Abstract
This article examines the regulatory function of the skeletal muscle, renal, and adrenergic systems in potassium homeostasis. The pathophysiologic bases of hypokalemia, systematic approach for an early diagnosis, and therapeutic strategy to avert life-threatening complications are highlighted. By promoting skeletal muscle uptake, intense physical exercise (post), severe trauma, and several toxins produce profound hypokalemia. Hypovolemia due to renal and extra-renal fluid losses and ineffective circulation activate secondary aldosteronism causing urinary potassium wasting. In addition to hypokalemic alkalosis, primary aldosteronism causes low-renin hypertension. Non-aldosterone mineralocorticoid activation leading to low-renin and low-aldosterone hypertension occurs in Liddle’s syndrome and apparent mineralocorticoid excess. Although there is enzymatic inhibition of cortisol synthesis in congenital adrenal hyperplasia, precursors of aldosterone produce low-renin hypokalemic hypertension. In addition to the glucocorticoid effect, hypercortisolism activates mineralocorticoid receptors in Cushing’s syndrome. Genetic mutations involving furosemide-sensitive Na+-K+-2Cl− co-transporters and thiazide-sensitive Na+-Cl− transporters result in (non-hypertensive) salt-wasting nephropathy. Proximal and distal renal tubular acidosis is associated with hypokalemia. Eating disorders causing hypokalemia include bulimia, laxative abuse, and diuretic misuse. Low urinary potassium (<15 mmol/day) and/or low urinary chloride (<20 mol/L) suggest a gastrointestinal pathology. Co-morbidity of hypokalemia with chronic pulmonary and cardiovascular diseases may increase the fatality rate.
Similar content being viewed by others
Introduction
Adequate distribution of a potassium cation (K+) in the body fluids is essential to maintain the physiologic function of all the human systems. Hypokalemia, defined as a plasma potassium concentration below 3.5 mmol/L, is a relatively common electrolyte disorder in children and adults [1•]. Apart from the direct impact of primary disease, physiologic responses to illness such as profuse sweating, diarrhea, and vomiting produce hypokalemia [2, 3]. In addition, common therapeutic interventions such as diuretic agents are frequent sources of a potassium deficit [4]. Consequently, hypokalemia occurs in about 20% of the hospitalized patients and accounts for a two-fold higher mortality rate [1•, 4]. In this review, we shall examine the potassium physiology based on the contribution from the gastrointestinal, musculoskeletal, endocrine, cardiovascular, and renal systems. We shall explore the etiologies of hypokalemia and examine the influence of potassium deficit on the clinical outcome of selected diseases. We have chosen this approach because the initial presentation of hypokalemia seldom occurs in isolation but is often appreciated in the context of a given pathology. Finally, a pragmatic approach to diagnosis if the initial presentation is hypokalemia and the required therapeutic strategy will be addressed.
Materials and Methods
We reviewed the relevant literature by conducting a PubMed search using the terms hypokalemia, potassium deficiency, and electrolyte disorders. Only the articles published in the English language were included. We retrieved available original articles, clinical trials, meta-analyses, case reports, and clinical case series on hypokalemia-related disorders.
Fundamentals of Potassium Homeostasis
The critical role of potassium in the human body is exemplified by its involvement in the Na+-K+-ATPase, an essential electrogenic enzyme that facilitates transcellular ion transport [5, 6]. Potassium, the most abundant intracellular cations (150 mmol/L), must be maintained at a precise concentration (within and out of the cells) to generate electrical gradient for basic physiologic function [1•, 5]. It is a co-factor to many essential enzymatic processes, and it maintains cellular integrity by preserving osmotic equilibrium [7, 8].
Skeletal Muscle and Potassium Homeostasis
Although long-term control of plasma potassium depends on the renal capacity for excretion, skeletal muscles are needed for a minute-to-minute regulation [9]. Skeletal muscles are the largest single repository of potassium in the body with a total content of 2600 mmol [200-fold of serum K+] [10]. In theory, activation of its numerous potassium channels can clear extracellular fluid (ECF) of its content within 25 s [10]. Primarily regulated by Na+-K+-ATPase, insulin, catecholamines, hyperkalemia, and alkalosis stimulate myocyte potassium uptake, while hypokalemia, hypertonicity, physical exercise, and acidemia maintain the extracellular content [1•, 5, 6, 11].
Hypokalemia Due to Intracellular Potassium Shift: Skeletal Muscle
Alteration in the physiochemical environment of skeletal muscles affects the total body potassium distribution. The occurrence of hypokalemia in these instances is frequently associated with predictable morbidity (Table 1).
Physical Exercise
Altered rate of skeletal muscle depolarization during an intense physical exercise doubles the arterial plasma potassium content in 1 min (40 mmol/min) [12]. To prevent the harmful effect of hyperkalemia, there is rapid diffusion of potassium into the dilated surrounding capillaries. Stress stimulation of adrenergic drive causes a massive myocyte re-uptake by upregulation of Na+/K+ pumps to produce a transient (post-exercise) hypokalemia [6, 10]. The rapid turnover of the plasma content of potassium has been implicated in the development of cardiac arrhythmia (and sudden death) during and after intense physical exercise [13]. A regular physical exercise program before a vigorous activity upregulates the Na+/K+ pump on skeletal myocytes and thereby prevents potentially harmful hyperkalemia [13]. In addition, the use of a β2-adrenergic agonist during intense physical activity minimizes hyperkalemia and prevents the development of a rebound (severe) hypokalemia [14•, 15].
Beta-Adrenergic Agonists
There is a dose-dependent development of hypokalemia that results from the therapeutic use of β2-adrenergic agonists [16]. The molecular basis for cellular uptake of potassium by the skeletal muscles is similar in all instances and will hereby be reviewed (Figure 1). The β2-adrenoceptor stimulates Gs protein to activate adenylyl cyclase, which in turn converts ATP to the cyclic AMP [15]. Cyclic AMP-dependent protein kinases phosphorylate phospholemman, an inhibitory regulatory protein of the Na+/K+-ATPase. There is a parallel activation of the mitogen-activated protein kinases which in turn stimulates Na+-K+-2Cl− co-transporters [15]. The inward movement of potassium depletes the ECF of its content to produce hypokalemia. Used in the treatment of asthma, a prevalent disease in most countries, β2-adrenergic agonists are commonly available in many households. A conventional dose of these agents could drop serum potassium by 0.4 to 0.6 mmol/L and has been associated with prolonged QT interval, ventricular arrhythmia, and sudden deaths [17, 18]. In addition, its liquid formulations, with lower efficacy but greater toxicity, are common sources of unintentional poisoning in children [19]. Due to a poor safety profile, clenbuterol, a long-acting β2-adrenergic agonist, is not approved for human use in many countries. Clenbuterol increases energy expenditure and promotes fatty acid oxidation, and it is therefore illegally used to enhance skeletal muscle mass by athletes [20•]. Occurrence of atrial fibrillation from profound hypokalemia has been reported in clenbuterol overdose [21].
