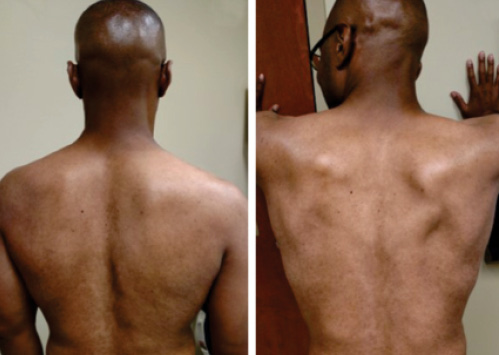
Figure 1. Inspection of the patient’s back at rest (A) and with pressing on the wall (B). Note the prominence of the scapular border and depressed shoulder on the right side.
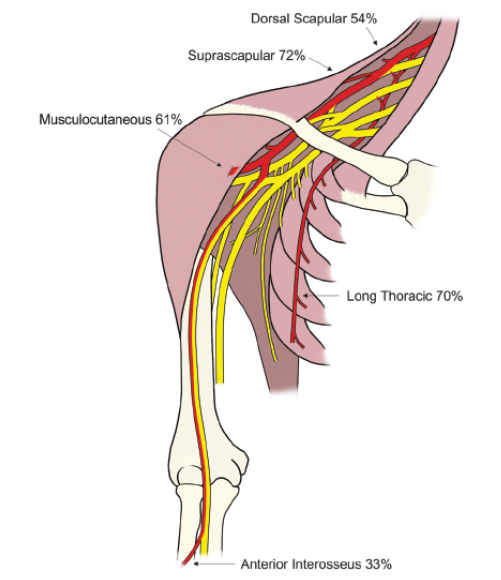
Figure 2. The brachial plexus with the regions that are commonly affected in red along with the percentage of NA patients that have involvement of each nerve.
A 49-year-old man presents to an electrodiagnostic (EMG) laboratory with a one-week history of new onset sudden right shoulder pain. He stated that he woke up with the pain one morning after an intense workout and that, “Now I know what 10/10 pain is; you are on the floor!” He went to the emergency room for treatment, was given pain medications, and then discharged. Two days after the pain episode he noted weakness in his right shoulder and difficulty abducting and flexing his right shoulder. Upon visual inspection, the patient’s right shoulder was depressed when compared to the left (Figure 1A). Additionally, the medial border of the right scapula appeared prominent posteriorly, which became more apparent when he pressed on the wall (Figure 1B). This finding is consistent with damage to the long thoracic nerve and subsequent serratus anterior muscle denervation, resulting in scapular winging.
The goal of this review is to characterize the approach to the patient described above. We review the anatomy and clinical aspects of Neuralgic Amyotrophy (NA) or Parsonage-Turner Syndrome with the goal of improving clinicians’ ability to diagnose and manage this potentially disabling and treatable condition. Neuralgic amyotrophy, or Parsonage-Turner Syndrome, is an idiopathic brachial plexopathy that is often mistaken for shoulder and neck pathology. Ahead, we will review the current state of knowledge regarding the anatomical and clinical aspects of this disorder with the goal of empowering clinicians with the information necessary to easily diagnose this potentially disabling and treatable condition.
Anatomy
The exact cause of NA is not clear. Immunological, mechanical and genetic factors have been implicated.1 The disorder is generally thought of as an autoimmune attack on the brachial plexus which is the neurological structure that connects the ventral (anterior) rami of the C5-T1 spinal nerves to the peripheral nerves of the upper limb. NA has a strong predilection for the upper (superior) trunk innervated muscles although the nerves affected are quite variable and the anterior interosseous nerve (AIN) is often affected as well. This can be very helpful in the diagnosis as very few entities can cause profound weakness in both the upper trunk and the AIN. The anatomy of the brachial plexus and percentage of each nerve involved2 is provided in Figure 2. Even though we typically think of NA as a plexopathy, involvement of the trapezius, phrenic nerve, recurrent laryngeal nerve and even cervical paraspinal muscles does not rule out NA. Finally, it is worth noting that a plexopathy such as NA can often result in much more profound weakness than a single level radiculopathy. For example, a C5 radiculopathy will not compromise all of the innervation to the biceps brachii because of the C6 contribution to this muscle. However, an upper trunk or lateral cord plexopathy can compromise 100 percent of the nerve fibers destined to the biceps and resulting in amyotrophy or even paralysis. Therefore, the clinician must always question the diagnosis of cervical radiculopathy when motor strength in an upper limb muscle is Medical Research Council (MRC) 3/5 or less on physical examination. It is also worth noting that NA can cause variable involvement of muscles innervated by the same nerve root. For example one can have complete paralysis of the brachialis with a relatively normal biceps brachii.
