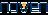Last update 16 May 2024
Zucapsaicin
Last update 16 May 2024
Overview
Basic Info
Drug Type Small molecule drug |
Synonyms (Z)-Capsaicin, Civamide, Civanex + [6] |
Target |
Mechanism TRPV1 agonists(Vanilloid receptor agonists) |
Therapeutic Areas |
Active Indication |
Inactive Indication |
Originator Organization |
Active Organization- |
Inactive Organization |
Drug Highest PhaseApproved |
First Approval Date CA (15 Jul 2010), |
RegulationOrphan Drug (US) |
Login to view First Approval Timeline
Structure
Molecular FormulaC18H27NO3 |
InChIKeyYKPUWZUDDOIDPM-VURMDHGXSA-N |
CAS Registry25775-90-0 |
Related
25
Clinical Trials associated with ZucapsaicinA Phase III, Double-Blind, Randomized, Vehicle-Controlled, Parallel-Group, Multicenter Evaluation of Civamide Nasal Solution 0.01% in the Prevention of Cluster Headaches During an Episodic Cluster Headache Period
The purpose of this study is to evaluate the safety and efficacy of intranasally administered civamide nasal solution in the prevention of cluster headaches during an episodic cluster headache period.
Start Date30 Nov 2023 |
Sponsor / Collaborator |
UW Withdraw From Tobacco Study: Enhancing and Evaluating Tobacco Withdrawal Assessment Psychometrics and Validity
It is of considerable scientific and clinical importance to assess tobacco withdrawal accurately since withdrawal severity is highly determinant of smoking cessation success. In addition, smoking cessation pharmacotherapy produces its effects on smoking abstinence by suppressing such symptoms. However, in order to ensure that a measure of tobacco withdrawal is sensitive to severe withdrawal, it is essential to examine a period of unmedicated abstinence. The current study aims to validate, and possibly enhance, a revised Wisconsin Smoking Withdrawal Scale long and brief version for use in research and clinical settings.
Two hundred adults who smoke cigarettes daily and report a desire to quit smoking will be enrolled. This is a treatment-delay, one-group clinical trial that is intended to enhance the assessment of tobacco withdrawal amongst participants who try to quit smoking with delayed use of cessation medication. Participants will not receive any pharmacotherapy during the first 1 week of their quit attempt and will initiate 8 weeks of combination nicotine replacement therapy (C-NRT; nicotine patch + nicotine mini-lozenge) starting 1 week past the target quit day (TQD). Participants will receive 4 counseling sessions as well (1 pre-quit, 3 post-quit). Participants will complete 4 weeks of ecological momentary assessment (EMA) smartphone surveys including a 2-week baseline (starting TQD-14) and 2-week post-TQD (1-week un-medicated, 1-week using C-NRT).
Two hundred adults who smoke cigarettes daily and report a desire to quit smoking will be enrolled. This is a treatment-delay, one-group clinical trial that is intended to enhance the assessment of tobacco withdrawal amongst participants who try to quit smoking with delayed use of cessation medication. Participants will not receive any pharmacotherapy during the first 1 week of their quit attempt and will initiate 8 weeks of combination nicotine replacement therapy (C-NRT; nicotine patch + nicotine mini-lozenge) starting 1 week past the target quit day (TQD). Participants will receive 4 counseling sessions as well (1 pre-quit, 3 post-quit). Participants will complete 4 weeks of ecological momentary assessment (EMA) smartphone surveys including a 2-week baseline (starting TQD-14) and 2-week post-TQD (1-week un-medicated, 1-week using C-NRT).
Start Date13 Jul 2021 |
Sponsor / Collaborator |
An Evaluator-blinded, Randomized Within-subject Repeat Insult Study to Evaluate Potential Skin Irritation and Sensitization of HP-5000 (Diclofenac Sodium Topical System) in Healthy Adults
This study will assess skin irritation and sensitization for HP-5000 patch in healthy subjects.
Start Date03 Jun 2021 |
Sponsor / Collaborator |
100 Clinical Results associated with Zucapsaicin
Login to view more data
100 Translational Medicine associated with Zucapsaicin
Login to view more data
100 Patents (Medical) associated with Zucapsaicin
Login to view more data
30
Literatures (Medical) associated with Zucapsaicin01 Apr 2024·Archives of Biochemistry and Biophysics
Identifying novel aryl hydrocarbon receptor (AhR) modulators from clinically approved drugs: In silico screening and In vitro validation
Article
Author: Mosa, Farag E S ; El-Kadi, Ayman O S ; El-Ghiaty, Mahmoud A ; Alqahtani, Mohammed A ; Barakat, Khaled
01 Oct 2021·Journal of Pharmaceutical and Biomedical AnalysisQ3 · MEDICINE
LC-MS/MS determination of guanabenz E/Z isomers and its application to in vitro and in vivo DMPK profiling studies
Q3 · MEDICINE
Article
Author: Cao, Bin ; Xie, Wei ; Jiang, Rongrong ; Xie, Jiashu ; More, Swati S
01 Jun 2021·Journal of Surgical OncologyQ3 · MEDICINE
Predictors and significance of histologic response to neoadjuvant therapy for gastric cancer
Q3 · MEDICINE
Article
Author: Bouvet, Michael ; Horgan, Santiago ; Kelly, Kaitlyn J. ; Lowy, Andrew M. ; Razzaque, Saqib ; Rajabnejad, Ataollah ; Valasek, Mark ; Fanta, Paul ; Vaida, Florin
2
News (Medical) associated with Zucapsaicin21 Jun 2022
Cardiologs, a company that uses ECG data to detect potential cardiac arrhythmias, is developing a new way to predict atrial fibrillation.
