Jan 9, 2022 via Shahriar Lahouti
CONTENTS
- Preface
- Anatomy of infarction
- Definition
- Diagnostic consideration
- Differential diagnosis of MI
- ECG findings in acute coronary occlusion
- ECG evolution in OMI
- ECG findings in OMI
- Risk stratification
- Management
- Universal initial measures
- Opioids
- Beta-blocker
- Nitroglycerin
- PCI
- Thrombolytic
- Antithrombotic drugs
- Anticoagulant
- Special circumstances
- Guideline links
- Further going
- References
Preface
The current practice and guidelines for ACS follows the STEMI vs. NSTEMI paradigm. However, this paradigm is suboptimal and should be updated to improve outcomes by identification of acute coronary occlusion (for which STEMI is a poor surrogate). Therefore, it is difficult to present a comprehensive overview of the management of ACS in such a way that the reader can emerge with the necessary skills to operate within the current standard of care while simultaneously understanding that there is room for improvement from that standard. This post is intended as a guide to myocardial infarction for the emergency physician. It is assumed that cardiology will be consulted in patients with more severe myocardial infarction (e.g. occlusive MI and more severe nonocclusive MI). Likewise it is assumed that cardiothoracic surgery will be consulted regarding patients who are potential candidates for CABG surgery.
Anatomy of infarction
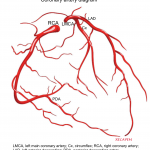
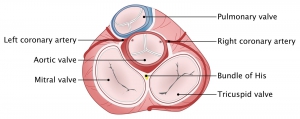
The right coronary artery (RCA) and the left main coronary artery (LMCA) extend from the aortic root 1. The left aortic sinus gives rise to the LMCA. The right aortic sinus gives rise to the RCA.
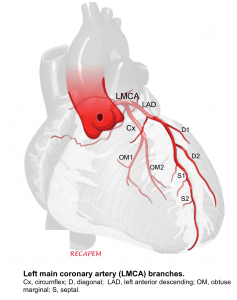
Left main coronary artery (LMCA). It branches into:
- Circumflex artery (Cx)- Gives off obtuse marginal branches (OM). Cx artery supplies blood to the left atrium; lateral and posterior of the left ventricle.
- Left anterior descending artery (LAD)- It gives off two branches; diagonal branches (D) and septal branches (S).
- LAD supplies the anterior and lateral of the left ventricle (via the diagonal branches) and the anterior of the septum (via septal branches). The apex of the heart is generally supplied by the “LAD”.
- The left anterior descending artery may extend even further in the case of a type 3 “wraparound left anterior descending artery,” which describes the left anterior descending artery wrapping around the apex and supplying the apex and inferior wall.
- LAD supplies the anterior and lateral of the left ventricle (via the diagonal branches) and the anterior of the septum (via septal branches). The apex of the heart is generally supplied by the “LAD”.
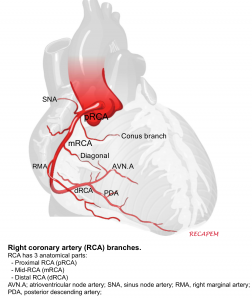
Right Coronary Artery (RCA)
The right coronary artery branches into:
- Sinoatrial nodal branch supplying sinoatrial node.
- Right marginal artery
- Posterior descending artery (PDA)
The right coronary artery supplies the right atrium, right ventricle and Inferior portion of both ventricles and posterior of the septum.
PDA: is the artery that supplies the posterior interventricular septum and atrioventricular node.
- It is supplied by RCA in 70% of the general population (called right dominant).
- In 10% of population, it is supplied by Cx (left dominant).
- In 20% of population, it is supplied by both RCA and Cx (called co-dominant).
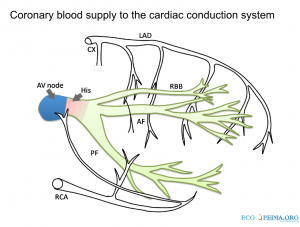
👉The coronary blockade can cause conduction block, on AV nodal, His or bundle branch level (the following figure).
- Sinus node is supplied by RCA.
- Atrioventricular node is supplied by RCA (in right dominant) individuals.
- Right bundle branch is supplied via LAD.
- Anterior fascicule (AF) supplied by LAD and posterior fascicule (PF) has dual blood supply via both PDA and LAD.
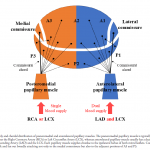
👉Coronary arterial supply to mitral papillary muscle (PM)
- Anterolateral PM: It receives dual blood supply (LAD and Cx) and therefore is relatively resistant to ischemia.
- Posteromedial PM: It receives single blood supply (PDA) and is more prone to injury from ischemia.
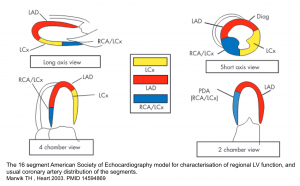
Echocardiography. The 16 segment model for determination of regional LV function and usual coronary artery distribution of segments is shown below.
ECG leads
Each ECG leads records underlying cardiac surface as shown below.
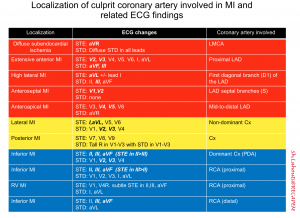
The ECG changes in MI is helpful to localize the involved coronary artery as shown below. Keep in mind that there are certain overlaps between coronary arteries territorial supply such as
- Anterolateral LV: supplied by both LAD and Cx
- Inferolateral LV: supplied by both Cx and RCA
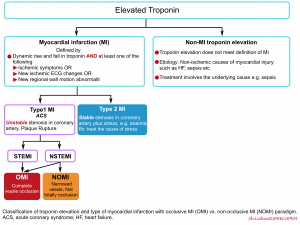
Definitions
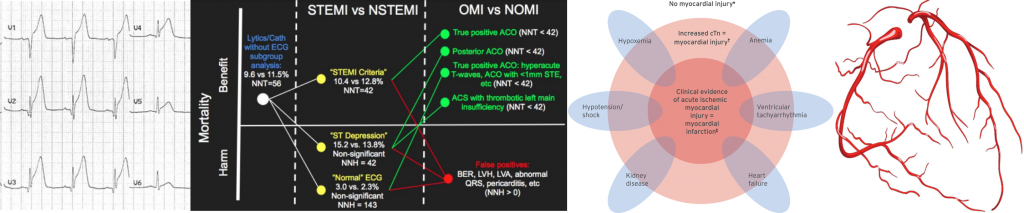
Criteria for Myocardial Injury
- Detection of an elevated cardiac troponin value above the 99th percentile URL is defined as myocardial injury.
- The injury is considered acute if there is a rise and/or fall of cardiac troponin values 2.
Clinical criteria for Myocardial Infarction (MI)
The term acute myocardial infarction should be used when there is a dynamic rise and fall in troponin (with at least one value above the 99th percentile of normal) and at least one of the following 2:
- Ischemic symptoms OR
- New ischemic ECG changes OR
- New regional wall motion abnormality in a pattern consistent with an ischemic etiology.
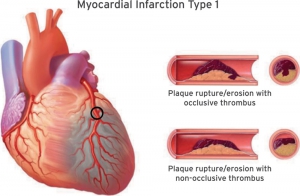
- AMI caused by atherothrombotic CAD and usually precipitated by atherosclerotic plaque disruption (rupture or erosion) 2. Differentiating Type 1 vs. Type 2 MI (below) is often challenging and subjective. Consideration of the following factors may be helpful:
- Amount of physiologic stress: Low
- Clinical context: Initial presentation is suggestive of acute coronary syndrome, e.g. spontaneous / sudden onset of chest pain
- Symptoms: Chest pain or anginal equivalent usually is present
- ECG findings: May be strongly diagnostic for occlusive MI
- Extent of troponin elevation: Larger troponin elevation is in favor of Type 1 MI.
- Echocardiographic finding: New RWMA or reduced EF.
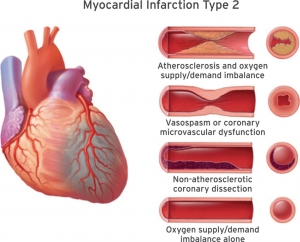
- Refers to patients with stable coronary artery stenosis who develop myocardial ischemia due to diminished myocardial perfusion (e.g. anemia, sustained bradyarrhythmia, respiratory failure, hypotension or shock) and/or increased myocardial demand (e.g. tachycardia, hypertension) 2. Differentiation Type 2 vs. Type 1 is often subjective. The following factors should be considered:
- Amount of physiologic stress: High (e.g. anemia, hypoxemia, hypoperfusion)
- Clinical context: Patients initially present with non-cardiac problems such as sepsis, hemorrhage.
- Ischemia is secondary to another primary problem.
- Symptoms: Often no symptoms of myocardial ischemia
- ECG findings: Nonspecific and minimal. ECG changes resolve with resolution of stress.
- Extent of troponin elevation: Usually moderate (however a severe fixed lesion such as left main stenosis can cause a marked troponin elevation due to stress)
- Echocardiographic finding: May show hyperkinetic underfilled heart without RWMA.