Clinical Presentation
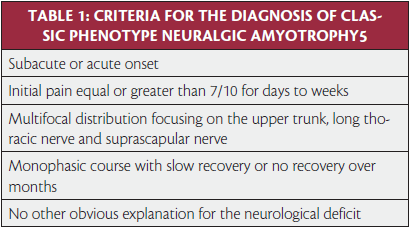
In 1964, United States Supreme Court Justice Potter Stuart famously proclaimed, “I know it when I see it,” with regard to questionable content in a motion picture.3 There are few neurological disorders that fit this description better than neuralgic amyotrophy. Once you have seen it or heard about it, you just have to know what questions to ask and what to look for.2 While NA is often thought of as rare condition with an annual incidence less than two per 100,000,4 more recent research has suggested a incidence as high as 1 per 1,000 when providers are “looking” for it.5 NA is characterized as sudden shoulder pain with subsequent patchy paresis in the upper extremity.6 Specific criteria for the diagnosis of NA have been defined by van Alfen (Table 1).
On history, it is important to ask the severity of the pain, the course of the illness, and history of preceding events such as viral infection, immunization, strenuous exercise, trauma, and operative procedures.7 Post-surgical NA is extremely difficult to recognize following neck and shoulder surgery when one would expect pain and weakness as a natural consequence of a surgical procedure.8 However, a sudden change of symptoms, such an acute increase and change in quality and localization of the point should raise a suspicion for NA. It is important to query orthopnea with exertional dyspnea to assess for phrenic nerve involvement. It is also helpful to obtain a family history of similar symptoms, as there is a hereditary form as well.
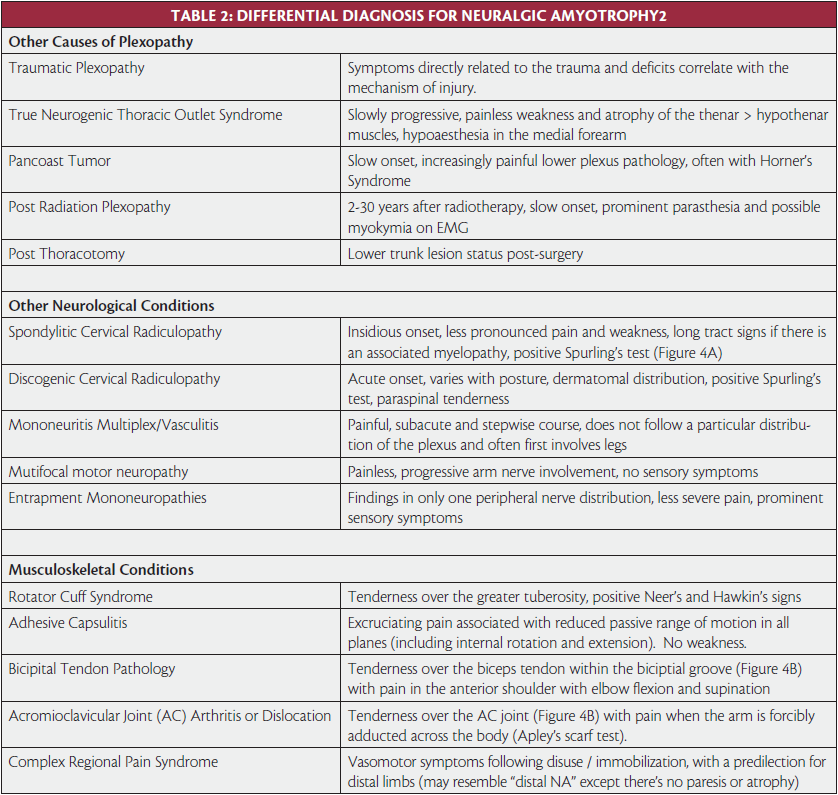
On physical examination, it is imperative to do a comprehensive motor, sensory, and reflex examination. It is important to examine the patient’s scapula for winging during a forward flexion-abduction movement of the shoulder. The long thoracic nerve innervates the serratus anterior and stabilizes the scapula against the chest wall. When the arms are flexed a compromised long thoracic nerve will result in the scapula displaced closer to the midline than the unaffected side.9 During abduction there will be lagging of scapular movement versus glenohumeral rotation and in the eccentric (downward or return) phase of forward flexion and abduction the scapula cannot be fixed against the chest wall, giving the typical winged appearance. At full rest the shoulder blade will be in a lateralized and more caudal position, although often the compensatory action of the trapezius, levator scapulae and pectoral muscles will cause the shoulder to be more adducted, elevated and ventralized than on the healthy side. The spinal accessory nerve innervates the trapezius, which elevates, adducts, and rotates the scapula. The trapezius functions simultaneously with the serratus anterior to allow for coordinated scapulothoracic movement to complement full shoulder range of motion. Therefore, with arm abduction, scapular winging will also be noted if the trapezius is compromised. Opposite to serratus weakness, the arm will now be “down and out”, because the healthy serratus will pull the shoulder blade ventrally. The patient may also have trapezius atrophy with resultant shoulder drop due to the weight of the arm.9
