At A Glance
- There are numerous retinal lesions, but some “usual suspects” are encountered frequently in the clinic.
- Knowing the presentations of these most common lesions can help clinicians refer promptly when necessary.
- This knowledge can also better equip us to understand when a lesion doesn’t fit the profile of one of the usual suspects.
A lesion is defined as “an abnormal change in structure of an organ or part due to injury or disease.”1 Lesions in the retina can occur from numerous etiologies. They may be tumorous, vascular, degenerative, inflammatory, traumatic, or even infectious.
There are more off-the-wall retinal lesions documented than anyone could ever fully keep track of, but on a daily basis we are much more likely to encounter certain “usual suspects.” It is important to know how to identify these common lesions and differentiate them from other conditions. In this article, I highlight some of the retina’s most common culprits.
POSTERIOR SEGMENT TUMOR: NEVUS
Choroidal nevus is the most common intraocular tumor. These are benign tumors composed of melanocytes, and they occur in about 3% to 10% of the population. As these are lesions in the choroid, they disappear or are much less evident with use of a red-free or green filter than with use of white light.
Although conversion of a nevus to choroidal melanoma is rare, patients should be monitored for signs of this occurrence. The following signs should draw concern for melanoma: thickness >2 mm, subretinal fluid, visual symptoms, orange pigment, margins ≤3 mm from the disc, ultrasonographic hollowness, absence of halo, and absence of drusen. These signs have been turned into the catchy pneumonic: To find small ocular melanoma, use helpful hints daily.2-4
OCT can be used to document nevus size and thickness and to evaluate for overlying drusen or subretinal fluid. Fundus autofluorescence (FAF) can be used to look for the presence of orange pigment. Nevi may create mottled autofluorescence due to disruption of the overlying retinal pigment epithelium (RPE), but orange pigment composed of lipofuscin will be highly hyperautofluorescent.3
Nevi may be confused with another frequently encountered lesion: congenital hypertrophy of the retinal pigment epithelium, or CHRPE. These flat lesions of the RPE will be visible with red-free or green filter. OCT will show atrophy of the outer retina overlying the lesion, and they will be hypoautofluorescent on FAF.5 Although the risk of conversion to a malignant lesion is not zero, it is significantly less than for nevi, and these lesions typically do not require as frequent observation (Figure 1).6
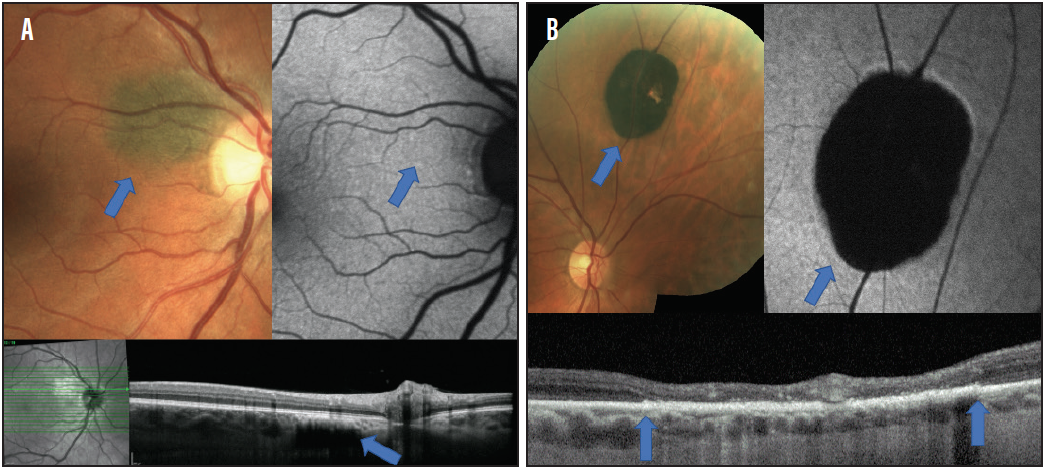
Figure 1. Small, flat nevus adjacent to the optic nerve (A). FAF (top right) shows no alteration to the FAF signal, demonstrating no presence of lipofuscin accumulation. OCT (bottom) confirms that the lesion is flat and located within the choroid. CHRPE shows dense hypo-autofluorescence on FAF (B). OCT (bottom) shows thickening of the RPE and atrophy of the outer retina without evidence of a choroidal lesion (blue arrows demonstrate the boundaries of the lesion). As CHRPE develop lacunae there is RPE atrophy.
