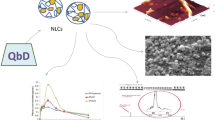
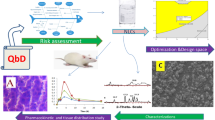
Abstract
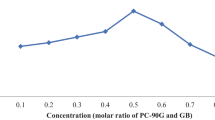
Raloxifene (RLX) is popularly indicated in treatment of osteoporosis and prevention of breast cancer. Owing to its poor aqueous solubility, high pre-systemic metabolism, intestinal glucuronidation, and P-glycoprotein (P-gp) efflux, however, it demonstrates low (< 2%) and inconsistent oral bioavailability. The current work, Quality by Design (QbD)-driven development of phospholipid-embedded nanostructured lipidic carriers (NLCs) of RLX, accordingly, was undertaken to potentiate its lymphatic uptake, augment oral bioavailability, and possibly reduce drug dosage. Factor screening and failure mode effect analysis (FMEA) studies were performed to delineate high-risk factors using solid lipid (glyceryl monostearate), liquid lipid (vitamin E), and surfactant (Tween 80). Response surface optimization studies were performed employing the Box-Behnken design. Mathematical and graphical methods were adopted to embark upon the selection of optimized NLCs with various critical quality attributes (CQAs) of mean particle size as 186 nm, zeta potential of − 23.6 mV, entrapment efficiency of 80.09%, and cumulative drug release at 12 h of 83.87%. The DSC and FTIR studies, conducted on optimized NLCs, indicated successful entrapment of drug into the lipid matrix. In vitro drug release studies demonstrated Fickian diffusion mechanism. In vivo pharmacokinetic studies in rats construed significant improvement in AUC0–72 h (4.48-folds) and in Cmax (5.11-folds), unequivocally indicating markedly superior (p < 0.001) oral bioavailability of RLX-NLCs vis-à-vis marketed tablet formulation. Subsequently, level “A” in vitro/in vivo correlation (IVIVC) was also successfully attempted between the percentages of in vitro drug dissolved and of in vivo drug absorbed at the matching time points. In vitro cytotoxicity and cellular uptake studies also corroborated higher efficacy and successful localization of coumarin-6-loaded NLCs into MG-63 cells through microfluidic channels.
Graphical abstract



















Similar content being viewed by others
Availability of data and materials
All the pertinent data generated or analyzed during these studies have been included in this article and its supplementary information files.
References
Pant A, Singh J, Barnwal RP, Singh G, Singh B. Theranostic approach for the management of osteoporosis. Crit Rev Ther Drug Carrier Syst. 2023;40(3):95–121.
Yavropoulou MP, Makras P, Anastasilakis AD. Bazedoxifene for the treatment of osteoporosis. Expert Opin Pharmacother. 2019;20(10):1201–10.
Sree KSN, Dengale SJ, Mutalik S, Bhat K. Raloxifene HCl–quercetin co-amorphous system: preparation, characterization, and investigation of its behavior in phosphate buffer. Drug Dev Ind Pharm. 2022;48(6):227–38.
Arbuthnot GN, Dalder BW, Hartauer KJ, Wayne DL, Stratford RE. Raloxifene salts and solvates, formulations containing same, and methods for their preparation. 1996;Hungarian Patent, HU9902750A2.
Vora D, Dandekar A, Bhattaccharjee S, Singh ON, Agrahari V, Peet MM, Doncel GF, Banga AK. Formulation development for transdermal delivery of raloxifene, a chemoprophylactic agent against breast cancer. Pharmaceutics. 2022;14(3):1–14.
Joshi A, Kaur J, Kulkarni R, Chaudhari R. In-vitro and ex-vivo evaluation of raloxifene hydrochloride delivery using nano-transfersome based formulations. J Drug Deliv Sci Technol. 2018;45:151–8.
Jain A, Sharma T, Kumar R, Katare OP, Singh B. Raloxifene-loaded SLNs with enhanced biopharmaceutical potential: QbD-steered development, in vitro evaluation, in vivo pharmacokinetics, and IVIVC. Drug Deliv Transl Res. 2022;12(5):1136–60.
