AT A GLANCE
- Subretinal delivery is now part of the surgical approach for several novel treatments, including approved and experimental gene therapies and even a subretinal prosthesis.
- If you do not lift the hyaloid, it will hinder fluid penetration through the retina when raising a bleb.
- Use the lowest pressure necessary to enter the subretinal space.
Subretinal delivery of therapeutic agents is a common technique employed for the displacement of submacular hemorrhage. It is now part of the surgical approach for several novel treatments, including approved and experimental gene therapy products and even a subretinal prosthesis (Prima, Pixium Vision).1-6 Clinical trials in dry and wet AMD and inherited retinal diseases (IRDs) that cause outer retinal degeneration are exploring the subretinal delivery of viral vectors containing various genes and even clustered, regularly interspaced, short palindromic repeats (CRISPR) gene editing mechanisms to repair the genetic defect or activate pathways aimed at preserving photoreceptors and/or retinal pigment epithelial (RPE) cells.7
Subretinal delivery involves iatrogenic detachment of the retina inside or outside of the macula and sometimes the fovea. While the subretinal delivery technique varies slightly between trials, the common goal is to detach the retina as safely as possible, deliver the agent without causing outer retina and RPE damage, and inject enough product to see tangible improvements in patients’ functional vision.8,9
In this article, I provide surgical pearls based on my team’s broad experience delivering therapeutic agents into the subretinal space for various retinal conditions, such as retinitis pigmentosa, choroideremia, achromatopsia, Leber congenital amaurosis, dry and wet AMD, as well as administration of the FDA-approved gene therapy voretigene neparvovec-rzyl (Luxturna, Spark Therapeutics).9
1. REMOVE THE HYALOID OVER THE INJECTION SITE
Use a staining agent (I prefer triamcinolone acetonide) to visualize the hyaloid and remove it over the treatment zone. If the posterior hyaloid looks like an adherent sheath, gently scrape it off with a membrane scraper (eg, Finesse Flex loop, Alcon) or aspirate with a soft tip to lift it from the retina before using the vitrector. If you do not lift the hyaloid, it will hinder fluid penetration through the retina when raising a bleb.
2. SELECT YOUR INJECTION SITE PREOPERATIVELY
Based on the study protocol, decide on your preferred injection site(s) along an easily recognizable vascular landmark. This will help locate the self-sealing penetration site if there are multiple injections administered into the same retinotomy. Typically, macular blebs are initiated along the superior temporal or inferior temporal arcade. Injecting at least 3 mm from the fovea is advisable because closer than 2 mm increases the risk of an intraoperative macular hole due to greater foveal stretch.10 Extramacular blebs for several ongoing AMD trials are positioned inferiorly; thus, select a site away from the optic nerve to allow for bleb expansion.
3. ALLOW THE INJECTION FLUID TO PENETRATE AND DETACH THE RETINA
The surgeon may assume that they must push the cannula through the retina to raise a bleb. However, the fluid that you are injecting penetrates the retina, not the cannula. In fact, the 38- or 41-gauge cannula is so fine that pushing it firmly against the retinal tissue will occlude the lumen and make raising the bleb more difficult. The two subretinal cannulas commonly used for subretinal delivery are the 23-gauge extendable 1270.ext DORC polytetrafluoroethylene cannula (Dutch Ophthalmic) that tapers to 41-gauge and 23-, 25-, and 27-gauge polypropylene PolyTip cannulas (MedOne Surgical) tapering to 38-gauge (equivalent to 41-gauge in other brands). PolyTip cannulas come in 2-mm or 5-mm lengths of the 38-gauge fiber.
Place the cannula against the retina gently to avoid blanching of the RPE. I use intraoperative OCT (iOCT) to confirm that I am not generating visible retinal and choroidal indentation; if I am, I lighten my touch by lifting my instrument slightly to open the lumen while depressing the pedal to allow the fluid jet to penetrate into the subretinal space.
Injecting a balanced salt solution (BSS) to initiate the retinal detachment with a tiny pre-bleb may be helpful when the virus volume available is small.8,10
4. USE THE LOWEST POSSIBLE INFUSION PRESSURE
I use the Viscous Fluid Control (VFC) function on the Constellation (Alcon) to perform the injection under pedal control. While the exact settings will depend on the vitrectomy machine being used, the surgeon should use the lowest pressure necessary to enter the subretinal space. I typically start at 10 mm Hg to 12 mm Hg and test the cannula outside of the eye to see a steady drip, not a stream. If I have trouble raising a bleb, I increase the pressure slowly, typically to 14 mm Hg or 16 mm Hg; I rarely go up to 18 mm Hg.
Once I see subretinal fluid forming, I continue injecting at a lower injection pressure to avoid outer retina and RPE damage. The injection pressure required to overcome the retina-RPE adhesion declines exponentially with bleb diameter; thus, it may be advisable to reduce the injection pressure once the bleb starts to expand.11
OCT and transmission electron microscopy show that the disruption of outer retinal layers and degeneration of outer segments in monkey eyes occur after subretinal BSS injection at 20 psi, but not at 6 psi. Moreover, migration and thickening of RPE cells were both seen at 20 psi.12 Because the severity of retinal damage is associated with the injection pressure, the subretinal injection should be performed at the lowest pressure possible.
If the study protocol allows, I bevel the tip of the subretinal cannula at 45° to help nick the internal limiting membrane and allow the stream to penetrate the retina easily.
5. COVER THE TARGET TREATMENT AREA
Once the surgeon has initiated the subretinal injection, the resulting bleb may unavoidably propagate away from the target zone. If the study protocol allows, I create more than one bleb to cover the desired treatment area. Some blebs expand in an anterior/posterior rather than a horizontal direction. Surgeons can consider using a fluid-air exchange to push and spread a high bleb laterally.
6. AVOID INJECTING SUBRETINAL AIR
While a few tiny bubbles do not cause visible negative effects, large air bubbles add extra volume into the subretinal space, displace the gene therapy from the target tissues, and may cause a macular hole or damage the retina or RPE. To avoid this potential complication, I load the virus into a microdose injection syringe (MedOne Surgical) through the front narrow end of the loading syringe rather than aspirating directly from a vial (Figure 1).8 I avoid aspirating air into the injection syringe and prime syringes and cannulas by pointing up to expel air bubbles.
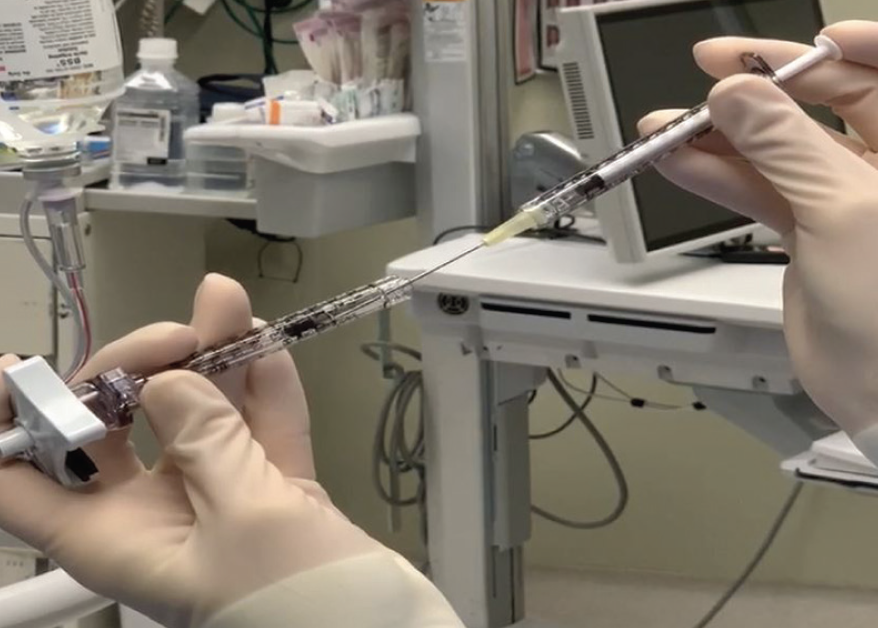
Figure 1. I load the therapeutic into the microinjector syringe through the front narrow end of the loading syringe to avoid air bubbles.
7. USE INTRAOPERATIVE OCT
In addition to visualizing excessive pressure on the retina during the injection, iOCT allows me to confirm delivery into the subretinal space, monitor for inadvertent suprachoroidal injection, map the extent of fluid across the treatment zone, and monitor the fovea to avoid excessive stretching or macular hole.13
I use an assistant to monitor the foveal contour during subretinal injection and stop injecting if the subretinal fluid is hydrating the central macula or approaching the fovea (when foveal detachment is not desired) or if the fovea everts during subfoveal delivery (Figure 2).

Figure 2. iOCT imaging shows a large subretinal bleb. Surgeons should monitor the fovea (blue arrows) to avoid excessive stretch or eversion.
9. ENSURE GOOD WRIST SUPPORT
Keep the cannula as steady as possible to avoid enlarging the retinotomy and to minimize reflux into the vitreous cavity. Reflux is difficult to visualize, although it likely occurs in every case to some degree.14 iOCT may show clues, such as the bleb rising and then flattening or never rising despite presumed subretinal injection. In addition to making it difficult to calculate the actual dose delivered, reflux increases inflammation if the vector is left in the vitreous.
10. CLEAN UP THE VITREOUS CAVITY
Depending on the agent and the surgical protocol, at the end of the case, I either perform a fluid-air exchange or irrigate the vitreous cavity for 1 to 2 minutes with BSS and trim any peripheral vitreous that may trap viral particles.
TAKEAWAYS
The key steps to successful subretinal delivery include: achieving posterior hyaloid detachment over the treatment zone, avoiding excessive pressure against the retina to let the fluid stream penetrate the neurosensory retina, monitoring the fovea or the treatment zone with iOCT, delivering the desired treatment volume, and removing refluxed virus from the vitreous cavity.
1. Haupert CL, McCuen BW 2nd, Jaffe GJ, et al. Pars plana vitrectomy, subretinal injection of tissue plasminogen activator, and fluid-gas exchange for displacement of thick submacular hemorrhage in age-related macular degeneration. Am J Ophthalmol. 2001;131(2):208-215.
2. Barnard AR, Groppe M, MacLaren RE. Gene therapy for choroideremia using an adeno-associated viral (AAV) vector. Cold Spring Harb Perspect Med. 2014;5(3):a017293.
3. Palanker DV, Le Mer Y, Hornig R, et al. Restoration of sight in geographic atrophy using a photovoltaic subretinal prosthesis. Invest Ophthalmol Vis Sci. 2019;60:970.
4. MacLaren RE, Bennett J, Schwartz SD. Gene therapy and stem cell transplantation in retinal disease: the new frontier. Ophthalmology. 2016;123(10S):S98-S106.
5. MacLaren RE, Groppe M, Barnard AR, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383(9923):1129-1137.
6. Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390(10097):849-860. Erratum in: Lancet. 2017;390(10097):848.
7. Guimaraes TAC, Georgiou M, Bainbridge JWB, Michaelides M. Gene therapy for neovascular age-related macular degeneration: rationale, clinical trials and future directions. Br J Ophthalmol. 2021;105(2):151-157.
8. Davis JL, Gregori NZ, MacLaren RE, Lam BL. Surgical technique for subretinal gene therapy in humans with inherited retinal degeneration. Retina. 2019;39(Suppl 1):S2-S8.
9. Gregori NZ, Davis JL. Surgical observations from the first 120 cases of subretinal gene therapy for inherited retinal degenerations [preprint published online December 18, 2020]. Retina.
10. Xue K, Groppe M, Salvetti AP, MacLaren RE. Technique of retinal gene therapy: delivery of viral vector into the subretinal space. Eye (Lond). 2017;31(9):1308-1316.
11. Kita M, Negi A, Kawano S, Honda Y, Maegawa S. Measurement of retinal adhesive force in the in vivo rabbit eye. Invest Ophthalmol Vis Sci. 1990;31(4):624-628.
12. Takahashi K, Morizane Y, Hisatomi T, et al. The influence of subretinal injection pressure on the microstructure of the monkey retina. PLoS One. 2018;13(12):e0209996.
13. Gregori NZ, Lam BL, Davis JL. Intraoperative use of microscope-integrated optical coherence tomography for subretinal gene therapy delivery. Retina. 2019;39(Suppl 1):S9-S12.
14. Hsu ST, Gabr H, Viehland C, et al. Volumetric measurement of subretinal blebs using microscope-integrated optical coherence tomography. Transl Vis Sci Technol. 2018;7(2):19.


-1_1674489004.jpg?auto=compress,format&w=70)
_1674488812.jpg?auto=compress,format&w=70)





















