A first seizure is potentially the most terrifying life event a person can experience. Questions about the ability to work, maintaining financial stability, driving, caregiving, or childbearing suddenly cloud future plans. Empathic attentiveness to an individual’s questions is an important aspect of seizure management. Exploration of personal and cultural understanding of seizures is also important in order to provide responsive counseling that mitigates potential discrimination and stigma from family, educators, and employers.
In the US, approximately 150,000 adults per year will present with a first unprovoked seizure.1 Up to 2% of all emergency room visits or 1 in every 100 emergency room visits in the US are for seizures.2 The lifetime incidence of seizures is approximately 10%, and 1 in 26 people in the US develop epilepsy.3 Approximately 3.4 million people in the US (1.2% of the population) and 70 million people, globally, have epilepsy.4,5 Epilepsy is the fourth most common neurologic condition.
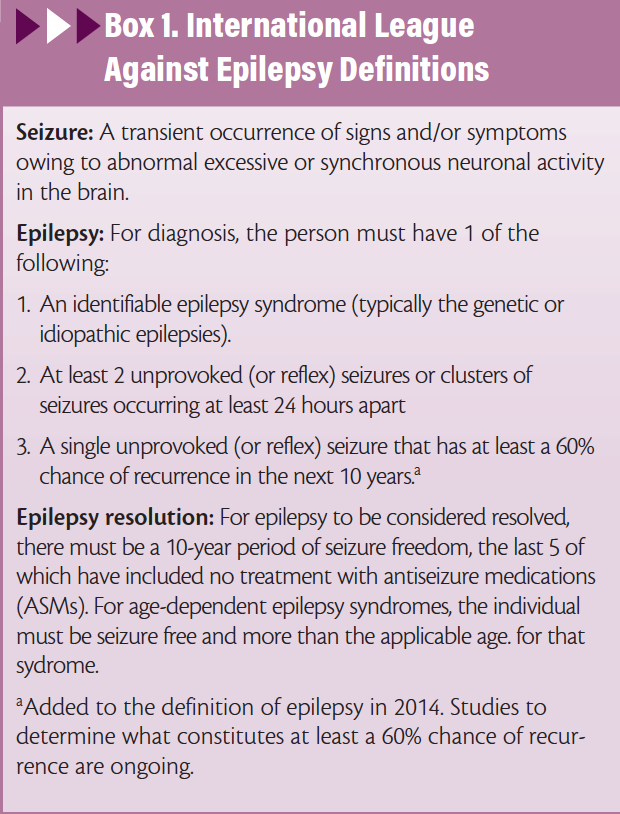
The established definitions of seizures and epilepsy are listed in Box 1.6 Determining the underlying cause of a seizure and distinguishing a seizure from epilepsy determines treatment decisions, prognosis, and counseling. Correct identification of epilepsy is a clinical care gap; there is up to a 3-year delay in antiseizure medication (ASM) initiation in 36.7% of people with newly diagnosed epilepsy, which may be larger when the 2014 definition of epilepsy is used in future studies.7
Differential Diagnosis
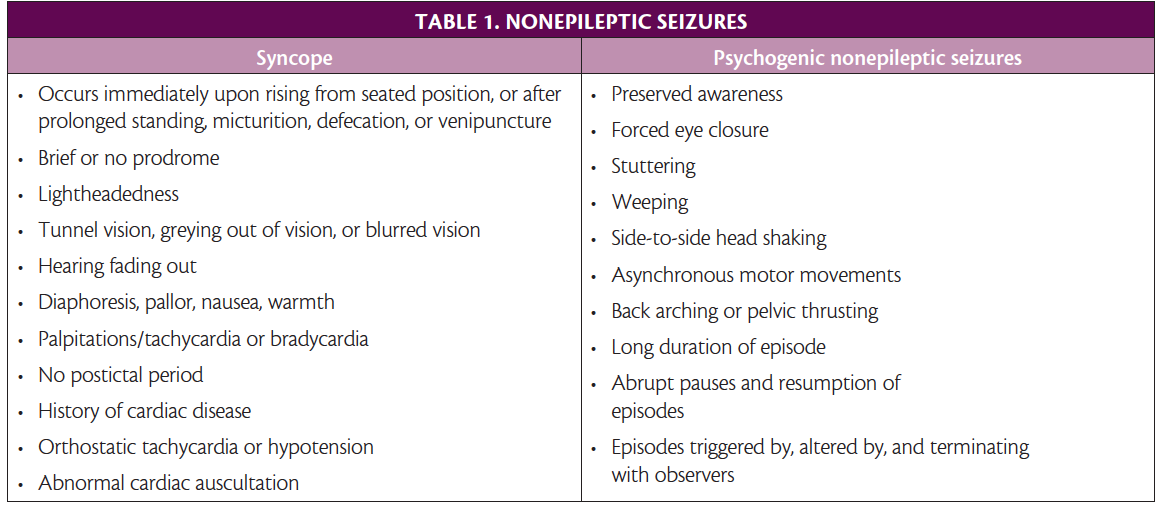
Transient loss of consciousness is one of the most common presenting complaints in people who may have had a seizure. The estimated lifetime incidence of transient loss of consciousness is as high as 50%.8 Over 90% of those presenting with transient loss of consciousness are found to have syncope, psychogenic nonepileptic seizures, and epileptic seizures.
Nonepileptic Diagnoses