Aldawsari HM, Negm AA. Potentiation of raloxifene cytotoxicity against MCF-7 breast cancer cell lines via transdermal delivery and loading on self-emulsifying nanoemulsions. Trop J Pharm Res. 2020;19(1):11–5.
Golmohammadzadeh S, Farhadian N, Biriaee A, Dehghani F, Khameneh B. Preparation, characterization and in vitro evaluation of microemulsion of raloxifene hydrochloride. Drug Dev Ind Pharm. 2017;43(10):1619–25.
Murthy A, Rao Ravi P, Kathuria H, Malekar S. Oral bioavailability enhancement of raloxifene with nanostructured lipid carriers. Nanomaterials (BaseI). 2020;10(6):1–17.
Patil PH, Belgamwar VS, Patil PR, Surana SJ. Solubility enhancement of raloxifene using inclusion complexes and cogrinding method. J Pharm. 2013;2013:1–9.
Jain A, Saini S, Kumar R, Sharma T, Swami R, Katare OP, Singh B. Phospholipid-based complex of raloxifene with enhanced biopharmaceutical potential: synthesis, characterization and preclinical assessment. Int J Pharm. 2019;571:1–15.
Tran TH, Poudel BK, Marasini N, Chi S-C, Choi H-G, Yong CS, Kim JO. Preparation and evaluation of raloxifene-loaded solid dispersion nanoparticle by spray-drying technique without an organic solvent. Int J Pharm. 2013;443(1–2):50–7.
Ağardan NBM, Değim Z, Yılmaz Ş, Altıntaş L, Topal T. The effectiveness of raloxifene-loaded liposomes and cochleates in breast cancer therapy. AAPS PharmSciTech. 2016;17:968–77.
Jain A, Kaur R, Beg S, Kushwah V, Jain S, Singh B. Novel cationic supersaturable nanomicellar systems of raloxifene hydrochloride with enhanced biopharmaceutical attributes. Drug Deliv Transl Res. 2018;8:670–92.
Musakhanian J, Rodier J-D, Dave M. Oxidative stability in lipid formulations: a review of the mechanisms, drivers, and inhibitors of oxidation. AAPS PharmSciTech. 2022;23(5):2–30.
Zakir F, Ahmad A, Farooq U, Mirza MA, Tripathi A, Singh D, Shakeel F, Mohapatra S, Ahmad FJ, Kohli K. Design and development of a commercially viable in situ nanoemulgel for the treatment of postmenopausal osteoporosis. Nanomedicine (Lond). 2020;15(12):1167–87.
Gadade DD, Pekamwar SS. Cyclodextrin based nanoparticles for drug delivery and theranostics. Adv Pharm Bull. 2020;10(2):166.
Huang Y, Dai W-G. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharmaceutica Sinica B. 2014;4(1):18–25.
Callender SP, Mathews JA, Kobernyk K, Wettig SD. Microemulsion utility in pharmaceuticals: implications for multi-drug delivery. Int J Pharm. 2017;526(1–2):425–42.
Jain S, Jain V, Mahajan S. Lipid based vesicular drug delivery systems. Adv Pharm. 2014;2014:1–12.
Elmowafy M, Shalaby K, Elkomy MH, Alsaidan OA, Gomaa HA, Abdelgawad MA, Mostafa EM. Polymeric nanoparticles for delivery of natural bioactive agents: recent advances and challenges. Polymers. 2023;15(5):1123.
Poovi G, Damodharan N. Lipid nanoparticles: a challenging approach for oral delivery of BCS class-II drugs. Future J Pharm Sci. 2018;4(2):191–205.
Zewail MB, Asaad GF, Swellam SM, Abd-Allah SM, Hosny SK, Sallah SK, Eissa JE, Mohamed SS, El-Dakroury WA. Design, characterization and in vivo performance of solid lipid nanoparticles (SLNs)-loaded mucoadhesive buccal tablets for efficient delivery of lornoxicam in experimental inflammation. Int J Pharm. 2022;624.
Dhiman N, Awasthi R, Sharma B, Kharkwal H, Kulkarni GT. Lipid nanoparticles as carriers for bioactive delivery. Front Chem. 2021;9.