Read more: Over-diagnosis and over-treatment of MI in critically ill patients(PulmCrit)
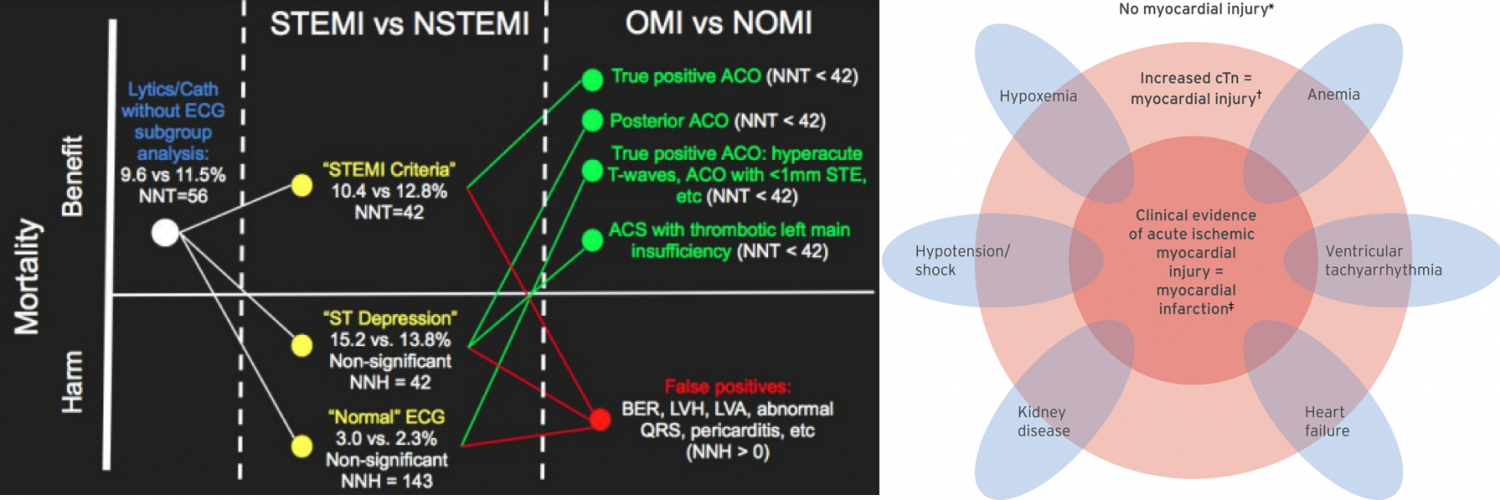
Traditionally, Type-I MI has been divided into patients with ST elevation (STEMI) vs. patients without ST elevation (NSTEMI). Arguably there’re several caveats in this paradigm:
- The number of mm of ST elevation was used to determine which patients had coronary artery occlusion.
- It has been recognized that many ECG patterns without ST elevation also reflected coronary artery occlusion (these patterns were termed STEMI-equivalents).
- The traditional “STEMI ECG criteria” are neither sensitive nor specific for the outcome that they are intended to identify acute coronary occlusion. It has been shown that 25% of NSTEMI patients have fully occluded culprit lesions.
Unfortunately at present, the ACC/AHA technically only recognizes one situation as a “STEMI equivalent”: Positive original Sgarbossa criteria in the setting of left bundle branch block!
-
- However, a wide variety of beliefs exist among different specialties, and guidelines regarding other conditions that constitute a STEMI equivalent (e.g. posterior STEMI, hyperacute T waves, de Winter’s T waves, modified Sgarbossa criteria, ST elevation in aVR associated with widespread ST depression, etc.).
Occlusion MI vs. Non-occlusion MI
More recently, a new nomenclature system has been proposed that encourages clinicians to be more thoughtful about various ECG patterns that reflect coronary artery occlusion:
- Occlusive MI (OMI): STEMI plus STEMI-equivalent patterns. It is virtually a type 1 ACS resulting in total occlusion or near-occlusion of the infarct-related vessel, with insufficient collateral circulation, such that full-thickness infarction will occur unless flow is restored immediately.
- Non-Occlusive MI (NOMI): is any MI (of any classification, types 1-5) that does not satisfy the description of occlusion MI above. ECG patterns are usually reflective of subendocardial ischemia.
OMI/NOMI paradigm: OMI manifesto, Dr. Smith and The OMI manifesto (EM:RAP). PDF Version of OMI Manifesto
Diagnostic consideration
Diagnosis of MI is complicated since no single risk factor, symptom, sign, or investigation finding in isolation has a high sensitivity or specificity for the diagnosis 3 4. Usual components of diagnosis include:
Clinical presentation
- Concerning features may include:
- Pain radiating to the shoulders, both arms, right arm/shoulder, neck, or jaw.
- Pain worse with exertion.
- Associated vomiting or diaphoresis.
Unusual presentations (e.g., MI without chest pain) are more likely in women, elderly, and/or diabetic patients. Presentations can vary broadly (e.g., nausea/vomiting, dyspnea, syncope, fatigue, jaw pain, epigastric pain).
ECG. Including baseline and serial ECGs
- Do Not merely rely on presence or absence of ST elevation for diagnosis of OMI.
- Diagnosis of MI is unfortunately vastly more complex than simply counting the number of millimeters of ST elevation in various leads (thereby invalidating the traditional STEMI/NSTEMI dichotomy). This is evolving into the NOMI/OMI conceptualization, which emphasizes more holistic ECG interpretation to detect a variety of patterns suggestive of coronary occlusion.
- Goal of ECG interpretation: It is not to secure a diagnosis with 100% certainty, rather, the goal of ECG interpretation is just to get a fairly reasonable concept of the diagnosis – to get pretty close. Definitive diagnosis will often require additional ECGs, echocardiography, and/or cardiac catheterization.
- How to interpret ECG in practice more efficiently?
- Context: Always interpret ECG in clinical context. i.e. “Is ECG obtained as a routine screening or is performed in patient suspected for MI”.
- Comparison: Always compare STAT ECG to baseline ECG (if available) as well as to serial ECGs over time.
- Rule of Proportionality: Implies that the finding on ECG should be compared to the overall ECG (e.g. 0.5 mm STE may be significant if the QRS complex is tiny).
Echocardiography. The role of echo in evaluation of patients with suspected MI include:
1.Diagnosis of MI: Regional wall motion abnormalities (RWMA) support the diagnosis.
- Sensitivity: While the presence of a “WMA” may help to increase the suspicion of MI, its absence should not necessarily be reassuring unless the echocardiogram is truly of perfect quality, performed with contrast, and read by a skilled echocardiographer.
- Specificity: The presence of “WMA” has a low specificity (since this may reflect a remote MI, myocarditis, or Takotsubo cardiomyopathy). Combining echo with ECG can improve specificity. To maximize efficiency, it’s often useful to start with ECG interpretation and then use echo to sort out different possibilities. For example:
- A patient presents with diffuse STE, concerning for either a very large occlusive MI or pericarditis. Echocardiography can readily differentiate these possibilities.
- A patient presents with right precordial T-wave inversion that is concerning for either Wellens pattern or a submassive PE. Echocardiography can rapidly evaluate the right ventricle, to assess the likelihood of a submassive PE.
- It is also important to remember that the “WMA” may be transiently present, corresponding with the dynamic nature of coronary occlusion and reperfusion. After reperfusion, the “WMA” may remain for hours to days to weeks (myocardial stunning), or it may resolve rapidly if the ischemia intensity and duration are minimal.
2.Complications of MI. For example papillary muscle rupture, myocardial rupture.
3.Hemodynamic status of the patient and risk stratification
4.Alternative diagnosis?
- RV dilation should always prompt consideration of PE (although other possibilities include right ventricular MI or chronic pulmonary hypertension).
- Aortic dilation and/or aortic valve regurgitation suggests aortic dissection.
Troponin. Including baseline and serial troponins.
- The myocardial injury may be acute, as evidenced by a newly detected dynamic rising and/or falling pattern of TnI values above the 99th percentile URL, or chronic, in the setting of persistently elevated Tn levels 2.
- Although elevated Tn values reflect injury to myocardial cells, they do not indicate the underlying pathophysiological mechanisms, and can arise following preload-induced mechanical stretch or physiological stresses in otherwise normal hearts.
- However, regardless of the mechanism, acute myocardial injury, when associated with a rising and/or falling pattern of Tn values with at least one value above the 99th percentile URL and caused by myocardial ischemia, is designated as an acute MI. Histological evidence of myocardial injury with myocyte death can be detected in clinical conditions associated with non-ischemic mechanisms of myocardial injury as well 2.
Differential diagnosis
Aortic dissection (AD)
- Symptoms: Chest pain (Sudden onset with rapid acceleration to maximal intensity, sharp/tearing/ripping, radiation to shoulder, may be migratory). May be associated with a variety of symptoms (e.g. abdominal pain, neurologic, syncope).
- ECG: Usually nonspecific. Aortic dissection may rarely cause occlusion of a coronary artery, producing an OMI-pattern ECG.
- AD is notorious to cause right sided STEMI; However it’s not true as it has been shown that left sided events can happen 5 .
- POCUS: Aortic root dilation, aortic regurgitation, pericardial effusion. Ultrasound of abdominal aorta, carotids, or femoral arteries may show a dissection flap.
- Other tests: CT angiography of aorta is diagnostic.
-
More on this here
PE causing pleural infarct with chest pain
- Symptoms: Sharp, pleuritic chest pain, hemoptysis
- ECG: Nonspecific
- POCUS: May show pleural effusion, small area of lung consolidation, and possibly DVT.
- Other test: D-dimer elevation. CT angiography is diagnostic.
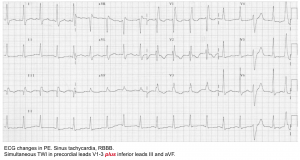
PE causing RV strain with myocardial ischemia
- Symptoms: May cause ischemic-quality chest pain due to hypoperfusion of the right ventricular free wall. It’s often associated with syncope or dyspnea.
