Interesting Science Videos
Introduction
Giemsa stain was a name adopted from a Germany Chemist scientist, for his application of a combination of reagents in demonstrating the presence of parasites in malaria.
It belongs to a group of stains known as Romanowsky stains. These are neutral stains made up of a mixture of oxidized methylene blue, azure, and Eosin Y and they performed on an air-dried slide that is post-fixed with methanol. Romanowsky stains are applied in the differentiation of cells, pathological examinations of samples like blood and bone marrow films and demonstration of parasites e.g malaria. There are four types of Romanoswsky stains:
- Giemsa stain
- Jenner Stain
- Wright stain
- May-Grunwald Stain
- Leishman stain
Objectives of Giemsa stain
- To accurately prepare the Giemsa stain stock solution
- To stain and identify blood cells
- To differentiate blood cells nuclei from the cytoplasm
Principle
Giemsa stain is a gold standard staining technique that is used for both thin and thick smears to examine blood for malaria parasites, a routine check-up for other blood parasites and to morphologically differentiate the nuclear and cytoplasm of Erythrocytes, leucocytes and Platelets and parasites.
Like any type of Romanowsky stains, it composed of both the Acidic and Basic dyes, in relation to affinities of acidity and basicity for blood cells. Azure and methylene blue, a basic dye binds to the acid nucleus producing blue-purple color. Eosin is an acidic dye that is attracted to the cytoplasm and cytoplasmic granules which are alkaline-producing red coloration. The stain must be buffered with water to pH 6.8 or 7.2, to precipitate the dyes to bind simple materials.
Classically, Giemsa stain is a differential stain which is made up of a combination of reagents (Azure, Methylene blue, and Eosin dye) used widely in cytogenetics and histopathology for the diagnosis of:
- Malaria, spirochetes and other blood parasites
- Chlamydia trachomatis inclusion bodies
- Borrelia spp
- Yersinia pestis
- Histoplasma spp
- Pneumocystis jiroveci cysts
Reagents Used
- Methanol
- Giemsa powder
- Glycerin
- Water (Buffer)
Procedure
Preparation of the Giemsa Stain Stock solution (500ml)
- Into 250ml of methanol, add 3.8g of Giemsa powder and dissolve.
- Heat the solution up to ~60oC
- Then, add 250ml of glycerin to the solution, slowly.
- Filter the solution and leave it to stand for about 1-2 months before use.
Preparation of Working solution
- Add 10ml of stock solution to 80ml of distilled water and 10ml of methanol
Staining procedure 1: Thin Film staining
- On a clean dry microscopic glass slide, make a thin film of the specimen (blood) and leave to air dry.
- dip the smear (2-3 dips) into pure methanol for fixation of the smear, leave to air dry for 30seconds
- Flood the slide with 5% Giemsa stain solution for 20-30 minutes.
- Flush with tap water and leave to dry
NOTE: In case of emergencies, leave the Giemsa stain solution for 5-10 minutes
Staining Procedure 2: Thick Film Staining
- Add a thick smear of blood and air dry for 1 hour on a staining rack.
- Dip the thick blood smear into diluted Giemsa stain (prepared by taking 1ml of the stock solution and adding to 49ml of phosphate buffer or distilled water, but the results may vary differently).
- Wash the smear by dipping in in buffered water of distilled water for 3-5 minutes
- Leave it to dry
Results
The Cytoplasm and cytoplasmic granules of blood cells appear red in color while the nucleus appears blue-purple in color.
The erythrocytes will appear pink in clour
- Eosinophils will have a blue-purple nucleus, a pale pink cytoplasm, and orange-red granules.
- Neutrophils will appear purple-red nucleus and a pink cytoplasm.
- Basophils will have a purple nucleus and bluish granules.
- Lymphocytes have a dark blue nucleus and a light blue cytoplasm.
- Monocytes will have a purple nucleus and a pink cytoplasm.
- Platelets will have purple granules.
