Hebius terrakarenorum, Hauser & Smits & David, 2022
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5116.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:9BF4E28B-2B86-4141-8E90-5F64AD346B7B |
|
DOI |
https://doi.org/10.5281/zenodo.6366308 |
|
persistent identifier |
https://treatment.plazi.org/id/03F83C50-114D-4D5E-FF74-FF01FDCF0E01 |
|
treatment provided by |
Plazi |
|
scientific name |
Hebius terrakarenorum |
| status |
sp. nov. |
Hebius terrakarenorum sp. nov.
Figs. 2–9 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 , 11A View FIGURE 11 , 12–18 View FIGURE 12 View FIGURE 13 View FIGURE 14 View FIGURE 15 View FIGURE 16 View FIGURE 17 View FIGURE 18
Amphiesma deschauenseei (juvenile), Plate 76 in Cox (1991).
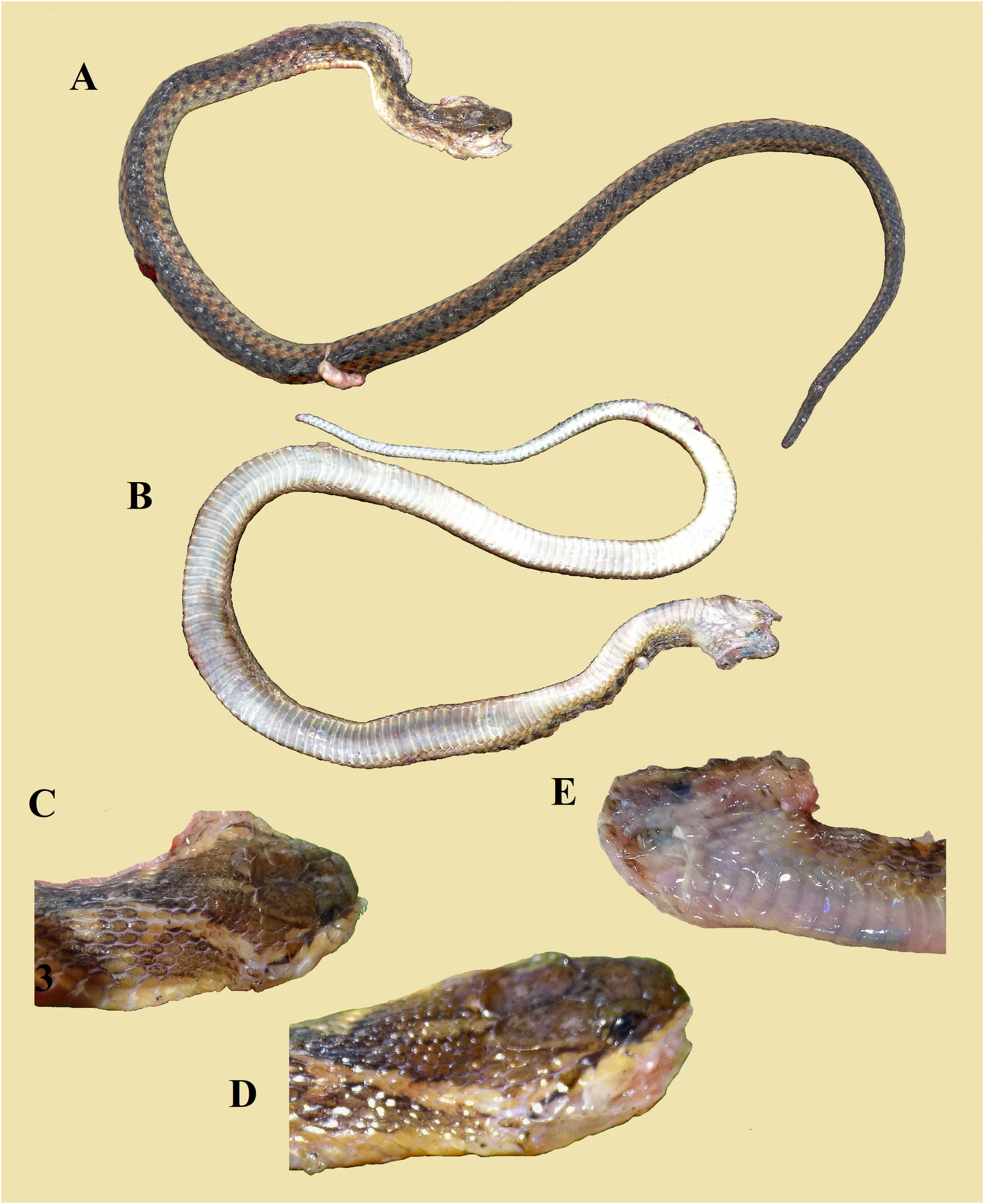
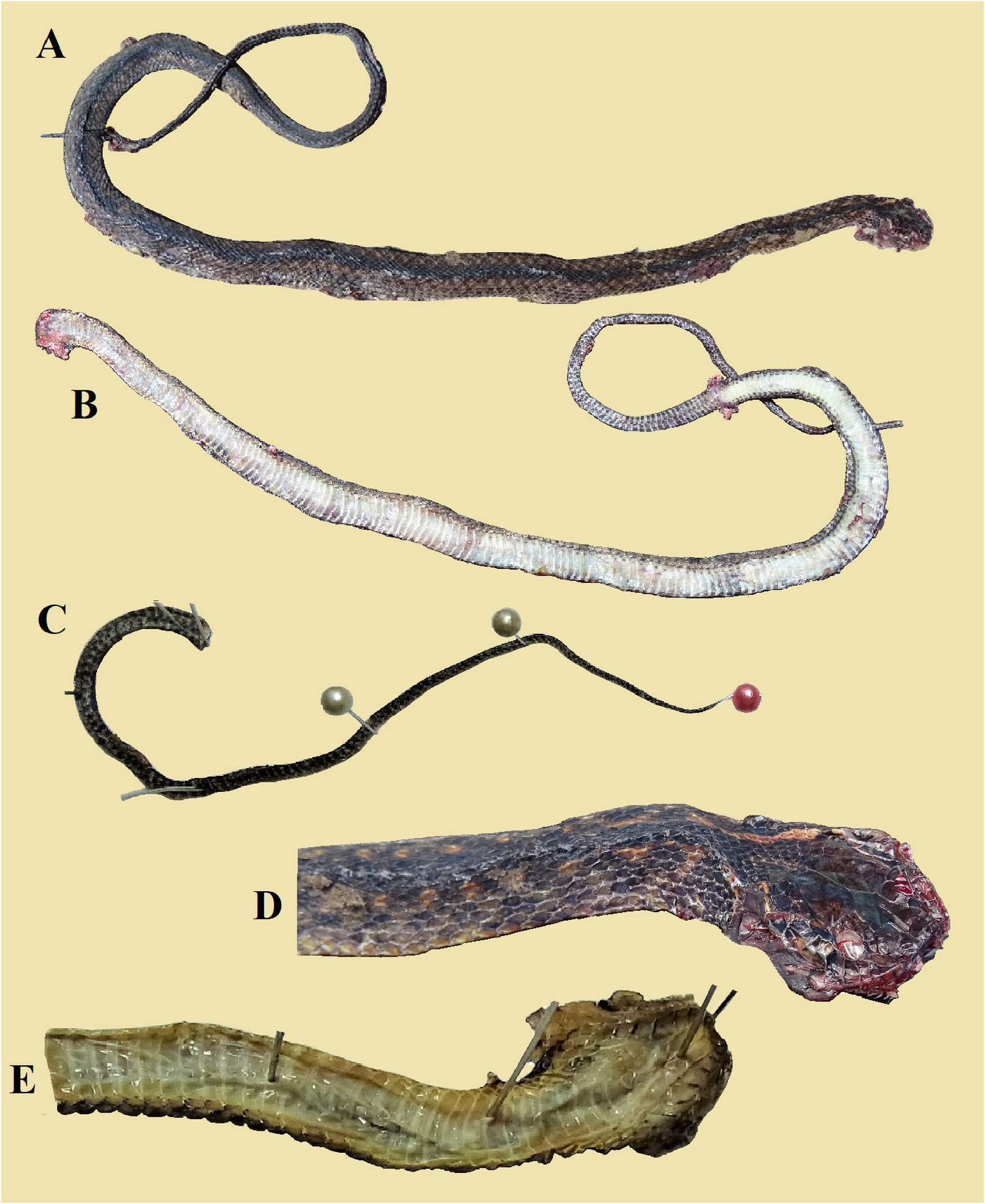
Holotype. QSMI 1686 , an adult male DOR from “Hw 1090, 8.2 km northeast of the bridge over the Mae Klong Khi River at Mae Klong Khi Village” (16.23661°N, 98.96871°E), elevation about 1,209 m, Umphang District , Tak Province, northwestern Thailand. Collected by Sjon Hauser on 17 October 2020. Status. Initially, the holotype was a relatively intact DOR specimen but part of its tail was missing. After complete examination and taking of photographs, the head was cut off and preserved in 95% ethanol, the rest of the body from neck to vent was skinned and the dried skin preserved dry. Parts of the tail and some muscle samples were also preserved in 95% ethanol. GoogleMaps
Paratypes (7 specimens). 1. QSMI 1687 . Male DOR from “Hw 1090, 16.4 km northeast of the bridge over the Mae Klong Khi River at Mae Klong Khi Village”, Umphang District, Tak Province; elevation about 1,200 m. Collected by Sjon Hauser on 5 October 2019 . 2. QSMI 1688 . Male DOR from “Hw 1090, 16.3 km northwest to the bridge over the Mae Klong Khi River at Mae Klong Khi Village”, Umphang District, Tak Province; elevation about 1, 200 m. Collected by Sjon Hauser on 5 October 2019 . 3. QSMI 1689 . Male DOR from “Hw1090 at the village of Rom Klao 4”, Phop Phra District, Tak Province; elevation about 1,100 m. Collected by Sjon Hauser on 3 October 2019 . 4. QSMI 1690 . DOR from “Hw1099 near the Stupa Monument and Viewpoint north of the village of Ban Musoe Pak Thang”, Omkoi District, Chiang Mai Province; elevation about 1,372 m. Collected by Sjon Hauser on 25 June 2019 . 5. QSMI 1691 . DOR from “Hw1099 near the southern entrance to the village of Ban Musoe Pak Thang”, Omkoi District, Chiang Mai Province; elevation about 1,275 m. Collected by Sjon Hauser on 24 June 2019 . 6. QSMI 1692 . Male DOR from “Hw 1090 5.4 km east of police checkpoint near (old) KM 49 marker”, Phop Phra District, Tak Province; elevation about 1,100 m. Collected by Sjon Hauser on 27 October 2018 . 7. QSMI 1693 . Male DOR from “Hw 1090 1.9 km south of Highways Depot, near Umpiam Refugee Camp”, Umphang District, Tak Province; elevation about 1,100m. Collected by Sjon Hauser on 20 October 2018 .