- ECG. may show:
- Tachycardia
- T-wave inversion involving the inferior leads and/or right precordial leads (V1-V4).
- 👉The presence of TWI in both leads III and V1 allows PE to be differentiated simply but accurately from ACS in patients with negative T waves in the precordial leads.
- Right axis deviation.
- Complete or partial right bundle branch block (RBBB).
- SI-QIII-TIII pattern.
- ST elevation in aVR and ST depression in lead I
- RV injury pattern with STE in V1-V3 and/or STD in V4-V6. More on this here.
- POCUS: RV dilation and RV systolic dysfunction. DVT study may be positive.
- Other tests: D-dimer and troponin is positive. CT angiography should be diagnostic.
- (sub)Massive PE can mimic acute MI.
- STEMI should cause RWMA involving the left ventricle and dysfunction of the left ventricle.
- Massive PE will cause RV dilation and usually an under-filled left ventricle (which is vigorously contracting).
Pericarditis
- Symptoms: Chest pain is often pleuritic, positional (worse lying flat) and may be sharp. May be associated with febrile illness.
- ECG: May show diffuse concave STE and PR depression throughout most of the limb leads (I, II, III, aVL, aVF) and precordial leads (V2-6). STD and PR elevation in lead aVR (± V1).
- POCUS: pericardial effusion may be present.
- Pericarditis is a diagnosis of exclusion. See more here.
Pneumothorax
- Symptoms: Dyspnea, sharp pleuritic chest pain
- ECG: Nonspecific
- POCUS: “Presence” of lung sliding and/or B-lines virtually exclude pneumothorax in that lung field (however absence of lung sliding and/or B-line is not specific for pneumothorax).
- Visualizing the junction between sliding lung and absent sliding is known as the “lung point sign” and is near 100% specific for pneumothorax
Takotsubo cardiomyopathy (aka. apical ballooning syndrome, or broken heart syndrome)
- Symptoms: Ischemic-quality chest pain is often preceded by emotional or physical stress. Dyspnea, arrhythmia, cardiogenic shock can be seen.
- ECG: STE is generally the first finding (usually greatest V2-V5). TWI may follow.
- POCUS: Typically shows apical hypokinesis. However, some patients may display a pattern of circumferential mid- or basal hypokinesis 21 (beyond a single epicardial vascular distribution).
- Other test: Elevated troponin. Cardiac catheterization needed to exclude MI.
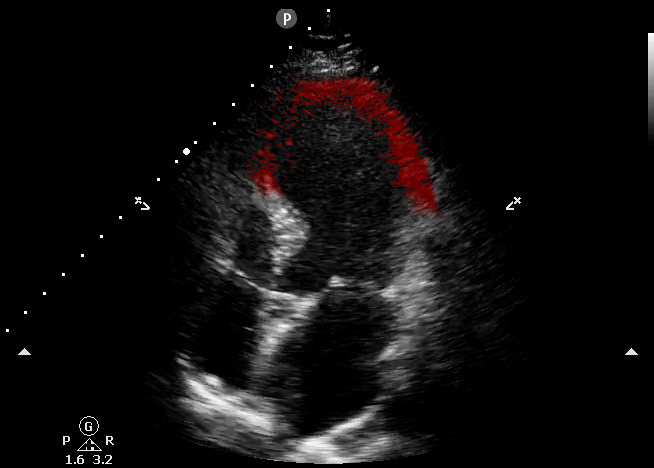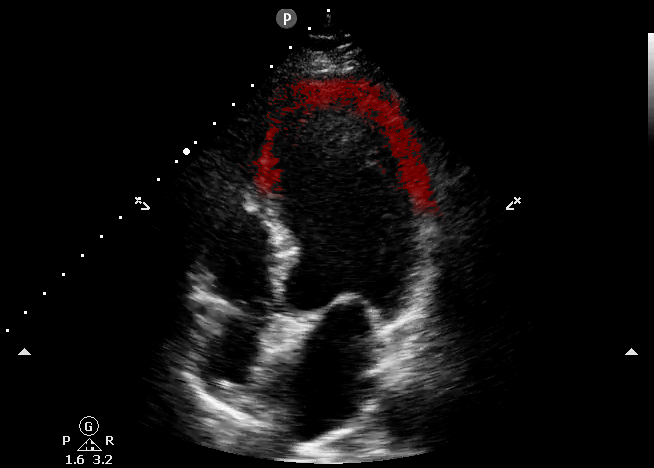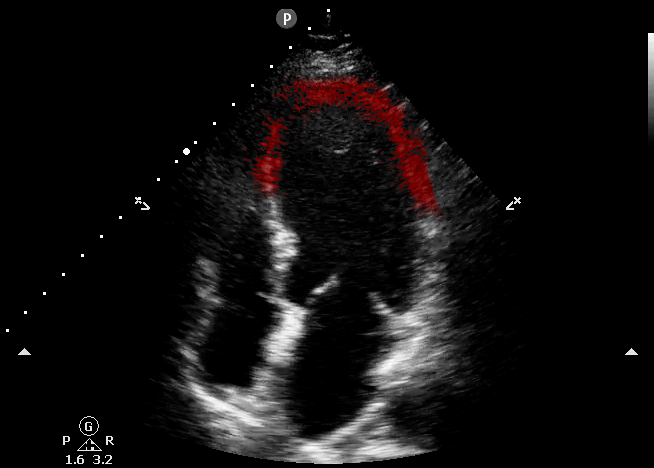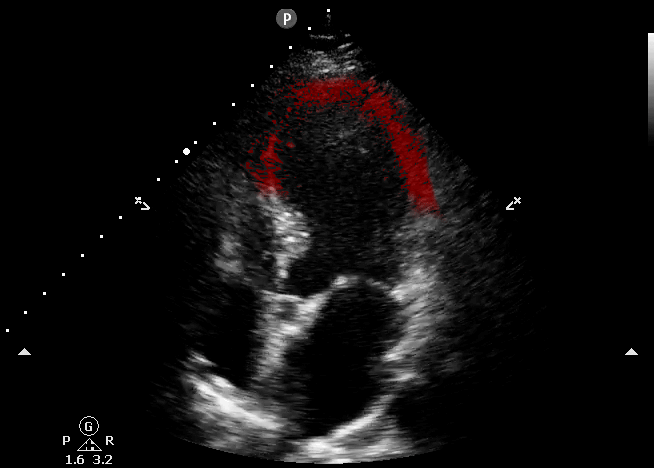
This echocardiography represents the classic appearance for Takotsubo Cardiomyopathy; akinetic apical septum and LV apex in the vascular territory of the LAD. This can be described as “ballooning” of the LV apex. The basilar septal segment is spared. From CORE ULTRASOUND.
ECG findings in acute coronary occlusion
The evolution of ECG findings in acute coronary occlusion is discussed here. Although several ECG patterns have been shown to be associated with acute occlusive MI, the hyperacute T-waves and ST elevation is explored below.
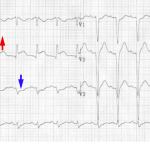
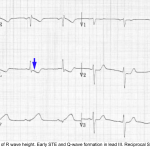
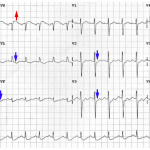
Hyperacute T-Wave
Hyperacute TW suggest ischemia which is either transmural or nearly transmural. Isolated hyperacute TW (without ST deviation) are often a harbinger of impending ST elevation. However, isolated hyperacute TW may also be seen following spontaneous thrombolysis that has recently led to resolution of ST elevation.
Characteristics of hyperacute TW
- Relatively symmetric, broad-based, with a relatively blunt peak. Straightened upslope (they should not be deeply concave). Tall in comparison to the RW.
- Involving multiple leads in an anatomic pattern:
- Hyperacute TW typically involve several contiguous leads.
- If only one lead looks hyperacute, this may be an anomaly.
- If all of the ECG leads look similar, this argues against hyperacute TW (suggesting hyperkalemia or early repolarization).
- May be associated with other ischemia features, for example:
- STE or STD (de Winter pattern).
- Loss of RW progression.
- Changed compared to baseline ECG.
Differential diagnosis of hyperacute TW
- Prominent T-waves as a reciprocal change to TWI
- Reperfused posterior MI may cause prominent right precordial TW.
- Reperfused high-lateral MI may cause large inferior TW.
- Reperfused inferior MI may cause large TW in aVL.
- Early repolarization
- TWs are tall and asymmetric.
- RW are also tall.
- Hyperkalemia
- TWs are narrow-based, pointy, symmetric, and tall.
- TWs are most marked in V2-V3, but the overall pattern is one of diffuse TW abnormality.
- May see other features of hyperkalemia (e.g. diminished P-waves, QRS widening). More on this here
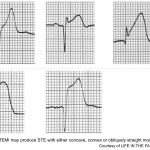
ST Elevation
Acute STEMI may produce ST elevation with either concave, convex or obliquely straight morphology.
The current formal “STEMI ECG criteria” include a new ST elevation measured at the J-point relative to the PQ junction, in two contiguous leads with the following cutoffs:
-
V2-V3: ≥2 mm in men ≥40 y old; ≥2.5 mm in men <40 y old, or ≥1.5 mm in women regardless of age.
-
Other leads: ≥1 mm.
DDX: STE may be seen in other conditions
- LBBB: ST segments and TW show “appropriate discordance” (i.e. they are directed opposite to the main vector of the QRS complex). This produces STE and upright TW in leads with a negative QRS complex.
- LVH: Causes a similar pattern of repolarization abnormalities as LBBB
- Ventricular paced rhythm
- LV aneurysm
- Pericarditis: widespread concave (“saddleback”) ST segment elevation with PR segment depression in multiple leads, typically involving I, II, III, aVF, aVL, and V2-6.