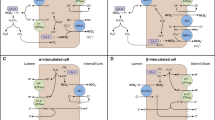
Cellular uptake of potassium due to adrenergic upregulation of Na+ -K+ pump on the skeletal muscle cell. Adrenaline or β-adrenergic agonists activate adrenoceptors on the skeletal muscle sarcolemma. The receptor is coupled to the stimulatory G-proteins, which in turn activate the adenylate cyclase enzyme (1). The enzyme converts adenosine triphosphate (ATP) to adenosine 3′,5′-cyclic monophosphate (3,5-cAMP). 3′,5′-cAMP can be degraded by phosphodiesterase (2). The activity of the c-AMP induces cAMP-dependent protein kinases (PKA) and mitogen-activated protein kinase (MAPK) pathways (3). PKA phosphorylates ryanodine receptors (RYR1) (4), transmembrane voltage-activated Ca2+ channels (5), and phospholamban (6). Both RYR1 and L-type Ca2+ channels release Ca2+ into the cytosol. Phosphorylation PLB reduces its binding to the sarcoendoplasmic reticulum (SR) Ca2+-ATPase, SERCA, and thereby decreases its Ca2+ transportation into the SR (6). Consequently, high cytosolic Ca2+ enhances myofiber contraction (7). PKA phosphorylates phospholamban, an inhibitory regulatory protein for Na+ /K+-ATPase, causing an increase in the exchange of Na+ for K+ by enhancing its affinity for both cations (8). Parallel activation of MAP kinase enhances the activity of sodium-potassium dichloride (NKCC2) cotransporter [Na-K-2Cl co-TX] (9). Exaggerated cellular uptake by the vast number of potassium-related channels on the skeletal muscles produces hypokalemia
Severe Trauma
Hypokalemia occurs in about 34.5% of patients with various degrees of trauma [22]. There is a positive correlation between the severity of trauma and the degree of hypokalemia [22]. An adaptive stimulation of catecholamine produces an elevation in plasma glucose, glucagon, and insulin [22, 23••]. Both insulin and catecholamines profoundly increase potassium cellular uptake [23••]. Consequently, a higher ratio of serum glucose and (plasma) potassium is predictive of greater mortality in patients with intracerebral hemorrhage [23••]. The control of intracranial hypertension (from trauma) with a pentobarbital-induced coma is frequently complicated by refractory hypokalemia [24]. This is due to an inhibition of the outward current of the voltage-dependent potassium channels on the skeletal muscles [25••]. An overzealous correction of hypokalemia frequently produces rebound hyperkalemia [24].
Diabetic Ketoacidosis (DKA)
Rarely, severe hypokalemia may complicate DKA with a potential for a fatal outcome [26, 27•]. At initial presentation, net potassium depletion may be underestimated due to extracellular displacement by insulin deficiency, hyperglycemia, and metabolic acidosis [28]. In addition to the potassium wasting from osmotic diuresis, the resultant hypovolemia stimulates secondary aldosteronism. Furthermore, insulin treatment with the resolution of hyperglycemia aggravates serum potassium depletion by increasing the cellular uptake. To forestall against severe hypokalemia, serum potassium (<3.3 mmol/L) often requires replenishment before the institution of insulin treatment [27•].
Drugs and Toxins
Overdose of certain therapeutic agents and poisonous substances may inadvertently produce severe hypokalemia that results in cardiac arrhythmia and death. Whereas β-adrenergic agonists, theophylline, and caffeine increase endogenous catecholamines, cationic barbiturates, barium chloride, and chloroquine impair potassium release by sarcolemma [29,30,31,32,33,34, 35•]. Hypokalemia may result with the therapeutic use of insulin or following an overdose in a suicidal attempt [31]. Apart from a direct insulin effect, hypoglycemia potentiates cellular potassium uptake by stimulating catecholamine release. A prolonged QT interval observed in this clinical setting may account for the observation of sudden (overnight) deaths in many diabetic patients [32]. Overdose of barium chloride in a suicidal attempt may produce severe hypokalemia and a fatal ventricular arrhythmia [33, 34]. There are also reports of incidental hypokalemia following widespread food poisoning due to industrial barium contamination of table salt [33]. In addition to the hypokalemia effect, chloroquine potentiates cardiac arrhythmia by inhibition of the atrioventricular conduction [35•]. Penicillin causes a dose-dependent adverse effect of hypokalemia [36••]. A non-absorbable anion, penicillin, promotes a potassium exchange for Na+ in the distal convoluted tubule (DCT) [36••].
Hypokalemic Periodic Paralysis (HPP)
HPP is a rare autosomal recessive (AR) genetic disorder (0.1 per 100,000) that is due to the genes encoding either voltage-gated Na+ or L-type Ca2+ channels of the skeletal muscle membrane [37]. There is a recurrent attack of a self-limiting (mostly) lower limb paralysis that results from an intracellular shift of potassium in response to a carbohydrate-rich diet or physical exercise [37]. The postulated mechanism of action is interference in the generation of an action potential by an aberrant gating pore current [37]. Thyrotoxic HPP is also rare but disproportionately occurs with greater frequency in men of Asian and Latin American ancestry. Close to 30% of those affected will have a mutation of the gene for inward rectifying Kir2.6 potassium channel [38].
Hypokalemic Alkalosis from Renal Losses: Aldosteronism
Secondary Aldosteronism
Stimulation of aldosterone is a major physiologic mechanism to defend against hypovolemia. A lower glomerular filtration rate (GFR) causes proximal renal sodium reabsorption [39••, 40]. A lower delivery of sodium and chloride to the macula densa enhances plasma renin, angiotensin II, and aldosterone secretions. Aldosterone activates the epithelial sodium channel (ENaC) on the DCT, thereby restoring the blood volume (Table 1 and Algorithm) [39••, 40]. To neutralize the generated negative luminal potential difference (PD), there is a preferential secretion of tubular hydrogen ions (H+) and a minimal K+ release [41]. Angiotensin-II stimulates basolateral Kir4.1, which in turn activates sodium chloride co-transporter (NCCT) and therefore reduces Na+ exchange for potassium secretion in the late DCT [41]. Paradoxically, the absence of angiotensin-II in primary aldosteronism aggravates potassium depletion [41].
Primary Aldosteronism
Primary aldosteronism (PA) is an autonomous secretion of aldosterone that is independent of renin, angiotensin II, sodium, and volume status [42]. Although many patients present as intractable or severe low-renin hypertension, personal experience suggests early diagnosis may be facilitated by a prompt evaluation of individuals exhibiting low-normal hypokalemia (and hypertension) [42]. Apart from injury of hypertension, there is direct end-organ damage by a persistent aldosterone receptor activation [43]. Surgical adrenalectomy is the treatment of choice for unilateral disease, while bilateral hyperplasia required lifelong mineralocorticoid inhibition [42].