Additional specimens (13). 1. QSMI XX- 13.11.01-21. “Hw 1090, 16.2 km northwest of the bridge over the Mae Klong Khi River at Mae Klong Khi Village”, Umphang District, Tak Province; elevation about 1,200 m. Collected by Sjon Hauser on 1 November 2013 . 2. QSMI 1694 . Living subadult from “south of Mae Wa Luang Village”, Tha Song Yang District, Tak Province; elevation about 750 m. Voucher photographs taken by Ton Smits on 24 September 2018 . 3. QSMI 1695 . DOR from “ Hw 1090, near KM 53 , 4 km east of Police Check Point (near KM 49 ), Phop Phra District, Tak Province; elevation about 1,100 m. Collected by Sjon Hauser on 10 September 2013 . 4. QSMI 1696 . DOR from “Hw 1090 3.5 km south of Highways Depot, near Umpiam Refugee Camp”, Umphang District, Tak Province; elevation about 1,150 m. Collected by Sjon Hauser on 11 September 2013 . 5. QSMI 1697 . DOR from “Hw 1090, 16.2 km northeast of the bridge over the Mae Klong Khi River at Mae Klong Khi Village”, Umphang District, Tak Province; elevation about 1,200 m. Collected by Sjon Hauser on 1 June 2015 . 6. QSMI 1698 . DOR from “Hw 1090 3.2 km south of Highways Depot”, Umphang District, Tak Province; elevation oabout 1,150 m. Collected by Sjon Hauser on 9 July 2016 . 7. SHPC 09.09.17-04. DOR from “Hw 108, 25 km east of Mae Sariang Town”, Mae Sariang District, Mae Hong Son Province; elevation about 1,000 m. Photographs taken by Sjon Hauser on 17 September 2009 . 8. SHPC 11.10.11-14. DOR from “Hw 1090 8 km south of Highway Depot”, Umphang District, Tak Province; elevation about 1,050 m. Voucher photographs taken by Sjon Hauser on 11 October 2011 . 9. SHPC 12.10.13-04. DOR from “Hw1090, 16 km northeast of the bridge over the Mae Klong Khi River at Mae Klong Khi Village”, Umphang District, Tak Province; elevation of about 1,200 m. Collected by Sjon Hauser on 13 October 2012 . 10. SHPC 15.10.21-10. DOR from “a little south of KM 86 , near the Umpiam Refugee Camp”, Umphang District, Tak Province; elevation about 1,150 m. Collected by Sjon Hauser on 21 October 2015 . 11. SHPC 17.09.09-07. DOR from “Hw105, 25 km north of Mae Salit Luang Village”, Tha Song Yang District, Tak Province; elevation about 600 m. Collected by Sjon Hauser on 9 September 2017. 12. QSMI 1701 . Living subadult/juvenile “next to a small stream in Khao Laem National Park, about 500 m from the park HQ,” Sangkhlaburi District, Kanchanaburi Province. Elevation about 300 m. Photographs taken by Ian Dugdale on 24 March 2017 . 13. IDPPA 2021-03 - 09 . Living adult resting on “stone in the middle of a stream, about 500 m north of the HQ, Thong Pha Phum National Park,” Thong Pha Phum District, Kanchanaburi Province. Elevation about 800 m. Photographs taken by Ian Dugdale on 9 March 2021 .
Diagnosis (based on the 21 available specimens). A medium-sized snake of the genus Hebius , defined by the combination of the following characters: (1) TaL/TOTL ratio 0.32–0.36 (n=12); (2) two preoculars; (3) two postoculars (the lower one much smaller than the upper one); (4) usually a single elongate anterior temporal followed by two posterior temporals, the upper one much larger than the lower one; (5) usually 9 supralabials, 5 th and 6 th in contact with eye, 8 th largest; (6) 159–171 ventrals plus 1–2 (rarely 3) preventrals; (7) cloacal plate divided; (8) 107–130 divided subcaudals; (9) dorsal scales in DSR 19-19-17 (rarely 19-17-17), dorsal keeling variable, most often 17 rows (out of 19) distinctly keeled at midbody, the enlarged scales of the 1 st DSR smooth; (10) reduction of dorsal scale rows from 19 to 17 rows by a reduction involving the 3 rd and 4 th DSR, namely 3 rd +4 th → 3 rd DSR on both sides within five successive ventral plates between the 79 th to 102 th ventral scale; (11) supralabials cream to pale brown broadly edged and dusted with dark grey; white blotches absent; (12) venter immaculate pinkish-cream except for the pale salmon corners of the ventral plates edged with grey and an adjacent cloudy grey spot; (13) dorsal coloration pattern variable, most commonly a brown ground color, darker on the top of the dorsum, with a dorsolateral series of some 80 distinct pale ochre-brown to orange-brown rounded blotches on row 6 extending from the neck to the tail with less distinct series of dark blotches below them on row 4 and above them on row 8; (14) a bicolored postocular streak to the corner of the jaw, where a characteristic pale ochre-brown pattern extends to the first pale blotch of the dorsolateral row.