Mitrović JR, Divović-Matović B, Knutson DE, Petković M, Djorović D, Randjelović DV, Dobričić VD, Đoković JB, Lunter DJ, Cook JM. High amount of lecithin facilitates oral delivery of a poorly soluble pyrazoloquinolinone ligand formulated in lipid nanoparticles: physicochemical, structural and pharmacokinetic performances. Int J Pharm. 2023:1–11.
Khurana RK, Gaspar BL, Welsby G, Katare OP, Singh KK, Singh B. Improving the biopharmaceutical attributes of mangiferin using vitamin E-TPGS co-loaded self-assembled phosholipidic nano-mixed micellar systems. Drug Deliv Transl Res. 2018;8:617–32.
Fricker G, Kromp T, Wendel A, Blume A, Zirkel J, Rebmann H, Setzer C, Quinkert R-O, Martin F, Müller-Goymann C. Phospholipids and lipid-based formulations in oral drug delivery. Pharm Res. 2010;27(8):1469–86.
Jain A, Sharma G, Kushwah V, Thakur K, Ghoshal G, Singh B, Jain S, Shivhare U, Katare O. Fabrication and functional attributes of lipidic nanoconstructs of lycopene: an innovative endeavour for enhanced cytotoxicity in MCF-7 breast cancer cells. Colloids Surf B. 2017;152:482–91.
Schubert MA, Harms M, Müller-Goymann CC. Structural investigations on lipid nanoparticles containing high amounts of lecithin. Eur J Pharm Sci. 2006;27(2–3):226–36.
Elmowafy M, Shalaby K, Elkomy M, Alruwaili NK, Mostafa EM, Afzal M, Alharbi KS, Mohammed EF, Ali HM, Salama A. Impact of highly phospholipid-containing lipid nanocarriers on oral bioavailability and pharmacodynamics performance of genistein. Pharm Dev Technol. 2022;27(4):435–47.
Liu D, Chen L, Jiang S, Zhu S, Qian Y, Wang F, Li R, Xu Q. Formulation and characterization of hydrophilic drug diclofenac sodium-loaded solid lipid nanoparticles based on phospholipid complexes technology. J Liposome Res. 2014;24(1):17–26.
Heiati H, Phillips NC, Tawashi R. Evidence for phospholipid bilayer formation in solid lipid nanoparticles formulated with phospholipid and triglyceride. Pharm Res. 1996;13:1406–10.
Salminen H, Helgason T, Aulbach S, Kristinsson B, Kristbergsson K, Weiss J. Influence of co-surfactants on crystallization and stability of solid lipid nanoparticles. J Colloid Interface Sci. 2014;426:256–63.
Komath S, Garg A, Wahajuddin M. Development and evaluation of chrysin-phospholipid complex loaded solid lipid nanoparticles-storage stability and in vitro anti-cancer activity. J Microencapsul. 2018;35(6):600–17.
Rathore C, Upadhyay NK, Sharma A, Lal UR, Raza K, Negi P. Phospholipid nanoformulation of thymoquinone with enhanced bioavailability: development, characterization and anti-inflammatory activity. J Drug Deliv Sci Technol. 2019;52:316–24.
Jansook P, Pichayakorn W, Ritthidej GC. Amphotericin B-loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carrier (NLCs): effect of drug loading and biopharmaceutical characterizations. Drug Dev Ind Pharm. 2018;44(10):1693–700.
Akanda M, Getti G, Douroumis D. In vivo evaluation of nanostructured lipid carrier systems (NLCs) in mice bearing prostate cancer tumours. Drug Deliv Transl Res. 2021:1–13.
Houacine C, Adams D, Singh KK. Impact of liquid lipid on development and stability of trimyristin nanostructured lipid carriers for oral delivery of resveratrol. J Mol Liq. 2020;316:1–50.
Thuy VN, Van TV, Dao AH, Lee B-J. Nanostructured lipid carriers and their potential applications for versatile drug delivery via oral administration. Open Nano. 2022;8.
Ye Q, Li J, Li T, Ruan J, Wang H, Wang F, Zhang X. Development and evaluation of puerarin-loaded controlled release nanostructured lipid carries by central composite design. Drug Dev Ind Pharm. 2021;47(1):113–25.
