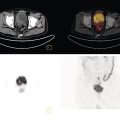
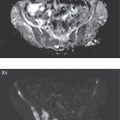
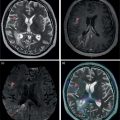
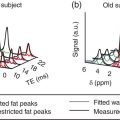
Mariano Grosso1, Michela Gabelloni2, Emanuele Neri2, and Giuliano Mariani1 1 Regional Center of Nuclear Medicine, Department of Translational Research and Advanced Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy 2 Diagnostic and Interventional Radiology, Department of Translational Research and Advanced Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy Benign gastrointestinal (GI) disorders include a host of conditions that can affect either the motility of the GI tract (functional disorders) and/or its macroscopic pathologic changes (structural diseases). Constipation and irritable bowel syndrome (IBS), two of the most common problems affecting the GI tract, are typical examples of functional disorders of the lower GI tract. Nevertheless, motility disorders can affect also the upper GI tract, the esophagus, and the stomach. Diseases involving macroscopic changes of the GI tract include conditions affecting the esophagus (e.g. diverticular disease, ulcerous disease associated with gastroesophageal reflux), the stomach and duodenum (primarily peptic ulcers, but also autoimmune atrophic gastritis), the small bowel (e.g. Crohn’s disease as a paradigm for inflammatory bowel disease, [IBD]), the colorectal tract (e.g. colon polyps, a benign but precancerous condition, and ulcerative colitis, another IBD condition), and the extreme lower end of the GI tract (e.g. hemorrhoids, anal fissures, perianal abscesses, anal fistulas, and perianal infections). More rare forms of colitis include ischemic colitis (caused by insufficient blood perfusion of the colon) and radiation colitis (after external beam radiation therapy). Additional structural diseases include GI bleeding (caused by different conditions) and ectopy of gastric mucosa in Meckel’s diverticulum. Diagnosis of these conditions relies on a wide spectrum of clinical information and often on the possibility of gaining direct visual access – through endoscopic procedures – to the GI tract, especially in conditions involving pathologic changes of the GI tract wall. On the other hand, a variety of noninvasive imaging procedures has been developed to characterize disorders of the GI tract, including both radiologic procedures and nuclear medicine procedures. This chapter will focus primarily on those benign GI disorders that can be investigated using either radiologic techniques and/or nuclear medicine techniques. These conditions currently include predominantly functional motility disorders of the GI tract, although either radiologic or nuclear medicine imaging procedures separately also play important roles in disorders involving structural pathologic changes of some portion(s) of the GI tract. The oral cavity, anatomically limited by the lips (anteriorly), the cheeks (laterally), the hard palate (superiorly), and the superior surface of the tongue and the muscles connected to the inner side of the mandible (inferiorly), is where the initial food shredding, mixing with saliva, and mastication take place; food is then directed through swallowing to pharynx (Figure 13.1). Being common to the digestive and respiratory tracts, the pharynx is in continuity with the esophagus, a muscular structure (average length 25 cm, diameter about 2.5 cm) whose distal end is located in the abdomen. The upper esophageal sphincter (UES) and the lower esophageal sphincter (LES) constitute the functional boundaries of the esophagus. Under physiologic conditions, the flow of material in the esophagus is unidirectional (top‐to‐bottom), granted by its concentric muscular configuration, and the tone of both the UES and the LES prevent the flow of air and the reflux of material from the stomach. Swallowing transports a food bolus from the mouth to the stomach through the esophagus, and this can be divided into three stages: oral, pharyngeal, and esophageal. Only the first stage is totally voluntary, the subsequent ones are reflexive and driven by both the peristaltic contractions of the posterior pharyngeal and esophageal musculature and the pressure gradient between the cervical and intrathoracic region. The last stage, by which the bolus enters the stomach, requires the coordination of the peristaltic pump with post‐swallowing relaxation of the LES. Overall, esophageal transit requires 2 seconds for liquids and 9 seconds for solids [1]. Figure 13.1 (a) Anatomic drawing shows the complex afferent and efferent neuroregulation of deglutition (sensory fibers in black, motor fibers in different colors) and cranial nerves