23 November 2023, by Shahriar Lahouti.
CONTENTS
- Key points
- Introduction
- Nomenclature and definition
- Epidemiology
- Pathophysiology and mechanism
- Risk factors
- Differential diagnosis
- Diagnostic evaluation
- Management
- Appendix
- Media
- References
Key points
Delirium is a geriatric syndrome characterized by an acute change in attention, awareness, and cognition that may last in a subacute form for weeks or even months, resulting in significant morbidity and mortality. It not only increases the risk of falls and prolonged hospitalization but also may result in irreversible cognitive decline and even death. Delirium is a manifestation of an underlying medical condition(s) that cannot be better explained by a pre-existing neurocognitive disorder and is often considered multifactorial. Multiple predisposing factors (e.g. pre-existing dementia) and precipitating factors (for example, infections, urinary retention, pain) for delirium have been described, with most patients having both types. There is no single treatment for delirium. Once it is diagnosed, finding and treating the underlying causes are key to resolving delirium. If the patient is dangerously agitated, pharmacologic agents such as antipsychotic medications should be used.
Introduction
Delirium is a common severe neurocognitive disorder characterized by the acute onset of inattention and other cognitive dysfunction (e.g. disorientation). They may also experience distressing symptoms of psychosis (e.g. hallucinations) and altered mood. Patients often have altered arousal, from reduced responsiveness at a near-coma level to severe agitation *.
Delirium is known by a variety of different terms, including altered mental status, acute mental status change, encephalopathy, agitation, altered level of consciousness, brain failure, and even psychosis *. Although these terms are descriptive of symptoms, they can be confusing to healthcare providers and can negatively affect clinical management *. For example, the use of the term ‘delirium’ may trigger specific management, whereas ‘septic encephalopathy’ may overlook mechanisms other than sepsis, such as metabolic alterations or drug side effects.
Nomenclature and definition
The consensus statement on nomenclature advocates using only two terms: acute encephalopathy and delirium *.
- They suggest that the terms acute confusional state, acute brain dysfunction, and acute brain failure should NOT be used in addition to the terms delirium and acute encephalopathy.
- The term altered mental status is not synonymous with delirium and should NOT be used.
| Acute Encephalopathy |
- This is a broad term, referring to a rapidly developing (usually within hours to a few days), diffuse pathobiological process that can lead to a clinical presentation of delirium, or coma, and may have additional features, such as seizures or extrapyramidal signs.
- Therefore, acute encephalopathy refers to the brain disorder underlying the delirium.
| Coma |
- Refers to a clinical state of severely depressed responsiveness defined by diagnostic systems such as the GCS * or FOUR score *.
| Delirium |
- Delirium is acute, generalized brain dysfunction leading to a clinical state characterized by a combination of features defined by DSM-5 *. The presence of delirium requires all the criteria to be met:
- Disturbance in attention (reduced ability to direct, focus, sustain, and shift attention) and awareness (reduced orientation to the environment or altered arousal).
- Disturbance develops acutely (in contrast to dementia) and tends to fluctuate in severity.
- At least one additional disturbance in cognition; “disorganized thinking” (memory deficit, disorientation, language, visuospatial ability, or perception).
- Not better explained by preexisting dementia.
- Not due to a severely reduced level of arousal or coma.
- Evidence of an underlying organic cause or causes.
| Subclinical Delirium This term is used to describe patients who have some delirium features but do not fulfill all criteria for a delirium diagnosis *,*. |
Additional features of delirium
Subtypes: Based on psychomotor behavioral disturbances, delirium is sub-typed into *:
- Hypoactive/quiet delirium
- Hypoactive symptoms: Apathy, staring, slowed movements and speech, ↓alertness, unawareness, and lethargy.
- Psychotic features are present in more than half of patients.
- Hypoactive delirium is the most common subtype in the emergency department *.
- It has a higher morality relative to the hyperactive subtype.
- In particular, this type may be easily missed or misdiagnosed *.
- Hyperactive/agitated delirium
- Hyperactive symptoms: Hypervigilance, restlessness, irritability, anger, combativeness, euphoria, fast motor responses, and speech, increased sympathetic activity, and impairment in sleep duration and architecture.
- The hyperactive subtype has the best prognosis *.
- Mixed delirium
- Periods of hyperactive and hypoactive delirium.
- The mixed subtype of delirium seems to have the worst prognosis *.
Other clinical features
- Emotional disturbances, including fear, depression, euphoria, or perplexity.
- Psychotic symptoms (hallucinations, delusions, paranoia).
- Sundowning (day-night reversal with hyperactivity at night) *.
Epidemiology
Delirium has primarily been studied in hospital settings. Nearly 30% of older medical patients experience delirium at some time during hospitalization. In general, delirium can be found wherever there are sick patients.
- Among critically ill patients in ICU (70-80%)*
- Hospice units (42%) *
- Postacute care settings (16%) *
- Emergency department (10%) *
Prognosis and outcomes
- Patients who develop delirium are at increased risk for a variety of poor outcomes including falls, debility, prolonged hospital stay, and increased risk of death *,*.
- Features of delirium that predict worse outcomes include:
Pathophysiology and mechanism
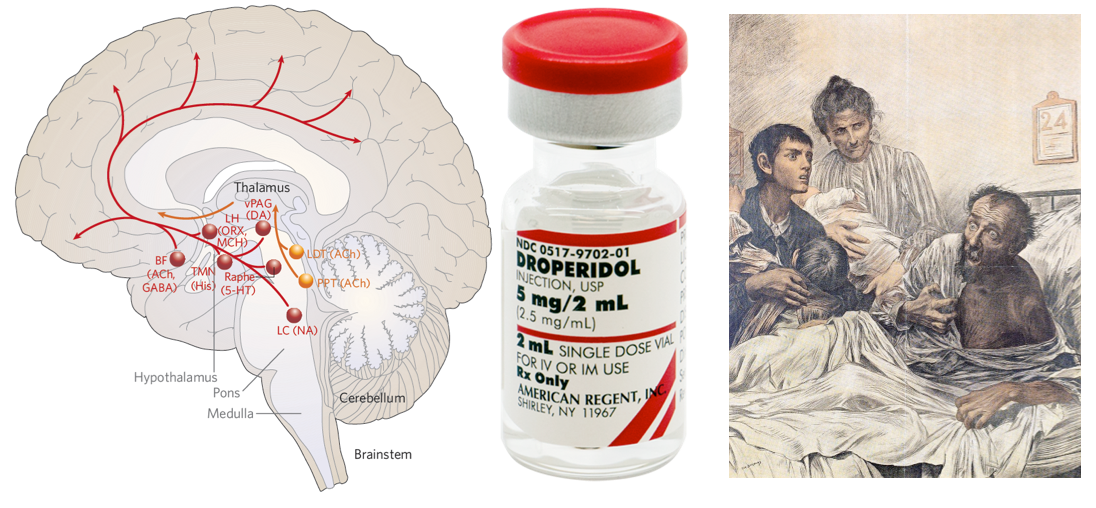
Background: Neurobiology of arousal and attention
- Key structures in maintaining an awake state and awareness involve the ascending reticular activating system (ARAS) from the mid-pontine tegmentum rostrally to the thalamus and the anterior cingulate regions, and cortical association area (precuneus and cuneus) *.
- The results of brainstem auditory evoked potential, somatosensory evoked potentials, and neuroimaging studies have supported an important role for subcortical (eg, thalamus, basal ganglia, and pontine reticular formation) as well as cortical structures in the pathogenesis of delirium *.
- These findings correlate with clinical reports that patients with subcortical strokes and basal ganglia abnormalities (including Parkinson’s disease) have a higher susceptibility to delirium.
Neurotransmitter imbalance
- Neurotransmitters such as acetylcholine, serotonin, gamma-aminobutyric acid (GABA), and dopamine become imbalanced in delirium, which results in the inability of delirious patients to process information and respond appropriately *.
- Acetylcholine
- It plays a key role in the pathogenesis of delirium *. Several studies support the cholinergic hypothesis of delirium.
- Anticholinergic drugs can cause delirium when given to healthy volunteers *.
- There is a correlation between cognitive impairment and anticholinergic drug serum levels. Elevated serum anticholinergic levels decrease when the delirium resolves *.
- Alzheimer’s disease, which is characterized by a loss of cholinergic neurons, increases the risk of delirium due to anticholinergic medications.
- Medical conditions precipitating delirium, such as hypoxia, hypoglycemia, and thiamine deficiency, decrease acetylcholine synthesis in the CNS *,*.
- Keep in mind the anticholinergic mechanism, since many drugs used by older adults (including several not traditionally viewed to have “anticholinergic effects”) can lead to detectable serum anticholinergic activity *.
- Psychotropic drugs, in particular, are likely to cause detectable serum anticholinergic activity at doses typically administered to older patients.
- It plays a key role in the pathogenesis of delirium *. Several studies support the cholinergic hypothesis of delirium.
- GABA
- GABA is the predominant inhibitory neurotransmitter in the CNS *.
- ⬆️GABAergic activity has been implicated in
- Hepatic encephalopathy (HE)*
- Elevated serum ammonia levels in HE contribute to ↑glutamate and glutamine levels; these amino acids are precursors to GABA.
- Withdrawal from benzodiazepine, and alcohol.
- Hepatic encephalopathy (HE)*
- Dopamine
- ⬆️ Dopaminergic activity has been implicated in causing delirium associated with bupropion toxicity.
- Symptomatic relief of delirium occurs with haloperidol, a potent dopamine blocker.
- Other
- Cerebrospinal fluid studies of patients with delirium reveal alterations in neuropeptides (eg, somatostatin), endorphins, and serotonin among others *, however, it is difficult to exclude the confounding effects of underlying illness or dementia.
Cerebral metabolic insufficiency
- The brain requires a large amount of energy, as either oxygen or glucose deficiency can markedly constrain brain function.
- Acute illnesses (precipitators of delirium) such as respiratory distress, and hemodynamic shock might impair brain metabolism in multiple ways.
Systemic inflammation
- Peripheral inflammation is a well-established trigger of delirium, but the precise mechanisms by which it disrupts brain function are not clearly understood.
- Several processes have been proposed in the pathogenesis of delirium in septic patients (sepsis-associated encephalopathy, “SAE”) shown in the figure below *.
- Moreover, inflammation may also contribute to delirium through the promotion of coagulation, which is common in sepsis.
Impaired neuronal network connectivity
- Neurochemical influences on the brain’s functional state are dependent on the neuroanatomical networks in which these neurotransmitters operate.
- Structural connectivity studies show that loss of integrity in the inter-hemispheric corpus callosum is associated with increased delirium duration *.
Risk factors
Delirium is a multifactorial disorder. Factors that increase the risk for delirium (figure below) can be classified into *:
- Predisposing factors: Premorbid conditions (i.e. patient’s characteristics) that increase baseline vulnerability to delirium.
- Precipitating factors: Factors relating to the presenting illness (i.e. causes of delirium, e.g. acute insult, injury, drugs).
- Advanced age >65 *
- History of delirium
- Frailty: Immobility, impairment in activities of daily living *
- Baseline cognitive impairment such as dementia, stroke, or Parkinson’s disease.
- Sensory impairment (e.g. hearing, visual impairment) *.
- Decreased oral intake: Dehydration, malnutrition *
- Depression and other psychiatric illnesses.
- Comorbidities (e.g. cardiovascular and renal disease).
- Illness severity (especially intubation).
- Alcohol abuse, illicit drugs, opioids, or benzodiazepine use.
Causes
Factors that may precipitate delirium are numerous and varied. Some common causes are listed below.
| Medication: Especially pay attention to: 🔸Drug-drug interaction 🔸New medications 🔸Polypharmacy (≥5 medications) 🔸Renally cleared medications plus acute kidney injury. |
- Medications with “predominant” anticholinergic effects *:
- Atropine
- Antisialagogue: Glycopyrrolate.
- Bladder antispasm: Oxybutynin, tolterodine, flavoxate.
- Used for irritable bowel syndrome: Dicyclomine, hyoscyamine.
- Antiemetic or antisialagogue: Scopolamine (hyoscine)
- Used for Parkinson’s disease: Benztropine, trihexyphenidyl *
- Anticholinergic eye drops: Atropine, cyclopentolate, homatropine.
- Inhaled bronchodilator: Ipratropium, tiotropium.
- Medications with “mixed” effects including anticholinergic effects *:
- First generation antihistamines: Chlorpheniramine, promethazine, cyproheptadine, dimenhydrinate, diphenhydramine, doxepin, hydroxyzine, clemastine, meclizine).
- TCA antidepressants: Amitriptyline, clomipramine, desipramine, doxepin, imipramine, nortriptyline.
- Muscle relaxants: (e.g. cyclobenzaprine, orphenadrine).
- Antipsychotics, especially:
- Low-potency typical antipsychotics e.g. chlorpromazine, fluphenazine, thioridazine.
- Atypical agents: Olanzapine, quetiapine.
- Dopamine agonists (antiparkinson): Amantadine, bromocriptine, levodopa, pergolide, pramipexole, ropinirole.
- Metoclopramide
- Sedative-hypnotics: Benzodiazepines, zolpidem, barbiturates.
- Analgesics: Opioid (esp. meperidine), NSAID.
- Skeletal muscle relaxers: Baclofen, cyclobenzaprine, tizanidine.
- Antiseizure medications: Carbamazepine, levetiracetam, phenytoin, valproate, vigabatrin.
- Other CNS-active agents: Phenothiazines, lithium, cholinesterase inhibitors (eg, donepezil), Interleukin 2, and disulfiram.
- Cardiovascular & hypertensive drugs: Digoxin, dihydropyridines calcium channel blockers e.g. amlodipine *, antiarrhythmic, β-blockers, clonidine, diuretics, methyldopa.
- Antimicrobial: Acyclovir, aminoglycosides, cefepime, fluoroquinolones, isoniazid, metronidazole, macrolide, rifampin, sulfonamides.
- Steroids.
- Medication side effects (e.g. hyperammonemia from valproic acid *,*, confusion from quinolones, serotonin syndrome *)
- Hypoglycemic agents.
| Toxicologic |
- Intoxication
- Almost any substance, including:
- Cardiovascular: Digoxin, antiarrhythmic, sympathomimetic, β-blocker, ⍺-2 adrenergic agonists (e.g. clonidine)
- Toxic alcohols (ethylene glycol, methanol), lithium, bupropion (norepinephrine/dopamine reuptake inhibitor), inhaled toxins e.g. carbon monoxide, cyanide, hydrogen sulfide, and salicylate intoxication.
- Almost any substance, including:
- Withdrawal from:
- Benzodiazepines, ethanol, opioids, gabapentin, baclofen, dexmedetomidine, serotonin-norepinephrine receptor inhibitors, e.g. duloxetine, venlafaxine.
| Infection |
- Sepsis (septic encephalopathy) *.
- Systemic infections
- Fever-related delirium
| Metabolic derangement |
- Electrolyte disturbance (⬆️ or ⬇️): Na, Ca, Mg, Phos.
- Endocrine disturbance (⬆️ or ⬇️): Thyroid, Parathyroid, Pancreas, Pituitary, Adrenal.
