What Is Flow Cytometry Light Scatter?
As a child, did you ever see a shadow puppet performance, or maybe make some with your hands to delight you and your friends? The idea is that you put something between a light source and the wall (like your hands) and the shadow becomes alive with shapes that can look like dogs, birds, wolves, and more.
If we look at the alignment of a typical flow cytometer, we have a light source on one side of the cells, and a series of detectors around the flow cell to capture the photons that are either emitted by the fluorochromes or that are scattered by the cells, as shown below in figure 1.
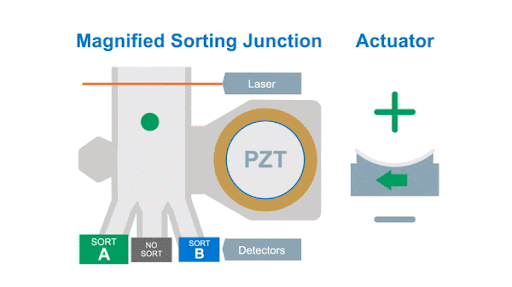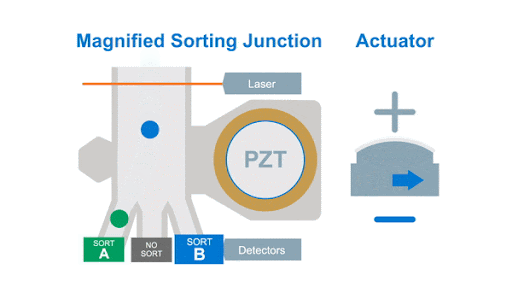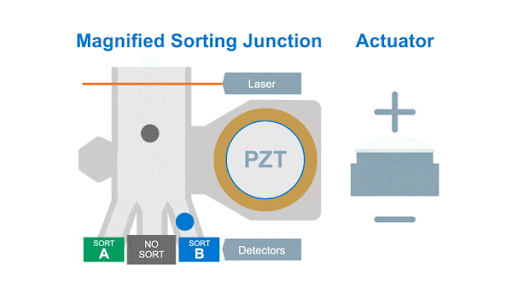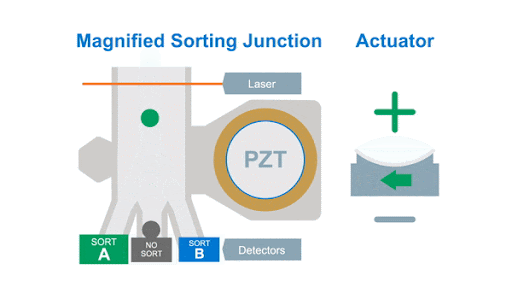
Figure 1: Optical arrangement of the WOLF Cell Sorter.
As with any flow cytometer, we can make two characteristic measurements of the physical properties of the cell, these are typically called forward scatter and side scatter, which are based on the location of the detector in relation to the laser interrogation point.
Forward Scatter
The forward scatter is located in line with the laser intercept and is typically considered a measure of the relative cell size. The side scatter is typically located perpendicular to the laser beam intercept and is used to measure the relative complexity of the cell. Taken together, these can be used to help characterize different cell populations at a macro level.
Looking at the forward scatter parameter, we are not actually measuring the shadow the cells casts on the detector. Instead, we measure something called Mie scatter, named after the German physicist who described it.
Briefly, it describes the light scattering by an object that is approximately equal to the incident light and is influenced by the refractive index of the particle and the medium. It is also influenced by the angle that the scattered light is measured at, which is vendor–dependent.
Figure 2 shows the idea of forward scatter. As a cell passes through the laser intercept, it can scatter light. This is measured by the in-line detector. Since the laser light is very bright, there is a bar that blocks the laser light from hitting the detector (black line in front of the detector). What is collected are the photons represented by the dashed lines. As shown in the bottom part of the figure, the larger the cell size, the more ‘intense’ the scatter, as described by Mie theory. Given the laser power is constant, the intensity is proportional to the scattering cross-sectional area.
