Abstract
Background and Objectives
Defibrotide is approved to treat severe veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) after haematopoietic cell transplantation in patients aged > 1 month in the European Union and for VOD/SOS with renal/pulmonary dysfunction post-haematopoietic cell transplantation in the United States. This meta-analysis estimated the incidence and risk of VOD/SOS after intravenous defibrotide prophylaxis using the published literature.
Methods
PubMed, Embase and Web of Science were searched through 30 November 2021 for defibrotide studies in VOD/SOS “prevention” or “prophylaxis,” excluding phase I studies, case reports, studies with fewer than ten patients and reviews.
Results
The search identified 733 records; 24 met inclusion criteria, of which 20 (N = 3005) evaluated intravenous defibrotide for VOD/SOS prophylaxis. Overall VOD/SOS incidence with intravenous defibrotide was 5%, with incidences of 5% in adults and 8% in paediatric patients. In eight studies with data on intravenous defibrotide prophylaxis vs controls (e.g. heparin, no prophylaxis), VOD/SOS incidence in controls was 16%. The risk ratio for developing VOD/SOS with defibrotide prophylaxis vs controls was 0.30 (95% confidence interval 0.12–0.71; p = 0.006).
Conclusions
This analysis suggests a low incidence of VOD/SOS following intravenous defibrotide prophylaxis, regardless of age group, and a lower relative risk for VOD/SOS with defibrotide prophylaxis vs controls in patient populations at high risk of VOD/SOS.
Similar content being viewed by others
This meta-analysis estimated the risk of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) after intravenous defibrotide prophylaxis. |
Twenty identified studies evaluated intravenous defibrotide for VOD/SOS prophylaxis. |
VOD/SOS incidence was 16% in controls and 5% with intravenous defibrotide prophylaxis. |
The risk ratio for developing VOD/SOS with defibrotide prophylaxis vs controls was 0.30. |
1 Introduction
Hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) is a rare, potentially life-threatening complication of haematopoietic cell transplantation (HCT) conditioning that may also occur after chemotherapy alone [1, 2]. Sustained exposure to chemotherapy and HCT conditioning regimens results in sinusoidal endothelial cell (EC) activation and damage, leading to a procoagulant and proinflammatory state [3, 4]. This EC dysfunction is hypothesised to contribute to hepatic VOD/SOS [1]. The incidence of VOD/SOS following HCT ranges from <5% with autologous HCT to approximately 10–15% with allogeneic HCT based on the presence of risk factors and use of a myeloablative conditioning regimen [5, 6]. Patient-related and transplantation-related risk factors for developing VOD/SOS include older and very young age, lower performance status, pre-existing liver disease, type of conditioning regimen, and type and number of transplants [5, 7, 8].
Defibrotide is a polydisperse mixture of predominantly single-stranded polydeoxyribonucleotide sodium salts [9]. In vitro, defibrotide has been shown to reduce EC activation, promote EC-mediated fibrinolysis and protect ECs from damage caused by chemotherapy [4, 10]. Defibrotide is approved for the treatment of severe hepatic VOD/SOS after HCT in patients older than 1 month of age in the European Union and for the treatment of adult and paediatric patients with hepatic VOD/SOS with renal or pulmonary dysfunction after HCT in the United States [9, 11]. The recommended dose for the treatment of VOD/SOS is 6.25 mg/kg every 6 hours (25 mg/kg/day), given as a 2-hour intravenous (IV) infusion [9, 11].
Several studies have found that defibrotide prophylaxis can reduce the incidence of VOD/SOS in high-risk patients [12,13,14]. Among these, a previous systematic review from Zhang et al. examining 13 studies from 2002 to 2010 showed an overall mean incidence of VOD/SOS after HCT of 4.7% (95% confidence interval [CI] 3.3–6.1) in patients receiving defibrotide prophylaxis vs 13.7% (95% CI 13.3–14.1; p < 0.005) in patients without defibrotide prophylaxis [14]. Among controlled trials, the relative risk of VOD/SOS was lower with defibrotide prophylaxis (risk ratio [RR] 0.47, 95% CI 0.31–0.73) [14].
