A stepwise and careful history helps identify the many causes of vertigo and dizziness. In the next issue, Part 2 covers the oculomotor and vestibular examinations.
First, Characterize Symptoms
Definitions
In 2009, the Barany Society published the first consensus classification of vestibular symptoms. Internal vertigo is a false or distorted sensation of self-motion including spinning, swaying, bobbing, tilting, bouncing, and sliding. External vertigo is a false or distorted sensation of the surroundings, excluding bidirectional motion, which is known as oscillopsia. The feeling of being unstable without a particular direction preference while sitting, standing, or walking is unsteadiness. Dizziness is a nonmotion sensation of disrupted spatial orientation. Purposefully, the definitions do not suggest a particular disease pathophysiology.1 Orthostatic hypotension and benign paroxysmal positional vertigo (BPPV), for instance, can both induce vertigo or dizziness,2,3 although the term vertigo will be used throughout this article to describe either symptom. Patients may have more than a single symptom at a time. For example, a common combination of symptoms in vestibular neuritis includes vertigo (due to semicircular canal imbalance) and oscillopsia (due to horizontal jerk nystagmus). Patients may also have symptoms that transition from one to another over time; for example, acute vertigo to chronic unsteadiness.
Direction
In some vestibular disorders (eg, vestibular paroxysmia), patients have directionally specific spinning that may be better recognized in vertigo than in external vertigo.4 Spinning vertigo that changes direction during a single event, is unique to Ménière’s disease and related to the phases of the attack—excitatory, inhibitory, or recovery.5 Understanding the direction of vertigo can occasionally help lateralize the disorder or better understand the pathophysiology.
Duration
Vertigo spells are brief, usually lasting seconds in patients with BPPV, vestibular paroxysmia, and cardiac arrhythmias. Patients with Ménière’s disease, vestibular migraine (VM), or transient ischemic attacks (TIAs) often present with vertigo spells lasting minutes to hours. In patients with with vestibular neuritis or central vestibular lesions from stroke or demyelination, vertigo lasts days to weeks. Patients with bilateral vestibular loss (BVL), uncompensated unilateral vestibular loss (UVL), chronic intoxication, or persistent postural perceptual dizziness (PPPD) often have months to years of symptoms.
In episodic conditions, asking the patient how long the specific vestibular symptom lasted (ie, dizziness or vertigo), rather than how long an attack lasted, can give a better estimate of duration. For example, a patient with BPPV may overestimate attack duration (eg, 5 minutes) because of persisting vegetative symptoms (eg, nausea, vomiting, and sweating) even when the spinning associated with BPPV lasted less than 1 minute.6
Second, Categorize Symptoms
Vestibular disorders can be grouped by presentation into acute, episodic, and chronic vestibular syndromes (AVS, EVS, and CVS, respectively). Patients with AVS present with more than 24 hours of continuous vertigo (lasting days to weeks and monophasic) with nausea/vomiting, imbalance, head motion intolerance, spontaneous nystagmus (eg, stroke or vestibular neuritis). Patients with EVS have similar symptoms and signs as AVS, lasting seconds to hours (eg, Ménière’s disease, VM). Patients with CVS have constant vestibular symptoms for weeks to years (eg, bilateral vestibular loss).