Involvement of the anterior interosseous nerve (AIN) with shoulder girdle weakness without trauma at onset is pathognomonic for NA. The Kiloh-Nevin sign is a physical examination finding of anterior interosseous neuropathy; the patient is asked to make an OK sign (Figure 3D) and is unable to flex the distal thumb and index finger interphalangeal joints. The patient will also have weakness when pronating the forearm with a flexed elbow (which prevents full pronator teres muscle strength so that the pronator quadratus becomes the major pronator of the arm); this can be tested by applying resistance against pronation.10
Excellent comprehensive protocols for the physical examination of a patient with suspected NA have been described previously.2,11 Also available is an exhaustive description of physical examination maneuvers assess almost all of the neuromuscular and musculoskeletal structures in the upper limb.12 Figures 3 and 4 demonstrate an abbreviated version of the neuromuscular and musculoskeletal physical examination, respectively. Figure 3C, testing of the elbow flexors, deserves special mention. Note how the examiner specifically isolates the elbow flexors by placing her arms above and below the joint. It is very important to use techniques such as this for “isolation” of muscles as weakness in the shoulder girdle can cause false positive “weakness” in muscles distal to the shoulder. When one is not careful, it is not possible to know whether the weakness comes from the elbow flexors, the shoulder girdle, the trunk muscles, or even the stability of the chair the patient is sitting in.
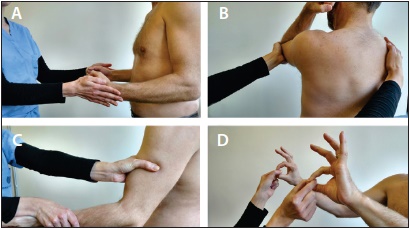
Figure 3. The brief neuromuscular examination of the patient with NA. A) Assessment of the external rotators of the shoulder can be done by having the patient hold their elbows flexed at 90 degrees. The patient is then told to resist while the examiner applies a force to internally rotate the shoulder. B) Assessment of the serratus anterior can be done by having the patient flex their elbow, flex and adduct their shoulder. The examiner then places a hand on the contralateral scapula and asks the patient to resist as a posterior force is applied to the elbow. Weakness is present if there is asymmetric scapular winging when compared to the contralateral side (as in Figure 5A). C) Assessment of the elbow flexors is accomplished by isolating the muscle with a hand above and below the joint and forcin the forearm down. D) Assessment of the AIN nerve is performed by having the patient make an “OK” and then trying to prevent the examiner from “breaking” the okay by pulling.
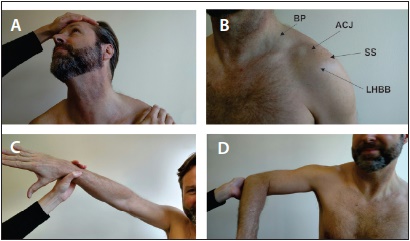
Figure 4. A brief assessment of the musculoskeletal structures includes the Spurling’s test. A) in which the neck is passively extended and then rotated and laterally deviated to the symptomatic side. A positive test is when the patient’s sensory symptoms are reproduced with axial compression. B) Palpation of the shoulder is a very easy way to assess for musculoskeletal pathology if one knows the surface anatomy. Common sources of shoulder pain include the rotator cuff syndrome (tendonopathy or tendon tear) with pain at the insertion of the supraspinatus, acromioclavicular joint pathology such as arthritis, and bicipital tendon pathology. The offending structure will often be tender when palpated on examination. The Neer’s (C) and Hawkin’s (D) signs assess for rotator cuff impingement due to tendon pathology. In the Neer’s sign the examiner performs passive internal rotation of the arm while in the Hawkin’s sign the examiner performs passive abduction and internal rotation of the arm. A positive sign is when there is shoulder pain with the movement.
