Fibromuscular dysplasia (FMD) is an idiopathic, noninflammatory, nonatherosclerotic vascular disease of small- to medium-sized arteries.1,2 Since it was first identified in 1938, FMD has been described in virtually every arterial bed but most commonly affects the renal and extracranial carotid arteries. This article focuses on cerebrovascular FMD, common neurologic symptoms associated with FMD, and the relationship between FMD and stroke.
Epidemiology
Because FMD is commonly identified in asymptomatic individuals, pinpointing the exact prevalence and natural history is difficult. According to the US FMD Registry, the median age at diagnosis is 51.9, 90% of people diagnosed with FMD are women, 72% have hypertension, and 60% have significant headaches.3 A minority of people with FMD report a close relative with FMD, although approximately 80% of first- or second-degree relatives have hypertension and 53.5% report a history of stroke. It is equally common for FMD to affect the renal and cerebrocervical arteries, with about a 65% coprevalence.3 Modern CT-angiography (CTA) and contrast-enhanced magnetic resonance angiography (MRA) have enhanced the radiographic characterization and diagnosis of FMD. Among individuals with cerebrocervical FMD, 95% have carotid involvement, often bilaterally, and 70% have involvement of the vertebral arteries.4
Pathophysiology and Genetics
The pathophysiology of FMD is poorly understood, although histologic evidence suggests a defective transformation of smooth muscle cell fibroblasts leads to degradation of the elastic laminae, aberrant collagen synthesis, and segmental fibroplasia.5 Adults most commonly have medial fibroplasia rather than other histologic subtypes.4,6 Strong evidence supports the heritability of FMD with high coprevalence of FMD in twins and first-degree relatives, although there are no known single-gene forms.4 Recent genome-wide association studies identified the first gene locus associated with FMD, a single nucleotide polymorphism (rs9349379) in the phosphatase and actin regulator 1 gene (PHACTR1) on chromosome 6.7 This variant is also associated with migraine, cervical artery dissection (CeAD), and spontaneous coronary artery dissection, and is inversely associated with atheromatous coronary artery disease and calcification.8 The PHACTR1 variant is known to regulate the expression of endothelin-1, an endogenous vasoconstrictor.9
Presentation and Diagnosis
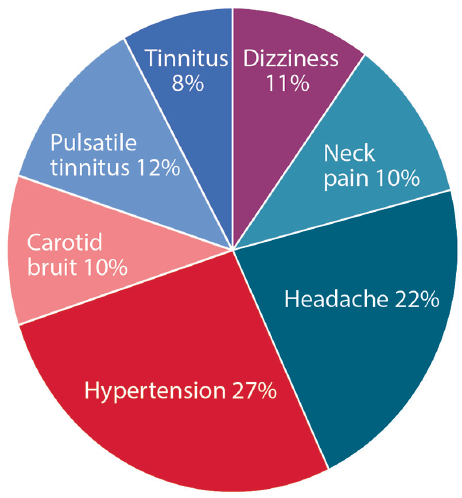
The clinical presentation of FMD depends on the affected vascular bed (Figure 1). In all locations, the 3 most common presenting symptoms include hypertension, headache, and pulsatile tinnitus.3 The most common presenting symptom of cerebrocervical FMD is pulsatile tinnitus, often described as whooshing in the ear. A carotid bruit may often be auscultated on physical exam, correlating with turbulent flow in the affected cervical arteries. In those affected by cerebrovascular FMD, 70% have headaches, with 30% or more characterized as migraine. People with cerebrocervical FMD may also be diagnosed concomitantly with cervical artery dissection, presenting with or without transient ischemic attack (TIA) or stroke.
Diagnosis and classification of FMD is based on angiographic appearance. Multifocal FMD accounts for 90% of cases and is characterized by a string-of-beads appearance representing alternating arterial dilation and constriction (Figure 2). Focal FMD accounts for 10% of cases and is characterized by a unifocal concentric or tubular smooth narrowing,10 most often in the middle and distal segments of the internal carotid artery and the V3-V4 segment of the vertebral arteries. Intracranial FMD is rare and more prevalent in children with intimal FMD subtypes.5 No particular imaging modality is required for diagnosis but CTA or MRA are used most frequently.10 Digital subtraction arteriography (DSA) is the standard imaging for diagnosis, but is more invasive, particularly for people with FMD who may be higher risk for iatrogenic dissection. Duplex ultrasonography can be useful for diagnosis and monitoring of carotid FMD. However, FMD often exists in the mid- or distal segment of the carotid arteries, and ultrasound is less sensitive for evaluating the cervical vertebral arteries.
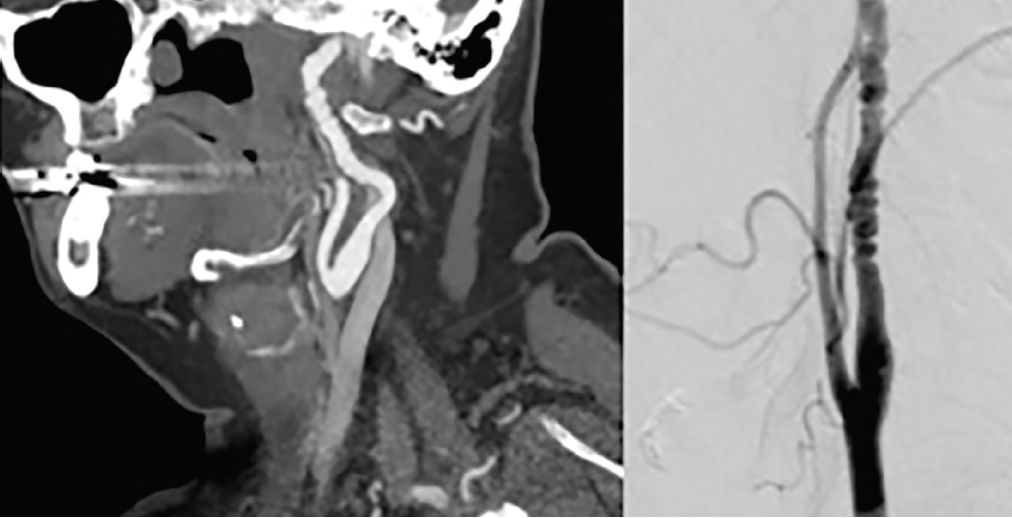
Figure 2. Carotid Artery Fibromuscular Dysplasia. A CT angiogram (left) and digital subtraction angiogram (right) demonstratebeading appearance with alternating stenoses and dilatation consistent with fibromuscular dysplasia (FMD) in the midcervical internal carotid artery.
Neurologic Manifestations
The observed natural history of FMD in asymptomatic incidentally diagnosed cases is relatively benign.1 However, in some cases the initial diagnosis of FMD is made in the setting of stroke, which as a manifestation of FMD, is most often because of concomitant cervical artery dissection (CeAD). Compared with the general population, FMD is associated with a higher prevalence of intracranial aneurysms, particularly in women. In the US FMD registry, over 40% of people with FMD report with associated CeAD or aneurysm, although this is likely an overestimation of the true natural history given referral and sampling bias in this population.11
Migraine Headaches. The most common neurologic manifestation of FMD is migraine. Nearly 80% of individuals with cerebrocervical FMD report headaches, most commonly migraine.12,13 The relationship between headache and FMD is poorly understood and likely multifactorial. Proposed mechanisms include nonlaminar cerebrovascular blood flow, uncontrolled hypertension, dysregulation of sympathetic regulation in the cervical artery wall, or heightened dural pain sensitivity. Refractory migraine may also manifest as a presenting symptom or residual sequela of CeAD.13 From a clinical perspective, transient neurologic symptoms in FMD resulting from migraine aura are often misdiagnosed as TIA and require careful adjudication to guide management.
Cervical Artery Dissection (CeAD). The definition of CeAD is the presence of a mural hematoma in the wall of a cervical (carotid or vertebral) artery resulting from either an intimal tear or direct bleeding within the wall secondary to a ruptured vasa vasorum.14 The estimated incidence of CeAD in the general population is 2 to 3 per 100,000, although the actual incidence is likely higher in people with FMD and other predisposing connective tissue vasculopathies.15,16 Coprevalence of CeAD and cerebrocervical FMD ranges from 5% to 15% in observational studies, and is higher in those presenting with multiple CeAD.5,10,17 Approximately 70% of CeAD presents with stroke or TIA that may often be delayed by days or weeks from the initial arterial dissection. Ischemic stroke most often results from thromboembolus formation or complete occlusion at the site of dissection. Rarely, a vertebral artery dissection extending into the intradural segment can manifest as subarachnoid hemorrhage (SAH).14
In addition, CeAD can present with other neurologic focal symptoms, including partial Horner syndrome (ie, ipsilateral ptosis and miosis) from disruption of ascending oculosympathetic tracts in the internal carotid artery. Uncommonly, lower cranial nerve (CN) palsies occur from related ischemia or injury the glossopharyngeal or vagus nerves. In contrast, vertebral artery dissection commonly presents with an acute vestibular syndrome, with or without posterior circulation stroke involving the posterior inferior cerebellar artery (PICA) territory and lateral medullary and/or cerebellar infarction. It is important to recognize that in CeAD, carotid or vertebral dissections often cause ipsilateral face or neck pain of moderate intensity and a nonspecific headache that can mimic migraine or cluster headache.14 Because of this association, the incidence of acute onset neck or face pain with any new focal neurologic symptoms in a person with FMD warrants urgent neurologic evaluation and cervical vascular imaging to rule out CeAD.
Intracranial Aneurysms. At any arterial site, FMD is associated with an increased prevalence of intracranial aneurysms (IA), particularly in women.18,19 In the US FMD Registry, 13% of women who had intracranial imaging had at least 1 IA and 4% had multiple IAs, although exact prevalence in the general population with FMD is unknown because of sampling bias in registry data. The risk of aneurysm rupture may be higher in patients with FMD at smaller sizes, although this is controversial. As in the general population, smoking is significantly associated with IA growth and formation in people with FMD.10,11,18 Whether individuals with FMD require more frequent angiographic monitoring for unruptured IAs is unclear.
Management
Management of cerebrocervical FMD is largely dependent on the presenting symptoms and any associated neurologic complications such as CeAD, migraine, or aneurysm. Whereas evidence-based guidelines are lacking, a careful understanding of the diagnostic approach to FMD and expected natural history guide expert opinion and appropriate patient counseling regarding their cerebrovascular risk.
Headache
Headache management in individuals with or without FMD is similar and largely driven by an accurate characterization of the headache subtype. Migraine management in those with or without FMD mirrors is also similar, considering both preventive strategies and abortive therapy when indicated. However, an important consideration in patients with FMD regards use of vasoactive abortive medications, such as ergots and triptans, which may introduce additional risk in patients already at risk for stroke or dissection.18 This is of particular concern for people with FMD and a concomitant history of spontaneous coronary artery dissection (SCAD), in whom vasoconstrictive medications such as triptans are absolute contraindication. Alternative abortive therapies for acute migraine in the setting of FMD may include antidopaminergic class (eg, prochlorperazine, metoclopramide), or a brief steroid taper. Botulinum toxin injections may also be efficacious for refractory chronic migraine in FMD. More recently, the approval of calcitonin gene-related peptide (CGRP) inhibitors offers yet another possibly effective strategy for treating migraine in FMD. Although clinical trials approving these new agents have revealed no significant cardiovascular or cerebrovascular side effects,20 further longitudinal observation and real-world practice are needed to verify this.
Cervical Artery Dissection
The median age for incidence of CeAD is in the early 40s, although people with FMD and other connective tissue vasculopathies may be at higher risk in younger ages. Stroke and TIA are associated with CeAD in approximately 70% of cases, and typically occur in the first few weeks postdissection. The incidence of stroke or TIA is often latent to arterial injury because the intramural hematoma expands and risk of intraluminal thromboembolism rises. As the vessel heals over weeks to months the risk of stroke decreases with an estimated long-term risk of recurrence ranging in incidence from 1% to 3%.21-23
There are no clinical trials investigating treatment of CeAD specific to FMD yet, so management is extrapolated from the treatment of CeAD at large. The CADISS studya compared 3 to 6 months of antiplatelet therapy with 3 to 6 months of anticoagulation with warfarin and showed no difference in stroke recurrence after the initial CeAD event. This study resulted in a class IIa, level B recommendation equating antiplatelet and anticoagulation for 3 to 6 months after CeAD.24 However, there is no report of the number of participants in this trial who also had FMD. There have also been no robust studies specifically studying the use of direct acting oral anticoagulants (eg, apixaban, rivaroxaban, or dabigatran) as early secondary prevention in CeAD and FMD. Nonetheless, antiplatelet therapy may be preferred because of safety and convenience, unless there is clear intraluminal thrombus or recurrent thromboembolic events despite antiplatelet therapy.
There are no randomized trials of endovascular treatment of CeAD in the setting of FMD. However, because vessel stenosis following CeAD typically recanalizes without the need for intervention, endovascular therapy should be reserved for those who have recurrent cerebral hypoperfusion or thromboembolic events despite optimized antithrombotic therapy.25 People with FMD may also be at higher risk for iatrogenic dissection with catheter-based interventions and this should be taken into consideration regarding periprocedural risk.
Those with FMD at risk for incident or recurrent CeAD are advised to avoid activities associated with extreme hyperextension or lateral rotation of the neck, especially chiropractic cervical spinal manipulation. Caution should be taken to cushion the neck during surgery, dental appointments, or other prolonged activities with forced hyperextension. Routine exercise and cardiovascular activities are not a contraindication, unless there is also an accompanying history of spontaneous coronary artery dissection requiring further moderation.
Ischemic Stroke
Occurrence of ischemic stroke in individuals with FMD in the absence of associated CeAD or other thromboembolic mechanism remains controversial. Nonetheless, management of acute stroke in patients with FMD should follow standard stroke guidelines.26 For instance, a known diagnosis of FMD is not a contraindication to fibrinolytic therapy or mechanical thrombectomy in otherwise eligible cases.
People with FMD and history of stroke should be on antiplatelet medication for secondary prevention, unless there is a competing stroke mechanism warranting anticoagulation such as paroxysmal atrial fibrillation or a hypercoagulable state. Notably, statins are not primarily indicated for secondary stroke prevention in people with CeAD in the setting of FMD, and indeed may have an inverse relationship with risk of arterial dissection.27,28
Asymptomatic FMD
Asymptomatic FMD when discovered incidentally has a favorable natural history. Regardless, consensus statements suggest 1-time vascular imaging from brain to pelvis, with either CTA or MRA, to screen for aneurysms, dissections, and FMD in other arterial beds.29 For people with FMD and no prior history of stroke, the long-term benefit of antiplatelet therapy for primary stroke prevention is unknown and should be weighed against the long-term bleeding risk.
Summary
A rare, noninflammatory nonatherosclerotic vasculopathy, FMD most commonly affects the renal and cervical arteries, is much more frequent in women, and is diagnosed either incidentally on vascular imaging or with symptoms of headache and whooshing tinnitus presenting in middle age. The incidence of stroke in FMD is primarily associated with CeAD and can be managed conservatively with antiplatelet therapy and avoidance of high-risk cervical exertion. Patients with FMD should also be considered for screening and monitoring of IA formation and growth. Further research (Figure 3) is needed to better understand the genetics, pathophysiology, and optimal treatment for symptomatic patients with FMD and to hopefully avoid preventable cerebrovascular complications.
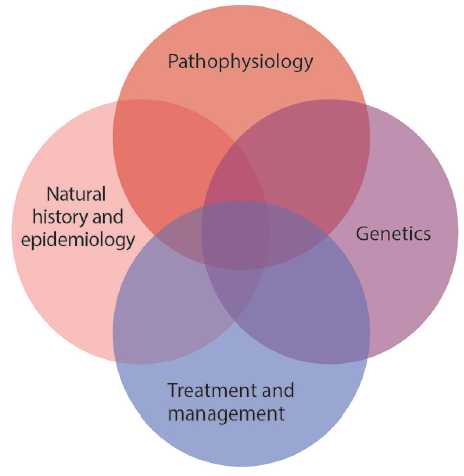
Figure 3. A number of research priorities will advance understanding of fibromuscular dysplasia (FMD), development of therapies and screening and surveillance strategies, and understanding of when to avoid intervention. Improved epidemiologic data regarding natural history of FMD, association with cardiovascular events (eg, stroke, dissection, and aneurysm), and risk factors are needed and it will be essential to understand the molecular pathophysiology of FMD and shared and distinct biology in relationship to other nonatherosclerotic arteriopathies. Understanding genetics of FMD through research on PHACTR1 and additional genetic contributors to FMD risk will support both of those goals. These priorities require investigation in cohort studies, family-based studies, genomic and other -omics investigation, and establishment of biobanks of blood, plasma, and vascular tissue.
a.
1. Olin JW, Gornik HL, Bacharach JM, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation. 2014;129(9):1048-1078.
2. Palubinskas AJ, Ripley HR. Fibromuscular hyperplasia in extrarenal arteries. Radiology. 1964;82:451-455.
3. Olin JW, Froehlich J, Gu X, et al. The United States Registry for Fibromuscular Dysplasia: results in the first 447 patients. Circulation. 2012;125(25):3182-3190.
4. Touze E, Oppenheim C, Trystram D, et al. Fibromuscular dysplasia of cervical and intracranial arteries. Int J Stroke. 2010;5(4):296-305.
5. Southerland AM, Meschia JF, Worrall BB. Shared associations of nonatherosclerotic, large-vessel, cerebrovascular arteriopathies: considering intracranial aneurysms, cervical artery dissection, moyamoya disease and fibromuscular dysplasia. Curr Opin Neurol. 2013;26(1):13-28.
