Gastrinoma and Zollinger-Ellison Syndrome
Michael D. Smith, MD
The Operative Review of Surgery. 2023; 1:168-174.
Table of Contents
Pathophysiology
Definitions
- Gastrinoma: Gastrin Secreting Neuroendocrine Tumor 1
- Results in Gastric Acid Hypersecretion 2
- Generally Originates in the Duodenum or Islet Cells of the Pancreas 1
- *See Pancreatic Neuroendocrine Tumor (PNET)
- Zollinger-Ellison Syndrome (ZES): Syndrome of Gastric Acid Hypersecretion Due to Gastrinoma 2
Distribution
- Duodenum – Most Common Location 1,3
- Sporadic Tumors: 50-88%
- MEN-1-Associated Tumors: 70-100%
- *Most Common in the First Portion
- Pancreas: 20-25% 4
- Other: 5-15% 1,5-7
- Stomach
- Jejunum
- Liver
- Biliary Tract
- Peripancreatic Lymph Nodes
- Ovary
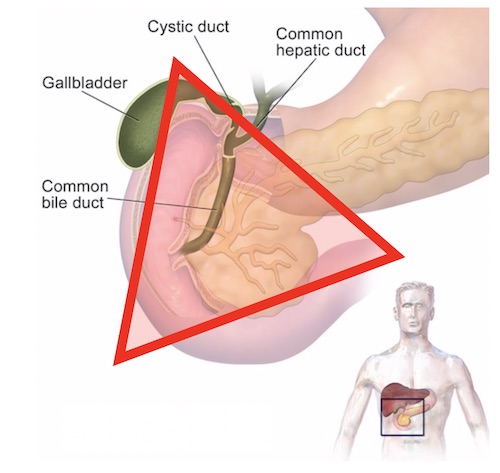
Gastrinoma (Passaro) Triangle
- Contain 60-90% of Tumors 9,10
- Borders:
- Junction of the Cystic & Common Bile Ducts
- Junction of the Second & Third Portions of the Duodenum
- Junction of the Neck & Body of the Pancreas
Size and Malignancy
- Duodenal Tumors: 4,10
- Usually Small in Size (< 1 cm) (60-90%)
- Liver Metastases are Uncommon (5%)
- Pancreatic Tumors: 4,10
- Usually Large (≥ 3 cm) (70%)
- Liver Metastases are Common (52%)
- Over Half Are Malignant (60-90%) at the Time of Diagnosis 10-12
- 20-30% are Associated with Multiple Endocrine Neoplasia Type 1 (MEN1) 3,13
- Most Common PNET in MEN-1 Syndrome 14
Epidemiology
- Average Age: 40 4
- 55-56% are Male 4
Gastrinoma (Passaro) Triangle 8
Presentation
Peptic Ulcer Disease
- Incidence: 73-98% 3,10
- The Most Common Presenting Symptom
- Due to Significantly Increased Basal Acid Output (BAO)
- Typically 4-Fold Higher, Can Be Over 10-Fold Higher 10
- Associated Symptoms:
- Abdominal Pain
- Bleeding
- Stricture
- Fistula
- Perforation
- Often Severe and Refractory to Initial Management with Proton Pump Inhibitors
- Ulcer Location: 15
- Proximal Duodenum: 75% – Most Common
- Distal Duodenum: 14%
- Jejunum: 11%
- *Often Occur More Distal in the Duodenum/Jejunum than Sporadic Ulcers
- Associated with Prominent Gastric Folds (94%) with Gastric Enterochromaffin-Like (ECL) Cell Hyperplasia 10
Additional Symptoms 3,10
- Heart Burn (52-55%)
- Diarrhea (60-75%)
- Weight Loss (7-53%)
- Nausea and Vomiting (20-30%)
Diagnosis
Diagnosis
- Diagnosis is Frequently Delayed 4-7 Years Because ZES is Such an Uncommon Cause of PUD 16-18
- Initial Screening: Elevated Fasting Serum Gastrin (FSG) and Measurement of Gastric pH 3,17,18
- Additional Testing if Initial Findings are Not Diagnostic:
- Secretin Stimulation Test
- Secretin Normally Shows Minimal Change but Induces a Marked Gastrin Increase in ZES