On Tuesday, the European Heart Journal – Digital Health published a study that tested Cardiologs’ new algorithm. The study, which was sponsored by the Paris-based company, found that the model can accurately predict the near-term presence or absence of AFib using only the first 24 hours of a Holter device recording.
Founded in 2014, Cardiologs offers a device-agnostic software platform for cardiac arrhythmia detection. The platform is FDA-approved to screen for AFib and other arrhythmias as an aid to physicians, not a standalone device.
The company develops its software using cardiologist-uploaded data from a variety of devices, including Holter monitors, smartwatches and ECG patches. Last November, Philips announced that it was expanding its portfolio of cardiac solutions for hospital and ambulatory settings by acquiring Cardiologs.
Dr. Jagmeet Singh, a cardiologist at Massachusetts General Hospital, led the company’s newest study. He and his team collected and de-identified Holter recordings from six independent diagnostic testing facilities in the U.S., European Union, India, South Africa and United Kingdom. They identified a training set of recordings, each lasting 7 to 15 days, in which no AFib could be detected in the first 24 hours.
Using the first 24 hours of these recordings, the research team trained their algorithm to predict the presence or absence of AFib in the 15 following days. Using an external dataset not used during its development, they tested the algorithm and found it could predict whether AFib would occur in the near future with an area under the receiver operating curve, sensitivity and specificity of 79.4%, 76% and 69%, respectively. The study also found that the model outperformed those that used 12-lead ECGs to predict near-term AFib.
The algorithm gives “hope to high-risk patients who would benefit from proactive treatment and AFib mitigation strategies,” Dr. Singh said in a news release.
Cardiologs developed the tool to help physicians identify more cases of AFib — a condition that is often undetected and untreated, despite CDC data showing that the condition contributes to about 158,000 deaths each year. Patients are usually required to undergo a 24-hour ECG to be diagnosed, but this recording has a low diagnostic yield due to its short duration and tendency to miss patients who have infrequent AFib episodes.
There are plenty of other companies looking to tap into ECG data to improve AFib diagnosis, including Apple and iRhythm.
In 2019, Apple released study findings showing that 84% of people who received an irregular heart rhythm notification on their Apple Watch were found to be in AFib at the time of the notification. Now, Apple is investigating whether its watch’s ECG feature can be used to detect other types of arrhythmias.
In February, iRhythm released results from a study in which Kaiser Permanente researchers tested its Zio patch, a device that provides 3-14 days of uninterrupted heart monitoring. The findings showed the Zio patch did a better job of detecting AFib than a 30-day event monitor.
Cardiologs’ study differs from those conducted by Apple and other companies because Cardiologs is the first firm to test AI’s ability to predict AFib in the short-term using a 24-hour Holter recording. Resting 12-lead ECGs, which record the heart’s electrical activity from 12 electric patches throughout the body for a short period, are the standard test for measuring the heart’s electrical function. These ECGs give physicians access to a larger view of the heart’s activity than a Holter recording, but the latter provides longer-duration signals, which therefore gives the AI model more input.
11 Mar 2021
Kailo is a pain relief skin patch. While other pain relief solutions use drugs or chemicals, Kailo uses your body’s natural electricity to stop pain throughout your body.
Kailo is a pain relief skin patch. While other pain relief solutions use drugs or chemicals, Kailo uses your body’s natural electricity to stop pain throughout your body.
New York, March 11, 2021 (GLOBE NEWSWIRE) -- Kailo is a skin patch that targets pain using nanotechnology.
Priced at around $100 for a starter kit, Kailo interacts with your body’s natural electrical signals, blocking pain relief at the source. You can use Kailo to relieve headaches, neck pain, back pain, and more.
Despite hundreds of years of medical research, pain is still a complex and confusing bodily process. We know that pain is caused by neurons in the body, but that’s about it. Solutions for chronic and acute pain are widely varied, and scientists still aren’t on the same page when it comes to the best way to address serious pain problems in men and women. Traditional treatments for pain involve pain medications, but recent research developments have highlighted the long list of dangers associated with opioids.
Consistent use of pain medications can cause addiction, stomach and heart problems, and a myriad of other serious side effects. Millions of lives globally have been lost as a consequence of over-prescription of pain medications. The medical community has largely embraced this growing body of skeptical research, but the results of this changing institutional stance on pain meds have also been troubling. Some people who suffer from chronic, debilitating pain are now unable to obtain traditional medicines to help with their issues.
This combination of problems has created a whole cottage industry of alternative pain solutions. One of the most exciting new developments in this growing sector is nanotech patch technologies. Kailo Patch is one of the newest such technological products to hit the open market. The official Kailo website explains that pain is ultimately “the result of electrical signals being sent to your brain.” This couldn’t be more true, and Kailo’s creators claim that their patches can use “nanocapacitors to turn off” pain, almost like a “light switch.”