Non-Aldosterone Mineralocorticoid Activities
Unlike PA, Liddle’s syndrome, licorice ingestion, and apparent mineralocorticoid excess (AME) cause hypertension and hypokalemic alkalosis but both plasma renin and serum aldosterone levels are suppressed [39••, 44••, 45]. Liddle’s syndrome is due to an autosomal dominant (AD) gain-in-function mutation of ENaC in the DCT. Although some patients respond to amiloride, intractable hypertension, accelerated kidney disease, and premature death from cerebrovascular accidents have been described [46]. With close to two decades of pediatric nephrology practice, I have encountered only one patient, a teenager. A presentation with a protracted course of severe resistant hypertension justified renal transplantation (despite a normal GFR) with a good clinical outcome [44••, 46]. Licorice contains glycyrrhizinic acid (occurs in herbal tea) which inhibits the enzymatic conversion of abundant cortisol to the inactive cortisone by 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) [39••, 45, 47]. Unlike the physiologic concentration, an excessive amount of cortisol can activate aldosterone receptors in the DCT [45]. Furthermore, there is a recent recognition of the inhibitory effects of antifungal posaconazole and itraconazole on 11β-HSD2 [48]. There is an AR mutation in the gene producing 11β-HSD2 in the syndrome of AME [44••, 49, 50••]. Prompt recognition and treatment may prevent fatal outcomes in early infancy [44••, 50••]. As in Liddle’s syndrome, hypertension may respond to amiloride; and renal transplantation is curative.
Congenital Adrenal Hyperplasia
The gene mutations of both 11β-hydroxylase (CYP11B1) and 17-alpha hydroxylase (CYP17A1) present as precocious puberty, hirsutism, hypokalemia, and hypertension in older children and teenagers [44••]. There is an enzymatic block of cortisol synthesis and a build-up of precursors of androgens and aldosterone [44••]. Treatment consists of glucocorticoid inhibition of ACTH stimulation of steroid synthesis.
Cushing’s Syndrome (CS)
Excessive adrenocorticotrophic hormone (ACTH) stimulation of adrenal steroids in pituitary adenoma and from a carcinoid tumor (in ectopic ACTH) may produce refractory hypokalemia and hypertension [51•, 52]. Rarely, a fatal cardiac arrhythmia may complicate such severe hypokalemia [53]. There is a recent description of a rare mutation of the gene [NR3C1] for glucocorticoid receptor in patients with similar hormonal profiles but who are lacking the physical features of CS [54]. Due to the small size, delayed localization of the tumor in ectopic CS increases fatality. The recent availability of [68Ga]-DOTATATE positron-emitting tomography which targets somatostatin receptors on neuroendocrine tumors may facilitate early diagnosis [55••].
Hypokalemic Alkalosis Due to Renal Loss (Without Hypertension)
A review of the renal control of potassium balance will be followed by the description of its dysregulation in the proximal tubule (PT), the thick ascending loop of Henle (TALH), and the DCT, respectively.
Potassium Control by the Proximal Tubules
Most of the potassium in the glomerular filtrates is passively reabsorbed across the paracellular pathway by solvent drag in the PT [56]. Its basolateral uptake by Na+-K+-ATPase pump is recycled by an outward basolateral potassium channel [56]. Potassium balance plays a crucial role in the regulation of acid-base homeostasis. During metabolic acidosis, there is an exchange of K+ for the extracellular H+, thereby providing a basis for the PT cell to generate NH3, and thereafter causes urinary excretion of net ammonium acids [57•]. Close to 100% of the filtered potassium is reabsorbed by PT and the TALH [56].
Potassium Physiology in the TALH
Apical transcellular co-transportation of sodium, potassium, and chloride ions (NKCC2) by the TALH is maintained by an apical potassium recycling [39••, 58]. The positive luminal PD created by the recycling enhances the paracellular transport of K+, Ca2+, and Mg2+ into the blood [39••, 58]. In addition, basolateral potassium uptake by the Na+/K+-ATPase is recycled by both the Kir4.1 channel and a K-Cl co-transporter. Basolateral chloride channels on the TALH and DCT, CIC-ka and CIC-kb, are also required to maintain a transepithelial equilibrium [39••, 58].
Bartter and Bartter-Like Syndrome
Type I BS results from a mutation of the gene that encodes NKCC2, while type II BS is due to mutation of the gene that produces ROMK channel [39••, 58, 59]. Types I and II are the most severe forms of BS and are characterized by polyhydramnios, newborn hypotension, and hypokalemic alkalosis [39••, 58]. Hypovolemia and delivery of unabsorbed sodium to the DCT provoke a secondary aldosteronism in all variants of BS [39••]. Type III BS is caused by mutations of the gene that produces the ClC-kb (chloride) channel, while type IV BS may be due to either a mutation in the BSND gene encoding barttin, a subunit of both the CLCKa and CLCKb channels, or a combined mutation in both the CLCNKB and CLCNKA genes [39••, 58]. Type III BS is less severe and manifests in older children, while type IV may present in infancy [58]. A gain-of-function mutation of a gene that encodes basolateral calcium-sensing receptor, CaSR, inactivates luminal NKCC2 on the TALH to produce type V BS [39••, 58]. Similarly, cationic molecules of aminoglycoside produce acquired BS by increasing the sensitivity of the CaSR (Figures 1 and 2) [39••, 58].
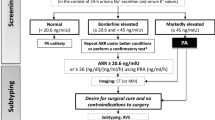
Approach to the diagnosis of hypokalemia using urinary potassium excretion as the initial parameter. (1) The first step in the evaluation of unexplained hypokalemia is to exclude pseudohypokalemia: traumatic venipuncture, cold ambient temperature, delayed laboratory processing, thrombocytosis, leukocytosis, and ethylenediamine tetra-acetic acid (EDTA) tubes; (2) urinary K:Cr ratio < 1.5 suggests nutritional deficiency, extra-renal losses, or intracellular shift. Causes of an intracellular shift are drugs, stress (catecholamines), periodic paralysis, and refeeding. Stool K is high (80–90 mmol/L) in diarrhea. Fecal chloride is elevated in congenital chloride diarrhea (>90 mmol/L); (3) if there is high renal K excretion and a normal anion gap acidosis: a diagnosis of RTA. A positive urine anion gap (Na + K-Cl) in distal RTA indicates a low NH4Cl excretion. A negative value occurs in proximal RTA (adequate urine NH4+). (4) High urine K loss and alkalosis suggest diuretic abuse/Bartter syndrome (urine Cl > 20 mmol/L) or gastric effluent (low urine Cl < 10 mmol/L). (5) If elevated urine K and normal or low blood pressure, associated high plasma renin and aldosterone levels suggest secondary aldosteronism. If there is hypertension, renovascular etiology and renin secreting tumor are more likely. (6) A low plasma renin and high serum aldosterone levels suggest primary aldosteronism and glucocorticoid-remediable aldosteronism. (7) A non-aldosterone mineralocorticoid activity produces low serum renin and low serum aldosterone levels; it occurs in Cushing’s syndrome (with high serum cortisol), Liddle’s syndrome (normal serum cortisol), and AME (with high serum cortisol). (8) Low plasma renin and low serum cortisol but elevated corticosterone and androgens are seen in 11β- or 17αhydroxylase deficiency. AME = apparent mineralocorticoid excess; BP = blood pressure; Cl = chloride; Cr = creatinine; EAST syndrome = epilepsy, ataxia, sensorineural deafness and tubulopathy; HELIX syndrome (hypohidrosis, electrolyte disturbances, hypolacrimia, ichthyosis, xerostomia) occurs in claudin 10b gene mutation in the tight unction of thick ascending loop of Henle; K= potassium; High PRA = High plasma renin activity > 4.3 ng/h; Low PRA = low plasma renin activity < 0.6 ng/h; PAC = plasma aldosterone concentration > 15 ng/dL; Low PAC = low plasma aldosterone concentration < 5 ng/dL; RTA = renal tubular acidosis
Potassium Physiology in the DCT
Renal secretion of potassium predominantly involves the DCT and the cortical collecting duct (CCD). The early section of the principal cell (DCT1) has apical NCCT, and the later part has ENaC (DCT2) [39••, 60]. Type A intercalated cells (IC) on the CCD has a luminal proton pump and H+-K+-ATPase, and type B IC has apical Cl−/HC03− exchanger (Pendrin) [39••, 57•, 62]. Sodium uptake by ENaC generates a lumen-negative PD, which in turn increases a basal amount of K+ recycle by the apical ROMK (Kir1.1) of the DCT2 [39••, 57•]. An apical Maxi-K with a larger capacitance is recruited to handle greater demand for K+ secretion (as in hyperkalemia and/or higher tubular flow) [39••, 61]. Recent studies suggest the basolateral K channel, Kir4.1, located on the DCT may be particularly important in the K regulation [63]. A low dietary potassium increases with-no-lysine kinase (WNK) while activating ste20-proline alanine-rich kinase (SPAK). The phosphorylated SPAK upregulates the activity of the NCCT. A lower sodium delivery to DCT2 reduces the expression of ENaC, reduces K secretion, and therefore corrects the potassium deficiency [63]. CCD contributes to acid-base regulation such that negative lumen created by ENaC through Na+ absorption enhances H+ secretion by type A IC [39••, 57•, 62].