participating in the system. (b) Summed image from dynamic recording of an oropharyngoesophageal radionuclide transit study (liquid bolus) allows clear identification of the various anatomic regions. Upper arrow indicates posterior mouth sphincter, lower arrow indicates UES, and the space between the arrows is the pharyngeal region. During scintigraphic acquisition, the patient stood facing the collimator surface for an anterior view of the chest, with head and neck tilted left. (Source: Reproduced from Mariani et al. [1]. © SNMMI). Indications for the test to be performed depend on the clinical conditions and the symptoms. Manometry, often performed after a barium swallow or endoscopy, is the gold standard for diagnosis of primary esophageal motility disorders in patients with dysphagia; these can include achalasia, scleroderma, diffuse esophageal spasm, hypertensive LES, and nonspecific esophageal motility disorders. Transit studies are indicated in conditions in which the use of manometry is prevented by its intrinsic limitations (i.e. it measures peristalsis only indirectly and the procedure itself can affect normal physiology), for instance in patients with dysphagia following surgery, with mechanical obstruction (neoplasia, scar), after radiotherapy, and in all those patients with poor or no compliance to manometry The examination does not require any specific preparation, except fasting over the prior 4–6 hours, and is performed via ingestion of a single or double radiologic contrast agent. Although barium sulfate contrast is the primary agent used, in patients at risk for aspiration or those who have undergone recent neck or esophageal surgery (in whom the examination is needed to rule out anastomotic leaks), barium is contraindicated and must be replaced with an iodinated contrast agent. Barium contrast can be prepared in different concentrations to obtain different consistencies and radiopacity. The examination begins with the patient standing up in the lateral oblique position and taking three consecutive swallows of barium suspension for evaluation of the proximal, mid, and distal portions of the thoracic esophagus, respectively. Further projections can be acquired with additional swallows and different decubitus positions to obtain a panoramic depiction of the esophageal morphology and function. In particular, the presence and degree of gastroesophageal reflux can be assessed by acquiring radiographs with the patient in the semiprone decubitus with Trendelenburg position, followed by a Valsalva maneuver to increase abdominal pressure. Fluoroscopic images are acquired in real time at a high frame rate (e.g. 30 frames per second) [2–5]. Patients with esophageal motility abnormalities often present with slowly progressing dysphagia for fluids and solids, sometimes associated with weight loss. The main causes of this condition are achalasia, diffuse esophageal spasm, and scleroderma. Achalasia is the most frequent primary motility disorder of the esophagus. Its etiology is unknown and its basic pathophysiologic mechanism is the inability of the LES to relax upon swallowing, associated with a complete loss of peristaltic coordination along the esophageal body. At the barium study, the esophagus is dilated (ranging from mild dilatation to megaesophagus in severe cases) with a tapered appearance of its distal tract (Figure 13.2). In the initial stages, the only sign may be stasis of contrast medium in the esophageal lumen with an air‐fluid level inside it, followed by rapid emptying by gravity when the hydrostatic pressure of the ingested barium column exceeds that of the LES, thus forcing its release and passage of the meal into the stomach. The main differential diagnosis is against pseudo‐achalasia, in which the esophagus is dilated with narrowing of its distal portion and of the gastroesophageal junction due to the presence of a tumor. Figure 13.2 Barium swallow study in a patient with achalasia (anterior–posterior projection): the esophagus is dilated with a tapered appearance of its distal tract. Figure 13.3 Barium swallow study in a patient with diffuse esophageal spasm. The esophagogram is characterized by multiple, spontaneous, and intermittent contractions giving rise to a segmented (“corkscrew”) appearance of the affected esophagus. Diffuse esophageal spasm is a motility disorder characterized by multiple, spontaneous, and intermittent contractions of the distal two thirds of the esophagus. Usually such contractions give rise to a segmented (“corkscrew”) appearance of the affected esophagus (Figure 13.3). Scleroderma is a connective tissue disorder characterized by atrophy of the esophageal