Zhao C, Liu Y, Fan T, Zhou D, Yang Y, Jin Y, Zhang Z, Huang Y. A novel strategy for encapsulating poorly soluble drug into nanostructured lipid carriers for intravenous administration. Pharm Dev Technol. 2012;17(4):443–56.
Telange DR, Sohail NK, Hemke AT, Kharkar PS, Pethe AM. Phospholipid complex-loaded self-assembled phytosomal soft nanoparticles: evidence of enhanced solubility, dissolution rate, ex vivo permeability, oral bioavailability, and antioxidant potential of mangiferin. Drug Deliv Transl Res. 2021;11:1056–83.
Telange DR, Nirgulkar SB, Umekar MJ, Patil AT, Pethe AM, Bali NR. Enhanced transdermal permeation and anti-inflammatory potential of phospholipids complex-loaded matrix film of umbelliferone: formulation development, physico-chemical and functional characterization. Eur J Pharm Sci. 2019;131:23–38.
Wang Q, Gong T, Sun X, Zhang Z. Structural characterization of novel phospholipid lipid nanoparticles for controlled drug delivery. Colloids Surf B. 2011;84(2):406–12.
Elsheikh MA, Elnaggar YS, Gohar EY, Abdallah OY. Nanoemulsion liquid preconcentrates for raloxifene hydrochloride: optimization and in vivo appraisal. Int J Nanomed. 2012:3787–3802
Shirazi AS, Varshochian R, Rezaei M, Ardakani YH, Dinarvand R. SN38 loaded nanostructured lipid carriers (NLCs); preparation and in vitro evaluations against glioblastoma. J Mater Sci Mater Med. 2021;32(7):1–12.
Shah B, Khunt D, Bhatt H, Misra M, Padh H. Intranasal delivery of venlafaxine loaded nanostructured lipid carrier: risk assessment and QbD based optimization. J Drug Deliv Sci Technol. 2016;33:37–50.
Fahmy R, Kona R, Dandu R, Xie W, Claycamp G, Hoag SW. Quality by design I: application of failure mode effect analysis (FMEA) and Plackett-Burman design of experiments in the identification of “main factors” in the formulation and process design space for roller-compacted ciprofloxacin hydrochloride immediate-release tablets. AAPS Pharm Scitech. 2012;13:1243–54.
Beg S, Saini S, Bandopadhyay S, Katare OP, Singh B. QbD-driven development and evaluation of nanostructured lipid carriers (NLCs) of olmesartan medoxomil employing multivariate statistical techniques. Drug Dev Ind Pharm. 2018;44(3):407–20.
Poonia N, Narang JK, Lather V, Beg S, Sharma T, Singh B, Pandita D. Resveratrol loaded functionalized nanostructured lipid carriers for breast cancer targeting: systematic development, characterization and pharmacokinetic evaluation. Colloids Surf B. 2019;181:756–66.
Yasir M, Zafar A, Noorulla KM, Tura AJ, Sara UVS, Panjwani D, Khalid M, Haji MJ, Gobena WG, Gebissa T. Nose to brain delivery of donepezil through surface modified NLCs: formulation development, optimization, and brain targeting study. J Drug Deliv Sci Technol. 2022;75:1–15.
Shete MB, Deshpande AS, Shende P. Enhancement of in-vitro anti-oral cancer activities of silymarin using dispersion of nanostructured lipid carrier in mucoadhesive in-situ gel. Int J Pharm. 2023;636:1–16.
Singh B, Pahuja S, Kapil R, Ahuja N. Formulation development of oral controlled release tablets of hydralazine: optimization of drug release and bioadhesive characteristics. Acta Pharm. 2009;59(1):1–13.
Garg B, Beg S, Kumar R, Katare OP, Singh B. Nanostructured lipidic carriers of lopinavir for effective management of HIV-associated neurocognitive disorder. J Drug Deliv Sci Technol. 2019;53:1–13.
Dolatabadi S, Karimi M, Nasirizadeh S, Hatamipour M, Golmohammadzadeh S, Jaafari MR. Preparation, characterization and in vivo pharmacokinetic evaluation of curcuminoids-loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs). J Drug Deliv Sci Technol. 2021;62:1–10.