Gitelman and Gitelman-Like Syndrome
(a) GS is due to AR mutations of the gene that encodes NCCT [39••, 58]. It is often milder than BS and predominantly occurs in older children and young adults [39••, 58]. A primary event may be an incidental laboratory discovery of hypokalemic alkalosis [39••]. An abundance of transcellular Ca2+ transport via TRPV5 produced the universal finding of hypocalciuria [39••]. Recently described AR rare variants of GS, both with peculiar physical features are HELIX and EAST syndromes [39••, 62,64,65]. The former is due to impaired paracellular Na+ absorption by TALH because of claudin 10b gene mutation, and the latter is due to a disorder of the Kir4.1 potassium channel on the DCT. Autoantibody formation in Sjögren’s syndrome may inactivate NCCT to produce an acquired form of GS [39••, 66]. In addition to oral supplementation, the use of indomethacin and amiloride may minimize renal electrolyte losses. I often avoided gastric bleeding complications by limiting the duration of indomethacin treatment to less than 5 years.
Diuretics, Hypomagnesemia, and Hypokalemia
In a retrospective study of over 58,000 in-hospital patients, drugs (mostly diuretics) accounted for 56% of hypokalemia [67]. Potassium loss is dose-dependent and may be minimized by sodium restriction. Diuretic aggravation of volume contraction in cardiac insufficiency propagates secondary aldosteronism and K+ loss. In addition to diuretic magnesium (Mg2+) wasting, a dietary deficiency frequently potentiates hypomagnesemia [68]. Mg2+ deficiency causes urinary K+ wasting by stimulating ROMK. Adequate repletion of potassium is not feasible in the presence of Mg2+ deficiency [68].
Hypokalemia in Renal Tubular Acidosis (RTA)
RTA is defined as a non-anion gap hyperchloremic metabolic acidosis that occurs in the presence of a normal or modestly reduced kidney function [57•]. Type I and type II RTA are frequently associated with hypokalemia. (i) Distal RTA (d-RTA) is due to a defect in H+ secretion (and therefore NH4+) by the IC of the DCT [57•]. Genetic mutations in children may lead to a deficiency of basolateral Cl−-HC03− exchanger, apical ATPase H+, and apical ATPase H+ pump on the DCT [57•]. Acquired form occurs in an adult due to medications (e.g., amphotericin B) and autoimmunity (Sjögren’s syndrome) [57•, 66]. Hypokalemia occurs partly because of K+ renal wasting from a decreased activity of the H+/K+-ATPase of the IC and in response to a secondary aldosteronism [57•, 69]. (ii) Proximal RTA is due to a reduction in the threshold for absorption of filtered HCO3− by the PT (in exchange for H+) which is normally set at about 25 mmol/L [57•]. This leads to the delivery of a larger amount of HCO3− to the DCT beyond the capacity for its reabsorption [57•]. A mutation of the gene for the Na+-HC03− exchanger produces isolated p-RTA. Delivery of Na+ load at DCT activates secondary aldosteronism and K+ wasting [57•].
Gastrointestinal Disorders and Hypokalemia
An average adult consumes a dietary potassium content of 80 mmol/day [70]. Gastrointestinal tracts (GI) absorb 75 mmol and close to 5 mmol is excreted in the feces. Although the exact mechanism is unclear, the extra-ordinary renal capacity to excrete the exact amount of daily GI absorption may be partly mediated by signal transduction from a sensor of dietary change (before there is a hormonal response) located in the splanchnic vascular bed [56, 71].
Nutritional Deficiency
Nutritional deficiency is unlikely to cause clinically significant hypokalemia. With a stool K+ content of 20–50 mmol/L, diarrhea is the commonest cause of hypokalemia in children. The principal driver of fatality from severe acute malnutrition is a co-morbidity with diarrhea potassium loss [72•]. Although there is a minimal K+ loss from gastric content (5–10 mmol/L) in emesis, a resultant alkalosis and secondary aldosteronism commonly produce hypokalemia [39••]. Nutritional rehabilitation after the anabolic phase of severe malnutrition may produce a potentially fatal refeeding syndrome [73]. The renewed supply of glucose causes hyperglycemia and exaggerated insulin response. Death from severe hypokalemia may result from respiratory muscle weakness and ventricular arrhythmia [74].
Eating Disorders
ED, a common disease in adolescents and young adults, occurs in 3 different forms including surreptitious vomiting, laxative abuse, and diuretic misuse [39••]. Life-threatening electrolyte changes are the major reasons for hospitalization [39••]. Instead of metabolic acidosis in acute diarrhea, laxative abuse causes a chronic volume loss which activates secondary aldosteronism [39••, 75]. Diuretic abuse with urinary losses of potassium, chloride, and sodium ions may mimic GS (see Figure 2). Toxicologic analysis of the urine for the offending diuretic agents may help confirm the diagnosis [75].
Other Gastrointestinal Potassium Losses
Secondary aldosteronism with kaliuresis aggravates gastric losses in pyloric stenosis [76]. Metabolic alkalosis in congenital chloride diarrhea causes potassium wasting [77]. Upregulation of colonic maxi-K occurs in secretory diarrhea of Ogilvie’s syndrome [78]. A vasoactive intestinal polypeptide (or gastrin) induces diarrheal potassium losses in neuroendocrine tumors [79, 80]. Finally, hypochloremic alkalosis produces hypokalemia following sweat chloride losses in cystic fibrosis [2, 81].
Hypokalemia in Cardiovascular Disorders (CVD)
Stress-induced activation of the sympathetic drive produces hypokalemia in congestive heart failure (CHF). A co-morbidity of hypokalemia and CVD is associated with a greater risk of ventricular fibrillation and sudden death [82•, 83]. The prevalence of hypokalemia in CHF ranges from 20 to 54%; the wide variation depends on the choice of therapeutic intervention [84, 85]. Treatment with insulin and diuretics lowers the prevalence but there is a greater incidence with the use of beta-blockers [84]. Diuretic use enhances volume contraction and secondary aldosteronism in CHF. Takotsubo cardiomyopathy, a recently described entity, manifests as an idiopathic coronary event and left ventricular hypertrophy [86]. An excessive catecholamine may account for the common finding of hypokalemia [86].