smooth muscle, leading to reduced or absent peristalsis of the distal two‐thirds of the esophagus, to incompetent LES, and dilation of the upper esophagus [3–5]. The use of esophageal transit scintigraphy to evaluate esophageal transit time per se is currently limited because the investigation is part of a more complex evaluation of the swallowing function and esophageal transit. In particular, oropharyngo‐esophageal scintigraphy (OPES) is useful for the evaluation of swallowing and esophageal motility disorders by allowing qualitative and semiquantitative evaluation of the various stages of swallowing, and represents a better diagnostic tool than esophageal transit scintigraphy alone to investigate the possible causes of dysphagia [6–9]. After fasting for at least 3 hours, the acquisition procedure starts with the patient standing in front of a gamma camera (equipped with a low‐energy, high‐resolution, parallel‐hole collimator, energy window 140 keV ± 10%), with the face tilted 80° to the left to include the entire region from the mouth to the epigastric area in the field of view. The patient keeps in the mouth a 10‐mL bolus of water labeled with 37 MBq (1 mCi) of 99mTc‐colloid, and is asked to swallow it 2 seconds after starting a dynamic acquisition for 60 seconds (0.125 s/frame, matrix 64 × 64, zoom 1). At the end of the dynamic scan, with the patient still in the same position, a 60‐second static image is acquired to detect any retention in the esophagus and/or tracheobronchial aspirate. The same test is repeated 30 minutes later, using a semisolid bolus (10 mL of a jelly‐based drink labeled with 37 MBq of 99mTc‐colloid), followed by 60 seconds of static acquisition, with the same protocol employed for the liquid bolus. Scintigraphy with a single swallow has poor sensitivity (44%) and specificity (71%), mainly due to aberrant swallows (which can also occur in healthy subjects) and intrasubject variability. This issue can be overcome by summing the images of six independent liquid bolus swallows at 30‐second intervals, and calculating both time parameters (mean time, mean transit time, and transit time) and retention parameters at particular time points (Figure 13.4). In adults this examination can be integrated with a high‐temporal‐resolution dynamic sequence with four or five Valsalva maneuvers (to increase intra‐abdominal pressure) performed over 120 seconds to detect the presence of gastroesophageal reflux [1]. Acquired images can be reviewed over time to detect aberrant swallowing patterns and quantitatively by drawing at least three regions of interests (ROIs) around the oral cavity, pharynx, and whole esophagus (Figure 13.5), to measure the rate of the oropharyngo‐esophageal transit and to identify retention. In turn, the esophagus can be further subdivided by drawing three ROIs around its upper, middle, and lower thirds, respectively. Quantitative indices can be defined as follows: The aspiration percentage (PA) can be calculated to quantify radioactivity in the larynx or in the tracheobronchial tract [11] by dividing the counts obtained in the aspiration area (RA) by the initial activity detected in the oral cavity before swallowing (activity time 1, AT1), subtracted from the residual activity after swallowing (activity time 2, AT2), by means of the following formula: An OPES study provides particularly helpful information in all the clinical conditions linked to mechanical and muscular disorders. For instance, nutcracker esophagus is associated with noncardiac chest pain or dysphagia and high‐amplitude peristaltic waves in the distal esophagus, caused by hypertensive LES that can show abnormally elevated tone at rest, exaggerated LES contraction, or incomplete LES relaxation. Diffuse esophageal spasm, described as simultaneous contractions induced by liquid swallows, can be scarcely detected by manometry, but it is always identified by multiple liquid‐bolus OPES. Classic achalasia, caused by degeneration of intramural neurons of the esophagus, causes a rise of the basal LES pressure, resulting in impaired esophageal clearance and delayed transit times, which can be detected during diagnosis (with a sensitivity close to 100%) and monitored after treatment (myotomy, pneumatic dilation, or botulinum toxin injections) using OPES. In scleroderma, impaired esophageal motility can be detected by OPES in otherwise asymptomatic patients as patients show a typical pattern of retention of radioactivity in the lower esophagus, which has a higher sensitivity compared to manometry and barium swallows. Neurogenic dysphagia is secondary to diseases of different parts of the central (e.g. supranuclear, motor