- Fasting Gastric Basal Acid Output (BAO) – Historical and Now Rarely Performed 3,19
- Secretin Stimulation Test
- Diagnostic Criteria: 10
- FSG > 10x Upper Limit of Normal and Gastric pH ≤ 2
- FSG < 10x Upper Limit of Normal and Secretin Stimulation Test Positive (≥ 120 pg/ml Increase)
- FSG < 10x Upper Limit of Normal and Elevated BAO (> 15 mEq/hr)
- Gastrin Measurement Requires Holding Any PPI for 3-7 Days Before Testing (PPIs Induce Hypergastrinemia) 20
- If Unsafe to Hold PPI (Life-Threatening Bleeding, etc.): Consider Somatostatin Receptor Imaging for Diagnosis & Localization
TNM Staging
- Same System Used for all Pancreatic Neuroendocrine Tumors 21
- *See Pancreatic Neuroendocrine Tumor (PNET)
Localization
- Initial Imaging: Noninvasive (CT or MRI)
- Somatostatin Receptor Imaging 22,23
- Consider if Initial Imaging Fails to Localize
- Options:
- Somatostatin (Octreotide) Receptor Scintigraphy (SRS) – Classic Test Used
- Functional PET Scan (Ga-68 DOTATATE) – Becoming More Prevalent with Higher Sensitivity
- If Noninvasive Imaging Fails: Invasive Imaging
- Endoscopic Ultrasound (EUS) – Generally Preferred Next Step 24
- Selective Arterial Secretin Stimulation with Hepatic Venous Sampling 25
- Selective Visceral Angiography
- Consider Surgical Exploration with Palpation or Intraoperative Ultrasound if High Suspicion but All Imaging Negative
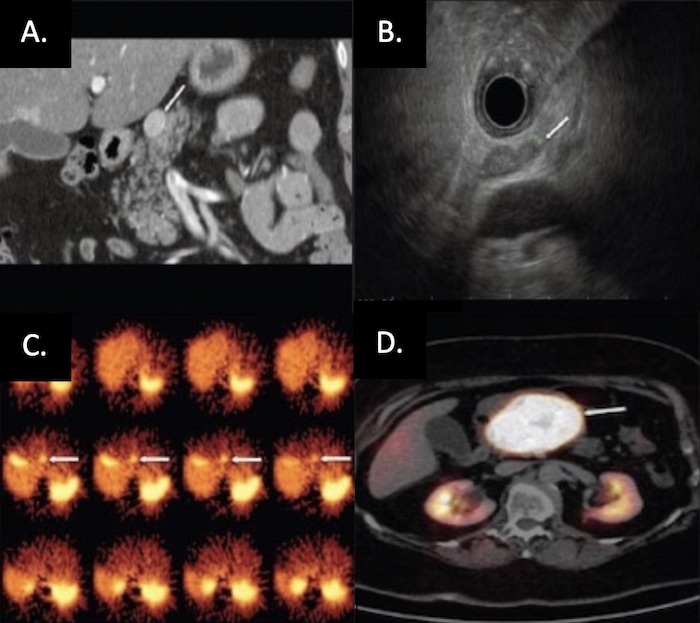
PNET on Imaging: (A) CT, (B) EUS, (C) SRS, (D) Functional PET 26
Treatment
Surgical Resection (Treatment of Choice)
- < 2-3 cm: Enucleation
- Additional Requirements:
- Single Lesion
- ≥ 2-3 mm From the Main Pancreatic Duct (Reduce Leak Risk)
- Well-Encapsulated
- No Local Invasion
- The Preferred Approach if Able
- Additional Requirements:
- > 2-3 cm: Surgical Resection
- Head/Neck: Pancreaticoduodenectomy
- Body/Tail: Distal Pancreatectomy (Concurrent Splenectomy if Malignancy is Suspected)
- Entire Pancreas: Total Pancreatectomy
Medical Management for Gastric Acid Hypersecretion
- Measures:
- High-Dose Proton Pump Inhibitor (PPI) – First Line 14,27,28
- Somatostatin Analogs (SSAs) if PPIs Fail 29,30
- Octreotide or Lanreotide
- Used Preoperatively or for Patients that are Not Surgical Candidates or in Unresectable Metastatic Disease
Liver-Directed Therapy
- Resection of Metastases if Able
- Radiofrequency Ablation (RFA) or Cryoablation 31,32
- Hepatic Artery Embolization 33
Additional Options in Surgically Unresectable Disease
- Chemotherapy 34,35
- Radiation Therapy 36,37
- Pancreatic Neuroendocrine Carcinomas Were Previously Considered to be Resistant to Radiation
References
- National Cancer Institute. NIH.