- Hypercarbia
- Hypoxemia
- Hypoglycemia, hyperglycemia including DKA, and hyperosmolar hyperglycemic state (HHS).
- Hyperosmolar and hypoosmolar states
- Inborn errors of metabolism: porphyria, Wilson disease, etc.
- Nutritional: Wernicke encephalopathy, vitamin B12 deficiency, possibly folate and niacin deficiencies
| Systemic organ failure |
- Cardiovascular
- Shock (hypoperfusion)
- Congestive encephalopathy *.
- PRES (Posterior Reversible Encephalopathy Syndrome).
- Hematologic
- Thrombocytosis
- Hypereosinophilia
- Leukemic blast cell crisis
- Polycythemia
- Thrombotic thrombocytopenic purpura (TTP).
- Liver failure: acute, chronic.
- Pathogenesis:
- Hepatic encephalopathy (causing postprandial confusion)
- Accumulation of hepatically cleared medications
- Pathogenesis:
- Pulmonary: Including hypercarbia and hypoxemia.
- Renal failure: acute, chronic.
- Pathogenesis:
- Uremia
- Electrolyte disturbance
- Accumulation of renally cleared medications e.g. digoxin.
- Dialysis disequilibrium syndrome (onset of toxic metabolic encephalopathy after dialysis)
- Pathogenesis:
| Brain disorder |
- CNS infections: encephalitis, meningitis, brain or epidural abscess.
- Autoimmune encephalitis.
- Ischemic stroke.
- Risk factors for delirium in the setting of stroke include preexisting cognitive impairment, infection, right hemispheric stroke, anterior circulation large vessel stroke, and greater stroke severity *.
- Space occupying lesions: Intracranial hemorrhage, tumor.
- Traumatic brain injury.
- Epileptic seizures, especially nonconvulsive status epilepticus (NCSE) *. More on this here.
| Physical |
- Pain (insufficient pain management, urinary retention, fecal impaction, etc. ) *
- Physical restraints, prolonged immobilization
- Sleep disruption, visual/hearing impairment.
- Surgery
- Trauma: trauma with systemic inflammatory response syndrome, fat embolism.
- Sleep deprivation: Frequent blood pressure or neurologic checks, uncontrolled pain, noisy environment.
- Bladder catheters.
- Hyperthermia, hypothermia.
Differential diagnosis (delirium mimics)
- Dementia
- Key features of patients with dementia that could differentiate them from delirious patients *:
- Intact attention and remote memory especially in the earlier stages of dementia.
- In practice, patients with dementia should be able to converse and perform some tests of attention such as counting backward from 20 or naming the months of the year or days of the week in reverse.
- Insidious and progressive, occurring over a much longer time (months to years).
- Not much fluctuation.
- Intact attention and remote memory especially in the earlier stages of dementia.
- Dementia with Lewy bodies (DLB) is similar to Alzheimer’s disease but can be more easily confused with delirium because fluctuations and visual hallucinations are common and prominent *.
- Key features of patients with dementia that could differentiate them from delirious patients *:
- Primary psychiatric illnesses
- Depression: The presence of dysphoria and absence of much fluctuation in severity are helpful distinguishing features of depression.
- Mania: is usually associated with a history of previous episodes of mania or depression.
- Schizophrenia: The delusions are usually highly systematized, the history is longer, and the sensorium is otherwise clear.
- Catatonia
- Catatonia is a motor dysregulation syndrome involving difficulty initiating or terminating actions *.
- This causes periods of physical rigidity, negativism, or stupor, although psychomotor hyperactivity may also occur *.
- Causes: It happens in the context of many underlying psychiatric and general medical disorders, e.g. anti-dopaminergic medications, viral encephalitis, multiple sclerosis, and Parkinson’s disease *, *.
- Catatonia may appear similar to delirium, however, these are fundamentally distinct processes:
- Delirium is predominantly an abnormality of attention, which largely reflects dysfunction of the cerebral cortex.
- 💡Psychomotor features of catatonia will be absent. The waxing and waning in delirium describes arousal/ orientation, whereas in catatonia it refers to motoric dysregulation *.
- Catatonia is predominantly an abnormality of motor regulation, which largely reflects dysfunction of the basal ganglia.
- Delirium is predominantly an abnormality of attention, which largely reflects dysfunction of the cerebral cortex.
- 💡Generally, catatonia has 3 types: Hypokinetic catatonia (causing stupor), hyperkinetic catatonia (causing agitation), and malignant catatonia (media below).
- Either hypokinetic or hyperkinetic catatonia may lead to autonomic instability, if severe enough. Catatonia which causes autonomic instability is defined as malignant catatonia.
- Catatonia is a motor dysregulation syndrome involving difficulty initiating or terminating actions *.
- Stupor and coma
- Abulia/akinetic mutism
- Profound failure of executive function (e.g. failure to speak, move, or eat), “lack of spontaneity”.
- Patients may respond to questions, but only in a very delayed fashion.
- Visual tracking may occur, with conjugate eye movements.
- Causes
- Damage to the medial frontal lobe(s) e.g. by tumor or trauma.
- Infarction of the ACA 📖
- Aphasia (various types especially Wernicke’s aphasia).
- Acute ischemic stroke can rarely mimic delirium:
- PCA (posterior cerebral artery) stroke 📖
- Bilateral infarctions involving the occipital region (Anton syndrome).
- Cortical blindness and confabulation might be confused with delirium. Careful examination will reveal a lack of vision.
- Inferomedial temporal lobe infarction may cause global amnesia in which the deficit is restricted to memory.
- Bilateral infarctions involving the occipital region (Anton syndrome).
- Inferior-division MCA stroke
- Patients may appear delirious in that they do not comprehend or obey and seem confused.
- In Wernicke’s aphasia, the problem is restricted to language, while other aspects of mental function are intact.
- In the inferior-division MCA stroke contralateral superior quadrantanopia is present. 📖
- Patients may appear delirious in that they do not comprehend or obey and seem confused.
- PCA (posterior cerebral artery) stroke 📖
- Nonconvulsive status epilepticus (NCSE)
- Patients with NCSE show no classic ictal features, but the following features should suggest the possibility of seizures *:
- Prominent bilateral facial twitching.
- Unexplained nystagmoid eye movements during obtunded periods.
- Spontaneous hiccups.
- Prolonged “postictal state”
- Automatisms (lip-smacking, chewing, or swallowing movements).
- Acute aphasia or neglect without a structural lesion.
- Diagnosis of NCSE requires an electroencephalogram (EEG). More on this here.
- NCSE should also be considered in the absence of these findings when the etiology of a confusional state remains obscure *.
- Patients with NCSE show no classic ictal features, but the following features should suggest the possibility of seizures *:
Diagnostic evaluation
There are two important aspects to the diagnostic evaluation of delirium:
- Recognizing that delirium is present.
- Identifying the underlying medical illness that has caused delirium.
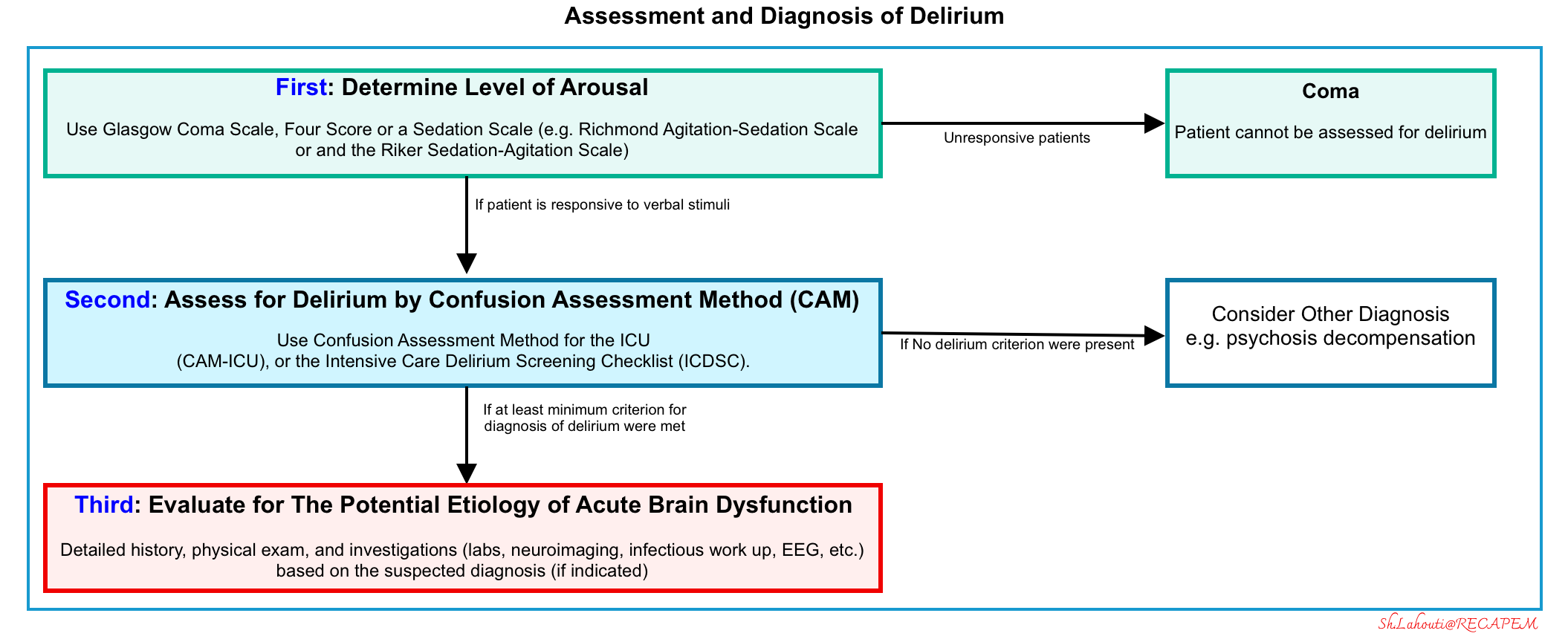
Recognizing delirium
- First, a patient’s level of arousal and content of consciousness need to be examined to evaluate for delirium.
- This can be performed through the use of the Glasgow Coma Scale, Four score, or a sedation scale.
- The Richmond Agitation-Sedation Scale 🧮 and the Riker Sedation-Agitation Scale 🧮 are commonly used tools that can be used to assess the level of arousal, including in patients on mechanical ventilation.
- If the patient is determined to be unresponsive, then he or she is in a comatose state and cannot be assessed for delirium.
- Second, assess for delirium using tools such as the Confusion Assessment Method (CAM), If the patient is responsive to verbal stimuli
- Evidence of acute change from baseline attention and awareness, which may fluctuate in presence and severity, is sought from the patient, caregivers, or staff who know the patient, from medical records, or, less commonly, from the clinician’s knowledge of the patient.
- Assessment tools
- The 4A’s test (4AT) is a 4-item test used in general hospital settings. A score ≥4 indicates delirium is likely. 🧮 MDCalc.
- A meta-analysis of 17 diagnostic test accuracy studies reported a pooled sensitivity and specificity of 88% *.
- Confusion Assessment Method (CAM)-based tools, such as the CAM, CAM- Intensive Care Unit (ICU) assess 4 features ( acute onset and fluctuating course, inattention, disorganized thinking, altered level of consciousness). 🧮MDCalc: CAM-ICU
- For delirium to be present according to CAM-based tools, features 1 and 2 must be present, plus either features 3 or 4.
- In a meta-analysis of 9 studies including 969 patients, the CAM-ICU had a pooled sensitivity of 80% and a pooled specificity of 96% *.
- The 4A’s test (4AT) is a 4-item test used in general hospital settings. A score ≥4 indicates delirium is likely. 🧮 MDCalc.
- After diagnosis, further assessment of the patient to obtain more details about the individual profile of delirium, including features such as delusions, hallucinations or mood changes is crucial because this information influences the specific management plan.
- Third: Diagnosis of delirium should prompt a thorough investigation into the potential etiology of the “acute brain dysfunction” that includes a detailed history, physical exam, and further laboratory and/or neuroimaging (if indicated). This is discussed below.
Identifying the underlying cause of delirium
Historical clues
- Helpful historical clues to identify the underlying etiology of delirium and confusion can be obtained from relatives:
- Recent febrile illness
- History of organ failure
- A medication list 💊
- History of alcoholism or drug abuse
- Recent depression.
- Recent events & procedures
- It is otherwise often difficult to impossible to obtain a history in the confused or uncooperative patient. As an example, myocardial infarction may cause sufficient confusion that the patient cannot relate to a history of chest pain.
Examination
- General exam
- Vital signs.
- Helpful findings in the general exam that may be suggestive of specific underlying causes of delirium
- Cyanosis: Chronic pulmonary disease
- Jaundice/ascites: Hepatic failure
- Cherry-red lips: CO poisoning
- A bitten tongue or posterior fracture-dislocation of the shoulder, signs of head injury: Epileptic seizures, nonconvulsive status epilepticus.
- Look for foci of infection
- 💡Note that sepsis may present as delirium without obvious fever (sometimes even with hypothermia) or localizing signs (eg, rebound tenderness from a perforated viscus).
- Neurologic exam
- The neurologic examination is often confounded by inattention and altered consciousness in patients with delirium.
- Pay attention to certain aspects of the neuro exam:
- Level of consciousness.
- Degree of attention or inattention.
- Visual fields, pupil exam.
- Cranial nerve and motor deficits.
- Note that the absence of a focal neurologic deficit does not exclude the possibility of focal or multifocal neurologic lesions as the cause of delirium
- Posterior cortical strokes, for example, can present as delirium with few findings other than hemianopia, and in some cases may present with no focal symptoms or signs.
- In the absence of an obvious cause for delirium, further testing including neuroimaging, LP, and EEG is indicated.
- The physical signs of metabolic/toxic delirium include multifocal myoclonus, asterixis, and postural action tremor.
- However, these are nonspecific findings and do not help establish any particular medical etiology within the metabolic/toxic category.
- Physical findings suggestive of drug exposure may be useful.
- For example, anticholinergic exposure would reveal increased temperature, flushed dry skin, tachycardia, and pupillary dilation on the exam (More on this 👉appendix)
Indications for more comprehensive investigations
- Delirium is common among critically ill patients, but not every patient requires an exhaustive workup.
- Some indications for more aggressive evaluation include:
- A rapid and major change in mental status.
- Delirium was the presenting problem at the hospital.
- It is important to note that most patients with delirium have more than one etiology. There are multifactorial etiologies contributing to delirium *.
- No obvious cause for delirium.
- No history of prior delirium/dementia.
Basic labs *
- Fingerstick glucose (STAT).
- CBC with differential (anemia, leukocytosis)
- Basic metabolic panel: (Hypo-/hypernatremia, hypercalcemia, uremia?) *
- ECG: Ischemic changes, arrhythmia, electrolyte abnormality
Further lab tests may be considered depending on context *:
- Liver function tests.
- Ammonia
- TSH.
- ABG/VBG (if hypercarbia is suspected).
- Infectious workup (e.g. urinalysis, blood cultures, chest X-ray).
- 💡Urine analysis frequently shows pyuria and bacteriuria in older adults. Consider alternative etiologies, especially in the absence of symptoms of UTI, pyelonephritis, or absence of signs of infection such as fever or leukocytosis *.