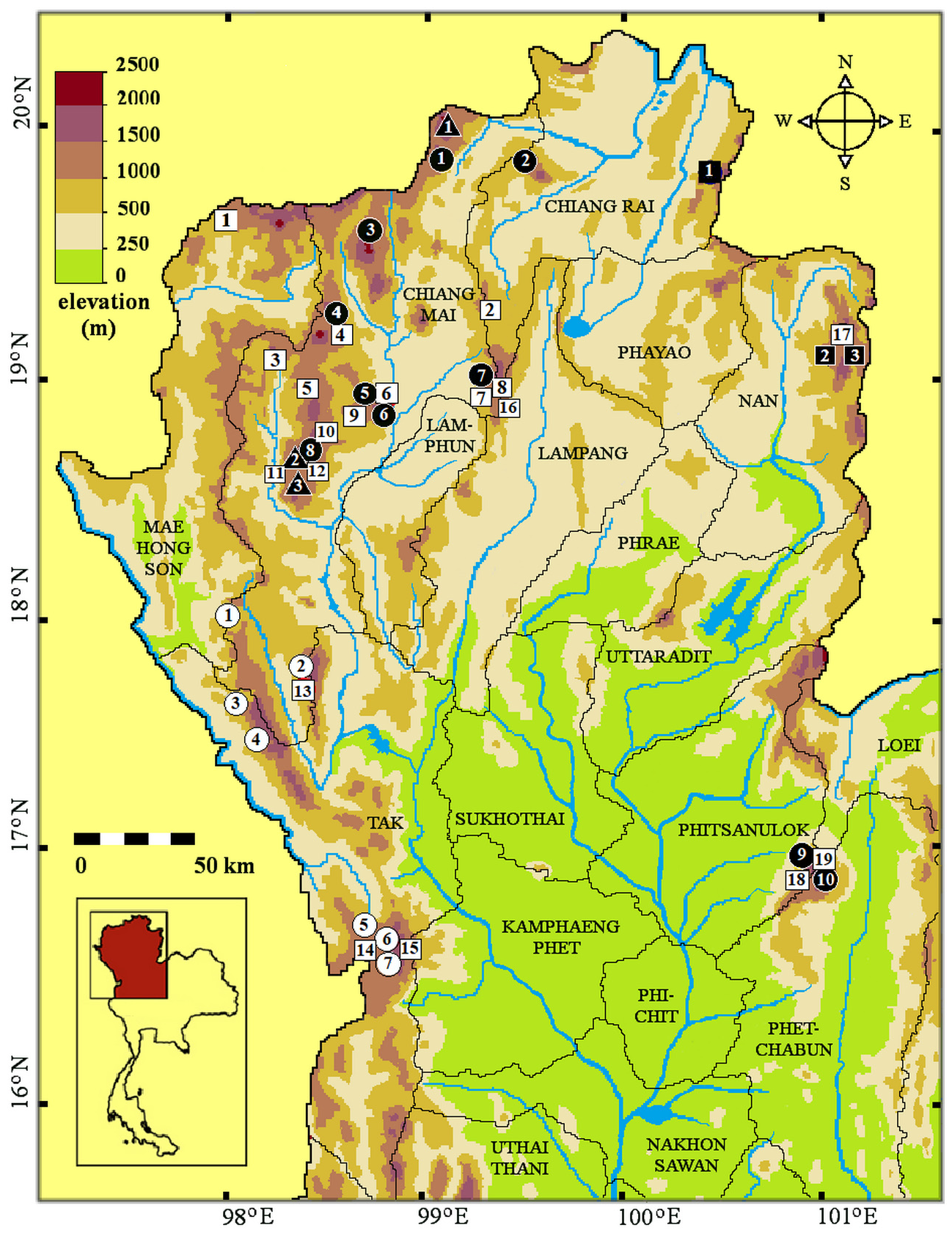
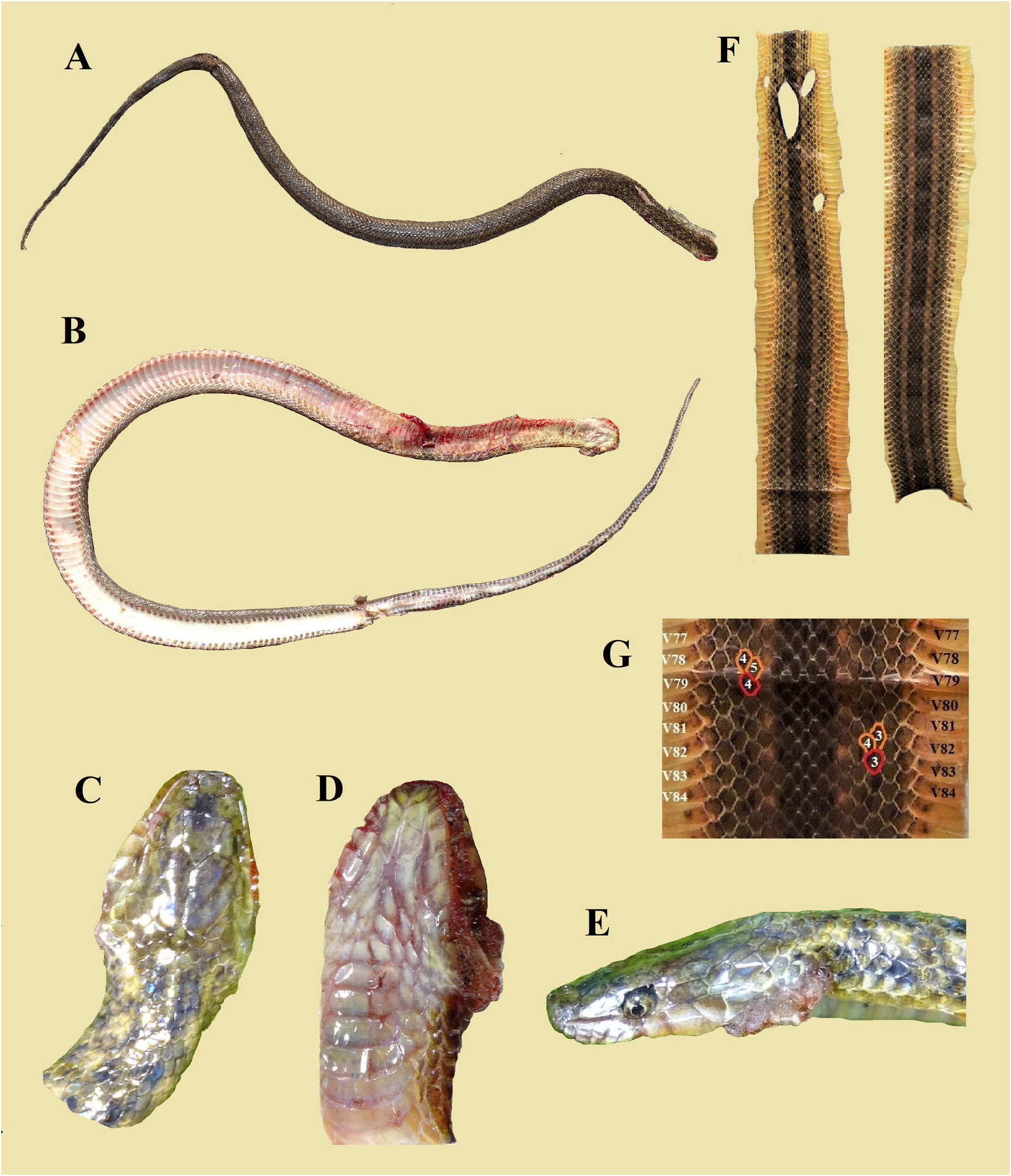
Description of the holotype. General morphology. A male specimen that weighed 31.4 gram, with SVL 425 mm and incomplete tail (TaL 162 mm). Head distinct from the neck; the body is quite round, not laterally compressed. Head length from tip of the snout to the neck (HL1) 21.4 mm; head moderately elongate (5.0% of SVL); HL2 from tip of the snout to posterior edges of the parietal shields 13.4 mm. Snout length (rostral tip to posterior edges of prefrontals) 4.1 mm, 1.8 times as long as horizontal diameter of the eye (of 2.3 mm); nares distinctly directed dorsolaterally on the snout, round, quite large (diameter 2.3 mm), 1.5 times as large as the distance between its lower margin and the margin of the lip (1.5 mm). Eye medium-sized with a round pupil. Tail broken, remaining proximal part cylindrical. Base of tail with distinct keels.
Size. SVL: 425 mm; TaL: not available; HL1: 21.4 mm; R: not available.
Dentition. Not available.
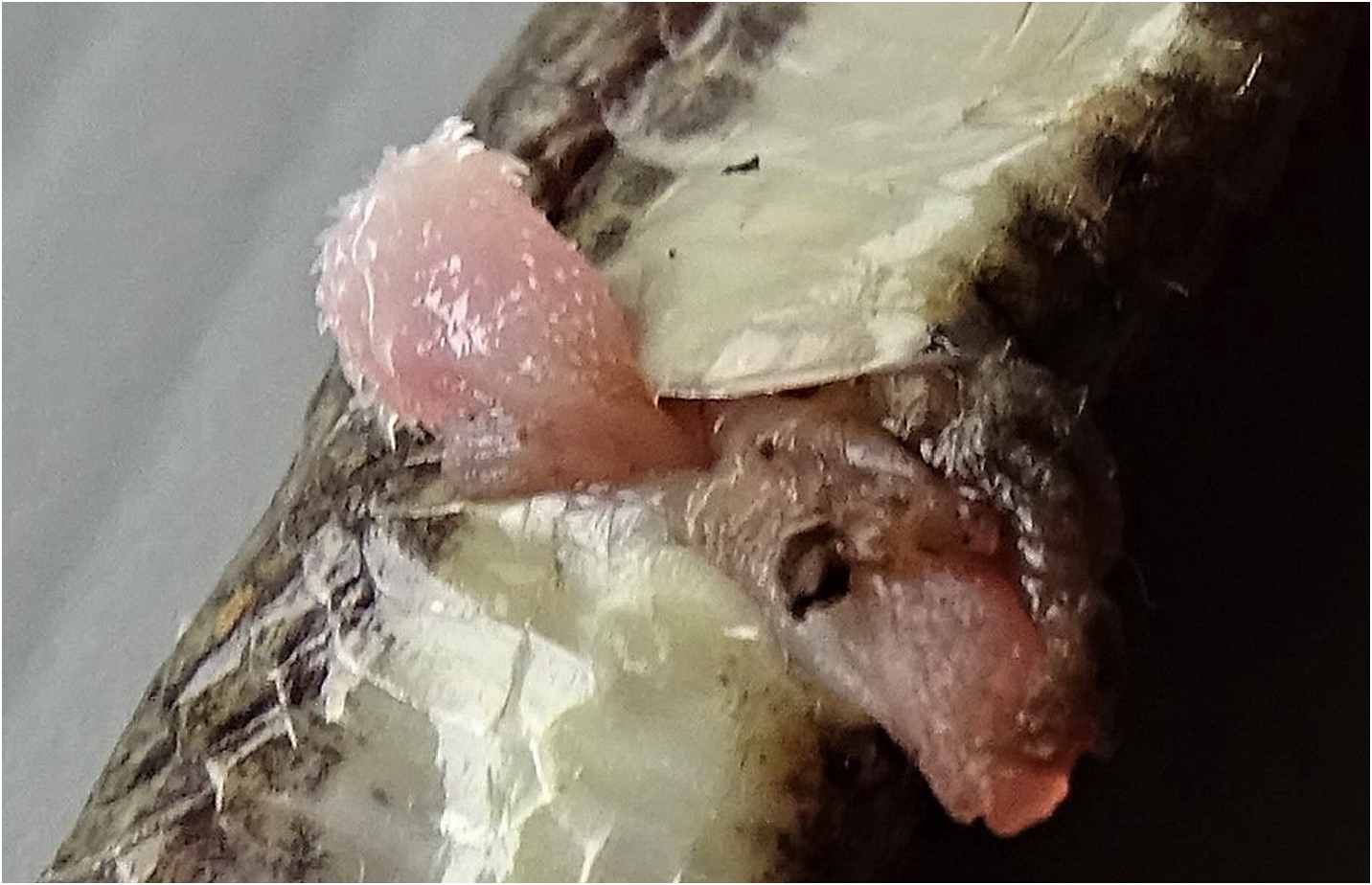
Hemipenes. The everted hemipenes are pinkish-white, short, single, not forked and bulbous; the organ is overall densely covered with tiny, slightly curved spines; spines at the tip of the organ are larger.