Syncope. Caused by cerebral hypoperfusion, syncope presents as an abrupt and complete loss of consciousness and postural tone followed by a rapid spontaneous recovery (Table 1). A nonepileptic convulsion may occur during syncope. Different types of syncope include orthostatic related, reflex (vasovagal being a common type), cardiac, and volume depletion. The American College of Cardiology and American Heart Association issue updated guidelines on managing syncope.9
Psychogenic Nonepileptic Seizures. Diagnosis of psychogenic nonepileptic seizures (PNES) can take up to 7 years from the initial event, underscoring the importance of carefully considering this diagnosis (Table 1).10 There is a high comorbidity of PNES and epilepsy; 10% to 40% of people with epilepsy have PNES. As many as 20% to 30% of people referred to epilepsy centers for medically refractory epilepsy have PNES.11 Risk factors include female sex (80%), childhood trauma, adult trauma, posttraumatic stress disorder, history of sexual abuse, military service, and concurrent medical diagnosis (especially asthma, chronic pain, and fibromyalgia). Diagnosis is made by a history consistent with PNES coupled with normal EEG findings during a typical PNES event. The most effective treatment is psychologic therapy, most commonly cognitive behavioral therapy 12
Epileptic Seizures
Epileptic seizures often have certain historical elements and stereotyped presenting semiologies. If the above diagnoses are lower on the differential diagnosis list, specific screening questions regarding the event (Box 2) may help determine whether an epileptic seizure occurred. Because it is rare for a person who had a seizure to be able to describe it accurately, it is important to ask any witnesses to the event the same questions. Clinical correlates are diverse, ranging from a barely perceptible microsecond loss of attention to oral or picking automatisms, focal twitch or dystonia, to a full body convulsion and transient loss of consciousness. After a seizure, there can be a postictal period that physiologically correlates with loss of neural function and often clinically correlates with absent or altered consciousness.