- Levels of medications the patient is on (e.g. lithium, digoxin, phenytoin, theophylline, valproic acid, phenobarbital).
- 💡Keep in mind that delirium can happen even with “therapeutic” levels of such agents as digoxin, lithium, or quinidine.
- Toxicologic workup {e.g. carboxyhemoglobin (CO poisoning), salicylates, acetaminophen, ethanol level}.
Neuroimaging
- Nonenhanced CT head may be considered for *:
- Patients presenting with delirium with unclear history.
- History of trauma.
- Significant anticoagulation *.
- Focal neurologic abnormality (note however that subdural hematoma can depress mental status without focal findings).
- Substantially reduced level of consciousness.
- MRI may provide additional information about a variety of conditions (especially CVA). Furthermore, brain MRI may suggest the underlying cause of toxic metabolic encephalopathy (shown below) *.
Lumbar puncture (LP) may be considered if
- Delirium is the chief complaint on admission to the hospital.
- Evidence of infection (fever, leukocytosis, nuchal rigidity).
- The presence of an obvious precipitating factor for CNS infection such as endocarditis or a history of neurosurgery *.
🔴Neuroimaging should be obtained before LP in patients with coma, focal signs, papilledema, or suspicion of increased ICP because of the very low but real risk of precipitating transtentorial herniation.
EEG should be considered if:
- History of seizure or active neurologic disorder that predisposes to seizure.
- Exam findings concerning for nonconvulsive status epilepticus, e.g. a bitten tongue or posterior fracture-dislocation of the shoulder, signs of head injury.
- Unexplained delirium despite other investigations.
Management
Delirium is an urgent medical condition, and prompt review of possible causes or inciting factors should be performed. Underlying causes such as pain, metabolic derangements, infection, and hypoxemia should be sought and treated. The management of delirium encompasses two broad categories:
- Prevention
- Pharmacologic treatment of dangerous agitation
An overview of diagnostic and therapeutic approaches to delirium is shown below.
| Prevention and Treatment of Delirium |
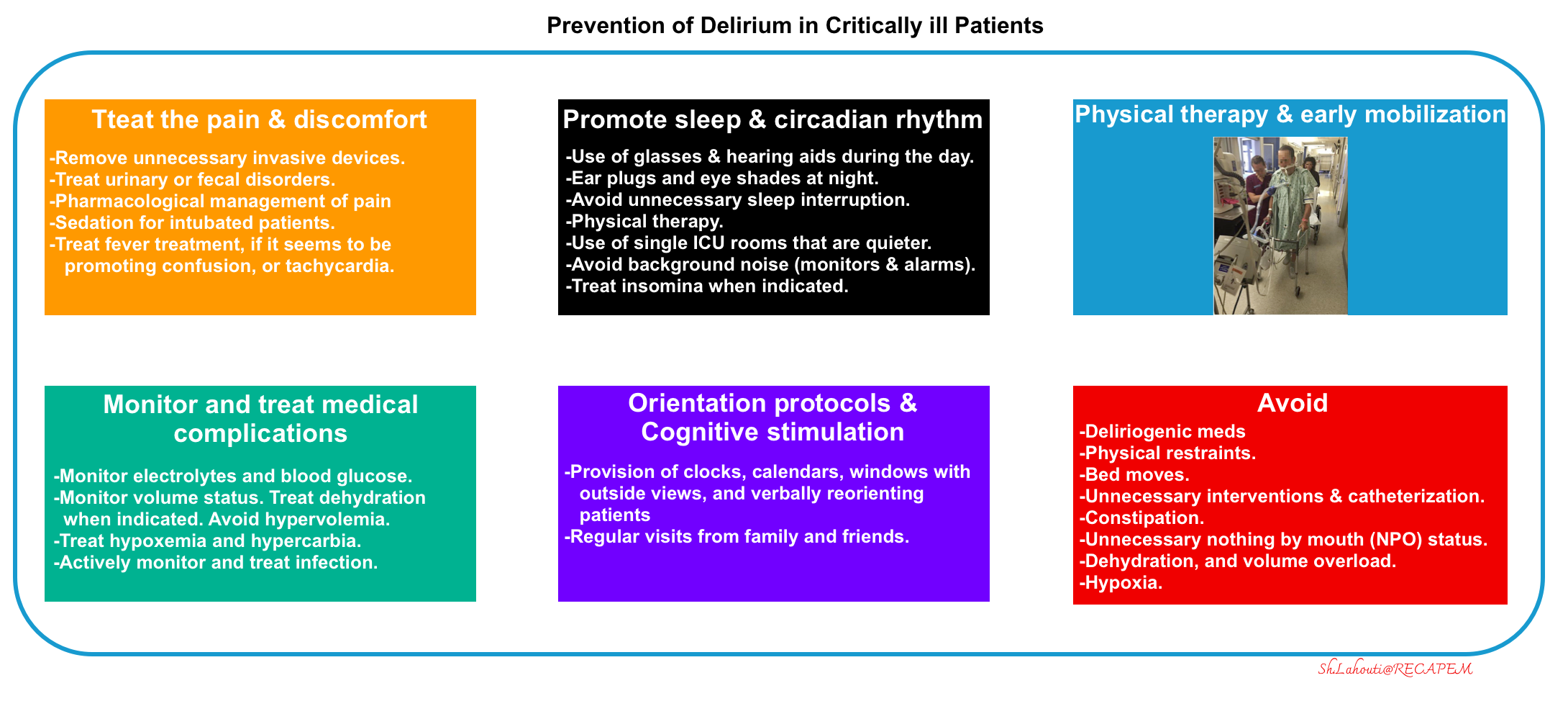
- Avoid and remove factors known to cause or aggravate delirium:
- Avoid deliriogenic medications
- ⚠️Benzodiazepines, in particular, are often implicated.
- In one systematic review, the authors concluded that benzodiazepines should be avoided in high-risk patients, while caution should be used in prescribing opioids, dihydropyridines, and antihistamines *.
- ⚠️Benzodiazepines, in particular, are often implicated.
- Maintain hydration.
- Environmental and supportive measures.
- Reduce noise, avoid restraints and disruption of the sleep-wake cycle, and early mobilization.
- Prevent and treat pain and discomfort (more on this below).
- Promote sleep and circadian rhythm
- Prevention *
- Stimulate during the day:
- Use the patient’s glasses and hearing aids if needed.
- Physical therapy.
- Open window shades.
- Limit stimulation at night:
- Using earplugs at night has been shown to avoid delirium.
- Decrease the frequency of vital sign monitoring at night if possible (especially blood pressure cuff cycling).
- Night-time noise should be reduced.
- Nursing and medical procedures, including the administration of medications, should be avoided during sleeping hours when possible.
- Use of single ICU rooms that are quieter.
- Stimulate during the day:
- Treat insomnia. Preferred medications are:
- Quetiapine 12.5-25 mg PO QHS might be the best agent.
- Trazodone 25-100 mg PO QHS
- Contraindications/side effects of note: QT prolongation, torsade de pointes, orthostatic hypotension, promotion of serotonin syndrome.
- Dexmedetomidine: Low-dose, nightly dexmedetomidine is probably the most effective agent to promote sleep and daytime wakefulness.
- If dexmedetomidine is used for insomnia or nocturnal agitation, it should be discontinued or aggressively down-titrated the following morning. Reserving dexmedetomidine for nocturnal use may promote the preservation of a normal circadian rhythm.
- Oral clonidine (0.2-0.3 mg QHS) may be considered as an alternative to dexmedetomidine for mild insomnia.
- Melatonin may still be utilized for the management of insomnia.
- ⚠️Avoid using of deliriogenic sedatives (e.g. benzodiazepine, diphenhydramine, or zolpidem).
- Prevention *
- Avoid deliriogenic medications
Treatment strategy once delirium is established:
- Identify and treat the underlying cause of delirium.
- Fever treatment.
- Management of pain & discomfort
- Appropriate sedation regimens.
Identify and treat the underlying cause of delirium
- Drugs
- Consider the etiologic role of newly initiated drugs, increased doses, interactions, over-the-counter drugs, and alcohol.
- Consider especially the role of high-risk drugs: lower the dose.
- Discontinue the deliriogenic drugs, or substitute a less psychoactive medication.
- Correct metabolic/electrolyte disturbances, especially:
- Infections: Evaluate and treat, especially urinary tract, respiratory tract, skin, and soft tissue.
- Intoxication/Withdrawal
Fever treatment
Background
- Fever in the ICU is defined as a temperature >38.3C *. However, a lower threshold for fever (>38/100.4) may be appropriate in some patients
- Immunocompromised patients (e.g. neutropenic).
- Elderly patients.
- Patients on scheduled acetaminophen or NSAIDs.
- Patients on ECMO or continuous renal replacement therapy.
- Hyperpyrexia: This is the term used for an extraordinarily high fever (>41.5°C), which can be seen in patients with severe infections but can also occur in patients with central nervous system (CNS) hemorrhages.
- Hyperthermia is defined as elevated temperature resulting from excess heat generation, rather than from an alteration of the hypothalamic set point and is suggested by the following clinical features:
- Extreme temperature elevation (temperatures >41C).
- Lack of rigors (in contrast to fever, that rigors can precede temperature spikes).
- Skin may be hot and dry (but not always).
- Antipyretics are ineffective.
🔴Infectious: Pneumonia, urinary tract infection, line infection, surgical site infection, etc.
🔴Non-infectious
1. Procedure-related: Febrile transfusion reaction, bronchoscopy, hemodialysis, postoperative fever (1-3 days post-surgery, usually <39C).
2. Medication-related: a)Drug fever. b)Medication-induced hyperthermia: Serotonin syndrome, neuroleptic malignant syndrome, malignant hyperthermia.
3. Sterile inflammation (pancreatitis, aspiration pneumonitis, ARDS).
4. Pulmonary embolism (usually low-grade fever, <39/102 *
5. Neurogenic fever (a.k.a. central fever): Subarachnoid hemorrhage, intracranial hemorrhage, traumatic brain injury *
6. Toxicologic a) Salicylate, tricyclic antidepressant, anticholinergic, sympathomimetic toxicity, thyroid hormones b)Withdrawals: Alcohol, benzodiazepines, baclofen
7. Miscellaneous
a)Malignancies (e.g. leukemia, lymphoma)
b)Systemic inflammatory disease (e.g. Polyarteritis nodosa, giant cell arteritis, inflammatory bowel disease)
c)Adrenal insufficiency
d)Thyroiditis
Deciding whether to treat fever in adults.
- Whether to treat fever and hyperpyrexia is often debated.
- Unlike fever and hyperpyrexia, there is no debate that hyperthermia should always be treated to rapidly reduce core temperature.
- When deciding whether to treat patients with fever, consideration of the adverse effects of pharmacologic and nonpharmacologic treatments must also be considered, as well as the possibility of masking temperature fluctuations that may provide valuable diagnostic or treatment-related information.
- It’s generally best to avoid antipyretics, as these will obscure the true fever curve in hospitalized adult patients.
- Indications for antipyretics:
- Severe fever (e.g. >40C).
- Neurologic injury (e.g. stroke, anoxic brain injury).
- Fever in patients with advanced age, underlying cardiac or pulmonary disease, or those on chemotherapy or checkpoint inhibitor therapy.
- Fever appears to cause clinical deterioration (fever can cause some patients to become delirious or tachycardia).
Treat pain & discomfort
Multimodal therapy
Multimodal therapy is a useful principle that can be applied to treat a variety of topics (e.g. pain, anxiety, hemodynamic support).
- It refers to using moderate doses of several different agents, in order to maximize efficacy while minimizing toxicity *.
- Using lower doses avoids toxicity from each medication, while the use of several medications provides synergistic efficacy.
- Different agents frequently work in a synergistic fashion (i.e. 1+1 = 3). Synergy allows moderate doses of several different agents to have a large combined impact.
- Using lower doses avoids toxicity from each medication, while the use of several medications provides synergistic efficacy.
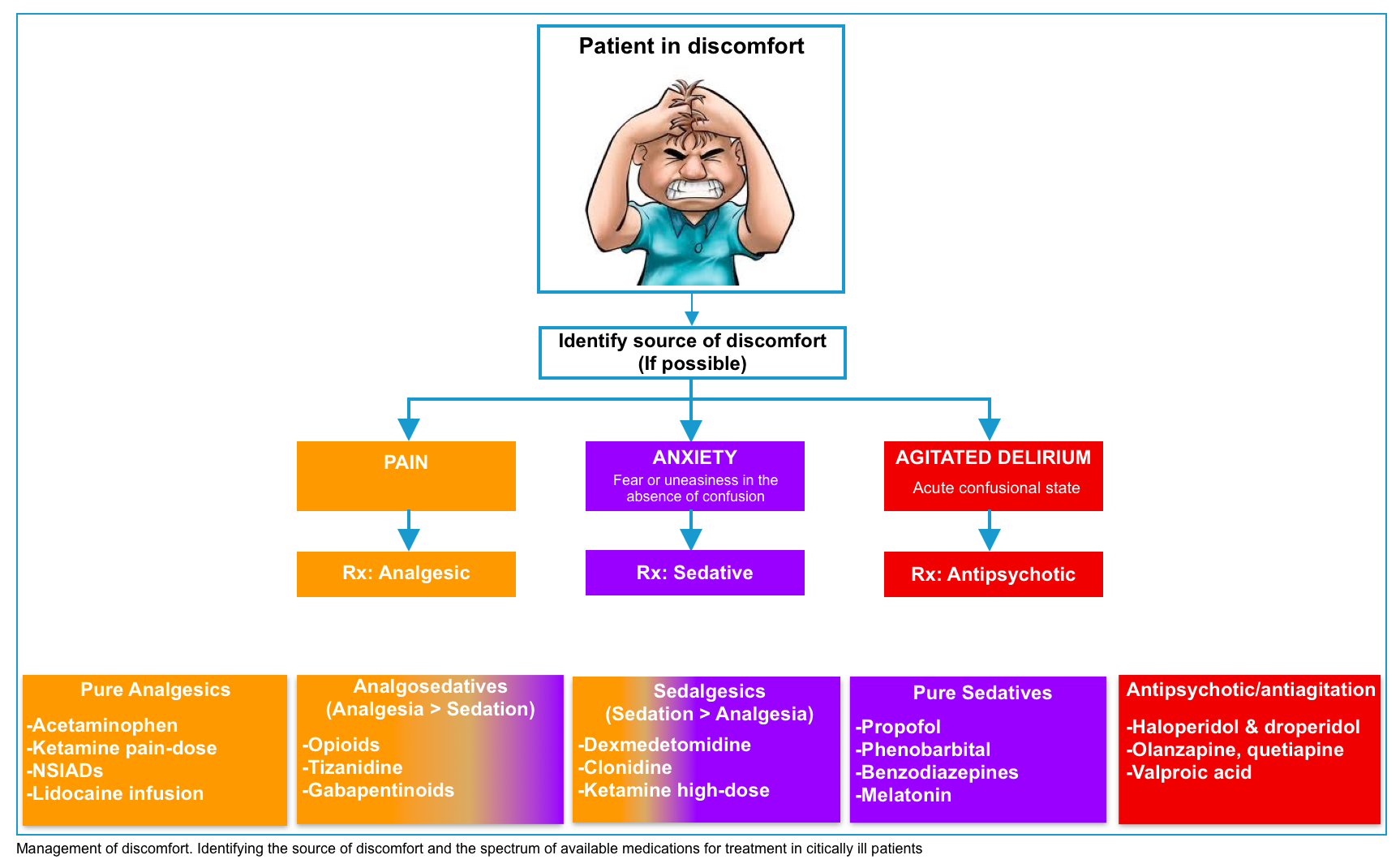
Target of treatment
- Ideally, an uncomfortable patient should be evaluated to determine the source of discomfort. This should be treated appropriately:
- Pain should be treated with an analgesic agent.