Wu L, Zhao L, Su X, Zhang P, Ling G. Repaglinide-loaded nanostructured lipid carriers with different particle sizes for improving oral absorption: preparation, characterization, pharmacokinetics, and in situ intestinal perfusion. Drug Delivery. 2020;27(1):400–9.
Ortiz AC, Yañez O, Salas-Huenuleo E, Morales JO. Development of a nanostructured lipid carrier (NLC) by a low-energy method, comparison of release kinetics and molecular dynamics simulation. Pharmaceutics. 2021;13(4):531–52.
Beg S, Raza K, Kumar R, Chadha R, Katare OP, Singh B. Improved intestinal lymphatic drug targeting via phospholipid complex-loaded nanolipospheres of rosuvastatin calcium. RSC Adv. 2016;6(10):8173–87.
Wu K-W, Sweeney C, Dudhipala N, Lakhani P, Chaurasiya ND, Tekwani BL, Majumdar S. Primaquine loaded solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and nanoemulsion (NE): effect of lipid matrix and surfactant on drug entrapment, in vitro release, and ex vivo hemolysis. AAPS Pharm Scitech. 2021;22:1–12.
Beg S, Katare OP, Saini S, Garg B, Khurana RK, Singh B. Solid self-nanoemulsifying systems of olmesartan medoxomil: formulation development, micromeritic characterization, in vitro and in vivo evaluation. Powder Technol. 2016;294:93–104.
Pant A, Sharma G, Saini S, Jain A, Barnwal RP, Singh G, Singh B. Quality by Design (QbD)‐steered development and validation of analytical and bioanalytical methods for raloxifene: application of Monte Carlo simulations and variance inflation factor. Biomed Chromatogr. 2023:1–20.
Hosny KM, Bahmdan RH, Alhakamy NA, Alfaleh MA, Ahmed OA, Elkomy MH. Physically optimized nano-lipid carriers augment raloxifene and vitamin D oral bioavailability in healthy humans for management of osteoporosis. J Pharm Sci. 2020;109(7):2145–55.
Singh B, Rani A, Ahuja N, Kapil R. Formulation optimization of hydrodynamically balanced oral controlled release bioadhesive tablets of tramadol hydrochloride. Sci Pharm. 2010;78(2):303–24.
Shadambikar G, Marathe S, Ji N, Almutairi M, Bandari S, Zhang F, Chougule M, Repka M. Formulation development of itraconazole PEGylated nano-lipid carriers for pulmonary aspergillosis using hot-melt extrusion technology. Int J Pharm: X. 2021;3:1–10.
Teeranachaideekul V, Müller RH, Junyaprasert VB. Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC)—effects of formulation parameters on physicochemical stability. Int J Pharm. 2007;340(1–2):198–206.
Uner B, Ozdemir S, Yildirim E, Yaba A, Tas C, Uner M, Ozsoy Y. Loteprednol loaded nanoformulations for corneal delivery: ex-vivo permeation study, ocular safety assessment and stability studies. J Drug Deliv Sci Technol. 2023;81:1–10.
Gera S, Pooladanda V, Godugu C, Swamy Challa V, Wankar J, Dodoala S, Sampathi S. Rutin nanosuspension for potential management of osteoporosis: effect of particle size reduction on oral bioavailability, in vitro and in vivo activity. Pharm Dev Technol. 2020;25(8):971–88.
Gera S, Sampathi S, Maddukuri S, Dodoala S, Junnuthula V, Dyawanapelly S. Therapeutic potential of naringenin nanosuspension: in vitro and in vivo anti-osteoporotic studies. Pharmaceutics. 2022;14(7):1–12.
Kammalla AK, Ramasamy MK, Inampudi J, Dubey GP, Agrawal A, Kaliappan I. Comparative pharmacokinetic study of mangiferin after oral administration of pure mangiferin and US patented polyherbal formulation to rats. AAPS PharmSciTech. 2015;16:250–8.
Varshosaz J, Dayani L, Chegini SP, Minaiyan M. Production of a new platform based on fumed and mesoporous silica nanoparticles for enhanced solubility and oral bioavailability of raloxifene HCl. IET Nanobiotechnol. 2019;13(4):392–9.