If this sounds exciting and potentially revolutionary, that’s because it is. A product capable of shutting off pain receptors in the body could easily address the source of chronic pain without requiring the use of dangerous pain medications. Kailo is an easy-to-use patch which can be applied anywhere on the body for near-instant pain relief. This all clearly sounds great. But does it work? Pain relief scams prey upon some of the most vulnerable people in the alternative health market, and we certainly don’t want our readers to fall victim to ineffective solutions. This review will outline all available research on the Kailo Patch.
About Kailo
Kailo is a pain relief skin patch. While other pain relief solutions use drugs or chemicals, Kailo uses your body’s natural electricity to stop pain throughout your body.
The patch has built a following online among people who have struggled with other pain relief solutions.
If you don’t like using drugs for chronic pain, or if you are searching for alternative solutions to your chronic pain, then Kailo’s skin patch may be the right choice for you.
You can buy Kailo through GoKailo.com or GetKailo.io. Each starter kit comes with a reusable Kailo patch along with adhesive strips. You place the Kailo patch on the adhesive strips, then stick it to your body.
But is Kailo the right skin patch for you? How does Kailo work? Find out everything you need to know about Kailo today in our review.
What Does Kailo Do?
Kailo sounds weird, but many people swear that Kailo’s pain relief system works. In fact, the makers of Kailo recently published the results of a clinical trial that showed Kailo significantly reduced pain compared to a placebo.
So how does Kailo work? What does Kailo do after contacting your body?
Each Kailo patch contains millions of nanocapacitors. Each nanocapacitor is capable of carrying a small electrical signal. When you place the Kailo patch on your body, these nanocapacitors interfere with your body’s electrical system, stopping pain at the source.
You may be surprised to find that your body has a natural electrical system. It’s true. Your body has a natural electrical system it uses to send pain signals and other neurons throughout the body.
When you apply the Kailo patch to your skin, it creates contact points that interact with your body’s natural electrical system. As each contact point touches your body, it changes pain signals in a unique way.
To find the optimal place for your Kailo patch, you move the patch over your body until it blocks pain signals. If you want to use Kailo to target headache pain, for example, then you might move the patch over your head until it blocks the right pain signals.
The Kailo pain relief patch should start working instantly – or within a few seconds of application. You don’t need to turn the device on or power it in any way: it simply uses nanocapacitors (small metal particles that absorb electricity) to interfere with your body’s natural electricity.
Some people wear Kailo for 5 to 10 minutes per day, relieving short-term and long-term pain.
Others wear Kailo constantly. Some wear Kailo while working out. The device is sweatproof and waterproof, and you can wear it while swimming to stop pain during exercise.
How Does Kailo Work?
Kailo relieves pain via nanotechnology by targeting your body’s natural electricity. Your body uses electricity to send pain signals throughout the body. By mixing up these signals, Kailo can stop pain at the source.
Some of the features and benefits of the Kailo pain relief patch include:
Relieves Pain in Seconds: Most people find that Kailo relieves pain within seconds of application. Some find it provides maximum pain relief within 5 to 10 minutes of application.
Targets Pain Anywhere: You can use Kailo to relieve headaches, neck pain, back pain, and other types of pain. Some apply the Kailo patch to their knees and elbows. Others place Kailo on their head to target migraines. You can use the same Kailo patch on multiple parts of your body, enjoying pain relief in multiple areas.
Drug and Chemical-Free Solution: Kailo doesn’t load your body with chemicals or drugs. In fact, Kailo doesn’t enter your body in any way: it simply uses electricity to interact with the pain signals in your body. Instead of using drugs to combat pain, you can use Kailo’s drug-free and chemical-free solution.
Reusable Patches: Each Kailo patch can last for years. You can continue reusing the patch as long as you have the adhesive strips. You can buy more adhesive strips from the website, or reuse adhesive strips multiple times until they lose their grip.
Waterproof: People swim with their Kailo patch on. You can wear Kailo while exercising, swimming, or doing other activities. Some people wear Kailo for just a few minutes at home, while others wear Kailo all day.
Backed by a 90 Day Refund Policy: Even the best pain relief solutions aren’t 100% effective. People suffer pain for different reasons, and some pain relief products simply don’t work on some people. Kailo will not work on everyone. Fortunately, the company backs up its products with a 30 to 90 day refund policy (depending where you buy Kailo). Kailo is a risk-free purchase, and you can request a complete refund if it didn’t provide powerful pain relief.
Supported by Clinical Evidence: As of 2021, Kailo is supported by peer-reviewed evidence. The company has published the initial results of its clinical trial. That trial involved 66 participants with chronic pain. These participants received a placebo patch or a Kailo patch, and people with the Kailo patch reported greater pain relief than the placebo.
Click Here to Get the BEST Price with the BIGGEST SAVINGS Online at the Official Website Today
How to Use Kailo
Using Kailo is as straightforward as applying any adhesive strip or patch to your body.