- Anxiety refers to fear or uneasiness in the absence of confusion. If medication is required, the optimal medication is a sedative (e.g. propofol or dexmedetomidine).
- Agitated delirium refers to an acute confusional state marked by agitation. If medication is required, the optimal therapy is an antipsychotic.
- However, in practice, it may be impossible to differentiate precisely between pain, anxiety, or agitated delirium.
- The ultimate goal is to keep the patient comfortable and calm while avoiding iatrogenic harm from medications.
Multimodal analgesia
- Multimodal analgesia has been shown to significantly reduce morbidity and mortality and the use of opioids in critically ill patients *.
- The analgesia should be targeted specifically at pain.
- Analgesics shouldn’t be used as nonspecific “calm down” medication for any agitated patient.
- The multimodal analgesia protocol involves systematic and periodic pain assessment of all patients, using validated and standardized pain scales:
- A numerical rating scale (NRS) or visual analog scale (VAS) for patients able to communicate
- A behavioral pain scale (BPS) for patients who could not be evaluated otherwise can help determine whether the patient is in pain.
- The use of analgesia is titrated to pain intensity.
- There are many regimens and designs for multimodal analgesia, however, there is no one-size-fits-all design for analgesia in critical patients. For example, patients with neuropathic pain may benefit from up-front initiation of a gabapentinoid, whereas most other patients probably won’t.
- The most popular strategy for critically ill patients is shown below:
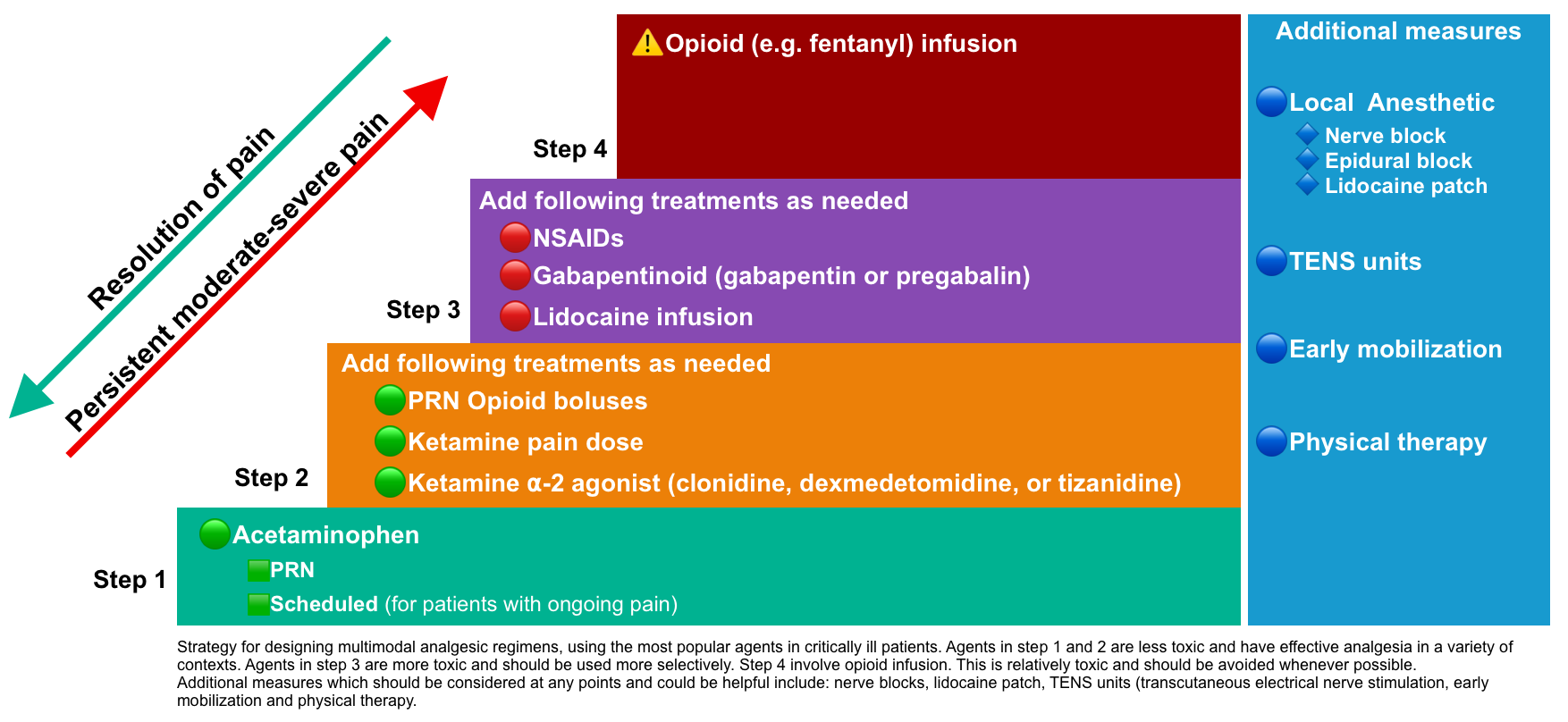
Step 1: Acetaminophen
- Pharmacology
- Acetaminophen (aka. paracetamol), is a centrally acting non-competitive reversible inhibitor of cyclooxygenase (COX) enzymes with analgesic and antipyretic effects but lacks anti-inflammatory activity compared to other COX inhibitors *.
- Metabolized hepatically
- Advantages: Both analgesic and antipyretic effects, safety profile, effective analgesia in a variety of contexts with benefits which may include reduced opioid requirements, reduced delirium, and avoidance of nausea/vomiting *,*,*.
- Contraindications and cautions
- Contraindicated in acute liver injury or decompensated cirrhosis.
- Dose reduction of up to 2 grams/day in patients with alcoholism, chronic liver disease, or low body weight (<50 kg) *.
- In neutropenia, acetaminophen might be avoided, to allow for early detection of neutropenic fever.
- It’s not a very potent analgesic.
- Indications / Usage
- Antipyretic (when indicated)
- Analgesia: It is used as the first level of the analgesic in the multimodal analgesic protocol (due to its safety, rather than its efficacy).
- Dosage
- The usual dose is 650-1,000 mg q6hr (up to 4 grams/day). For patients with ongoing pain this should be scheduled, to provide a baseline level of analgesia.
Step 2: Opioids PRN
- They are often used as second-line therapy, mostly due to convenience.
- For more stable patients with moderate pain who can take oral medications, oral opioids may be preferable.
- The most important aspect of opioid administration is dose titration, rather than the selection of any particular drug.
- PRN bolus-dose opioids will often be required for the management of critically ill patients.
- Opioid toxicity increases substantially with the use of a continuous opioid infusion, so these should be avoided whenever possible.
- Agents
- Complications
- Delirium
- Respiratory suppression
- GI: Nausea/vomiting, gastroparesis, ileus, and colonic pseudo-obstruction which may lead to colonic perforation.
- Opioid dependence and withdrawal.
Step 2: Ketamine infusion +/- clonidine/dexmedetomidine
- Ketamine
- Pain-dose ketamine provides a mild-moderate amount of analgesia.
- Ketamine has been shown to reduce opioid requirements and decrease the incidence of nausea/vomiting *,*.
- Ketamine may also attenuate the development of opioid tolerance and opioid-induced hyperalgesia, thereby making co-administered opioids safer and more effective*,*.
- Dose: The typical dosing range is 0.1-0.3 mg/kg/h.
- However, guidelines suggest that doses up to 1 mg/kg/h may be used, with close monitoring for psychomimetic side effects *.
- Dexmedetomidine/clonidine
- Dexmedetomidine or clonidine synergizes with ketamine to improve analgesia *.
- Dexmedetomidine or clonidine also prevents the psychomimetic side effects of ketamine, thereby further enhancing the safety of ketamine *,*.
- Contraindications: Patients with bradycardia, heart block, or hypotension.
- Dexmedetomidine dose:
- Do not give bolus doses. Start the infusion at a high rate (e.g. 1-1.4 mcg/kg/min) and down-titrate as the drug takes effect (within an hour). Infusion rate at 0-1.4 mcg/kg/hr.
- Clonidine dose
- Multimodal analgesia: Start at 0.1-0.2 mg q12h. May increase to 0.3-0.4mg q8h.
Step 3: NSAIDs
- NSAIDs are generally not preferred in critically ill patients due to risks of renal failure, GI hemorrhage, and platelet dysfunction.
- NSAIDs may be considered selectively for patients who meet all the following criteria:
- The patient is on no other nephrotoxic medications.
- Renal function is good and stable.
- No sources of hemodynamic instability or impaired perfusion.
- Absence of cirrhosis or inflammatory bowel diseas
- No history of GI ulceration/bleeding.
- No active hemorrhage or severe coagulopathy.
- IV ketorolac 💉 takes effect faster than oral agents.
Step 3: Gabapentinoids
- Pharmacology
- These drugs don’t actually interact with GABA receptors (they function to inhibit voltage-dependent calcium channels).
- Gabapentin has a half-life of 5-7 hours, which is prolonged in renal dysfunction.
- Pregabalin may have a faster onset, with 90% bioavailability and peak concentration within an hour.
- Indications: Predominantly for neuropathic pain e.g. Guillain-Barre Syndrome, diabetic neuropathy, spinal cord injury, post-herpetic neuralgia, pain due to subarachnoid hemorrhage; post-stroke central pain.
- Contraindications and cautions
- Side effects include somnolence, respiratory depression, hypoactive delirium, and myoclonus.
- Risks are compounded by renal dysfunction and other CNS-suppressive medications.
- Rapid discontinuation of gabapentinoids can cause withdrawal.
- Patients who were on these medications prior to ICU admission should generally be continued on them (with dose adjustment as needed based on renal function).
Step 4: Fentanyl infusion
- Fentanyl infusions should be avoided whenever possible, for many reasons:
- Over several days patients will become tolerant to opioids, which may subsequently cause withdrawal when fentanyl is discontinued *.
- Fentanyl accumulates in adipose tissue, causing the drug to remain after the infusion is stopped, leading to delayed awakening *.
- A 25 mcg/hr fentanyl infusion is equivalent to 120 mg of oxycodone per day.
- Unless the patient has some extraordinary source of pain, 25-50 mcg/hr of fentanyl should be sufficient.
- High-dose fentanyl infusions can exacerbate pain, a phenomenon known as opioid-induced hyperalgesia*.
- If a fentanyl infusion is used, attempts should be made on a regular basis to reduce the dose.
Sedation for intubated patients
- Intubated patients will generally require a sedative infusion
- Sedative regimens that focus on targeted arousal levels and light sedation have positively affected the rates of delirium.
- In contrast, exposure to sedative medications and deeper levels of sedation have been associated with an increased risk of delirium.
- Sedation should therefore be titrated to the minimal required amount to keep the patient comfortable and safe but awake.
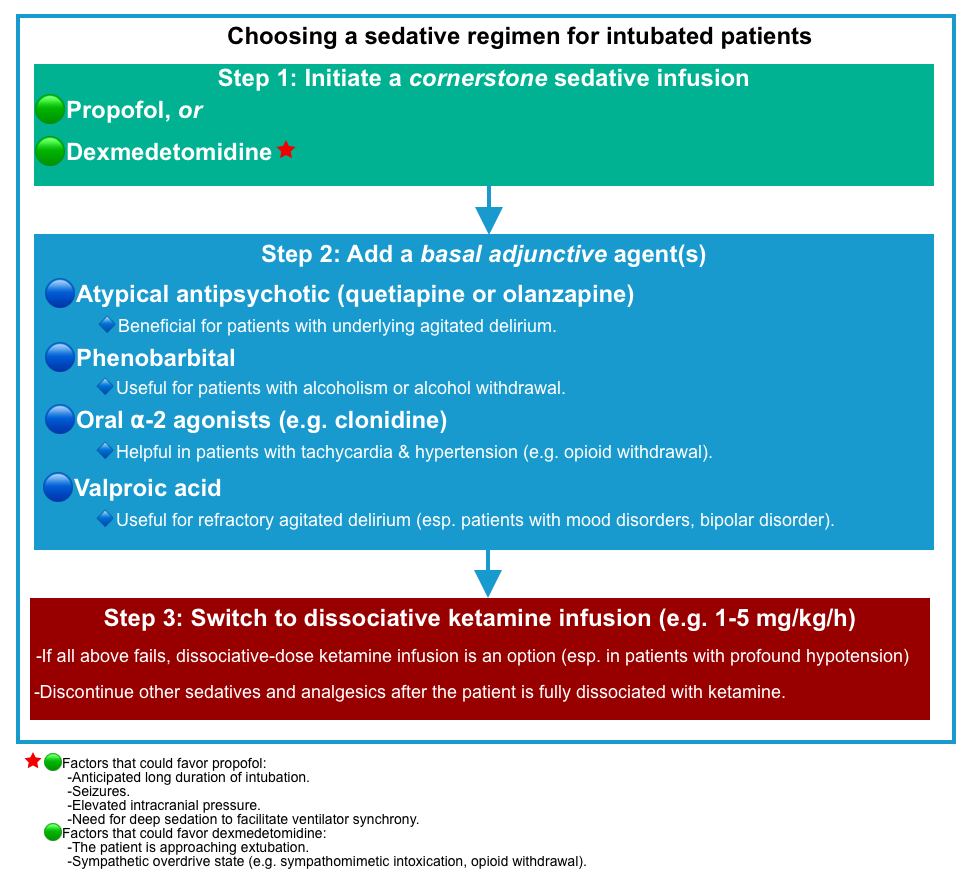
- Choosing a sedative regimen for an intubated patient may involve the following steps
- Step 1:Initiate a cornerstone sedative infusion.
- Agent of choice: Propofol or dexmedetomidine.
- Step 2: Addition of a basal adjunctive agent(s)
- Fundamental concept
- Basal agents may be used to reduce the required dose of propofol or dexmedetomidine.
- Decreasing the dose of propofol or dexmedetomidine may avoid complications with these agents (e.g. hemodynamic instability, propofol infusion syndrome, or dexmedetomidine tolerance/withdrawal).
- Basal agents are required to achieve control of refractory agitation.
- Basal agents may be used to reduce the required dose of propofol or dexmedetomidine.
- Agents: Atypical antipsychotics (quetiapine or olanzapine), phenobarbital, oral ⍺-2 agonist (e.g. clonidine), valproic acid.