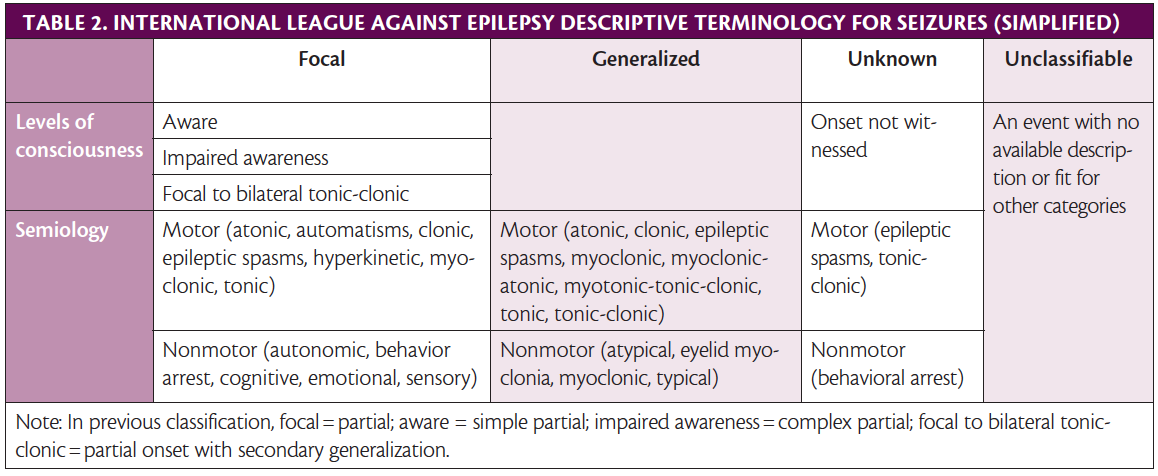
The 2017 International League Against Epilepsy (ILAE) guide-lines for describing seizures are also a helpful diagnostic tool. A shared descriptive terminology for seizures and epilepsy types allows for more accurate diagnosis and treatment (Table 2).13,14
Distinguishing whether a seizure is an acute symptomatic seizure, unprovoked seizure, or an epilepsy syndrome helps determine treatment decisions, prognosis, and counseling.
Symptomatic vs Unprovoked Seizures
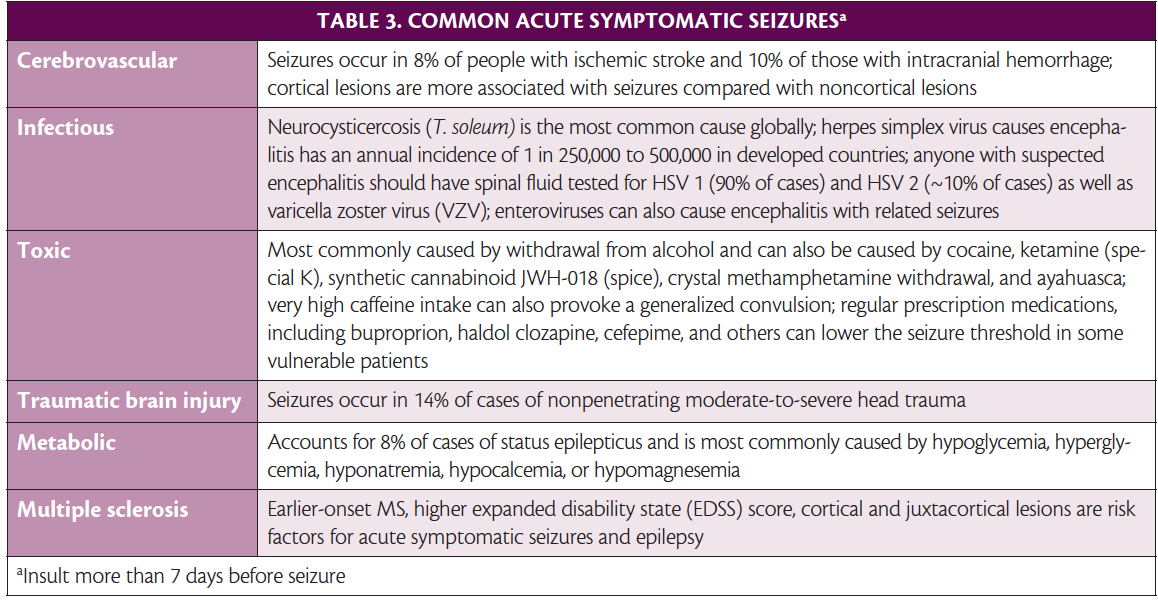
Acute Symptomatic Seizures. Acute symptomatic seizures occur at the time or within 7 days of an identifiable acute event. Incidence is 29 to 39 per 100,000 people annually and acute symptomatic seizures comprise 34% to 50% of reported afebrile seizures. The most common causes are cerebrovascular disease, central nervous system infections, traumatic brain injuries (TBIs), acute drug exposures or withdrawals, and metabolic insults (Table 3).1,15-24 Other acute causes include intracranial surgery, anoxic brain insult, and active multiple sclerosis (MS) or other autoimmune diseases of the central nervous system (CNS). A careful history and appropriate imaging, laboratory tests, and drug screens are helpful in diagnosis.