- ZOLLINGER RM, ELLISON EH. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann Surg. 1955 Oct;142(4):709-23; discussion, 724-8.
- Metz DC, Cadiot G, Poitras P, Ito T, Jensen RT. Diagnosis of Zollinger-Ellison syndrome in the era of PPIs, faulty gastrin assays, sensitive imaging and limited access to acid secretory testing. Int J Endocr Oncol. 2017;4(4):167-185.
- Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore). 2006 Nov;85(6):295-330.
- Norton JA, Alexander HR, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Possible primary lymph node gastrinoma: occurrence, natural history, and predictive factors: a prospective study. Ann Surg. 2003 May;237(5):650-7; discussion 657-9.
- Jensen RT, Cadiot G, Brandi ML, de Herder WW, Kaltsas G, Komminoth P, Scoazec JY, Salazar R, Sauvanet A, Kianmanesh R; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95(2):98-119.
- Norton JA, Foster DS, Blumgart LH, Poultsides GA, Visser BC, Fraker DL, Alexander HR, Jensen RT. Incidence and Prognosis of Primary Gastrinomas in the Hepatobiliary Tract. JAMA Surg. 2018 Mar 1;153(3):e175083.
- Blaus B. Wikimedia Commons (License: CC BY-SA 4.0) – Altered Image
- Stabile BE, Morrow DJ, Passaro E Jr. The gastrinoma triangle: operative implications. Am J Surg. 1984 Jan;147(1):25-31.
- Jensen RT, Ito T. Gastrinoma. 2020 Nov 21. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext
- Cingam SR, Botejue M, Hoilat GJ, Karanchi H. Gastrinoma. 2022 Sep 26. In: StatPearls
- Qin, R. (2015). Gastrinoma: Evidence of Surgical Treatment. In Pancreatic Cancer, Cystic Neoplasms and Endocrine Tumors (eds H.G. Beger, A. Nakao, J.P. Neoptolemos, S.Y. Peng and M.G. Sarr).
- Norton JA. Neuroendocrine tumors of the pancreas and duodenum. Curr Probl Surg. 1994 Feb;31(2):77-156.
- Jensen RT, Fraker DL. Zollinger-Ellison syndrome. Advances in treatment of gastric hypersecretion and the gastrinoma. JAMA. 1994 May 11;271(18):1429-35.
- Meko JB, Norton JA. Management of patients with Zollinger-Ellison syndrome. Annu Rev Med. 1995;46:395-411.
- Jensen RT, Gardner JD. Gastrinoma. In: Go VLW, DiMagno EP, Gardner JD et al. eds. The Pancreas: Biology, Pathobiology and Disease. New York: Raven Press Publishing Co.; 1993:931-978.
- Ito T, Cadiot G, Jensen RT. Diagnosis of Zollinger-Ellison syndrome: Increasingly difficult. World J Gastroenterol. 2012;18:5495–5503.
- Metz DC. Diagnosis of the Zollinger-Ellison syndrome. Clin Gastroenterol Hepatol. 2012;10:126–130.
- Roy PK, Venzon DJ, Feigenbaum KM, et al. Gastric secretion in Zollinger-Ellison syndrome: correlation with clinical expression, tumor extent and role in diagnosis – A prospective NIH study of 235 patients and review of the literature in 984 cases. Medicine(Baltimore). 2001;80:189–222.
- Mendelson AH, Donowitz M. Catching the Zebra: Clinical Pearls and Pitfalls for the Successful Diagnosis of Zollinger-Ellison Syndrome. Dig Dis Sci. 2017 Sep;62(9):2258-2265.
- Bergsland EK, Woltering EA, Rindo G. Neuroendocrine tumors of the pancreas. In: AJCC Cancer Staging Manual, 8th ed, Amin MB (Ed), AJCC, Chicago 2017. p.407. Corrected at 4th printing, 2018.
- Deppen SA, Liu E, Blume JD, Clanton J, Shi C, Jones-Jackson LB, Lakhani V, Baum RP, Berlin J, Smith GT, Graham M, Sandler MP, Delbeke D, Walker RC. Safety and Efficacy of 68Ga-DOTATATE PET/CT for Diagnosis, Staging, and Treatment Management of Neuroendocrine Tumors. J Nucl Med. 2016 May;57(5):708-14.