PERIPHERAL RETINAL DEGENERATION: LATTICE DEGENERATION
Technically, the most common type of peripheral retinal degeneration is microcystoid degeneration, but lattice degeneration is a more clinically significant finding that occurs in about 10% of the population. Patients with lattice degeneration have increased risk for retinal detachment (RD). Although the overall risk is low (around 1%), lattice degeneration and atrophic retinal holes are responsible for about 30% of RDs.7
Lattice degeneration can lead to trouble in multiple ways. Lattice represents thin areas of retina, and these regions often have dense vitreous adhesions.8,9 As patients age and undergo posterior vitreous detachment (PVD), areas of lattice degeneration are prime locations for retinal breaks to occur. Patients with lattice degeneration should be thoroughly educated about symptoms of acute PVD such as flashes of light and new-onset floaters. Patients should be carefully examined and monitored during acute PVD events.
In addition, patients with lattice degeneration are at increased risk of developing atrophic retinal holes that may lead to slowly progressive RD without symptoms until more central vision is affected.10 These patients should be educated to monitor their peripheral vision and report any incidence of new shadows or curtains in their peripheral vision. They should also be monitored carefully with dilated fundus examination to look for subclinical RDs.
The benefit of prophylactic photocoagulation for individuals with lattice degeneration is disputed because of the low incidence of complications from these lesions. Most often, lattice degeneration without holes is monitored without treatment unless there is some increased cause for concern such as a history of RD in the fellow eye. Some may elect to refer patients who have atrophic retinal holes to a retina specialist. Retinal holes with associated subretinal fluid should be referred for evaluation and possible treatment. Retinal tears associated with lattice degeneration should also be referred for treatment (Figures 2 and 3).7
LESION MISTAKEN FOR RD: WHITE WITHOUT PRESSURE
White without pressure (WWOP) is a common peripheral retina finding often seen in young patients with myopia. The etiology of WWOP is not well understood. Although WWOP presents no increased risk for RD, it is often mistaken for RD or peripheral retinoschisis. WWOP is a flat lesion, but it can be deceiving, giving an optical illusion of elevation.11
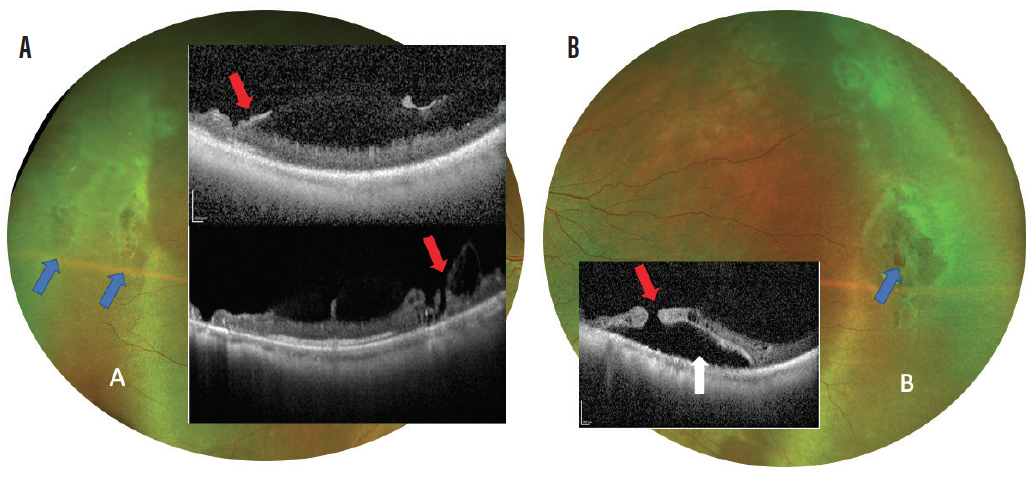
Figure 2. OCT taken through areas of lattice degeneration (blue arrows) shows retinal thinning and regions of dense vitreous attachment to the retina (A; red arrows). These factors combined lead to increased risk of operculated breaks and tears within lattice degeneration as seen in Figure 3. OCT taken through an atrophic retinal hole within lattice shows the presence of the retinal hole (B; red arrow) and presence of surrounding subretinal fluid (white arrow). Continued expansion of this fluid can lead to insidious development of retinal detachment as seen in Figure 3.