- Fundamental concept
- Step 3: Switching to dissociative ketamine infusion (when all above medications fail)
- A dissociative-dose ketamine infusion (e.g. 1-5 mg/kg/hour) is an option when all above medications fail (esp. in patients with profound hypotension, which limits the ability to give sedatives (e.g., propofol, alpha-2 agonists, or phenobarbital) *.
- Step 1:Initiate a cornerstone sedative infusion.
Dexmedetomidine: Sedalgesics (sedation > analgesia)
- Pharmacology
- It is a highly selective, centrally acting ⍺-2-agonist with anxiolytic, sedative, and some analgesic effects.
- Infusion takes ~30-60 min to reach equilibrium levels.
- Excretion is mostly renal.
- Advantages
- When used for sedation during mechanical ventilation, it has improved delirium outcomes in RCT when compared to lorazepam *, midazolam *, propofol, and morphine.
- Lack of respiratory depression.
- Therefore it is safe to use in non-intubated patients as well as throughout the weaning process (unlike propofol, which must be shut off before extubation). This is an excellent option for patients who develop anxiety and tachypnea whenever sedation is lifted, making it difficult to extubate them *.
- Titratable infusion, which can be discontinued easily.
- Patients often remain arousable while on dexmedetomidine (so this may be used in situations requiring frequent neurologic examinations) *.
- Disadvantages
- It may cause hypotension due to bradycardia (especially when bolused).
- This issue can be circumvented with an infusion of low-dose epinephrine or dobutamine if the use of dexmedetomidine is critical.
- Avoid boluses of dexmedetomidine, as these can cause bradycardia and hemodynamic instability.
- Rather, the infusion may be started at a high rate (e.g. 1-1.4 mcg/kg/min) and down-titrated as the drug takes effect (within an hour).
- Prolonged uninterrupted use (>3-5 days) may cause tolerance and subsequent withdrawal after dexmedetomidine is discontinued *.
- It does not provide very deep levels of sedation.
- However, deep sedation isn’t usually preferred among critical patients, it may be desirable in some situations such as patients undergoing intubated prone ventilation.
- Delayed onset of action, so it’s not a good option for acute, dangerous agitation.
- It may cause hypotension due to bradycardia (especially when bolused).
- Indications
- Dosing 💉
- Do not give boluses of dexmedetomidine as it may cause bradycardia and hemodynamic collapse.
- Rather, the infusion may be started at a high rate (e.g. 1-1.4 mcg/kg/min) and down-titrated as the drug takes effect (within an hour).
- Infusion rate at 0-1.4 mcg/kg/hr.
Propofol: Pure sedative
- Pharmacology
- Propofol is a GABA-receptor agonist. Pure sedative with anticonvulsant properties.
- Advantages
- Pharmacokinetic profile, with fast recovery even after prolonged sedation *.
- Anticonvulsive
- Dose-dependent reduction of ICP
- Disadvantages
- Hemodynamic instability (↓MAP)
- This can be counteracted with an infusion of low-dose phenylephrine (e.g. ~0-80 mcg/min) or norepinephrine (e.g. ~0-8 mcg/min).
- Lack of analgesic effect
- Risk of propofol infusion syndrome 📖.
- Hypertriglyceridemia
- Hemodynamic instability (↓MAP)
- Indications
- Sedative for intubated patients
- First-line sedative to treat intracranial hypertension *.
- First choice sedative for status epilepticus unresponsive to antiepileptic drugs 📖.
- Postcardiac arrest, when rapid awakening is important *.
- Dosing 💉
- Sedation for intubated patients
- Initial: 5 mcg/kg/min; increase by 5 to 10 mcg/kg/min q10 min until goal sedation level is achieved.
- Usual maintenance dose: 5 to 50 mcg/kg/min
- Sedation for intubated patients
| Pharmacologic Treatment of Dangerous Agitation |
The principle of delirium treatment involves a multimodal approach.
- Treat the underlying cause(s) of delirium
- Active management of delirium requires consideration of both non-pharmacological and pharmacological options.
- Pharmacologic treatment of delirium is indicated to manage symptoms that are acutely dangerous (dangerous agitation).
- Generally, when pharmacologic treatment is indicated, the use of low-dose, short-acting agents is recommended.
Antipsychotic drugs
- Antipsychotic drugs interact with several different receptors, causing variable different side effects (table in appendix).
- Antipsychotic classes:
- Typical antipsychotics such as haloperidol, predominantly block the D2 dopamine receptor.
- They have most strong antagonistic effect on D2 dopamine receptors among antipsychotics and a relatively high risk of extrapyramidal symptoms.
- They can cause dystonia or akathisia (disturbing restlessness).
- The severity of extrapyramidal symptoms can be assessed:🧮 MODIFIED SIMPSON-ANGUS SCALE (MSAS)
- Prolonged administration can cause tardive dyskinesia, so these aren’t preferred as maintenance antipsychotics.
- The neuroleptic malignant syndrome can occur (rarely).
- They can cause dystonia or akathisia (disturbing restlessness).
- They have most strong antagonistic effect on D2 dopamine receptors among antipsychotics and a relatively high risk of extrapyramidal symptoms.
- Atypical antipsychotics (e.g. quetiapine, risperidone, olanzapine) block the 5-HT-2A receptor, with less pronounced blockade of the D2 dopamine receptor.
- A balance of activity against 5-HT2a receptors and D2 receptors reduces the risk of extrapyramidal side effects among atypical antipsychotics.
- Anticholinergic effects may further reduce the rate of extrapyramidal side effects.
- Typical antipsychotics such as haloperidol, predominantly block the D2 dopamine receptor.
- General concepts on antipsychotic use
- Indications for use in the context of delirium:
- Symptomatic treatment of dangerous agitation.
- The severity of agitation can be assessed: 🧮 MDCalc: Richmond Agitation-Sedation Scale (RASS)
- Treatment of insomnia
- Symptomatic treatment of dangerous agitation.
- 💡Antipsychotics are not beneficial in patients with hypoactive delirium *.
- Response variability
- Patients may respond variably to different agents.
- If a patient isn’t responding favorably to one agent, it may be better to switch to another agent rather than continuing to up-titrate the dose.
- Dosage
- The dose and duration should be limited as much as possible.
- Optimal dose ranges for ICU patients are largely undefined. Overly aggressive up-titration may increase the risk of neuroleptic malignant syndrome.
- Among outpatients or patients in the general ward, antipsychotics are generally started at a low dose and up-titrated. This may be a rational approach among critically ill patients as well.
- Overly aggressive up-titration may increase the risk of neuroleptic malignant syndrome.
- In a controlled setting (e.g. ICU), higher doses can be used especially for younger patients with more severe agitation.
- Antipsychotic Dose Conversion Calculator🧮
- Indications for use in the context of delirium:
| EPS/TD | Sedation | Anticholinergic side effect | Orthostatic hypotension | QTc prolongation | Consideration | |
| Haloperidol | +++ | + | +/- | – | PO: ++ IV:+++ | ⛔️CI: PD, LBD, known QTc prolongation |
| Quetiapine | -/+ | +++ | +++ | ++ | ++ | ✅Advantage: Lowest risk of EPS, low risk of TdP. ✅Can be used for PD. ✅Can be used as a long-term maintenance agent. ⚠️Dose adjustment for hepatic dysfunction. 👉May cause orthostatic hypotension. |
| Risperidone | +++ | ++ | + | + | ++ | ⛔️CI: PD, LBD, known QTc prolongation ⚠️Highest risk of EPS among atypical antipsychotics ⚠️Dose reduction in hepatic or renal insufficiency. ✅Less sedating among atypical antipsychotics. |
| Olanzapine | ++ | ++ | ++ | + | + | ✅Advantage: No risk of TdP. ⚠️Relative CI: Parkinson, DLB ⚠️Dose adjustment in renal, hepatic dysfunction |
Butyrophenones (haloperidol & droperidol)
Advantages
- Immediately available on most units
- Can be administered IV/IM.
- More easily dose-titrated than most other antipsychotics.
- Hemodynamically stable agents.
- Do not suppress respiration (allowing them to be used for non-intubated patients).
Contraindications and toxicity
- ⚠️QTc prolongation and torsade de pointes can occur.
- ⛔️These agents are contraindicated in patients with known QTc prolongation. (If this is a problem, consider using olanzapine, which doesn’t affect QT).
- ⚠️Extrapyramidal symptoms can occur.
- ⛔️These agents are contraindicated in patients with Parkinson’s disease, or Lewy Body Dementia (LBD).
- Butyrophenones can cause dystonia or akathisia (disturbing restlessness). This generally isn’t a major problem but must be identified and treated appropriately. Akathisia must not be misdiagnosed as “agitation” and treated with escalating doses of antipsychotic agents.
- Prolonged administration can cause tardive dyskinesia, so these aren’t preferred as a maintenance antipsychotic (consider quetiapine or olanzapine instead).
- Neuroleptic malignant syndrome can occur (rarely).
Use in agitated delirium
- These agents are a traditional mainstay of initial therapy for dangerous agitation *.
- These agents may not be ideal for longer-term management of delirium, due to a relatively high risk of extrapyramidal symptoms.
- If ongoing maintenance therapy is needed, an atypical antipsychotic (e.g. quetiapine or olanzapine) is generally preferable, with IV haloperidol or IV droperidol utilized for PRN management of breakthrough agitation.
- Continuous or prophylactic dosing is not recommended.
Haloperidol
- Route
- IV route is usually utilized and preferred among the critically ill.
- This carries a lower risk of dose-stacking or extrapyramidal side effects, though with a higher risk of torsade de pointes.
- With IV administration, onset occurs in ~5-20 minutes.
- IM route can be used.
- IV route is usually utilized and preferred among the critically ill.
- Dosage for agitation
- The initial dosage depends on the severity of agitation, the patient’s age (young vs. elderly group), and the setting in which medication is given, for example, a higher dosage may be used in a closely monitored setting e.g. ICU where the goals and sedation needs are different.
Droperidol
- Droperidol is ~2-3 times more potent than haloperidol *. Rapid onset of 3 to 10 minutes, is advantageous in severely agitated violent patients.
- Onset begins within ~10 minutes when given IM. Half-life is ~2 hours, with a duration of action of ~2-4 hours.
- Metabolized in the liver.
- Route: IM or IV
- Dosage: 💉
- Wait ~20 minutes before re-dosing.
Sedating atypical antipsychotics
(Quetiapine, olanzapine, risperidone)
General advantages
- Generally, less extrapyramidal side effects (as compared to prolonged, high-dose haloperidol).
- Thus, gradual escalation of scheduled atypical antipsychotics as a haloperidol-sparing agent may lead to an overall safer antipsychotic regimen.
- May be continued outside of the ICU (for patients with ongoing delirium).
- A useful combination is often PRN IV haloperidol plus scheduled atypical antipsychotics.
Olanzapine 💉
- Route: PO, or IM, or IV
- The typical starting dose for agitation is 2.5–5 mg IM/IV. May use higher doses for younger and more severe agitation, e.g. 10mg IV.
- Olanzapine is the preferred agent for managing agitation associated with thyroid storm *.
- Maintenance therapy for agitated delirium: 5-20 mg PO daily, in the early evening.
- Advantages
- Olanzapine doesn’t cause torsade de pointes. So it can be used in patients with prolonged QTc.
- PO olanzapine can be used as maintenance therapy for agitated delirium.
- Contraindication, cautions
- Olanzapine can cause extrapyramidal symptoms (though with a lower risk than typical antipsychotics).
- ⚠️Thus, Parkinson’s disease and Lewy Body Dementia are a relative contraindication.
- ⚠️Dose adjustment in elderly, or patients with hepatic and/or renal dysfunction.
- Olanzapine can cause extrapyramidal symptoms (though with a lower risk than typical antipsychotics).
Quetiapine 💊
- Advantages
- Lowest risk of extrapyramidal side effects (compared to other antipsychotics).
- For patients with Parkinson’s disease, quetiapine may be the antipsychotic of choice.
- Quetiapine can slightly prolong the QTc interval, but the risk of torsade de pointes seems to be extremely low.
- Lowest risk of extrapyramidal side effects (compared to other antipsychotics).
- Contraindications, cautions
- ⚠️Adjust for hepatic dysfunction.
- Quetiapine may cause hypotension due to the antagonism of alpha-adrenergic receptors (more so than olanzapine).
- This may cause orthostatic hypotension for mobile patients.
- Quetiapine is very sedating, which could be useful for patients on mechanical ventilation.
- Indication and usage
- Insomnia: Seven-hour half-life allows effects to wear off overnight.
- Longer-term maintenance agent for agitation (especially during mechanical ventilation)
- Quetiapine is very sedating, which could be useful for patients on mechanical ventilation.
- Dosage
- Typical starting dose for agitation is 25–50 mg PO q12 hours.
- Can be used as a long-term maintenance agent for agitation.
- Typical starting dose for insomnia is 12.5–25 mg PO QHS.
- Typical starting dose for agitation is 25–50 mg PO q12 hours.
Risperidone 💊
- Contraindication and cautions
- The highest risk of extrapyramidal symptoms among atypical antipsychotics.
- ⛔️Contraindicated in patients with Parkinson’s disease or Lewy Body Dementia.
- QTc prolongation and torsade de pointes can occur.
- ⛔️Risperidone is contraindicated in patients with known QTc prolongation.
- ⚠️Dose reduction in hepatic or renal insufficiency.
- The highest risk of extrapyramidal symptoms among atypical antipsychotics.
- Advantage and usage
- It can be used as an oral maintenance agent in agitated delirium, perhaps as a second-line agent.
- Less sedating effect than other atypical antipsychotics (e.g. quetiapine, olanzapine).
- This could be useful in non-intubated patients with intermittent agitation due to an underlying psychiatric disorder.
- Typical starting dose: ≤ 1 mg PO
Dexmedetomidine
- This is a centrally acting alpha-2-agonist with anxiolytic, sedative, and some analgesic effects.
- Dexmedetomidine is an easily titratable agent with a short half-life.
- Use in delirious patients
- Controlling nocturnal agitation (“sundowning”) and promoting sleep at night.
- It can be used to treat nocturnal agitation and promote sleep at night. The following day, dexmedetomidine can be titrated off, thereby promoting wakefulness during the day *
- The short half-life of dexmedetomidine avoids the sundowning cycle wherein a patient gets tons of medications for agitation at 2 AM, sleeps for 18 hours, and then wakes up and gets agitated the following night.
- Controlling nocturnal agitation (“sundowning”) and promoting sleep at night.
Benzodiazepines
- They are primarily metabolized by the liver, and many have active metabolites prolonging their duration of action.
- Disadvantages
- Benzodiazepines might be the most deleriogenic sedative agent.
- Benzodiazepines have been shown to increase the duration of mechanical ventilation (when compared to dexmedetomidine or propofol) *.
- Benzodiazepines may cause paradoxical agitation *,*.
- This may lead to a vicious spiral of increased benzodiazepine use, leading to obtundation.
- Ongoing exposure to benzodiazepines may lead to tolerance, with subsequent withdrawal.
- Lorazepam infusions tend to cause propylene glycol intoxication.
- Midazolam may accumulate over time in adipose tissue, especially in patients with renal or hepatic dysfunction or due to various drug-drug interactions.
- Benzodiazepines might be the most deleriogenic sedative agent.