A convenient strategy is to employ a 2-layer approach to acute and episodic clinical syndromes (Figures 1-4).1
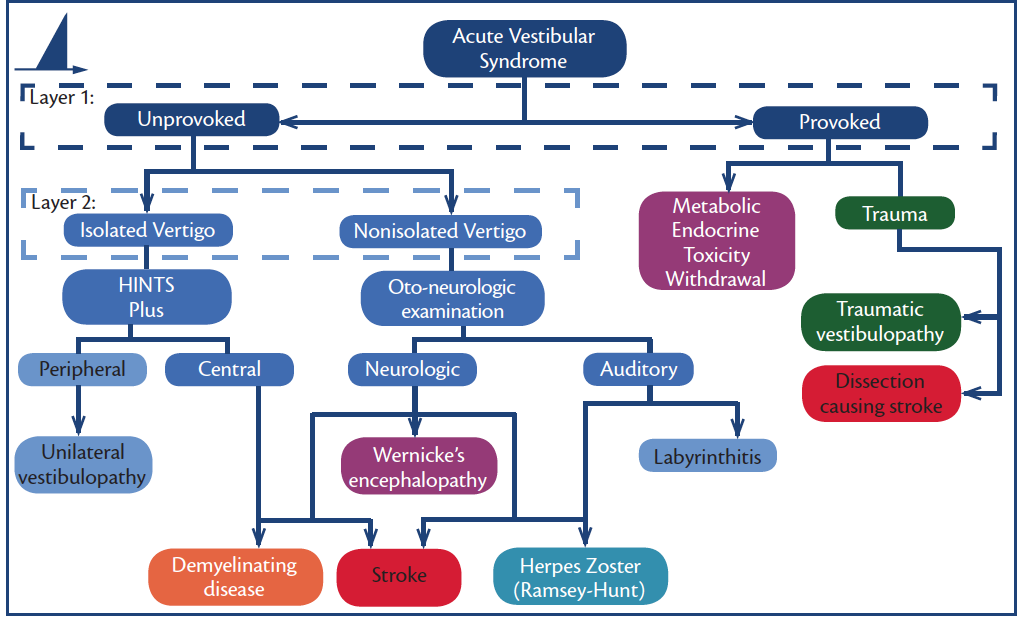
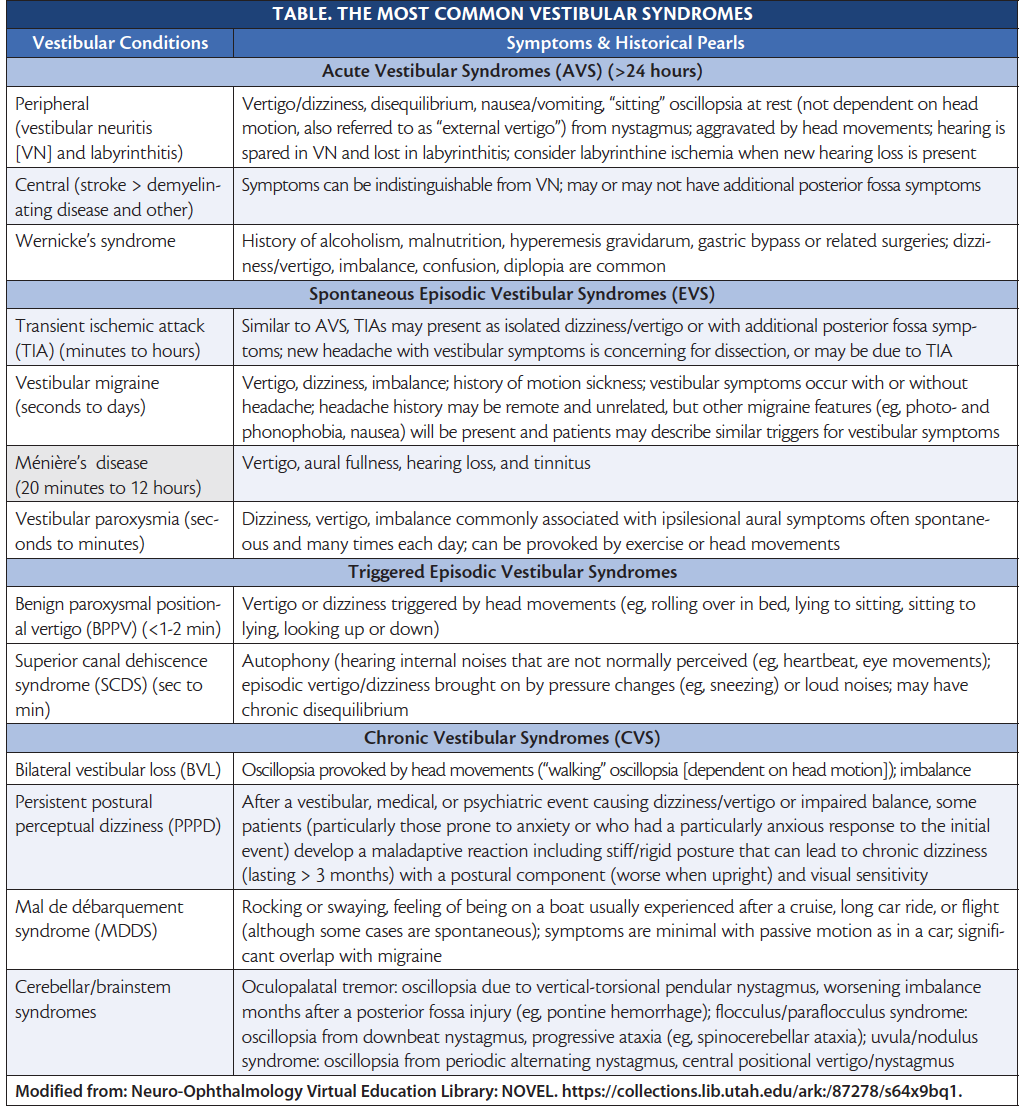
Figure 1. Acute vestibular syndromes (AVS). Note: HINTS Plus is a mnemonic for head impulse, nystagmus, test for skew, plus new unilateral hearing loss.