In contrast, a 2015 Cochrane systematic review concluded that there was insufficient evidence to indicate a reduction in the incidence of VOD/SOS or mortality with defibrotide prophylaxis; however, only one randomised controlled defibrotide trial was analysed and the authors acknowledged further evaluation was needed through high-quality, randomised controlled trials [15]. An ongoing phase III, prospective study (ClinicalTrails.gov Identifier: NCT02851407) of defibrotide for VOD/SOS prophylaxis recently stopped enrolment after meeting the protocol-defined criteria for futility, suggesting a low probability of meeting the primary endpoint of demonstrating a significant 30-day VOD/SOS-free survival difference with the sample size estimates used; analyses are ongoing and results are not yet reported [16].
In addition to patient-related and transplantation-related risk factors of VOD/SOS, some approved antitumour therapies, such as gemtuzumab ozogamicin and inotuzumab ozogamicin, have been shown to contribute to an increased risk of VOD/SOS [17]. Given the variety of factors that may place a patient at high risk of VOD/SOS, there is a need to better understand the utility of IV defibrotide prophylaxis for VOD/SOS post-HCT. The goal of this systematic literature review and meta-analysis was to provide a current estimate of the overall incidence and risk of developing VOD/SOS after IV defibrotide prophylaxis using the published literature, as an update to the Zhang et al. analysis [14].
2 Methods
2.1 Search Strategy and Selection Criteria
A systematic search of PubMed (MEDLINE), ClinicalTrials.gov, Google Scholar, Web of Science and Embase, which was used to search for abstracts (e.g. EBMT, Blood and Marrow Transplantation, American Society for Hematology and European Hematology Association) from database inception through 30 November 2021, was performed per a prespecified and clearly defined protocol based on Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. The search terms for all databases were “prevention” or “prophylaxis” of defibrotide in VOD/SOS; search fields were limited to the title or abstract and to articles published in the English language. Duplicate results from these searches were removed.
Defibrotide studies of adult or paediatric patients, including controlled trials, observational or retrospective studies, retrospective or post hoc analyses, and case reports with ten or more patients were eligible for inclusion in the meta-analysis. Phase I studies, case reports, studies with fewer than ten patients and review articles were excluded.
2.2 Data Analysis
The full text of the selected studies and conference abstracts were assessed for study design, sample size, dose, route of administration, treatment duration and control comparators. Publications were evaluated for the presence of data on endpoints of interest, which included incidence of VOD/SOS, incidence of severe/very severe VOD/SOS, overall adverse events, bleeding and/or haemorrhagic events and site of bleeding (if reported).
All studies with relevant data were included in the meta-analysis. Pooled VOD/SOS incidence estimates with 95% CIs were calculated using a random-effects model after Freeman–Tukey double arcsine transformation. The Mantel–Haenszel method and random-effects modelling (Stata 14.2 software) were used for overall incidence rates and RRs, respectively. Interstudy heterogeneity was assessed with Cochrane Q and I2 tests (with significant heterogeneity indicated by p < 0.10 or I2 ≥ 50%). For analyses by patient age, only studies specifying adult or paediatric data were included. All reported p-values were nominal. Safety results were not pooled because of differences in adverse event reporting among studies. The quality of the data was assessed based on study design parameters, such as retrospective vs prospective design, number of sites and size of the study population.
3 Results
3.1 Literature Search Results
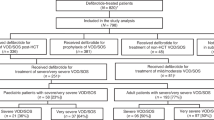
A total of 733 records were identified in the search (Fig. 1), and 24 met inclusion criteria for the analysis. Of these 24 studies, 20 (N = 3005) reported on IV defibrotide for VOD/SOS prophylaxis, including eight adult studies, six paediatric studies and six studies with both age groups or in which age was not specified. Of these 20 studies, 14 reported on VOD/SOS severity based on the investigator’s assessment. Four of the studies did not specify the mode by which defibrotide was administered; therefore, these studies were not included in the subanalysis by dose. This analysis included prospective cohort studies and case series; retrospective case series, studies and chart reviews; and phase II and III open-label, randomised controlled studies. Details pertaining to data quality are included in Table 1, which summarises key features of each study’s design.