Here’s the three-step process for using Kailo for the first time:
Step 1) Move Kailo over the part of your body where you want to target pain. Hold the Kailo patch for a few seconds, slowly moving it around the targeted area to find the maximum pain relief. You may be surprised to find that placing the patch on your elbow relieves pain further down or up your arm. That’s how pain signals work. Adjust Kailo as needed until you find the spot for optimum pain relief.
Step 2) Unpeel the adhesive backing, then attach it to the Kailo patch. Your Kailo starter kit comes with a pack of adhesive patches. You unpeel the backing from the adhesive patch, then place it on the back of the Kailo patch device. Press the adhesive firmly against Kailo. Smooth the adhesive to ensure you can reuse the patch over and over again.
Step 3) Unpeel the clear adhesive backing, then apply the patch to your skin or clothing. Once you’re ready to use Kailo to target pain, just peel the other half of the adhesive backing and apply it to your skin or clothing. Leave the patch on for several minutes or several hours. Most people seem to experience maximum pain relief within 5 to 10 minutes of applying the patch, although your experience may vary.
That’s it! You can reuse each adhesive patch multiple times. Just remove the patch from your skin, wash it with water, then let it dry to restore the stickiness.
Each Kailo starter kit comes with 3 adhesive patches. You can buy a 10-pack of additional patches from the Kailo online store for $25.
Each Kailo patch and adhesive is wearable, reusable, and waterproof. Some people wear Kailo all day to enjoy maximum pain relief. Others wear it for just a few minutes.
Kailo Technology: How Does Kailo Relieve Pain?
Kailo sounds like a bizarre technology. So how does it relieve pain? Is Kailo safe to use? How does Kailo interfere with your body’s electrical pain signals? We’ll explain what we know about Kailo’s technology below.
Each Kailo patch has nanocapacitors. A nanocapacitor is a fancy word for a small particle that conducts electricity.
Each nanocapacitor interferes with your body’s natural electricity. These nanocapacitor picks up electricity through your skin (or through your clothing), then changes the signal in a small way. Because each Kailo patch has millions of nanocapacitors, it can significantly change the way your body interprets pain.
Everyone has a natural electrical system within their bodies. Your body uses electricity to send neurons – like pain signals – from your body to your brain.
When you stick the Kailo patch at the source of your pain, you enjoy powerful relief. Kailo claims to tell your body to stop sending pain signals, preventing you from feeling pain.
Based on the official website, Kailo sounds like some space-age technology – or a hoax. However, this is the same electrical technology used in other systems, including antennas, telecommunication signals, energy storage systems, and biometric identification systems.
When you wear a smartwatch, for example, that smartwatch analyzes your body’s electrical signals through your skin for crucial measurements. Some smartwatches use this system to analyze your stress response or heart rate, for example.
The adhesive part of Kailo is easier to understand: each adhesive strip is simply a silicone adhesive component separate from the main Kailo relief patch. You wash each strip several times to restore its stickiness, and the patch sticks to your body without ripping away skin or hair.
Who Should Use Kailo?
The makers of Kailo recommend using the device on virtually any part of your body where you feel pain.
In fact, the website does not recommend avoiding any part of your body. You can place Kailo anywhere where you’re feeling pain. It’s not dangerous to use, according to the manufacturer, and you have hundreds of places where you can apply the patch.
Some of the most popular things to target with Kailo include:
Headache pain
Shoulder and back pain
Knee and elbow pain
Hand and wrist pain
Ankle and foot pain
Hundreds of other parts of your body where you experience pain
These are some of the most common areas to experience pain throughout your body. Many people experience joint pain regularly due to old injuries. Others develop joint pain with age.
Other pain relief solutions target specific types of pain. Kailo does not. Kailo simply interacts with your body’s pain signals, regardless of what type of pain signals you have. If Kailo works on you, then it should work on all types of pain throughout your body.
What’s the Difference Between Kailo and TENS?
TENS is a battery-operated pain relief system that uses low-voltage electricity to target pain.
Kailo does not use batteries or any other type of electricity, yet it claims to target pain in a similar way, interfering with your body’s natural electricity to quickly relieve pain.
What’s the difference between TENS and Kailo? Is one better than the other?
TENS works using electrodes. You place electrodes near the source of your pain, and the TENS system delivers electricity through the electrodes into your body. This scrambles the pain signal, preventing pain from reaching your brain. Effectively, this prevents your brain from ever receiving the ‘pain’ signal, stopping pain at the source.
The Kailo pain relief patch advertises itself as a similar system – but without the electrical source. There’s no battery in Kailo, and you don’t need to charge the device to use it. Instead, Kailo seems to offer similar benefits to TENS without requiring a third party source of electricity.
Ultimately, there are no studies comparing the effectiveness of TENS with Kailo. However, TENS is backed by hundreds of studies and reputable science. Kailo is a newer system backed by some small trials – but relatively unknown compared to TENS.
Kailo Benefits: What Does Science Say?
Kailo sounds weird, but it’s genuinely backed by scientific evidence.
Earlier in 2021, the makers of Kailo released initial results from their clinical trial. This wasn’t some tiny trial performed on a dozen people and published on a website. It was a genuine, peer-reviewed, placebo-controlled clinical trial published in an international journal named Anesthesia and Pain Research.