Diagnostic Studies
Before discussing electrodiagnosis, it is important to recognize that NA is a clinical diagnosis. Since nerve conduction studies of proximal nerves are often difficult to perform and sensory NCS can give normal results even in clinically affected regions,13 confirmation of the diagnosis mainly relies on needle EMG of the clinically affected muscles. A suggested protocol for an electrodiagnostic evaluation of a patient with suspected NA is listed in Table 3. Note that the protocol deviates from what one might use for a suspected radiculopathy, which is why a detailed history and physical is so important in the diagnosis of NA. The protocol can be altered based upon the physical examination findings. Needle examination of the serratus anterior can be challenging without ultrasound due to the fact that it is quite easy to accidentally enter the latissimus dorsi, abdominal muscles, or worst of all, pleura. The assessment of the flexor pollicus longus should also be done under ultrasound guidance if possible because there is a 0.5cm gap between the radial artery and the median nerve in this region. The author has unfortunately caused an iatrogenic median nerve injury when targeting this muscle. However, since the clinical picture is often typical and the differential diagnosis limited, the utility of electrodiagnostic testing mainly rests in excluding other pathology, such as radiculopathy, mononeuropathy, or mononeuritis multiplex.
Key Practice Points
Neuralgic Amyotrophy should be suspected in any patient with sudden onset, severe continuous pain in the shoulder or arm followed by weakness and numbness and muscle wasting.
If diagnostic testing such as MRI or electrodiagnostic (EMG) studies will delay treatment, the patient should be treated empirically with high dose steroids when there are no contraindications.
While neuromuscular ultrasound is well established for evaluation of entrapment neuropathies,14 ultrasound of brachial plexus pathology is an emerging field. Recent research by Aranyi, et al. has characterized the extremely heterogenous findings one encounters in ultrasound imaging of the brachial plexus.15 From clinical experience we find that NA patients can show focal abnormalities on nerve US scanning of the interscalene plexus and proximal limb nerves, showing focal nerve thickening of the nerve and especially the perineural connective tissue layers. Another helpful sonographic finding is atrophy and increased hyperechogenicity of muscles such as the infraspinatus which can result from chronic dennervation. Once one develops experience with ultrasound examination technique and anatomy, neuromuscular ultrasound can be done efficiently and be very helpful in equivocal cases. The major caveat is that ultrasound is not helpful for excluding most other neurological disorders in the differential. Therefore, the authors recommend ultrasound as an adjuvant to electrodiagnosis and not a standalone diagnostic test.
A fully integrated diagnostic approach to the diagnosis of NA is illustrated in Figure 5 combining elements of history, physical examination, and ultrasound. In figure 5C, notice how helpful ultrasound side to side comparison can be in terms of identifying anatomical alterations in the plexus. Almost always, patients can recognize the difference between the affected and normal plexus and find it very helpful to “see” what has been causing their pain and weakness for the first time. Also, sonopalpation (applying transducer pressure) to the plexus in the supraclavicular region (root of the neck) can reproduce pain in a sort of sonographic “Tinel’s sign” of the plexus.
Finally, it is important to understand the pitfalls of MRI in NA. Given the low specificity of MRI of the cervical spine and shoulder, it is not uncommon for patients to have false positive imaging of their shoulder16 or cervical spine17 which may erroneously lead a clinician to believe that cervical spine or rotator cuff pathology is the culprit for the weakness and symptoms. This can result in unnecessary treatment that will at best have no benefit and at worst exacerbate the condition. It is incumbent upon the treating physician to clinically correlate imaging findings to prevent unnecessary surgical interventions.