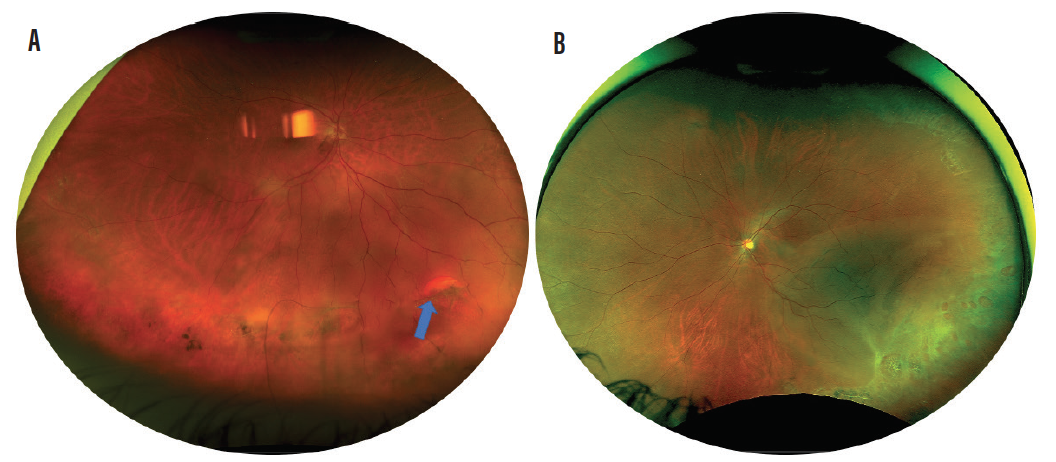
Figure 3. Retinal detachment from retinal tear (blue arrow) occurring during acute PVD even in patient with lattice degeneration (A). Retinal detachment occurring from multiple atrophic retinal holes in patient with lattice degeneration (B).
WWOP and RD can be located anywhere in the peripheral retina, but peripheral retinoschisis is most often bilateral and located temporally, with the infratemporal quadrant being its most common locus.12 If there is presence of a pigment demarcation line around the lesion, there should be increased suspicion for RD (Figure 4). Peripheral retinoschisis should be monitored due to increased risk of RD. RDs must be referred for treatment.