- Advantages
- More favorable hemodynamic profile (hemodynamically stable).
- Anticonvulsive properties.
- They have a very limited role in the treatment of delirium. Possible indications of benzodiazepine may be limited to
- Agitation caused by alcohol or substance abuse.
- Management of moderate-severe alcohol withdrawal.
- Bupropion intoxication
- Ketamine re-emergence, procedural sedation.
- Patients who are chronically on benzodiazepines as a home medication.
- Palliative sedation
- Status epilepticus
- When antipsychotic medications are contraindicated:
- History of seizure: Antipsychotics lower the seizure threshold.
- Anti-cholinergic intoxication.
- Neuroleptic malignant syndrome.
- Prolonged QTc: Benzodiazepines are the first choice. Olanzapine is the second choice.
- 🧮Equivalent Benzodiazepine Calculator
Specific condition
Agitation associated with sympathomimetic intoxication
- The traditional belief that antipsychotics may promote hyperthermia or seizures is debunked.
- Sympathomimetic intoxication increases brain dopamine activity, therefore antipsychotics may work to antagonize dopamine signaling and clear the agitation.
- Available evidence suggests that antipsychotics are safe and effective *, *.
- In an RCT involving 92 emergency department patients with methamphetamine-related agitation, who were randomized to treatment with 10 mg droperidol, 10 mg olanzapine, or a combination of 5 mg droperidol plus 5 mg midazolam (all administered intravenously). The droperidol/midazolam combination therapy was more rapidly effective. However, there were more adverse events in the combination therapy group (although they were generally minor) *.
- Possible treatment options
- Antipsychotic monotherapy strategy
- Droperidol or olanzapine 10 mg IV, or IM, followed by an additional 5-10 mg as needed.
- Haloperidol might work as well, but haloperidol is about half as potent as droperidol/olanzapine. So this would require higher doses of haloperidol to be effective.
- Antipsychotic/benzodiazepine combination strategy
- This will work more rapidly to control the agitation.
- Midazolam 5mg + Droperidol 5mg (or haloperidol 10mg, if droperidol isn’t available) *.
- Lorazepam should be avoided here due to a slow onset of action.
- Antipsychotic monotherapy strategy
Agitation associated with serotonin syndrome
- Front-line agents are theoretically cyproheptadine and dexmedetomidine.
- Benzodiazepines are the first choice in patients with seizures.
- For patients with very mild agitation who can take oral medication, cyproheptadine may be trialed (with the understanding that it takes a while to work).
- For patients who are unable to take oral medication or need more immediate sedation, dexmedetomidine may be preferable.
- For patients with seizures who aren’t intubated, benzodiazepines make sense.
- For an intubated patient, dexmedetomidine or propofol could be used.
Agitation due to neuroleptic malignant syndrome (NMS)
- NMS with malignant catatonia: Benzodiazepines may help control agitation and also promote muscle relaxation.
- A usual dose may be ~2 mg IV q8hr of lorazepam.
- There is a risk that benzodiazepines could cause or promote the development of delirium.
- Management of agitation
- Treatment options may include:
- Central alpha 2 agonists (e.g.dexmedetomidine, clonidine).
- Valproate is probably a reasonable treatment for hyperactive delirium in this context.
- ⛔️Avoid antipsychotics.
- Treatment options may include:
Delirium due to salicylate intoxication
- CNS glucose levels are often lower than the blood level.
- Glucose should be given to patients with altered mental status, even if their serum glucose levels are normal (e.g. 1-2 ampules of D50W).
- The target glucose level is unclear, but it may be reasonable to maintain the glucose >80-100 mg/dL (>4.4-5.5 mM).
- Glucose infusion should be considered in severe intoxication.
- Most patients will receive an infusion of isotonic bicarbonate which is formulated in D5W, which should suffice.
Agitation due to anti-cholinergic intoxication
- Non-medication therapies
- If bladder distension is driving agitation, this should be managed with Foley catheter placement.
- Consider dimming the room lights, as pupil dilation may cause photophobia.
- Physostigmine 💉
- Benzodiazepines
Agitation due to bupropion intoxication
- Benzodiazepines are front-line therapy (which could also potentially reduce the risk of seizure).
Appendix
Receptor-binding profile of antipsychotic medications
Media
References
1. Wilson JE, Mart MF, Cunningham C, Shehabi Y, Girard TD, MacLullich AMJ, Slooter AJC, Ely EW. Delirium. Nat Rev Dis Primers. 2020 Nov 12;6(1):90. doi: 10.1038/s41572-020-00223-4. Erratum in: Nat Rev Dis Primers. 2020 Dec 1;6(1):94. PMID: 33184265.
2. Morandi A, et al. Understanding international differences in terminology for delirium and other types of acute brain dysfunction in critically ill patients. Intensive Care Med. 2008 Oct;34(10):1907-15. doi: 10.1007/s00134-008-1177-6. Epub 2008 Jun 18. PMID: 18563387.
3. Slooter AJC, et al. Updated nomenclature of delirium and acute encephalopathy: statement of ten Societies. Intensive Care Med. 2020 May;46(5):1020-1022. doi: 10.1007/s00134-019-05907-4. Epub 2020 Feb 13. PMID: 32055887; PMCID: PMC7210231.
4. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974 Jul 13;2(7872):81-4. doi: 10.1016/s0140-6736(74)91639-0. PMID: 4136544
5. Wijdicks EF, Bamlet WR, Maramattom BV, Manno EM, McClelland RL. Validation of a new coma scale: The FOUR score. Ann Neurol. 2005 Oct;58(4):585-93. doi: 10.1002/ana.20611. PMID: 16178024.
6. Pandharipande PP, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013 Oct 3;369(14):1306-16. doi: 10.1056/NEJMoa1301372. PMID: 24088092; PMCID: PMC3922401.
7. Cole M, McCusker J, Dendukuri N, Han L. The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003 Jun;51(6):754-60. doi: 10.1046/j.1365-2389.2003.51255.x. PMID: 12757560.
8. Ouimet S, et al. Subsyndromal delirium in the ICU: evidence for a disease spectrum. Intensive Care Med. 2007 Jun;33(6):1007-13. doi: 10.1007/s00134-007-0618-y. Epub 2007 Apr 3. Erratum in: Intensive Care Med. 2007 Sep;33(9):1677. Bergeon, Nicolas [corrected to Bergeron, Nicolas]. PMID: 17404704.
9. Stagno D, Gibson C, Breitbart W. The delirium subtypes: a review of prevalence, phenomenology, pathophysiology, and treatment response. Palliat Support Care. 2004 Jun;2(2):171-9. doi: 10.1017/s1478951504040234. PMID: 16594247.
10. Han JH, Zimmerman EE, Cutler N, Schnelle J, Morandi A, Dittus RS, Storrow AB, Ely EW. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009 Mar;16(3):193-200. doi: 10.1111/j.1553-2712.2008.00339.x. Epub 2009 Jan 20. PMID: 19154565; PMCID: PMC5015887.
11. Lee S, Angel C, Han JH. Succinct Approach to Delirium in the Emergency Department. Curr Emerg Hosp Med Rep. 2021;9(2):11-18. doi: 10.1007/s40138-021-00226-9. Epub 2021 Mar 18. PMID: 33758677; PMCID: PMC7971395.
12. Stagno D, Gibson C, Breitbart W. The delirium subtypes a review of prevalence, phenomenology, pathophysiology, and treatment response. Palliat Support Care. 2004 Jun;2(2):171-9. doi: 10.1017/s1478951504040234. PMID: 16594247.
13. Bliwise DL. What is sundowning? J Am Geriatr Soc. 1994 Sep;42(9):1009-11. doi: 10.1111/j.1532-5415.1994.tb06598.x. PMID: 8064089
14. McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003 May;51(5):591-8. doi: 10.1034/j.1600-0579.2003.00201.x. PMID: 12752832
15. Lawlor PG, Gagnon B, Mancini IL, Pereira JL, Hanson J, Suarez-Almazor ME, Bruera ED. Occurrence, causes, and outcome of delirium in patients with advanced cancer: a prospective study. Arch Intern Med. 2000 Mar 27;160(6):786-94. doi: 10.1001/archinte.160.6.786. PMID: 10737278.
16. Elie M, Rousseau F, Cole M, Primeau F, McCusker J, Bellavance F. Prevalence and detection of delirium in elderly emergency department patients. CMAJ. 2000 Oct 17;163(8):977-81. PMID: 11068569; PMCID: PMC80546.
17. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010 Jul 28;304(4):443-51. doi: 10.1001/jama.2010.1013. PMID: 20664045.
18. Setters B, Solberg LM. Delirium. Prim Care. 2017 Sep;44(3):541-559. doi: 10.1016/j.pop.2017.04.010. PMID: 28797379.
19. Jackson TA, Wilson D, Richardson S, Lord JM. Predicting outcome in older hospital patients with delirium: a systematic literature review. Int J Geriatr Psychiatry. 2016 Apr;31(4):392-9. doi: 10.1002/gps.4344. Epub 2015 Aug 24. PMID: 26302258.
20. Cole MG, et al. Partial and No Recovery from Delirium in Older Hospitalized Adults: Frequency and Baseline Risk Factors. J Am Geriatr Soc. 2015 Nov;63(11):2340-8. doi: 10.1111/jgs.13791. Epub 2015 Oct 30. PMID: 26515438.
21. Young GB, Pigott SE. Neurobiological basis of consciousness. Arch Neurol. 1999 Feb;56(2):153-7. doi: 10.1001/archneur.56.2.153. PMID: 10025420.
22. Trzepacz PT. The neuropathogenesis of delirium. A need to focus our research. Psychosomatics. 1994 Jul-Aug;35(4):374-91. doi: 10.1016/S0033-3182(94)71759-X. PMID: 7916159.
23. Hsieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. 2008 Jul;63(7):764-72. doi: 10.1093/gerona/63.7.764. PMID: 18693233; PMCID: PMC2917793
24. Campbell N, Boustani M, Limbil T, Ott C, Fox C, Maidment I, Schubert CC, Munger S, Fick D, Miller D, Gulati R. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging. 2009;4:225-33. doi: 10.2147/cia.s5358. Epub 2009 Jun 9. PMID: 19554093; PMCID: PMC2697587.
25. Mach JR Jr, Dysken MW, Kuskowski M, Richelson E, Holden L, Jilk KM. Serum anticholinergic activity in hospitalized older persons with delirium: a preliminary study. J Am Geriatr Soc. 1995 May;43(5):491-5. doi: 10.1111/j.1532-5415.1995.tb06094.x. PMID: 7730529.
26. Gibson GE, Jope R, Blass JP. Decreased synthesis of acetylcholine accompanying impaired oxidation of pyruvic acid in rat brain minces. Biochem J. 1975 Apr;148(1):17-23. doi: 10.1042/bj1480017. PMID: 1156396; PMCID: PMC1165501
27. Gibson G, Barclay L, Blass J. The role of the cholinergic system in thiamin deficiency. Ann N Y Acad Sci. 1982;378:382-403. doi: 10.1111/j.1749-6632.1982.tb31213.x. PMID: 7044229.
28. Chew ML, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008 Jul;56(7):1333-41. doi: 10.1111/j.1532-5415.2008.01737.x. Epub 2008 May 26. PMID: 18510583
29. Schafer DF, Jones EA. Hepatic encephalopathy and the gamma-aminobutyric-acid neurotransmitter system. Lancet. 1982 Jan 2;1(8262):18-20. doi: 10.1016/s0140-6736(82)92559-4. PMID: 6119414
30. Trzepacz PT. The neuropathogenesis of delirium. A need to focus our research. Psychosomatics. 1994 Jul-Aug;35(4):374-91. doi: 10.1016/S0033-3182(94)71759-X. PMID: 7916159.
31. Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012 Oct;8(10):557-66. doi: 10.1038/nrneurol.2012.183. Epub 2012 Sep 18. PMID: 22986430.
32. Van Montfort SJT, et al. . Brain network disintegration as a final common pathway for delirium: a systematic review and qualitative meta-analysis. Neuroimage Clin. 2019;23:101809. doi: 10.1016/j.nicl.2019.101809. Epub 2019 Apr 3. PMID: 30981940; PMCID: PMC6461601.
33. LaHue SC, Douglas VC. Approach to Altered Mental Status and Inpatient Delirium. Neurol Clin. 2022 Feb;40(1):45-57. doi: 10.1016/j.ncl.2021.08.004. PMID: 34798974; PMCID: PMC9469667.
34. Marcantonio ER. Delirium in Hospitalized Older Adults. N Engl J Med. 2017 Oct 12;377(15):1456-1466. doi: 10.1056/NEJMcp1605501. PMID: 29020579; PMCID: PMC5706782
35. Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008 Mar 10;168(5):508-13. doi: 10.1001/archinternmed.2007.106. PMID: 18332297
36. Walker V. Severe hyperammonaemia in adults not explained by liver disease. Ann Clin Biochem. 2012 May;49(Pt 3):214-28. doi: 10.1258/acb.2011.011206. Epub 2012 Feb 20. PMID: 22349554
37. Zhao L, Walline JH, Gao Y, Lu X, Yu S, Ge Z, Zhu H, Li Y. Prognostic Role of Ammonia in Critical Care Patients Without Known Hepatic Disease. Front Med (Lausanne). 2020 Oct 22;7:589825. doi: 10.3389/fmed.2020.589825. PMID: 33195354; PMCID: PMC7642587
38. Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012 Oct;8(10):557-66. doi: 10.1038/nrneurol.2012.183. Epub 2012 Sep 18. PMID: 22986430
39. Beaubien-Souligny W, Cavayas YA, Denault A, Lamarche Y. First step toward uncovering perioperative congestive encephalopathy. J Thorac Cardiovasc Surg. 2020 Jul 2:S0022-5223(20)31087-4. doi: 10.1016/j.jtcvs.2020.02.146. Epub ahead of print. PMID: 32624312.
40. Oldenbeuving AW, de Kort PL, Jansen BP, Algra A, Kappelle LJ, Roks G. Delirium in the acute phase after stroke: incidence, risk factors, and outcome. Neurology. 2011 Mar 15;76(11):993-9. doi: 10.1212/WNL.0b013e318210411f. Epub 2011 Feb 9. PMID: 21307355.
41. Veran O, Kahane P, Thomas P, Hamelin S, Sabourdy C, Vercueil L. De novo epileptic confusion in the elderly: a 1-year prospective study. Epilepsia. 2010 Jun;51(6):1030-5. doi: 10.1111/j.1528-1167.2009.02410.x. Epub 2009 Dec 1. PMID: 20002146.
42. Sampson EL, West E, Fischer T. Pain, and delirium: mechanisms, assessment, and management. Eur Geriatr Med. 2020 Feb;11(1):45-52. doi: 10.1007/s41999-019-00281-2. Epub 2020 Jan 9. PMID: 32297242.
43. McKhann GM, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011 May;7(3):263-9. doi: 10.1016/j.jalz.2011.03.005. Epub 2011 Apr 21. PMID: 21514250; PMCID: PMC3312024
44. McKeith IG, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017 Jul 4;89(1):88-100. doi: 10.1212/WNL.0000000000004058. Epub 2017 Jun 7. PMID: 28592453; PMCID: PMC5496518.