3.2 Incidence of VOD/SOS
With IV defibrotide prophylaxis, the overall incidence of VOD/SOS among the 20 studies was 5% (95% CI 3–8; I2 = 75.85%; p < 0.01; Fig. 2A). In studies reporting results in either adult or paediatric patients, the incidences of VOD/SOS were 5% (95% CI 3–8; I2 = 36.16%; p = 0.13) and 8% (95% CI 6–11; I2 = 25.25%; p = 0.24), respectively (Fig. 3). Among eight studies using IV defibrotide prophylaxis that also included data from control groups (e.g. heparin or no prophylaxis), the incidence of VOD/SOS with control treatment was 16% (95% CI 7–28). The RR for developing VOD/SOS with defibrotide prophylaxis vs control was 0.30 (95% CI 0.12–0.71; p = 0.006; I2 = 75%; Fig. 4).
Incidence of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) with intravenous (IV) defibrotide prophylaxis in adult (A) and paediatric (B) patients [6, 12, 18, 24,25,26,27, 29, 30, 32, 33, 36, 38, 41, 42]. CI confidence interval, ES effect size. Five studies with IV defibrotide prophylaxis included patients of both age groups or did not specify age
Risk ratio of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) vs controls in intravenous (IV) defibrotide prophylaxis studies that included a control arm [6, 12, 13, 29, 33, 35, 41, 42] CI confidence interval. Note: Weights are from the random-effects analysis. aControl was no prophylaxis. bControl was heparin. cControl was without defibrotide. dControl was standard of care
The overall incidence of severe/very severe VOD/SOS with IV defibrotide prophylaxis was 2% (95% CI 0–4; I2 = 69.46%; p < 0.01; Fig. 2B) in the 14 studies reporting disease severity based on the investigator’s assessment. In the four studies that reported data from control groups, the incidence of severe/very severe VOD/SOS with control treatment was 8% (95% CI 2–15); the RR for developing severe/very severe VOD/SOS with defibrotide prophylaxis vs control was 0.59 (95% CI 0.31–1.14; p = 0.12).
3.3 Safety
While safety data were not pooled because of differences in reporting, defibrotide safety results in individual studies were generally consistent with the known safety profile of defibrotide in the prophylactic setting (Table 2). Among studies that reported bleeding, the largest study in adults (N = 63) described grade 2 or 3 bleeding events in 22% of those receiving IV defibrotide prophylaxis [18]. Similarly, the largest paediatric study (N = 356) reported a cumulative haemorrhage incidence of 22% for those receiving IV defibrotide prophylaxis (and 21% for controls) [12].
4 Discussion
VOD/SOS is a life-threatening complication of HCT. A number of factors may place a patient at high risk of developing VOD/SOS, including age, primary disease, type of conditioning regimen, and type and number of HCTs [5, 8, 17]. In addition, exposure to some antitumor agents, such as gemtuzumab ozogamicin and inotuzumab ozogamicin, has also been shown to increase the risk of VOD/SOS [17]. Thus, there is a medical need for therapies that could reduce the risk of VOD/SOS. In this meta-analysis of defibrotide use for the prevention of VOD/SOS, the overall incidence of VOD/SOS following prophylaxis with IV defibrotide was low (at 5%) and was comparable in adults (5%) and in paediatric patients (8%). There was a lower relative risk of developing VOD/SOS with defibrotide vs controls such as heparin or no prophylaxis. Similarly, the incidence of severe/very severe VOD/SOS with IV defibrotide prophylaxis was low (at 2%), and the relative risk of VOD/SOS was lower with defibrotide vs control treatment.