- Early repolarization: ER pattern is a normal variant commonly seen in young, healthy patients. Most literature defines ER pattern as being present on the ECG when there is J-point elevation of ≥1 mm in ≥2 contiguous inferior and/or lateral leads 6. ECG features include:
- Widespread concave STE, most prominent in the mid-to-left precordial leads (V2-5)
- Remember that the STE of ER is not constant. In particular, it may be diminished with exercise, sympathetic tone, and heart rate, and may be increased when the heart rate is slower.
- There is often notching of the J-point; the “fish-hook” pattern.
- Prominent, slightly asymmetrical T waves that are concordant with the QRS complex
- Widespread concave STE, most prominent in the mid-to-left precordial leads (V2-5)
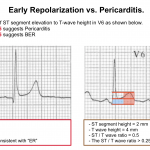
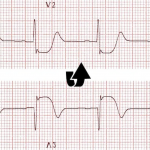
👉Early repolarization (ER) vs. Pericarditis: Pericarditis can be difficult to differentiate from “ER”, as both conditions are associated with concave ST elevation.
- One useful clue to distinguish between these two entities is to look at the ST segment / T wave ratio and the Fish Hook Pattern.
- In ER, the TW amplitude is more prominent than pericarditis. Calculate the height of ST segment elevation and compare to the TW height in lead V6. STE to TW height ratio in V6 < 0.25 (see below)
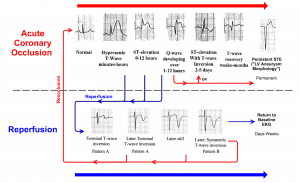
ECG evolution in OMI
The progression of ECG findings seen during acute coronary occlusion and reperfusion is shown below (adapted from OMI manifesto, Dr. Smith blog).
Prototypical evolution during acute occlusion
-
The earliest ECG sign of occlusion is QT lengthening, followed by hyperacute T wave, which signals a viable but ischemic myocardium.
-
Then, as the ischemia progresses, the ST segment begins to elevate.
-
As the amount of viable myocardium diminishes with infarction (irreversible cell death or myocardial stunning), the ST segment begins to fall.
-
As the myocardium irreversibly infarcts, Q waves form.
-
Q waves may also form in salvageable myocardium. This is especially true in anterior MI, where Q waves may form early and are reversible with early reperfusion.
-
ECG evidence of reperfusion
- The resolution of hyperacute T waves and ST segment elevation (>70%).
- The progression of terminal, then full, T-wave inversion over the course of hours to days.
Anterior OMI
- STE
- Most often seen in V2-V4
- Coved STE favors ischemia if seen (but anterior OMI can also cause concave STE).
- TW changes
- Hyperacute T-waves may be seen.
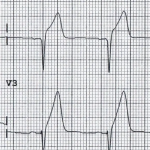
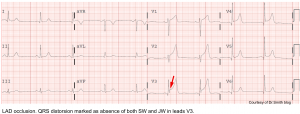
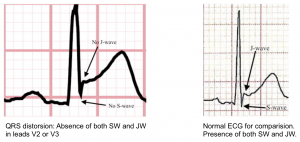
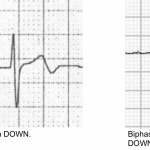
- Terminal QRS distortion in V2-V3 may occur (results from broad, malignant T-waves that invade the QRS complex and obliterate any S- or J-waves). See the following figure.
- Loss of RW progression
- QW changes: may emerge rapidly (these are NOT a contraindication to revascularization).
- Reciprocal changes: STD anywhere other than in aVR or V1 favors OMI.
👉Terminal QRS distortion: defined as emergence of J-point > 50% of the RWs in leads with qR configuration or disappearance of the S-wave in leads with Rs configuration.
- Terminal QRS distortion simply identified as absence of both S-wave or J-wave in either lead V2-V3. (figure below).
- Terminal QRS distortion is very specific for “LAD” occlusion. When differential diagnosis is acute LAD occlusion vs. early repolarization, terminal QRS distortion is 100% specific for LAD occlusion.
Anterior OMI vs. Early Repolarization
In the presence of anterior STE of at least >1 mm, the following approach may help differentiate anterior OMI versus early repolarization. Presence of “ANY” of the following features suggest anterior OMI:
- STE >5 mm.
- Any convex STE in V2-V6.
- Inferior reciprocal changes.
- Anterior ST depression.
- Any Q-wave in V2-V4.
- Any TWI in V2-V6, and/or aVL.
- Terminal QRS distortion in V2 or V3 (distortion is defined as lack of either an S-wave or a J-wave in that lead. see above).
If none of the above features is present, Smith’s formula can be used to differentiate OMI versus early repolarization.
- Caution: This formula is designed only to sort out OMI vs. early repolarization. It will not differentiate OMI from other MI mimics (e.g. LVH or pericarditis).
👉Avoid diagnosis of “ER” in patients older than 50 y/o or patients with cardiovascular risk factors.
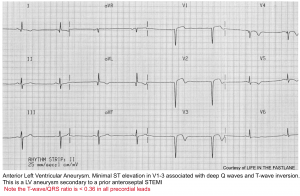
Anterior OMI vs. LV aneurysm
The features below may help sort out acute anterior OMI versus chronic aneurysm. For patients presenting late in the course of MI (e.g. >6 hours after chest pain onset), this distinction will become blurred.
Typical findings in LV aneurysm
- Deep Q-waves in V1-V4 (usually with at least one QS wave in these leads).
- Coved STE in V1-V4.
- Often causes some TWI, which is typically shallow.
Features that suggest acute anterior MI (rather than solely an aneurysm)
- Any tall upright T-wave in V1-V4, with T-wave >36% the size of the QRS complex 8.
- Choose one lead with positive TW in V1-4. Calculate the height of the TW and compare to QRS height. A TW:QRS ratio of >0.36 is in favor of new anterior OMI.
- Other ischemic findings on the ECG, for example:
- STE in leads without deep Q-waves (especially aVL).
- Reciprocal STD.
High lateral OMI
ECG findings of lateral OMI
- STE in aVL +/- Lead I.
- STE is often <1 mm (especially with low QRS voltages).
- Inferior reciprocal changes.
- STD in lead III (+/- STD in II and aVF) – may be the most obvious abnormality.
- TWI may occur as a reciprocal of hyperacute T-waves in lateral leads (more worrisome if this occurs in leads with a positive QRS complex).
Culprit vessels
- Occlusion of the first diagonal branch (D1) of the LAD may produce isolated STE in I and aVL
- Occlusion of the left circumflex artery may cause STE in I, aVL along with leads V5-6.
DDx of high lateral OMI
- Anterior aneurysm involving the lateral wall.
- Pericarditis (suggested by more diffuse STE and lack of reciprocal STD).
- Early repolarization (suggested by large complexes with proportionally unimpressive ST deviation, lack of hyperacute T-waves, benign early repolarization morphology in anterior leads).
- Right ventricular hypertrophy (suggested by tall R-wave in V1, terminal S-wave in lead I).
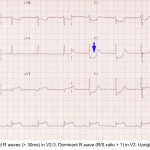
Posterior OMI
Posterior OMI is usually associated with inferior OMI and/or lateral OMI; though it can happen alone (5-10% of OMIs).
ECG findings
- Precordial STD >1 mm that is maximal in V1-V4.
- Normally there is slight STE in V2-V3, so any STD is worrisome.
- This differentiates posterior OMI from subendocardial ischemia (wherein STD is usually maximal ~V4-V6).
- Tall R-waves (>30msec) in the right precordial leads.
- This represents a Q-wave equivalent.
- It is not invariably present, nor is it necessarily an early finding.
- Abnormal T-waves in the right precordium.
- TWI may occur as a mirror-image of hyperacute posterior T-waves.
- Prominent upright T-waves can occur as a mirror-image of posterior TWI (due to reperfusion).
- Ischemia in the inferior or lateral leads.
- Posterior ischemia often occurs together with inferior or lateral ischemia.
- Ischemic morphology using the mirror test.
- The mirror test inverts the precordial ECG, to mimic what you would see using posterior leads. This is probably about as good as getting an ECG with posterior leads (V7-V9), and it’s much faster.
- Using paper: flip the ECG over and look through it as shown below.
DDx of posterior OMI
- RV hypertrophy may cause a tall R-wave in the right precordial leads and a strain pattern in V1-V3 with STD.
- Other features of RV hypertrophy may suggest this diagnosis (e.g., terminal S-wave in lead I, TWI in lead III).
- Digoxin or hypokalemia may cause scooped STD in the right precordial leads. Clues to suggest digoxin or hypokalemia:
- STD is diffusely present throughout the ECG (including the inferior leads).
- Other features:
- Hypokalemia: Prominent U-wave.
- Digoxin: short QTc, unusual rhythms.
- Subendocardial ischemia (NOMI):
- Maximal STD in ~V2-V4 favors posterior OMI, whereas maximal STD in ~V4-V6 favors subendocardial ischemia.
- If seen, posterior OMI is suggested by:
- Tall R-waves in the right precordium.
- Upright T-waves in the right precordium (however, TWI in the right precordium can be consistent with either posterior OMI or subendocardial ischemia).
- Anterior de Winter T-wave pattern
- Suggested by lack of a tall R-wave and broad/hyperacute T-waves.
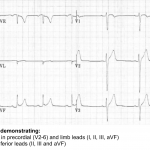
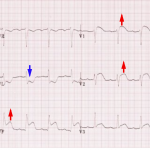
Inferior OMI
Core features of inferior OMI
- STE in inferior leads (👉caution: STE can be subtle).