Electrophysiology of Hypokalemia
Hypokalemia produces a more negative resting membrane potential, and, in electrical diastole, it reduces excitability by increasing the threshold for the generation of the action potential [87, 88]. Internalization of the potassium channel IKr and downregulation of the IKs expression reduce the phase 3 outward K+ current with a prolongation of repolarization [87]. The development of after-depolarizations produces ventricular arrhythmias [87, 88]. The prolonged repolarization causes a reduction in the amplitude of the T-waves on the electrocardiogram [87]. There is also a prominent U-wave and depression of the ST segment. There is a fusion of T- and U-waves in severe hypokalemia. The reduced electrical conduction causes a longer duration of QRS, atrioventricular block, peaked p-wave, and a prolonged P–R interval [87].
COVID-19 and Hypokalemia
COVI-19 infection, originating from China in early 2020, caused a worldwide pandemic with an extraordinary case fatality rate. Hypokalemia, a common finding in COVID-19 infection, is predictive of critical illness and manifests EKG indices that are supportive of greater susceptibility to ventricular arrhythmia [89, 90].
The Clinical Approach in the Diagnosis and Treatment of Hypokalemia
-
a)
Diagnosis
A systematic approach is necessary to unravel diagnosis in patients who present with initial hypokalemia. As depicted in the algorithm (Fig. 2), a determination of urinary potassium excretion differentiates renal from extra-renal losses (and intracellular shifts) [1•, 91]. However, despite enteral losses in vomiting and chronic diarrhea, urinary potassium may be elevated due to secondary aldosteronism. Elevated urinary K excretion should be stratified by acid-base status: non-anion gap acidosis suggests RTA, while alkalosis indicates salt-losing nephropathy [1•, 91]. Kaliuresis, elevated plasma renin, and serum aldosterone occur in secondary aldosteronism (no hypertension), while low serum renin with high serum aldosterone (and hypertension) supports a diagnosis of PA [42]. Low serum renin and low serum aldosterone (and hypertension) are seen in Cushing’s disease (elevated serum cortisol), Liddle’s syndrome (normal serum cortisol), and congenital adrenal hyperplasia [44••, 45,46,47,48,49, 50••, 51•, 52].
-
b)
Treatment
Hypokalemia is often a reflection of an underlying pathology that warrants early treatment. Correction of alkalosis may restore normal serum potassium. Oral supplementation is often adequate in patients with serum potassium levels between 2.5 and 3.5 mmol/L [1•, 91]. Urgent intravenous treatment is undertaken if serum K <2.5 mmol/L, and/or if associated with EKG changes. A frequent assessment of serum K during and after therapy may be necessary to avoid exceeding a target of 4–5 mmol/L [1•, 91, 92]. Mg2+ depletion must be replaced for the successful treatment of hypokalemia [93].
In summary, exposure to toxins, diuretic treatment, stress-induced catecholamines, and hypovolemic stimulation of secondary aldosteronism are major reasons for hypokalemia in hospitalized patients. Hypokalemia causes cardiac arrhythmia and is associated with a higher death rate in patients with asthma, diabetes, and cardiovascular disease. Systematic evaluation of incidental hypokalemia may unravel covert disorders including renal tubular acidosis, primary aldosteronism, and Gitelman syndrome.
References and Recommending Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Kardalas E, Paschou SA, Anagnostis P, Muscogiuri G, Siasos G, Vryonidou A. Hypokalemia: a clinical update. Endocr Connect. 2018;7(4):R135–46. https://doi.org/10.1530/EC-18-0109 A comprehensive review of the etiology, presentation, and therapeutic strategy of hypokalemia.
Cao Y, Donaldson R, Lee D. “Summer hypokalemia” as an initial presentation of cystic fibrosis in a morbidly obese African American adult: case report. BMC Nephrol. 2020;21(1):462. https://doi.org/10.1186/s12882-020-02130-y.
Ikuta S, Yasui C, Kawanaka M, Aihara T, Yoshie H, Yanagi H, et al. Watery diarrhea, hypokalemia and achlorhydria syndrome due to an adrenal pheochromocytoma. World J Gastroenterol. 2007;13(34):4649–52. https://doi.org/10.3748/wjg.v13.i34.4649.
Collins AJ, Pitt B, Reaven N, Funk S, McGaughey K, Wilson D, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46(3):213–21. https://doi.org/10.1159/000479802.
Clausen MJ, Poulsen H. Sodium/Potassium homeostasis in the cell. Met Ions Life Sci. 2013;12:41–67. https://doi.org/10.1007/978-94-007-5561-1_3.
Pirkmajer S, Chibalin AV. Na, K-ATPase regulation in skeletal muscle. Am J Physiol Endocrinol Metab. 2016;311(1):E1–31. https://doi.org/10.1152/ajpendo.00539.2015.
Ying WZ, Aaron KJ, Sanders PW. Sodium and potassium regulate endothelial phospholipase C-gamma and Bmx. Am J Physiol Ren Physiol. 2014;307(1):F58–63. https://doi.org/10.1152/ajprenal.00615.2013.
Kahle KT, Rinehart J, Lifton RP. Phosphoregulation of the Na-K-2Cl and K-Cl cotransporters by the WNK kinases. Biochim Biophys Acta. 2010;1802(12):1150–8. https://doi.org/10.1016/j.bbadis.2010.07.009.
McDonough AA, Thompson CB, Youn JH. Skeletal muscle regulates extracellular potassium. Am J Phys. 2002;282:F967–74. https://doi.org/10.1152/ajprenal.00360.2001.
Clausen T. Hormonal and pharmacological modification of plasma potassium homeostasis. Fundam Clin Pharmacol. 2010;24(5):595–605. https://doi.org/10.1111/j.1472-8206.2010.00859.x.
Aronson PS, Giebisch G. Effects of pH on potassium: new explanations for old observations. J Am Soc Nephrol. 2011;22(11):1981–9. https://doi.org/10.1681/ASN.2011040414.
Medbø JI, Sejersted OM. Plasma potassium changes with high intensity exercise. J Physiol. 1990;421:105–22. https://doi.org/10.1113/jphysiol.1990.sp017935.
Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343(19):1355–61. https://doi.org/10.1056/NEJM200011093431902.
• Atanasovska T, Smith R, Graff C, Tran CT, Melgaard J, Kanters JK, et al. Protection against severe hypokalemia but impaired cardiac repolarization after intense rowing exercise in healthy humans receiving salbutamol. J Appl Physiol (1985). 2018;125(2):624–33. https://doi.org/10.1152/japplphysiol.00680.2017 Article highlights the impact of β2-adrenergic agonist stimulation in the protection against post physical exercise hypokalemia. It validates the important role of skeletal muscle in the regulation of potassium homeostasis.
Cairns SP, Borrani F. β-Adrenergic modulation of skeletal muscle contraction: key role of excitation-contraction coupling. Rev J Physiol. 2015;593(21):4713–27. https://doi.org/10.1113/JP270909.