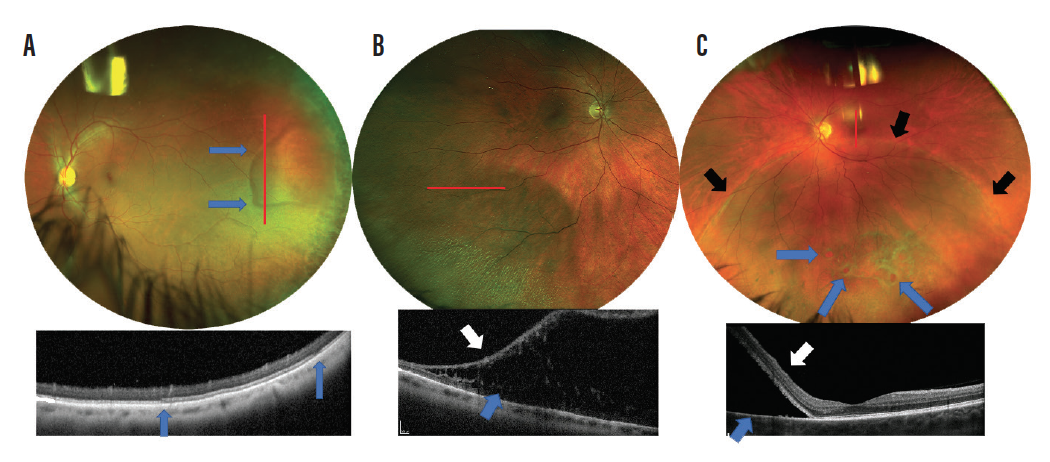
Figure 4. Red lines show region of OCT scans. Region of WWOP with distinct borders (A). OCT shows a flat lesion without retinal elevation. Within WWOP there is thickening and increased reflectivity of the external limiting membrane. The blue arrows demonstrate the boundaries of the lesion. Peripheral retinoschisis is shown splitting the inner retina (white arrow) and outer retina (B; blue arrow). Inferior retinal detachment from atrophic retinal holes (C; blue arrows). OCT shows complete separation of the neurosensory retina (white arrow) from the RPE (blue arrow). There is also a subtle pigment demarcation line around the entire edge of the lesion (black arrows).
SYSTEMIC DISEASE MANIFESTATION: DIABETES
Diabetes is at the top of the list for far too many negative health effects. One of them is that it is the most common cause of blindness in working age adults.13 Numerous lesions present in patients with diabetic retinopathy. Physicians should be on the lookout for lesions including microaneurysms, retinal hemorrhages, exudates, cotton wool spots, intraretinal microvascular abnormalities (IRMA), retinal neovascularization, iris neovascularization, preretinal hemorrhage, and vitreous hemorrhage.
Patients with mild nonproliferative retinopathy (NPDR) will present with few microaneurysms or retinal hemorrhages. Presence of cotton wool spots or exudates puts patients into the category of moderate NPDR.13 In addition, individuals with macular exudates must be evaluated carefully for the presence of diabetic macular edema (Figure 5).
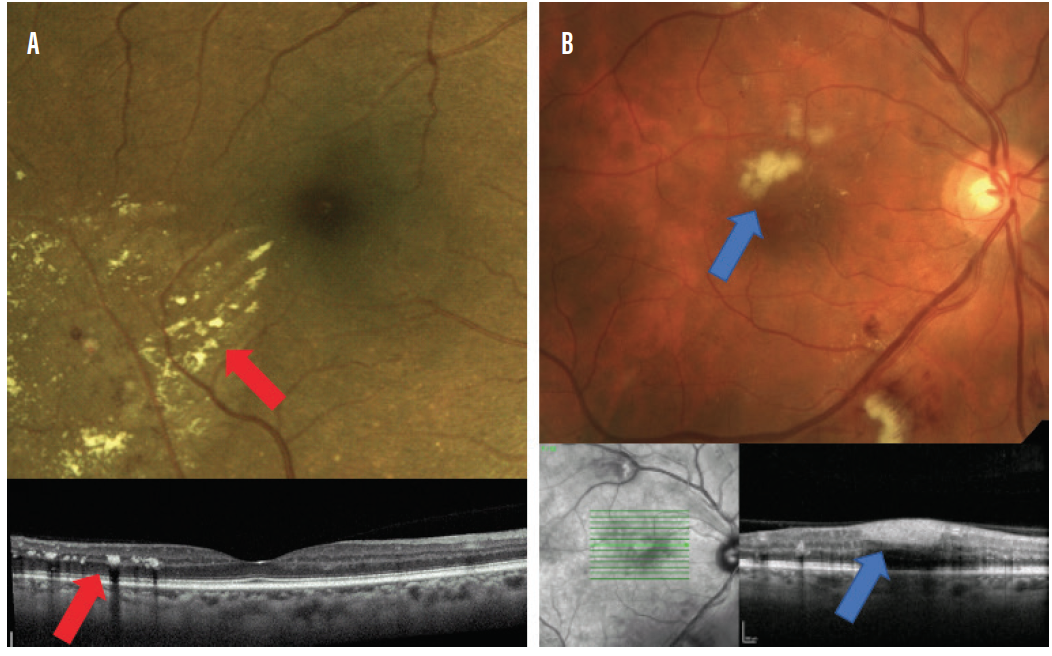
Figure 5. Lesions to be on the lookout for in moderate NPDR: retinal exudation (A; red arrows) and cotton wool spots (B; blue arrows).