45. Fink M, Taylor MA. The catatonia syndrome: forgotten but not gone. Arch Gen Psychiatry. 2009 Nov;66(11):1173-7. doi: 10.1001/archgenpsychiatry.2009.141. PMID: 19884605.
46. Apetauerova D, Patel PA, Burns JD, Lerner DP. Movement Disorder Emergencies. Neurol Clin. 2021 May;39(2):615-630. doi: 10.1016/j.ncl.2021.01.005. Epub 2021 Mar 31. PMID: 33896535
47. Denysenko L, et al. Catatonia in the medically ill: Etiology, diagnosis, and treatment. The Academy of Consultation-Liaison Psychiatry Evidence-Based Medicine Subcommittee Monograph. Ann Clin Psychiatry. 2018 May;30(2):140-155. PMID: 29697715
48. Edinoff AN, et al. Catatonia: Clinical Overview of the Diagnosis, Treatment, and Clinical Challenges. Neurol Int. 2021 Nov 8;13(4):570-586. doi: 10.3390/neurolint13040057. PMID: 34842777; PMCID: PMC8628989
49. Saddawi-Konefka D, Berg SM, Nejad SH, Bittner EA. Catatonia in the ICU: an important and underdiagnosed cause of altered mental status. a case series and review of the literature*. Crit Care Med. 2014 Mar;42(3):e234-41. doi: 10.1097/CCM.0000000000000053. PMID: 24275514.
50. Saddawi-Konefka D, Berg SM, Nejad SH, Bittner EA. Catatonia in the ICU: an important and underdiagnosed cause of altered mental status. a case series and review of the literature*. Crit Care Med. 2014 Mar;42(3):e234-41. doi: 10.1097/CCM.0000000000000053. PMID: 24275514.
51. Veran O, Kahane P, Thomas P, Hamelin S, Sabourdy C, Vercueil L. De novo epileptic confusion in the elderly: a 1-year prospective study. Epilepsia. 2010 Jun;51(6):1030-5. doi: 10.1111/j.1528-1167.2009.02410.x. Epub 2009 Dec 1. PMID: 20002146.
52. Gusmao-Flores D, Salluh JI, Chalhub RÁ, Quarantini LC. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care. 2012 Jul 3;16(4):R115. doi: 10.1186/cc11407. PMID: 22759376; PMCID: PMC3580690
53. Soiza RL, Myint PK. The Scottish Intercollegiate Guidelines Network (SIGN) 157: Guidelines on Risk Reduction and Management of Delirium. Medicina (Kaunas). 2019 Aug 15;55(8):491. doi: 10.3390/medicina55080491. PMID: 31443314; PMCID: PMC6722546
54. Scottish Intercollegiate Guidelines Network (SIGN). Risk Reduction and Management of Delirium. Edinburgh: SIGN; 2019. (SIGN publication; no.157). [March 2019]. Available from URL: http://www.sign.ac.uk..https://www.sign.ac.uk/media/1423/sign157.pdf (2019) Accessed 8 June 2020.
55. de Oliveira AM, Paulino MV, Vieira APF, McKinney AM, da Rocha AJ, Dos Santos GT, Leite CDC, Godoy LFS, Lucato LT. Imaging Patterns of Toxic and Metabolic Brain Disorders. Radiographics. 2019 Oct;39(6):1672-1695. doi: 10.1148/rg.2019190016. PMID: 31589567
56. Metersky ML, Williams A, Rafanan AL. Retrospective analysis: are fever and altered mental status indications for lumbar puncture in a hospitalized patient who has not undergone neurosurgery? Clin Infect Dis. 1997 Aug;25(2):285-8. doi: 10.1086/514531. PMID: 9332525
57. Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing. 2011 Jan;40(1):23-9. doi: 10.1093/ageing/afq140. Epub 2010 Nov 9. PMID: 21068014.
58. Devlin JW, et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018 Sep;46(9):e825-e873. doi: 10.1097/CCM.0000000000003299. PMID: 30113379.
59. Hajiesmaeili MR, Safari S. Pain management in the intensive care unit: do we need special protocols? Anesth Pain Med. 2012 Spring;1(4):237-8. doi: 10.5812/aapm.4523. Epub 2012 Apr 1. PMID: 24904806; PMCID: PMC4018715.
60. American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012 Feb;116(2):248-73. doi: 10.1097/ALN.0b013e31823c1030. PMID: 22227789.
61. de Souza RLP Sr, Abrão J, Garcia LV, Vila Moutinho S, Wiggers E, Cagnoni Balestra A. Impact of a Multimodal Analgesia Protocol in an Intensive Care Unit: A Pre-post Cohort Study. Cureus. 2022 Mar 3;14(3):e22786. doi: 10.7759/cureus.22786. PMID: 35371872; PMCID: PMC8971103
62. Wampole CR, Smith KE. Beyond Opioids for Pain Management in Adult Critically Ill Patients. J Pharm Pract. 2019 Jun;32(3):256-270. doi: 10.1177/0897190019834479. Epub 2019 Mar 7. PMID: 30845871
63. Subramaniam B, et al. Effect of Intravenous Acetaminophen vs Placebo Combined With Propofol or Dexmedetomidine on Postoperative Delirium Among Older Patients Following Cardiac Surgery: The DEXACET Randomized Clinical Trial. JAMA. 2019 Feb 19;321(7):686-696. doi: 10.1001/jama.2019.0234. Erratum in: JAMA. 2019 Jul 16;322(3):276. PMID: 30778597; PMCID: PMC6439609
64. Lee Y, Yu J, Doumouras AG, Ashoorion V, Gmora S, Anvari M, Hong D. Intravenous Acetaminophen Versus Placebo in Post-bariatric Surgery Multimodal Pain Management: a Meta-analysis of Randomized Controlled Trials. Obes Surg. 2019 Apr;29(4):1420-1428. doi: 10.1007/s11695-019-03732-8. PMID: 30726545
65. Memis D, Inal MT, Kavalci G, Sezer A, Sut N. Intravenous paracetamol reduced the use of opioids, extubation time, and opioid-related adverse effects after major surgery in intensive care unit. J Crit Care. 2010 Sep;25(3):458-62. doi: 10.1016/j.jcrc.2009.12.012. Epub 2010 Mar 1. PMID: 20189753.
66. Imani F, Motavaf M, Safari S, Alavian SM. The therapeutic use of analgesics in patients with liver cirrhosis: a literature review and evidence-based recommendations. Hepat Mon. 2014 Oct 11;14(10):e23539. doi: 10.5812/hepatmon.23539. PMID: 25477978; PMCID: PMC4250965.
67. Buchheit JL, Yeh DD, Eikermann M, Lin H. Impact of Low-Dose Ketamine on the Usage of Continuous Opioid Infusion for the Treatment of Pain in Adult Mechanically Ventilated Patients in Surgical Intensive Care Units. J Intensive Care Med. 2019 Aug;34(8):646-651. doi: 10.1177/0885066617706907. Epub 2017 May 3. PMID: 28468568
68. Moitra VK, Patel MK, Darrah D, Moitra A, Wunsch H. Low-Dose Ketamine in Chronic Critical Illness. J Intensive Care Med. 2016 Mar;31(3):216-20. doi: 10.1177/0885066615587868. Epub 2015 May 28. PMID: 26025196.
69. Schwenk ES, et al. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Acute Pain Management From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018 Jul;43(5):456-466. doi: 10.1097/AAP.0000000000000806. PMID: 29870457.
70. Nitta R, Goyagi T, Nishikawa T. Combination of oral clonidine and intravenous low-dose ketamine reduces the consumption of postoperative patient-controlled analgesia morphine after spine surgery. Acta Anaesthesiol Taiwan. 2013 Mar;51(1):14-7. doi: 10.1016/j.aat.2013.03.003. Epub 2013 May 3. PMID: 23711600
71. Handa F, Tanaka M, Nishikawa T, Toyooka H. Effects of oral clonidine premedication on side effects of intravenous ketamine anesthesia: a randomized, double-blind, placebo-controlled study. J Clin Anesth. 2000 Feb;12(1):19-24. doi: 10.1016/s0952-8180(99)00131-2. PMID: 10773503.
72. Cohen SP, et al. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Chronic Pain From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018 Jul;43(5):521-546. doi: 10.1097/AAP.0000000000000808. PMID: 29870458.
73. Wanzuita R, et al. Replacement of fentanyl infusion by enteral methadone decreases the weaning time from mechanical ventilation: a randomized controlled trial. Crit Care. 2012 Dec 12;16(2):R49. doi: 10.1186/cc11250. PMID: 22420584.
74. Reardon DP, Anger KE, Szumita PM. Pathophysiology, assessment, and management of pain in critically ill adults. Am J Health Syst Pharm. 2015 Sep 15;72(18):1531-43. doi: 10.2146/ajhp140541. PMID: 26346209
75. Lyons PJ, Rivosecchi RM, Nery JP, Kane-Gill SL. Fentanyl-induced hyperalgesia in acute pain management. J Pain Palliat Care Pharmacother. 2015 Jun;29(2):153-60. doi: 10.3109/15360288.2015.1035835. PMID: 26095487
76. Adams CD, et al. Analgesia and Sedation Strategies in Mechanically Ventilated Adults with COVID-19. Pharmacotherapy. 2020 Dec;40(12):1180-1191. doi: 10.1002/phar.2471. Epub 2020 Nov 20. PMID: 33068459.
77. Pandharipande PP, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007 Dec 12;298(22):2644-53. doi: 10.1001/jama.298.22.2644. PMID: 18073360.
78. Lin N, Han R, Zhou J, Gelb AW. Mild Sedation Exacerbates or Unmasks Focal Neurologic Dysfunction in Neurosurgical Patients with Supratentorial Brain Mass Lesions in a Drug-specific Manner. Anesthesiology. 2016 Mar;124(3):598-607. doi: 10.1097/ALN.0000000000000994. PMID: 26756518
79. Tobias JD. Dexmedetomidine: Are There Going to be Issues with Prolonged Administration? J Pediatr Pharmacol Ther. 2010 Jan;15(1):4-9. PMID: 22477787; PMCID: PMC3017404
80. Ammar MA, et al. Sedation, Analgesia, and Paralysis in COVID-19 Patients in the Setting of Drug Shortages. J Intensive Care Med. 2021 Feb;36(2):157-174. doi: 10.1177/0885066620951426. Epub 2020 Aug 26. PMID: 32844730.
81. Opdenakker O, Vanstraelen A, De Sloovere V, Meyfroidt G. Sedatives in neurocritical care: an update on pharmacological agents and modes of sedation. Curr Opin Crit Care. 2019 Apr;25(2):97-104. doi: 10.1097/MCC.0000000000000592. PMID: 30672819
82. Skrobik Y, Duprey MS, Hill NS, Devlin JW. Low-Dose Nocturnal Dexmedetomidine Prevents ICU Delirium. A Randomized, Placebo-controlled Trial. Am J Respir Crit Care Med. 2018 May 1;197(9):1147-1156. doi: 10.1164/rccm.201710-1995OC. PMID: 29498534
83. Girard TD, et al. Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness. N Engl J Med. 2018 Dec 27;379(26):2506-2516. doi: 10.1056/NEJMoa1808217. Epub 2018 Oct 22. PMID: 30346242.
84. Gilchrist NA, Asoh I, Greenberg B. Atypical antipsychotics for the treatment of ICU delirium. J Intensive Care Med. 2012 Nov-Dec;27(6):354-61. doi: 10.1177/0885066611403110. Epub 2011 Mar 25. PMID: 21441282.
85. Satoh T, et al. 2016 Guidelines for the management of thyroid storm from The Japan Thyroid Association and Japan Endocrine Society (First edition). Endocr J. 2016 Dec 30;63(12):1025-1064. doi: 10.1507/endocrj.EJ16-0336. Epub 2016 Oct 15. PMID: 27746415.
86. Girard TD, et al. Risk factors for post-traumatic stress disorder symptoms following critical illness requiring mechanical ventilation: a prospective cohort study. Crit Care. 2007;11(1):R28. doi: 10.1186/cc5708. PMID: 17316452.
87. Zaal IJ, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. 2015 Dec;41(12):2130-7. doi: 10.1007/s00134-015-4063-z. Epub 2015 Sep 24. PMID: 26404392
88. Fraser GL, et al. Benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adults: a systematic review and meta-analysis of randomized trials. Crit Care Med. 2013 Sep;41 (9 Suppl 1): S30-8. doi: 10.1097/CCM.0b013e3182a16898. PMID: 23989093.
89. Mancuso CE, Tanzi MG, Gabay M. Paradoxical reactions to benzodiazepines: literature review and treatment options. Pharmacotherapy. 2004 Sep;24(9):1177-85. doi: 10.1592/phco.24.13.1177.38089. PMID: 15460178
90. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed, APA Press, Washington, DC 2013.
91. Richards JR, Derlet RW. Another dogma dispelled? Antipsychotic treatment of sympathomimetic toxicity. Am J Emerg Med. 2019 Dec;37(12):2256-2257. doi: 10.1016/j.ajem.2019.05.013. Epub 2019 May 6. PMID: 31088749
92. Connors NJ, Alsakha A, Larocque A, Hoffman RS, Landry T, Gosselin S. Antipsychotics for the treatment of sympathomimetic toxicity: A systematic review. Am J Emerg Med. 2019 Oct;37(10):1880-1890. doi: 10.1016/j.ajem.2019.01.001. Epub 2019 Jan 6. PMID: 30639129
93. Yap CYL, et al. Intravenous midazolam-droperidol combination, droperidol or olanzapine monotherapy for methamphetamine-related acute agitation: subgroup analysis of a randomized controlled trial. Addiction. 2017 Jul;112(7):1262-1269. doi: 10.1111/add.13780. Epub 2017 Feb 28. PMID: 28160494.