Scheinin M, Koulu M, Laurikainen E, Allonen H. Hypokalaemia and other non-bronchial effects of inhaled fenoterol and salbutamol: a placebo-controlled dose-response study in healthy volunteers. Br J Clin Pharmacol. 1987;24(5):645–53. https://doi.org/10.1111/j.1365-2125.1987.tb03224.x.
Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: A meta-analysis. Chest. 2004;125:2309–21. https://doi.org/10.1378/chest.125.6.2309.
Wong CS, Pavord ID, Williams J, Britton JR, Tattersfield AE. Bronchodilator, cardiovascular, and hypokalaemic effects of fenoterol, salbutamol, and terbutaline in asthma. Lancet. 1990;336:1396–9. https://doi.org/10.1016/0140-6736(90)93099-b.
Prior JG, Cochrane GM, Raper SM, Ali C, Volans GN. Self-poisoning with oral salbutamol. Br Med J (Clin Res Ed). 1981;282(6280):1932. https://doi.org/10.1136/bmj.282.6280.1932.
• Jessen S, Solheim SA, Jacobson GA, Eibye K, Bangsbo J, Nordsborg NB, Hostrup M. Beta 2 -adrenergic agonist clenbuterol increases energy expenditure and fat oxidation, and induces mTOR phosphorylation in skeletal muscle of young healthy men. Drug Test Anal. 2020;12(5):610–8. https://doi.org/10.1002/dta.2755 Article promotes awareness of the potential fatal cardiovascular toxicity from illegal use of clenbuterol by athletes and body builders. There is also a therapeutic potential to apply a less toxic version of this agent in the metabolic control of obesity.
Daubert GP, Mabasa VH, Leung VWY, Aaron C. Acute clenbuterol overdose resulting in supraventricular tachycardia and atrial fibrillation. Case Rep J Med Toxicol. 2007;3(2):56–60. https://doi.org/10.1007/BF03160909.
Beal AL, Scheltema KE, Beilman GJ, Deuser WE. Hypokalemia following trauma. Shock. 2002;18(2):107–10. https://doi.org/10.1097/00024382-200208000-00002.
•• Wu XY, Zhuang YK, Cai Y, Dong XQ, Wang KY, Du Q, et al. Serum glucose and potassium ratio as a predictive factor for prognosis of acute intracerebral hemorrhage. J Int Med Res. 2021;49(4):3000605211009689. https://doi.org/10.1177/03000605211009689 A readily available laboratory test (serum glucose-potassium ratio) may allow assessment of adverse prognosis in head injury. Assessment of the risk benefit of therapeutic intervention may improve clinical outcome.
Awad M, Bonitz J, Pratt A. Pentobarbital Induced Hypokalemia: A Worrying Sequela. Int J Surg Case Rep. 2020;71:323–6. https://doi.org/10.1016/j.ijscr.2020.05.032.
•• Aytuluk HG, Topcu H. Severe hypokalemia and rebound hyperkalemia during barbiturate coma in patients with severe traumatic brain injury. Neurocirugia (Astur: Engl Ed). 2020;31(5):216–22. https://doi.org/10.1016/j.neucir.2019.12.003 Article provides awareness of a frequent occurrence of rebound hypokalemia when using pentobarbital-induced coma to control intracranial hypertension. Pentobarbital coma should be used only as the last resort in traumatic brain injury to avoid development of cardiac arrhythmia due to severe hypokalemia.
Usher-Smith JA, Thompson M, Ercole A, Walter FM. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia. 2012;55(11):2878–94. https://doi.org/10.1007/s00125-012-2690-2.
• Syed Z, Kimball T, Ismail M. Successful use of renal replacement therapy for refractory hypokalemia in a diabetic ketoacidosis patient. Case Rep Crit Care. 2019;2019:6130694. https://doi.org/10.1155/2019/6130694 Article suggests a possible application of continuous renal replacement therapy to control intractable hypokalemia.
Davis SM, Maddux AB, Alonso GT, Okada CR, Mourani PM, Maahs DM. Profound hypokalemia associated with severe diabetic ketoacidosis. Pediatr Diabetes. 2016;17(1):61–5. https://doi.org/10.1111/pedi.12246.
Meglasson MD, Hazelwood RL. Adrenergic regulation of insulin secretion from the chicken pancreas in vitro. Gen Comp Endocrinol. 1984;56(1):82–9. https://doi.org/10.1016/0016-6480(84)90064-9.
Higbee MD, Kumar M, Galant SP. Stimulation of endogenous catecholamine release by theophylline: a proposed additional mechanism of action for theophylline effects. J Allergy Clin Immunol. 1982;70(5):377–82. https://doi.org/10.1016/0091-6749(82)90028-8.
Arem R, Zoghbi W. Insulin overdose in eight patients: insulin pharmacokinetics and review of the literature. Case Rep Med. 1985;64(5):323–32. https://doi.org/10.1097/00005792-198509000-00004.
Murphy NP, Ford-Adams ME, Ong KK, Harris ND, Keane SM, Davies C, et al. Prolonged cardiac repolarisation during spontaneous nocturnal hypoglycaemia in children and adolescents with type 1 diabetes. Diabetologia. 2004;47(11):1940–7. https://doi.org/10.1007/s00125-004-1552-y.
Tao H, Man Y, Shi X, Zhu J, Pan H, Qin Q, Liu S. Inconceivable hypokalemia: A case report of acute severe barium chloride poisoning. Case Rep Med. 2016;2016:2743134. https://doi.org/10.1155/2016/2743134.
Walter SJ, Shirley DG, Folkerd EJ, Unwin RJ. Effects of the potassium channel blocker barium on sodium and potassium transport in the rat loop of Henle in vivo. Exp Physiol. 2001;86(4):469–74. https://doi.org/10.1113/eph8602210.
• Hughes DA. Acute chloroquine poisoning: A comprehensive experimental toxicology assessment of the role of diazepam. Br J Pharmacol. 2020;177(21):4975–89. https://doi.org/10.1111/bph.15101 Chloroquine induces hypokalemia and potentiates the development of cardiac arrhythmia. Caution is necessary when using diazepam for supportive care because of its prolongation of QTc interval. Combined administration of diazepam and adrenaline may aggravate adverse effect of hypokalemia with chloroquine overdose.
•• van der Heijden CDCC, Duizer ML, Fleuren HWHA, Veldman BA, Sprong T, Dofferhoff ATSM, et al. Intravenous flucloxacillin (and perhaps other penicillins) treatment is associated with a high incidence of hypokalaemia. Br J Clin Pharmacol. 2019;85:2886–90. https://doi.org/10.1111/bcp.13969 Hypokalemia due to flucloxacillin is dose dependent and may be more common than appreciated in hospitalized patients.
Cannon SC. Sodium Channelopathies of Skeletal Muscle. Handb Exp Pharmacol. 2018;246:309–30. https://doi.org/10.1007/164_2017_52.
Phillips L, Trivedi JR. Skeletal Muscle Channelopathies. Neurotherapeutics. 2018;15:954–65. https://doi.org/10.1007/s13311-018-00678-0.
•• Bamgbola OF, Ahmed Y. Differential diagnosis of perinatal Bartter, Bartter and Gitelman syndromes. Clin Kidney J. 2020;14(1):36–48. https://doi.org/10.1093/ckj/sfaa172 An excellent review that highlights pathophysiology of Bartter and Gitelman syndromes in the context of hypokalemic metabolic alkalosis. Clinical application of information provided was strengthened by comparison of the salt losing nephropathy with common differential diagnoses.