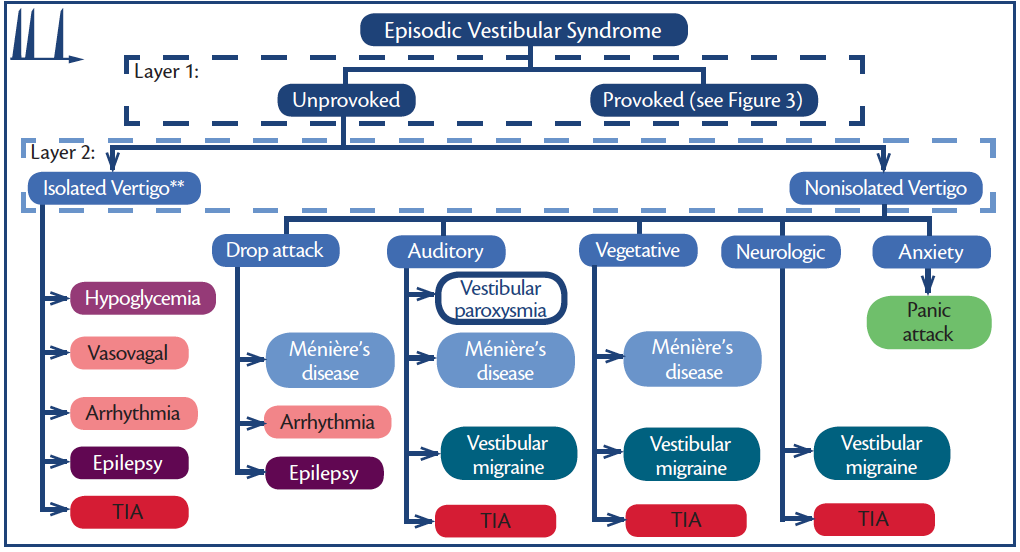
Figure 2. Episodic vestibular syndromes (EVS)—unprovoked. **Each of these disorders may or may not be isolated (eg, aura is common in seizure and diaphoresis in hypoglycemia.) Abbreviation: TIA, transient ischemic attack.
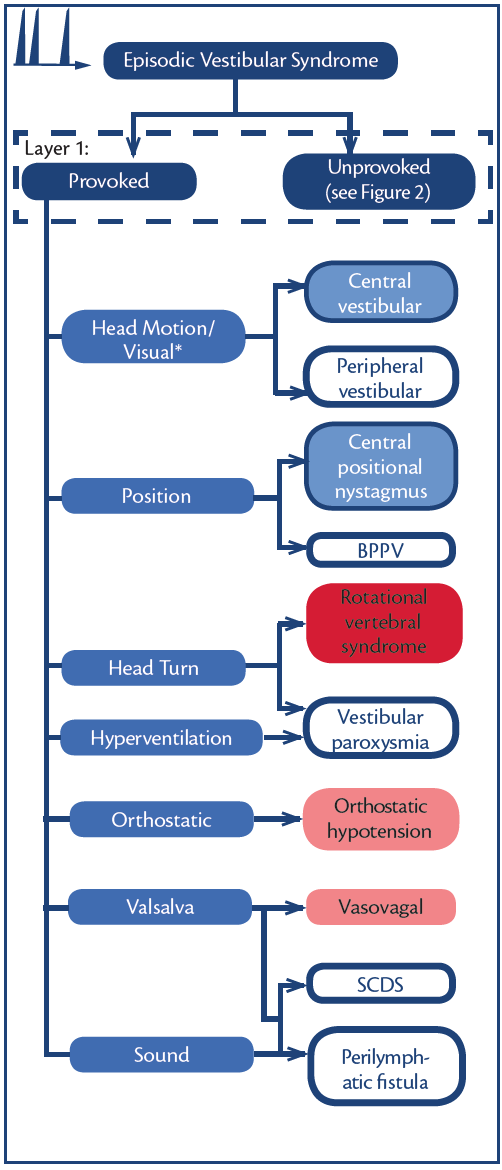
Figure 3. Episodic vestibular syndromes (EVS)—provoked. * Visual vertigo following any vestibular disorder. Abbreviations: BPPV, benign paroxysmal positional vertigo; SCDS, superior canal dehiscence syndrome.
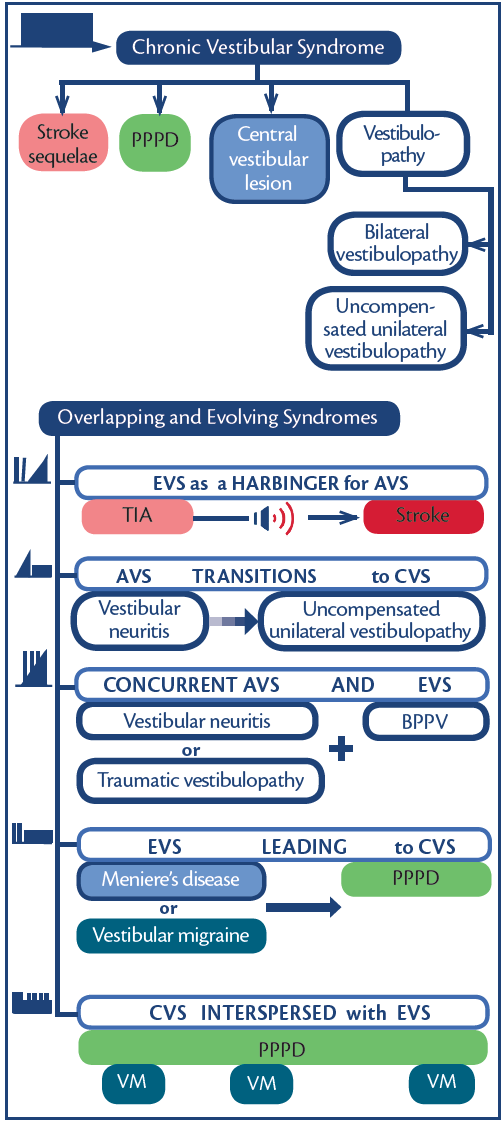
Figure 4. Chronic vestibular syndromes and overlapping/evolving syndromes. Abbreviations: AVS, acute vestibular syndrome; BPPV, benign paroxysmal positional vertigo, CVS, chronic vestibular syndrome; EVS, episodic vestibular syndrome; PPPD, persistent postural perceptual dizziness; TIA, transient ischemic attack; VM, vestibular migraine.
1. First Layer: Are symptoms provoked or unprovoked?
2. Second Layer: Do vestibular symptoms occur in isolation or are there additional neurologic or audiologic symptoms?
Core clinical syndromes commonly overlap and evolve as do symptoms (Figure 4, Table).7

Third, Identify Symptom Triggers
Head Motion, Position, Orthostatic Change, or Exertion
Head Motion. In a patient with a vestibular disorder (peripheral or central), a normal head movement can lead to a faulty estimation of movement. Symptoms are experienced during or time-locked with the head movement.
Position. Attacks of vertigo and dizziness in patients with BPPV or central positional nystagmus occur in response to changes in the gravitational vector during movements such as looking down-then-up, bending over, or rolling over in bed. Symptoms are triggered by the head movement.
Orthostatic Change. Triggered by changing from a seated to standing position or from lying to seated, orthostatic vertigo and may be related to a neurologic condition (eg, multiple system atrophy), medications (eg, antihypertensives), hypovolemia, or presyncope. If symptoms are triggered by standing up from sitting when there is no change in spatial orientation of the head with respect to gravity (eg, standing from a chair without moving the head), orthostatic hypotension is favored. Symptoms triggered by going from sitting to lying or rolling over in bed (both cause a change in gravitational vector), in contrast, favor BPPV.
Exertion. Exertion-related vertigo may be caused simply by activities leading to head movements that trigger positional or head motion-induced symptoms. If vertigo occurs with exertion when the head is stationary, however, cardiopulmonary disorders should be considered. Additionally, hyperventilation and changes in cerebrospinal fluid (CSF) pH that occur with strenuous activity may also lead to 8th cranial nerve hyperexcitability in vestibular paroxysmia or with acoustic neuroma.
Head Turning and Eye Position
Head Turning. Some patients with vestibular paroxysmia may experience short spells of vertigo with head turn (to the right or left) in the upright position, because they may have more neurovascular contact with certain head positions. Rotational vertebral artery occlusion syndrome should be considered when protracted head turn induces stereotypical vertigo attacks.4

Eye Position. A particular eye position may bring on symptoms. For example, monocular oscillopsia can be induced by down and medial gaze in superior oblique myokymia.
Sound, Valsalva, and External Ear Pressure Changes
Patients with a third-window syndrome (superior canal dehiscence syndrome [SCDS], perilymph fistula, or enlarged vestibular aqueduct syndrome) have inner ear bony structure deficits. Changes in intracranial or middle ear pressure or loud sound (Tulio phenomenon) often lead to inappropriate excitation or inhibition of a semicircular canal, causing vertigo and nystagmus. In SCDS, excitatory stimuli for the anterior (also known as superior) canal include Valsalva against pinched nostrils (eg, blowing the nose), positive pressure in the external auditory canal (EAC) (eg, inserting a wet finger into the EAC) or a loud sound. The resultant nystagmus will be downbeat-torsional (top poles beating toward the affected ear). Inhibitory stimuli include Valsalva against a closed glottis (eg, heavy objects lifting, coughing, straining, and laughing) or negative pressure in the EAC (eg, pulling out a wet finger from the EAC). The resultant nystagmus will be upbeat-torsional (top poles beating toward the unaffected ear).9 A history of head trauma including barotrauma and blast injury is a known risk factor.10 In comparison to patients with third-window syndromes, vertigo spells in Chiari malformation or situational and vasovagal syncope may be driven by closed-glottis Valsalva maneuvers but are not triggered by loud sound or middle ear pressure changes.

Complex Visual Environments, Passive Self-Motion, or Illusions of Passive Self-Motion
Loss or distortion of vestibular input may cause increased reliance on visual information for balance, which is thought to be the genesis of most visual vertigo. Complex visual environments include patterned wallpaper or carpets or a busy grocery store. In patients who overrely on visual input, there is commonly impaired compensation for moving scenes, (eg, passive self-motion on a car, bus, train, or plane) or illusions of passive self-motion (eg, video games on large screens, 3D movies, virtual reality headsets or looking at traffic) that can cause spatial disorientation. Abnormal visual dependency can cause anxiety specific to open spaces or shopping centers and ultimately agoraphobia. Visual tasks that require fixation on small target (eg, mobile devices or reading a book) may be a trigger in patients with PPPD.11
Walking on Uneven Surfaces or in the Dark
Understanding that normal balance relies upon visual, proprioceptive, and vestibular inputs can help the clinician establish which system(s) are impaired depending on the specific condition(s) that worsen balance. Asking about situations with down-regulation of visual cues (walking in the dark) and disrupted somatosensory cues (walking on uneven surfaces) can have localizing value.
A history of oscillopsia while walking (ie, head movement dependent) will favor BVL,12 whereas an abnormal general neurologic exam in a patient with imbalance may suggest a nonvestibular etiology (eg, diminished vibration/proprioception sensation and hyporeflexia in polyneuropathy). In elderly patients, imbalance is often multifactorial, and related to a combination of orthopedic, neuropathic, visual, proprioceptive, and vestibular impairments. Recovery from vestibular neuritis may be suboptimal because of combined abnormal visual dependency and anxiety, dispite objective vestibular function tests showing recovery of function.13
Fourth, Assess Associated Symptoms
Vegetative Symptoms: Nausea and Vomiting
When it’s not clear whether a patient with acute prolonged or episodic symptoms has a vestibular or nonvestibular etiology, the presence of vegetative symptoms suggest a vestibular disorder.
Auditory Symptoms: Deafness, Tinnitus and Aural Fullness
Ischemic auditory symptoms. New unilateral hearing loss in a patient with AVS is concerning for anterior inferior cerebellar artery (AICA) ischemia. The inner ear is particularly susceptible to ischemic injury because it is supplied by an end artery, the internal auditory artery (IAA). The most common mechanism of IAA territory infarction is thrombotic stenosis of the parent vessel, usually the AICA, or the origin of the AICA in the basilar artery. Spells of vertigo associated with auditory symptoms (eg, tinnitus or unilateral hearing loss) that last for minutes can represent AICA TIAs that precede stroke. Patients with transient vestibular or auditory symptoms and vascular risk factors should have head and neck vascular imaging and brain MRI.14
Nonischemic auditory symptoms. Clinically, labyrinthitis resembles vestibular neuritis but can be differentiated by the presence of acute unilateral hearing loss. In patients with acute or chronic middle ear infection or meningitis, abrupt audiovestibular symptoms are concerning for bacterial labyrinthitis. Abrupt-onset audiovestibular symptoms with ipsilesional peripheral facial paresis and vesicular rash (may involve the auricle, EAC, and tympanic membrane) is concerning for herpes zoster (Ramsay-Hunt syndrome).