Treatment
Many cases of NA are diagnosed months after the initial insult. Unfortunately, patients generally do not benefit from the typical neuropathic analgesics such as calcium-channel alpha 2-delta ligands, tricyclic antidepressants, and selective serotonin norepinephrine inhibitors. Given the severity of the pain, opioid analgesia may be necessary immediately after presentation. If a patient is diagnosed within the first week, patients may benefit from a course of oral steroids. A reasonable protocol for adults was found to be 60mg of prednisone (1mg/kg per day) for one week, tapering with 10mg per day during the second week.2 We recommend that providers not wait for electrodiagnostic confirmation of the disorder if it will delay treatment. Intravenous Immunoglobulin (IVIg) has also been proposed as a treatment,18 although there is not enough evidence to recommend its use at this time. Ultimately the best treatment is rehabilitative / allied health care therapy. However, Physiotherapists (Physical Therapists) with experience in treating NA are hard to come by given the perceived rarity of the disorder, and unfortunately standard physical therapy can be ineffective in over 50 percent of patients. An integrated approach focusing on addressing scapular stability and increased fatigability with therapists well versed in NA is recommended.19 Unfortunately, the natural history of NA is less favorable than what is described in the textbooks with 2/3 of patients exhibiting pain and functional impairments three years after onset.6
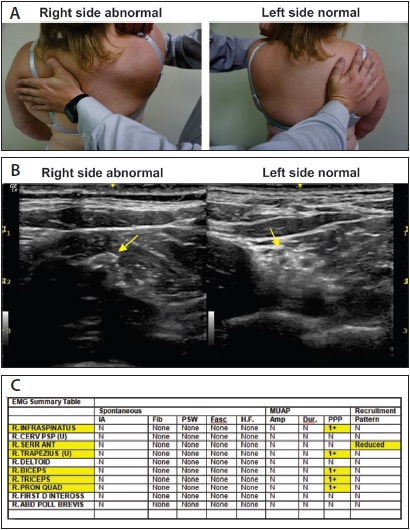
Figure 5. An integrated approach to the diagnosis of NA in a 39 year old woman a history of 10/10 shoulder pain following a surgical repair of a labral tear several months prior. A) Scapular winging with manual muscle testing of the right serratus anterior. B) Cross sectional neuromuscular ultrasound of the brachial plexus at the supraclavicular level. The left side shows the normal appearance of the brachial plexus with hypoechoic (dark) circles surrounded by hyperechoic (white) perineural fat. On the right side, there is loss of the normal nerve architecture. C) Needle electrode (EMG) findings reveal chronic dennervation and reinnervation of multiple nerves as manifested by polyphasic potentials (PPP) in muscles innervated from the upper trunk and one muscle (pronator quadratus) innervated by the AIN.
Resolution of Case
The patient described above was started on high dose steroids and returned to clinic two weeks later with much improved strength and pain. He was caught early enough to receive treatment, which, unfortunately, is an uncommon occurrence given that the average time from symptom onset to diagnosis is over two months, and steroids are only thought to be effective within the first week after symptom onset.6 He also received a comprehensive home exercise program by a therapist with extensive training in rehabilitation of the shoulder focused on strengthening of the shoulder girdle musculature.
Future Directions and Conclusion
Further elucidating the pathogenesis of the disease and causative agents is an ongoing area of investigation. A high index of suspicion by physicians and careful monitoring of the disorder by epidemiologists may be very helpful in elucidating potential infectious etiologies. For example, Hepatitis E20 and Dengue Fever21 have been implicated in the disorder. There have been reports of epidemics of NA such as an increased incidence in Czechoslovakia from 1949 to 1953 associated with a Coxsackie virus type A2 and an outbreak from April to June 1997 among a Native American Indian population in the southwestern United States. We are currently looking into an increased incidence of NA in Eastern North Carolina, United States which started in May 2015 and continues at the time of this writing. A heightened vigilance as well as education of primary care providers and emergency physicians has the potential to greatly benefit this patient population through early diagnosis and treatment. n
John W. Norbury, MD is Clinical Assistant Professor of Physical Medicine and Rehabilitation and Director of the ECU/Vidant Electrodiagnostic Laboratory at East Carolina University in Greenville, NC.
Nens van Alfen, MD, PhD is Medical Director of CNP laboratory at Radboud University Medical Center in Nijmegen, Netherlands. She has co-authored several studies on NA.
Kelly Harrell, PhD, MPT is an Adjunct Assistant Professor of Physical Medicine and Rehabilitation at East Carolina University.
Carrie McShane, MD is a Resident Physician at The Brody School of Medicine at East Carolina University.