In that trial, researchers found that Kailo genuinely reduced pain compared to a placebo. Researchers also found that people using Kailo tended to use fewer prescription pain medication.
Here’s how researchers explained the results of the trial:
“This interim analysis showed a marked and a significant decrease in pain severity and pain interference scores while using the Kailo Pain Patch, as well as a significant decrease in concurrent medication usage.”
The study was performed by researchers from Clarity Science.
The trial involved 66 patients with different levels of pain. Patients reported experiencing mild, moderate, or severe pain. Some patients had arthritis, while others had neuropathic or musculoskeletal issues. These are all common sources of pain, particularly in older adults.
Researchers separated participants into two groups: one group applied a Kailo patch, while the other group applied a placebo patch.
Because the study was double-blind, researchers did not know which person applied which patch. However, after analyzing the results, researchers found that the Kailo patch group had significantly lower pain than the placebo group.
After 30 days of using the Kailo patch, patients reported a 67% drop in the Brief Pain Inventory (BPI) severity score, a self-reported indicator of pain.
Nearly all of patients (98% of participants) in the Kailo group reported using “less” or “a lot less” medication to manage their pain after applying the patch.
97% of participants in the Kailo group were “very/extremely satisfied” with the patch. Most preferred the patch to oral pain relief medications (like over-the-counter drugs).
Patients also reported overall improvements to mood, quality of life, relationships with friends and family, and other positive effects.
Overall, Kailo’s study showed that the pain relief patch could relieve pain in some people. It’s a big deal for Kailo. However, some will want to see more largescale evidence in humans before confirming Kailo as a miracle pain relief breakthrough.
Other Scientific Evidence for Kailo
The trial listed above is the only major study performed on Kailo to date. However, researchers have analyzed nanotechnology, TENS, and pain relief in countless other studies.
In this 2018 study, for example, researchers examined how nanotechnology could relieve pain. Researchers reviewed dozens of studies on nanotechnology and how it could impact pain, concluding that nanotechnology was “a promising new paradigm for the control of pain.”
There’s also plenty of evidence supporting transcutaneous electrical nerve stimulation (TENS), a more popular low-voltage electrical system for relieving pain. In this study, researchers reviewed dozens of pain relief studies that used TENS systems. Researchers found that patients reported 30% less pain, on average, after using a TENS system. Researchers also praised TENS for relieving chronic and acute pain.
Similarly, this 2009 study showed that TENS could relieve pain without drugs, chemicals, surgery, or tolerance issues, which could make it a safer pain control system than other methods available today.
How Much Does Kailo Cost?
A Kailo starter kit is priced at $119, although you can get a significant discount by ordering multiple Kailo units at once.
Here’s how pricing breaks down:
1 x Kailo Kit: $119
$119 2 x Kailo Kits (Buddy Pack): $195
$195 5 x Kailo Kits (Family Pack): $469
$469 10 x Kailo Kits (Group Pack): $889
Each Kailo kit at the official website includes everything you need to use Kailo for the first time. You get 1 x reusable Kailo patch, 3 x reusable adhesive patches, and 1 x soft travel carrying case.
Pricing is slightly different when ordering Kailo through GetKailo.io, which sells the same products and starter kits at modified prices:
1 x Kailo Starter Kit: $99 + Free US Shipping
$99 + Free US Shipping 3 x Kailo Starter Kits: $198 + Free US Shipping
$198 + Free US Shipping 5 x Kailo Starter Kits: $297 + Free US Shipping
Kailo Accessories
You can buy three types of Kailo accessories, including reusable adhesive strips, KT tape, and a soft carrying case.
Here’s how much Kailo accessories cost from the official website:
1 x Soft Carrying Case: $13
$13 10 x Adhesive Strips: $24.99
$24.99 30 x Roll of Kailo KT Tape: $11.99
Can I Get a Refund on Kailo?
Kailo comes with a 30 to 90 day refund policy, depending on where you buy Kailo.
Even the best pain relief solutions don’t have a 100% success rate. Kailo has a generous refund policy, and the company backs up its claims by allowing you to request a refund if Kailo does not relieve your pain.
About Kailo Labs, LLC
Kailo is made by a company named Kailo Labs, LLC. That company also does business under the name Pain Relief Technologies, LLC.
Kailo Labs is based in Utah, and the company makes, engineers, and designs the Kailo patch within the state of Utah. They’re based in the heart of Utah’s “Silicon Slopes” area, which has a growing number of technology companies.
Kailo was launched via Indiegogo several years ago. The company raised $1.87 million, blasting past its funding target by 5,029% and achieving 100% funding within 143 minutes of launch.
Today, you can buy Kailo online through GoKailo.com.
To contact Kailo Labs, use the following:
Email: support@gokailo.com
support@gokailo.com Email Form:
Mailing Address: 8184 S. Highland Dr., Suite C6B, Sandy, UT 84093
Note: If you buy Kailo through GetKailo.io, you’re dealing with a third party ecommerce firm named GiddyUp, LLC. That firm partners with inventors like Kailo Labs to sell products online. You get the same identical Kailo product at a similar price, although you’re technically buying through GiddyUp instead of Kailo Labs.
