Hartmann’s Solution
Hartmann’s Solution is one of the most commonly administered intravenous crystalloid solutions for perioperative care and circulatory resuscitation.
The electrolyte composition of Hartmann’s solution (Alexis Frank Hartmann – 1932) is similar to Ringer’s solution (Sydney Ringer – 1882) first formulated to maintain cellular function. Hartmann’s solution has been adapted by adding sodium lactate to more closely resemble the ionic composition of plasma, creating a ‘balanced salt solution’ that reduces transmembrane ion and water shifts post-transfusion.
Hartmann’s solution is also known as: Hartmann’s; Compound Sodium Lactate (CSL); sodium lactate solution; Ringer-Locke’s solution; Ringer-lactate; Lactated Ringer’s Solution (LRS).
Ingredients
Crystalloid solution containing Potassium chloride; Sodium chloride; Calcium chloride dihydrate; Sodium lactate in various concentrations. The exact make up is determined by country and manufacturer.
In Australia the most common constituents for Compound Sodium Lactate includes sodium lactate (3.17g/L), sodium chloride (6.0g/L), potassium chloride (400 mg/L) and calcium chloride dihydrate (270mg/L) in water for injections.
| Contents mmol.L-1 | 0.9% NaCl UK/AUS | Hartmann’s UK/AUS | Plasmalyte |
|---|---|---|---|
| Na+ | 150/154 | 130/131 | 140 |
| Cl– | 150/154 | 111/112 | 98 |
| K+ | 5/5.4 | 5 | |
| Ca2+ | 2 | 2 | |
| Mg2+ | 1.5 | ||
| Lactate | 29/28 | ||
| Acetate | 27 | ||
| Gluconate | 23 | ||
| pH | 5.0 | 5-7 | 6-7.4 |
| Osmolality mOsm/kg | 300 | 254 | 294 |
How does it work?
Hartmann’s solution acts as a source of both glucose and alkalinising agent in the form of bicarbonate that is generated from lactate metabolism.
Metabolism of the lactate in Hartmann’s solution is by either gluconeogenesis or oxidation.
Gluconeogenesis (70%): Lactate undergoes gluconeogenesis, predominantly in the liver and to a lesser extent in the kidneys. Lactate is first converted to pyruvate (via pyruvate kinase), then oxaloacetate and phosphoenol pyruvate (via phosphoenolpyruvate carboxykinase). In normal healthy adults there is a transient increase in blood glucose which provokes an appropriate insulin response.
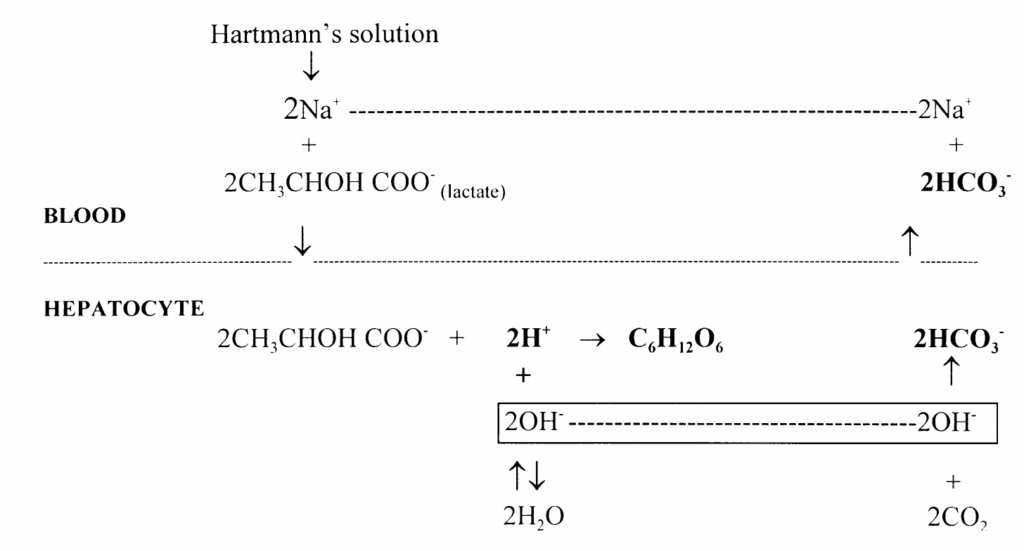
Oxidation (30%): Lactate undergoes oxidation predominantly in the hepatocytes, but also in the kidneys as well as cardiac and skeletal muscle under certain physiological conditions. H+ ions are consumed in lactate oxidation to CO2 and H2O
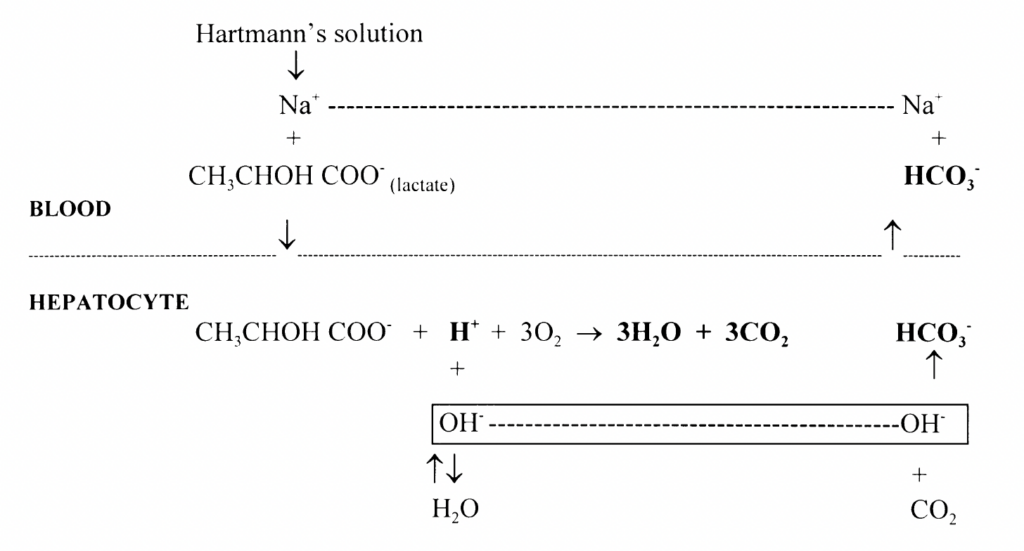
Hydrogen ions are consumed in both gluconeogenesis and oxidation of lactate. Both reactions contribute to the alkalinising effect of Hartmann’s solution by producing a relative excess of bicarbonate ions (excess OH– combines with CO2 to form HCO3 )
In normal adults the production of both glucose and bicarbonate is gradual and produces small changes in their plasma concentrations, easily corrected by the usual homeostatic mechanisms.