Daniel Moore, MD is Professor and Chair of Physical Medicine and Rehabilitation at East Carolina University.
Steven Mandel, MD is a Clinical Professor of Neurology at Hofstra North Shore LIJ School of Medicine, Hempstead, NY.
Acknowledgements: Alan Branigan for his assistance in the development of the figures.
1. van Alfen N. Clinical and pathophysiological concepts of neuralgic amyotrophy. Nat Rev Neurol. 2011;7:315-22.
2. van Alfen N. The trouble with neuralgic amyotrophy. Practical Neurology. 2006;6:298-307.
3. Stewart P. Jacobellis v. Ohio 378 U.S. 184. 1964.
4. Beghi E, Kurland LT, Mulder DW, Nicolosi A. Brachial plexus neuropathy in the population of rochester, minnesota, 1970-1981. Ann Neurol. 1985;18:320-3.
5. van Alfen N, van Eijk JJ, Ennik T, et al. Incidence of neuralgic amyotrophy (parsonage turner syndrome) in a primary care setting--a prospective cohort study. PLoS One. 2015;10:e0128361.
6. van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129:438-50.
7. Smith CC, Bevelaqua AC. Challenging pain syndromes: Parsonage-turner syndrome. Phys Med Rehabil Clin N Am. 2014;25:265-77.
8. Verhasselt S, Schelfaut S, Bataillie F, Moke L. Postsurgical parsonage-turner syndrome: A challenging diagnosis. Acta Orthop Belg. 2013;79:20-4.
9. Preston D.C. Shapiro B. Proximal neuropathies of the shoulder and arm. In: Electromyography and neuromuscular Disorders clincial-electrophysiologic correlations. 3rd ed. New York: Elsevier Saunders; 2013:494.
10. Preston D.C. Shapiro B. Proximal median neuropathy. In: Electromyography and neuromuscular disorders clinical electrophysiologic correlations. 3rd ed. New York: Elsevier Saunders; 2013:291.
11. Schreiber AL, Abramov R, Fried GW, Herbison GJ. Expanding the differential of shoulder pain: Parsonage-turner syndrome. J Am Osteopath Assoc. 2009;109:415-22.
12. Miller A, Heckert KD, Davis BA. The 3 minute musculoskeletal and peripheral nerve exam. 1st ed. New York: Demos Medical; 2009.
13. van Alfen N, Huisman WJ, Overeem S, van Engelen BG, Zwarts MJ. Sensory nerve conduction studies in neuralgic amyotrophy. Am J Phys Med Rehabil. 2009;88:941--6.
14. Norbury JW, Cartwright MS, Walker FO, Gutierrez C, Moore DP, Mandel S. Ultrasonographic evaluation of entrapment neuropathies in the upper limb. Pract Neurol. 2011;10:38-44.
15. Aranyi Z, Csillik A, Devay K, et al. Ultrasonographic identification of nerve pathology in neuralgic amyotrophy: Enlargement, constriction, fascicular entwinement, and torsion. Muscle Nerve. 2015. 52:503-11.
16. Gill TK, Shanahan EM, Allison D, Alcorn D, Hill CL. Prevalence of abnormalities on shoulder MRI in symptomatic and asymptomatic older adults. Int J Rheum Dis. 2014;17:863-71.
17. Healy JF, Healy BB, Wong WH, Olson EM. Cervical and lumbar MRI in asymptomatic older male lifelong athletes: Frequency of degenerative findings. J Comput Assist Tomogr. 1996;20:107-12.
18. Naito KS, Fukushima K, Suzuki S, et al. Intravenous immunoglobulin (IVIg) with methylprednisolone pulse therapy for motor impairment of neuralgic amyotrophy: Clinical observations in 10 cases. Intern Med. 2012;51:1493-500.
19. Cup EH, Ijspeert J, Janssen RJ, et al. Residual complaints after neuralgic amyotrophy. Arch Phys Med Rehabil. 2013;94:67-73.
20. Dartevel A, Colombe B, Bosseray A, et al. Hepatitis E and neuralgic amyotrophy: Five cases and review of literature. J Clin Virol. 2015;69:156-64.
21. Verma R, Sharma P, Khurana N, Sharma LN. Neuralgic amyotrophy associated with dengue fever: Case series of three patients. J Postgrad Med. 2011;57:329-31.