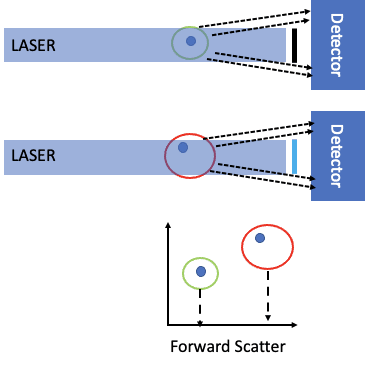
Figure 2: Forward scatter in a flow cytometer.
As mentioned, this is also dependent on the refractive index of the particle, so that given a individual cell with a refractive index of approximately 1.3611, a polystyrene bead, of the same size, with a refractive index of 1.6052 (at 488 nm) will have a different scatter profile, therefore, one must be careful to overinterpret the forward scatter measurement.
Side Scatter
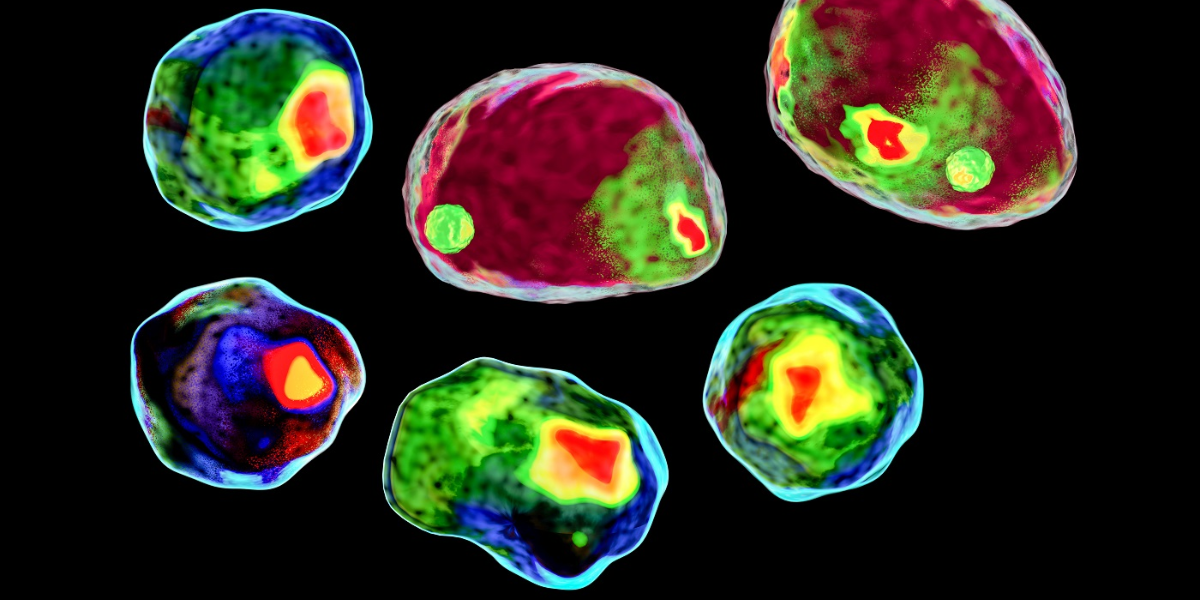
The other physical parameter that we can measure is the side angle scatter, which represents the scattering from the internal complexity of the cell of interest. This is influenced by cellular components such as the nucleus, granules and the like. If one compares a lymphocyte3 to a basophil4 to a neutrophil.5 As shown below, it is easy to see how this side scatter parameter could be used to identify these major PBMC subsets.
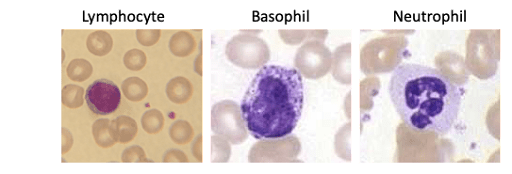
Figure 3: Wright-Giemsa staining of different PBMC based on increasing complexity.