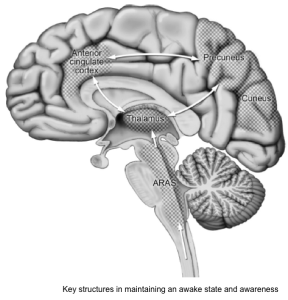
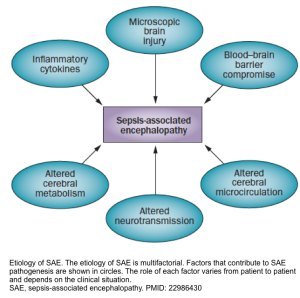
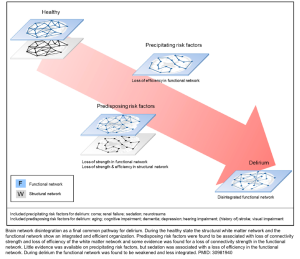
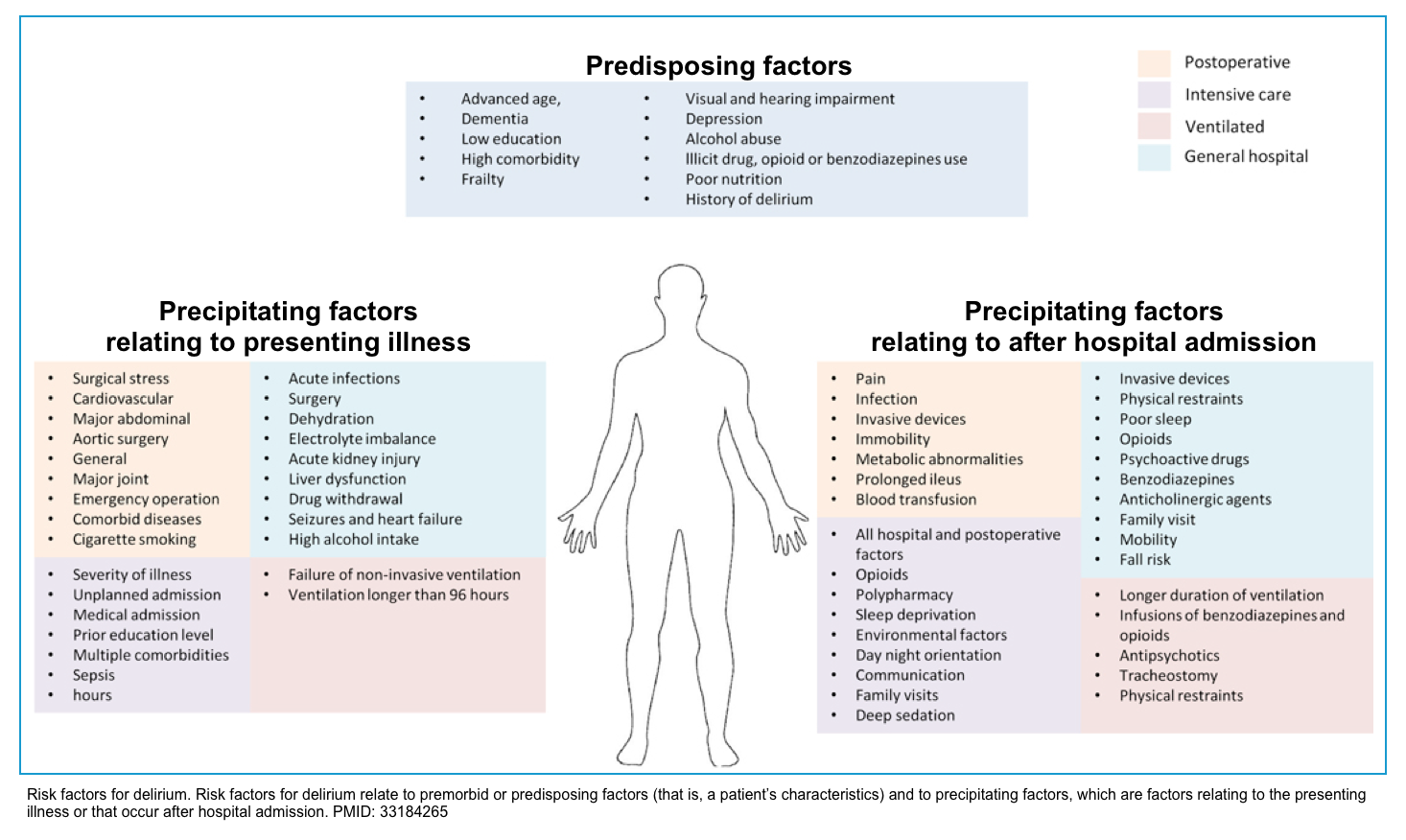
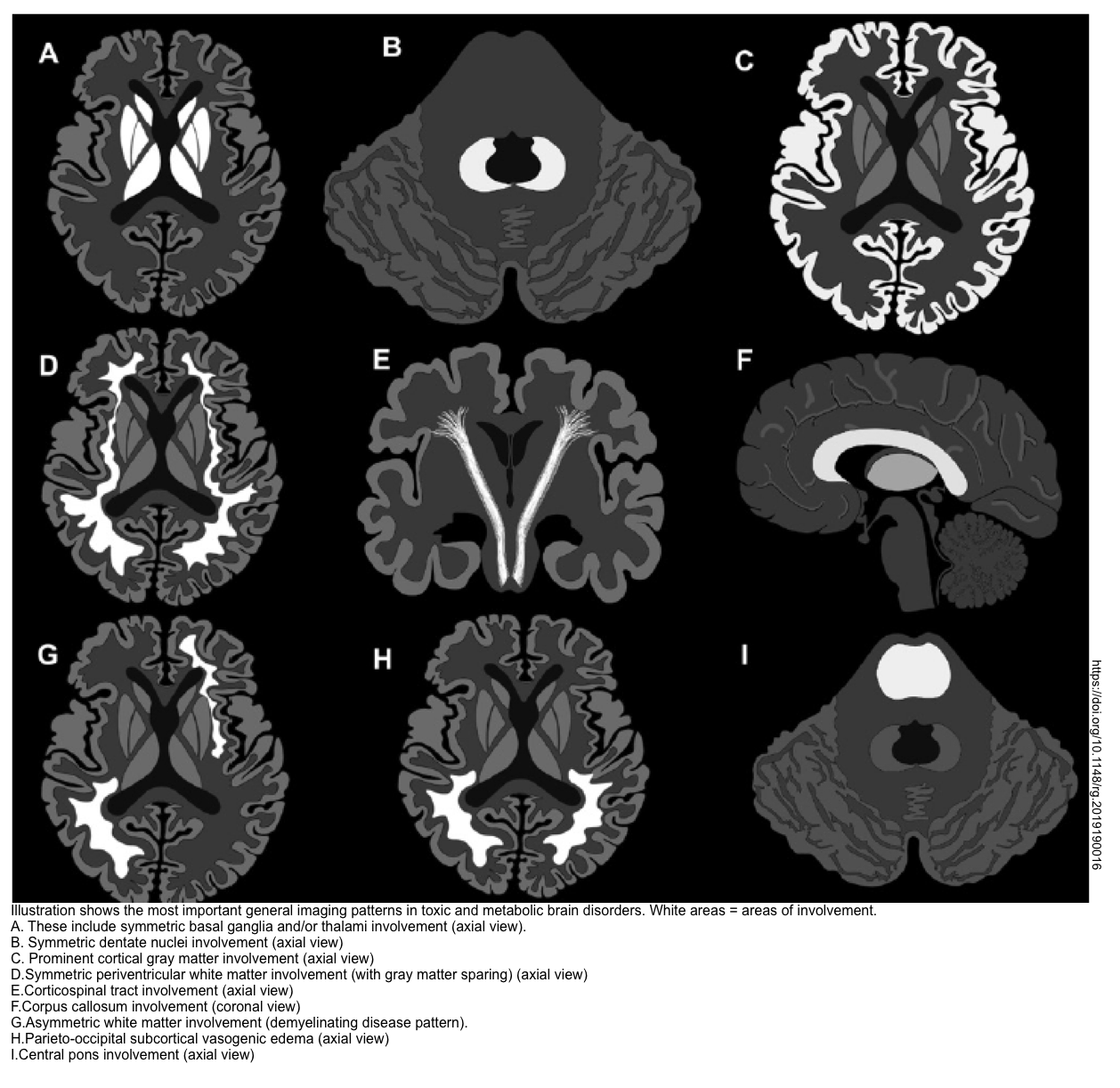
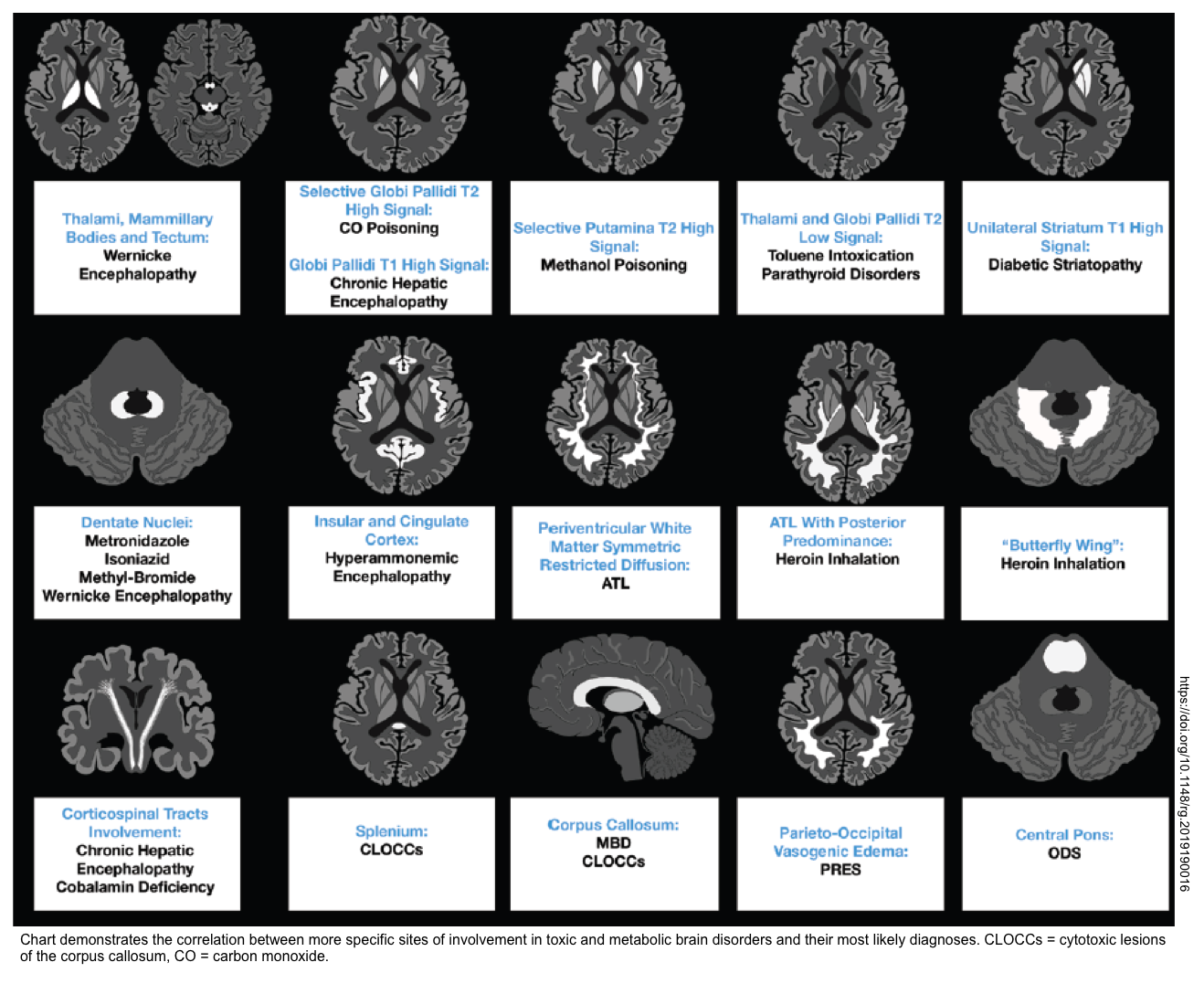
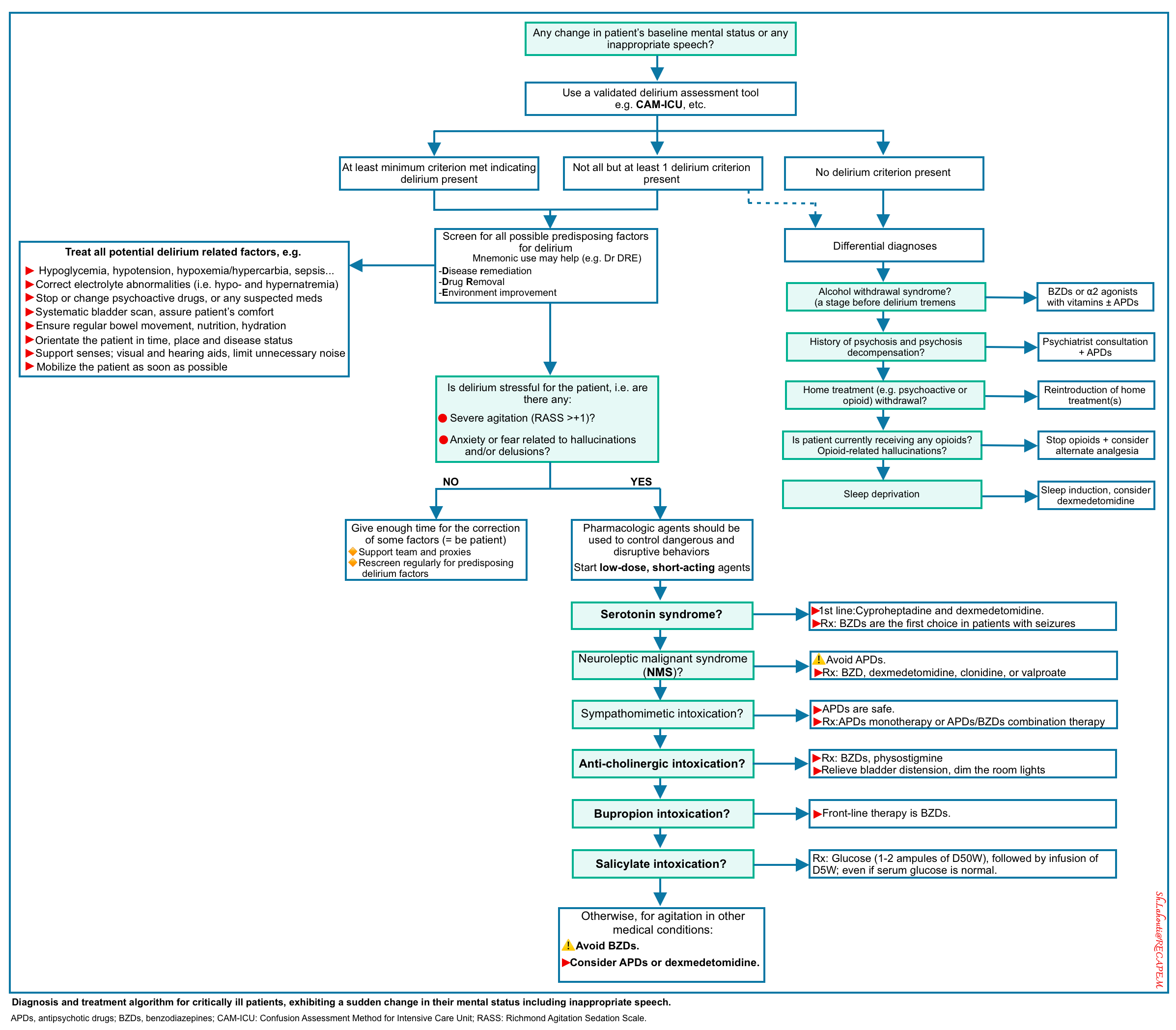


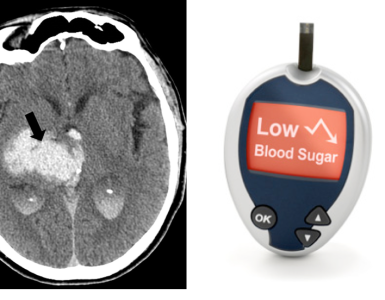
Add comment