Loffing J, Zecevic M, Féraille E, Kaissling B, Asher C, Rossier BC, et al. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Ren Physiol. 2001;280:F675–68. https://doi.org/10.1152/ajprenal.2001.280.4.F675.
Yoshitomi K, Shimizu T, Taniguchi J, Imai M. Electrophysiological characterization of rabbit distal convoluted tubule cell. Pflugers Arch. 1989;414:457–63. https://doi.org/10.1007/BF00585057.
Vaidya A, Mulatero P, Baudrand R, Adler GK. The expanding spectrum of primary aldosteronism: Implications for diagnosis, pathogenesis, and treatment. Endocr Rev. 2018;39(6):1057–88.
Rocha R, Stier CT Jr, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, et al. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141(10):3871–8. https://doi.org/10.1210/endo.141.10.7711.
•• Raina R, Krishnappa V, Das A, Amin H, Radhakrishnan Y, Nair NR, et al. Overview of monogenic or mendelian forms of hypertension. Front Pediatr. 2019;7:263. https://doi.org/10.3389/fped.2019.00263 Excellent review of diseases of monogenic inheritance that cause hypertension and hypokalemic alkalosis, highlighting pathophysiology and treatment modality.
Mumford E, Unwin RJ, Walsh SB. Liquorice, Liddle, Bartter or Gitelman—how to differentiate? Nephrol Dial Transplant. 2019;34:38–9. https://doi.org/10.1093/ndt/gfy199.
Botero-Velez M, Curtis JJ, Warnock DG. Liddle’s syndrome revisited -- A disorder of sodium reabsorption in the distal tubule. N Engl J Med. 1994;330(3):178–81. https://doi.org/10.1056/NEJM199401203300305.
Gallacher SD, Tsokolas G, Dimitropoulos I. Liquorice-induced apparent mineralocorticoid excess presenting in the emergency department. Clin Med (Lond). 2017;17(1):43–5. https://doi.org/10.7861/clinmedicine.17-1-43.
Beck KR, Telisman L, van Koppen CJ, Thompson GR 3rd, Odermatt A. Molecular mechanisms of posaconazole- and itraconazole-induced pseudohyperaldosteronism and assessment of other systemically used azole antifungals. J Steroid Biochem Mol Biol. 2020;199:105605. https://doi.org/10.1016/j.jsbmb.2020.105605.
Yau M, Haider S, Khattab A, Ling C, Mathew M, Zaidi S, et al. Clinical, genetic, and structural basis of apparent mineralocorticoid excess due to 11β-hydroxysteroid dehydrogenase type 2 deficiency. Proc Natl Acad Sci U S A. 2017;114(52):E11248–56. https://doi.org/10.1073/pnas.1716621115.
•• He X, Modi Z, Else T. Hereditary causes of primary aldosteronism and other disorders of apparent excess mineralocorticoid activity. Gland Surg. 2020;9(1):150–8. https://doi.org/10.21037/gs.2019.11.20 A comprehensive discussion of the pathophysiology, clinical presentation, diagnosis, and management of low-renin hypertension from excessive mineralocorticoid activity. Described recent discoveries of several pathogenic variants of ion channels involved in the etiology of familial aldosteronism.
• Fan L, Zhuang Y, Wang Y, Liu X, Liu D, Xiang B, et al. Association of hypokalemia with cortisol and ACTH levels in Cushing's disease. Ann N Y Acad Sci. 2020;1463(1):60–6. https://doi.org/10.1111/nyas.14205 A study showing the pathogenic role (dose-dependent mineralocorticoid) effect of cortisol rather than ACTH in the etiology of hypokalemia due to Cushing disease.
Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A. Complications of Cushing's syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4(7):611–29. https://doi.org/10.1016/S2213-8587(16)00086-3.
Al Argan R, Saskin A, Yang JW, D'Agostino MD, Rivera J. Glucocorticoid resistance syndrome caused by a novel NR3C1 point mutation. Endocr J. 2018;65(11):1139–46. https://doi.org/10.1507/endocrj.EJ18-0135.
•• Grigoryan S, Avram AM, Turcu A. Functional imaging in ectopic Cushing syndrome. Curr Opin Endocrinol Diab Obes. 2020;27(3):146–54. https://doi.org/10.1097/MED.0000000000000541 Discuss the technique and performance of a recently approved high-resolution functional imaging modality that targets the abundant somatostatin receptors on ectopic neuroendocrine tumors. [Ga]-DOTATATE positron emission tomography scan has a better performance than the conventional diagnostic tool for localization of ectopic Cushing syndrome. Early diagnosis provides opportunity for prompt surgical intervention and greater survival rate.
Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ. 2016;40(4):480–90. https://doi.org/10.1073/pnas.1716621115.
• Palmer BF, Kelepouris E, Clegg DJ. Renal tubular acidosis and management strategies: A narrative review. Rev Adv Ther. 2021;38(2):949–68. https://doi.org/10.1007/s12325-020-01587-5 An excellent review with updated information on the pathophysiology of all variants of renal tubular acidosis.
Seyberth HW, Weber S, Komhoff M. Bartter’s and Gitelman’s syndrome. Curr Opin Pediatr. 2017;29:179–86. https://doi.org/10.1097/00041552-199407000-00015.
Ares GR, Caceres PS, Ortiz PA. Molecular regulation of NKCC2 in the thick ascending limb. Am J Physiol Ren Physiol. 2011;301:F1143–59. https://doi.org/10.1152/ajprenal.00396.2011.
Hebert SC, Mount DB, Gamba G. Molecular physiology of cation-coupled Cl cotransport: the SLC12 family. Pflugers Arch. 2004;447:580–93. https://doi.org/10.1007/s00424-003.
Pluznick JL, Sansom SC. BK channels in the kidney: role in Kþ secretion and localization of molecular components. Am J Physiol Ren Physiol. 2006;291:F517–29. https://doi.org/10.1152/ajprenal.00118.2006.
Carraro-Lacroix LR, Malnic G. Acid-base transport by the renal distal nephron. J Nephrol. 2010;23(Suppl 16):S19–27.
Wang WH. Basolateral Kir4.1 activity in the distal convoluted tubule regulates K secretion by determining NCC activity. Curr Opin Nephrol Hypertens. 2016;25(5):429–35. https://doi.org/10.1097/MNH.0000000000000248.
Breiderhoff T, Himmerkus N, Stuiver M, Mutig K, Will C, Meij IC, et al. Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc Natl Acad Sci U S A. 2012;109(35):14241–6. https://doi.org/10.1073/pnas.1203834109.
Freudenthal B, Kulaveerasingam D, Lingappa L, Shah MA, Brueton L, Wassmer E, et al. KCNJ10 mutations disrupt function in patients with EAST syndrome. Nephron Physiol. 2011;119(3):p40–8. https://doi.org/10.1159/000330250.
Gu X, Su Z, Chen M, Xu Y, Wang Y. Acquired Gitelman syndrome in a primary Sjogren syndrome patient with a SLC12A3 heterozygous mutation: a case report and literature review. Nephrol. 2017;22:652–5. https://doi.org/10.1111/nep.13045.