In patients with metabolic acidosis, there is a slow and steady production of bicarbonate which is of potential benefit. Bicarbonate produced has a half life of 10-15 minutes, with excess being excreted by the kidneys.
Note: some critically ill patients are obligate excretors of acidic urine. The additional bicarbonate load cannot be excreted and may render the patient alkalotic with increased renal potassium loss…
History of Hartmann’s solution or Ringer’s lactate
Sydney Ringer (1835-1910) was a British clinician, physiologist and pharmacologist. He studied the effects of electrolytes on cardiac and involuntary muscles. In particular the actions of various inorganic salts on the behaviour of the heart. In 1882 discovered a solution of salts dissolved in water to create an isotonic solution relative to the body fluids of an animal.
The discovery of Ringers solution was an accident. Ringer’s lab assistant mistakenly substituted tap water for distilled water, in experiments they were performing on frogs hearts. Ringer noticed that the experiments with tap water yielded different results from those with distilled water; he deduced this was due to the fact tap water contained inorganic substances.
I discovered, that the saline solution which I had used had not been prepared with distilled water, but with pipe water supplied by the New River Water Company. As this water contains minute traces of various inorganic substances, I at once tested the action of saline solution made with distilled water and I found that I did not get the effects described in the paper referred to. It is obvious therefore, that the effects I had obtained are due to some of the inorganic constituents of the pipe water.
Ringer 1883
The ability of a small volume of Ringer solution to sustain an excised frog heart-nerve preparation enabled Otto Loewi and Sir Henry Hallett Dale to propose the chemical transmission of nerve impulses For this, Loewi and Dale received the 1936 Nobel Prize in Physiology or Medicine.
A mixture containing 100cc saline [0.75% NaCl], 5cc sodium bicarbonate [0.5%], 5cc calcium chloride [1 in 1082, i.e. approximately 0.1% Ca], with 1 cc potassium chloride [1%] makes an excellent artificial circulating fluid, for with this mixture the heart will continue beating perfectly.
Ringer 1883
Alexis Frank Hartmann (1898-1964) was an American pediatrician and clinical biochemist. His research work centred around treating acidosis in sick children. In the 1930’s there were several problems complicating the use of sodium bicarbonate to treat acidosis. The process of sterilisation was laborious; the solution was highly irritant and made administration difficult; and sodium bicarbonate administration often produced too rapid a correction of the acidosis potentially resulting in a profound alkalosis.
An alternative to sodium bicarbonate was needed:
…if sodium bicarbonate were properly administered along with other indicated therapeutic measures, severe acidosis could be much more effectively treated…however, if given intravenously in amounts large enough to insure effectiveness, it tended to produce too rapid a change in the reaction of the body fluids, and often resulted in an almost immediate shift from uncompensated acidosis to uncompensated alkalosis.
In order to overcome those objections the mixture of sodium lactate and hypotonic Ringer’s solution was devised. The conversion of sodium lactate into sodium bicarbonate, it was felt, would be sufficiently slow to permit maintenance of the proper bicarbonate ratio which would lessen the danger of alkalosis and possibly even permit quantitative restoration of diminished body fluid sodium bicarbonate by a single dose. Sodium lactate, alone or combined with Ringer’s solution, is non-irritating, stable and can be sterilized readily by boiling.
Hartmann and Senn, 1932
References
Original articles
- Ringer S. A further Contribution regarding the influence of the different Constituents of the Blood on the Contraction of the Heart. J Physiol. 1883 Jan; 4(1): 29–42 [Ringer’s solution]
- Hartmann AF. Chemical Changes Occurring in the Body as the Result of Certain Diseases. I. The Effects of Diarrhea, Vomiting, Dehydration and Oliguria on the Acid-Base Balance of the Plasma of Infants with Mastoiditis. Am J Dis Child. 1928;35(4):557-575
- Hartmann AF, Senn MJE. Studies in the metabolism of sodium r-lactate. I. Response of normal human subjects to the intravenous injection of sodium r-lactate J. Clin. Invest. 1932: 11(2): 327-335
Eponymous terms
- Lee JA. Sydney Ringer (1834-1910) and Alexis Hartmann (1898-1964). Anaesthesia. 1981 Dec;36(12): 1115-21
- White SA, Goldhill DR. Is Hartmann’s the solution? Anaesthesia. 1997 May;52(5):422-7.
- Barlow J. Intravenous Fluids. Part One

Critical Care
Compendium
Doctor currently working in South Wales, training in anaesthetics. Graduated Leeds University with MB ChB with BSc in microbiology in relation to medicine. Special interests in emergency medicine, critical care and anaesthetics