- Hope TA, Bergsland EK, Bozkurt MF, Graham M, Heaney AP, Herrmann K, Howe JR, Kulke MH, Kunz PL, Mailman J, May L, Metz DC, Millo C, O’Dorisio S, Reidy-Lagunes DL, Soulen MC, Strosberg JR. Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors. J Nucl Med. 2018 Jan;59(1):66-74.
- Rösch T, Lightdale CJ, Botet JF, Boyce GA, Sivak MV Jr, Yasuda K, Heyder N, Palazzo L, Dancygier H, Schusdziarra V, et al. Localization of pancreatic endocrine tumors by endoscopic ultrasonography. N Engl J Med. 1992 Jun 25;326(26):1721-6.
- Thom AK, Norton JA, Doppman JL, Miller DL, Chang R, Jensen RT. Prospective study of the use of intraarterial secretin injection and portal venous sampling to localize duodenal gastrinomas. Surgery. 1992 Dec;112(6):1002-8; discussion 1008-9.
- Kartalis N, Mucelli RM, Sundin A. Recent developments in imaging of pancreatic neuroendocrine tumors. Ann Gastroenterol. 2015 Apr-Jun;28(2):193-202. (License: CC BY-NC-SA 3.0)
- Hirschowitz BI, Simmons J, Mohnen J. Clinical outcome using lansoprazole in acid hypersecretors with and without Zollinger-Ellison syndrome: a 13-year prospective study. Clin Gastroenterol Hepatol. 2005 Jan;3(1):39-48.
- Metz DC, Comer GM, Soffer E, Forsmark CE, Cryer B, Chey W, Pisegna JR. Three-year oral pantoprazole administration is effective for patients with Zollinger-Ellison syndrome and other hypersecretory conditions. Aliment Pharmacol Ther. 2006 Feb 1;23(3):437-44.
- Daniels LM, Khalili M, Morano WF, Simoncini M, Mapow BC, Leaf A, Bowne WB. Case report: optimal tumor cytoreduction and octreotide with durable disease control in a patient with MEN-1 and Zollinger-Ellison syndrome-over a decade of follow-up. World J Surg Oncol. 2019 Dec 9;17(1):213.
- Aerts M, Reynaert H. Disease Control on Lanreotide Autogel® 120 mg in a Patient with Metastatic Gastrinoma: A Case Report. Case Rep Gastroenterol. 2017 Dec 6;11(3):616-623.
- Deol ZK, Frezza E, DeJong S, Pickleman J. Solitary hepatic gastrinoma treated with laparoscopic radiofrequency ablation. JSLS. 2003 Jul-Sep;7(3):285-9.
- Grandhi MS, Lafaro KJ, Pawlik TM. Role of Locoregional and Systemic Approaches for the Treatment of Patients with Metastatic Neuroendocrine Tumors. J Gastrointest Surg. 2015 Dec;19(12):2273-82.
- Chen JX, Rose S, White SB, El-Haddad G, Fidelman N, Yarmohammadi H, Hwang W, Sze DY, Kothary N, Stashek K, Wileyto EP, Salem R, Metz DC, Soulen MC. Embolotherapy for Neuroendocrine Tumor Liver Metastases: Prognostic Factors for Hepatic Progression-Free Survival and Overall Survival. Cardiovasc Intervent Radiol. 2017 Jan;40(1):69-80.
- Kouvaraki MA, Ajani JA, Hoff P, Wolff R, Evans DB, Lozano R, Yao JC. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004 Dec 1;22(23):4762-71.
- Prakash L, Bhosale P, Cloyd J, Kim M, Parker N, Yao J, Dasari A, Halperin D, Aloia T, Lee JE, Vauthey JN, Fleming JB, Katz MH. Role of Fluorouracil, Doxorubicin, and Streptozocin Therapy in the Preoperative Treatment of Localized Pancreatic Neuroendocrine Tumors. J Gastrointest Surg. 2017 Jan;21(1):155-163.
- Contessa JN, Griffith KA, Wolff E, Ensminger W, Zalupski M, Lawrence TS, Ben-Josef E. Radiotherapy for pancreatic neuroendocrine tumors. Int J Radiat Oncol Biol Phys. 2009 Nov 15;75(4):1196-200.
- Strosberg J, Hoffe S, Gardner N, Choi J, Kvols L. Effective treatment of locally advanced endocrine tumors of the pancreas with chemoradiotherapy. Neuroendocrinology. 2007;85(4):216-20.