and sensory nuclei) or peripheral nervous system (e.g. nerves, muscle cells and spindles) involved in the swallowing process as a result of conditions such as stroke, brain tumors, brain injury, bulbar and pseudobulbar paralysis, and neurodegenerative diseases (e.g. amyotrophic lateral sclerosis, multiple sclerosis, Parkinson’s disease, Huntington’s disease, myasthenia, myopathies) (Figure 13.6) [12, 13]. OPES, with its lack of invasiveness and excellent tolerability, allows for the functional, objective, and qualitative–quantitative study of swallowing in patients with neurogenic dysphagia, with sensitivity and overall validity values (97.9% and 86.7%, respectively) akin to videoendoscopy in oropharyngeal dysphagia [14]. Figure 13.4 Oropharyngo‐esophageal transit scintigraphy (OPES) in a healthy volunteer. (a) ROIs drawn on the oral cavity, pharynx, and esophagus on the summed image obtained with the dynamic acquisition. Corresponding activity–time curves for oral transit (b), pharyngeal transit (c), and esophageal transit (d). (e) Condensed image obtained by collating side by side each frame of the dynamic acquisition. (Source for (a)–(d): Reproduced from Aghakhanyan et al. [10]/with permission of Springer Nature). Figure 13.5 OPES in a patient with dermatomyositis and dysphagia. (a) Condensed image of the dynamic acquisition using multiple liquid boluses that depict the presence of multiple swallows for each deglutition. (b) The static image acquired after swallowing liquid boluses reveals persistent pharyngeal retention associated with right tracheobronchial inhalation. (Source: Reproduced with permission from Aghakhanyan G, Fiasconaro E, Tardelli E, Grosso M, Paglianiti I. Functional imaging for benign conditions of the gastrointestinal tract. In: Volterrani D, Erba PA, Carrió I, Strauss HW, Mariani G, eds. Nuclear Medicine Textbook – Methodology and Clinical Applications. Cham, Switzerland: Springer Nature Switzerland; 2019:809–840 [10]). As most recently summarized by Maurer et al. [15], there are some clinical scenarios where the use of esophageal transit scintigraphy (often associated with the evaluation of gastroesophageal reflux) is most appropriate. These scenarios include dysphagia and quantification of response to therapy (the latter following treatment for achalasia); in turn, the underlying causes of dysphagia include achalasia, scleroderma, diffuse esophageal spasm, hypertensive LES, nonspecific motility disorder, and esophageal outflow obstruction. On the other hand, esophageal transit scintigraphy may be appropriate in cases of suspected aspiration, rumination, gastroesophageal reflux, and evaluation of patients pre‐ and postfundoplication. Radionuclide investigations have virtually no role in the diagnostic workup of benign esophageal disorders implying macroscopic changes of the esophagus. In this domain, a major role is played by the direct approach based on endoscopy. In addition to endoscopy, barium swallow videofluoroscopy plays an important role in defining the severity and extent of structural abnormalities of the esophagus. Figure 13.6 OPES with liquid bolus (a) and semisolid bolus (b) in a patient with amyotrophic lateral sclerosis (ALS), showing delay of pharyngeal transit. This confirms the neurogenic component of dysphagia in ALS, which affects in particular the oropharyngeal phase. The residual activity is about 14% with the liquid bolus (b) and 52% with the semisolid bolus (c). In the same patient, selected sequential frame images (d) reveal marked retention of the radiolabeled bolus at the oropharyngeal level. Several structural abnormalities of the esophagus can be observed during barium swallow studies; the most frequent of such abnormalities include esophageal diverticula, esophageal narrowing and stenosis, ab‐extrinsic compression, infections, and tumors. Esophageal diverticula are outpouchings originating from the esophageal wall. They can be true (when they include all parietal layers) or false (consisting of herniation of the mucosa and submucosa only through the muscular layer). In general, they can be classified into traction diverticula (formed as a result of external traction forces on the esophageal wall) or pulsion diverticula (secondary to increased intraluminal pressure). On barium swallow studies they appear as contrast‐filled pouches communicating with the esophageal lumen. Zenker’s diverticulum is an acquired pulsion diverticulum formed by mucosal and submucosal herniation through the Killian triangle, an area of focal weakness of the hypopharynx at the transition between the upper fibers of the cricopharyngeal and inferior pharyngeal constrictor