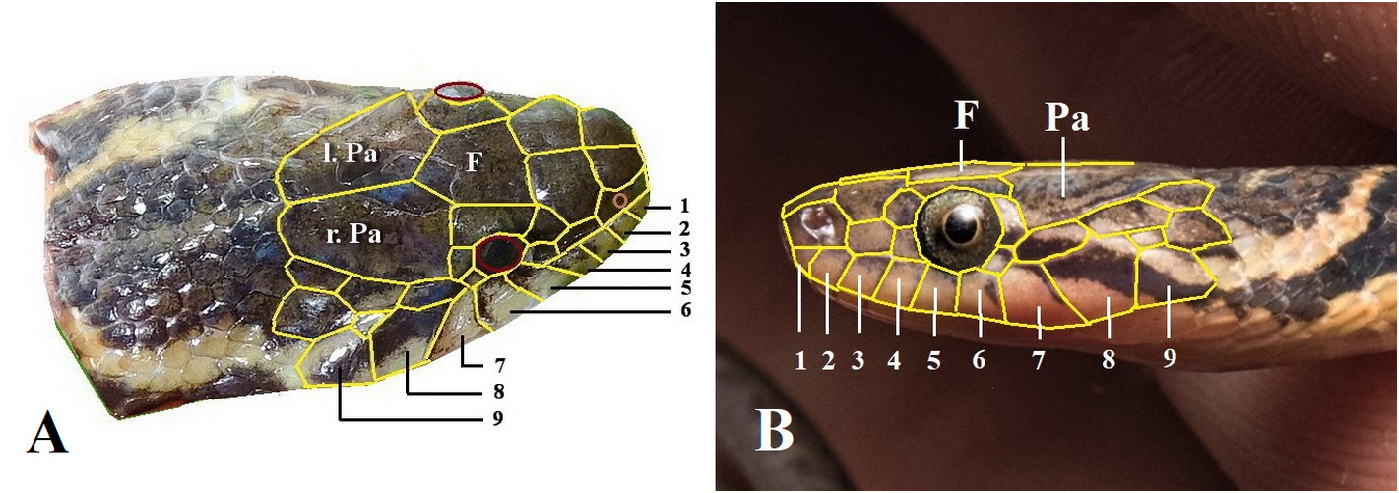
Body scalation. DSR: 19-19-17 scales, scales rhombohedral, not notched at their distal end, with moderately strong keels at midbody, except for the smooth, slightly enlarged scales of the 1 st DSR; vertebral scales not enlarged; scales around the base of the tail distinctly keeled, not strongly keeled; the reduction from 19 to 17 dorsal scale rows takes place between the 95 th and 98 th ventral plate; at level V96 on the right side, the 3 rd scale row fuses with 4 th row fuse to become row 3; and at level V97 on the left side scale row 3 and 4 fuse to become row 3; 168 ventral plates + 2 preventrals; SC not available (tail truncated), all paired; cloacal plate divided.
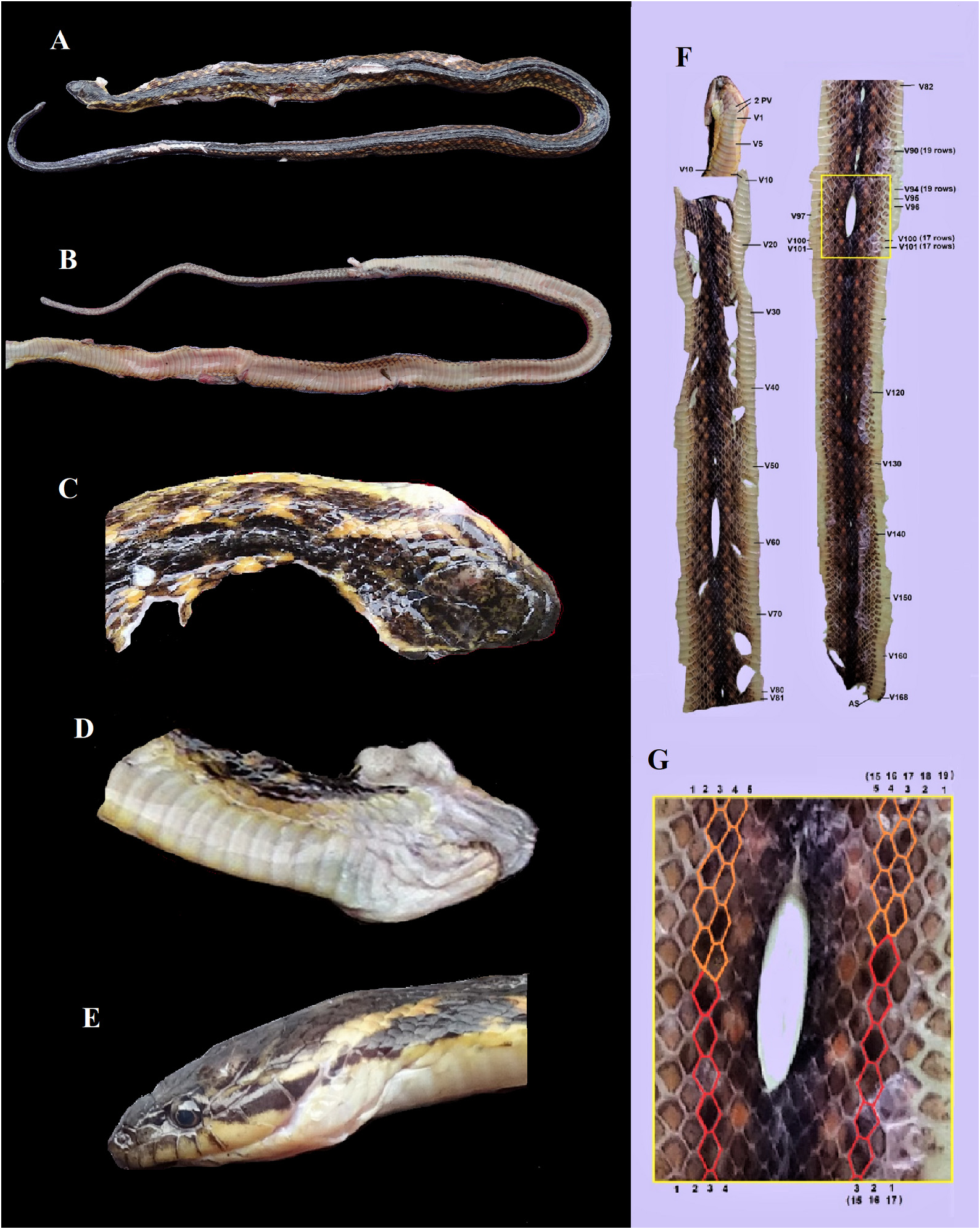
Head scalation. Arrangement of upper head scales complete, including 2 internasals, 2 prefrontals, 2 supraoculars, 1 frontal, and two parietals. Rostral wider than high, not visible from above; nasals subrectangular, distinctly elongate, divided above and below the quite large, central naris; internasals subtriangular, in broad contact with each other, wider than long, narrowing anteriorly; 2 prefrontals, subrectangular, much larger than internasals; frontal hardly longer than wide, longer than prefrontal, the anterior edge perpendicular to the longitudinal axis. Parietals large, suture between them barely longer than the frontal; 2/2 preoculars; 2/2 postoculars, upper one much larger than lower one; left loreal trapezoid, a little longer than high; right loreal rectangular, nearly twice as long as high; 9/9 supralabials, 5 th and 6 th in contact with eye, 7 th and 8 th largest; 5/5 temporals in formula 1 + 2 + 2, anterior temporal elongate, disk-shaped/ flattened hexagonal, upper temporal of second row much larger than lower one (both sides); infralabials much damaged posteriorly on both sides, 1 st to 5 th IL in contact with anterior shin shields; posterior shin shields longer than anterior ones.
Coloration of freshly killed holotype and its dried skin.
Head and neck. The upper side of the head and neck is dark brown with some greyish vermiculation. Supralabials are cream or pale ochre-brown with their edges dark grey and surroundings peppered with grey. The eye is round, quite large, with a round, black pupil with a yellow halo; the iris is pale brown mottled and peppered with black. An oblique, bicolored postocular streak (upper side pale yellowish-brown or yellow-ochre, lower side dark brown) traverses the anterior temporal shield and the 8 th and 9 th supralabials, dividing these shields in a pale- and dark-colored section. At the corner of the upper jaw the dark part of the streak fuses with the dark brown ground color of the neck, the pale ochre part meanders in the characteristic pattern of a curved bar with knobs towards the midline of the neck and fuses with the first, most anterior pale blotch of the dorsolateral row of blotches.
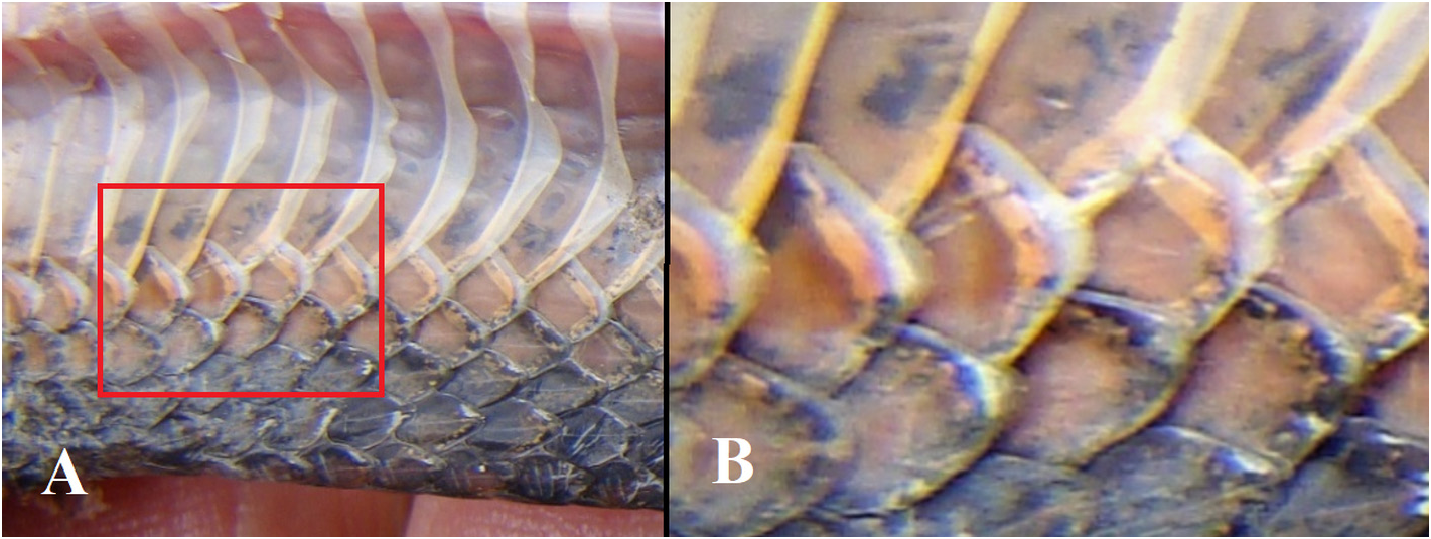
Dorsal side of the body and tail. The dorsum is dark brown in the central nine scale rows, gradually a little lighter greyish-brown in the lateral rows, with the enlarged outermost row distinctly paler, its centre ochre-brown, edged by grey. A dorsolateral series of roundish pale ochre- or orange-brown blotches the size of one scale (plus parts of the surrounding scales) stretches from the neck to the tail on row 6, each spot separated from the next one by one brown scale (see Figs. 3 View FIGURE 3 , 5A View FIGURE 5 ). These dorsolateral blotches are aligned on a diffuse stripe, slightly paler than the dark brown ground color; the blotches are all of similar size and distinct until the very posterior end of the body where they become smaller and indistinct and fade away on the tail; another series of lateral dark brown blotches on dorsal row 4 below each ochre- or orange-brown spot. About 80 blotches are distinct in each row. The upper side of the tail is uniformly dark brown.
Lower side of head and neck, body and tail. Infralabials and chin shields are white to cream. The venter immaculate pinkish-white/cream except for the corners of the ventral plates which are salmon, laterally edged or peppered with grey; there is a cloudy grey spot near the corner. Anteriorly these spots are rather vague, cloudy-amoeboid, posteriorly they are slightly larger, denser, darker and more distinct.
The lower side of the (incomplete) tail is densely peppered with grey, most densely at the lateral sides of the (divided) subcaudals.
Habitat. The holotype was collected on a road traversing low montane evergreen forest. Next to the road tiny streams of rain water run off were hidden in the dense vegetation ( Fig. 10A View FIGURE 10 ). Some 50 m south of the site, a little fast-flowing stream cascades into a pool that drains under the road onto a steep mountain slope on the other side. Around the pool bushes of large, 1–2 m high members of the Zingeriberaceae and Phlogacanthus curviflorus (Wallich), and also stands of bamboo. The forest around the pool is inhabited by numerous other snake species, including Hebius khasiensis, Psammodynastes pulverulentus (H. Boie in F. Boie), Sibynophis collaris (Gray), Lycodon chapaensis (Angel & Bourret) , previously Lycodon septentrionalis (Günther) (see Wang et al. 2021), Pareas hamptoni (Boulenger), Pareas macularius Theobald, Rhabdophis nigrocinctus (Blyth) and Trimeresurus popeiorum Smith.
Description of the paratypes and variation. A summary of morphological and meristic data of the seven paratypes is given in Table 1 View TABLE 1 . For photographs of these paratypes, see Figures 12–18 View FIGURE 12 View FIGURE 13 View FIGURE 14 View FIGURE 15 View FIGURE 16 View FIGURE 17 View FIGURE 18 . Morphological data, often incomplete, of twelve additional, much damaged DORs or living specimens are summarized in Appendix 2.
Variation of the head scalation and coloration of the head
The head scalation of the paratypes is similar to that of the holotype. Differences occur in the number of supralabials, of temporals and the shape of the loreal.
There are always 2 preoculars (on both sides); there are nearly always 2 postoculars (on both sides) but in a few cases (3 out of 18 occurrences) it appeared that the two were fused to one; in a few specimens (3 out of 17) a side of the head has 10 supralabials (vs. 9 in the holotype and in most of the paratypes); the loreal is rectangular, usually longer than high, the ratio length/height varying from 1.2 to 2.0. In one specimen, the loreal is slightly trapezoid-shaped; there is mostly 1 elongated anterior temporal followed by 2 posterior temporals (in 14 out of 17 occurrences), the upper one much larger than the lower one. However, the second row of posterior temporals shows more variation (2, 3 or 4) or damage means the number of shields hard to count accurately.
The supralabials are nearly always edged or peppered with grey, but there is considerable variation in the degree of this pigmentation between the specimens, e.g. sometimes the peppering extends to cover nearly half of the shield. The postocular streak and its characteristic extension towards dorsal row 6 of the neck is distinct and conspicuous in more than half of the specimens, but in other specimens (pale morphs) it was vague or could not be clearly distinguished.
Variation of the dorsal scale row reduction of 19 to 17 rows
The skins of seven specimens were quite complete and the dorsal scale row reductions of from 19 to 17 rows could be determined accurately. In two specimens this reduction was on one or on both sides aberrant in the sense that a reduction was reversed by a division to the original rows, and subsequently two or three scale lengths posteriorly there was again a fusion which resulted in the stable lower number of rows. These reductions and divisions are summarized below.
QSMI 1686 (holotype) left side : V097 - V098 =3+4→3 right side: V096 - V097 =3+4→3
QSMI 1688 (paratype 2) left side : V093 - V094 =3+4→3 right side: V093 - V094 =3+4→3
QSMI 1689 (paratype 3) left side : V079 - V080 =4+5→4 right side: V082 - V083 =3+4→3
QSMI 1690 (paratype 4) left side : V070 - V071 =3+4→3 right side: V073 - V074 =3+4→3 V075 - V076 =3→3+4 V078 - V079 =3+4→3
QSMI 1691 (paratype 5) left side : V102 - V103 =3+4→3 right side: V102 - V103 =3+4→3
QSMI 1693 (paratype 7) left side : V094 - V095 =3+4→3 V095 - V096 =3→4+5 V096 - V097 =4+5→4 right side: V095 - V096 =4+5→4 V096 - V097 =4→4+5 V097 - V098 =4+5→4
QSMI 1698 (additional 7) left side: V089-V090=3+4→3 right side: V088-V089=3+4→3
Variation of the dorsal pattern of coloration
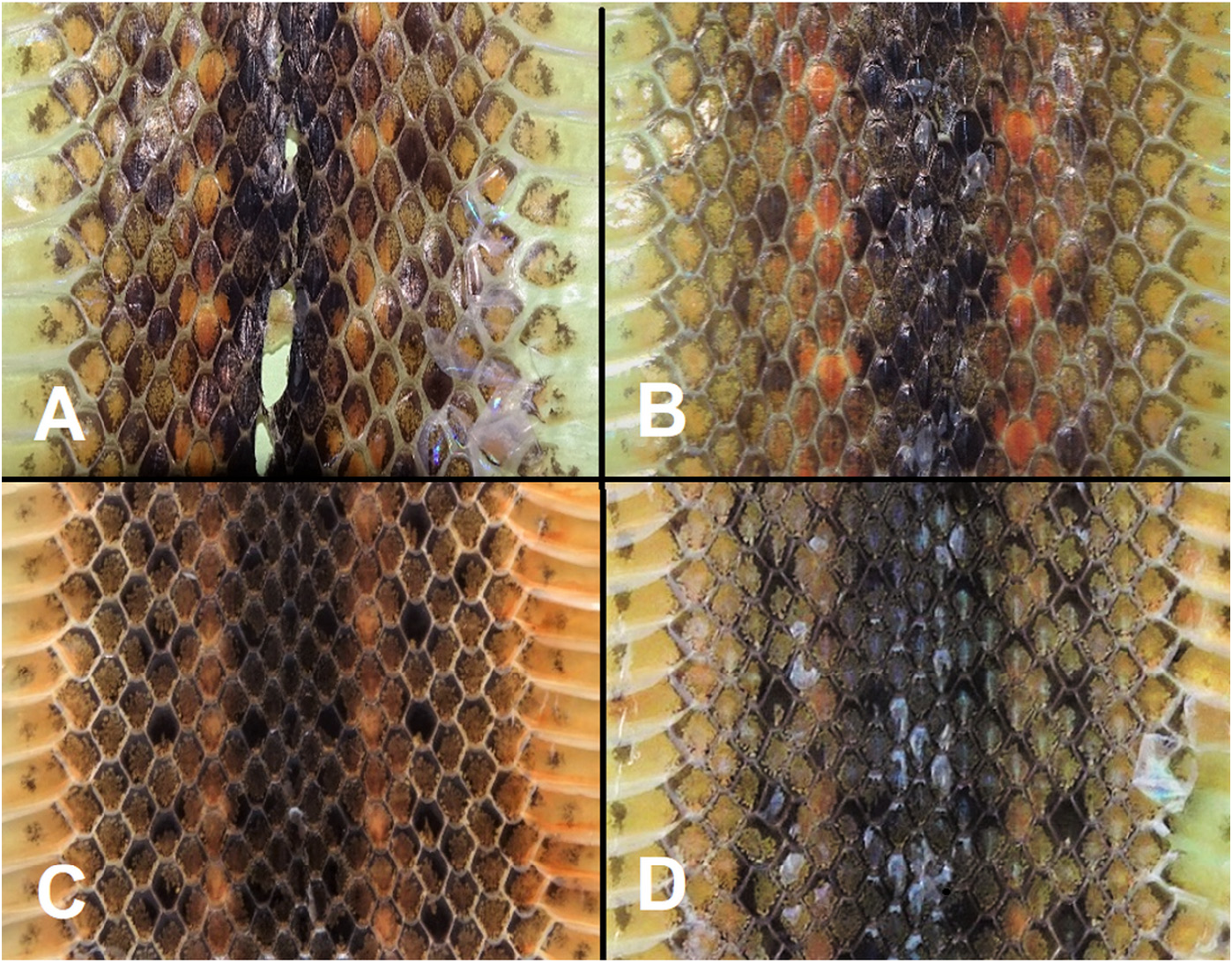
The dorsolateral row of pale ochre/orange blotches on the 6 th DSR is by far the most conspicuous pattern in a majority of specimens. This row extends from the neck to the tail without fusing into a stripe, but it is situated on a vague continuous stripe darker than the blotches but lighter than the background color. Adjacent contrasting dark blotches on the 4 th and 8th dorsal scale rows, distinctly darker than the brown ground color, accentuate the pale blotches on both sides. In some specimens, these dark blotches become more conspicuous, while at the same time the pale blotches have faded to a considerable degree (see Fig. 5D View FIGURE 5 of the skin of specimen QSMI 1690 from Omkoi). In other specimens, the overall color is much paler, such as pale greenish bronze-brown, without any conspicuous pale blotches on the paler dorsolateral stripe, which remains clearly visible, and with only numerous, rather inconspicuous darker spots present, as is illustrated in Fig. 6A View FIGURE 6 for specimen QSMI 1693 from Umphang District. In a few specimens, the dorsum is nearly uniformly pale greyish-brown without distinct spots and with only a remnant of the pale dorsolateral stripe, as is illustrated in Fig. 6B View FIGURE 6 for specimen QSMI 1695 from Phop Phra District. In both pale morphs, however, traces of the full-developped coloration pattern of a series of blotches can be seen. In these pale morphs the postocular streak is generally either vague or absent, whereas the underside of the tail is nearly white (vs. dark grey in the more colorful specimens with pronounced blotches). However, in these pale morphs the cloudy spots near the corners of the ventral plates are always distinct.
Coloration pattern of the venter
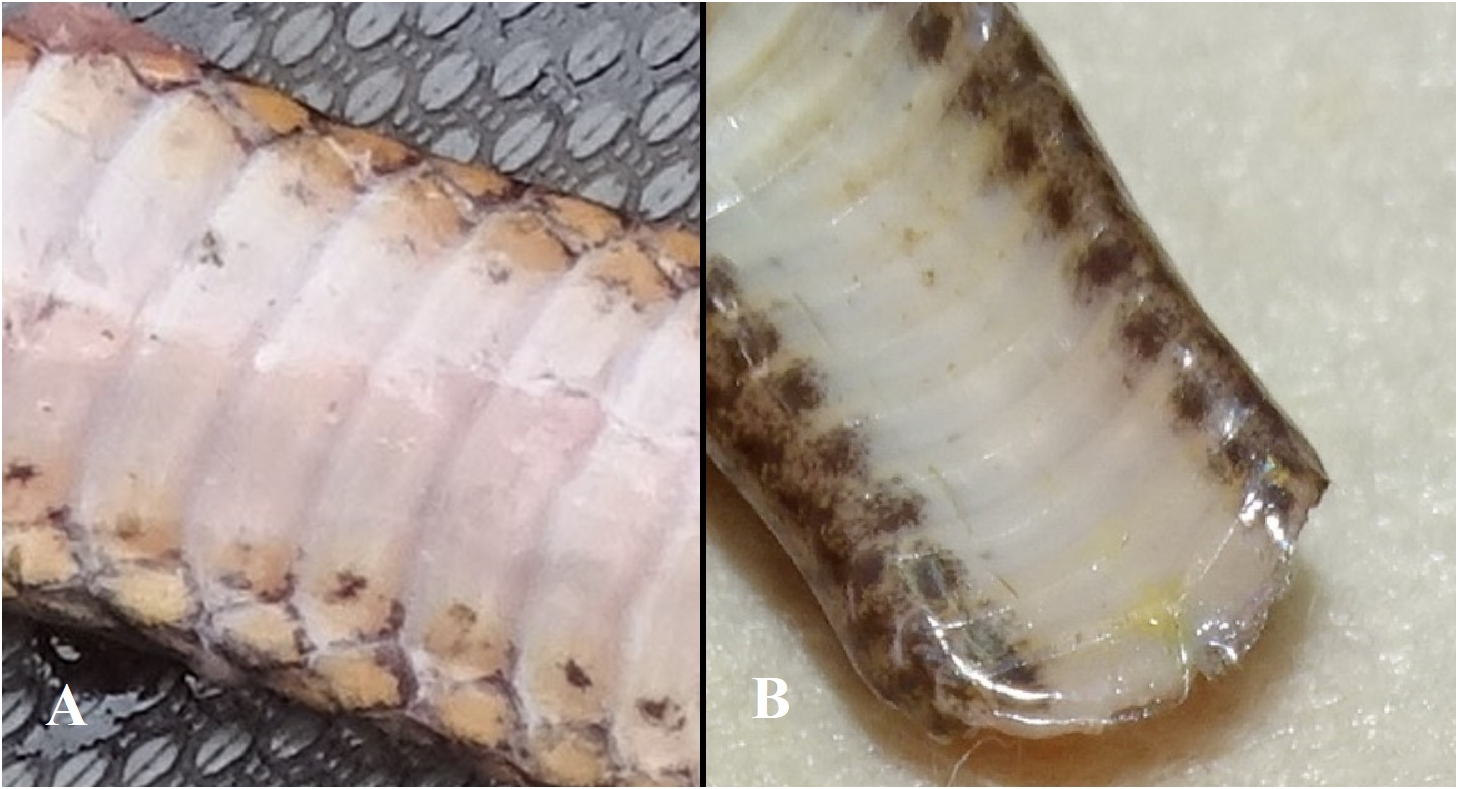
The color of the ventral plates is pinkish-white with the corners of these plates pale salmon. The pale salmon color of the corners usually extends to the first dorsal row (adjacent to the ventral plates). At the border of the pale salmon corner of the ventral plate and their pinkish-white central part is an inconspicuous greyish-brown spot that is cloudy and usually amoeboid-shaped or a vague short streak (see Fig. 7A View FIGURE 7 ). This pattern is best shown anteriorly, whereas posteriorly the pinkish-white background color of the spots may change to a very pale brownish-white and the spots often become darker, denser and more closely set in the corner of the ventral plate, but never intensely dark (see Fig. 7B View FIGURE 7 ).
Salmon pigment accumulations. A peculiar characteristic of the coloration pattern of the species consists of the salmon (pale orange) color covering the corners of the ventral plates and the first row of dorsal scales, sometimes extending to the second row, in the ventrolateral area of the anterior part of the body. This pigmentation is strongly concentrated along the edges of these plates and scales. We recorded these accumulations in a good number of the DOR-specimens, but they are particularly conspicuous in specimen SHPC11.10.11-14 (see Fig. 8A and 8B View FIGURE 8 ).
These pigment accumulations also appear to be present in specimen QSMI 1694, a living individual from Tha Song Yang District, Tak Province.
Hemipenes. We everted specimens of specimens QSMI 1686 and QSMI 1693. They show that the hemipenis of this species is short, single, not forked and bulbous; the proximal part is short (or less prone to be everted following pressure on the base of the tail) with some large basal spines; the distal part is slightly pear-shaped and densely covered with many short, curved spines, their tip pointing downwards in the direction of the vent; among these tiny spines may be seen a number of much larger spines similarly curved and directed downwards; spines at the tip of the organ are larger. The sulcus is divided near the base but this forking is just short and cannot be distinguished more proximally where it seems to “disappear” amidst the spines ( Fig. 9 View FIGURE 9 ). Lips of the sulcus are not prominent.
Etymology. The species nomen terrakarenorum is a modern Latin noun meaning “Land of the Karen”. It refers to the Karen People, the major inhabitants of the mountains throughout the hitherto known range of the new species. This species nomen is a noun at the plural genitive case, not an adjective.
We suggest the colloquial names: Karenland Keelback (English), Hébius du Pays Karen (French) , Karenland Wassernatter (German) and Ngu Lai Sap Thin Kariang (Thai). It is likely that the species also occurs in ‘Karen country’, namely in the mountainous part of eastern Myanmar adjacent to the Thai-Myanmar border, which remains a herpetologically extremely under-explored region.
Distribution and habitat. This species is definitely known from the provinces of Chiang Mai, Mae Hong Son, Tak and Kanchanaburi (see Fig. 1 View FIGURE 1 ). Mae Sariang District, Mae Hong Son Province, is the northernmost locality where we have recorded a specimen. Nevertheless, it is possible that the range extends further northward in Mae La Noi, Khun Yuan and even Mueang districts of Mae Hong Son Province, which still are herpetologically poorly surveyed areas. The type locality of the species is at the edge of the mountains just north to Umphang valley. It is most likely that the species occurs in mountain streams south of that valley. The most southern localities are 120 to 150 km to south in Kanchanaburi Province, in central Thailand, beyond the northern Thai region. A picture of a juvenile Hebius taken by Jarujin Nabhitabhata and identified as H. deschauenseei in Cox (1991) is probably a misidentification and instead is a juvenile H. terrakarenorum sp. nov. This specimen might have originated from Uthai Thani Province (south of Umphang) as Uthai Thani was mentioned both in Cox (1991), Cox et al. (2012) and Chan-ard et al. 2015 (see also David et al. 2021) as part of the range of H. deschauenseei , without reference to a preserved specimen or voucher photographs. To us Uthai Thani Province seems unlikely as home of H. deschauenseei as we have never come across it to the south of Doi Inthanon National Park, in spite of quite extensive field surveys in Chiang Mai’s Omkoi District and in much of Tak Province.
Biology. Nearly all specimens were found next to fast-flowing mountain streams. For examples of three different habitats, see Fig. 10. A View FIGURE 10 majority of the road-killed specimens were male and many of them were collected in October, which suggests that the main mating season might be at the end of the rainy season in October. This might indicate that beyond the mating season these snakes rarely venture beyond the direct surroundings of the streams. A living specimen captured and handled by the second author was docile and did not strike or bite, which is in line with the behavior of other Hebius species known from Thailand and elsewhere.
Comparisons of Hebius terrakarenorum spec. nov. with other species.
In many characters, Hebius terrakarenorum sp. nov. is similar to H. igneus David, Vogel, Nguyen, Orlov, Pauwels, Teynié & Ziegler, 2021 . It can be distinguished from the latter by (1) the immaculate pinkish-cream venter except for the grey-edged corners of the ventral plates and a modest, cloudy grey dot in or near that corner (vs. three or four parallel rows, often fused, of very distinct blackish blotches largely darkening the ventral surface in H. igneus ), (2) the distinctly but moderately keeled scales at the tail base (vs. strongly keeled in H. igneus ), (3) the row of pale ochre dorsal blotches that remain separate posteriorly (vs. usually fused into a lateral stripe posteriorly of midbody in H. igneus ), and (4) the reduction of 19 → 17 DSR in H. terrakarenorum follows a different pattern to that of H. igneus . In H. terrakarenorum the reduction of DSR 3+4 → 3 is on both sides within a space of no more than 5 ventral plates, so there is only a short section of less than 3 ventral plates of the total body length that is spanned by 18 dorsal rows, but in H. igneus the reduction implies DSR 4+5 → 4 at V85–98 on the left and at V100–103 on the right, so the part of the body spanned by 18 dorsal scale rows can be as long as 15 successive ventral plates, see David et al. (2021). The number of ventrals of the new species (159–171, n=19) is not significantly different from the Chinese and Vietnamese populations of H. igneus (159–165, n=9, David et al. 2021), but lower than in the Hebius igneus population of eastern North Thailand (168–178, n=5, see results).
H. terrakarenorum sp. nov. resembles H. deschauenseei (Taylor) but can be distinguished from the latter by (1) the pattern of two rows of modest, cloudy dots on the otherwise immaculate cream belly (vs. three parallel rows of very distinct dark blotches partly blackening the venter in H. deschauenseei ), (2) the higher number of 159–171 ventral plates (vs. 148–163 in H. deschauenseei ) and (3) the usual 2 rows of ochre- or orange-brown dorsal spots extending towards the tail (vs. only spotted anteriorly).
H. terrakarenorum sp. nov. superficially resembles Hebius clerki (Wall) from Northern India and North Myanmar, as the dorsum of both species is usually adorned with two rows of distinct pale brown blotches and both have a postocular streak that extends to the neck in a characteristic way. However, there are many differences that easily distinguish these species apart, as follows: (1) Hebius terrakarenorum sp. nov. has a longer tail with a relative tail length R of 0.32–0.36 (vs. 0.26–0.33 in H. clerki ), (2) a higher number of subcaudal pairs of 107–130 (vs. 63–77 in H. clerki ), (3) a usual number of 9 supralabials (vs. mostly 8 in H. clerki ), (4) the presence of 1 anterior temporal (vs. 1 or 2 in H. clerki ), and (5) the cloudy amoeboid spots in/near the corner of the ventral plates (vs. dark streaklike spots in H. clerki ).
H. terrakarenorum sp. nov. can be distinguished from its sympatric congener Hebius khasiensis (Boulenger) by (1) its pale brown/cream supralabials (vs. supralabials with large ovoid or triangle-shaped, ivory blotches in H. khasiensis ), (2) the greyish-brown cloudy, amoeboid spots in/near the corners of the ventral plates (vs. dark brown, rectangular and closely set in the corners in H. khasiensis ), (3) the much higher number of ventral plates of 159–171 (vs. 139–149, in western Thailand) and (4) upper dorsal scales moderately keeled (vs. strongly keeled in H. khasiensis ).