Many of the studies included in this meta-analysis specified the inclusion of patients at high risk for developing VOD/SOS and, thus, with a potential need for VOD/SOS prophylaxis. High risk of VOD/SOS was defined in a variety of ways across the publications but was generally based upon patient-related factors (e.g. primary disease) or HCT-related factors (e.g. conditioning regimen or transplant type). For example, the randomised controlled phase III study by Corbacioglu et al. included patients with one or more of the following risk factors for VOD/SOS: pre-existing liver disease; second myeloablative HCT; allogeneic HCT for leukaemia beyond the second relapse; conditioning with busulfan and melphalan; previous treatment with gemtuzumab ozogamicin; and diagnoses of inherited haemophagocytic lymphohistiocytosis, adrenoleukodystrophy or osteopetrosis [12]. VOD/SOS was generally diagnosed and graded using Baltimore or modified Seattle criteria. These criteria utilise the presence of hyperbilirubinaemia, ascites, hepatomegaly and weight gain as the primary basis for diagnosis [7, 19]. Use of VOD/SOS diagnostic and severity grading criteria in the studies in this meta-analysis is reasonable, given the time at which the studies were conducted. As diagnostic and grading criteria have evolved to include more sensitive measures of disease, the observed incidence of VOD/SOS has increased; for instance, an up to four-fold increase in the incidence of VOD/SOS was seen with the transition from Baltimore to modified Seattle criteria [20]. In the more recent adult and paediatric EBMT criteria, additional factors are considered and the severity of VOD/SOS is based on multiple elements, including liver enzyme and bilirubin levels, international normalised ratio for coagulation, ascites, weight gain, renal function, encephalopathy, persistent refractory thrombocytopaenia and pulmonary function [5, 21]. As use of these more recent, sensitive VOD/SOS diagnostic criteria becomes more widespread, leading to a greater recognition of conditions such as anicteric or late-onset VOD/SOS, it is possible that the incidence of VOD/SOS post-HCT will increase [22, 23].
Our findings are consistent with previous studies that have demonstrated a benefit of defibrotide prophylaxis in patients with VOD/SOS [12,13,14]. In a randomised phase III trial of defibrotide prophylaxis in paediatric patients, VOD/SOS occurred by 30 days post-HCT in 22 (12%) of 180 patients treated with defibrotide vs 35 (20%) of 176 control patients (risk difference −7.7%, 95% CI −15.3 to −0.1; Z test for competing risk analysis p = 0.0488; log-rank test p = 0.0507) [12]. In a large retrospective study (N = 237), a multivariate analysis demonstrated that defibrotide prophylaxis had a beneficial impact on the day 100 cumulative incidence of VOD/SOS post-HCT (hazard ratio 7.5 × 10-7; 95% CI 1.8 × 10-7–3.2 × 10-6); p < 0.00001) [13]. The previous systematic review from Zhang et al. reported an overall mean incidence of VOD/SOS after HCT of 4.7% (95% CI 3.3–6.1) in patients receiving defibrotide prophylaxis vs 13.7% (95% CI 13.3–14.1; p < 0.005) in patients without defibrotide prophylaxis [14]. The relative risk of VOD/SOS was also lower with defibrotide prophylaxis among controlled trials (RR 0.47, 95% CI 0.31–0.73) [14].
Compared with the Zhang et al. analysis [14], this meta-analysis was able to include 11 more studies evaluating IV defibrotide in 1775 more patients, bringing the total number of patients included in this meta-analysis to 3005. This number is impactful when considering that VOD/SOS is a rare condition. In addition, the inclusion of more recent studies (conducted from 2012 through 2021) captures more current clinical practice. Despite these differences, the overall incidences of VOD/SOS in the defibrotide and control groups were similar between our analysis and the Zhang et al. analysis. Also similar to our analysis, the Zhang et al. study concluded that there was a lower relative risk of VOD/SOS with defibrotide prophylaxis than with controls (RR 0.47, 95% CI 0.31–0.73). This is in contrast to a 2015 Cochrane Report on prophylaxis for VOD/SOS post-HCT, in which the authors stated that there was insufficient evidence to support prophylactic defibrotide use; however, only one trial was included and the quality of evidence for those statements was low [15].
This analysis is limited by the small number of controlled peer-reviewed studies. Some of the studies included in the analysis were congress abstracts with limited detail; however, we made efforts to contact the authors and gather additional details, with variable results. Variations in the diagnosis and classification of VOD/SOS and its severity, along with different defibrotide doses and durations of treatment, may complicate the comparison of results across studies. Importantly, data from large, prospective, randomised controlled trials were included in the current analysis; however, there were a number of small analyses that were only reported as congress abstracts. Because of the rare nature of VOD/SOS and the reality that few researchers study this disease, there is a limited number of studies to assess, and many of those summarised in this report are small retrospective studies. Therefore, we did not analyse the effect of the quality of data on the meta-analysis results, and a formal bias assessment was not conducted.