- Should generally see STE in two inferior leads.
- STD in aVL (+/- Lead I).
- If no STD in aVL, the diagnosis should be questioned. STD in aVL may theoretically be obscured by simultaneous lateral ischemia (with STE in V5 or V6). However, evidence shows that STD occurs in 99% of inferior OMI.
- TWI in aVL may also support the diagnosis (this is the mirror image of hyperacute T-waves in the inferior leads).
Culprit lesion in inferior OMI
- Inferior OMI can happen due to RCA occlusion (80%) or less commonly occluded Cx (10%). See below.
Additional features that can be seen in inferior OMI
- Right precordium:
- RV MI may cause STE in V1.
- Manifestations of posterior ischemia (~V2-V4).
- ST depression or flattening.
- T-wave abnormalities.
- STE in V5, V6 may occur in left circumflex occlusions.
- In inferior MI, always systematically evaluate for possible RV myocardial infarction.
ECG mimics of IMI
- LVH.
- Can be very difficult to sort out.
- Features that may support inferior MI:
- STE disproportionately large compared to preceding S-waves.
- Ischemic features in other territories (e.g., right precordial STD).
- Old inferior MI
- Early repolarization. Clues that suggest benign early repolarization:
- Benign early repolarization may also be present in precordial leads.
- Benign-appearing morphology (e.g., concave STE, J-waves).
- Absence of STD in aVL.
- PE can cause STE in inferior leads and V1, with reciprocal changes in aVL.
- Diffuse and prominent TWI in the inferior and anterior leads may help suggest PE.
Right Ventricular OMI (RVMI)
- Diagnosis of RVMI is important since the supportive management is different (more on this here).
- RVMI most commonly happen in the context of inferior OMI, but rarly it can happen alone.
- In both conditions (either in inferior MI or isolated RVMI); it almost always occurs due to a proximal RCA occlusion.
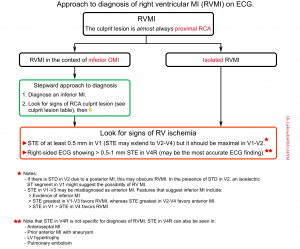
- Stepward approach to diagnosis of RVMI on ECG is shown in figure below.
RVMI in the context of inferior MI
- Diagnose an inferior MI.
- Look for signs of an RCA culprit lesion (table below👇).
- Look for evidence of RV ischemia
- STE of at least 0.5 mm in V1 suggests RVMI (STE may extend to V2-V4, but it should be maximal in V1-V2).
- Right-sided ECG showing >0.5-1 mm STE in V4R may be the most accurate ECG finding
👉When in doubt, POCUS may be the fastest way to evaluate RV function.
Isolated RVMI. This may occur in two situations:
- Isolated RV marginal branch occlusion.
- Old inferior MI with a new proximal occlusion of the right coronary artery (the inferior wall is already scarred, so only the RV MI causes ST elevation).
- ECG: The main finding is STE in V1-V3 (which will tend to be misdiagnosed as anterior MI).
OMI in the context of LBBB or paced rhythm
A new LBBB in the context of acute MI implies a large infarction, which is potentially an indication for catheterization. However, in practice, many patients present with a chronic LBBB, so the finding of LBBB alone is nonspecific. For patients with either LBBB or an RV-paced rhythm, ischemia may be diagnosed using the Smith-modified Sgarbossa criteria as listed below.
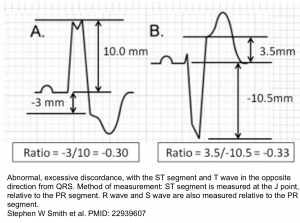
Smith-Modified Sgarbossa Criteria (OMI is revealed if ANY criteria is met, even in just a SINGLE lead) 7:
- Concordant STE of at least 1 mm in any lead.
- Concordant STD of at least 1 mm in V1-V3.
- Excessive discordant STE or discordant STD in any lead that is >1 mm and also >25% of the depth of the preceding R-wave or S-wave, respectively (figure below). However, this doesn’t apply in extreme tachycardia, pulmonary edema, hyperkalemia, or hypertension (if these conditions are present, then treat them and repeat the ECG)
Other features that may support the presence of ischemia:
- Marked convexity of the ST segment (especially in the precordial leads).
- T-wave inversion in leads with a negative QRS (concordant TWI).
- Substantial qualitative changes compared to the baseline ECG.
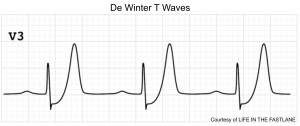
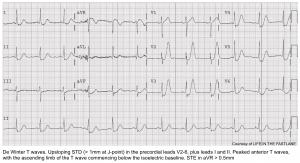
De Winter pattern
This pattern reflects nearly complete LAD occlusion and should be treated as an occlusive MI (e.g. with cath lab activation). However, a de Winter pattern can also occur in the inferior or lateral territories, with similar implications.
Classically defined as a combination of the following:
- Tall, hyperacute T-waves in the precordial leads.
- Up-sloping STD >1 mm in the precordial leads.
- Absence of STE in the precordial leads.
- Normal QRS duration.
- Often, mild STE is seen in aVR
Abnormalities are often maximal around V3. 9
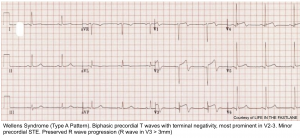
Wellens pattern
Wellens patterns reflect transient occlusion of the “LAD” with spontaneous reperfusion. Clinically, this is often associated with a history of chest pain which has currently resolved.
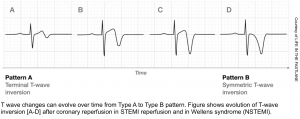
Wellens pattern A refers to biphasic TW, suggestive of very recent occlusion (figure above). Over time, Wellens pattern A will evolve into deep, symmetric TW inversion (pattern B).
- Wellens pattern B is less immediately worrisome, because it implies that the vessel has managed to remain open for a bit longer, without re-occluding.
Wellens patterns do not indicate an active occlusive MI, but they imply the presence of a high-grade proximal LAD lesion that needs to be taken very seriously. One series found that most patients with Wellens syndrome who didn’t undergo revascularization went on to develop an anterior OMI within a few weeks. Patients with Wellens pattern should not undergo stress testing, as this may trigger an acute MI. 9
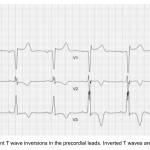
ECG criteria for Wellens pattern
- TWI in V2-V3 (and possibly extending out as far as V5).
- Preservation of the RW (if the RW is replaced by deep Q-waves, this suggests anterior aneurysm).
- Evolution over time (in particular, Wellens A is never a static pattern).
DDx of inverted T-waves
- Normal finding in children
- TWI in the right precordial leads (V1-3) are a normal finding in children, representing the dominance of right ventricular forces.
- TWI in the right precordial leads may persist into adulthood (called “persistent juvenile T-wave pattern”). TWIs are asymmetric, shallow (<3mm) and usually limited to leads V1-3
- LBBB: Produces TWIs in the lateral leads I, aVL and V5-6.
- LVH: Produces TWIs in the lateral leads I, aVL, V5-6 (left ventricular ‘strain’ pattern), with a similar morphology to that seen in LBBB.
- RBBB: Produces TWIs in the right precordial leads V1-3.
- RVH: Produces TWI in the right precordial leads V1-3 (right ventricular ‘strain’ pattern) and also the inferior leads (II, III, aVF).
- PE: Acute right heart strain (e.g. secondary to massive PE) produces a similar pattern to RVH, with TWIs in V1-3 and inferior leads.
- Raised intracranial pressure (e.g. via SAH): Produces widespread deep TWIs with a bizarre morphology.
- Hypertrophic cardiomyopathy (HCM):
- The chief abnormality associated with HCM is left ventricular hypertrophy (LVH), of variable degree from mild hypertrophy (13-15 mm) to extreme myocardial thickening (30-60 mm).
- The most commonly observed pattern is asymmetrical thickening of the anterior interventricular septum (aka. asymmetrical septal hypertrophy). Typical ECG findings include: deep, narrow (“dagger-like”) Q waves in lateral (I, aVL, V5-6) +/- inferior (II, III, aVF) leads.
- Other less common pattern of LVH include apical hypertrophy (10%). The classic ECG finding in apical HCM is giant T-wave inversion in the precordial leads.
DDx of biphasic TW
- Wellens syndrome Type 1: T waves go up (initial deflection positive) then down (terminal deflection is negative).
- Hypokalemia: T waves go down then up.
Subendocardial ischemia pattern
This pattern reveals diffuse, severe subendocardial ischemia of the entire myocardium and it does not indicate active coronary vessel occlusion.
ECG features of subendocardial ischemia
- Diffuse STD, which is maximal in V4-V6 and lead II.
- No evidence of focal OMI (e.g. no STE in leads other than aVR and V1).
- If very severe, this may be accompanied by STE in aVR (and sometimes V1 as well). Cause of STE in AVR: Two possible mechanisms:
- Diffuse subendocardial ischemia, with STD in the lateral leads producing reciprocal change in aVR (most common)
- Infarction of the basal septum, i.e. a STEMI involving aVR
- The basal septum is supplied by the first septal perforator artery (a very proximal branch of the LAD), so ischemia / infarction of the basal septum would imply involvement of the proximal LAD.
Clinical significance of severe subendocardial ischemia with STE in aVR
- This pattern signifies diffuse, severe subendocardial ischemia of the entire myocardium.