IRMA, which represents damaged retinal capillaries, essentially looks like small, squiggly blood vessels. These can be difficult to see clinically, and spotting them requires careful evaluation. Patients with clinically visible IRMA should be staged as having severe NPDR (Figure 6). Patients who have significant retinal hemorrhages in all four quadrants or two quadrants of venous beading should also be staged as having severe NPDR.13 Referral to a retina specialist should be considered for those with severe NPDR.
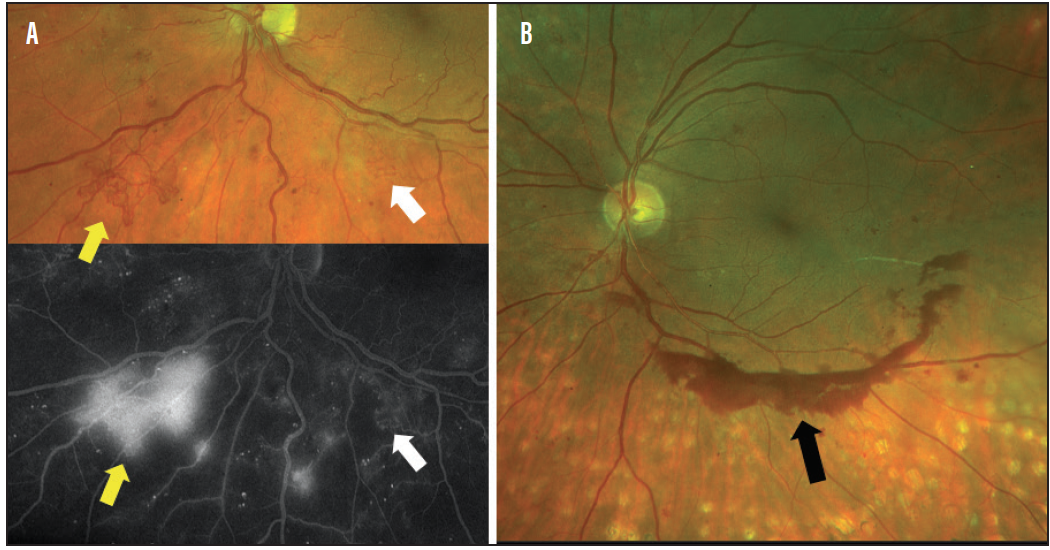
Figure 6. Lesions to be on the lookout for in severe NPDR and PDR: retinal neovascularization that leaks on fluorescein angiography (FA) (A; yellow arrows), IRMA that does not leak on FA (white arrows), and preretinal hemorrhage (B; black arrow), indicating active neovascularization elsewhere in a patient with prior panretinal photocoagulation.
Any presence of retinal neovascularization puts a patient into the stage of proliferative diabetic retinopathy (PDR). Patients with PDR should be referred for treatment due to high risk of vision loss (Figure 6).13 Patients with diabetic retinopathy who present with either vitreous or preretinal hemorrhage should raise high suspicion for PDR. In this state, neovascularization grows on the surface of the retina, and when it bleeds it creates either preretinal or vitreous hemorrhage (Figure 6).