5 Conclusions
This meta-analysis suggests a low incidence of VOD/SOS following IV defibrotide prophylaxis at 5%, regardless of age group (5% in adults; 8% in paediatric patients), and a lower relative risk of 0.30 for VOD/SOS with defibrotide prophylaxis vs controls in studies that included a control arm. These results support a potential benefit of defibrotide prophylaxis for the prevention of VOD/SOS in both adult and paediatric patients. An ongoing phase III study of defibrotide prophylaxis (NCT02851407) recently stopped enrolment after meeting the protocol-defined futility criteria; when the final results are available, this study will provide additional context for understanding the role of defibrotide in VOD/SOS prophylaxis. In addition, use of the most recent diagnostic and grading criteria to better identify and understand high-risk patient populations will provide more context on the utility of prophylactic therapy in these patients.
References
Fan CQ, Crawford JM. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J Clin Exp Hepatol. 2014;4:332–46.
Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16:157–68.
Richardson PG, Corbacioglu S, Ho VT, Kernan NA, Lehmann L, Maguire C, et al. Drug safety evaluation of defibrotide. Expert Opin Drug Saf. 2013;12:123–36.
Richardson PG, Carreras E, Iacobelli M, Nejadnik B. The use of defibrotide in blood and marrow transplantation. Blood Adv. 2018;2:1495–509.
Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016;51:906–12.
Soyer N, Gunduz M, Tekgunduz E, Deveci B, Ozdogu H, Sahin HH, et al. Incidence and risk factors for hepatic sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of Turkish Hematology Research and Education Group (ThREG). Transfus Apher Sci. 2020;59:102827.
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–67.
Valla DC, Cazals-Hatem D. Sinusoidal obstruction syndrome. Clin Res Hepatol Gastroenterol. 2016;40:378–85.
Defitelio (defibrotide sodium) [prescribing information]. Palo Alto, CA: Jazz Pharmaceuticals, Inc. 2016. https://pp.jazzpharma.com/pi/defitelio.en.USPI.pdf. Accessed 2 Apr 2019.
Falanga A, Vignoli A, Marchetti M, Barbui T. Defibrotide reduces procoagulant activity and increases fibrinolytic properties of endothelial cells. Leukemia. 2003;17:1636–42.
Defitelio (defibrotide sodium) [summary of product characteristics]. Villa Guardia, Italy: Genium SpA. 2018. https://www.ema.europa.eu/documents/product-information/defitelio-epar-product-information_en.pdf. Accessed 2 Apr 2019.
Corbacioglu S, Cesaro S, Faraci M, Valteau-Couanet D, Gruhn B, Rovelli A, et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomised controlled trial. Lancet. 2012;379:1301–9.
Chalandon Y, Simonetta F, Dantin C, Koutsi A, Mamez AC, Beauverd Y, et al. Defibrotide shows efficacy in the prevention of sinusoidal obstruction syndrome (SOS) after allogeneic hematopoietic stem cell transplantation: a retrospective study on 237 patients. European Society for Blood and Marrow Transplantation (EBMT); 25–29 March 2017; Marseille.
Zhang L, Wang Y, Huang H. Defibrotide for the prevention of hepatic veno-occlusive disease after hematopoietic stem cell transplantation: a systematic review. Clin Transplant. 2012;26:511–9.
Cheuk DK, Chiang AK, Ha SY, Chan GC. Interventions for prophylaxis of hepatic veno-occlusive disease in people undergoing haematopoietic stem cell transplantation. Cochrane Database Syst Rev. 2015;27:CD009311.
Jazz Pharmaceuticals. Jazz Pharmaceuticals stops enrollment in phase 3 study evaluating defibrotide for the prevention of veno-occlusive disease [press release]. 2020. https://www.prnewswire.com/news-releases/jazz-pharmaceuticals-stops-enrollment-in-phase-3-study-evaluating-defibrotide-for-the-prevention-of-veno-occlusive-disease-301049642.html. Accessed 9 Oct 2020.