- Clinical causes include
- Left main coronary artery (LMCA) stenosis
- Proximal left anterior descending artery (LAD) stenosis
- Severe triple vessel disease
- Hypoxia or hypotension for example after resuscitation from cardiac arrest.
- Diffuse ischemia may be caused in various ways:
- It can reflect acute plaque rupture (Type I MI) with partial occlusion of the vessel. This is a very scary scenario, because there is a lot of myocardium in jeopardy if the stenosis progresses to an acute occlusion.
- It can reflect stable coronary stenosis plus acute physiological stress (Type-2 MI).
- Management depends on clinical context:
- If context suggests a Type I MI (e.g. patient presenting with chest pain): treat medically for MI and pursue prompt catheterization. However, be cognizant that patients will often have severe coronary artery disease that cannot be treated by percutaneous coronary intervention. Therefore, consider holding P2Y12 inhibitors, since CABG may be needed.
-
If context suggests a Type-2 MI (e.g. patient presenting with gastrointestinal hemorrhage): treat the underlying cause of stress and follow the ECGs carefully to ensure resolution of ischemia.
Clinical significance of milder subendocardial ischemia without STE in aVR
- Nonocclusive MI of any vessel causing subendocardial ischemia will tend to create this same ECG pattern. (For some reason, subendocardial ischemia doesn’t localize on ECG.)
- This is perhaps the most classic ECG pattern associated with nonocclusive MI (NOMI).
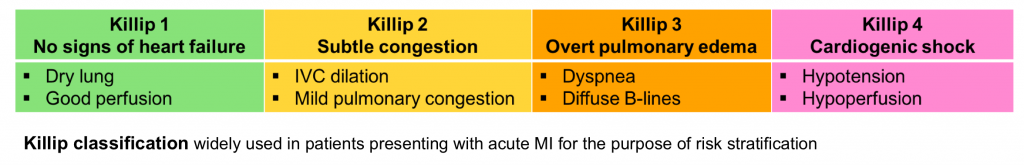
Risk stratification
Risk stratification and hemodynamic status can be rapidly determined at the bedside. This may be very helpful regarding disposition and management. For example, the presence of severely reduced LV systolic function suggests that the patient may respond poorly to beta-blockers (management of cardiogenic shock here.)
- Vitals
- Shock index (HR/SBP) > ~ 0.8 suggests significant instability and possible shock.
- Narrow pulse pressure (less than 25% of SBP) may suggest low cardiac output.
- MAP <65 mmHg or significant drop from baseline is concerning for hemodynamic instability.
- Hemodynamically significant bradycardia (e.g. HR< ~30) should always raise concern for shock (even if BP is maintained by compensatory systemic vasoconstriction).
- Skin perfusion
- Cool extremities, mottling and prolonged capillary refill are suggestive for low cardiac output.
- Echo
- RV, LV size and function?
- Mitral and aortic valve function (exclude severe regurgitation)
- IVC size and collapsibility?
- Pericardial effusion?
- Lung ultrasound
- B-Lines? bilateral and diffuse anterior B-lines suggest elevated pulmonary capillary wedge pressure (can be a sign of cardiogenic pulmonary edema).
Management
Universal initial measures
- Establish IV access, perform a 12-lead ECG, and initiate cardiac monitoring.
- Target hemoglobin >8 mg/dL. 10
- Supplemental oxygen only for patients with hypoxemia, titrated to a target of normoxia (e.g. target saturation 92-97%).11
- Target potassium >3.5 mEq/L and magnesium >2 mEq/L (avoids arrhythmia).
- Maximum medical management of ACS refers to administration of aspirin + P2Y12 inhibitor +/- anticoagulant (as per cardiology discretion/institutional protocol) +/- nitroglycerin (if not contraindicated).
Opioid
- Avoid opioid unless the decision to proceed with immediate reperfusion therapy has already been made. then opioids may be administered to alleviate symptoms during the time between evaluation and definitive reperfusion.
Beta-blocker
-
STEMI: it is reasonable to administer IV beta blockers at the time of presentation to patients with STEMI and no contraindications to their use who are hypertensive or have ongoing ischemia.
-
NSTEMI: (Oral) beta blockers recommended for all ACS within the first 24 h without contraindications, but there is no strong evidence or recommendation that beta blockers should be started in the ED.
Contraindications
- Bradycardia (heart rate <60 bpm).
- Heart block (PR interval >240 ms, or second/third degree heart block); most commonly seen in RCA occlusions.
- Heart failure (e.g. cardiogenic pulmonary edema).
- Cardiogenic shock, or risk factors for developing shock (e.g. HR >110, SBP< 120, or Shock index > ~0.8).12
- Clinical and echocardiographic risk stratification may help predict which patients will tolerate beta-blockade.
Tachycardia is a relative contraindication to beta-blockade. That may be implausible, but compensatory tachycardia may reflect underlying instability which indicates an inability to tolerate beta-blockers.
Dosing
- IV beta-blockade is generally achieved with sequential 5-mg doses of metoprolol (up to a total of 15 mg, as tolerated/required).
- For most patients, starting an oral beta-blocker within 24 hours is adequate. Preferred agents are metoprolol or carvedilol.
Nitroglycerin
There’s no robust evidence supporting the use of nitroglycerine in STEMI in the reperfusion era. Nevertheless, nitroglycerine may be useful for symptom management (e.g. relief from anginal chest pain, management of hypertension, and treatment of pulmonary edema).
Contraindications
- Hypotension.
- Aortic stenosis (relative contraindication).
- RV infarction.
- Recent use of phosphodiesterase inhibitors:
- Sildenafil or vardenafil within 24 hours.
- Tadalafil within 48 hours.
Dosing
- Initially may be given as a sublingual tablet of 0.4 mg q5 minutes (up to three doses).
- If the patient responds favorably, may transition to a nitroglycerine infusion (e.g. 10-50mcg/min for ongoing chest pain)
PCI
General indications for emergent PCI
- Occlusive MI.
- Hemodynamic instability (including shock, or new/worsening heart failure attributable to the acute MI).
- Electrical instability (ventricular tachycardia or ventricular fibrillation).
- Recurrent or persistent ischemia (chest pain or ECG findings of ischemia) that is refractory to medical therapy.
- (If unsure about need for PCI: optimize medically, obtain serial ECGs & echocardiography.)
- 👉 PCI CheatSheet-PDF
Relative contraindications to PCI
- Severe renal failure.
- Patients at high risk of bleeding (after stenting, patient must remain on dual antiplatelet therapy).
- Patients who are unlikely to be compliant with dual antiplatelet therapy.
- Low life expectancy (e.g., severe dementia, advanced malignancy with poor oncologic prognosis, extremely elderly).
- Patients with a history of prior CABG may benefit less from PCI. More on this here.
Non-occlusive MI (NOMI) :early invasive strategy vs. conservative strategy
- Patients with NOMI without indications for emergent PCI (as listed above) may be treated either with an early invasive strategy (e.g. catheterization within 24 hours) or with a conservative strategy (including medical management followed by a stress test, with PCI performed only if there is recurrent spontaneous ischemia or a positive stress test).
- An early invasive strategy generally reduces the risk of recurrent myocardial ischemia or rehospitalization for acute coronary syndrome. However, meta-analysis involving 10,150 patients found no difference in the risk of mortality after one year (4.3% vs. 4.4%). ESC 2020 guidelines likewise state that an early invasive strategy doesn’t reduce all-cause mortality in the overall population of patients with NSTEMI.
- When unclear, the decision to pursue PCI will be made in conjunction with cardiology consultation. This should take numerous factors into account (e.g. the specifics of ECG and echocardiography findings, renal function, indicators of risk due to myocardial ischemia, other active medical problems, and patient preferences).
Maximal medical management for patients undergoing primary PCI
- This will be determined by cardiology. The typical regimen includes
- Aspirin: 162-325 mg PO (non-enteric-coated, chewable)
- P2Y12 Inhibitor: Ticagrelor 180mg or Clopidogrel 600 mg PO as per cardiology.
- Unfractionated heparin: 60-70 units/kg (max 4,000-5,000 units) IV push loading dose, then 12-15 units/kg/h (max 1,000 units/h) infusion. Following successful PCI, heparin is discontinued (leaving the patient on aspirin and P2Y12 inhibitor).
- Aspirin: 162-325 mg PO (non-enteric-coated, chewable)
- For patients with ECGs suggestive of surgical coronary disease (e.g. diffuse ST depression with STE in aVR), it may be wise to initially hold dual antiplatelet therapy in anticipation of the possibility of CABG surgery. A potent P2Y12 inhibitor may be initiated in the catheterization laboratory, if coronary anatomy is amenable to PCI (either prasugrel or ticagrelor).
Thrombolytic for STEMI
-
If it will be >90-120 min total time from the first medical contact to PCI, or >90 min from STEMI diagnosis to PCI, and
-
Symptoms <12 h, or between 12-24 h if “a large area of the myocardium is at risk or hemodynamic instability is present”, or ECG shows high “acuteness” (large upright T waves, persistent R waves, persistent ST elevation).