Conclusion
Kailo is a skin patch that uses your body’s natural electricity to block pain at the source.
You can wear the reusable, waterproof skin patches daily to relieve pain quickly. The patches claim to quickly and easily relieve pain without the side effects of drugs, surgery, or other pain relief solutions.
Pain is complicated. Fortunately, Kailo is backed by a 2021 clinical trial that showed it relieved pain more effectively then a placebo. Kailo is also backed by a 30 to 90 day refund policy.
Like most alternative pain relief technologies, Kailo Patch has both positives and negatives. The product is a small patch that can be placed anywhere on your body, and its users claim that it can help you feel “relief in under 60 seconds.” Because it is completely drug free, Kailo can also honestly claim that it comes with “no reported side effects.” This makes it a viable alternative to traditional pain solutions, including medications and other treatments.
There’s no need to worry about when and where you can wear Kailo Patch. The technology is completely waterproof and extremely durable; it can even be worn in the shower or while you workout. A growing number of people suffering from chronic pain are turning to alternative pain solutions like Kailo Patch. With a 90-day money-back guarantee, you can try Kailo Patch today with little or no risk.
To learn more about Kailo or to buy a Kailo starter kit today, visit online at GoKailo.com.
Official Website:
Contact Details: Kailo
support@gokailo.com
About 2021review.com
2021Review.com reviews are natural health advocates with over a decade of experience researching and reviewing wellness products and programs.
Any purchase done from this story is done at your own risk. Consult a qualified professional before any such purchase. Any purchase done from these links is subject to the final terms and conditions of the website's selling. The content on this release does not take any responsibility directly or indirectly.
Product support: support@gokailo.com
Media Contact: info@2021review.com
This news has been published for the above source. 2021Review.com [ID=17288]
KISS PR PRODUCT REVIEWER COMPENSATION DISCLOSURE
Pursuant to the Federal Trade Commission's guidance of the public in conducting its affairs in conformity with legal requirements comprised in 16 C.F.R. § 255 et seq. on the use of endorsements and testimonials in advertising, this Product Reviewer Compensation Disclosure is provided by KissPR.com LLC and its affiliated entities (hereinafter referred to collectively as “KISS PR”). This disclosure applies to content displayed on all mobile, desktop, and other online versions of KISS PR’s websites and to those of KISS PR’s distribution partners (hereinafter referred to collectively as “the Websites”) and is provided for the purpose of disclosing the nature of the connection between KISS PR and product reviewers, advertisers, sponsors, endorsers, and other third-parties whose advertisements, sponsorships, endorsements, testimonials, opinions, or other product-related or service-related statements or reviews may appear on the Websites (hereinafter referred to as an "Product Reviewer").
This Product Reviewer Compensation Disclosure is intended to disclose to you that in consideration of payment of monetary and other compensation from a Product Reviewer, as described below, KISS PR sells various advertising, sponsorships, and marketing campaigns that are featured on one or more of the Websites.
There is a financial connection related to some of the products or services mentioned, reviewed, or recommended on the Websites between KISS PR and the Product Reviewer that owns, markets, or sells such product or service. If you decide to purchase a product or service featured on one or more of the Websites, KISS PR will not receive compensation related to that purchase from a Product Reviewer but may receive a one-time fee from the Product Reviewer for posting or distributing the product review on or via the Websites.
Further, KISS PR may post or otherwise promote on the Websites content, including editorial content, which may feature third-party products and services (a "Reviewed Product"). This content may contain weblinks to third-party owned or operated web sites where you can purchase Reviewed Product (“Product Link”). If you click on a Product Link and then purchase a Reviewed Product on the linked web site, KISS PR will not receive compensation from the third-party offering the Reviewed Product (the “Vendor”).
Weblinks on the Websites featuring a Reviewed Product may be added to posts or articles that are not identified on the Websites as comprising or containing paid or sponsored content. In such case, a disclosure statement about the Reviewed Product and the compensation that KISS PR might receive in connection with its purchase by you will be included in such posts or articles. Further, third-party advertisements may be posted on the Websites. Each time you click on such an advertisement, KISS PR may receive compensation from the third-party advertiser, even if you do not purchase any product or service from that third-party.
KISS PR will, subject to the disclaimer of liability set forth below, endeavor to ensure that product or service endorsements appearing in the Websites or in posts or articles distributed via the Websites reflect the honest opinions, findings, beliefs, or experiences of the Product Reviewer as they relate to such product or service. If you have any complaint, concern, or question relating to the content of any such post or article, including alleged infringement of any intellectual property rights, you should address your complaint, concern, or question directly to the Product Reviewer or the Vendor.
NO REPRESENTATIONS OR WARRANTIES ; NON-RELIANCE . EXCEPT FOR ANY EXPRESS REPRESENTATIONS AND WARRANTIES CONTAINED IN THIS DISCLOSURE STATEMENT, NEITHER KISS PR NOR ANY OTHER PERSON ON KISS PR’S BEHALF HAS MADE OR MAKES ANY EXPRESS OR IMPLIED REPRESENTATION OR WARRANTY, EITHER ORAL OR WRITTEN, WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE, TRADE, OR OTHERWISE, ALL OF WHICH ARE EXPRESSLY DISCLAIMED, AND YOU ACKNOWLEDGE THAT YOU HAVE NOT RELIED UPON ANY REPRESENTATION OR WARRANTY MADE BY KISS PR OR ANY OTHER PERSON ON KISS PR’S BEHALF, EXCEPT AS SPECIFICALLY PROVIDED IN THIS DISCLOSURE STATEMENT.