Du X, Gao N, Song X. Bioadhesive polymer/lipid hybrid nanoparticles as oral delivery system of raloxifene with enhancive intestinal retention and bioavailability. Drug Delivery. 2021;28(1):252–60.
Shah NV, Seth AK, Balaraman R, Aundhia CJ, Maheshwari RA, Parmar GR. Nanostructured lipid carriers for oral bioavailability enhancement of raloxifene: design and in vivo study. J Adv Res. 2016;7(3):423–34.
zur Mühlen A, Schwarz C, Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery–drug release and release mechanism. Eur J Pharm Biopharm. 1998;45(2):149–155.
Saha I, Palak A, Rai VK. Relevance of NLC-gel and microneedling-assisted tacrolimus ointment against severe psoriasiform: in vitro dermal retention kinetics, in vivo activity and drug distribution. J Drug Deliv Sci Technol. 2022;71:1–16.
Saini S, Sharma T, Jain A, Kaur H, Katare OP, Singh B. Systematically designed chitosan-coated solid lipid nanoparticles of ferulic acid for effective management of Alzheimer’s disease: a preclinical evidence. Colloids Surf B Biointerfaces. 2021;205:111838.https://doi.org/10.1016/j.colsurfb.2021.111838.
Garg B, Katare O, Beg S, Lohan S, Singh B. Systematic development of solid self-nanoemulsifying oily formulations (S-SNEOFs) for enhancing the oral bioavailability and intestinal lymphatic uptake of lopinavir. Colloids Surf B. 2016;141:611–22.
Garg NK, Sharma G, Singh B, Nirbhavane P, Tyagi RK, Shukla R, Katare OP. Quality by Design (QbD)-enabled development of aceclofenac loaded-nano structured lipid carriers (NLCs): an improved dermatokinetic profile for inflammatory disorder (s). Int J Pharm. 2017;517(1–2):413–31.
Sharma T, Katare OP, Jain A, Jain S, Chaudhari D, Borges B, Singh B. QbD-steered development of biotin-conjugated nanostructured lipid carriers for oral delivery of chrysin: role of surface modification for improving biopharmaceutical performance. Colloids Surf B Biointerfaces. 2021;197:111429. https://doi.org/10.1016/j.colsurfb.2020.111429.
Kesharwani P, Md S, Alhakamy NA, Hosny KM, Haque A. QbD enabled azacitidine loaded liposomal nanoformulation and its in vitro evaluation. Polymers. 2021;13(2):250–68.
Lin X, Li X, Zheng L, Yu L, Zhang Q, Liu W. Preparation and characterization of monocaprate nanostructured lipid carriers. Colloids Surf A. 2007;311(1–3):106–11.
Alhalmi A, Amin S, Khan Z, Beg S, Al Kamaly O, Saleh A, Kohli K. Nanostructured lipid carrier-based codelivery of raloxifene and naringin: formulation, optimization, in vitro, ex vivo, in vivo assessment, and acute toxicity studies. Pharmaceutics. 2022;14(9):1–27.
Okonogi S, Riangjanapatee P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formulations for topical administration. Int J Pharm. 2015;478(2):726–35.
Rehman M, Madni A, Ihsan A, Khan WS, Khan MI, Mahmood MA, Ashfaq M, Bajwa SZ, Shakir I. Solid and liquid lipid-based binary solid lipid nanoparticles of diacerein: in vitro evaluation of sustained release, simultaneous loading of gold nanoparticles, and potential thermoresponsive behavior. Int J Nanomed. 2015;10:2805–14.
Jain K, Sood S, Gowthamarajan K. Optimization of artemether-loaded NLC for intranasal delivery using central composite design. Drug Delivery. 2015;22(7):940–54.
da Silva MG, de Godoi KRR, Gigante ML, Cardoso LP, Ribeiro APB. Nanostructured lipid carriers for delivery of free phytosterols: effect of lipid composition and chemical interesterification on physical stability. Colloids Surf A Physicochem Eng. 2022;640:1–13.