-
Tenecteplase (TNK-tPA)
-
Reconstitute a 50-mg vial in 10 mL sterile water (5 mg/mL)
-
Single weight-based bolus, IV push over 5 sec
-
<60 kg: 30 mg
-
60-69 kg: 35 mg
-
70-79 kg: 40 mg
-
80-89 kg: 45 mg
-
>90 kg: 50 mg
-
-
Alteplase (tPA)
-
90-min weight-based infusion
-
>67 kg: Infuse 15 mg IV over 1-2 min; then, 50 mg over 30 min; then, 35 mg over the next 60 min (ie, 100 mg over 1.5 h)
-
≤67 kg: Infuse 15 mg IV over 1-2 min; then, 0.75 mg/kg (max 50 mg) over 30 min; then, 0.5 mg/kg (max 35 mg) over 60 min
-
Maximal medical management for STEMI patients receiving thrombolytic
- The typical regimen includes
- Aspirin: 162-325 mg PO (non-enteric-coated, chewable)
- P2Y12 Inhibitor: Clopidogrel 300 mg PO
-
Enoxaparin:
-
<75 y old: 30 mg IV bolus, then by 1 mg/kg subcutaneous (SC) (max 100 mg for the first dose) in 15 min
-
≥75 y old: No bolus, 0.75 mg/kg SC (max 75 mg for the first dose)
-
For creatinine clearance <30, give 1 mg/kg q24h (not 12 h)
-
Continue throughout hospitalization
-
-
Transfer to a PCI-capable facility should be carried out after the administration of thrombolytics.
-
Immediate PCI is indicated if there is no ECG evidence of reperfusion after thrombolytics (Rescue PCI).
-
After thrombolytics, at the receiving institution:
-
Routine immediate angiography +/- PCI in high-risk patients
-
Extensive STE, new LBBB, previous MI, Killip class ≥2, LV ejection fraction <35%
-
-
Routine angiography +/- PCI for all others within 24 h.
-
Absolute Contraindications
1. Any prior ICH
2. Known structural cerebral vascular lesion (eg, arteriovenous malformation)
3. Known malignant intracranial neoplasm (primary or metastatic)
4. Ischemic stroke within 3 mo
5. EXCEPT acute ischemic stroke within 4.5 h
6. Suspected aortic dissection
7. Active bleeding or bleeding diathesis (excluding menses)
8. Significant closed-head or facial trauma within 3 mo
9. Intracranial or intraspinal surgery within 2 mo
10. Severe uncontrolled hypertension (unresponsive to emergency therapy)
11. For streptokinase, prior treatment within the previous 6 mo
Relative contraindications
1. History of chronic, severe, poorly controlled hypertension
2. Significant hypertension on presentation (SBP >180 mm Hg or DBP >110 mm Hg)
3. History of prior ischemic stroke >3 mo
4. Dementia
5. Known intracranial pathology not covered in absolute contraindications
6. Traumatic or prolonged (>10 min) CPR
7. Major surgery (<3 wk)
8. Recent (within 2 to 4 wk) internal bleeding
9. Noncompressible vascular punctures
10. Pregnancy
11. Active peptic ulcer
12. Oral anticoagulant therapy
Aspirin
Aspirin has been robustly demonstrated to cause mortality benefit following MI, and is considered the most important antithrombotic agent in MI.
Indications: Should be given immediately to any patient with definite or probable Type-I MI (unless contraindicated).
Dosing
- Loading dose: 325 mg of non-enteric-coated aspirin (e.g. four uncoated 81-mg tablets).
- Maintenance dose: 81 mg enteric-coated aspirin daily (higher doses are not more effective, but carry greater bleeding risk).
Contraindications
- Active bleeding or very high risk of bleeding.
- Aspirin allergy (in which case ticagrelor or clopidogrel may be used instead; if clopidogrel is utilized then a 600-mg loading dose should be given to achieve therapeutic levels sooner).
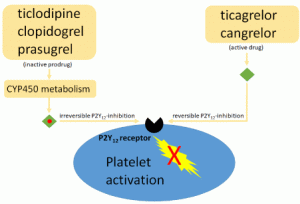
P2Y12 inhibitors
All patients undergoing PCI will require a P2Y12 inhibitor. These inhibitors must be given as soon as possible to achieve onset by the time of PCI (PCI results in heightened platelet reactivity). The following discussion is focused on patients undergoing medical management.
Role of P2Y12 inhibitors in medical management
- Adding a P2Y12 inhibitor has benefit even in patients who aren’t undergoing PCI (in terms of fewer myocardial infarctions and potentially reduced mortality).
- Evidence supporting the use of P2Y12 inhibitors is more robust that evidence supporting the use of heparin anticoagulation.
-
General consideration
-
Thienopyridine, a prodrug, requires conversion to its active metabolite form and, therefore, has unpredictable effects.
- For patients on oral anticoagulation, clopidogrel may be favored over ticagrelor.
-
-
Dose
-
Loading dose of 300 mg or 600 mg, followed by 75 mg daily.
- 600-mg loading dose takes effect more rapidly (2 hours), compared to 300 mg loading dose (~6 hours).
- 300-mg is often used for medical management. A 600-mg loading dose has been demonstrated to be superior to 300 mg prior to PCI.
-
-
General consideration
-
Does not require metabolic activation (unlike clopidogrel), thus has a faster onset.
-
Ticagrelor works more consistently than clopidogrel (which may have slow absorption and erratic metabolism, depending on individual CYP2C19 activity).
-
Reversible inhibition (unlike clopidogrel) enhances the rate of the offset of action but is also more risky in non-compliant patients.
- Ticagrelor has better ischemic outcomes than clopidogrel.
- Ticagrelor should be considered for MI patients who aren’t taking oral anticoagulation and aren’t at high risk of bleeding 13.
- Ticagrelor is safer if the patient ultimately needs CABG (shorter-acting).
-
- Dose: 180-mg loading dose, followed by 90 mg BID.
- Ticagrelor may cause nonexertional dyspnea as a side effect. Aminophylline may be utilized to transiently reverse dyspnea or bradycardia due to ticagrelor 14.
Anticoagulation
Anticoagulation for patients undergoing PCI
-
- Angiography and PCI must always be in the setting of anticoagulation to avoid immediate procedural thrombosis.
- European Society of Cardiology lists unfractionated heparin as class I and enoxaparin as class IIa.
- Avoid fondaparinux due to the increased incidence of PCI catheter thrombosis.
- Following successful PCI, unfractionated heparin is discontinued (leaving the patient on aspirin and P2Y12 inhibitor).
Anticoagulation in other patients
The rational use of heparin is as a temporary bridge to stabilize coronary stenosis prior to PCI. There isn’t evidence to support anticoagulation in patients who aren’t planned to undergo catheterization. However, short-term anticoagulation may be reasonable in patients who are evolving rapidly and might become candidates for catheterization.
- The traditional practice of providing heparin anticoagulation for 48 hours as “medical management” without subsequent PCI is not evidence-based and needs to be abandoned.
1st-line anticoagulant: Fondaparinux 2.5 mg sq daily
- The ESC 2020 NSTEMI guidelines recommend fondaparinux as the first-line anticoagulant for patients not undergoing immediate catheterization (i.e. patients being medically managed, or receiving delayed PCI) 13 .
- Compared to enoxaparin, fondaparinux caused less bleeding and reduced mortality in the OASIS-5 trial.
- Fondaparinux is contraindicated in renal dysfunction (GFR <20 ml/min).
- If fondaparinux is used alone, this will lead to an increase in catheter-related thrombi among patients undergoing PCI. However, this problem may be avoided by the addition of a single heparin bolus during the PCI procedure.
2nd line anticoagulant: Low molecular-weight heparin (LMWH)
- It is useful when full anticoagulation is required for another reason (e.g. atrial fibrillation or pulmonary embolism).
- LMWH has more predictable pharmacology than unfractionated heparin (UFH), often making it safer and more effective. Indeed, meta-analyses have found that LMWH is superior to UFH in myocardial infarction.
- The usual dose is enoxaparin 1 mg/kg sq q12hr (contraindicated if GFR <30 ml/min).
3rd line anticoagulant: Unfractionated heparin (UFH) infusion
- It is the only available option for patients with GFR<20 ml/min (who cannot be treated with fondaparinux or enoxaparin).
- UFH infusions have the highest risk of bleeding complications (compared to either fondaparinux or LMWH).
Special circumstances
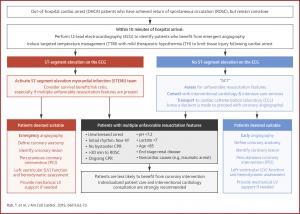
ACS in cardiac arrest
-
Attempt defibrillation for ventricular fibrillation (VF)/ventricular tachycardia (VT) and initiate high-quality cardiopulmonary resuscitation.
-
Consider transvenous pacemaker placement for significant bradycardia contributing to cardiogenic shock refractory to standard advanced cardiac life support (ACLS) and transcutaneous pacing when there will be a significant delay to the catheter lab.
-
VF/VT refractory to multiple defibrillations and standard ACLS:
-
Ensure optimal pad placement.
- Consider esmolol
- Consider veno-arterial extracorporeal membrane oxygenation (ECMO) followed by emergency PCI in patients deemed salvageable with a good baseline quality of life.
-
Which Cardiac Arrest Patients with Return of Spontaneous Circulation Require the Catheterization Lab?
- All patients with STEMI on ECG require the catheterization lab.
- Patients with initial shockable rhythm who do not have diagnostic STE but occlusion MI is suspected (i.e. even in the absence of STEMI) catheterization lab activation is recommended. (see the following algorithm for risk stratification of comatose cardiac arrest patients). 15
ACS in cardiogenic shock
- The differential of cardiogenic shock in the setting of ACS is based on pump failure (LV and/or right ventricle), and mechanical complications (papillary rupture, free wall rupture, etc.) 18 .
- ACS with resulting cardiogenic shock is an indication for emergency PCI, regardless of ECG findings or the time since the onset of symptoms.
-
The current guidelines give a class I, level B recommendation for emergency PCI of STEMI in cardiogenic shock, regardless of the time since onset.