If reversible causes are avoided and there is no permanent brain damage, recurrence is unlikely. Individuals with acute symptomatic seizures from CNS infection, TBI, or stroke, however, were 8.9 times more likely to die in the first 30 days compared with patients who had a first unprovoked seizure. Beyond 30 days, the 10 year mortality rate for acute symptomatic seizure was equivalent to a first unprovoked seizure. Furthermore, the acute symptomatic seizure patients were 80% less likely to have subsequent seizures.25
Unprovoked Seizures. A single seizure or a cluster of seizures in a 24-hour peroid with no identifiable trigger is termed unprovoked. Incidence of unprovoked seizures is approximately 42 to 61 per 100,000 annually, and a standardized mortality ratio (SMR) of 2.3 has been reported.1 Some unprovoked seizures will have history, physical, labs, imaging, and EEG that are unrevealing. Typically, starting an ASM is not warranted in this situation.
Alternatively, unprovoked seizures can be remote symptomatic seizures with an underlying anatomic insult from more than 7 days earlier. Prior ischemic strokes, intracranial hemorrhage, CNS infection, TBI, PML, brain neoplasms, neurodegenerative diseases, paraneoplastic processes, and autoimmune disease can be associated with remote symptomatic seizure. Approximately 43-45% of unprovoked remote symptomatic seizures will have a recurrent seizure within 2 years.26 The underlying pathology influences recurrence rates. Ischemic strokes may lead to 25 times increased risk of recurrence whereas intracranial hemorrhage increases risk of recurrence in the next year tenfold.19,21 Recurrence risk is 7 times higher over a 10-year period with CNS infection.27 The 5-year cumulative seizure probability is 0.7% for mild TBI, 1.2% for moderate TBI, and 10% for severe TBI.27 Studies suggest people who serve in the military have increased likelihood of post-traumatic epilepsy (PTE) and that 86% of veterans who have a single posttraumatic seizure will have another within 2 years.24 This suggests posttraumatic seizures may meet the new criteria for epilepsy (a single unprovoked or reflex seizure with a 60% or greater chance of recurrence in the next 10 years) and warrant ASM initiation after the first posttraumatic unprovoked seizure.
Individuals who had a first unprovoked seizure should be informed they have a 21% to 45% chance of recurrent seizure within the next 2 years with higher likelihood in the first year.28 If they have a history of stroke, head trauma, epileptiform EEG, significant abnormal results on head imaging, and/or nocturnal seizures, their risk is increased further. The recent update to the definition of epilepsy (a single unprovoked or reflex seizure with a 60% or greater chance of recurrence in the next 10 years) raises questions of whether ASM treatment should be initiated. Understanding what supports a 60% chance of recurrence, however, is undetermined and an area requiring further research. After a second seizure, recurrence risk increases to 57% at 1 year, 61% at 2 years, and 73% at 5 years.31
Epilepsy Syndromes. Diagnosis of epilepsy, including generalized genetic epilepsies and idiopathic generalized epilepsies (eg, childhood absence, juvenile absence, juvenile myoclonic) is reliant on a history of previously unrecognized stereotypic seizures, family history, EEG findings, imaging, laboratory test findings, and, if available, genetic testing. For all new diagnoses of epilepsy, the risk of sudden unexpected death in epilepsy (SUDEP) should be discussed. Rates of SUDEP increase proportionally to severity of epilepsy: 0.9 to 2.3 per 1000 patient-years in the community vs 3.2 to 5.9 in medically refractory epilepsy, and 6.3 to 9.3 in people who are candidates for epilepsy surgery.22,32
Management and Counseling
Initiating Antiseizure Medications
Initiating ASM treatment after a first seizure is likely to reduce relative risk for seizure recurrence in the next 2 years by 35% but does not necessarily improve quality of life. Treatment with ASMs does not affect risk of SUDEP,32 nor does timing of ASM treatment affect chance of seizure freedom 3 years after an initial seizure. Typically, starting an ASM is not warranted in this situation. If a second unprovoked seizure occurs, the diagnosis of epilepsy is made, and ASMs should be initiated.
The decision to start ASMs should be an integrative individualized discussion that considers personal preferences and lifestyle. Maintaining employment, caring for family members, and driving may play a large role in the decision. Driving, in particular, is a critical discussion because seizure freedom for a certain period of time may need to be established to maintain licensure, even after a first seizure, depending on variable state regulations. The Epilepsy Foundation maintains a searchable State Driving Laws Database that includes professional reporting mandates and minimum seizure freedom requirements.33
Risks of Antiseizure Medications
Adverse side effects of ASMs occur in 7% to 31% of people treated, depending on which drug is used. Most are mild and reversible. Women of childbearing age taking ASMs need counseling on risk of birth defects and access to birth control if desired. They should also be informed that with good seizure control and coordination with their health care team, they can still make a plan for conception if that is a goal.
Comorbid Conditions, Lifestyle, and Psychosocial Factors
Major depression and suicidality are frequent comorbidities of epilepsy. Screening for and documenting any depression and thoughts of suicide or homicide should be done reflexively and reassessed frequently for everyone who has had a seizure. If ASMs are initiated, this assessment is critical because some ASMs may worsen depression and suicidal ideation. A low threshold for supportive services referral should be maintained.
Seizure threshold can be lowered by stress, including sleep deprivation, nutrition deprivation, extreme heat, and extreme emotional distress. People should be counseled to avoid these situations, if possible. Counseling for safety precautions includes avoiding bathtubs, swimming alone, going to heights, and operating heavy machinery.
The psychosocial impact of seizures and epilepsy should be explored, including how seizures and/or epilepsy may affect their interpersonal relationships, education or employment, and lifestyle. Some cultures or belief systems ostracize seizures and this possibility should be explored with each individual. Again, a low threshold to refer to supportive services should be observed. There are many resources and communities online via social media as well as profesional societies with resources, incluing the ILAE, the American Epilepsy Society, and the Epilepsy Foundation. The Epilepsy Foundation aslo has local chapters with information on local support group meetings.
1. Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49(S1):8-12.
2. Martindale JL, Goldstein JN, Pallin DJ. Emergency department seizure epidemiology. Emerg Med Clin North Am. 2011;29(1):15-27.
3. Hesdorffer DC, Logroscino G, Benn EK, Katri N, Cascino G, Hauser WA. Estimating risk for developing epilepsy: a population-based study in Rochester, Minnesota. Neurology. 2011;76(1):23-27.
4. Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy—United States, 2015. MMWR. 2017;66:821-825.
5. Ngugi AK, Kariuki SM, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Incidence of epilepsy: a systematic review and meta-analysis. Neurology. 2011;77(10):1005-1012.
6. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475-482.
7. Kalilani L, Faught E, Kim H, et al. Assessment and effect of a gap between new-onset epilepsy diagnosis and treatment in the US. Neurology. 2019;92(19):e2197-e2208.
8. Westby M, Davis S, Bullock I, et al. Transient loss of consciousness (‘blackouts’) management in adults and young people. London, United Kingdom: National Clinical Guideline Centre for Acute and Chronic Conditions, Royal College of Physicians; 2010. https://www.ncbi.nlm.nih.gov/books/NBK63820/ Accessed August 30,2019.
9. Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2017;14(8):e155-e217.
10. Reuber M, Fernández G, Bauer J, Helmstaedter C, Elger CE. Diagnostic delay in psychogenic nonepileptic seizures. Neurology. 2002;58(3):493-495.