Paice BJ, Paterson KR, Onyanga-Omara F, Donnelly T, Gray JM, Lawson DH. Record linkage study of hypokalaemia in hospitalized patients. Postgrad Med J. 1986;62(725):187–91. https://doi.org/10.1136/pgmj.62.725.187.
Swaminathan R. Magnesium metabolism and its disorders. Clin Biochem Rev. 2003;24(2):47–66.
• Kallistrou E, Architha NN, Pal SK, Oyibo SO. Severe hypokalemia secondary to transient distal renal tubular acidosis in a previously healthy woman. Case Rep Cureus. 2021;13(1):e12765. https://doi.org/10.7759/cureus.12765 Although proximal renal tubular acidosis (RTA) is often self-limiting, distal RTA are known for their permanence. This is an interesting observation demonstrating the possibility of a transient manifestation of distal RTA.
Youn JH, Alicia A, McDonough AA. Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol. 2009;71:381–401. https://doi.org/10.1152/ajpcell.00464.2007.
Greenlee M, Wingo CS, McDonough AA, Youn JH, Kone BC. Narrative review: evolving concepts in potassium homeostasis and hypokalemia. Ann Intern Med. 2009;150(9):619–25. https://doi.org/10.7326/0003-4819-150-9-200905050-00008.
• Alasad SMS, Salih OAM, Hassan M. Insight into potassium’s role in childhood mortality due to severe acute malnutrition. Sudan J Paediatr. 2019;19(1):44–51. https://doi.org/10.24911/SJP.106-1513711620 This article validates the view that the insidious process of malnutrition makes it unlikely to cause severe hypokalemia that would have resulted in death. The acute drop in the serum potassium that occurs with a co-morbid watery diarrhea greatly increase the odd of a fatal outcome.
Reber E, Friedli N, Vasiloglou MF, Schuetz P, Stanga Z. Management of Refeeding Syndrome in Medical Inpatients. J Clin Med. 2019;8(12):2202. https://doi.org/10.3390/jcm8122202.
Aubry E, Friedli N, Schuetz P, Stanga Z. Refeeding syndrome in the frail elderly population: prevention, diagnosis and management. Clin Exp Gastroenterol. 2018;11:255–64. https://doi.org/10.2147/CEG.S136429.
Mehler PS, Rylander M. Bulimia Nervosa – medical complications. J Eat Disord. 2015;3:12. https://doi.org/10.1186/s40337-015-0044-4 eCollection 2015.
Peters B, Oomen MW, Bakx R, Benninga MA. Advances in infantile hypertrophic pyloric stenosis. Expert Rev Gastroenterol Hepatol. 2014;8:533–41. https://doi.org/10.1586/17474124.2014.903799.
Konishi K, Mizuochi T, Yanagi T, Watanabe Y, Ohkubo K, Ohga S, et al. Clinical features, molecular genetics, and long-term outcome in congenital chloride diarrhea: a nationwide study in Japan. J Pediatr. 2019;214:151–57.e6. https://doi.org/10.1016/j.jpeds.2019.07.039.
Ram P, Goyal A, Lu M, Sloan J, McElhaugh W. Use of Aldosterone Antagonist to Treat Diarrhea and Hypokalemia of Ogilvie's Syndrome. Case Rep Gastrointest Med. 2016;2016:1207240. https://doi.org/10.1155/2016/1207240.
Belei OA, Heredea ER, Boeriu E, Marcovici TM, Cerbu S, Mărginean O, et al. Verner-Morrison syndrome. Literature review. Romanian J Morphol Embryol. 2017;58(2):371–6.
Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore). 2006;85(6):295–330. https://doi.org/10.1097/01.md.0000236956.74128.76.
Ratjen F, Bell SC, Rowe SM, Goss CH, Quittner AL, Bush A. Cystic fibrosis. Nat Rev Dis Primers. 2015;1:15010. https://doi.org/10.1038/nrdp.2015.10.
• Ferreira JP, Butler J, Rossignol P, Pitt B, Anker SD, Kosiborod M, et al. Abnormalities of Potassium in Heart Failure: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(22):2836–50. https://doi.org/10.1016/j.jacc.2020.04.021 A detailed review of the role of potassium dysregulation in the clinical outcome of congestive heart failure.
Francis GS. The relationship of the sympathetic nervous system and the renin-angiotensin system in congestive heart failure. Am Heart J. 1989;118:642–8. https://doi.org/10.1016/0002-8703(89)90291-3.
Skogestad J, Aronsen JM. Hypokalemia-Induced Arrhythmias and Heart Failure: New Insights and Implications for Therapy. Front Physiol. 2018;9:1500. https://doi.org/10.3389/fphys.2018.01500.
Collins AJ, Pitt B, Reaven N, Funk S, McGaughey K, Wilson D, et al. Association of Serum Potassium with All-Cause Mortality in Patients with and without Heart Failure, Chronic Kidney Disease, and/or Diabetes. Am J Nephrol. 2017;46:213–21. https://doi.org/10.1159/000479802.
Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of Takotsubo Syndrome. Circulation. 2017;135(24):2426–41. https://doi.org/10.1161/circulationaha.116.027121.
El-Sherif N, Turitto G. Electrolyte disorders and arrhythmogenesis. Cardiol J. 2011;18(3):233–45.
Pezhouman A, Singh N, Song Z, Nivala M, Eskandari A, Cao H, et al. Molecular basis of Hypokalemia-Induced ventricular fibrillation. Circulation. 2015;132(16):1528–37. https://doi.org/10.1161/circulationaha.115.016217.
Alfano G, Ferrari A, Fontana F, Perrone R, Mori G, Ascione E, et al. Modena Covid-19 Working Group (MoCo19). Hypokalemia in Patients with COVID-19. Clin Exp Nephrol. 2021;25(4):401–9. https://doi.org/10.1007/s10157-020-01996-4.
Yenerçağ M, Arslan U, Doğduş M, Günal Ö, Öztürk ÇE, Aksan G, et al. Evaluation of electrocardiographic ventricular repolarization variables in patients with newly diagnosed COVID-19. J Electrocardiol. 2020;62:5–9. https://doi.org/10.1016/j.jelectrocard.2020.07.005.
Lim S. Approach to hypokalemia. Acta Med Ind. 2007;39(1):56–64.
Wang X, Han D, Li G. Electrocardiographic manifestations in severe hypokalemia. J Int Med Res. 2020;48(1):1–7. https://doi.org/10.1177/0300060518811058.
Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol. 2007;18(10):2649–52. https://doi.org/10.1681/ASN.2007070792.
Mohib O, Papleux E, Remmelink M, Gottignies P, De Bels D. An ectopic Cushing's syndrome as a cause of severe refractory hypokalemia in the ICU. Acta Clin Belg. 2020;1-6. https://doi.org/10.1080/17843286.2020.1734162.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Oluwatoyin Fatai Bamgbola declares that there is no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric Nephrology
Rights and permissions
About this article
Cite this article
Bamgbola, O.F. Review of the Pathophysiologic and Clinical Aspects of Hypokalemia in Children and Young Adults: an Update. Curr Treat Options Peds 8, 96–114 (2022). https://doi.org/10.1007/s40746-022-00240-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40746-022-00240-3