-
The current guidelines recommend (I,B) fibrinolytics for STEMI patients with cardiogenic shock who are unsuitable candidates for either PCI or CABG.
- Emergency PCI is superior to thrombolytics in STEMI with cardiogenic shock, and emergency PCI is superior to “medical stabilization” with delayed PCI in this group.
-
Adjunct therapies to emergency reperfusion in ACS with cardiogenic shock 18:
-
Optimize the preload with fluids/blood as necessary based on hematocrit and fluid tolerance.
-
Consider intubation, mechanical ventilation, and paralysis to reduce the demand for cardiac output during the delay to definitive management.
-
Vasopressors in cardiogenic shock: Norepinephrine is first-line, then consider adding dobutamine for persistent low cardiac output after norepinephrine
-
Correct and prevent dysrhythmias (VT and VF).
-
- Consider veno-arterial ECMO to restore perfusion and prevent cardiac arrest while arranging for emergency PCI if the patient is worsening despite the above therapies.
Guideline Link
- 2017 ESC guidelineS for STEMI
- 2020 ESC guidelines for NSTEMI
- 2018 ESC guidelines for myocardial revascularization
- 2020 NICE guidelines for ACS
Further going
Acute References
1. Ogobuiro I, Wehrle CJ, Tuma F. Anatomy, Thorax, Heart Coronary Arteries. [Updated 2021 Jul 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534790/
2. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018 Nov 13;138(20):e618-e651. doi: 10.1161/CIR.0000000000000617. Erratum in: Circulation. 2018 Nov 13;138(20):e652. PMID: 30571511
3. Jung J, Bord S. Emergency department management of non-ST-segment elevation myocardial infarction. Emerg Med Pract. 2020 Jan;22(1):1-24. Epub 2020 Jan 1. PMID: 31855327
4. Fanaroff AC, Rymer JA, Goldstein SA, Simel DL, Newby LK. Does This Patient With Chest Pain Have Acute Coronary Syndrome?: The Rational Clinical Examination Systematic Review. JAMA. 2015 Nov 10;314(18):1955-65. doi: 10.1001/jama.2015.12735. PMID: 26547467
5. Kawahito K, Adachi H, Murata S, Yamaguchi A, Ino T. Coronary malperfusion due to type A aortic dissection: mechanism and surgical management. Ann Thorac Surg. 2003 Nov;76(5):1471-6; discussion 1476. doi: 10.1016/s0003-4975(03)00899-3. PMID: 14602269
6. Patton KK, Ellinor PT, Ezekowitz M, Kowey P, Lubitz SA, Perez M, Piccini J, Turakhia M, Wang P, Viskin S; American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Functional Genomics and Translational Biology. Electrocardiographic Early Repolarization: A Scientific Statement From the American Heart Association. Circulation. 2016 Apr 12;133(15):1520-9. doi: 10.1161/CIR.0000000000000388. Epub 2016 Mar 7. PMID: 27067089
7. Smith SW, Dodd KW, Henry TD, Dvorak DM, Pearce LA. Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ratio in a modified Sgarbossa rule. Ann Emerg Med. 2012 Dec;60(6):766-76. doi: 10.1016/j.annemergmed.2012.07.119. Epub 2012 Aug 31. Erratum in: Ann Emerg Med. 2013 Oct;62(4):302. PMID: 22939607
8. Miranda DF, Lobo AS, Walsh B, Sandoval Y, Smith SW. New Insights Into the Use of the 12-Lead Electrocardiogram for Diagnosing Acute Myocardial Infarction in the Emergency Department. Can J Cardiol. 2018 Feb;34(2):132-145. doi: 10.1016/j.cjca.2017.11.011. Epub 2017 Nov 29. PMID: 29407007
9. Tzimas G, Antiochos P, Monney P, Eeckhout E, Meier D, Fournier S, Harbaoui B, Muller O, Schläpfer J. Atypical Electrocardiographic Presentations in Need of Primary Percutaneous Coronary Intervention. Am J Cardiol. 2019 Oct 15;124(8):1305-1314. doi: 10.1016/j.amjcard.2019.07.027. Epub 2019 Jul 27. PMID: 31455501
10. Ducrocq G, Gonzalez-Juanatey JR, Puymirat E, Lemesle G, Cachanado M, Durand-Zaleski I, Arnaiz JA, Martínez-Sellés M, Silvain J, Ariza-Solé A, Ferrari E, Calvo G, Danchin N, Avendaño-Solá C, Frenkiel J, Rousseau A, Vicaut E, Simon T, Steg PG; REALITY Investigators. Effect of a Restrictive vs Liberal Blood Transfusion Strategy on Major Cardiovascular Events Among Patients With Acute Myocardial Infarction and Anemia: The REALITY Randomized Clinical Trial. JAMA. 2021 Feb 9;325(6):552-560. doi: 10.1001/jama.2021.0135. PMID: 33560322; PMCID: PMC7873781
11. Stub D, Smith K, Bernard S, Nehme Z, Stephenson M, Bray JE, Cameron P, Barger B, Ellims AH, Taylor AJ, Meredith IT, Kaye DM; AVOID Investigators. Air Versus Oxygen in ST-Segment-Elevation Myocardial Infarction. Circulation. 2015 Jun 16;131(24):2143-50. doi: 10.1161/CIRCULATIONAHA.114.014494. Epub 2015 May 22. PMID: 26002889.
12. Wei Z, Bai J, Dai Q, Wu H, Qiao S, Xu B, Wang L. The value of shock index in prediction of cardiogenic shock developed during primary percutaneous coronary intervention. BMC Cardiovasc Disord. 2018 Oct 1;18(1):188. doi: 10.1186/s12872-018-0924-z. PMID: 30285644; PMCID: PMC6167806
13. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM; ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021 Apr 7;42(14):1289-1367. doi: 10.1093/eurheartj/ehaa575. Erratum in: Eur Heart J. 2021 May 14;42(19):1908. Erratum in: Eur Heart J. 2021 May 14;42(19):1925. Erratum in: Eur Heart J. 2021 May 13;: PMID: 32860058.
14. Minner SA, Simone P, Chung BB, Shah AP. Successful Reversal of Bradycardia and Dyspnea With Aminophylline After Ticagrelor Load. J Pharm Pract. 2018 Feb;31(1):112-114. doi: 10.1177/0897190016680978. Epub 2016 Dec 5. PMID: 27920235
15. Rab T, Kern KB, Tamis-Holland JE, Henry TD, McDaniel M, Dickert NW, Cigarroa JE, Keadey M, Ramee S; Interventional Council, American College of Cardiology. Cardiac Arrest: A Treatment Algorithm for Emergent Invasive Cardiac Procedures in the Resuscitated Comatose Patient. J Am Coll Cardiol. 2015 Jul 7;66(1):62-73. doi: 10.1016/j.jacc.2015.05.009. PMID: 26139060.
16. Lemkes JS, Janssens GN, van der Hoeven NW, Jewbali LSD, Dubois EA, Meuwissen M, Rijpstra TA, Bosker HA, Blans MJ, Bleeker GB, Baak R, Vlachojannis GJ, Eikemans BJW, van der Harst P, van der Horst ICC, Voskuil M, van der Heijden JJ, Beishuizen A, Stoel M, Camaro C, van der Hoeven H, Henriques JP, Vlaar APJ, Vink MA, van den Bogaard B, Heestermans TACM, de Ruijter W, Delnoij TSR, Crijns HJGM, Jessurun GAJ, Oemrawsingh PV, Gosselink MTM, Plomp K, Magro M, Elbers PWG, van de Ven PM, Oudemans-van Straaten HM, van Royen N. Coronary Angiography after Cardiac Arrest without ST-Segment Elevation. N Engl J Med. 2019 Apr 11;380(15):1397-1407. doi: 10.1056/NEJMoa1816897. Epub 2019 Mar 18. PMID: 30883057.
17. Millin MG, Comer AC, Nable JV, Johnston PV, Lawner BJ, Woltman N, Levy MJ, Seaman KG, Hirshon JM. Patients without ST elevation after return of spontaneous circulation may benefit from emergent percutaneous intervention: A systematic review and meta-analysis. Resuscitation. 2016 Nov;108:54-60. doi: 10.1016/j.resuscitation.2016.09.004. Epub 2016 Sep 15. PMID: 27640933.
18. Holger Thiele, E Magnus Ohman, Suzanne de Waha-Thiele, Uwe Zeymer, Steffen Desch, Management of cardiogenic shock complicating myocardial infarction: an update 2019, European Heart Journal, Volume 40, Issue 32, 21 August 2019, Pages 2671–2683, https://doi.org/10.1093/eurheartj/ehz363.
19. Xi H, Yu RH, Wang N, Chen XZ, Zhang WC, Hong T. Serum potassium levels and mortality of patients with acute myocardial infarction: A systematic review and meta-analysis of cohort studies. Eur J Prev Cardiol. 2019 Jan;26(2):145-156. doi: 10.1177/2047487318780466. PMID: 31060369.
20. Kieboom BC, Niemeijer MN, Leening MJ, van den Berg ME, Franco OH, Deckers JW, Hofman A, Zietse R, Stricker BH, Hoorn EJ. Serum Magnesium and the Risk of Death From Coronary Heart Disease and Sudden Cardiac Death. J Am Heart Assoc. 2016 Jan 22;5(1):e002707. doi: 10.1161/JAHA.115.002707. PMID: 26802105; PMCID: PMC4859391.
21. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss HP, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck KH, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KE, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med. 2015 Sep 3;373(10):929-38. doi: 10.1056/NEJMoa1406761. PMID: 26332547.




Add comment