11. Jirsch JD, Ahmed SN, Maximova K, Gross DW. Recognition of psychogenic nonepileptic seizures diminishes acute care utilization. Epilepsy Behav. 2011;22(2):304-307.
13. Kanemoto K, LaFrance WC, Duncan R, et al. PNES around the world: Where we are now and how we can close the diagnosis and treatment gaps-an ILAE PNES Task Force report. Epilepsia Open. 2017;2(3):307-316.
14. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522-530.
15. Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512-521.
16. Ferlazzo E, Gasparini S, Beghi E, et al. Epilepsy in cerebrovascular diseases: review of experimental and clinical data with meta-analysis of risk factors. Epilepsia. 2016;57(8):1205-1214.
17. Chiquete E, es-Madrid S, Alonso-Vanegas M, a-Ramos G, Cant-Brito C. Acute seizures and 12-month outcome after acute ischemic stroke. Abstract 1.337 presented at American Epilepsy Society Annual Meeting; November 29-December 3, 2012; San Diego, CA. https://www.aesnet.org/meetings_events/annual_meeting_abstracts/view/16489. Accessed August 30, 2019.
18. Brigo F, Schneider M, Wagenpfeil G, et al. Intravenous thrombolysis with tPA and cortical involvement increase the risk of early poststroke seizures: results of a case-control study. Epilepsy Behav. 2019: pii: S1525-5050(19)30250-1.
19. Biffi A, Rattani A, Anderson CD, et al. Delayed seizures after intracerebral haemorrhage. Brain. 2016;139(Pt 10):2694-2705.
20. White AC, Coyle CM, Rajshekhar V, et al. Diagnosis and treatment of neurocysticercosis: 2017 clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am J Trop Med Hyg. 2018;98(4):945-966.
21. Whitley RJ. Herpes simplex encephalitis: adolescents and adults. Antiviral Res. 2006;71(2-3):141-148.
22. Hung C, Chen JW. Treatment of post-traumatic epilepsy. Curr Treat Options Neurol. 2012;14(4):293-306.
23. Nardone R, Brigo F, Trinka E. Acute symptomatic seizures caused by electrolyte disturbances. J Clin Neurol. 2016;12(1):21-33.
24. Lee H, Mizrahi MA, Hartings JA, et al. Continuous electroencephalography after moderate to severe traumatic braininjury. Crit Care Med. 2019;47(4):574–582.)
25. Hesdorffer DC, Benn EK, Cascino, GD and Hauser WA. Is a first acute symptomatic seizure epilepsy? Mortality and risk for recurrent seizure. Epilepsia. 2009;50:1102-1108.
26. Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure Neurology. 1991;41(7):965.
27. Gasparini S, Ferlazzo E, Ascoli M, et al. Risk factors for unprovoked epileptic seizures in multiple sclerosis: a systematic review and meta-analysis. Neurol Sci. 2017;38(3):399-406.
28. Nowacki TA, Jirsch JD. Evaluation of the first seizure patient: key points in the history and physical examination. Seizure. 2017;49:54-63.
29. Miskin DP, Herman ST, Ngo LH, Koralnik IJ. Predictors and characteristics of seizures in survivors of progressive multifocal leukoencephalopathy. J Neurovirol. 2016;22(4):464-471.
30. Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015;84(16):1705-1713.
31. Hauser WA, Rich SS, Lee JR, Annegers JF, Anderson VE. Risk of recurrent seizures after two unprovoked seizures. N Engl J Med. 1988;338:429-434.
32. Devinsky O, Friedman D, Duckrow RB, et al. 2016. Sudden unexpected death in epilepsy in patients treated with brain-responsive neurostimulation. Epilepsia. 2018;59(3):555-561.)
33. Epilepsy Foundation. Living with epilepsy: driving and transportation. State driving laws database. https://www.epilepsy.com/driving-laws. Accessed August 30, 2019.
CH reports no disclosures.