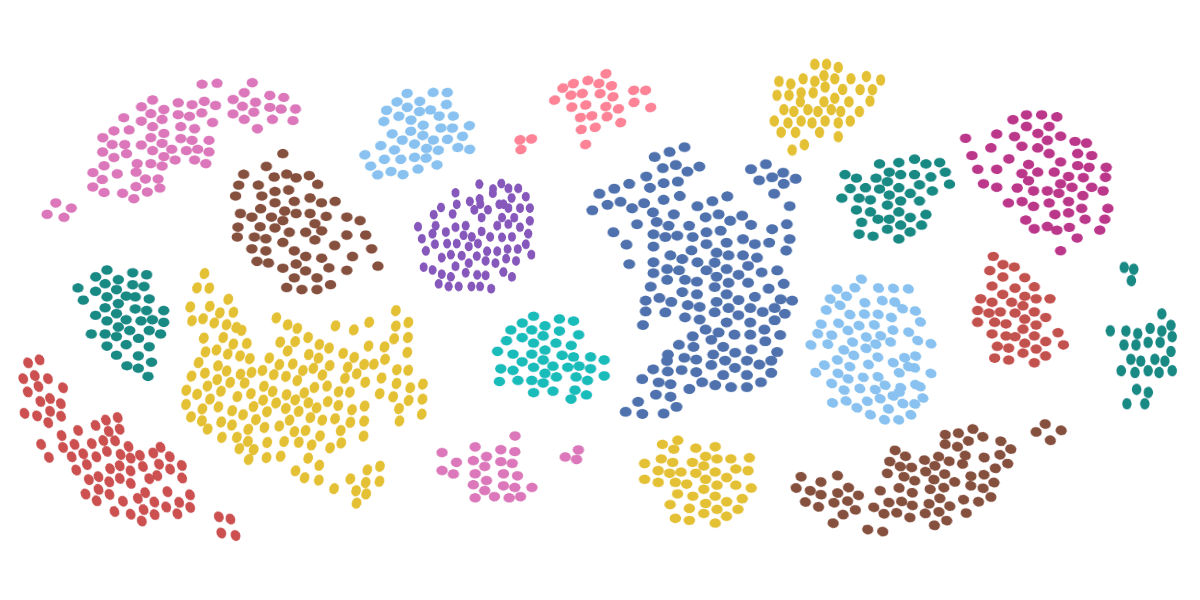
The power of measuring these physical parameters by flow cytometry light scatter becomes apparent when they are combined. It is possible to provide a first order identification of the cell subsets in a sample. This is shown below in Figure 4.
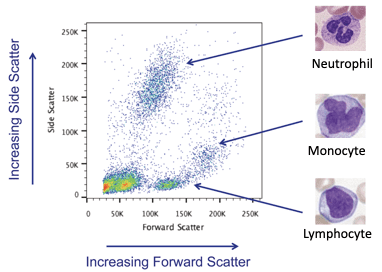
Figure 4: Identification of cellular subsets using physical parameters.
From https://www.drugtargetreview.com/article/6339/flow-cytometry-not-just-a-box-in-the-lab/
While these examples have shown separating heterogeneous populations from a complex mixture (blood), these parameters are useful even for otherwise homogeneous cell populations such as from cell culture. The scatter parameters can be used to identify small debris in the culture, as well as larger aggregations of cells. As flow cytometry is a single-cell technique, getting rid of these aggregates is an important step to ensure high-quality data for analysis or cell sorting.
In conclusion, flow cytometrists use the physical parameters of forward and side angle scatter to roughly describe the cell populations in their sample. Taken together, they are important components of the analysis workflow and can lead to greater characterization of the sample.
Sources:
- Zhang, Qinnan et al. “Quantitative refractive index distribution of single cell by combining phase-shifting interferometry and AFM imaging.” Scientific reports vol. 7,1 2532. 31 May. 2017, doi:10.1038/s41598-017-02797-8
- Stefka Nikolova Kasarova, Nina Georgieva Sultanova and Christo Dimitrov Ivanov et al. Analysis of the dispersion of optical plastic materials. Optical Materials. Vol. 29(11):1481-1490. DOI: 10.1016/j.optmat.2006.07.010
- Stefka Nikolova Kasarova, Nina Georgieva Sultanova and Christo Dimitrov Ivanov et al. Analysis of the dispersion of optical plastic materials. Optical Materials. Vol. 29(11):1481-1490. DOI: 10.1016/j.optmat.2006.07.010
- By User CS99 at German Wikipedia – German Wikipedia, Public Domain, https://commons.wikimedia.org/w/index.php?curid=349590
- By User CS99 at German Wikipedia – German Wikipedia, Public Domain, https://commons.wikimedia.org/w/index.php?curid=349598