Lohan S, Sharma T, Saini S, Singh A, Kumar A, Raza K, Kaur J, Singh B. Galactosylated nanoconstructs of berberine with enhanced biopharmaceutical and cognitive potential: a preclinical evidence in Alzheimer’s disease. J Drug Deliv Sci Technol. 2021;66:1–10.
Ortiz AC, Yañez O, Salas-Huenuleo E, Morales JO. Development of a nanostructured lipid carrier (NLC) by a low-energy method, comparison of release kinetics and molecular dynamics simulation. Pharmaceutics. 2021;13(4):1–21.
Yang S-J, Chang C-H, Young T-H, Wang C-H, Tseng T-H, Wang M-L. Human serum albumin-based nanoparticles alter raloxifene administration and improve bioavailability. Drug Delivery. 2022;29(1):2685–93.
Muzio LL, Santarelli A, Orsini G, Memè L, Mattioli-Belmonte M, De Florio I, Gatto R, Gallusi G, Nocini P, Bertossi D. MG63 and MC3T3-E1 osteoblastic cell lines response to raloxifene. Eur J Inflamm. 2013;11(3):797–804.
Babanejad N, Farhadian A, Omrani I, Nabid MR. Design, characterization and in vitro evaluation of novel amphiphilic block sunflower oil-based polyol nanocarrier as a potential delivery system: raloxifene-hydrochloride as a model. Mater Sci Eng C. 2017;78:59–68.
Iemsam-Arng J, Ketchart O, Rattana-Amron T, Wutikhun T, Tapaneeyakorn S. Modified NLC-loaded coumarin for pharmaceutical applications: the improvement of physical stability and controlled release profile. Pharm Dev Technol. 2016;21(8):1015–22.
Soni NK, Sonali L, Singh A, Mangla B, Neupane YR, Kohli K. Nanostructured lipid carrier potentiated oral delivery of raloxifene for breast cancer treatment. Nanotechnology. 2020;31(47):1–47.
Shah VP, Amidon GL. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability, Pharm Res 12, 413–420, 1995—Backstory of BCS. AAPS J. 2014;16:894–898.
Sharma T, Jain A, Kaur R, Saini S, Katare OP, Singh B. Supersaturated LFCS type III self-emulsifying delivery systems of sorafenib tosylate with improved biopharmaceutical performance: QbD-enabled development and evaluation. Drug Deliv Transl Res. 2020;10:839–61.
Acknowledgements
The authors enunciate their thankfulness to the Department of Science & Technology, New Delhi, India, for providing requisite fiscal grants for the research work. The generosity of Stat-Ease Inc., USA, is also gratefully acknowledged for gifting premium and annual licenses of Design Expert® software 12.0 version to the corresponding author (B.S.) and his team, as an Award of Distinction for his stellar contribution in QbD-steered research work for systematic development of novel drug delivery systems and for analytical method development and validation of varied drugs.
Funding
Department of Science & Technology, New Delhi, India, for the requisite funding to the first author (A.P.) to perform the present work as a DST-INSPIRE Research Fellow (IF170942) availing the facilities of the University Institute of Pharmaceutical Sciences for the present research work.
Author information
Authors and Affiliations
Contributions
Anjali Pant: data curation, formal analysis, funding acquisition, investigation, methodology, validation, writing—original draft. Gajanand Sharma: methodology and validation. Sumant Saini: software, validation, original draft review. Gurjeet Kaur: cell line studies and validation. Atul Jain: validation and editing. Anil Thakur: In vivo experimental studies. Bhupinder Singh: conceptualization, visualization, project administration, resources, software, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The In vivo studies were carried out in female Wistar rats (220–280 g), after obtaining the requisite permission from the Institutional Animal Ethics Committee (IAEC) of the Panjab University, Chandigarh, India, vide their letter no. PU/45/99/CPCSEA/IAEC/2021/463. All institutional and national guidelines for the care and use of laboratory animals were followed.
Consent for publication
Yes.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pant, A., Sharma, G., Saini, S. et al. QbD-driven development of phospholipid-embedded lipidic nanocarriers of raloxifene: extensive in vitro and in vivo evaluation studies. Drug Deliv. and Transl. Res. 14, 730–756 (2024). https://doi.org/10.1007/s13346-023-01427-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-023-01427-3