Corbacioglu S, Jabbour EJ, Mohty M. Risk factors for development of and progression of hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Biol Blood Marrow Transplant. 2019;25:1271–80.
Picod A, Bonnin A, Battipaglia G, Giannotti F, Ruggeri A, Brissot E, et al. Defibrotide for sinusoidal obstruction syndrome/veno-occlusive disease prophylaxis in high-risk adult patients: a single-center experience study. Biol Blood Marrow Transplant. 2018;24:1471–5.
Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–83.
Corbacioglu S, Carreras E, Ansari M, Balduzzi A, Cesaro S, Dalle JH, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2018;53:138–45.
Mahadeo KM, Bajwa R, Abdel-Azim H, Lehmann LE, Duncan C, Zantek N, et al. Diagnosis, grading, and treatment recommendations for children, adolescents, and young adults with sinusoidal obstructive syndrome: an international expert position statement. Lancet Haematol. 2020;7:e61–72.
Ragoonanan D, Khazal SJ, Wang J, Payne A, Kohorst M, Harden A, et al. Improved detection of sinusoidal obstructive syndrome using pediatric-AYA diagnostic criteria and severity grading. Bone Marrow Transplant. 2021;56:175–84.
Szmit Z, Gorczynska E, Mielcarek-Siedziuk M, Ussowicz M, Owoc-Lempach J, Kalwak K. Veno-occlusive disease in children and adolescents after hematopoietic stem cell transplantation: did the modified Seattle criteria fit the characteristics of pediatric population? Adv Clin Exp Med. 2020;29:339–44.
Antmen B, Sasmaz I, Karagun B, Erkman H, Serbest M, Turksoy D. B317-Defibrotide prophylaxis and treatment of hepatic veno-occlusive disease after pediatric allogeneic hematopoietic stem cell transplantation. Presented at: 45th Annual Meeting of the European Society for Blood and Marrow Transplantation; 24–27 March 2019; Frankfurt.
Bonini A, Imovilli A, Ghirarduzzi A, Silingardi M, Pilia A, Gugliotta L, et al. Defibrotide low-dose continuous infusion after allogeneic stem cell transplantation as prophylaxis for veno-occlusive disease of the liver. Blood. 2010;116:3483.
Bonnin A, Devaux C, Baylatry MT, Vekhoff A, Ruggeri A, Lapusan S, et al. PT014: Prophylaxis of sinusoidal obstruction syndrome after allogeneic stem cell transplantation in high-risk adult patients. Int J Clin Pharm. 2016;38:573–4.
Calore E, Rossin S, Pillon M, Tumino M, Mainardi C, Toffolutti T, et al. Veno occlusive disease of the liver after hematopoietic stem cell transplantation (HSCT) in pediatric patients: Padova experience. Bone Marrow Transplant. 2015;50:S212.
Cappelli B, Chiesa R, Evangelio C, Biffi A, Roccia T, Frugnoli I, et al. Absence of VOD in paediatric thalassaemic HSCT recipients using defibrotide prophylaxis and intravenous busulphan. Br J Haematol. 2009;147:554–60.
Corbacioglu S, Honig M, Lahr G, Stohr S, Berry G, Friedrich W, et al. Stem cell transplantation in children with infantile osteopetrosis is associated with a high incidence of VOD, which could be prevented with defibrotide. Bone Marrow Transplant. 2006;38:547–53.
Dignan F, Gujral D, Ethell M, Evans S, Treleaven J, Morgan G, et al. Prophylactic defibrotide in allogeneic stem cell transplantation: minimal morbidity and zero mortality from veno-occlusive disease. Bone Marrow Transplant. 2007;40:79–82.
Giglio F, Xue E, Lazzari L, Greco R, Clerici TD, Marktel S, et al. P170: Prophylaxis with defibrotide in adults at very high risk of veno-occlusive disease: results in 11 patients. Presented at: 45th Annual Meeting of the European Society for Blood and Marrow Transplantation; 24–27 March 2019; Frankfurt.
Gray JC, Marshall L, Qureshi A, Ridwan R, Sankpal S, Ethell M, et al. Defibrotide as prophylaxis for veno-occlusive disease in children undergoing allogenic and autologous stem cell transplantation. Bone Marrow Transplant. 2008:S298–9.