DEGENERATIVE DISEASE: AGE-RELATED MACULAR DEGENERATION
Age-related macular degeneration (AMD) is the most common cause of vision loss in older patients in the United States.14 A shocking statistic published a few years ago noted that primary eye care physicians have overlooked findings of AMD in 25% of patients.15
Drusen are the hallmark lesion of AMD. Especially in at-risk populations, retinal examinations should be carefully performed so as not to overlook these lesions. Drusen that are larger than 125 µm (about the width of a retinal vessel as it crosses the disc margin) are considered large, and large drusen constitute the diagnosis of intermediate AMD (Figure 7). Pigmentary abnormalities are also considered a hallmark of intermediate stage AMD (Figure 7).
![<p>Figure 7. Lesions to be on the lookout for in AMD that increase risk of conversion to advanced AMD: large-sized drusen (A). Drusen larger than 125 µm or about the width of a major vessel as it crosses over the disc margin [black arrow] are large drusen. The red arrow points to multiple drusen that are easily large in size. Pigmentary alterations (B; red arrow).</p>](https://core4-cms.imgix.net/issue-1794/1120_Sub1_Fig7.png)
Figure 7. Lesions to be on the lookout for in AMD that increase risk of conversion to advanced AMD: large-sized drusen (A). Drusen larger than 125 µm or about the width of a major vessel as it crosses over the disc margin [black arrow] are large drusen. The red arrow points to multiple drusen that are easily large in size. Pigmentary alterations (B; red arrow).
These two findings increase the risk of conversion to advanced stages of AMD. Patients with these conditions require frequent monitoring and heavy patient education about at-home vision monitoring. Patients with intermediate AMD can also benefit from vitamin supplementation, as demonstrated in the AREDS trials.16,17
Advanced AMD can either be exudative, with the development of choroidal neovascularization (CNV), or nonexudative, with the development of geographic atrophy (GA). Early detection of exudative AMD is crucial so that treatment for maintenance of central vision can be initiated with anti-VEGF injections.
Any patient with signs of intra- or subretinal fluid, retinal hemorrhage (particularly subretinal hemorrhage), exudation, or retinal thickening should be carefully evaluated for the potential of CNV (Figure 8). Detection of CNV is most often aided now by OCT imaging. OCT angiography can also be highly useful, and occasionally intravenous fluorescein angiography or indocyanine green angiography may still be employed.18-20
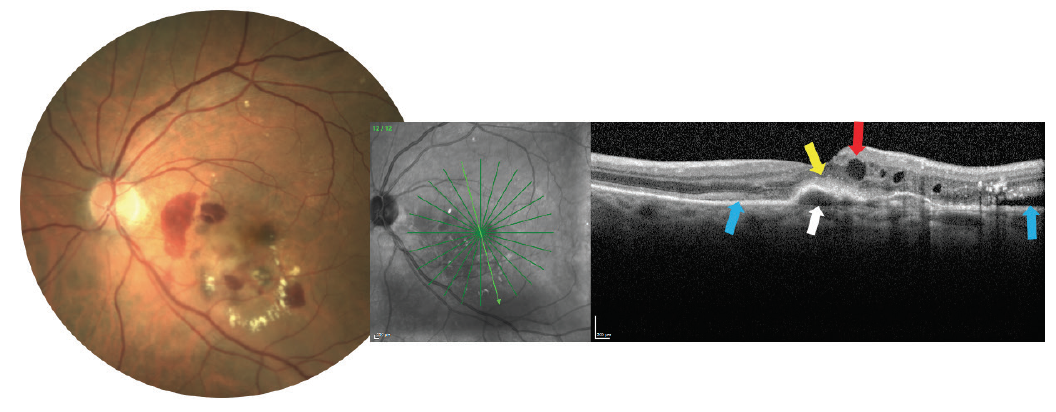
Figure 8. A choroidal neovascular membrane lesion may produce some or all of the following findings: hemorrhage and exudate (as seen on the photograph), pigment epithelial detachment (white arrow), subretinal hyperreflective material (yellow arrow overlying the pigment epithelial detachment), intraretinal fluid (red arrow), or subretinal fluid (blue arrows).