muscles; hence, it can be considered as an outpouching of the hypopharynx in close proximity to the UES. On barium studies it can be seen on lateral radiographs as a contrast‐filled outpouching located posterior to the cervical esophagus and possibly plunging into the mediastinum (Figure 13.7). The Killian–Jamieson diverticulum is a pulsion diverticulum of the anterolateral wall of the cervical esophagus, just below the cricopharyngeal muscle. It originates from an area of anatomical weakness called the Killian–Jamieson space, through which the lower laryngeal nerve passes. Due to its position, it can be seen on barium swallow studies on frontal views, and it can be either transient or persistent. Esophageal candidiasis is the most common cause of infectious esophagitis in patients with immune deficiencies. This infection produces whitish and slightly raised plaques that can extend to the pharynx or tongue; in the esophagus they resemble small mucous nodules that are usually lined up longitudinally (Figure 13.8). This presentation accounts for their radiological appearance on double contrast swallow studies as linear or irregular filling defects that have a longitudinal orientation and are separated by normal mucosa. Figure 13.7 Barium swallow study in a patient with Zenker’s diverticulum, showing retention of the contrast medium in a large diverticulum. Double‐contrast esophagography has high sensitivity (up to 90%) for detecting gastroesophageal reflux disease because it allows the depiction of mucosal abnormalities that can be missed on single‐contrast examinations. The morphofunctional abnormalities related to gastroesophageal reflux disease include the following features: Edema and inflammation are usually present in the lower third or lower half of the esophagus, resulting in a finely nodular or granular appearance characterized by weakly, poorly defined radiolucent areas. Superficial ulcers and erosions can also occur in the distal esophagus, and manifest with a pointed, linear or star‐like configuration surrounded by a halo of edematous mucosa, radiated folds or sacculated areas of the adjacent esophageal wall. Less frequently, a hallmark of gastroesophageal reflux disease can be represented by a single ulcer in the posterior wall of the esophagus due to acid reflux occurring overnight in the patient sleeping in the supine position. Other patterns include fold thickening associated with edema and inflammation, or a single thickened fold mimicking a smooth polyp that originates from the gastroesophageal junction and extends into the distal esophagus. In this latter case, endoscopy is required to rule out neoplastic disease [2, 4, 5]. Figure 13.8 Barium swallow study in a patient with esophageal candidiasis, showing longitudinal irregular filling defects separated by normal mucosa. Esophagogastroscopy currently constitutes the mainstay for investigating and characterizing structural benign disease of the stomach, such as gastric ulcers or similar conditions. Therefore, this section will only consider functional gastric disorders, chiefly involving abnormalities of gastric emptying. In this regard, although in the past the barium sulfate meal has also been used to evaluate the gastric emptying time, this technique has currently been virtually totally replaced with evaluation based on the more reliable approach of radiolabeled meal(s) [16], as detailed below. The stomach is divided into two anatomic regions: the fundus, proximally, and the antrum, distally. Gastric emptying depends on the characteristics of the meal (volume, amount of carbohydrates, fats) and on hormones such as cholecystokinin and gastrin. The main clinical indication for gastric emptying studies is the failure to find an adequate explanation for symptoms such as nausea, vomiting or abdominal fullness using the conventional radiographic or endoscopic examinations [9]
13
Correlative Imaging of Benign Gastrointestinal Disorders
Oropharyingo‐esophageal Tract
Anatomy and Physiology

Imaging to Investigate Functional Motility Swallowing Disorders
Clinical Indications
Radiologic Evaluation of Esophageal Transit by Barium Swallow Videofluoroscopy
Main Motility Disorders Investigated with Barium Swallow Videofluoroscopy


Radionuclide Evaluation of Oropharyngo‐esophageal Transit
Patient Preparation and Scintigraphic Acquisition
Qualitative and Quantitative Image Processing



Structural Abnormalities of the Esophagus

Radiologic Evaluation of Structural Abnormalities of the Esophagus


Benign Gastric Diseases
Functional Disorders of Gastric Emptying
Anatomy and Physiology
Clinical Indications
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree