition to soil (O-2013) (Orr et al. 1977b). Von Arx (1977) examined some of these strains and noted that they were variable in culture morphology, peridial hyphal formation and ascospore size. He considered the Gymnoascoideus (in the literature often as “Gymnoascoides”) intermediate between Arachniotus and Gymnoascus. Phylogenetically, the nearest neighbor of G. petalosporus is G. boliviensis (98% ITS similarity between AJ315828 and AB361639; 526 bp), which differs by its brown ascomata and ascospores without equatorial thickening (Guarro et al. 1992b). Gymnoascus boliviensis has been reported to form clusters of asci and lack discrete ascocarps on sterilized soil supplemented with hair. The presence of interwoven peridial hyphae and ascospores in a petaloid pattern and limited growth at 37 °C have also been reported (Guarro et al. 1992b). Since the type strain clustered in Gymnoascoideus, the following combination was proposed for Gymnoascus boliviensis:
Gymnoascoideus boliviensis (Guarro, Ulfig & De Vroey) Kandemir & de Hoog, comb. nov. – MycoBank MB842903. Basionym: Gymnoascus boliviensis Guarro, Ulfig & De Vroey—Mycotaxon 45: 317, 1992; isotype: FMR 3793 = IMI 351986 from soil of chicken yard, Bolivia, 1972 by De Vroey & Villavicencio.
Gymnoascus verrucosus Sharma & Singh—IMA Fungus 4(2): 178, 2013; holotype: AMH 9454; ex-type culture: NFCCI 2672, from soil, India.
The species is described with verruculose ascospores with small tubercles over the entire surface. The type strain was found as a separate branch and with its neighbors, G. petalosporus and G. boliviensis, at considerable distance (89% ITS identity to HM991270 and AJ315828, respectively). Gymnoascus verrucosus can easily be distinguished from Gymnoascoideus species by its ascospores, which are pale yellow, smooth and without an equatorial rim, and by the presence of peridial appendages, which are absent in Gymnoascoideus spp. (Orr et al. 1977b; Guarro et al. 1992b; Sharma and Singh 2013). Based on both morphological and molecular differences, G. verrucosus is not included in Gymnoascoideus and requires nomenclatural revision.
The other species of the family Gymnoascaceae
Pseudoarachniotus reticulatus Kuehn & Goos—Mycologia 52(1): 40, 1960 ≡ Amauroascus reticulatus (Kuehn & Goos) v. Arx—Persoonia 6(3): 375, 1971 ≡ Amaurascopsis reticulata (Kuehn & Goos) Guarro & Gené—Mycotaxon 45: 174, 1992 ≡ Mallochia reticulata (Kuehn & Goos) Solé, Cano & Guarro—Mycol. Res. 106(4): 395, 2002; ex-type culture: CBS 392.61 = ATCC 14045 = IMI 84358, from rhizosphere soil of Musa sapientum, USA, R.D. Goos, 1959.
Kuehn and Goos (1960) characterized Pseudoarachniotus reticulatus by the absence of ascocarps, globose and reticulate ascospores, usually red-capped hyphae among tufts of asci, and peripheral extensions on the ascospores. In the present study, the type strain was placed in a cluster containing a strain identified as M. reticulata (IFO 9196) by Solé et al. (2002a). More detailed molecular and morphological examinations are needed to establish the relationship between these two strains. The habitat choice of the rhizosphere indicates that P. reticulatus is not oligotrophic, such that, compared to bulk soil, the rhizosphere has higher nutrient availability (McNear 2013).
Pseudoarthropsis cirrhata (Oorschot & de Hoog) Stchigel, Rodr.-Andr. & Cano—IMA Fungus 27(1): 47, 2021 ≡ Arthropsis cirrhata Oorschot & de Hoog—Mycotaxon 20: 130, 1984; ex-type culture: CBS 628.83, from brick wall, The Netherlands.
The genus Arthropsis is typified with Arthropsis truncata (UAMH 4430, from dead leaves, Peru; Sigler et al. 1982a) in Sordariomycetes (Giraldo et al. 2014). However, two species, A. hispanica and A. cirrhata, are accommodated within Onygenales (Fig. 1). Similar to A. hispanica, A. cirrhata shows basipetal septation to form enteroarthric conidia. However, the arthroconidia of A. cirrhata are light yellowish-orange, and the fungus shows restricted growth at 25 °C, with a maximum growth temperature of 30 °C (van Oorschot and de Hoog 1984). During our study, only an LSU sequence was available, and phylogeny showed A. cirrhata in Clade 9, Gymnoascaceae remote from A. hispanica (incertae sedis), the other member described in Arthropsis (Supplementary Figures 1 and 2). Rodríguez-Andrade et al. (2021) also analysed ITS and LSU sequences of A. cirrhata and proposed a new genus Pseudoarthropsis for A. cirrhata.
Gymnoascus halophilus Zhou & Cai—Mycologia 108(1): 185, 2016. Holotype: HMAS 246110; ex-holotype culture: CGMCC 3.17579 = LC4751, from salt lake sediment, China.
Ascomata are discrete or confluent, and peridial hyphae are surrounded by clumps of asci and lack appendages. Optimal growth occurs at 25 °C, and growth can still be observed near 40 °C. This species is unable to grow on media without additional salt and tolerates NaCl up to 22.5% (Zhou et al. 2016). Therefore, it is an obligate halophilic fungus. However, phylogenetically, it is not found in Group 9-II, which includes species predominantly isolated from saline environments. Based on the ITS phylogeny (Fig. 10), G. halophilus formed a single cluster in the family, whereas in the maximum likelihood tree, this cluster was found at the base of Group 9-IV, Narasimhella (Fig. 10). Further studies are required to establish the correct nomenclature for G. halophilus (ex-type culture: CGMCC3.17579) as well as G. verrucosus (ex-type culture: NFCCI 2672).
Kraurogymnocarpa trochleospora (Kuehn & Orr) Udagawa & Uchiyama—Mycoscience 42(3): 281, 2001 ≡ Pseudoarachniotus trochleosporus Kuehn & Orr—Mycologia 64(1): 58, 1972 ≡ Arachniotus trochleosporus (Kuehn & Orr) Udagawa—Nippon Kingakukai Kaiho 38(3): 154, 1997; ex-type culture: CBS 591.71 = ATCC 18900 = NRRL A-16936 = DPG 71 = UAMH 3416 = UAMH 10101, from soil, USA.
Pseudoarachniotus trochleosporus was described as lacking discrete ascocarps, mostly with globose ascospores with an external rim, roughened walls, lenticular to pulley-shaped in side view and grooved on the lateral axis, with crests on each side of the groove. The name K. lenticulospora was based on morphological comparison with K. lenticulospora (Udagawa and Uchiyama 2001). In the present study, the species was found to be a sister branch to Diploospora rosea in ITS analyses (Fig. 10), while multilocus data analyses revealed D. rosea as incertae sedis (Fig. 1). The latter species is both phylogenetically (82% ITS identity to NR_111882.1) and morphologically (by enteroarthric conidial formation) quite different from K. trochleospora. Moreover, in contrast to the xerophilic nature of D. rosea, K. trochleospora prefers sandy-clay and clay soils, which have higher water-holding capacities than most other soil types (Orr and Kuehn 1972; O’Geen 2013).
Clade 10 Ajellomycetaceae Untereiner, Scott & Sigler.
Type genus: Ajellomyces McDonough & Lewis; type species: Ajellomyces dermatitidis McDonough & Lewis—Mycologia 60: 77, 1968; ex-type culture: CBS 674.68 = ATCC 18188 = UAMH 3539, from human patient, USA, E.S. McDonough, 1968.
The family Ajellomycetaceae is based on the genus Ajellomyces, which was described for several sexual states of members of this family. Many Ajellomycetaceae members are causative agents of systemic diseases in mammals. The type species of Ajellomyces is Ajellomyces dermatitidis, the sexual state of Blastomyces dermatitidis, the main agent of human and canine blastomycosis. Other Ajellomyces species have been found by in vitro mating in Histoplasma, Emergomyces (formerly Emmonsia) and Helicocarpus. Today, separate sexual names have been abandoned, and the genus Ajellomyces has become superfluous.
Clade 10 contains thermally dimorphic fungi, including Blastomyces (type species B. dermatitidis; Gilchrist and Stokes 1898), Emergomyces (type species E. pasteurianus; Dukik et al. 2017), Emmonsiellopsis (type species E. terrestris; Marin-Felix et al. 2015), Helicocarpus (type species H. griseus; Marin-Felix et al. 2015), Histoplasma (type species H. capsulatum; Darling 1906), and Paracoccidioides (type species P. brasiliensis; Almeida 1930). Another genus, Emmonsia (type species E. parva; Ciferri and Montemartini 1959), was also described in Ajellomycetaceae, but later, the species of this genus were reclassified into Blastomyces and Emergomyces based on the results of multiphasic approaches, including multilocus sequencing and morphological and physiological data (Jiang et al. 2018, 2020).
Lacazia loboi was described with the basionym Paracoccidioides loboi, which was invalidly published (Taborda et al. 1999a). The phylogenetic position of L. loboi was found amidst Paracoccidioides species (Fig. 12). Although phylogenetically close, the process of yeast cell formation in tissue is different in the two genera, i.e., unilateral versus multilateral. In addition, Lacazia contains melanin in its cell wall (Taborda et al. 1999b) and causes only (sub)cutaneous infections, whereas Paracoccidioides does not contain melanin and is an agent of disseminated infections (Taborda et al. 1999a). All available records of L. loboi were obtained from tissue samples, since the fungus has not been cultured in vitro. Although the histological features of lobomycosis in humans and dolphins are similar, the fungus was reported to differ in morphology between the two hosts (Haubold et al. 2000). In the present study, Lacazia clustered with Paracoccidioides (with > 88% ITS identity; Fig. 12), but the phylogenetic distance between the genera remained significant (Fig. 1). In accordance with the available data (Cowan 1993; Simose-Lopes et al. 1993; Haubold et al. 1998, 2000; Taborda et al. 1999a; Esperón et al. 2012; Schaefer et al. 2016), the etiological agents of lobomycosis in humans and dolphins are likely to be different species. Vilela and Mendoza (2018) proposed the name Paracoccidioides brasiliensis var. cetii to describe the uncultivated fungus causing cutaneous disease in dolphins and used the name L. loboi to describe the etiological agents of the same disease in humans. Recently, Vilela et al. (2021) accepted Paracoccidioides brasiliensis var. ceti as Paracoccidioides cetii and Lacazia loboi as Paracoccidioides loboi. Several ITS sequences from dolphins (AF035674 and AF035675) and plants (GU361967 and FJ037740) were recorded in GenBank as L. loboi but might be considered contaminants or misidentifications. The phylogenetic structure of Clade 10 was inconsistent between the ITS and LSU analyses. In LSU analysis, the family Eremascaceae was found in Clade 10, whereas it formed a separate clade in ITS and multilocus analyses (Figs. 1 and 12; Supplementary Figures 1 and 2).
Phylogenetic tree of Ajellomycetaceae based on ITS sequences obtained with Bayesian analysis and maximum likelihood analysis using the TNe + G4 + I + F model in IQ-TREE-ML. Branch values of ≥ 90% for Bayesian probability and ≥ 80% for maximum likelihood and type species of the genera are shown in bold. Arthroderma flavescens and Arthroderma vespertilii were used as outgroup species. Please note that rDNA ITS is not sufficient to resolve genera in Ajellomycetaceae, as shown in the figure and in previous studies (Dukik et al. 2017; Jiang et al. 2018). For detailed information about the classification of the genera in Ajellomycetaceae, see Sepúlveda et al. 2017; Hrycyk et al. 2018; Jiang et al. 2018; Muñoz et al. 2018 and Vilelea et al. 2021
Clade 11 Spiromastigoidaceae Guarro, Cano & Stchigel.
≡ Spiromastixaceae Guarro, Cano & Stchigel—Mycoses 57(7): 425, 2014 (invalid name, orthographic variant) ≡ Spiromastigaceae Hirooka, Tanney & Seifert—Mycologia 108(1): 135, 2016 (based on the type genus Spiromastix).
Type genus: Spiromastigoides Doweld—Index Fungorum 30: 1, 2013 ≡ Spiromastix Kuehn & Orr—Mycologia 54(2): 160, 1962; type species: Spiromastigoides warcupii (Kuehn & Orr) Doweld ≡ Spiromastix warcupii Kuehn & Orr—Mycologia 54(2): 160, 1962; ex-type culture: CBS 576.63 = ATCC 14964 = IFO 31795 = MUCL 9856 = NRRL A-10679 = QM 9683, from wheat field soil, Australia, J.H. Warcup, 1959, as Myxotrichum sp.
Spiromastix was originally described as a member of the family Gymnoascaceae, with the type species Spiromastix warcupii (Kuehn and Orr 1962). It was found to be unique because of its curved but never completely coiled peridial appendages (Kuehn and Orr 1962). However, since the name Spiromastix had already been applied to algae (Perfil'ev 1929), it was changed to Spiromastigoides by Doweld (2013). Rizzo et al. (2014) proposed the order “Spiromastixales” (orthographic variant) based on LSU D1-D2 sequence analysis. The species have discrete or confluent, brown ascomata covered with orange-brown, curved or twisted appendages; eight-spored, subglobose, ovoid to ellipsoidal asci; and smooth-walled, yellowish to pale brown ascospores (Rizzo et al. 2014). However, Hirooka et al. (2016) reduced this to the family level as Spiromastigaceae in Onygenales using ITS and concatenated 18S + 28S data. This conclusion was supported by Stchigel et al. (2017), who changed the family name to Spiromastigoidaceae because of its link with Spiromastigoides. Since Spiromastigoidaceae is typified with the type genus Spiromastigoides, it is maintained here. In the current study, the family comprises the genera Sigleria, Spiromastigoides and Pseudospiromastix, as reflected in our LSU and multilocus data analyses (Fig. 1; Supplementary Figures 1 and 2), although with ITS data, Sigleria grouped separately, close to Clade 14 (Supplementary Figures 3 and 4).
All members of Spiromastigoides are known to be cellulolytic and nonkeratinolytic, except Spiromastigoides pyramidialis, which has been reported to be weakly keratinolytic (Currah 1994; Hirooka et al. 2016). Human and animal infections have been suggested for S. albida and S. asexualis because of culture from biopsy specimens, and they are considered potential opportunistic pathogens (Rizzo et al. 2014; Stchigel et al. 2017). While all members of the genus have hemolytic and urease activity, only S. albida and S. asexualis also have lipolytic activity (Stchigel et al. 2017; Rizzo et al 2014). Phylogenetically, these two species are placed far apart (Figs. 1 and 13), suggesting that lipolysis emerged more than once during the separation of the species or that it might be an atavism.
Phylogenetic tree of Spiromastigoidaceae based on ITS sequences obtained with Bayesian analysis and maximum likelihood analysis using the TPM3u + G4 + F + I model on IQ-TREE. Branch values of ≥ 90% for Bayesian probability and ≥ 80% for maximum likelihood and type species of the genera are shown in bold. Arthroderma ciferrii and Arthroderma eboreum are used as outgroup species
For two species, S. saturnispora (Uchiyama et al. 1995b, ex-type culture: CBM BF54422, from meadowy soil, Indonesia) and S. sphaerospora (Udagawa and Uchiyama 1999b; ex-type culture: SUM 3128, from sports field, Japan), material was not available for study (Hirooka et al. 2016). The asexual morph of Spiromastigoides was reported in the literature either as malbranchea- or chrysosporium-like (Hirooka et al. 2016). During our analyses, the type strain of Malbranchea gypsea, CBS 134.77, was found among Spiromastigoides species. This strain was reported to be cellulolytic, not keratinolytic, and it was recovered from lesions on the spleens and livers of mice after experimental passages (Orr 1972; Sigler and Carmichael 1976). Rodríguez-Andrade et al. (2021) proposed Spiromastigoides gypsea for M. gypsea.
Spiromastigoides gypsea (Sigler & Carmichael) Stchigel, Rodr.-Andr. & Cano – IMA Fungus 12: 25, 2021. Basionym: Malbranchea gypsea Sigler & Carmichael—Mycotaxon 4(2): 455, 1976; ex-type culture: CBS 134.77 = ATCC 35432 = UAMH 1975, Herb. IMI 211203 from soil, USA, G.F. Orr, 1964.
A strain deposited as Chrysosporium pseudomerdarium (CBS 279.77) in the CBS collection by J.W. Carmichael was found in Clade 11 (Fig. 13); however, the ex-type culture of this species (CBS 631.79 = IMI 282432) clusters outside Spiromastigoidaceae (Tanney et al. 2015). Similarities between ITS and LSU loci of S. warcupii (CBS 576.63) and C. pseudomerdarium CBS 279.77 were found to be 94% and 97%, respectively. Therefore, CBS 279.77 was provisionally identified as Spiromastigoides sp.
Sigleria Hirooka, Tanney & Seifert—Mycologia 108(1): 140, 2016; type species: Sigleria carmichaelii Hirooka, Tanney & Seifert; ex-type culture: CBS 138264 = DAOM 250076, from house dust, Micronesia.
The genus Sigleria is represented by two species, Sigleria amendiana (Hirooka et al. 2016; ex-type culture: CBS 138357 = DAOM 588476) and Sigleria carmichaelii. Both species were isolated from house dust. Although they are morphologically similar to Spiromastigoides species, conidiophores of Sigleria tend to show opposite branching, whereas Spiromastigoides species have a predominantly unilateral branching pattern (Hirooka et al. 2016). The most distinguishing characteristic of Sigleria is its limited ability to utilize nitrate as a nitrogen source. Furthermore, growth rates on malt extract agar (MEA) are slow, and ascomata have not been observed (Hirooka et al. 2016). Morphological differences between the two genera were supported by phylogenetic results (Figs. 1 and 13). Interestingly, S. carmichaelii was often found to co-occur with Wallemia species on MEA with high sugar content (Hirooka et al. 2016).
Pseudospiromastix Guarro, Stchigel & Cano –Mycologia 108(1): 135, 2016 (validated by Hirooka et al. 2016); type species: Pseudospiromastix tentaculata (Guarro, Gené & De Vroey) Guarro, Stchigel & Cano ≡ Spiromastix tentaculata Guarro, Gené & De Vroey—Mycotaxon 46: 308, 1993; ex-type culture: CBS 184.92 = FMR 3842 = IMI 351264 = MUCL 40569 = UAMH 7098, from soil, Somalia, J. Guarro, 1968.
The genus Pseudospiromastix thus far contains only P. tentaculata, which differs from other members of the family by its ascomata bearing undulate appendages with swollen ends (Guarro et al. 1993). As in Spiromastigoides species, P. tentaculata can use nitrate as its sole nitrogen source (Hirooka et al. 2016).
Clade 12 Incertae sedis
In the present study, Clade 12 was treated separately because incompatible results were obtained for members of the clade. The monotypic genus Polytolypa (Scott et al. 1993) is represented by its type species Polytolypa hystricis (ex-type culture: UAMH 7299, from porcupine dung, Canada). It is nonkeratinolytic, unable to grow at 37 °C and possesses gymnothecia with helical peridial appendages, globose, irregularly disposed asci and apricot yellow, smooth to minutely roughened ascospores, and a chrysosporium-like anamorph (Scott et al. 1993). Morphological characteristics have been reported similar to Helicocarpus griseus (Untereiner et al. 2002). Based on the results of LSU locus analysis and whole genome analysis, P. hystricis was reported as a basal clade of Ajellomycetaceae (Untereiner et al. 2002; Muñoz et al. 2018), whereas it was found to be close to Spiromastigoidaceae by ITS locus analysis (Hirooka et al. 2016). In the current multilocus study, the type strain was found to be a basal clade with Spiromastigoidaceae, together with Chrysosporium chiropterorum (ex-type culture: MUCL 45495, from bat fur, France; Beguin et al. 2005) and Chrysosporium lobatum (ex-type culture: CBS 666.78, from mouse hair, Russia; Scharapov 1978) (Fig. 1). The ITS identity of ex-type cultures P. hystricis UAMH 7299 and C. chiropterorum MUCL 45495 was found to be 91%. Chysosporium lobatum (CBS 666.78) showed 90% ITS similarity with P. hystricis. Nevertheless, more detailed genome analyses including members from closely related families, i.e., Spiromastigoidaceae and Ascosphaeraceae, combined with physiological and morphological data, are required to conclusively determine the taxonomic position of Polytolypa.
Clade 13 Ascosphaeraceae Olive & Spiltoir.
= Synascomycetaceae Varitchak—Le Botaniste 25: 365, 1933 (invalid, ICBN Art. 36; see Cooke and Hawksworth 1970).
= Pericystaceae Bessey—Morph. Tax. Fungi, p. 352, 1950 (invalid, ICBN Art. 36; see Cooke and Hawksworth 1970).
Type genus: Ascosphaera Olive & Spiltoir—Mycologia 47(2): 242, 1955; type species: Ascosphaera apis (Maasen ex Claussen) Olive & Spiltoir—Mycologia 47(2): 242, 1955 ≡ Pericystis apis Maasen ex Claussen—Mitt. Biol. Bundes Anst. Land- u. Forstw. 16: 51‒58, 1916.
= Pericystis apis var. major Prökschl & Zobl—Arch. Mikrobiol. 18(2): 200, 1953 ≡ Ascosphaera apis var. major Prökschl & Zobl ex Olive & Spiltoir—Mycologia 47(2): 243, 1955.
= Pericystis apis var. minor Prökschl & Zobl—Arch. Mikrobiol. 18(2): 200, 1953.
Neotype designated here: Herb. No: CBS H-9145, dried culture isolated from Apis mellifera, Baarn, The Netherlands, J.B. Pannebakker, 1969. ex-type culture: CBS 534.69 = IHEM 3748.
The family Synascomycetaceae was proposed by Varitchak (1933) to describe fungi that have a sexual reproduction organ called the synascus, i.e., a single large vesicle with an acellular membranous peridium containing masses of “endospores” (spore balls). Since the fungi have reduced gametangia, Pericystis was concluded to be related to Plectascales and primitive Ascomycetes (Bessey 1950; Spiltoir and Olive 1955; Berbee and Taylor 1992; Geiser et al. 2006).
The name Pericystis was changed to Ascosphaera by Spiltoir and Olive (1955) because it was already in use for red algae (Pericystis Agardh 1847). Although the authors reclassified the genus and designated Ascosphaera apis as the type species, no type material was indicated. Ascosphaera was later reclassified in the family Ascosphaeraceae in the order Ascosphaerales (Gäumann 1964; invalid, ICBN Art. 37) and validated by Benny and Kimbrough (1980). Subsequently, Ascosphaera, Bettsia (Skou 1972) and Arrhenosphaera (Stejskal 1974) were included in the family. Arrhenosphaera, represented by the type species Arrhenosphaera craneae, was isolated from pollen and larvae in a comb of Apis mellifera in Venezuela (Stejskal 1974). However, no type was indicated, and the studied material was not known to be preserved; therefore, this name is considered doubtful. Although Bettsia also produces spore cysts and shares the same niche as saprophytic Ascosphaera species (Skou 1972; Wynns 2015), it is found in Leotiomycetes, phylogenetically far from Ascosphaera (Pitt et al. 2013). Currently, Ascosphaeraceae is classified in Onygenales as a monophyletic family with the single genus Ascosphaera (Fig. 1), and it is described with spore cysts with a double-layered wall and spore balls formed by groups of asci covered by a single membrane (Wynns et al. 2013).
Ascosphaeraceae comprises a group of fungi with a very specific ecology. Members of the family have thus far only been isolated from pollen, bee larvae/cadavers or nest cells (Supplementary Table 1; Fig. 4). They can also grow in habitats related to other pollenivorous insects and have a high sugar content (Wynns et al. 2013). Only Ascosphaera atra, a solitary bee-related saprobe, has been isolated from grass silage, apparently outside the bee habitat (Skou 1986). Some species are bee pathogens. Ascosphaera apis and A. aggregata are of special interest because of their aetiologies of chalkbrood disease in economically important bee species (Aronstein and Murray 2010; Pitts-Singer and Cane 2011). Available ecological data in this study showed that most Ascosphaera species are associated with solitary bees in the Megachilidae family (n = 24/28; Fig. 14) rather than with economically important social bees. Megachilidae have a worldwide distribution, which is matched by the widely distributed family Ascosphaeraceae (Marinho et al. 2018; Wynns et al. 2013).
Combination of data for the host preference, pathogenicity and phylogeny of Ascosphaeraceae members. Phylogenetic tree based on ITS sequences obtained by Bayesian analysis and maximum likelihood analysis using the TNe + G4 + F model on IQ-TREE. Branch values of ≥ 90% for Bayesian probability and ≥ 80% for maximum likelihood and type species of the genera are shown in bold. The designated neotype is indicated in red. Bettsia alvei was used as the outgroup
The presence of simple sac-like fruitbodies is a common feature among bee-related fungi and is shared by Ascosphaera (Onygenales; Spiltoir and Olive 1955), Bettsia (Leotiales; Wynns 2015) and Eremascus (Onygenales; Gueidan et al. 2008; Wijayawardene et al. 2017; Wynns 2015). This characteristic seems to have emerged more than once in the course of evolution and may have an ecological adaptation to this specific habitat and dispersion of propagules (Wynns 2015). In contrast to Ascosphaera and Bettsia, Eremascus is described with completely naked asci (Wynns 2015). Rudimentary development or near-absence of gymnothecia has also been observed in other onygenalean genera, such as Narasimhella and Pseudoarachniotus (von Arx 1986; Orr et al. 1977a; de Hoog et al. 2020). In the present study, Chrysosporium alvearium Liu & Cai (Zhao et al. 2018) (ex type culture: LC11684), originating from hive-stored pollen, was also found to be a member of the Onygenales (Fig. 1; Supplementary Figures 1‒4) and has been reclassified above in Keratinophyton (Fig. 7).
Clade 14 Eremascaceae Engler & Gilg.
Type genus: Eremascus Eidam; type species: Eremascus albus Eidam—Beitr. Biol. Pfl. 3: 385; 1883. Neotype designated here: CBS 975.69, isolated by H.J. Phaff, from an unknown source, USA, deposited by W.D. Gray.
The family Eremascaceae (Engl. & Gilg 1924) was introduced with the type species Eremascus albus (Eidam 1883) and Eremascus fertilis. The family was found in Onygenales, close to Ascosphaeraceae, by several researchers (Gueidan et al. 2008; Johnston et al. 2015; Wijayawardene et al. 2017), while it was recorded as incertae sedis in the Index Fungorum database (access date February 09, 2022). Eremascus albus is a very rare species (Eidam 1883; Harrold 1950; Paugh and Gray 1969), sharing with Wallemia and Sigleria an obligate osmophilic nature in habitats with low water activity due to high sucrose content. The second species, E. fertilis Stoppel (1907), also thrived in an osmotic habitat (apple and red currant jelly) but was morphologically different (Kreger-van Rij et al. 1974). Molecular studies have shown that E. fertilis can be classified in Leotiomycetes, remote from E. albus, and was reclassified as Skoua fertilis (Wynns 2015). Eremascus terrestris Asthana & Mahmud (1944), from a betel vine (Piper betle) in a garden in India, has also been included in Eremascus, but no type was indicated, and original material is not known to be preserved; therefore, this name is considered doubtful. Since type material is not known to exist for E. albus, the following neotype is indicated here: CBS 975.69, isolated by H.J. Phaff, USA.
In our phylogenetic studies, the cluster Eremascaceae comprises E. albus and Dactylodendron pinicola. Typification of the latter genus was established with Arthrographis kalrae in Eremomycetaceae, which is a genus of Dothideomycetes (Giraldo et al. 2014; Hernández-Restrepo et al. 2020). Arthrographis alba (non-Eremascus albus) is also found in Onygenales (Clade 3, incertae sedis) and is synonymized with Leucothecium emdenii. The reduced characteristics of ascoma morphology, with naked asci and a preference for high-osmotic habitats, are similar to those of Ascosphaeraceae.
Discussion
Different interpretations of the morphological characteristics of Onygenales isolates have led to divergent approaches for the classification of taxa in this fungal order. While the implementation of molecular taxonomic approaches provides faster identification, a higher level of differentiation increases the number of recognized taxa. This is particularly obvious in the classical dimorphic agents of systemic disease, which have been treated as monotypic genera for many decades but today comprise clusters of closely related species (Sepúlveda et al. 2017; Jiang et al. 2020; Mavengere et al. 2020). However, the application of the One Fungus=One Name concept and multiphasic approaches comprising molecular, morphological, clinical and physiological characteristics of species should lead to abandoning many names to simplify nomenclature. To this end, the stabilization of reference points by indicating the type materials of the species is essential. In the present study, a multilocus phylogeny of Onygenales was presented with the indication of the type of materials where possible, and conclusions were compared with ecological, physiological and morphological parameters.
The order Onygenales is particularly known for its ecological association with mammals, either via dung or keratin, or via pathogenicity, which can be superficial or systemic. The majority of the species included have environmental sexual phases with elaborate gymnothecia and invariably thallic conidiogenesis. Generic delimitations based solely on morphology have been problematic, as was demonstrated in the family Gymnoascaceae, where numerous species have been recombined over time in nearly the same list of generic names (Fig. 11).
Some species in the order Onygenales share characteristics with members of adjacent orders; therefore, the determination of the borderline of the order is needed. However, this can be challenging because the effects of the chosen methods, number of samples, number of genes and outgroup choice may be crucial (Li et al. 2021). For instance, the phylogenetic position of the genus Arachnomyces was debated; it was classified in Onygenales (Abbott et al. 1996; Malloch and Cain 1970) before a separate order, Arachnomycetales, was established (Gibas et al. 2002a). Similarly, the genus Ascosphaera was classified in Ascosphaerales together with Eremascus (Geiser and LoBuglio 2001), but later, both genera were included in Onygenales (Geiser et al. 2006). In the present study, using Aspergillus fumigatus as an outgroup (Eurotiales), the position of Arachnomycetales was confirmed as an individual order (Wijayawardene et al. 2020). Morphological differences in the ascomata structures of Ascosphaera and Eremascaceae were supported by phylogenetic results, and they were kept in separate families within the order Onygenales.
Taxonomic methods have changed from the morphological to the molecular level such that morphology, physiology and ecology data have become supporter parameters rather than the main determinants of taxonomy. This process is likely to continue to become more detailed. Thus, changes in nomenclature remain inevitable. Taxon sampling and inclusion of both type species and related genera are particularly essential to minimize changes and stabilize nomenclature for longer periods. In addition, providing molecular data for the isolates and making them publicly available is also important to prevent taxonomic disagreements. For example, Chlamydosauromyces was introduced as a new genus (Sigler et al. 2002b) because molecular data of its closest relative, Neogymnomyces, were not available at that time, even though it had been described earlier. Whole-genome data of the strain CBS 281.48 of Onygena corvina are available in databases (Huang et al. 2015); however, as confirmed in the present study, this strain represented an Arthroderma species (Garces et al. 2019). This misidentification led to another one: Pectinotrichum chinense was described using the misidentified Onygena corvina CBS 281.48 as the reference without using the generic type, P. llanense or any member of Arthrodermataceae (Zhang et al. 2017b).
Calculation of the supported/unsupported ratio, together with high support values for all clades, was shown to be effective in finding the “best tree”, i.e., the tree with the most resolved backbone for the members of Chaetothyriales (Quan et al. 2020); therefore, this approach was followed in the current study. However, the groups variously formed supported clades, and hence, the ratio of supported/unsupported clades increased, while the backbone of the trees became less resolved, as in the ITS phylogeny (Table 3; Supplementary Figure 3).
The present study also aimed to investigate whether the ecological parameters can assist in defining groups within the Onygenales at the family level and whether the interpretation of these parameters can provide a model to estimate the course of evolution in this order. Evolution from geophilic lifestyles to anthropophilic association with Homo sapiens is an intuitively logical order of events. However, evolution may be ongoing at any branch of the evolutionary tree, and thus, none of the clades has priority for an ultimate position; ancestral reconstruction is therefore applicable within branches, using ecological traits of environmental lifestyle. Significant ecological traits that determine evolution in Onygenales are osmophily, thermophily, cellulolysis, eutrophism, oligotrophism, keratinolysis, and thermal dimorphism (Fig. 3). In particular, osmophily, keratinolysis and thermal dimorphism are highly exceptional in the fungal kingdom, and therefore, reemergence in the course of evolution is less likely. These characteristics are pronounced in the osmophilic, keratinolytic and dimorphic families, namely, Ascosphaeraceae, Arthrodermataceae and Ajellomycetaceae, respectively (Fig. 3). While thermal dimorphism is present in all members of the Ajellomycetaceae, albeit sometimes with reluctance (Jiang et al. 2018), it is shared by Coccidioides at a remote position in Onygenaceae. By combining the results of the phylogenetic analyses in the present study and genome analyses performed by Muñoz et al. (2018), it may be hypothesized that pathogenic Ajellomycetaceae evolved from a nonthermophilic and nondimorphic ancestor, which encountered a mammalian host (e.g., armadillo) and developed several mechanisms (i.e., producing yeast-like forms in vivo) to adapt to a pathogenic lifestyle.
Pathogenic Ajellomycetaceae, along with Onygenaceae members such as Coccidioides, Amauroascus, Uncinocarpus and Byssoonygena, have elevated levels of proteases and carbohydrate-active enzymes, whereas Helicocarpus griseus, a nonpathogenic geophilic member of Ajellomycetaceae, still has more carbohydrate-active enzymes related to plant cell degradation in its genome (Muñoz et al. 2018). The relationship between nonpathogenic Helicocarpus and pathogenic, systemic Ajellomycetaceae may be comparable to that of Uncinocarpus, which is known to be the closest genus to Coccidioides but is nonpathogenic and nondimorphic. These data suggest that thermal dimorphism may have occurred independently in Ajellomycetaceae and Onygenaceae or that the other dimorphic species between these two groups no longer exist. Nevertheless, why this characteristic is restricted to only Coccidioides in Onygenaceae and the molecular background of the relationship between Uncinocarpus and Coccidioides are unknown.
The majority of the Onygenaceae and Gymnoascaceae species are found in association with dung and in different types of soil, while only a few species have been reported from plant debris. Comparing the dung types from which members of both these families were isolated, Gymnoascaceae members were mainly found in herbivore dung, while Onygenaceae members were often isolated from carnivore dung (Supplementary Table 1). Carnivore dung is more nutrient-rich than herbivore dung (Frank et al. 2017), providing suitable habitat for keratinophilic species because it may also contain hair of prey. Environments such as dung and agricultural soil provide wide opportunities for fungi with respect to nutrition (Marinari et al. 2000; Frank et al. 2017), but this requires proper metabolic mechanisms for competition since these habitats are colonized by a plethora of microbes. On the other hand, some onygenalean fungi prefer poor environments where few competing microbes reside but where they need efficient nutrient scavenging. Remarkably, these two opposite habitat choices can be seen among members of the family Gymnoascaceae, suggesting that this group may contain the starting points of several evolutionary lines.
Niche adaptation requires metabolic and reproductive efficiency; therefore, morphological and physiological characteristics may be informative for habitat choice and evolutionary processes. In the present study, gymnothecium with reticuloperidium was found to be the most common ascoma type. Compared to the closed structure of a cleistothecium, loosely composed gymnothecia can be disrupted, easily enhancing spore dispersal, while the ascomal appendages may be helpful for attachment to animal surfaces (Currah 1985), such as the impressive comb-like appendages of Ctenomyces that attach to feathers.
In onygenalean fungi, two main ways of feeding are encountered: keratinolytic and cellulolytic. Families in ancestral positions, or those that drifted in other directions, such as Ajellomycetaceae and Spiromastigoidaceae, were found to lack keratinase, which is one of the main characteristics of the relatively derived family Arthrodermataceae. Cellulolytic activity in Onygenales seems to have been lost in most clades, as noted earlier in genomic comparisons (Muñoz et al. 2018).
While most of the species in Onygenales are confined to environmental sources, some are established pathogens for humans and/or other mammals, sometimes with a restricted host range. Coccidioides, Blastomyces, Emergomyces, Histoplasma and Paracoccidioides are all dimorphic environmental pathogens with a double life cycle (de Hoog et al. 2018). Onygenaceae, especially Group V, contain species that consistently cause skin infections in reptiles, and they are only extremely rare on other hosts. Reptile association is found in several phylogenetically separated genera, including Aphanoascella, Emydomyces, Nannizziopsis, Paranannizziopsis, and Ophidiomyces. Although Arthrodermataceae is known as the main family in Onygenales with keratinolytic ability and the ability to cause skin infections in mammals, there is only a single report (Rees 1967a) on isolates obtained from the skin of a skink (Egernia sp.) and a monitor lizard (Varanus sp.) infected by Arthroderma cuniculi and Paraphyton cookei, respectively. Similarly, despite the regular occurrence of members of Gymnoascaceae on feather remains, only a single dermatophyte, Lophophyton gallinae, occurs on fowl (de Hoog et al. 2020). Keratin- and lipid-associated differences between mammalian, reptilian and avian skin may cause the selection of keratinophilic fungi. For instance, the presence of α-keratin has been shown in all vertebrates, whereas β-keratin is exclusively found in reptiles and birds (Greenwold et al. 2014). In reptiles, β-keratin provides strength and hardness (Rutland et al. 2019). The introduction of a reptile host during evolution may have caused the divergence of keratinolytic Onygenaceae and later Arthrodermataceae from a common ancestor. In humans, long-chain fatty acids in sebum have been thought to have a fungistatic effect (Khosravi et al. 2016; Hay et al. 2017), a role in preventing water loss from skin, as well as pro- and anti-inflammatory functions (Makrantonaki et al. 2011). Since the effect of sebum increases with age, sebum activity is considered an important factor in the occurrence of tinea capitis caused by T. violaceum (Su et al. 2019). Similarly, avian epidermal lipids provide antimicrobial functions in addition to their roles in evaporative cooling and permeability (Menon and Menon 2000).
Several groups in Onygenales, such as Arachniotus in Gymnoascaceae, and members of Spiromastigoidaceae, Ascosphaeraceae and Eremascaceae show an osmophilic tendency. Among these groups, Ascosphaeraceae are remarkable because of the unique habitat choice. Although the morphology and taxonomy of the members of this family have been well studied, little is known about their enzymatic abilities. Studies with Ascosphaera apis showed that this species does not possess many lytic enzymes, including chitinases, which can be found in other insect pathogens (Gochnauer and Margetts 1979; Heath 1982). Given that members can also be isolated from cocoons, which are made of silk and composed of fibroin and sericin, different types of proteolytic properties might play a role in these bee-specialist fungi. Similar to dung-associated fungi, Ascosphaera spores depend on their passage through an animal gut. However, the spores of Ascosphaera species germinate in the midgut of larvae, and subsequently, fungal hyphae break through the outer cuticula of larvae (Holloway et al. 2012; Wynns et al. 2013; Heath 1982). The biochemical mechanisms of anaerobic spore germination and the aerobic hyphal growth cycle of pathogenic Ascosphaera species are currently not well understood. As in the Neogymnomycetaceae clade, multilocus analyses revealed a high degree of specialization (emergence of many species in a short time) in the Ascosphaeraceae clade, suggesting that evolution might have occurred within a relatively short timeframe in these groups.
A similar conclusion can be reached for the whole order based on the divergence time estimations, and Onygenales can be considered a relatively recent (103 Mya) evolved order compared to Chaetothyriales (151 Mya; Quan et al. 2020) and Hypocreales (137 Mya; Fonseca et al. 2020) orders that also contain pathogen species. Nonkeratinolytic basal families appeared earlier than keratinophilic members of the order, suggesting that cellulolytic and osmophilic abilities might be ancestral characteristics. In contrast, thermal dimorphism, as in Coccidioides species and members of Ajellomycetaceae, can be a result of independent adaptation.
In the present study, some of the phylogenetic trees showed different topologies, particularly in Gymnoascaceae and Onygenaceae. However, these differences were affected by the number of distantly related samples used in the analyses, which necessitated shortening of the sequence length, with concomitant loss of information. Another pitfall was the presence of incomparable sequences obtained from public databases. Although the universal barcode for fungi is the ITS sequence (Stielow et al. 2015), it is often necessary to use alternative universal primers for genes that have multiple primer locations, such as TEF1, TEF3, RP60S, and RPB2, to appropriately differentiate species from each other (Stielow et al. 2015; Thines et al. 2018). This approach might also lend more reproducibility to further studies.
Considering that many species with special ecology, such as Nannizziopsis, Auxarthronopsis, Sigleria and Spiromastigoides, have been discovered only during the last decade, our results likely suffer from taxon sampling effects. In addition, molecular, morphological and physiological data are insufficient for the majority of species, hampering the establishment of degrees of intraspecific variation. Ongoing collection from divergent types of environments, increasing the sample size and observed variation, and using a polyphasic methodology may lead to changes in the chosen boundaries. However, consistent re-estimation of these boundaries can provide a better understanding of the phylogeny and evolutionary process of this fascinating order.
Even though the Onygenales are a recent order, they have been on Earth longer than human existence, and at some point, some members encountered animals and humans. Similar to some other fungi, onygenalean members either gained new abilities or used their ready-made abilities to adapt and survive on these new hosts (Casadevall et al. 2003; Naranjo-Ortiz and Gabaldón 2019). Almost all families in the order have members that can be found on skin and nails, which can cause asymptomatic or symptomatic infections, or members that are able to grow at 37 °C and cause systemic infections (Fig. 4). This is particularly important because environmental and host alterations can lead to the emergence of new fungal pathogens, which can be either currently unknown species or species that are currently known as nonpathogens. Similar to the emergence of Nannizziopsis species, Ophidiomyces ophidiocola (Sigler et al. 2013), Trichophyton benhamiae (Čmoková et al. 2020), T. indotineae (Kano et al. 2020) and T. quinckeanum (Uhrlaß et al. 2018), we will likely face new fungal pathogens as a result of the increased captivity of wild animals, changes in the environmental temperature and continued human activity involving soil and animals. Although temperature adaptation is not the only criterion for fungal virulence, soil fungi, especially those that can grow at 37 °C, might have an increased potential to be encountered because they also have an opportunity to undergo selection by their ongoing interactions with other soil microorganisms. Therefore, the order Onygenales continues to deserve close attention.
References
Abarca ML, Martorell J, Castellá G, Ramis A, Cabañes FJ (2009) Dermatomycosis in a pet inland bearded dragon (Pogona vitticeps) caused by a Chrysosporium species related to Nannizziopsis vriesii. Vet Dermatol 20(4):295–299. https://doi.org/10.1111/j.1365-3164.2009.00736.x
Abbott SP, Sigler L, Currah RS (1996) Delimitation, typification and taxonomic placement of the genus Arachnomyces. Syst Ascom 14:70–85
Agardh JG (1847) Nya alger från Mexico. Öfversigt af Kongl. Vetenskaps-Adademiens Förhandlingar, Stockholm 4:5–17
Ajello A, Cheng SLY (1967) A new geophilic Trichopyton. Mycologia 59(2):255–263. https://doi.org/10.2307/3756798
Almeida FD (1930) Differences between the etiological agents of coccidioidal granuloma in the United States and in Brazil. New genus for the Brazilian fungus. C r Seances Soc Biol 105:315–316
Amselem J, Cuomo CA, van Kan JA et al (2011) Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet 7(8):e1002230. https://doi.org/10.1371/journal.pgen.1002230
Anderson DL, Gibbs AJ, Gibson NL (1998) Identification and phylogeny of spore-cyst fungi (Ascosphaera spp.) using ribosomal DNA sequences. Mycol Res 102(5):541–547. https://doi.org/10.1017/s0953756297005261
Anderson DL, Gibson NL (1998) New species and isolates of spore-cyst fungi (Plectomycetes: Ascosphaerales) from Australia. Aust Syst Bot 11(1):53–72
Apinis AE (1964) Revision of British Gymnoascaceae. Mycol Pap 96:1–56
Apinis AE (1968) Relationships of certain keratinophilic Plectascales. Mycopathol Mycol Appl 35(2):97–104. https://doi.org/10.1007/BF02049572
Apinis AE (1970) Concerning Rollandina. Trans Br Mycol Soc 55(3):499–502
Apinis AE, Clark BM (1974) Neoxenophila foetida gen. et sp. nov., a new keratinophilic ascomycete. Trans Brit Mycol Soc 63(2):261–265. https://doi.org/10.1016/S0007-1536(74)80171-3
Apinis AE, Rees RG (1976) An undescribed keratinophilic fungus from southern Queensland. Trans Br Mycol Soc 67(3):522–524. https://doi.org/10.1016/S0007-1536(76)80188-X
Armaleo D, Müller O, Lutzoni F et al (2019) The lichen symbiosis reviewed through the genomes of Cladonia grayi and its algal partner Asterochloris glomerata. BMC Genomics 20(1):605. https://doi.org/10.1186/s12864-019-5629-x
Aronstein KA, Murray KD (2010) Chalkbrood disease in honey bees. J Invertebr Pathol 103(Suppl 1):S20-29. https://doi.org/10.1016/j.jip.2009.06.018
Asthana RP, Mahmud KA (1944) Preliminary note on a new species of Eremascus. Curr Sci 13(11):285–286
Baert F, Stubbe D, D’hooge E, Packeu A, Hendrickx M, (2020) Updating the taxonomy of dermatophytes of the BCCM/IHEM Collection according to the new standard: a phylogenetic approach. Mycopathologia 185(1):161–168. https://doi.org/10.1007/s11046-019-00338-7
Baranetzky J (1872) Entwicklungsgeschichte Des Gymnoascus Reessii. Bot Zeitung 30:145–160
Barker BM (2018) Coccidioidomycosis in animals. In: Seyedmousavi S, de Hoog GS, Guillot J, Verweij PE (eds) Emerging and epizootic fungal infections in animals. Springer, New York, pp 81–114
Baroncelli R, Amby DB, Zapparata A, Sarrocco S, Vannacci G, Le Floch G, Harrison RJ, Holub E, Sukno SA, Sreenivasaprasad S, Thon MR (2016) Gene family expansions and contractions are associated with host range in plant pathogens of the genus Colletotrichum. BMC Genomics 17:555. https://doi.org/10.1186/s12864-016-2917-6
Beguin H, Larcher G, Nolard N, Chabasse D (2005) Chrysosporium chiropterorum sp. nov., isolated in France, resembling Chrysosporium state of Ajellomyces capsulatus (Histoplasma capsulatum). Med Mycol 43(2):161–169. https://doi.org/10.1080/13693780400006096
Beguin H, Pyck N, Hendrickx M, Planard C, Stubbe D, Detandt M (2012) The taxonomic status of Trichophyton quinckeanum and T. interdigitale revisited: a multigene phylogenetic approach. Med Mycol 50(8):871–882. https://doi.org/10.3109/13693786.2012.684153
Benedek T (1964) New dermatophyte, Thallomicrosporon kuehnii Benedek, 1963. Mycopath Mycol Appl 23:85–98. https://doi.org/10.1007/BF02049263
Benedek T (1969) Parthenogenetic production of fertile cleistothecia in Nannizzia incurvata Stockd. Under the influence of Chrysosporium species. Mycopathol Mycol Appl 37:193–214. https://doi.org/10.1007/BF02051354
Benjamin RK (1956) A new genus of Gymnoascaceae with a review of the other genera. Aliso 3(3):301–328
Benny GL, Kimbrough JW (1980) A synopsis of the orders and families of Plectomycetes with keys to genera. Mycotaxon 12(1):1–91
Berbee ML, Taylor JW (1992) Two Ascomycete classes based on fruiting-body characters and ribosomal DNA sequence. Mol Biol Evol 9(2):278–284. https://doi.org/10.1093/oxfordjournals.molbev.a040719
Berbee ML, Taylor JW (2001) Fungal Molecular Evolution: gene trees and geologic time. In: McLaughlin DJ, McLaughlin EG, Lemke PA (eds) Systematics and evolution. The Mycota (A comprehensive treatise on fungi as experimental systems for basic and applied research), vol 7B. Springer, Berlin
Berkeley MJ (1860) Outlines of British Fungology. Lovell Reeve, London
Berkeley MJ, Broome CE (1851) Notices of British fungi (538–614). Ann Mag Nat Hist 7:176–189
Bessey EA (1950) Morphology and taxonomy of fungi. The Blakiston Company, Toronto
Bissett J (1988) Contribution toward a monograph of the genus Ascosphaera. Can J Bot 66(12):2541–2560
Bissett J, Duke G, Goettel M (1996) Ascosphaera acerosa sp. nov. isolated from alfalfa leavutting bee, with a key to the species of Ascosphaera. Mycologia 88(5):797‒803. https://doi.org/10.1080/00275514.1996.12026717
Böhme H (1967) Arthroderma gertleri sp. nov., die perfekte Form von Trichophyton vanbreuseghemii Rioux, Jarry et Juminer. Mykosen 10(6):247‒252
Bolognesi G, Chiurco GA (1925) Malbranchea bolognesii-chiurcoi Vuill., Pollacci & Nann. Archivi di Biologia 1, Nr.5–6:14
Borman AM, Szekely A, Fraser M, Lovegrove S, Johnson EM (2018) A novel dermatophyte relative, Nannizzia perplicata sp. nov., isolated from a case of tinea corporis in the United Kingdom. Med Mycol 57(5):548‒556. https://doi.org/10.1093/mmy/myy099
Borowiec ML (2016) AMAS: A fast tool for alignment manipulation and computing of summary statistics. Peer J 4:e1660. https://doi.org/10.7717/peerj.1660
Bowman BH, White TJ, Taylor JW (1996) Human pathogenic fungi and their close nonpathogenic relatives. Mol Phylogenet Evol 6:89–96. https://doi.org/10.1006/mpev.1996.0061
Brasch J, Beck-Jendroschek V, Voss K, Yurkov A, Gräser Y (2019) Arthroderma chiloniense sp. nov. isolated from human stratum corneum: Description of a new Arthroderma species. Mycoses 62:73–80. https://doi.org/10.1111/myc.12850
Brasch J, Gräser Y (2005) Trichophyton eboreum sp. nov. isolated from human skin. J Clin Microbiol 43(10):5230–5237. https://doi.org/10.1128/JCM.43.10.5230-5237.2005
Brown EM, McTaggart LR, Zhang SX, Low DE, Stevens DA, Richardson SE (2013) Phylogenetic analysis reveals a cryptic species Blastomyces gilchristii, sp. nov. within the human pathogenic fungus Blastomyces dermatitidis. PLoS One 8(3):e59237. https://doi.org/10.1371/journal.pone.0059237
Burmester A, Shelest E, Glöckner G, Heddergott C, Schindler S, Staib P, Heidel A, Felder M, Petzold A, Szafranski K, Feuermann M, Pedruzzi I, Priebe S, Groth M, Winkler R, Li W, Kniemeyer O, Schroeckh V, Hertweck C, Hube B, White TC, Platzer M, Guthke R, Heitman J, Wöstemeyer J, Zipfel PF, Monod M, Brakhage AA (2011) Comparative and functional genomics provide insights into the pathogenicity of dermatophytic fungi. Genome Biol 12(1):R7. https://doi.org/10.1186/gb-2011-12-1-r7
Caldwell M, Nydam R, Palci A, Apesteguía S (2015) The oldest known snakes from the Middle Jurassic-Lower Cretaceous provide insights on snake evolution. Nat Commun 6:5996. https://doi.org/10.1038/ncomms6996
Campbell CK, Borman AM, Linton CJ, Bridge PD, Johnson EM (2006) Arthroderma olidum, sp. nov. A new addition to the Trichophyton terrestre complex. Med Mycol 44(5):451–459. https://doi.org/10.1080/13693780600796538
Cano J, Guarro J (1989) Two new keratinophilic Ascomycetes from Spain. Stud Mycol 31:60–67
Cano J, Guarro J (1990) The genus Aphanoascus. Mycol Res 94(3):355–377. https://doi.org/10.1016/S0953-7562(09)80361-4
Cano J, Guarro J (1994) Studies on keratinophilic fungi. III. Chrysosporium Siglerae sp. nov. Mycotaxon 51:75–80
Cano J, Guarro J, Castaneda Ruiz RF (1994) Studies on keratinophilic fungi. IV. Bifidocarpus, a new genus of the Eurotiales. Mycotaxon 52(1):53–57
Cano J, Sagués M, Barrio E, Vidal P, Castañeda RF, Gené J, Guarro J (2002a) Molecular taxonomy of Aphanoascus and description of two new species from soil. Stud Mycol 47:153–164
Cano J, Solé M, Pitarch LB, Guarro J (2002b) Castanedomyces australiensis gen. nov., sp. nov., a keratinophilic fungus from Australian soil. Stud Mycol 47:165–172
Cano J, Solé M, Pitarch LB, Guarro J (2002c) Pseudoamauroascus, a new genus of the Onygenales (Ascomycota). Stud Mycol 47:173–179
Cano J, Ulfig K, Guillamon J, Vidal P, Guarro J (1997) Studies on keratinophilic fungi. IX: Neoarachnotheca gen. nov. and a new species of Nannizziopsis. Antonie Van Leeuwenhoek 72:149–158. https://doi.org/10.1023/A:1000394118040
Cardoso MA, Tambor JH, Nobrega FG (2007) The mitochondrial genome from the thermal dimorphic fungus Paracoccidioides brasiliensis. Yeast 24(7):607–616. https://doi.org/10.1002/yea.1500
Carmichael JW (1962) Chrysosporium and some other aleuriosporic Hyphomycetes. Can J Bot 40:1137–1181. https://doi.org/10.1139/b62-104
Carrero LL, Niño-Vega G, Teixeira MM, Carvalho MJ, Soares CM, Pereira M, Jesuino RS, McEwen JG, Mendoza L, Taylor JW, Felipe MS, San-Blas G (2008) New Paracoccidioides brasiliensis isolate reveals unexpected genomic variability in this human pathogen. Fungal Genet Biol 45(5):605–612. https://doi.org/10.1016/j.fgb.2008.02.002
Casadevall A, Steenbergen JN, Nosanchuk JD (2003) “Ready made” virulence and “dual use” virulence factors in pathogenic environmental fungi–the Cryptococcus neoformans paradigm. Curr Opin Microbiol 6(4):332–337. https://doi.org/10.1016/s1369-5274(03)00082-1
Castellani A (1937) A preliminary report on two pathogenic fungi: Trichophyton dankaliense n. sp.; Sporotrichum anglicum n. sp. J Trop Med Hyg 40:313–318
Castellani A, Chalmers AJ (1919) Manual of Tropical Medicine, 3rd edn (London). https://doi.org/10.5962/bhl.title.84653
Chen KH, Miadlikowska J, Molnár K, Arnold AE, U’Ren JM, Gaya E, Gueidan C, Lutzoni F (2015) Phylogenetic analyses of eurotiomycetous endophytes reveal their close affinities to Chaetothyriales, Eurotiales, and a new order – Phaeomoniellales. Mol Phylogenet Evol 85:117–130. https://doi.org/10.1016/j.ympev.2015.01.008
Choi JS, Gräser Y, Walther G, Peano A, Symoens F, de Hoog GS (2012) Microsporum mirabile and its teleomorph Arthroderma mirabile, a new dermatophyte species in the M. cookei clade. Med Mycol 50(2):161–169. https://doi.org/10.3109/13693786.2011.594456
Ciferri R, Azevedo PC, Campos S, Carneiro LS (1956) Taxonomy of Jorge Lobo’s disease fungus. Publ Do Inst De Micol Da Univ Do Rec 53:1–21
Ciferri R, Montemartini A (1959) Taxonomy of Haplosporangium parvum. Mycopathol Mycol Appl 10(4):303–316. https://doi.org/10.1007/BF02051638
Clements FE, Shear CL (1954) The genera of fungi. Hafner, New York
Čmoková A, Kolařík M, Dobiáš R, Hoyer LL, Janouškovcová H, Kano R, Kuklová I, Lysková P, Machová L, Maier T, Mallátová N, Man M, Mencl K, Nenoff P, Peano A, Prausová H, Stubbe D, Uhrlaß S, Větrovský T, Wiegand C, Hubka V (2020) Resolving the taxonomy of emerging zoonotic pathogens in the Trichophyton benhamiae complex. Fungal Divers 104:333–387. https://doi.org/10.1007/s13225-020-00465-3
Čmoková A, Rezaei-Matehkolaei A, Kuklová I, Kolařík M, Shamsizadeh F, Ansari S, Gharaghani M, Miňovská V, Najafzadeh MJ, Nouripour-Sisakht S, Yaguchi T, Zomorodian K, Zarrinfar H, Hubka V (2021) Discovery of new Trichophyton members, T. persicum and T. spiraliforme spp. nov., as a cause of highly inflammatory tinea cases in Iran and Czechia. Microbiol Spectr 31;9(2):e0028421. https://doi.org/10.1128/Spectrum.00284-21
Cooke MC (1875) British fungi (cont). Grevillea 4(30):66–69
Cooke MC, Ellis JB (1879) New Jersey Fungi. Grevillea 8(45):11–16
Cooke WB, Hawksworth DL (1970) A preliminary list of families proposed for fungi (including the lichens). Commonwealth Mycological Institute Paper No. 121
Corda ACJ (1833) Deutschlands flora, Abt. III. Die Pilze Deutschlands 3(13):65–96
Cowan DF (1993) Lobo’s disease in a bottlenose dolphin (Tursiops truncatus) from Matagorda Bay, Texas. J Wildl Dis 29:488–489. https://doi.org/10.7589/0090-3558-29.3.488
Crane JL, Schoknecht JD (1986) Revision of Torula and Hormiscium species. New names for Hormiscium undulatum, Torula equina, and Torula convolvuli. Mycologia 78(1):86–91. https://doi.org/10.2307/3793381
Crous PW, Wingfield MJ, Burgess TI et al. (2017) Fungal Planet description sheets: 558–624. Persoonia 38:240–384 (145). https://doi.org/10.3767/003158517X698941
Crous PW, Wingfield MJ, Chooi YH et al. (2020) Fungal Planet description sheets: 1042–1111. Persoonia 44:301–459 (159). https://doi.org/10.3767/persoonia.2020.44.11
Crous PW, Wingfield MJ, Guarro J et al. (2013) Fungal Planet description sheets: 154–213. Persoonia 31(1):188–296 (109). https://doi.org/10.3767/003158513X675925
Crous PW, Wingfield MJ, Lombard L et al. (2019) Fungal Planet sheets 951–1041. Persoonia 43:223–425 (203). https://doi.org/10.3767/persoonia.2019.43.06
Crous PW, Wingfield MJ, Richardson DM et al. (2016) Fungal Planet description sheets: 400–468. Persoonia 36:316–458 (143). https://doi.org/10.3767/003158516X692185
Currah RS (1985) Taxonomy of the Onygenales: Arthrodermataceae, Gymnoascaceae, Myxotrichaceae and Onygenaceae. Mycotaxon 24:1–216
Currah RS (1988) An annotated key to the genera of the Onygenales. Syst Ascomycetum 7(1):1–12
Currah RS (1994) Peridial morphology and evolution in the prototunicate ascomycetes. In: Hawksworth DL (ed) Ascomycete systematics: problems and perspectives in the nineties. Plenum Press, New York, pp 281–293
Currah RS, Abbott SP, Sigler L (1996) Arthroderma silverae sp. nov. (Onygenales, Arthrodermataceae) and Chrysosporium vallenarense (Hyphomycetes), keratinophilic fungi from arctic and montane habitats. Mycol Res 100(2):195–198. https://doi.org/10.1016/S0953-7562(96)80121-3
Darling ST (1906) A protozoön general infection producing pseudotubercles in the lungs and focal necrosis in the liver, spleen and lymph nodes. JAMA 46:1283–1285. https://doi.org/10.1001/jama.1906.62510440037003
Davies WL (1938) The composition of cheese rinds. J Dairy Res 9(3):342–346. https://doi.org/10.1017/S0022029900002612
Dawson CO (1963) Two new species of Arthroderma isolated from soil from rabbit burrows. Sabouraudia 2(3):185–191. https://doi.org/10.1080/00362176385190301
Dawson CO, Gentles JC (1961) The perfect states of Keratinomyces ajelloi Vanbreuseghem, Trichophyton terrestre (Durie): Frey and Microsporum nanum Fuentes. Sabouraudia 1(1):49–57. https://doi.org/10.1080/00362176285190111
de Bary A (1884) Vergleichende Morphologie und Biologie der Pilze, Mycetozoen, und Bacterien. Engelmann, Leipzig, p 213
de Hoog GS, Ahmed SA, Danesi P, Guillot J, Gräser Y (2018) Distribution of pathogens and outbreak fungi in the fungal kingdom. In: Seyedmousavi S, de Hoog GS, Guillot J, Verweij PE (eds) Emerging and epizootic fungal infections in animals. Springer, New York, pp 3–17
de Hoog GS, Dukik K, Monod M, Packeu A, Stubbe D, Hendrickx M, Kupsch C, Stielow JB, Freeke J, Göker M, Rezaei-Matehkolaei A, Mirhendi H, Gräser Y (2017) Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 182(1–2):5–31. https://doi.org/10.1007/s11046-016-0073-9
de Hoog GS, Guarro J, Gené J, Ahmed S, Al-Hatmi AMS, Figueras MJ, Vitale RG (2020) Atlas of Clinical Fungi the ultimate benchtool for diagnostics, 4th edition. Foundation Atlas of Clinical Fungi, Hilversum, The Netherlands.
de Hoog S, Zalar P, van den Ende BG, Gunde-Cimerman N (2005) Relation of halotolerance to human-pathogenicity in the fungal tree of life: An overview of ecology and evolution under stress. In: Gunde-Cimerman N, Oren A, Plemenitaš A (eds) Adaptation to life at high salt concentrations in Archaea, Bacteria, and Eukarya. Cellular origin, life in extreme habitats and astrobiology, vol 9. Springer, Dordrecht
de Respinis S, Tonolla M, Pranghofer S, Petrini L, Petrini O, Bosshard PP (2013) Identification of dermatophytes by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Med Mycol 51(5):514–521. https://doi.org/10.3109/13693786.2012.746476
de Vries GA (1964) Keratinophilic fungi. Ann Soc Beige Mgd Trop 44(4–5):795–803
de Vries GA (1969) Das problem: Aphanoascus Zukal oder Anixiopsis Hansen. Mykosen 12(2):111–122. https://doi.org/10.1111/j.1439-0507.1969.tb03437.x
de Vroey C (1966) Etude mycologique de Gymnoascaceae isolées de lésions cutanées et de la terre en République de Somalie; note préliminaire. Ann Soc Belge Med Trop 46:457–464
Del Rocío R-M, Pérez-Huitrón MA, Ocaña-Monroy JL, Frías-De-León MG, Martínez-Herrera E, Arenas R, Duarte-Escalante E (2016) The habitat of Coccidioides spp. and the role of animals as reservoirs and disseminators in nature. BMC Infect Dis 16(1):550–558. https://doi.org/10.1186/s12879-016-1902-7
Desjardins CA, Champion MD, Holder JW et al (2011) Comparative genomic analysis of human fungal pathogens causing paracoccidioidomycosis. PLoS Genet 7(10):e1002345. https://doi.org/10.1371/journal.pgen.1002345
Desmazières JBHJ (1827) Observations sur le Sporendonema casei, nouveau genre des Mucédinées. Ann Sci Nat Bot 11:246–249
Ditmar LPF (1813) Deutschlands Flora. Abt III Die Pilze Deutschlands 1(1):1–34
Doveri F, Pecchia S, Vergara M, Sarrocco S, Vannacci G (2012) A comparative study of Neogymnomyces virgineus, a new keratinolytic species from dung, and its relationships with the Onygenales. Fungal Divers 52(1):13–34. https://doi.org/10.1007/s13225-011-0120-2
Doweld A (2013) Index Fungorum 30:1
Dukik K, de Hoog GS, Stielow JB, Freeke J, van den Ende BG, Vicente VA, Menken SBJ, Ahmed SA (2020) Molecular and phenotypic characterization of Nannizzia (Arthrodermataceae). Mycopathologia 185(1):9–35. https://doi.org/10.1007/s11046-019-00336-9
Dukik K, Muñoz JF, Jiang Y, Feng P, Sigler L, Stielow JB, Freeke J, Jamalian A, Gerrits van den Ende B, McEwen JG, Clay OK, Schwartz IS, Govender NP, Maphanga TG, Cuomo CA, Moreno LF, Kenyon C, Borman AM, de Hoog S (2017) Novel taxa of thermally dimorphic systemic pathogens in the Ajellomycetaceae (Onygenales). Mycoses 60(5):296–309. https://doi.org/10.1111/myc.12601
Dutta BG, Ghosh GR, Orr GF, Kuehn HH (1963) Soil Fungi from Orissa (India). II. A new species of Pseudoarachniotus. Mycologia 55(6):775–780. https://doi.org/10.1080/00275514.1964.12018096
Dutta BG, Ghosh GR, Orr GF, Kuehn HH (1964) Soil fungi from Orissa (India). III. A new species of Pseudoarachniotus with punctate spores. Mycologia 56(2):153–157. https://doi.org/10.1080/00275514.1964.12018096
Eidam E (1880) Beitrag zur Kenntnis der Gymnoasceen. Beitr Biol Pflanz 3:267–305
Eidam E (1883) Zur Kenntnis der Entwickelung bei den Ascomyceten. Beitr Biol Pflanz 3:377–433
Eidam E (1886) Untersuchungen über die Femilie der Gymnoascaceen. Jber Schles Ges Vaterl Kultur 64:162
Emmons CW (1954) Isolation of Myxotrichum and Gymnoascus from the lungs of animals. Mycologia 46(3):334–338. https://doi.org/10.1080/00275514.1954.12024372
Emmons CW, Ashburn LL (1942) The isolation of Haplosporangium parvum n. sp. and Coccidioides immitis from wild rodents. Publ Health Rep 57:1715–1727
Engler A, Gilg E (1924) Syllabus der Pflanzenfamilien: eine Übersicht über das ganze Pflanzensystem mit besonderer Berücksichtigung der Medizinal- und Naturpflanzen nebst einer Übersicht über die Florenreiche und Florengebiete der Erde zum Gebrauch bei Vorlesungen und Studien über spezielle und medizinisch-pharmazeutische Botanik, 9th edn. Gebrüder Borntraeger, Berlin
Esperón F, García-Párraga D, Bellière EN, Sánchez-Vizcaíno JM (2012) Molecular diagnosis of lobomycosis-like disease in a bottlenose dolphin in captivity. Med Mycol 50(1):106–109. https://doi.org/10.3109/13693786.2011.594100
Fisher MC, Koenig GL, White TJ, Taylor JW (2002) Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia 94(1):73‒84. https://doi.org/10.1080/15572536.2003.11833250
Fonseca PLC, Badotti F, De-Paula RB, Araújo DS, Bortolini DE, Del-Bem LE, Azevedo VA, Brenig B, Aguiar ERGR, Góes-Neto A (2020) Exploring the relationship among divergence time and coding and non-coding elements in the shaping of fungal mitochondrial genomes. Front Microbiol 11:765. https://doi.org/10.3389/fmicb.2020.00765
Frank K, Brückner A, Hilpert A, Heethoff M, Blüthgen N (2017) Nutrient quality of vertebrate dung as a diet for dung beetles. Sci Rep 7:12141. https://doi.org/10.1038/s41598-017-12265-y
Franklinos LHV, Lorch JM, Bohuski E, Rodriguez-Ramos Fernandez J, Wright ON, Fitzpatrick L, Petrovan S, Durrant C, Linton C, Baláž V, Cunningham AA, Lawson B (2017) Emerging fungal pathogen Ophidiomyces ophiodiicola in wild European snakes. Sci Rep 7(1):3844. https://doi.org/10.1038/s41598-017-03352-1
Fries EM (1829) Systema Mycologicum, vol 3. Sumptibus Ernesti Mauritii, Greifswald. https://doi.org/10.5962/bhl.title.5378
Fuckel L (1870) Symbolae mycologicae. Beiträge zur Kenntniss der Rheinischen Pilze. Jahrb Nassau Ver Naturkd 23–24:1–459. https://doi.org/10.5962/bhl.title.47117
Gallo JG, Woods M, Graham RM, Jennison AV (2017) A severe transmissible Majocchi’s granuloma in an immunocompetent returned traveler. Med Mycol Case Rep 18:5–7. https://doi.org/10.1016/j.mmcr.2017.07.003
Garces HG, Yamauchi DH, Theodoro RC, Bagagli E (2020) PRP8 Intein in Onygenales: distribution and phylogenetic aspects. Mycopathologia 185(1):37–49. https://doi.org/10.1007/s11046-019-00355-6
Garg AK (1966) Isolation of dermatophytes and other keratinophilic fungi from soils in India. Sabouraudia 4(4):259–264. https://doi.org/10.1080/00362176685190571
Gäumann E (1964) Die Pilze: Grundzüge ihrer Entwicklungsgeschichte und Morphologie. Birkhäuser, Basel
Ge X, Sun C, Feng Y, Wang L, Peng J, Che Q, Gu Q, Zhu T, Li D, Zhang G (2019) Anthraquinone derivatives from a marine-derived fungus Sporendonema casei HDN16–802. Mar Drugs 17(6):E334. https://doi.org/10.3390/md17060334
Geiser DM, Gueidan C, Miadlikowska J, Lutzoni F, Kauff F, Hofstetter V, Fraker E, Schoch CL, Tibell L, Untereiner WA, Aptroot A (2006) Eurotiomycetes: Eurotiomycetidae and Chaetothyriomycetidae. Mycologia 98(6):1053–1064. https://doi.org/10.3852/mycologia.98.6.1053
Geiser DM, LoBuglio KL (2001) The monophyletic Plectomycetes: Ascosphaerales, Onygenales, Eurotiales. In: McLaughlin DJ, McLaughlin EG, Lemke PA (eds) The Mycota: systematics and evolution. Springer, Berlin, pp 201–220
Gené J, Guillamón JM, Ulfig K, Guarro J (1996) Studies on keratinophilic fungi. X. Arthrographis alba sp. nov. Can J Microbiol 42:1185–1189. https://doi.org/10.1139/m96-152
Genise JF, Bellosi ES, Sarzetti LC, Krause JM, Dinghi PA, Sánchez MV, Umazano AM, Puerta P, Cantil LF, Jicha BR (2020) 100 Ma sweat bee nests: Early and rapid codiversification of crown bees and flowering plants. PLoS ONE 15(1):e0227789. https://doi.org/10.1371/journal.pone.0227789
Ghosh GR, Orr GF, Kuehn HH (1963) A new genus of the Gymnoascaceae with a rudimentary peridium. Mycopath Mycol Appl 21:36–44. https://doi.org/10.1007/BF02053253
Ghosh GR, Sur B (1985) Keratinophilic fungi from Orissa, India III-Two New Records. Kavaka 12(2):63–69
Gibas CF, Sigler L, Summerbell RC, Currah RS (2002a) Phylogeny of the genus Arachnomyces and its anamorphs and the establishment of Arachnomycetales, a new eurotiomycete order in the Ascomycota. Stud Mycol 47:131–139
Gibas CF, Sigler L, Summerbell RC, Hofstader SL, Gupta AK (2002b) Arachnomyces kanei (anamorph Onychocola kanei) sp. nov., from human nails. Med Mycol 40(6):573–580. https://doi.org/10.1080/mmy.40.6.573.580
Gilchrist C, Stokes WR (1898) A case of pseudolupus vulgaris caused by a Blastomyces. J Exp Med 3:53–78. https://doi.org/10.1084/jem.3.1.53
Giraldo A, Gené J, Sutton DA, Madrid H, Cano J, Crous PW, Guarro J (2014) Phylogenetic circumscription of Arthrographis (Eremomycetaceae, Dothideomycetes). Persoonia 32:102–114. https://doi.org/10.3767/003158514X680207
Gochnauer TA, Margetts VJ (1979) Properties of honeybee larvae killed by chalkbrood disease. J Apic Res 18(3):212–216. https://doi.org/10.1080/00218839.1979.11099971
Gräser Y, de Hoog S, Summerbell RC (2006) Dermatophytes: recognizing species of clonal fungi. Med Mycol 44(3):199–209. https://doi.org/10.1080/13693780600606810
Gräser Y, El Fari M, Vilgalys R, Kuijpers AF, De Hoog GS, Presber W, Tietz H (1999a) Phylogeny and taxonomy of the family Arthrodermataceae (dermatophytes) using sequence analysis of the ribosomal ITS region. Med Mycol 37(2):105–114. https://doi.org/10.1080/02681219980000171
Gräser Y, Kuijpers AF, Presber W, de Hoog GS (1999b) Molecular taxonomy of Trichophyton mentagrophytes and T. tonsurans. Med Mycol 37(5):315–330
Gräser Y, Kuijpers AF, Presber W, de Hoog GS (2000) Molecular taxonomy of the Trichophyton rubrum complex. J Clin Microbiol 38(9):3329–3336. https://doi.org/10.1128/JCM.38.9.3329-3336.2000
Greenwold MJ, Bao W, Jarvis ED, Hu H, Li C, Gilbert MT, Zhang G, Sawyer RH (2014) Dynamic evolution of the alpha (α) and beta (β) keratins has accompanied integument diversification and the adaptation of birds into novel lifestyles. BMC Evol Biol 14:249–265. https://doi.org/10.1186/s12862-014-0249-1
Grigoraki L (1924) Contribution à l’Étude des Dermatophytes. C R Hebd Seances Acad Sci Paris 179:1423–1425
Grigoraki L (1925) Récherches cytologiques et taxonomiques sur les dermatophytes et quelques autres champignons parasites. Ann Sci Nat Bot 7:165–444
Grove WB (1916) New or noteworthy fungi V. J Bot 54:217–223
Guarro J, Cano J, de Vroey CH (1991) Nannizziopsis (Ascornycotina) and related genera. Mycotaxon 42:193–200
Guarro J, Gené J, De Vroey CH (1992a) Amaurascopsis, a new genus of Eurotiales. Mycotaxon 45:171–178
Guarro J, Gené J, De Vroey CH (1993) A new species of Spiromastix from Africa. Mycotaxon 46:307–313
Guarro J, Punsola L, Cano J (1987a) Byssoonygena ceratinophila, gen. et sp. nov. a new keratinophilic fungus from Spain. Mycopathologia 100(3):159–161. https://doi.org/10.1007/BF00437042
Guarro J, Punsola L, Figueras MJ (1987b) Brunneospora reticulata, gen. et sp. nov. A keratinophilic ascomycete from Spain. Persoonia 13(3):387–390
Guarro J, Ulfig K, De Vroey C (1992b) A new keratinophilic Gymnoascus from bolivian soil. Mycotaxon 45:317–322
Guého E, de Vroey C (1986) A new species of Anixiopsis. Can J Bot 64(10):2207–2210. https://doi.org/10.1139/b86-294
Gueidan C, Villaseñor CR, de Hoog GS, Gorbushina AA, Untereiner WA, Lutzoni F (2008) A rock-inhabiting ancestor for mutualistic and pathogen-rich fungal lineages. Stud Mycol 61:111–119. https://doi.org/10.3114/sim.2008.61.11
Guin K, Chen Y, Mishra R, Muzaki SRB, Thimmappa BC, O’’Brien CE, Butler G, Sanyal A, Sanyal K, (2020) Spatial intercentromeric interactions facilitated the emergence of evolutionary new centromeres. Elife 9:e58556. https://doi.org/10.7554/eLife.58556
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59(3):307–321. https://doi.org/10.1093/sysbio/syq010
Gupta AK, Foley KA, Versteeg SG (2017) New antifungal agents and new formulations against dermatophytes. Mycopathologia 182(1–2):127–141. https://doi.org/10.1007/s11046-016-0045-0
Hainsworth S, Kučerová I, Sharma R, Cañete-Gibas CF, Hubka V (2021) Three-gene phylogeny of the genus Arthroderma: basis for future taxonomic studies. Med Mycol 59(4):355–365. https://doi.org/10.1093/mmy/myaa057
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hambleton S, Egger KE, Currah RS (1998) The genus Oidiodendron: species delimitation and phylogenetic relationships based on nuclear ribosomal DNA analysis. Mycologia 90(5):854–868. https://doi.org/10.1080/00275514.1998.12026979
Hammer BW, Gilman JC (1944) Red mold on blue cheese. J Dairy Sci 27:413–418. https://doi.org/10.3168/jds.S0022-0302(44)92617-2
Hansen EC (1877) De danske Gjodningssvampe (Fungi fimicoli danici). Vidensk Meddel Dansk Naturhist Foren Kjøbenhavn 38:207–354
Hansen EC (1897) Anixiopsis stercoraria E. Chr. Hansen. Bot Zeitung 55:127–132
Haridas S, Albert R, Binder M et al (2020) 101 Dothideomycetes genomes: a test case for predicting lifestyles and emergence of pathogens. Stud Mycol 96:141–153. https://doi.org/10.1016/j.simyco.2020.01.003
Harrold CE (1950) Studies in the genus Eremascus: I. The rediscovery of Eremascus albus Eidam and some new observations concerning its life-history and cytology. Ann Bot 14(54):127–148
Hartmann M (1912) Blastosporidium. Zentralblatt Für Bakteriologie, Parasitenkunde, Infektionskrankheiten Und Hygiene, Abteilungen I:254
Haubold EM, Aronson JF, Cowan DF, McGinnis MR, Cooper CR Jr (1998) Isolation of fungal rDNA from bottlenose dolphin skin infected with Loboa loboi. Med Mycol 36(5):263–267
Haubold EM, Cooper CR Jr, Wen JW, McGinnis MR, Cowan DF (2000) Comparative morphology of Lacazia loboi (syn. Loboa loboi) in dolphins and humans. Med Mycol 38(1):9‒14. https://doi.org/10.1080/mmy.38.1.9.14
Hay RJ (2017) Tinea capitis: current status. Mycopathologia 182(1–2):87–93. https://doi.org/10.1007/s11046-016-0058-8
Heath LAF (1982) Development of chalkbrood in a honeybee colony: a review. Bee World 63(3):119–130. https://doi.org/10.1080/0005772X.1982.11097876
Heidemann S, Monod M, Gräser Y (2010) Signature polymorphisms in the internal transcribed spacer region relevant for the differentiation of zoophilic and anthropophilic strains of Trichophyton interdigitale and other species of T. mentagrophytes sensu lato. Br J Dermatol 162(2):282–295. https://doi.org/10.1111/j.1365-2133.2009.09494.x
Hermet A, Mounier J, Keravec M, Vasseur V, Barbier G, Jany JL (2014) Application of capillary electrophoresis single-stranded conformation polymorphism (CE-SSCP) analysis for identification of fungal communities in cheese. Food Microbiol 41:82–90. https://doi.org/10.1016/j.fm.2014.01.013
Hernández-Restrepo M, Giraldo A, van Doorn R, Wingfield MJ, Groenewald JZ, Barreto RW, Colmán AA, Mansur PSC, Crous PW (2020) The Genera of Fungi - G6: Arthrographis, Kramasamuha, Melnikomyces, Thysanorea, and Verruconis. Fungal Syst Evol 6:1–24. https://doi.org/10.3114/fuse.2020.06.01
Hill AG, Sandy JR, Begg A (2019) Mycotic dermatitis in juvenile freshwater crocodiles (Crocodylus johnstoni) caused by Nannizziopsis crocodili. J Zoo Wildl Med 50(1):225–230. https://doi.org/10.1638/2018-0133
Hirooka Y, Tanney JB, Nguyen HDT, Seifert KA (2016) Xerotolerant fungi in house dust: taxonomy of Spiromastix, Pseudospiromastix and Sigleria gen. nov. in Spiromastigaceae (Onygenales, Eurotiomycetes). Mycologia 108(1):135–156. https://doi.org/10.3852/15-065
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: Improving the ultrafast bootstrap approximation. Mol Biol Evol 35(2):518–522. https://doi.org/10.1093/molbev/msx281
Holloway BA, Sylvester HA, Bourgeois L, Rinderer TE (2012) Association of single nucleotide polymorphisms to resistance to chalkbrood in Apis mellifera. J Apic Res 51(2):154–163. https://doi.org/10.3896/IBRA.1.51.2.02
Hrycyk MF, Garcia Garces H, Bosco SMG, de Oliveira SL, Marques SA, Bagagli E (2018) Ecology of Paracoccidioides brasiliensis, P. lutzii and related species: infection in armadillos, soil occurrence and mycological aspects. Med Mycol 56(8):950–962. https://doi.org/10.1093/mmy/myx142
Huang Y, Busk PK, Herbst FA, Lange L (2015) Genome and secretome analyses provide insights into keratin decomposition by novel proteases from the nonpathogenic fungus Onygena corvina. Appl Microbiol Biotechnol 99(22):9635–9649. https://doi.org/10.1007/s00253-015-6805-9
Hubka V, Dobiášová S, Dobiáš R, Kolařík M (2014) Microsporum aenigmaticum sp. nov. from M. gypseum complex, isolated as a cause of tinea corporis. Med Mycol 52(4):387–396. https://doi.org/10.1093/mmy/myt033
Hubka V, Dobiasova S, Lyskova P, Mallatova N, Chlebkova J, Skorepova M, Kubatova A, Dobias R, Chudickova M, Kolarik M (2013) Auxarthron ostraviense sp. nov., and A. umbrinum associated with nondermatophytic onychomycosis. Med Mycol 51(6):614‒624. https://doi.org/10.3109/13693786.2013.770608
Hubka V, Nissen CV, Jensen RH, Arendrup MC, Cmokova A, Kubatova A, Skorepova M, Kolarik M (2015) Discovery of a sexual stage in Trichophyton onychocola, a presumed geophilic dermatophyte isolated from toenails of patients with a history of T. rubrum onychomycosis. Med Mycol 53(8):798–809. https://doi.org/10.1093/mmy/myv044
Ito T, Nakagiri A (1995) Amauroascus purpureus, a new species of the Amauroascaceae (Ascomycotina). Mycotaxon 55:347–352
Jiang JR, Cai L, Liu F (2017) Oligotrophic fungi from a carbonate cave, with three new species of Cephalotrichum. Mycology 8:164–177. https://doi.org/10.1080/21501203.2017.1366370
Jiang Y, Dukik K, Muñoz JF, Sigler L, Schwartz IS, Govender NP, Kenyon C, Feng P, van den Ende BG, Stielow JB, Stchigel AM, Lu H, de Hoog S (2018) Phylogeny, ecology and taxonomy of systemic pathogens and their relatives in Ajellomycetaceae (Onygenales): Blastomyces, Emergomyces, Emmonsia, Emmonsiellopsis. Fungal Divers 90:245–291. https://doi.org/10.1007/s13225-018-0403-y
Jiang Y, Tsui CKM, Ahmed SA, Hagen F, Shang Z, Gerrits van den Ende AHG, Verweij PE, Lu H, de Hoog GS (2020) Intraspecific diversity and taxonomy of Emmonsia crescens. Mycopathologia 185(4):613–627. https://doi.org/10.1007/s11046-020-00475-4
Joardar V, Abrams NF, Hostetler J, Paukstelis PJ, Pakala S, Pakala SB, Zafar N, Abolude OO, Payne G, Andrianopoulos A, Denning DW, Nierman WC (2012) Sequencing of mitochondrial genomes of nine Aspergillus and Penicillium species identifies mobile introns and accessory genes as main sources of genome size variability. BMC Genomics 13:698. https://doi.org/10.1186/1471-2164-13-698
Johnston PR, Nguyen HD, Park D, Hirooka Y (2015) Harorepupu aotearoa (Onygenales) gen. sp. nov.; a threatened fungus from shells of Powelliphanta and Paryphanta snails (Rhytididae). IMA Fungus 6(1):135‒143. https://doi.org/10.5598/imafungus.2015.06.01.08
Jong SC, Davis EE (1975) The imperfect state of Aphanoascus. Mycologia 67:1143–1147. https://doi.org/10.1080/00275514.1975.12019857
Kalyaanamoorthy S, Minh BQ, Wong KFT, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589. https://doi.org/10.1038/nmeth.4285
Kamyschko OP (1967) De specie nova generis Pseudoarachniotus Kuehne terra isolata. Novisti Sist Niz Rast 4:224–225
Kandemir H, Dukik K, Hagen F, Ilkit M, Gräser Y, de Hoog GS (2020) Polyphasic discrimination of Trichophyton tonsurans and T. equinum from humans and horses. Mycopathologia 185(1):113–122. https://doi.org/10.1007/s11046-019-00344-9
Kano R, Kimura U, Kakurai M, Hiruma J, Kamata H, Suga Y, Harada K (2020) Trichophyton indotineae sp. nov.: A new highly terbinafine-resistant anthropophilic dermatophyte species. Mycopathologia 185(6):947–958. https://doi.org/10.1007/s11046-020-00455-8
Kano R, Yoshida E, Yaguchi T, Hubka V, Anzawa K, Mochizuki T, Hasegawa A, Kamata H (2014) Mating type gene (MAT1-2) of Trichophyton verrucosum. Mycopathologia 177(1–2):87–90. https://doi.org/10.1007/s11046-013-9722-4
Kasuga T, White TJ, Taylor JW (2002) Estimation of nucleotide substitution rates in Eurotiomycete fungi. Mol Biol Evol 19(12):2318–2324. https://doi.org/10.1093/oxfordjournals.molbev.a004056
Kenyon C, Bonorchis K, Corcoran C, Meintjes G, Locketz M, Lehloenya R, Vismer HF, Naicker P, Prozesky H, van Wyk M, Bamford C, du Plooy M, Imrie G, Dlamini S, Borman AM, Colebunders R, Yansouni CP, Mendelson M, Govender NP (2013) A dimorphic fungus causing disseminated infection in South Africa. N Engl J Med 369(15):1416–1424. https://doi.org/10.1056/NEJMoa1215460
Khosravi AR, Shokri H, Vahedi G (2016) Factors in etiology and predisposition of adult tinea capitis and review of published literature. Mycopathologia 181(5–6):371–378. https://doi.org/10.1007/s11046-016-0004-9
Khurana A, Sardana K, Chowdhary A (2019) Antifungal resistance in dermatophytes: recent trends and therapeutic implications. Fungal Genet Biol 132:103255. https://doi.org/10.1016/j.fgb.2019.103255
Kirk PM (2014) Nomenclatural novelties. Index Fungorum 120:1–1
Klinger EG, James RR, Youssef NN, Welker DL (2013) A multigene phylogeny provides additional insight into the relationships between several Ascosphaera species. J Invertebr Pathol 112(1):41–48. https://doi.org/10.1016/j.jip.2012.10.011
Kochhar S, Gupta VS, Sethi HS, Capoor MR (2018) A rare case of keratomycosis due to Myriodontium keratinophilum. Delhi J Ophthalmol 29:54–56. https://doi.org/10.7869/djo.399
Kreger-van Rij NJW, Veenhuis M, Leemburg-van der Graaf CA (1974) Ultrastructure of hyphae and ascospores in the genus Eremascus Eidam. Antonie Van Leeuwenhoek 40:533–542. https://doi.org/10.1007/BF00403817
Krivanec K, Janecková V, Otcenásek M (1977) Arthroderma melis spec. nov. - a new dermatophyte species isolated from badger burrows in Czechoslovakia. Ceská Mykologie 31(2):91–99
Kuehn HH (1957) Observations on Gymnoascaceae. V. Developmental morphology of two species representing a new genus of the Gymnoascaceae. Mycologia 49(5):694–706. https://doi.org/10.1080/00275514.1957.12024681
Kuehn HH (1958) A preliminary survey of the Gymnoascaceae I. Mycologia 50(3):417–439. https://doi.org/10.2307/3756079
Kuehn HH (1960) Observations on Gyrnnoascaceae. VIII. A new species of Arthroderma. Mycopathol Mycol Appl 13:189–197
Kuehn HH, Goos RD (1960) Observations on Gymnoascaceae. VII. A new species of Pseudoarachniotus from Honduras soil. Mycologia 52(1):40–46. https://doi.org/10.2307/3756249
Kuehn HH, Orr GF (1959) Observations on Gymnoascaceae. VI. A new species of Arachniotus. Mycologia 51(6):864–870. https://doi.org/10.1080/00275514.1959.12024867
Kuehn HH, Orr GF (1962) A new genus of Gymnoascaceae. Mycologia 54(2):160–167. https://doi.org/10.1080/00275514.1962.12024988
Kuehn HH, Orr GF (1963) Observations on Gymnoascaceae, IX. A new species of Pseudoarachniotus from soil-burial beds. Mycopath Mycol Appl 19:257–264. https://doi.org/10.1007/BF02051256
Kuehn HH, Orr GF (1964) Arachniotus ruber (van Tieghem) Schroeter. Trans Br Mycol Soc 47(4):553–558. https://doi.org/10.1016/S0007-1536(64)80034-6
Kuehn HH, Orr GF, Ghosh GR (1961) A new and widely distributed species of Pseudoarachniotus. Mycopathol Mycol Appl 14:215–229. https://doi.org/10.1007/BF02057328
Kuehn HH, Orr GF, Ghosh GR (1964a) A new species of Petalosporus. Mycopathol Mycol Appl 23(1):29–35. https://doi.org/10.1007/BF02049181
Kuehn HH, Tubaki K, Orr GF (1964b) Arachniotus aureus. Mycologia 56(6):863–872. https://doi.org/10.1080/00275514.1964.12018177
Kumar B, Trivedi P, Mishra AK, Pandey A, Palni LMS (2004) Microbial diversity of soil from two hot springs in Uttaranchal Himalaya. Microbiol Res 159(2):141–146. https://doi.org/10.1016/j.micres.2004.01.004
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874. https://doi.org/10.1093/molbev/msw054
Kushwaha RKS, Agrawal SC (1977) Chrysosporium crassitunicatum sp. nov., a new keratinophilic fungus. Trans Br Mycol Soc 68(3):464‒467. https://doi.org/10.1016/S0007-1536(77)80209-X
La Touche CJ (1968) Apinisia graminicola gen. et sp. nov. Trans Br Mycol Soc 51(2):283‒285. https://doi.org/10.1016/S0007-1536(68)80062-2
Labuda R, Bernreiter A, Hochenauer D, Kubátová A, Kandemir H, Schüller C (2021) Molecular systematics of Keratinophyton: the inclusion of species formerly referred to Chrysosporium and description of four new species. IMA Fungus 12(1):17. https://doi.org/10.1186/s43008-021-00070-2
Leclerc MC, Philippe H, Guého E (1994) Phylogeny of dermatophytes and dimorphic fungi based on large subunit ribosomal RNA sequence comparisons. J Med Vet Mycol 32(5):331–341. https://doi.org/10.1080/02681219480000451
Letunic I, Bork P (2019) Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res 47(W1):W256–W259. https://doi.org/10.1093/nar/gkz239
Lewis ER, Bowers JR, Barker BM (2015) Dust devil: the life and times of the fungus that causes Valley Fever. PLoS Pathog 11(5):e1004762. https://doi.org/10.1371/journal.ppat.1004762
Li W, Metin B, White TC, Heitman J (2010) Organization and evolutionary trajectory of the mating type (MAT) locus in dermatophyte and dimorphic fungal pathogens. Eukaryot Cell 9(1):46–58. https://doi.org/10.1128/EC.00259-09
Li Y, Steenwyk JL, Chang Y, Wang Y, James TY, Stajich JE, Spatafora JW, Groenewald M, Dunn CW, Hittinger CT, Shen XX, Rokas A (2021) A genome-scale phylogeny of the kingdom Fungi. Curr Biol 31(8):1653-1665.e5. https://doi.org/10.1016/j.cub.2021.01.074
Liang JD, Han YF, Du W, Liang ZQ, Li ZZ (2009) Chrysosporium linfenense: a new Chrysosporium species with keratinolytic activity. Mycotaxon 110:65–71. https://doi.org/10.5248/110.65
Liu TT, Hu DM, Liu F, Cai L (2013) Polyphasic characterization of Plectosphaerella oligotrophica, a new oligotrophic species from China. Mycoscience 54(5):387–393. https://doi.org/10.1016/j.myc.2013.01.003
L’Ollivier C, Ranque S (2017) MALDI-TOF-Based dermatophyte identification. Mycopathologia 182(1–2):183–192. https://doi.org/10.1007/s11046-016-0080-x
Lorch JM, Knowles S, Lankton JS, Michell K, Edwards JL, Kapfer JM, Staffen RA, Wild ER, Schmidt KZ, Ballmann AE, Blodgett D, Farrell TM, Glorioso BM, Last LA, Price SJ, Schuler KL, Smith CE, Wellehan JFX, Blehert DS (2016) Snake fungal disease: An emerging threat to wild snakes. Philos Trans R Soc Lond B Biol Sci 371:20150457. https://doi.org/10.1098/rstb.2015.0457
Lorch JM, Minnis AM, Meteyer CU, Redell JA, White JP, Kaarakka HM, Muller LK, Lindner DL, Verant ML, Shearn-Bochsler V, Blehert DS (2015) The fungus Trichophyton redellii sp. nov. Causes skin infections that resemble white-nose syndrome of hibernating bats. J Wildl Dis 51(1):36–47. https://doi.org/10.7589/2014-05-134
Maddison WP, Maddison DR (2019) Mesquite: a modular system for evolutionary analysis. Version 3.61. http://www.mesquiteproject.org
Makrantonaki E, Ganceviciene R, Zouboulis C (2011) An update on the role of the sebaceous gland in the pathogenesis of acne. Dermatoendocrino 3(1):41–49. https://doi.org/10.4161/derm.3.1.13900
Malloch D, Cain RF (1971) New genera of Onygenaceae. Can J Bot 49(6):839–816. https://doi.org/10.1139/b71-124
Maran AG, Kwong K, Milne LJ, Lamb D (1985) Frontal sinusitis caused by Myriodontium keratinophilum. Br Med J (clin Res Ed) 290:207. https://doi.org/10.1136/bmj.290.6463.207
Marangoni PRD, Robl D, Berton MAC, Garcia CM, Bozza A, Porsani MV, do Rocio Dalzoto P, Vicente VA, Pimentel IC, (2013) Occurrence of sulphate reducing bacteria (SRB) associated with biocorrosion on metallic surfaces in a hydroelectric power station in Ibirama (SC) – Brazil. Braz Arch Biol Technol 56(5):801–805. https://doi.org/10.1590/S1516-89132013000500011
Marinari S, Masciandaro G, Ceccanti B, Grego S (2000) Influence of organic and mineral fertilizers on soil biological and physical properties. Bioresour Technol 72(1):9–17. https://doi.org/10.1016/S0960-8524(99)00094-2
Marin-Felix Y, Stchigel AM, Cano-Lira JF, Sanchis M, Mayayo E, Guarro J (2015) Emmonsiellopsis, a new genus related to the thermally dimorphic fungi of the family Ajellomycetaceae. Mycoses 58(8):451–460. https://doi.org/10.1111/myc.12336
Marinho D, Muniz DB, Azevedo GG (2018) Nesting biology of three Megachile (Hymenoptera: Megachilidae) species from Eastern Amazonia, Brazil. Rev Bras Entomol 62(2):97–106. https://doi.org/10.1016/j.rbe.2018.03.002
Martinez DA, Oliver BG, Gräser Y et al (2012) Comparative genome analysis of Trichophyton rubrum and related dermatophytes reveals candidate genes involved in infection. Mbio 3(5):e00259-12. https://doi.org/10.1128/mBio.00259-12
Martinez-Rossi NM, Bitencourt TA, Peres NTA, Lang EAS, Gomes EV, Quaresemin NR, Martins MP, Lopes L, Rossi A (2018) Dermatophyte resistance to antifungal drugs: mechanisms and prospectus. Front Microbiol 9:1108. https://doi.org/10.3389/fmicb.2018.01108
Massee G, Salmon E (1902) Researches on coprophilous fungi. II Ann Bot 16(61):57–93
Mathur PN, Thirumalachar MJ (1970) Studies on some Pseudoarachniotus species from India. Mycopathol Mycol Appl 40(2):97–105
Matruchot L, Dassonville C (1899) Recherches experimentales sur une dermatomycoses des poules et sur son parasite. Rev Gen Bot 11:429–444
Mavengere H, Mattox K, Teixeira MM, Sepúlveda VE, Gomez OM, Hernandez O, McEwen J, Matute DR (2020) Paracoccidioides genomes reflect high levels of species divergence and little interspecific gene flow. Mbio 11(6):e01999-20. https://doi.org/10.1101/2020.07.16.204636
Mazza S, Parodi S (1928) Sur une mycose du chaco produite par le Pseudococcidioides mazzai da Fonseca. CR Congr Int Me´d Trop Hyg Le Caire 3: 995‒1003
McNear DH Jr (2013) The Rhizosphere - roots, soil and everything in between. Nature Education Knowledge 4(3):1
Meerupati T, Andersson KM, Friman E, Kumar D, Tunlid A, Ahrén D (2013) Genomic mechanisms accounting for the adaptation to parasitism in nematode-trapping fungi. PLoS Genet 9(11):e1003909. https://doi.org/10.1371/journal.pgen.1003909
Menon GK, Menon J (2000) Avian epidermal lipids: functional considerations and relationship to feathering. Am Zool 40(4):540–552. https://doi.org/10.1093/icb/40.4.540
Metin B, Heitman J (2017) Sexual reproduction in dermatophytes. Mycopathologia 182(1–2):45–55. https://doi.org/10.1007/s11046-016-0072-x
Milochevitch S (1935) Une nouvelle espèce pathogène de Ctenomyces, Ctenomyces bossæ n. sp. Ann Parasitol Hum Comp 13(6):559‒567. https://doi.org/10.1051/parasite/1935136559
Minh BQ, Hahn MW, Lanfear R (2020a) New methods to calculate concordance factors for phylogenomic datasets. Mol Biol Evol 37(9):2727–2733. https://doi.org/10.1093/molbev/msaa106
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R (2020b) IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol 37(5):1530–1534. https://doi.org/10.1093/molbev/msaa015
Minnis AM, Lindner DL (2013) Phylogenetic evaluation of Geomyces and allies reveals no close relatives of Pseudogymnoascus destructans, comb. nov., in bat hibernacula of eastern North America. Fungal Biol 117(9):638‒649. https://doi.org/10.1016/j.funbio.2013.07.001
Mirarab S, Nguyen N, Guo S, Wang LS, Kim J, Warnow T (2015) PASTA: ultralarge multiple sequence alignment for nucleotide and amino-acid sequences. J Comput Biol 22(5):377–386. https://doi.org/10.1089/cmb.2014.0156
Mirhendi H, Makimura K, de Hoog GS, Rezaei-Matehkolaei A, Najafzadeh MJ, Umeda Y, Ahmadi B (2015) Translation elongation factor 1-α gene as a potential taxonomic and identification marker in dermatophytes. Med Mycol 53(3):215–224. https://doi.org/10.1093/mmy/myu088
Monod M (2019) Antifungal resistance in dermatophytes: Emerging problem and challenge for the medical community. J Mycol Med 29(4):283–284. https://doi.org/10.1016/j.mycmed.2019.100913
Mortimer RK, Johnston JR (1986) Genealogy of principal strains of the yeast genetic stock center. Genetics 113(1):35–43. https://doi.org/10.1093/genetics/113.1.35
Moskaluk A, VandeWoude S (2022) Two novel species of Arthroderma isolated from domestic cats with dermatophytosis in the United States. Med Mycol 60(2): myac001. https://doi.org/10.1093/mmy/myac001
Moustafa AF (1973) Arachniotus desertorum sp. nov. Trans Br Mycol Soc 61(2):392–393
Muñoz JF, Farrer RA, Desjardins CA, Gallo JE, Sykes S, Sakthikumar S, Misas E, Whiston EA, Bagagli E, Soares CM, Teixeira MM, Taylor JW, Clay OK, McEwen JG, Cuomo CA (2016) Genome diversity, recombination, and virulence across the major lineages of Paracoccidioides. mSphere 1(5):e00213–16. https://doi.org/10.1128/mSphere.00213-16
Muñoz JF, McEwen JG, Clay OK, Cuomo CA (2018) Genome analysis reveals evolutionary mechanisms of adaptation in systemic dimorphic fungi. Sci Rep 8(1):4473–4485. https://doi.org/10.1038/s41598-018-22816-6
Murat C, Payen T, Noel B et al (2018) Pezizomycetes genomes reveal the molecular basis of ectomycorrhizal truffle lifestyle. Nat Ecol Evol 2(12):1956–1965. https://doi.org/10.1038/s41559-018-0710-4
Naranjo-Ortiz MA, Gabaldón T (2019) Fungal evolution: major ecological adaptations and evolutionary transitions. Biol Rev Camb Philos Soc 94(4):1443–1476. https://doi.org/10.1111/brv.12510
Neafsey DE, Barker BM, Sharpton TJ et al (2010) Population genomic sequencing of Coccidioides fungi reveals recent hybridization and transposon control. Genome Res 20(7):938–946. https://doi.org/10.1101/gr.103911.109
Nguyen LT, Schmidt AH, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32(1):268–274. https://doi.org/10.1093/molbev/msu300
Nierman WC, Pain A, Anderson MJ et al (2005) Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438(7071):1151–1156. https://doi.org/10.1038/nature04332.Erratum(2006)in:Nature439(7075):502
Nováková A, Kolařík M (2010) Chrysosporium speluncarum, a new species resembling Ajellomyces capsulatus, obtained from bat guano in caves of temperate Europe. Mycol Progress 9(2):253–260. https://doi.org/10.1007/s11557-009-0634-0
O’Geen AT (2013) Soil water dynamics. Nat Educ Knowl 4(5):9
Ohkura M, Fitak RR, Wisecaver JH, DeBlasio D, Niazi F, Egholm M, Rounsley SD, Kodira CD, Orbach MJ (2017) Genome sequence of Ophidiomyces ophiodiicola, an emerging fungal pathogen of snakes. Genome Announc 5(30):e00677-e717. https://doi.org/10.1128/genomeA.00677-17
Orr GF (1970) Neogymnomyces, a new genus of the Gymnoascaceae. Can J Bot 48(6):1061–1066. https://doi.org/10.1139/b70-153
Orr GF (1972) Recovery of several arthroaleuriosporous fungi from mice following intraperitoneal inoculation. Tech Rep Desert Test Center, Dugway, DTC Proj. No. TN-72–542
Orr GF (1976) Kuehniella, a new genus of the Gymnoascaceae. Mycotaxon 4(1):171–178
Orr GF (1977a) New Gymnoascaceae. Mycotaxon 6(1):33–42
Orr GF (1977b) Another genus of the Gymnoascaceae with swollen septa on peridial elements. Mycotaxon 5(1):283–290
Orr GF, Ghosh GR, Roy K (1977a) The Genera Gymnascella, Arachniotus, and Pseudoarachniotus. Mycologia 69(1):126–163. https://doi.org/10.1080/00275514.1977.12020039
Orr GF, Kuehn HH (1963) The genus Ctenomyces Eidam. Mycopathol Mycol Appl 21(3–4):321–333. https://doi.org/10.1007/BF02052585
Orr GF, Kuehn HH (1972) Notes on Gymnoascaceae II. Some Gymnoascaceae and keratinophilic fungi from Utah. Mycologia 64(1):55–72. https://doi.org/10.1080/00275514.1972.12019235
Orr GF, Kuehn HH, Plunkett OA (1963a) A new genus of the Gymnoascaceae with swollen peridial septa. Can J Bot 41(10):1439–1456
Orr GF, Kuehn HH, Plunkett OA (1963b) The genus Gymnoascus Baranetzky. Mycopath Mycol Appl 21(1):1–18. https://doi.org/10.1007/BF02053249
Orr GF, Kuehn HH, Varsavsky E (1965) The Genus Amauroascus. Mycopathol Mycol Appl 25:100–108. https://doi.org/10.1007/BF02049616
Orr GF, Roy K, Ghosh GR (1977b) Gymnoascoideus, a new genus of the Gymnoascaceae. Mycotaxon 5(2):459–469
Ota M, Langeron M (1923) Nouvelle classification des dermatophytes. Ann Parasitol Hum Comp 1:305–336
Otčenášek M, Dvořák J (1975) Ecological classification of dermatophytes. Mycoses 18(10):425–434. https://doi.org/10.1111/j.1439-0507.1975.tb03521.x
Padhye AA, Carmichael JW (1972) Arthroderma insingulare sp. nov., another Gymnoascaceous state of the Trichophyton terrestre complex. Sabouraudia 10(1):47‒51. https://doi.org/10.1080/00362177285190101
Pan S, Sigler L, Cole GT (1994) Evidence for a phylogenetic connection between Coccidioides immitis and Uncinocarpus reesii (Onygenaceae). Microbiology 140(6):1481–1494. https://doi.org/10.1099/00221287-140-6-1481
Partridge EC, Morgan-Jones G (2002) Notes on Hyphomycestes LXXXIX concerning the Genus Sphaerosporium. Mycotaxon 84:69–77
Patouillard NT (1905) Rollandina, nouveau genre de Gymnoascées. Bull Trimest Soc Mycol Fr 21:81–83
Patouillard NT (1907) Champignons nouveaux du Tonkin. Bull Trimest Soc Mycol Fr 23(1):69–79
Paugh RL, Gray WD (1969) Studies on the growth of the osmophilic fungus Eremascus albus. Mycologia 61(2):281–288. https://doi.org/10.1080/00275514.1969.12018731
Pchelin IM, Zlatogursky VV, Rudneva MV, Chilina GA, Rezaei-Matehkolaei A, Lavnikevich DM, Vasilyeva NV, Taraskina AE (2016) Reconstruction of phylogenetic relationships in dermatomycete genus Trichophyton Malmsten 1848 based on ribosomal internal transcribed spacer region, partial 28S rRNA and beta-tubulin genes sequences. Mycoses 59:566–575. https://doi.org/10.1111/myc.12505
Peck CH (1884) Report of the Botanist, 1882. Annual Report on the New York State Museum of Natural History 35:125–164
Perfil’ev BW (1929) Spiromastix verrucosus nov. g.n.sp.n.fam. - Vertreter eines neuen Typus der Flagellaten. Mikrobiol Zurn (leningrad) 9:132–137
Persinoti GF, Martinez DA, Li W et al (2018) Whole-Genome analysis illustrates global clonal population structure of the ubiquitous dermatophyte pathogen Trichophyton rubrum. Genetics 208(4):1657–1669. https://doi.org/10.1534/genetics.117.300573
Persoon CH (1799) [1796] Observatines mycologicae 2: Apud Petrum Phillippum Wolf, Lipsiae
Peterson SW (2000) Phylogenetic relationships in Aspergillus based on rDNA sequence analysis. In: Samson RA, Pit JI (eds) Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Harwood Academic Publishers, The Netherlands, pp 323–355
Peterson SW, Sigler L (1998) Molecular genetic variation in Emmonsia crescens and Emmonsia parva, etiologic agents of adiaspiromycosis, and their phylogenetic relationship to Blastomyces dermatitidis (Ajellomyces dermatitidis) and other systemic fungal pathogens. J Clin Microbiol 36(10):2918–2925. https://doi.org/10.1128/JCM.36.10.2918-2925.1998
Pitt JI, Lantz H, Pettersson OV, Leong SL (2013) Xerochrysium gen. nov. and Bettsia, genera encompassing xerophilic species of Chrysosporium. IMA Fungus 4(2):229‒241. https://doi.org/10.5598/imafungus.2013.04.02.08
Pitts-Singer TL, Cane JH (2011) The alfalfa leaf cutting bee, Megachile rotundata: the world’s most intensively managed solitary bee. Annu Rev Entomol 56:221–237. https://doi.org/10.1146/annurev-ento-120709-144836
Pore RS, Tsao GC, Plunkett OA (1965) A new species of Arthroderma established according to biological species concepts. Mycologia 57(6):969–973. https://doi.org/10.1080/00275514.1965.12018287
Procházka E, Franko F, Poláková S, Sulo P (2012) A complete sequence of Saccharomyces paradoxus mitochondrial genome that restores the respiration in S. cerevisiae. FEMS Yeast Res 12(7):819–830. https://doi.org/10.1111/j.1567-1364.2012.00833.x
Punsola L, Guarro J (1984) Keratinomyces ceretanicus sp. nov., a psychrophilic dermatophyte from soil. Mycoptahologia 85:185–190. https://doi.org/10.1007/BF00440951
Qin X, Tang JC, Li DS, Zhang QM (2012) Effect of salinity on the bioremediation of petroleum hydrocarbons in a saline-alkaline soil. Lett Appl Microbiol 55(3):210–217. https://doi.org/10.1111/j.1472-765X.2012.03280.x
Quan Y, Muggia L, Moreno FL, Wang M, Al-Hatmi AMS, da Silva MN, Shi D, Deng S, Ahmed S, Hyde KD, Vicente VA, Kang Y, Stielow BJ, de Hoog S (2020) A re-evaluation of the Chaetothyriales using criteria of comparative biology. Fungal Divers 103:47–85. https://doi.org/10.1007/s13225-020-00452-8
Quélet L (1875) Les champignons du Jura et des Vosges. Hachette Livre, Paris
Rainwater KL, Wiederhold NP, Sutton DA, Garner MM, Maguire C, Sanders C, Gibas C, Cano JF, Guarro J, Stchigel AM (2019) Novel Paranannizziopsis species in a Wagler’s viper (Tropidolaemus wagleri), tentacled snakes (Erpeton tentaculatum), and a rhinoceros snake (Rhynchophis boulengeri) in a zoological collection. Med Mycol 57(7):825–832. https://doi.org/10.1093/mmy/myy134
Rajeev S, Sutton DA, Wickes BL, Miller DL, Giri D, Van Meter M, Thompson EH, Rinaldi MG, Romanelli AM, Cano JF, Guarro J (2009) Isolation and characterization of a new fungal species, Chrysosporium ophiodiicola, from a mycotic granuloma of a black rat snake (Elaphe obsoleta obsoleta). J Clin Microbiol 47(4):1264–1268. https://doi.org/10.1128/JCM.01751-08
Rammeloo J (1982) Onygena mutata Quélet, an earlier name for Amauroascus verrucosus (Eidam) Schroeter (Gymnoascaceae). Bull Jard Bot Natl Belg 52(1/2):241–243. https://doi.org/10.2307/3668059
Randhawa HS, Sandhu RS (1963) On the occurrence of two new species of Trichophyton in Indian soils. Mycopathol Mycol Appl 20(3–4):225–238. https://doi.org/10.1007/BF02089211
Randhawa HS, Sandhu RS (1964) Keratinophyton terreum gen. nov., sp. nov., a keratinophilic fungus from soil in India. Sabouraudia 3(3): 251–257. https://doi.org/10.1080/00362176485190421
Redman RS, Litvintseva A, Sheehan KB, Henson JM, Rodriguez R (1999) Fungi from geothermal soils in Yellowstone National Park. Appl Environ Microbiol 65(12):5193–5197. https://doi.org/10.1128/AEM.65.12.5193-5197.1999
Rees RG (1967a) Keratinophilic fungi from Queensland: I Isolation from Animal Hairs and Scales. Sabouraudia 5(3):165–172. https://doi.org/10.1080/00362176785190351
Rees RG (1967b) Arthroderma flavescens sp. nov. Sabouraudia 5(1):206–208. https://doi.org/10.1080/00362176785190401
Remak R (1845) Diagnostische und pathogenetische Untersuchungen in der Klinik von Schönlein:193
Rixford E, Gilchrist TC (1896) Two cases of protozoan (coccidioidal) infection of the skin and other organs. Johns Hopkins Hospital Rep 1:209–268
Rizzo L, Sutton DA, Wiederhold NP, Thompson EH, Friedman R, Wickes BL, Cano-Lira JF, Stchigel AM, Guarro J (2014) Isolation and characterisation of the fungus Spiromastix asexualis sp. nov. from discospondylitis in a German Shepherd dog, and review of Spiromastix with the proposal of the new order Spiromastixales (Ascomycota). Mycoses 57(7):419–428. https://doi.org/10.1111/myc.12178
Rodríguez-Andrade E, Cano-Lira JF, Wiederhold N, Pérez-Cantero A, Guarro J, Stchigeet AM (2021) A revision of malbranchea-like fungi from clinical specimens in the United States of America reveals unexpected novelty. IMA Fungus 12:25. https://doi.org/10.1186/s43008-021-00075-x
Rodríguez-Andrade E, Stchigel AM, Guarro J, Cano-Lira JF (2020) Fungal diversity of deteriorated sparkling wine and cork stoppers in Catalonia. Spain Microorganisms 8(1):12. https://doi.org/10.3390/microorganisms8010012
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12):1572–1574. https://doi.org/10.1093/bioinformatics/btg180
Ropars J, Cruaud C, Lacoste S, DuPont J (2012) A taxonomic and ecological overview of cheese fungi. Int J Food Microbiol 155(3):199–210. https://doi.org/10.1016/j.ijfoodmicro.2012.02.005
Rostrup E (1894) Mykologiske Meddelelser (IV) Botanisk Tidsskrift. J Bot 19:44–46
Routien JB (1967) A new species of Anixiopsis. Mycologia 59(3):475–481. https://doi.org/10.1080/00275514.1967.12018440
Rutland CS, Cigler P, Kubale V (2019) Reptilian skin and its special histological structures. In: Rutland CS, Kubale V (eds) Veterinary Anatomy and Physiology. IntechOpen. https://doi.org/10.5772/intechopen.84212
Sabouraud R (1907) Sur 1 “eczema marginatum” de hebra “trichophytie inguinale” et son parasite (Epidermophyton inguinale, Sabouraud). Arch Med Exp Anat Pathol 19(565–586):737–766
Saccardo PA (1882) Fungi Gallici lecti a Cl viris Brunaud P, Gillet CC, Letendre Abb, Malbranche A, Therry J. Libert d Series IV Michelia 2(8):583–648
Saccardo PA (1913) Notae Mycologicae Series XVI. Ann Mycol 11(4):312–325
Samarakoon MC, Hyde KD, Hongsanan S, McKenzie EHC, Ariyawansa HA, Promputtha I, Zeng XY, Tian Q, Liu JK (2019) Divergence time calibrations for ancient lineages of Ascomycota classification based on a modern review of estimations. Fungal Divers 96:285–346. https://doi.org/10.1007/s13225-019-00423-8
Samson RA, Polonelli L (1978) Myriodontium keratinophilum, gen. et sp.nov. Persoonia 9(4):505‒509
Samson RA, Yilmaz N, Houbraken J, Spierenburg H, Seifert KA, Peterson SW, Varga J, Frisvad JC (2011) Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud Mycol 70:159–183. https://doi.org/10.3114/sim.2011.70.04
Sarrocco S, Diquattro S, Baroncelli R et al (2015) A polyphasic contribution to the knowledge of Auxarthron (Onygenaceae). Mycol Progress 14(11):112
Scaramuzza N, Diaferia C, Berni E (2015) Monitoring the mycobiota of three plants manufacturing Culatello (a typical Italian meat product). Int J Food Microbiol 203:78–85. https://doi.org/10.1016/j.ijfoodmicro.2015.02.034
Schaefer AM, Reif JS, Guzmán EA, Bossart GD, Ottuso P, Snyder J, Medalie N, Rosato R, Han S, Fair PA, McCarthy PJ (2016) Toward the identification, characterization and experimental culture of Lacazia loboi from Atlantic bottlenose dolphin (Tursiops truncatus). Med Mycol 54(6):659–665. https://doi.org/10.1093/mmy/myw011
Scharapov VM (1974) Species fungorum Ceratinophilorum novae et in URSS primum inventae. Novosti Sist Vyssh Rast 11:266–271
Scharapov VM (1978) Species Chrysosporii Cda. novae. Nov Sist Nizs Rast 15:141–149
Schoch CL, Ciufo S, Domrachev M, Hotton CL, Kannan S, Khovanskaya R, Leipe D, Mcveigh R, O’Neill K, Robbertse B, Sharma S, Soussov V, Sullivan JP, Sun L, Turner S, Karsch-Mizrachi I (2020) NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database (Oxford) 2020:baaa062. https://doi.org/10.1093/database/baaa062
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Fungal Barcoding Consortium (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. PNAS 109(16):6241–6246. https://doi.org/10.1073/pnas.1117018109
Schroeter J (1893) Die Pilze Schlesiens. Fam. Gymnoascacei. In: Cohn F (ed) Krypt-Fl Schles 3(2), J.U. Kern’s Verlag, Breslau, pp 129‒2.
Schumacher HCF (1803) Enumeratio Plantarum, in Partibus Sællandiae Septentrionalis et Orientalis Crescentium 2:477. https://doi.org/10.5962/bhl.title.150042
Schwartz IS, Wiederhold NP, Hanson KE, Patterson TF, Sigler L (2019) Blastomyces helicus, a new dimorphic fungus causing fatal pulmonary and systemic disease in humans and animals in western Canada and the United States. Clin Infect Dis 68(2):188–195. https://doi.org/10.1093/cid/ciy483
Scott JA, Malloch DW, Gloer JB (1993) Polytolypa, an undescribed genus in the Onygenales. Mycologia 85(3):503–508. https://doi.org/10.1080/00275514.1993.12026300
Scott JA, Untereiner WA (2004) Determination of keratin degradation by fungi using keratin azure. Med Mycol 42:239–246. https://doi.org/10.1080/13693780310001644680
Sepúlveda VE, Márquez R, Turissini DA, Goldman WE, Matute DR (2017) Genome sequences reveal cryptic speciation in the human pathogen Histoplasma capsulatum. Mbio 8(6):e01339-17. https://doi.org/10.1128/mBio.01339-17
Shang Y, Xiao G, Zheng P, Cen K, Zhan S, Wang C (2016) Divergent and convergent evolution of fungal pathogenicity. Genome Biol Evol 8(5):1374–1387. https://doi.org/10.1093/gbe/evw082
Shao M, Sun C, Liu X, Wang X, Li W, Wei X, Li Q, Ju J (2020) Upregulation of a marine fungal biosynthetic gene cluster by an endobacterial symbiont. Commun Biol 3(1):527–537. https://doi.org/10.1038/s42003-020-01239-y
Sharma R, Gräser Y, Singh SK (2013) Auxarthronopsis, a new genus of Onygenales isolated from the vicinity of Bandhavgarh National Park, India. IMA Fungus 4:89–102. https://doi.org/10.5598/imafungus.2013.04.01.09
Sharma R, Shouche Y (2018) Nannizzia graeserae sp. nov., a new dermatophyte of geophilic clade isolated from vicinity of a barbershop in India. Kavaka 50:14–20
Sharma R, Shouche YS (2020) Diversity of onygenalean fungi in keratin-rich habitats of Maharashtra (India) and description of three novel taxa. Mycopathologia 185(1):67–85. https://doi.org/10.1007/s11046-019-00346-7
Sharma R, Singh SK (2013) A new species of Gymnoascus with verruculose ascospores. IMA Fungus 4(2):177–186. https://doi.org/10.5598/imafungus.2013.04.02.03
Sharpton TJ, Stajich JE, Rounsley SD, Gardner MJ, Wortman JR, Jordar VS, Maiti R, Kodira CD, Neafsey DE, Zeng Q, Hung CY, McMahan C, Muszewska A, Grynberg M, Mandel MA, Kellner EM, Barker BM, Galgiani JN, Orbach MJ, Kirkland TN, Cole GT, Henn MR, Birren BW, Taylor JW (2009) Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res 19(10):1722–1731. https://doi.org/10.1101/gr.087551.108
Sigler L (2013) Ophidiomyces Sigler, Hamleton & Paré, gen. nov. IF550166 and IF550167. In Nomenclatural novelties: Lynne Sigler. Index Fungorum no.19. http://www.indexfungorum.org/Publications/Index%20Fungorum%20no.19.pdf
Sigler L, Carmichael JW (1976) Taxonomy of Malbranchea and some other Hyphomycetes with arthroconidia. Mycotaxon 4(2):349–488
Sigler L, Congly H (1990) Toenail infection caused by Onychocola canadensis gen. et sp. nov. J Med Vet Mycol 28(5):405–417
Sigler L, Dunn MT, Carmichael JW (1982a) Arthrocristula and Arthropsis, two new hyphomycetes with dematiaceous arthroconidia. Mycotaxon 15:409–419
Sigler L, Flis AL, Carmichael JW (1998) The genus Uncinocarpus (Onygenaceae) and its synonym Brunneospora: new concepts, combinations and connections to anamorphs in Chrysosporium, and further evidence of relationship with Coccidioides immitis. Can J Bot 76(9):1624–1636. https://doi.org/10.1139/b98-110
Sigler L, Gauer PK, Lichtwardt RW, Carmichael JW (1979) Renispora flavissima, a new gymnoascaceous fungus with tuberculate conidia. Mycotaxon 10(1):133–141
Sigler L, Guarro J, Punsola L (1986) New keratinophilic species of Chrysosporium. Can J Bot 64(6):1212–1215. https://doi.org/10.1139/b86-164
Sigler L, Hambleton S, Flis AL, Pare JA (2002a) Auxarthron teleomorphs for Malbranchea filamentosa and Malbranchea albolutea and relationships within Auxarthron. Stud Mycol 1(47):111–122
Sigler L, Hambleton S, Paré JA (2002b) Chlamydosauromyces punctatus gen. & sp. nov. (Onygenaceae) from the skin of a lizard. Stud Mycol 47:123–130
Sigler L, Hambleton S, Paré JA (2013) Molecular characterization of reptile pathogens currently known as members of the Chrysosporium anamorph of Nannizziopsis vriesii complex and relationship with some human-associated isolates. J Clin Microbiol 51(10):3338–3357. https://doi.org/10.1128/JCM.01465-13
Sigler L, Lacey J, Carmichael JW (1982b) Two new species of Malbranchea. Mycotaxon 15:465–471
Silvestro D, Bacon CD, Ding W, Zhang Q, Donoghue PCJ, Antonelli A, Xing Y (2021) Fossil data support a pre-Cretaceous origin of flowering plants. Nat Ecol Evol 5:449–457. https://doi.org/10.1038/s41559-020-01387-8
Simose-Lopes PC, Paula GS, Both MC, Xavier FM, Scaramello AC (1993) First case of lobomycosis in a bottlenose dolphin from southern Brazil. Mar Mammal Sci 9:329–331. https://doi.org/10.1111/j.1748-7692.1993.tb00462.x
Skou JP (1972) Ascosphaerales. Friesia 10(1–2):1–24
Skou JP (1975) Two new species of Ascosphaera and notes on the conidial state of Bettsia alvei. Friesia 11(1):62–74
Skou JP (1979) A new homothallic species of Ascosphaera. Friesia 11(5):265–271
Skou JP (1982) Ascosphaera asterophora species nova. Mycotaxon 14(1):149–159
Skou JP (1986) Notes on the habitats, morphology and taxonomy of spore cyst fungi. Apimondia 30:260–264
Skou JP (1988) Japanese species of Ascosphaera. Mycotaxon 31(1):173–190
Skou JP (1992) A series of xerophilic Chrysosporium species. Mycotaxon 43:237–259
Skou JP, King J (1984) Ascosphaera osmophila sp. nov. an Australian spore cyst fungus. Aust J Bot 32(3):225–231
Solé M, Cano J, Guarro J (2002a) Molecular phylogeny of Amauroascus, Auxarthron, and morphologically similar onygenalean fungi. Mycol Res 106(4):388–396. https://doi.org/10.1017/S0953756202005750
Solé M, Cano J, Pitarch LB, Stchigel AM, Guarro J (2002b) Molecular phylogeny of Gymnoascus and related genera. Stud Mycol 47:141–152
Solé M, Cano J, Stchigel AM, Guarro J (2002c) Two new species of Auxarthron morphologically and genetically close to A. kuehnii. Stud Mycol 47:103–111
Song J, Liang JF, Mehrabi-Koushki M et al (2019) Fungal Systematics and Evolution: FUSE 5. Sydowia 71:141–245. https://doi.org/10.12905/0380.sydowia71-2019-0141
Spegazzini C (1911) Mycetes Argentinenses (Series V). Anales Del Museo Nacional De Historia Natural Buenos Aires, Ser 3(13):329–467
Spiltoir C, Olive L (1955) A reclassification of the genus Pericystis Betts. Mycologia 47(2):238–244. https://doi.org/10.2307/3755414
Staats M, van Kan JA (2012) Genome update of Botrytis cinerea strains B05.10 and T4. Eukaryot Cell 11(11):1413–1414. https://doi.org/10.1128/EC.00164-12
Stchigel AM, Cano J, Mac Cormack W, Guarro J (2001) Antarctomyces psychrotrophicus gen. et sp. nov., a new ascomycete from Antarctica. Mycol Res 105(3):377‒382. https://doi.org/10.1017/S0953756201003379
Stchigel AM, Sutton DA, Cano-Lira JF, Cabañes FJ, Abarca L, Tintelnot K, Wickes BL, García D, Guarro J (2013) Phylogeny of chrysosporia infecting reptiles: proposal of the new family Nannizziopsiaceae and five new species. Persoonia 31:86–100. https://doi.org/10.3767/003158513X669698
Stchigel AM, Sutton DA, Cano-Lira JF, Wiederhold N, Guarro J (2017) New species Spiromastigoides albida from a lung biopsy. Mycopathologia 182(11):967–978. https://doi.org/10.1007/s11046-017-0179-8
Steenwyk JL, Buida TJ III, Li Y, Shen X-X, Rokas A (2020) ClipKIT: a multiple sequence alignment trimming software for accurate phylogenomic inference. PLoS Biol 18(12):e3001007. https://doi.org/10.1371/journal.pbio.3001007
Stejskal M (1974) Arrhenosphaera cranei, gen. et sp. nov., A beehive fungus found in Venezuela. J Apic Res 13:(1):39‒45. https://doi.org/10.1080/00218839.1974.11099757
Stielow JB, Lévesque CA, Seifert KA et al (2015) One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia 35:242–263. https://doi.org/10.3767/003158515X689135
Stockdale PM (1961) Nannizzia incurvata gen. nov., sp. nov., a perfect state of Microsporum gypseum (Bodin) Guiart & Grigorakis. Sabouraudia 1:41–48. https://doi.org/10.1080/00362176285190101
Stockdale PM (1963) The Microsporum gypseum complex (Nannizzia incurvata Stockd., N. gypsea (Nann.) comb. nov., N. fulva sp. nov.). Sabouraudia 3(1):114‒126. https://doi.org/10.1080/00362176485190161
Stockdale PM (1967) Nannizzia persicolor sp. Nov., the perfect state of Trichophyton persicolor Sabouraud. Sabouraudia 5(4):355–359. https://doi.org/10.1080/00362176785190641
Stoppel R (1907) Eremascus Fertilis Nov Spec. Flora (regensburg) 97:332–346. https://doi.org/10.1016/S0367-1615(17)32769-6
Su H, Packeu A, Ahmed SA, Al-Hatmi AMS, Blechert O, İlkit M, Hagen F, Gräser Y, Liu W, Deng S, Hendrickx M, Xu J, Zhu M, de Hoog S (2019) Species distinction in the Trichophyton rubrum complex. J Clin Microbiol 57(9):e00352-e419. https://doi.org/10.1128/JCM.00352-19
Sugiyama M, Mikawa T (2001) Phylogenetic analysis of the nonpathogenic genus Spiromastix (Onygenaceae) and related onygenalean taxa based on large subunit ribosomal DNA sequences. Mycoscience 42:413–421. https://doi.org/10.1007/BF02464337
Sugiyama M, Ohara A, Mikawa T (1999) Molecular phylogeny of onygenalean fungi based on small subunit ribosomal DNA (SSU rDNA) sequences. Mycoscience 40(3):251–258. https://doi.org/10.1007/BF02463962
Sugiyama M, Summerbell R, Mikawa T (2002) Molecular phylogeny of onygenalean fungi based on small subunit (SSU) and large subunit (LSU) ribosomal DNA sequences. Stud Mycol 47:5–23
Suh SO, Grosso KM, Carrion ME (2018) Multilocus phylogeny of the Trichophyton mentagrophytes species complex and the application of matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) mass spectrometry for the rapid identification of dermatophytes. Mycologia 110(1):118–130. https://doi.org/10.1080/00275514.2018
Summerbell RC (2000) Form and function in the evolution of dermatophytes. In: Kushwaha RKS and Guarro J (eds). Biology of Dermatophytes and other Keratinophilic Fungi. Rev Iberoam Micol 17:30–43
Summerbell RC, Moore MK, Starink-Willemse M, Van Iperen A (2007) ITS barcodes for Trichophyton tonsurans and T. equinum. Med Mycol 45(3):193–200. https://doi.org/10.1080/13693780601087614
Sun BD, Zhou YG, Chen AJ, Houbraken J (2019) Phylogeny and a new species of the genus Arachnomyces (Arachnomycetaceae). Phytotaxa 394(1):89–97. https://doi.org/10.11646/phytotaxa.394.1.6
Sutton DA, Marín Y, Thompson EH, Wickes BL, Fu J, García D, Swinford A, de Maar T, Guarro J (2013) Isolation and characterization of a new fungal genus and species, Aphanoascella galapagosensis, from carapace keratitis of a Galapagos tortoise (Chelonoidis nigra microphyes). Med Mycol 51(2):113–120. https://doi.org/10.3109/13693786.2012.701767
Taborda PR, Taborda VA, McGinnis MR (1999a) Lacazia loboi gen. nov., comb. Nov., the etiologic agent of lobomycosis. J Clin Microbiol 37(6):2031–2033. https://doi.org/10.1128/JCM.37.6.2031-2033.1999a
Taborda VBA, Taborda PRO, McGinnis MR (1999b) Constitutive melanin in the cell wall of the etiologic agent of lobomycosis. Rev Inst Med Trop Sao Paulo 41:9–12. https://doi.org/10.1590/S0036-46651999000100003
Takahashi E (1968) Silica as a nutrient to the rice plant. Japan Agr Res Quart 3:1–4
Takashio M (1982) Nannizzia corniculata sp. nov., the perfect state of Microsporum boullardii. Mycotaxon 14(1):383–389
Tamura K, Battistuzzi FU, Billing-Ross P, Murillo O, Filipski A, Kumar S (2012) Estimating divergence times in large molecular phylogenies. PNAS 109(47):19333–19338. https://doi.org/10.1073/pnas.1213199109
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30(12):2725–2729. https://doi.org/10.1093/molbev/mst197
Tamura M, Kasuga T, Watanabe K, Katsu M, Mikami Y, Nishimura K (2002) Phylogenetic characterization of Histoplasma capsulatum strains based on ITS region sequences, including two new strains from Thai and Chinese patients in Japan. Nippon Ishinkin Gakkai Zasshi 43(1):11–19. https://doi.org/10.3314/jjmm.43.11
Tang C, Kong X, Ahmed SA, Thakur R, Chowdhary A, Nenoff P, Uhrlass S, Verma SB, Meis JF, Kandemir H, Kang Y, de Hoog GS (2021) Taxonomy of the Trichophyton mentagrophytes/T. interdigitale species complex harboring the highly virulent, multiresistant genotype T. indotineae. Mycopathologia 186(3):315–326. https://doi.org/10.1007/s11046-021-00544-2
Tanney JB, Nguyen HD, Pinzari F, Seifert KA (2015) A century later: rediscovery, culturing and phylogenetic analysis of Diploöspora rosea, a rare onygenalean hyphomycete. Antonie Van Leeuwenhoek 108(5):1023–1035. https://doi.org/10.1007/s10482-015-0555-7
Teixeira Mde M, Barker BM, Stajich JE (2019) Improved reference genome sequence of Coccidioides immitis strain WA_211, isolated in Washington State. Microbiol Resour Announc 8(33):e00149-e219. https://doi.org/10.1128/MRA.00149-19
Teixeira Mde M, Theodoro RC, Oliveira FF, Machado GC, Hahn RC, Bagagli E, San-Blas G, Soares Felipe MS (2014) Paracoccidioides lutzii sp. nov:. biological and clinical implications. Med Mycol 52(1):19–28. https://doi.org/10.3109/13693786.2013.794311
Thaxter R (1914) New or peculiar Zygomycetes 3: Blakeslea, Dissophora and Haplosporangium, nova genera. Bot Gazette Crawfordsville 58:353–366
Thines M, Crous PW, Aime MC, Aoki T, Cai L, Hyde KD, Miller AN, Zhang N, Stadler M (2018) Ten reasons why a sequence-based nomenclature is not useful for fungi anytime soon. IMA Fungus 9(1):177–183. https://doi.org/10.5598/imafungus.2018.09.01.11
Thirumalachar MJ, Mathur PN (1966) Narasimhella, a new genus of the Gymnoascaceae. Sydowia 19:184–186
Tintelnot K, de Hoog GS, Antweiler E, Losert H, Seibold M, Brandt MA, Gerrits van den Ende AHG, Fisher MC (2007) Taxonomic and diagnostic markers for identification of Coccidioides immitis and Coccidioides posadasii. Med Mycol 45(5):385–393. https://doi.org/10.1080/13693780701288070
Turissini DA, Gomez OM, Teixeira MM, McEwen JG, Matute DR (2017) Species boundaries in the human pathogen Paracoccidioides. Fungal Genet Biol 106:9–25. https://doi.org/10.1016/j.fgb.2017.05.007
Uchiyama S, Kamiya S, Udagawa SI (1995a) A new species of Nannizziopsis from New Jersey soil. Mycoscience 36(2):205–209. https://doi.org/10.1007/BF02268559
Uchiyama S, Kamiya S, Udagawa SI (1995b) Spiromastix saturnispora, a new species from Indonesian soil. Mycoscience 36(3):353–357. https://doi.org/10.1007/BF02268612
Udagawa S, Takada M (1973) The rediscovery of Aphanoascus cinnabarinus. J Jap Bot 48:21–26
Udagawa S, Uchiyama S (1999a) Taxonomic studies on new or critical fungi of nonpathogenic Onygenales 1. Mycoscience 40(3):277–290. https://doi.org/10.1007/BF02463965
Udagawa S, Uchiyama S (1999b) Taxonomic studies on new or critical fungi of nonpathogenic Onygenales 2. Mycoscience 40(3):291–305. https://doi.org/10.1007/BF02463966