GA unfortunately has no true treatment, but detection is important for management of patients’ visual needs. OCT imaging and FAF are useful for tracking GA and for providing patient education (Figure 9).21,22
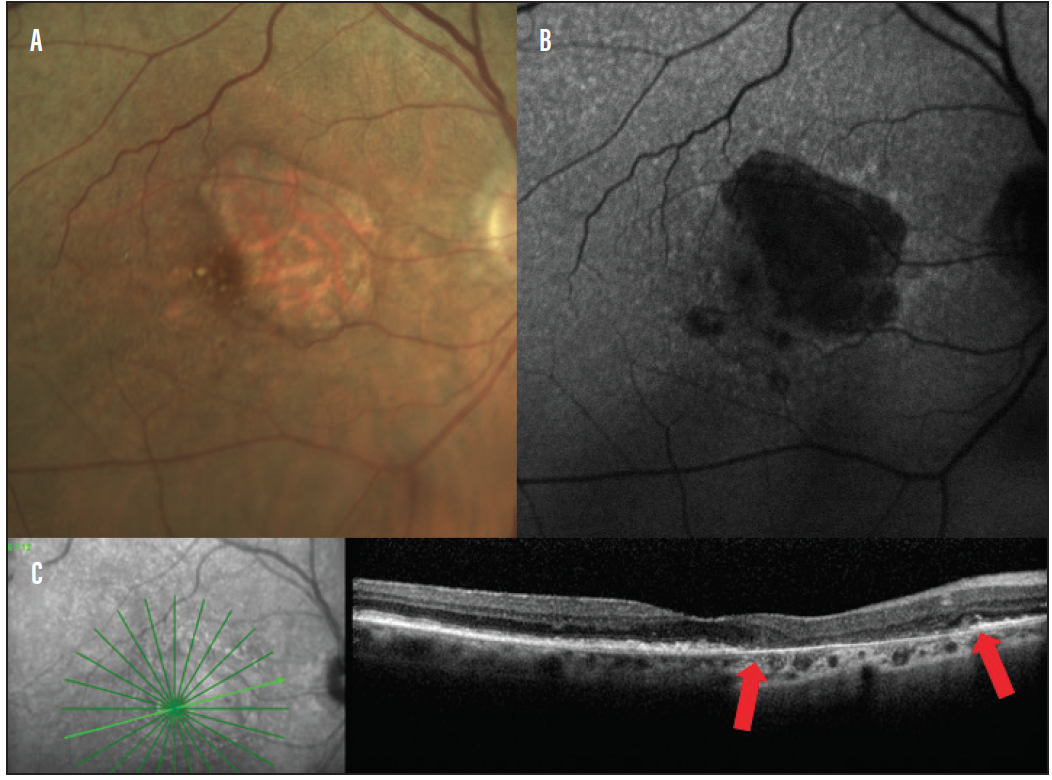
Figure 9. A lesion of GA photography (A) FAF (B) shows hypoautofluorescence in the area of GA. OCT (C) shows outer retinal atrophy in the region of GA (lesion is between the red arrows).
BE ON THE LOOKOUT
Use the guidance presented here to be on the lookout for these common retinal culprits. A firm knowledge of the presentation of these lesions allows clinicians to achieve better diagnosis and management. In addition, it better equips us to understand when a lesion doesn’t fit the profile of one of the usual suspects, so we can then dive into less commonly encountered conditions.
- 1. Definition of lesion. Merriam-Webster dictionary online. www.merriam-webster.com/dictionary/lesion. Accessed October 18, 2020.
- 2. Shields CL, Furuta M, Berman EL, et al. Choroidal nevus transformation into melanoma: Analysis of 2514 consecutive cases. Arch Ophthalmol. 2009;127(8):981-987.