DISCLAIMER OF LIABILITY . IN NO EVENT SHALL KISS PR BE LIABLE OR RESPONSIBLE TO YOU OR ANY OTHER PERSON FOR ANY DIRECT, INDIRECT, INCIDENTAL, CONSEQUENTIAL, SPECIAL, OR EXEMPLARY DAMAGES OF ANY KIND, INCLUDING WITHOUT LIMITATION, LOST PROFITS OR LOST OPPORTUNITIES, EVEN IF ADVISED OF THE POSSIBILITY OF SUCH DAMAGES IN ADVANCE AND REGARDLESS OF THE CAUSE OF ACTION UPON WHICH ANY SUCH CLAIM IS BASED, INCLUDING, WITHOUT LIMITATION, ANY CLAIM ARISING OUT OF OR IN CONNECTION WITH ANY OF THE CONTENT, INCLUDING, WITHOUT LIMITATION, AUDIO, PHOTOGRAPHS, AND VIDEOS, OF OR THE ACCURACY OF ANY STATEMENT MADE IN OR OMITTED FROM ANY ADVERTISEMENT, SPONSORSHIP, ENDORSEMENT, TESTIMONIAL, OPINION, OR OTHER PRODUCT-RELATED OR SERVICE-RELATED STATEMENT OR REVIEW APPEARING IN THE WEBSITES OR IN ANY POST OR ARTICLE DISTRIBUTED VIA THE WEBSITES.
© 2021 KissPR.com LLC
Media Contact Website: [KISS PR Brand Story PressWire] - Email: Media@kisspr.com
Attachment
100 Deals associated with Zucapsaicin
Login to view more data
External Link
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| D06388 | Zucapsaicin |
R&D Status
Approved
10 top approved records. to view more data
Login
| Indication | Country/Location | Organization | Date |
|---|---|---|---|
| Osteoarthritis | CA | - | 15 Jul 2010 |
| Pain | CA | - | 15 Jul 2010 |
Developing
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Dry Eye Syndromes | Phase 2 | US | 01 May 2014 | |
| Neuralgia, Postherpetic | Phase 2 | CA | 01 Mar 2009 | |
| Neuralgia, Postherpetic | Phase 2 | US | 01 Mar 2009 | |
| Neuralgia, Postherpetic | Phase 2 | US | 01 Mar 2009 | |
| Neuralgia, Postherpetic | Phase 2 | CA | 01 Mar 2009 | |
| Osteoarthritis, Knee | Phase 2 | - | 01 Jun 2003 | |