Hasenkamp J, Conradi I, Wulf G, Jung W, Truemper L, Glass B. Prevention of veno-occlusive disease in hematopoietic stem cell transplantation due to defibrotide prophylaxis. Blood. 2004;104:1139.
Joshi R, Kerridge I, Grace S, et al. Prophylactic defibrotide for the prevention of hepatic veno-occlusive disease (VOD) in hematopoietic stem cell transplantation. Blood. 2002;2002:413A.
Kikuta A, Fukuda T, Ohashi K, Taniguchi S, Asano-Mori Y, Horibe K, et al. Defibrotide for treatment of hepatic veno-occlusive disease following hematopoietic stem cell transplantation: results from the Japanese prophylactic use, phase II, randomized trial. Presented at: 44th Annual Meeting of the EBMT; 18–21 March 2018; Lisbon.
Milone G, Poidomani M, Coppoletta S, Mauro E, Marturano E, Crispi F, et al. Defibrotide in prevention of liver toxicity in patients at high risk of VOD after HSC transplantation. Blood. 2008;112:3275.
Milone G, Spina P, Berritta D, Parisi M, Leotta S, Cupri A, et al. Defibrotide as phophylaxis of liver toxicity during allogeneic HSC transplantation in acute leukemia patients. Haematologica. 2014;99(Suppl. 1):428.
Mohty M, Bourhis JH, Faraci M, et al. Defibrotide prophylaxis for veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) after haematopoietic cell transplantation (HCT): analysis of a multicentre, multinational, prospective, observational registry study (EBMT PASS). Presented at: 47th Annual Meeting of the EBMT; 14–17 March 2021; virtual.
Pasqualini C, Cros G, Dufour C, Abbou S, Dourthe M-E, Rigaud C, et al. Defibrotide prophylaxis of veno-occlusive disease in children with high-risk neuroblastoma treated with busulfan-melphalan high-dose chemotherapy and autologous stem cell transplantation. Bone Marrow Transplant. 2016;51:S260.
Qureshi A, Marshall L, Lancaster D. Defibrotide in the prevention and treatment of veno-occlusive disease in autologous and allogeneic stem cell transplantation in children. Pediatr Blood Cancer. 2008;50:831–2.
Roh YY, Hahn SM, Kim HS, Ahn WK, Han JH, Kwon S, et al. Efficacy of low dose and short duration defibrotide prophylaxis for hepatic veno-occlusive disease after autologous haematopoietic stem cell transplantation. Bone Marrow Transplant. 2020;56:411–8.
Tekgunduz E, Akpinar S, Bozdag SC, Tetik A, Kocubaba S, Cinarsoy M, et al. Effectiveness of defibrotide in the prevention of VOD among patients receiving allogeneic hematopoetic cell transplantation: a retrospective single center experience. Blood. 2012;120:4508.
Wass EN, Lovejoy B, Glover N, Zhao Q, Sosa W, Chavan R. Prevention of veno-occlusive disease (VOD) with defibrotide in high-risk pediatric hematopoietic stem cell transplant patients. Biol Blood Marrow Transplant. 2018;24:S289.
Acknowledgements
Medical writing and editorial assistance were provided by Nancy Tang, PharmD, of Cello Health Communications/SciFluent Communications, Inc., and were financially supported by Jazz Pharmaceuticals.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding for this study was provided by Jazz Pharmaceuticals.
Conflicts of Interest
SC has consulted for and received honoraria from Gentium/Jazz Pharmaceuticals. OT and SA have consulted for Jazz Pharmaceuticals.
Ethics approval
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
All of the data in this systematic review were taken from the published literature.
Code Availability
Not applicable.
Authors’ Contributions
SC, OT and SA designed the meta-analysis. OT and SA performed the meta-analysis and analysed and verified the data. SC, OT and SA were involved in the interpretation of the data, and provided critical input, review and approval to submit the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Corbacioglu, S., Topaloglu, O. & Aggarwal, S. A Systematic Review and Meta-Analysis of Studies of Defibrotide Prophylaxis for Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome. Clin Drug Investig 42, 465–476 (2022). https://doi.org/10.1007/s40261-022-01140-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01140-y