Head injuries, especially those including temporal bone trauma, can cause hearing loss with or without vestibular symptoms. In patients with a recent temporal bone fracture, barotrauma, or stapes surgery, co-occurrence of episodic vertigo and unilateral hearing loss may suggest a perilymphatic fistula.10 Supranormal bone thresholds and a low-frequency conductive hearing loss in the presence of normal tympanometry are associated audiologic findings of SCDS.9
Progressive peripheral facial nerve weakness with auditory symptoms (eg, tinnitus and hearing loss) suggests a neoplastic process in the middle ear (eg, glomus body tumor), internal auditory canal, or cerebellopontine angle (eg, vestibular and facial schwannoma or metastasis).15,16
Fluctuating sensorineural hearing loss in the low-to-medium frequency (> 30 dB, < 2000 Hz) range with tinnitus and aural fullness occur in association with spontaneous episodic vertigo in patients with Ménière’s disease. With recurrent attacks, hearing loss is often progressive.5

Cogan’s syndrome has similar presentation to Ménière’s disease (episodic vertigo with fluctuating hearing loss) with a more fulminant course and interstitial keratitis.17 Other inflammatory disorders that may involve the inner ear include polyarteritis nodosa, systemic lupus, Sjögren syndrome, Vogt-Koyanagi-Harada syndrome, granulomatosis with polyangiitis, and relapsing polychondritis.18 Patients with presyncope may experience muffled sounds. Patients with VM may have a mild feeling of aural fullness and tinnitus.19
Migraine Headache
Episodes of VM typically last 5 minutes to 72 hours and are accompanied by a variety of sensations including dizziness, vertigo, and/or unsteadiness that are often aggravated by head movements and visual stimulation. Associated migraine features may include headache (unilateral onset, throbbing or pounding in quality, moderate to severe in intensity and worsening by routine physical activity), phonophobia (bilateral sound discomfort), photophobia and visual aura, and nausea.19 Patients with migraine may experience associated migraine features before, during, or after vestibular symptoms. Commonly, patients are overwhelmed by vestibular symptoms and devalue the associated headache or nonheadache migraine features during the acute phase.19
Neurologic Symptoms
Neurologic symptoms of diplopia, dysarthria, numbness, weakness, hiccups, vision loss, or clouded consciousness should be assessed. Although stroke is the most common cause of central AVS, multiple sclerosis and Wernicke’s encephalopathy also should be considered.
In addition to nonischemic causes of a lower motor neuron facial palsy discussed, the presence of vertigo and a 7th nerve palsy should also raise suspicion for an AICA territory infarction involving the root entry zone and 7th nerve fascicle.14
Inflammatory disorders (eg, granulomatosis with polyangiitis, lupus erythematosus, or sarcoidosis) and infectious diseases (eg, tuberculosis, fungal infection, or syphilis) are often subacute and may present as focal (inflammatory infiltrate or abscess) or diffuse (multiple cranial neuropathy) neurologic deficits. Autoimmune encephalitis (paraneoplastic or non-paraneoplastic) also has a subacute temporal profile.
Autophony
A patient with SCDS may report hearing an echo of their own voice, footsteps, or movements of the jaws and eyes while chewing or looking around, respectively. Autophony is also a core clinical symptom in patients with a patulous eustachian tube; however, these patients will note an echo of their own breath sounds that patients with SCDS will not.9
Anxiety
Anxiety is a common comorbidity of all vestibular disorders and may adversely influence recovery. Of note, spells of dizziness or vertigo may result from a panic attack, agoraphobia, traumatic stress disorders, or generalized anxiety. Patients with anxiety-related dizziness are less likely to report external vertigo or significant vegetative symptoms.
Last,in Cases of Isolated Vertigo
The most common diagnoses of AVS are vestibular neuritis and stroke (about 5%-10% of cases), which can be indistinguishable with history and general neurologic examination. Even MR with diffusion-weighted imaging (DWI) in the first 24 to 48 hours of an attack may be falsely negative in 6% to 21% of strokes.20 Approximately 29% of patients with vertigo caused by a posterior circulation stroke reported a preceding isolated episode of vertigo that lasted minutes.21 Transient isolated vertigo attacks are potentially a warning sign for strokes, and like any other transient neurologic attack, vascular imaging (CT angiography or MRA) should be considered.22 Because there is potential for acute MRI results to be false negative, CT-perfusion may be helpful in some cases if posterior fossa ischemia is suspected.20
Conclusion
By approaching the vestibular history methodically, an accurate diagnosis can usually be made, or the differential diagnosis at least narrowed substantially. Using symptom type alone to diagnose dizzy or vertiginous patients is an ineffective approach and often leads to the wrong diagnosis and inappropriate testing. Once timing and triggers are understood, categorization into acute, episodic, or chronic vestibular syndromes can be done and a targeted exam chosen. That examination will then confirm the diagnosis and/or inform further audiovestibular testing or neuroimaging. The approach to the vestibular and ocular motor examination will be discussed in Part 2 in the next issue of Practical Neurology.
1. Bisdorff A, Von Brevern M, Lempert T, Newman-Toker DE. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res. 2009;19(1-2):1-13.
2. Choi JH, Seo JD, Kim MJ, et al. Vertigo and nystagmus in orthostatic hypotension. Eur J Neurol. 2015;22(4):648-655.
3. Von Brevern M, Bertholon P, Brandt T, et al. Benign paroxysmal positional vertigo: diagnostic criteria. J Vestib Res. 2015;25(3-4):105-117.
4. Strupp M, Lopez-Escamez JA, Kim JS, et al. Vestibular paroxysmia: diagnostic criteria. J Vestib Res. 2016;26(5-6):409-415.
5. Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Menière’s disease. J Vestib Res. 2015;25(1):1-7.
6. Bronstein AM, Lempert T. Symptoms and syndromes in the patient with dizziness or unsteadiness. In Bronstein AM (ed): Oxford Textbook of Vertigo and Imbalance. Oxford, United Kingdom: Oxford University Press; 2013: 354.
7. Bisdorff A. Vestibular symptoms and history taking. Handb Clin Neurol. 2016;137:83-90.
8. Edlow JA, Newman-Toker DE, Savitz SI. Diagnosis and initial management of cerebellar infarction. Lancet Neurol. 2008;7(10):951-964.
9. Ward BK, Carey JP, Minor LB. Superior canal dehiscence syndrome: lessons from the first 20 years. Front Neurol. 2017;8:177.
10. Minor LB. Labyrinthine fistulae: pathobiology and management. Curr Opin Otolaryngol Head Neck Surg. 2003;11(5):340-346.
11. Staab JP, Eckhardt-Henn A, Horii A, et al. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): consensus document of the Committee for the Classification of Vestibular Disorders of the Bárány Society. J Vestib Res. 2017;27(4):191-208.
12. Strupp M, Kim JS, Murofushi T, et al. Bilateral vestibulopathy: diagnostic criteria consensus document of the Classification Committee of the Bárány Society. J Vestib Res. 2017;27(4):177-189.
13. Cousins S, Kaski D, Cutfield N, et al. Predictors of clinical recovery from vestibular neuritis: a prospective study. Ann Clin Transl Neurol. 2017;4(5):340-346.
14. Choi KD, Lee H, Kim JS. Ischemic syndromes causing dizziness and vertigo. Handb Clin Neurol. 2016;137:317-40.
15. Lees KA, Tombers NM, Link MJ, et al. Natural history of sporadic vestibular schwannoma: a volumetric study of tumor growth. Otolaryngol Head Neck Surg. 2018;159(3):535-542.
16. West N, Sass H, Møller MN, Cayé-Thomasen P. Facial nerve schwannomas presenting with vestibular dysfunction: a case series. Acta Neurochir (Wien). 2018;160(12):2315-2319.
17. Gluth MB, Baratz KH, Matteson EL, Driscoll CL. Cogan syndrome: a retrospective review of 60 patients throughout a half century. Mayo Clin Proc. 2006;81(4):483-488.
18. Girasoli L, Cazzador D, Padoan R, et al. Update on vertigo in autoimmune disorders, from diagnosis to treatment. J Immunol Res. 2018;2018:5072582.
19. Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res. 2012;22(4):167-172.
20. Choi JH, Oh EH, Park MG, et al. Early MRI-negative posterior circulation stroke presenting as acute dizziness. J Neurol. 2018;265(12):2993-3000.
21. Grad A, Baloh RW. Vertigo of vascular origin. Clinical and electronystagmographic features in 84 cases. Arch Neurol. 1989;46(3):281-284.
22. Kattah JC. Use of HINTS in the acute vestibular syndrome. an overview. Stroke Vasc Neurol. 2018;3(4):190-196.
Disclosure
The authors have no financial or other relationships relevant to this content to disclose.