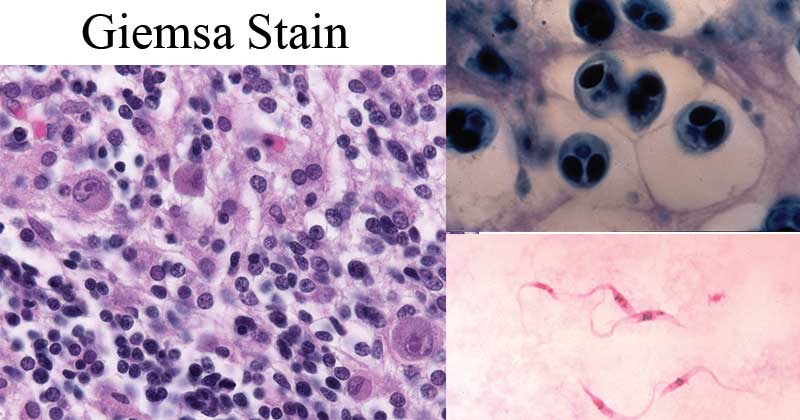
Image Source: Wikipedia
Interpretation
Azure and methylene blue, a basic dye binds to the acid nucleus producing blue-purple color. Eosin is an acidic dye that is attracted to the cytoplasm and cytoplasmic granules which are alkaline-producing red-orange coloration.
Applications Giemsa stain
- Giemsa stain is specific for the phosphate groups of DNA. It attaches itself to regions of DNA with high amounts of adenine-thymine bonding.
- Giemsa stain is used in Giemsa banding (G-banding), to stain chromosomes and it is often used to create a diagrammatic representation of chromosomes (idiogram).
- Being a differential stain, Giemsa stain can be used to study the adherence of pathogenic bacteria to human cells, differentiating human cells as purple and bacterial cells as pink.
- It can be used for histopathological diagnosis of malaria and some spirochete and protozoan blood parasites.
- It is also used in Wolbach’s tissue stain i.e staining hematopoietic tissue and for the identification of bacteria and rickettsia
- Giemsa stain is a classic blood film stain for peripheral blood smears and bone marrow specimens. Red Blood Cells stain pink, platelets stain a light pale pink, lymphocyte cytoplasm stains sky blue, monocyte cytoplasm stains pale blue, and leukocyte nuclear chromatin stains magenta.
- Giemsa stain is also used to visualize chromosomes, identifying chromosomal anomalies like translocation and rearrangement,
- Giemsa stains the fungus Histoplasma, Chlamydia bacteria, and can be used to identify Mast cells.
Advantages
- Readily available, easy to prepare, maintain and use
Limitations
- Working Giemsa stain must be prepared shortly before use.
References
- Staining techniques: Giemsa by Kathleen P Freeman, Karen L Gerber: Vetstream
- Paramedic World; Hematology Practicals/Giemsa staining Technique
- How Romanowsky stains work and why they remain valuable – including a proposed universal Romanowsky staining mechanism and a rational troubleshooting scheme by Horobin RW./ncbi.nlm.nih.gov
Internet Sources
- 13% – https://en.wikipedia.org/wiki/Giemsa_stain
- 3% – http://pathonet.com/pathonet/education-stainings
- 1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC540181/
- 1% – https://clinicalgate.com/preparation-and-staining-methods-for-blood-and-bone-marrow-films/
- <1% – https://www.researchgate.net/publication/24346194_Histopathology_for_the_diagnosis_of_infectious_diseases
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1453983/
- <1% – https://chlorine.americanchemistry.com/Science-Center/Chlorine-Compound-of-the-Month-Library/Methylene-Blue-Part-2-The-Chemists-Indicator/
- <1% – https://answers.yahoo.com/question/index?qid=20080712002122AAAhrqK

Great
Very nice job tnx
Good job congrats
Good work
This is really interesting, so detailed, thank you Soo much for such a journal
it is a grt one tanx
Interested in this site more update
God bless you
It is really good thank you
Very elaborative and educative
Good work
good work
I thought the acidic dyes were azure and eosin?
Hello, Azure is a basic dye, and Eosin is an acidic dye.
Very Interesting
Send more updates on staining procedure technics.