- 3. Shields CL, Dalvin LA, Ancona-Lezama D, et al. Choroidal nevus imaging features in 3,806 cases and risk factors for transformation into melanoma in 2,355 cases: The 2020 Taylor R. Smith and Victor T. Curtin Lecture. Retina. 2019;39(10):1840-1851.
- 4. Marous CL, Shields CL, Yu MD, Dalvin LA, Ancona-Lezama D, Shields JA. Malignant transformation of choroidal nevus according to race in 3334 consecutive patients. Indian J Ophthalmol. 2019;67(12):2035-2042.
- 5. Fung AT, Pellegrini M, Shields CL. Congenital hypertrophy of the retinal pigment epithelium: Enhanced-depth imaging optical coherence tomography in 18 cases. Ophthalmology. 2014;121(1):251-256.
- 6. Moulin AP, Zografos L, Schalenbourg A. RPE adenocarcinoma arising from a congenital hypertrophy of the RPE (CHRPE) treated with proton therapy. Klin Monbl Augenheilkd. 2014;231(4):411-413.
- 7. Flaxel CJ, Adelman RA, Bailey ST, et al. Posterior vitreous detachment, retinal breaks, and lattice degeneration preferred practice pattern. Ophthalmology. 2020;127(1):P223-P258.
- 8. Kothari A, Narendran V, Saravanan VR. In vivo sectional imaging of the retinal periphery using conventional optical coherence tomography systems. Indian J Ophthalmol. 2012;60(3):235-239.
- 9. Manjunath V, Taha M, Fujimoto JG, Duker JS. Posterior lattice degeneration characterized by spectral domain optical coherence tomography. Retina. 2011;31(3):492-496.
- 10. Tillery WV, Lucier AC. Round atrophic holes in lattice degeneration: an important cause of phakic retinal detachment. Trans Am Acad Ophthalmol Otolaryngol. 1976;81(3 Pt 1):509-518.
- 11. Fawzi AA, Nielsen JS, Mateo-Montoya A, et al. Multimodal imaging of white and dark without pressure fundus lesions. Retina. 2014;34(12):2376-2387.
- 12. Stehouwer M, Tan SH, van Leeuwen TG, Verbraak FD. Senile retinoschisis versus retinal detachment, the additional value of peripheral retinal OCT scans (SL SCAN-1, Topcon). Acta Ophthalmol. 2014;92(3):221-227.
- 13. Wong TY, Sun J, Kawasaki R, et al. Guidelines on Diabetic Eye Care: The International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings. Ophthalmology. 2018;125(10):1608-1622.
- 14. Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137(3):486-495.
- 15. Neely DC, Bray KJ, Huisingh CE, Clark ME, McGwin G, Owsley C. Prevalence of undiagnosed age-related macular degeneration in primary eye care. JAMA Ophthalmol. 2017;135(6):570-575.
- 16. Kassoff A, Kassoff J, Buehler J, et al. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417-1436.
- 17. Chew EY, Clemons TE, SanGiovanni JP, et al. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005-2015.
- 18. Kanski JJ, Bowling B. Clinical Ophthalmology: A Systematic Approach. 7th ed. Elsevier; 2011. 611-628.
- 19. Gualino V, Tadayoni R, Cohen SY, et al. Optical coherence tomography, fluorescein angiography, and diagnosis of choroidal neovascularization in age-related macular degeneration. Retina. 2019;39(9):1664-1671.
- 20. Nikolopoulou E, Lorusso M, Micelli Ferrari L, et al. Optical coherence tomography angiography versus dye angiography in age-related macular degeneration: sensitivity and specificity analysis. Biomed Res Int. 2018;2018:6724818.
- 21. Fleckenstein M, Issa PC, Helb HM, et al. High-resolution spectral domain-OCT imaging in geographic atrophy associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49(9):4137-4144.
- 22. Choudhry N, Giani A, Miller JW. Fundus autofluorescence in geographic atrophy: a review. Semin Ophthalmol. 2010;25(5-6):206-213.