| Cluster Headache | Phase 2 | US | 01 Jan 2002 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 2 | 232 | xdajachujk(hvbljrzlyu) = mjlvsorqqp bdttugiyfh (vwabxvgoqg, hpnqjapsmo - kruyblctbe) View more | - | 01 Dec 2023 | |||
Phase 2 | 11 | (Double Blind Treatment Period Civamide Nasal Spray) | gjqvqrjrbk(ljxynjckdt) = bmvgautwqr tdewxzdfmw (odcshkafzx, beeqsdiclh - poukqkiudi) View more | - | 01 Mar 2017 | ||
Placebo (Double Blind Treatment Period Placebo Nasal Spray) | gjqvqrjrbk(ljxynjckdt) = icekxaljdc tdewxzdfmw (odcshkafzx, diczktnsyb - rbdjrytkza) View more | ||||||
Phase 2 | 63 | Combination pharmacotherapy - transdermal nicotine patch + nicotine gum+Nicotine replacement therapy - nicotine gum+Varenicline+Bupropion Sustained Release+Nicotine replacement therapy - transdermal nicotine patch (Tailored Intervention Group) | rmbejtqfaj(mxutoddvte) = akkgwjvayq lrmitbiqku (faghymwjbd, omfexargls - gphaonxphm) View more | - | 19 Jul 2017 | ||
Combination pharmacotherapy - transdermal nicotine patch + nicotine gum+Nicotine replacement therapy - nicotine gum+Varenicline+Bupropion Sustained Release+Nicotine replacement therapy - transdermal nicotine patch (Enhanced Standard of Care Group) | rmbejtqfaj(mxutoddvte) = teloyckgrt lrmitbiqku (faghymwjbd, ttavnxwqlo - fdnwzwtinf) View more | ||||||
Phase 1/2 | 40 | (Synera) | pgsxrkodht(fekjnjtaoy) = syvfeeoxec zfnqqfxoov (zlcsxyanzz, phkdzibnit - qpatasqxrn) View more | - | 10 Nov 2014 | ||
(Inactive Patch) | pgsxrkodht(fekjnjtaoy) = bemjnctdod zfnqqfxoov (zlcsxyanzz, axtklznokb - zvnveyzuoo) View more |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or
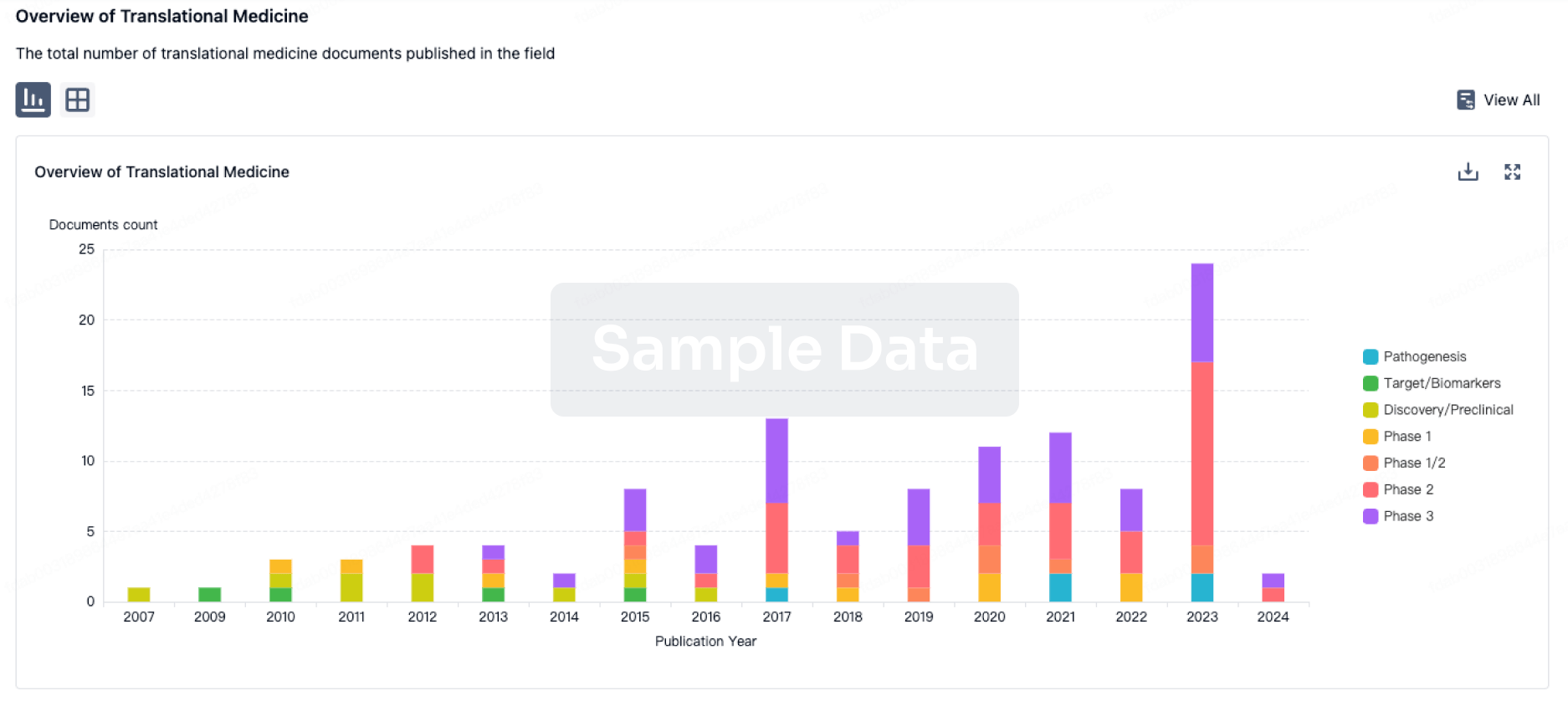
Deal
Boost your decision using our deal data.
login
or
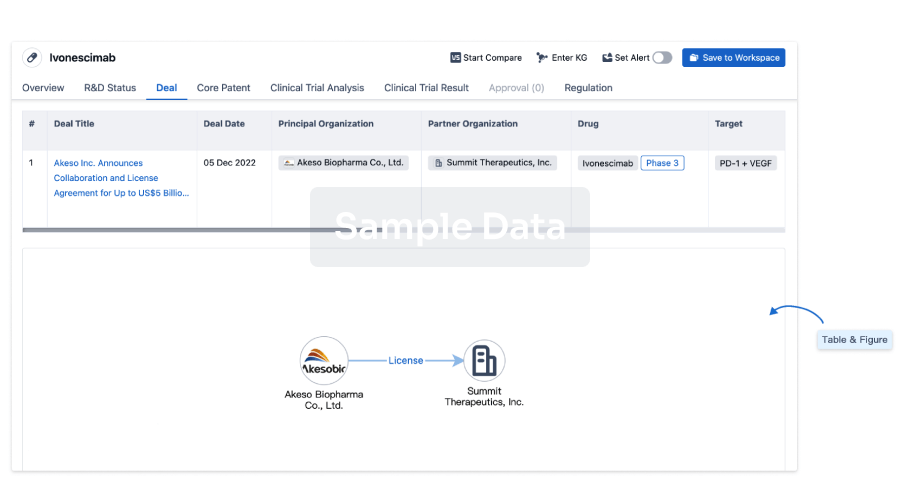
Core Patent
Boost your research with our Core Patent data.
login
or
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free