Udagawa S, Uchiyama S (2000) Taxonomic studies on new or critical fungi on nonpathogenic Onygenales 3. Mycoscience 41(4):303–311. https://doi.org/10.1007/BF02463943
Udagawa S, Uchiyama S (2001) Taxonomic studies on new or critical fungi of nonpathogenic Onygenales 4. Mycoscience 42(3):281–287. https://doi.org/10.1007/BF02463920
Udagawa S, Uchiyama S (2002) The genus Mallochia (Amauroascaceae). Stud Mycol 47:181–188
Uhrlaß S, Schroedl W, Mehlhorn C, Krüger C, Hubka V, Maier T, Gräser Y, Paasch U, Nenoff P (2018) Molecular epidemiology of Trichophyton quinckeanum – a zoophilic dermatophyte on the rise. J Dtsch Dermatol Ges 16(1):21–32. https://doi.org/10.1111/ddg.13408
Ulfig K, Gené J, Guarro J (1995) Studies on keratinophilic fungi VI. A new Arthropsis (Fungi imperfecti) from marine sediments. Mycotaxon 54:281–286
Ulfig K, Guarro J, Cano J, Gené J, Vidal P, Figueras MJ, Lukasik W (1998) A preliminary study of the occurrence of actidione–resistant fungi in sediments of Catalonian River mouths (Spain). I Keratinolytic Fungi and Related Onygenales. Mycopathologia 141:143–151. https://doi.org/10.1023/A:1006978032246
Untereiner W, Scott J, Naveau F, Bachewich J (2002) Phylogeny of Ajellomyces, Polytolypa and Spiromastix (Onygenaceae) inferred from rDNA sequence and nonmolecular data. Stud Mycol 47:25–35
Untereiner WA, Scott JA, Naveau FA, Sigler L, Bachewich J, Angus A (2004) The Ajellomycetaceae, a new family of vertebrate-associated Onygenales. Mycologia 96(4):812–821. https://doi.org/10.1080/15572536.2005.11832928
Valero C, Gago S, Monteiro MC, Alastruey-Izquierdo A, Buitrago MJ (2018) African histoplasmosis: new clinical and microbiological insights. Med Mycol 56(1):51–59. https://doi.org/10.1093/mmy/myx020
van Beyma FH (1942) Beschreibung einiger neuer Pilzarten aus dem Centraalbureau voor Schimmelcultures, Baarn (Nederland). VII. Mitteilung. Antonie Van Leeuwenhoek 8:105–122
van den Brink J, Samson RA, Hagen F, Boekhout T, de Vries RP (2012) Phylogeny of the industrial relevant, thermophilic genera Myceliophthora and Corynascus. Fungal Divers 52:197–207. https://doi.org/10.1007/s13225-011-0107-z
van Dyke MCC, Teixeira MM, Barker BM (2019) Fantastic yeasts and where to find them: the hidden diversity of dimorphic fungal pathogens. Curr Opin Microbiol 52:55–63. https://doi.org/10.1016/j.mib.2019.05.002
van Oorschot CAN (1980) A revision of Chrysosporium and allied genera. Stud Mycol 20. Elsevier, Amsterdam
van Oorschot CAN, de Hoog GS (1984) Some Hyphomycetes with thallic conidia. Mycotaxon 20(1):129–132
van Oorschot CAN, Piontelli E (1985) Chrysosporium vallenarense sp. nov. Persoonia 12(4):487–488
van Tieghem P (1877) Sur le développement de quelques Ascomycètes II. Bull Soc Bot France 24:157–161
Vanbreuseghem R (1952) Interêt theorique et practique d’un nouveau dermatophyte isolée du sol: Keratinomyces ajeIloi gen. nov., sp. nov. Bull Acad R Belg 38:1068–1077
Varitchak B (1933) Deuxième contribution a étude du développement des Ascomycetes. L’évolution nucleaire dans le sac sporifère de Pericystis apis Maassen et sa signification pour la phylogenie des Ascomycetes. Le Botaniste 25:343–391
Varsavsky E, Ajello L (1964) The perfect and imperfect forms of a new keratinophilic fungus, Arthroderma ciferrii sp. nov. Rivista Patol Veg 4:339–350
Varsavsky E, Orr GF (1971) A new genus of the Gymnoascaceae. Mycopathol Mycol Appl 43(2):229–234. https://doi.org/10.1007/BF02051726
Vidal P, Sanchez-Puelles JM, Milan D, Guarro J (2000a) Chrysosporium fluviale, a new keratinophilic species from river sediments. Mycol Res 104(2):244–250. https://doi.org/10.1017/S0953756299001082
Vidal P, Ulfig K, Valmaseda M, Guarro J (1999) Studies on keratinophilic fungi. XI. Chrysosporium undulatum sp. nov. Antonie Van Leeuwenhoek 75:171–182. https://doi.org/10.1023/A:1001734015104
Vidal P, Valmaseda M, Vinuesa MÁ, Guarro J (2002) Two new species of Chrysosporium. Stud Mycol 47:199–210
Vidal P, Vinuesa MA, Sánchez-Puelles JM, Guarro J (2000b) Phylogeny of the anamorphic genus Chrysosporium and related taxa based on rDNA internal transcribed spacer sequences. Rev Iberoam Micol 17:22–29
Vilela R, Huebner M, Vilela C, Vilela G, Pettersen B, Oliveira C, Mendoza L (2021) The taxonomy of two uncultivated fungal mammalian pathogens is revealed through phylogeny and population genetic analyses. Sci Rep 11(1):18119. https://doi.org/10.1038/s41598-021-97429-7
Vilela R, Mendoza L (2018) Paracoccidioidomycosis ceti (Lacaziosis/Lobomycosis) in dolphins. In: Seyedmousavi S, de Hoog GS, Guillot J, Verweij PE (eds) Emerging and epizootic fungal infections in animals. Springer, New York, pp 177–199
Vilela R, Rosa PS, Belone AF, Taylor JW, Diório SM, Mendoza L (2009) Molecular phylogeny of animal pathogen Lacazia loboi inferred from rDNA and DNA coding sequences. Mycol Res 113(Pt 8):851–857. https://doi.org/10.1016/j.mycres.2009.04.007
von Arx JA (1971) On Arachniotus and related genera of the Gymnoascaceae. Persoonia 6(3):371–380
von Arx JA (1977) Notes on Gymnoascaceae. Persoonia 9(3):393–400
von Arx JA (1986) The Ascomycete genus Gymnoascus. Persoonia 13:173–183
von Arx JA, Samson RA (1973) Two new genera of the Eurotiales. Persoonia 7(3):377–380
von Arx JA, Samson RA (1986) Mallochia a new genus of the Eurotiales. Persoonia 13(2):185–188
von Schweinitz LD (1832) Synopsis fungorum in America boreali media degentium. Trans Am Philos Soc 4(2):141–316
von Willdenow CL (1787) Florae Berolinensis Prodromus. Kessinger, Montana
Vu D, Groenewald M, de Vries M, Gehrmann T, Stielow B, Eberhardt U, Al-Hatmi A, Groenewald JZ, Cardinali G, Houbraken J, Boekhout T, Crous PW, Robert V, Verkley GJM (2019) Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud Mycol 92:135–154. https://doi.org/10.1016/j.simyco.2018.05.001
Wainwright M, Al-Wajeeh K, Grayston S (1997) Effect of silicic acid and other silicon compounds on fungal growth in oligotrophic and nutrient-rich media. Mycol Res 101(8):933–938. https://doi.org/10.1017/S0953756297003560
Wallroth CFW (1833) Flora Cryptogamica. Germaniae 2:289
Wanderley Costa IP, Maia LC, Cavalcanti MA (2012) Diversity of leaf endophytic fungi in mangrove plants of northeast Brazil. Braz J Microbiol 43(3):1165–1173. https://doi.org/10.1590/S1517-838220120003000044
Wang H, Xu Z, Gao L, Hao B (2009) A fungal phylogeny based on 82 complete genomes using the composition vector method. BMC Evol Biol 9:195–208. https://doi.org/10.1186/1471-2148-9-195
Wang P, Kenyon C, de Hoog S, Guo L, Fan H, Liu H, Li Z, Sheng R, Yang Y, Jiang Y, Zhang L, Xu Y (2017) A novel dimorphic pathogen, Emergomyces orientalis (Onygenales), agent of disseminated infection. Mycoses 60(5):310–319. https://doi.org/10.1111/myc.12583
Wang Z, Johnston PR, Takamatsu S, Spatafora JW, Hibbett DS (2006) Toward a phylogenetic classification of the Leotiomycetes based on rDNA data. Mycologia 98(6):1065–1075. https://doi.org/10.1080/15572536.2006.11832634
Wijayawardene NN, Hyde KD, Al-Ani LKT et al (2020) Outline of fungi and fungus-like taxa. Mycosphere 11(1):1060–1456. https://doi.org/10.5943/mycosphere/11/1/8
Wijayawardene NN, Hyde KD, Rajeshkumar KC et al (2017) Notes for genera: Ascomycota. Fungal Divers 86:1–594. https://doi.org/10.1007/s13225-017-0386-0
Wolfe BE, Button JE, Santarelli M, Dutton RJ (2014) Cheese rind communities provide tractable systems for in situ and in vitro studies of microbial diversity. Cell 158(2):422–433. https://doi.org/10.1016/j.cell.2014.05.041
Woodburn DB, Miller AN, Allender MC, Maddox CW, Terio KA (2019) Emydomyces testavorans, a new genus and species of onygenalean fungus isolated from shell lesions of freshwater aquatic turtles. J Clin Microbiol 57(2):e00628-e718. https://doi.org/10.1128/JCM.00628-18
Wu Y, Yang J, Yang F, Liu T, Leng W, Chu Y, Jin Q (2009) Recent dermatophyte divergence revealed by comparative and phylogenetic analysis of mitochondrial genomes. BMC Genomics 10:238–252. https://doi.org/10.1186/1471-2164-10-238
Wynns AA (2015) Convergent evolution of highly reduced fruiting bodies in Pezizomycotina suggests key adaptations to the bee habitat. BMC Evol Biol 15:145–156. https://doi.org/10.1186/s12862-015-0401-6
Wynns AA, Jensen AB, Eilenberg J (2013) Ascosphaera callicarpa, a new species of bee-loving fungus, with a key to the genus for Europe. PLoS ONE 8(9):e73419. https://doi.org/10.1371/journal.pone.0073419
Wynns AA, Jensen AB, Eilenberg J, James R (2012) Ascosphaera subglobosa, a new spore cyst fungus from North America associated with the solitary bee Megachile rotundata. Mycologia 104(1):108–114. https://doi.org/10.3852/10-047
Yang J, Wang L, Ji X et al (2011) Genomic and proteomic analyses of the fungus Arthrobotrys oligospora provide insights into nematode-trap formation. PLoS Pathog 7(9):e1002179. https://doi.org/10.1371/journal.ppat.1002179
Yang Y, Ye Q, Li K, Li Z, Bo X, Li Z, Xu Y, Wang S, Wang P, Chen H, Wang J (2017) Genomics and comparative genomic analyses provide insight into the taxonomy and pathogenic potential of novel Emmonsia pathogens. Front Cell Infect Microbiol 7:105. https://doi.org/10.3389/fcimb.2017.00105
Yilmaz N, Visagie CM, Houbraken J, Frisvad JC, Samson RA (2014) Polyphasic taxonomy of the genus Talaromyces. Stud Mycol 78:175–341. https://doi.org/10.1016/j.simyco.2014.08.001
Yue JX, Li J, Aigrain L, Hallin J, Persson K, Oliver K, Bergström A, Coupland P, Warringer J, Lagomarsino MC, Fischer G, Durbin R, Liti G (2017) Contrasting evolutionary genome dynamics between domesticated and wild yeasts. Nat Genet 49(6):913–924. https://doi.org/10.1038/ng.3847
Zaki SM, Mikami Y, El-Din AAK, Youssef YA (2005) Keratinophilic fungi recovered from muddy soil in Cairo vicinities. Egypt Mycopathologia 160(3):245–251. https://doi.org/10.1007/s11046-005-0143-x
Zhan P, Dukik K, Li D, Sun J, Stielow JB, Gerrits van den Ende B, Brankovics B, Menken SBJ, Mei H, Bao W, Lv G, Liu W, de Hoog GS (2018) Phylogeny of dermatophytes with genomic character evaluation of clinically distinct Trichophyton rubrum and T. violaceum. Stud Mycol 89:153–175. https://doi.org/10.1016/j.simyco.2018.02.004
Zhang T, Victor TR, Rajkumar SS, Li X, Okoniewski JC, Hicks AC, Davis AD, Broussard K, LaDeau SL, Chaturvedi S, Chaturvedi V (2014) Mycobiome of the bat white nose syndrome affected caves and mines reveals diversity of fungi and local adaptation by the fungal pathogen Pseudogymnoascus (Geomyces) destructans. PLoS ONE 29;9(9):e108714. https://doi.org/10.1371/journal.pone.0108714. Erratum in: PLoS One 2014;9(12):e116149
Zhang YW, Chen WH, Zou X, Han YF, Liang ZQ (2016) A new Chrysosporium species from sparrow’s nest in Shanxi Province. Mycosystema 35(11):1337–1343. https://doi.org/10.13346/j.mycosystema.160166
Zhang YW, Zeng GP, Zou X, Han YF, Liang ZQ, Qiu SY (2017a) Two new keratinophilic fungal species. Phytotaxa 303(2):173–180. https://doi.org/10.11646/phytotaxa.303.2.7
Zhang ZF, Liu F, Zhou X, Liu XZ, Liu SJ, Cai L (2017b) Culturable mycobiota from Karst caves in China, with descriptions of 20 new species. Persoonia 39:1–31. https://doi.org/10.3767/persoonia.2017.39.01
Zhang ZF, Zhou SY, Eurwilaichitr L, Ingsriswang S, Raza M, Chen Q, Zhao P, Liu F, Cai L (2021) Culturable mycobiota from Karst caves in China II, with descriptions of 33 new species. Fungal Divers 106:29–136. https://doi.org/10.1007/s13225-020-00453-7
Zhang ZY, Han YF, Chen WH, Liang ZQ (2019) Phylogeny and taxonomy of three new Ctenomyces (Arthrodermataceae, Onygenales) species from China. MycoKeys 47:1–16. https://doi.org/10.3897/mycokeys.47.30740
Zhang ZY, Zou X, Han YF, Liang ZQ (2017c) Microsporum guizhouense, a new species of the genus Microsporum. Mycosystema 36(5):535–541. https://doi.org/10.13346/j.mycosystema.160259
Zhao YZ, Zhang ZF, Cai L, Peng WJ, Liu F (2018) Four new filamentous fungal species from newly collected and hive-stored bee pollen. Mycosphere 9(6):1089–1116. https://doi.org/10.5943/mycosphere/9/6/3
Zhou N, Zhang Y, Liu F, Cai L (2016) Halophilic and thermotolerant Gymnoascus species from several special environments. China Mycologia 108(1):179–191. https://doi.org/10.3852/15-086
Zukal H (1890) Ueber einige neue Pilzformen und über das Verhältniss der Gymnoascen zu den übrigen Ascomyceten. Ber Dtsch Bot Ges 8:295–296. https://doi.org/10.1111/j.1438-8677.1890.tb06010.x
Acknowledgements
We would like to thank Vit Hubka and Cony Decock for providing some of the sequences used in the study for Arthroderma and Sphaerosporium species. Also, we would like to thank the anonymous reviewers for their valuable comments and suggestions, which helped us to improve the quality of this manuscript. H.K. was supported by the Scientific and Technological Research Council of Turkey (TUBITAK) 2214-A International Research Fellowship Programme for PhD Students (No: 1059B141801143). The preliminary findings of the research were presented at the ISHAM Workshop Onygenales, September 10–11, 2020, Prague, Czech Republic. The research reported in this publication was part of the long-term goals of the ISHAM Working Group Onygenales.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Handling Editor: Jian-Kui Liu.
Supplementary Information
Below is the link to the electronic supplementary material.
13225_2022_506_MOESM2_ESM.pdf
Supplementary Fig. 1 Phylogenetic tree of the species based on Group I LSU sequences (n = 409), obtained with the TIM3e+G4+I+F model in IQ-TREE-ML. Aspergillus fumigatus was used as the outgroup, and Arachnomycetales was found outside the order. Supplementary file2 (PDF 106 KB)
13225_2022_506_MOESM3_ESM.pdf
Supplementary Fig. 2 Phylogenetic tree of the species based on Group II LSU sequences of type species (n = 269), obtained with the TIM3e+G4+I+F model in IQ-TREE-ML. Aspergillus fumigatus was used as the outgroup, and Arachnomycetales was found outside the order. Supplementary file3 (PDF 737 KB)
13225_2022_506_MOESM4_ESM.pdf
Supplementary Fig. 3 Phylogenetic tree of the species based on Group I ITS sequences (n = 488), obtained with the GTR+G4+I+F model in IQ-TREE-ML. Aspergillus fumigatus was used as an outgroup, and Arachnomycetales was found outside the order. Supplementary file4 (PDF 418 KB)
13225_2022_506_MOESM5_ESM.pdf
Supplementary Fig. 4 Phylogenetic tree of the species based on Group II ITS sequences (n = 324), obtained with the GTR+G4+I+F model in IQ-TREE-ML. Aspergillus fumigatus was used as the outgroup, and Arachnomycetales was found outside the order.Supplementary file5 (PDF 617 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kandemir, H., Dukik, K., de Melo Teixeira, M. et al. Phylogenetic and ecological reevaluation of the order Onygenales. Fungal Diversity 115, 1–72 (2022). https://doi.org/10.1007/s13225-022-00506-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-022-00506-z