6. Stanley JC, Gewertz BL, Bove EL, Sottiurai V, Fry WJ. Arterial fibrodysplasia. Histopathologic character and current etiologic concepts. Arch Surg. 1975;110(5):561-566.
7. Kiando SR, Tucker NR, Castro-Vega LJ, et al. PHACTR1 is a genetic susceptibility locus for fibromuscular dysplasia supporting Its complex genetic pattern of inheritance. PLoS Genet. 2016;12:e1006367.
8. Di Monaco S, Georges A, Lengele JP, Vikkula M, Persu A. Genomics of fibromuscular dysplasia. Int J Mol Sci. 2018;19(5):pii:E1526.
9. Gupta RM, Hadaya J, Trehan A, et al. A genetic variant associated with five vascular diseases is a distal regulator of endothelin-1 gene expression. Cell. 2017;170(3):522-533,e15.
10. Touze E, Southerland AM, Boulanger M, et al. Fibromuscular dysplasia and its neurologic manifestations: a systematic review. JAMA Neurol. 2019;76(2):217-226.
11. Kadian-Dodov D, Gornik HL, Gu X, et al. Dissection and aneurysm in patients with fibromuscular dysplasia: findings from the U.S. Registry for FMD. J Am Coll Cardiol. 2016;68(2):176-185.
12. Mettinger KL, Ericson K. Fibromuscular dysplasia and the brain. I. Observations on angiographic, clinical and genetic characteristics. Stroke. 1982;13:46-52.
13. O’Connor SC, Poria N, Gornik HL. Fibromuscular dysplasia: an update for the headache clinician. Headache. 2015;55(5):748-755.
14. Debette S, Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol. 2009;8(7):668-678.
15. Giroud M, Fayolle H, Andre N, et al. Incidence of internal carotid artery dissection in the community of Dijon. J Neurol Neurosurg Psychiatry. 1994;57(11):1443.
16. Lee VH, Brown RD, Jr., Mandrekar JN, Mokri B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. 2006;67(10):1809-1812.
17. Bejot Y, Aboa-Eboule C, Debette S, et al. Characteristics and outcomes of patients with multiple cervical artery dissection. Stroke. 2014;45(1):37-41.
18. Cloft HJ, Kallmes DF, Kallmes MH, Goldstein JH, Jensen ME, Dion JE. Prevalence of cerebral aneurysms in patients with fibromuscular dysplasia: a reassessment. J Neurosurg. 1998;88(3):436-440.
19. Lather HD, Gornik HL, Olin JW, et al. Prevalence of intracranial aneurysm in women with fibromuscular dysplasia: a report from the US registry for fibromuscular dysplasia. JAMA Neurol. 2017;74(9):1081-1107.
20. Favoni V, Giani L, Al-Hassany L, et al. CGRP and migraine from a cardiovascular point of view: what do we expect from blocking CGRP? J Headache Pain. 2019;20(1):27.
21. Schievink WI, Mokri B, O’Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994;330(6):393-397.
22. Kennedy F, Lanfranconi S, Hicks C, et al. Antiplatelets vs anticoagulation for dissection: CADISS nonrandomized arm and meta-analysis. Neurology. 2012;79(7):686-689.
23. Debette S. Pathophysiology and risk factors of cervical artery dissection: what have we learnt from large hospital-based cohorts? Curr Opin Neurol. 2014;27(1):20-28.
24. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet. Neurol 2015;14(4):361-367.
25. Peng J, Liu Z, Luo C, et al. Treatment of cervical artery dissection: antithrombotics, thrombolysis, and endovascular therapy. Biomed Res Int. 2017;2017:3072098.
26. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418.
27. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236.
28. Debette S, Metso T, Pezzini A, et al. Association of vascular risk factors with cervical artery dissection and ischemic stroke in young adults. Circulation. 2011;123(14):1537-1544.
29. Gornik HL, Persu A, Adlam D, et al. First international consensus on the diagnosis and management of fibromuscular dysplasia. Vasc Med. 2019;24(2):164-189.
AMS receives research support from the NIH (NINDS, NHLBI), AHA/ASA, the Coulter Translational Research Fund, and Diffusion Pharmaceuticals, Inc. I also provide expert legal consultation in vascular neurology cases.
BBW is a deputy editor for the journal Neurology, receives funding from the NIH (current NINDS,NCATS and prior NINDS, NHGRI) and the Australian-American Fulbright Commission, and reports no commercial support.