H. terrakarenorum sp. nov. can be distinguished from Hebius bitaeniatus (Wall) by (1) most usually spotted dorsal pattern (vs. two distinct unbroken ochre-brown lateral stripes edged with black in H. bitaeniatus ), (2) its modest keeling (vs. nearly all rows strongly keeled in H. bitaeniatus ), (3) the cloudy, amoeboid ventral spots adjacent to the corners of the ventral plates (vs. dark brown short stripes in H. bitaeniatus ) and (4) its higher number of subcaudal pairs (107–130 vs. 80–100 in H. bitaeniatus ).
H. terrakarenorum sp. nov. can be distinguished from Hebius groundwateri (Smith) by (1) its higher number of 159–171 ventral plates (vs. 147–154 in H. groundwateri ), (2) its divided cloacal plate (vs. entire in H. groundwateri ) and (3) the number of 19 rows of dorsal scales at midbody (vs. 17 in H. groundwateri ).
Among other species, H. terrakarenorum sp. nov. can be distinguished from Hebius boulengeri (Gressitt) , Hebius leucomystax (David, Bain, Nguyen, Orlov, Vogel, Vu & Ziegler) , and Hebius inas (Laidlaw) by its pale brown supralabials (vs. supralabials with ivory white blotches in H. boulengeri and H. inas , and entirely white in H. leucomystax ) and moderately strong dorsal keels (vs. strong dorsal keels in H. boulengeri , H. leucomystax and H. inas ).
The pinkish-white belly and the 19 rows of dorsal scales at midbody distinguish H. terrakarenorum sp. nov. from Hebius venningi (Wall) , Hebius nigriventer (Wall) , Hebius taronensis (Smith) , Hebius chapaensis (Bourret) and Hebius annamensis (Bourret) that have 17 or 15 dorsal rows at midbody) and a dark venter, except H. annamensis in which the venter is largely pale. These species have recently been revised by David et al. (2021).
H. terrakarenorum sp. nov. can be distinguished from Hebius modestus (Günther) by its higher number of 159–171 ventral scales (vs. 143–163 in H. modestus ), a distinct postocular streak (vs. absent in H. modestus ) and the light-colored pinkish-white belly (vs. a mostly pale brown, edged with dark grey venter in H. modestus ).
H. terrakarenorum sp. nov. can be distinguished by its 19 rows of dorsal scales at midbody from H. atemporalis (Bourret) , H. sauteri (Boulenger) , H. arquus (David and Vogel) , H. frenatus (Dunn), H. sarawacensis (Günther) , all with 17 rows at midbody.
H. terrakarenorum sp. nov. can be distinguished from Hebius metusia (Inger, Zhao, Shaffer & Wu) by the absence of sharp, well-defined dorsolateral stripes (vs. present in H. metusia )
H. terrakarenorum sp. nov. can be distinguished from Hebius andreae (Ziegler and Le) from Vietnam by its lower number (159–171) of ventral plates (vs. 179–180 in H. andreae ) and higher number (107–130) of subcaudal pairs (vs. 99–103 in H. andreae ). Moreover, the new species has 2 pre- and 2 postoculars (vs. 1 and 3 respectively in H. andreae ), and it lacks a conspicuous dorsal pattern of pale, black-edged crossbars on the anterior body (vs. present in H. andreae ).
H. terrakarenorum sp. nov. can be distinguished from Hebius octolineatus (Boulenger) , H. septemlineatus (Schmidt) and Hebius parallelus (Boulenger) by the absence of distinct dorsolateral stripes (vs. present in H. octolineatus and H. parallelus ).
H. terrakarenorum sp. nov. is differentiated from Hebius flavifrons (Boulenger) and Hebius nicobariensis (Sclater) by its divided cloacal plate (vs. entire in H. flavifrons and H. nicobariensis ).
H. terrakarenorum sp. nov. differs from Hebius sangzhiensis Zhou, Qi, Lu, Lyu & Li in Zhou, Sun, Qi, Lu, Lyu, Wang, Li & Ma by its larger number (107–130) of subcaudal pairs (vs. 81–82 in H. sangzhiensis ), higher (0.32–0.36) relative tail length (vs. 0.24–0.27 in H. sangzhiensis ) and the number of 9 supralabials (vs. 8 or 7 in H. sangzhiensis ).
H. terrakarenorum sp. nov. can be separated from Hebius beddomei (Günther) , H. kerinciensis (David & Das), Hebius petersii (Boulenger) and Hebius viperinus (Schenkel) by having more ventral scales, 159–171, vs. 140–150 in H. beddomei , 140 in H. kerinciensis, 134–150 in H. petersii , and 101 in H. viperinus .
H. terrakarenorum sp. nov. can be distinguished from Hebius concelarus (Malnate) , Hebius ishigakiensis (Malnate & Munsterman) and Hebius optatus (Hu and Zhao) by the absence of dorsal crossbars (vs. distinct crossbars in H. concelarus , H. ishigakiensis and H. optatus ).
H. terrakarenorum sp. nov. is different from Hebius celebicus (Peters & Doria) and H. sarasinorum (Boulenger) by its number of DSRs of 19-19(17)-17 (vs. 15-15- 15 in both H. celebicus and H. sarasinorum ), higher number (159–171) of ventral plates (vs. 125–150 in H. celebicus and 137–146 in H. sarasinorum ) and much higher number (107–130) of subcaudal pairs (vs. 45–75 in H. celebicus and 64–75 in H. sarasinorum ).
H. terrakarenorum sp. nov. can be distinguished from Hebius craspedogaster (Boulenger) by its dorsolateral series of pale brown blotches (vs. a series of small, black-edged, white spots in H. craspedogaster ) and cloudy, distinctly spaced ventral spots (vs. ventral spots that are almost in contact with each other in H. craspedogaster ).
H. terrakarenorum sp. nov. is different from Hebius johannis (Boulenger) by its usual pattern of two rows of dorsolateral blotches and the moderate but distinct keels of the dorsal scales (vs. a variegated or reticulated dorsal pattern and smooth or weakly keeled scales in H. johannis ).
H. terrakarenorum sp. nov. is different from Hebius lacrima Purkayastha & David by the absence of distinct white blotches on the supralabials (vs. present in H. lacrima ).
H. terrakarenorum sp. nov. is different from Hebius miyajimae (Maki) by a series of distinct pale blotches (vs. well-defined dorsolateral stripes in H. miyajimae ).
H. terrakarenorum sp. nov. is different from Hebius popei (Schmidt) by the absence of distinct dorsolateral stripes and the relatively high number of 159–171 ventral plates (vs. presence of dorsolateral stripes and 130–142 ventral plates).
H. terrakarenorum sp. nov. can be distinguished from Hebius pryeri (Boulenger) by its brown dorsal ground color with a dorsolateral series of pale blotches (vs. blackish with pale crossbars in H. pryeri ).
H. terrakarenorum sp. nov. is different from Hebius sanguineus (Smedley) from West Malaysia by its dorsal color pattern of brown with dorsolateral rows of pale blotches (vs. brick red flanks and brown reticulated upper part of the dorsum in H. sanguineus ) and higher number (159–171) of ventral plates (vs. 140–155 in H. sanguineus )
H. terrakarenorum sp. nov. is different from H. vibakari (H. Boie) by its pale brown supralabials (vs. large white roundish blotches in H. vibakari ) and the dorsum with series of blotches (vs. nearly unpatterned in H. vibakari )
H. terrakarenorum sp. nov. is distinguished from Hebius yanbianensis Liu, Zhong, Wang, Liu & Guo by the presence of 2 postoculars (vs. 3 in H. yanbianensis ), its long tail with a TaL/TOTL ratio of 0.32–0.36 (vs. 0.26 in H. yanbianensis ), its high number of 107–130 subcaudals pairs (vs. 90 in H. yanbianensis ) and its rows of cloudy ventral spots (vs. intense dark, closely set blotches in H. yanbianensis ).
Three species placed until recently in the genus Hebius have now be referred to two other genera (see Das et al. 2020; Lalronunga et al. 2020; Deepak et al. 2021). H. terrakarenorum sp. nov. differs from Herpetoreas pealii (Sclater) by having more (159–171) ventral scales (vs. 142–144 in Herpetoreas pealii . Hebius terrakarenorum sp. nov. differs from Herpetoreas xenura (Wall) by its paired subcaudals (vs. single subcaudals in Herpetoreas xenura ). Finally, Hebius terrakarenorum sp. nov. differs from Amphiesma monticola (Jerdon) by its number of ventral plates, 159–171 vs. 136–144 in A. monticola , and a much distinct dorsal pattern.
Morphological data used in the comparisons above have been taken from Boulenger (1890; 1893), Das et al. (2020), David et al. (2005; 2007; 2013; 2015a-b, 2021), Deepak et al. (2021), De Lang & Vogel (2005), Kaitio & Mamoru (2016), Lalronunga et al. (2020), Liu et al. (2018), Purkayastha & David (2019), Smith (1943), Zhou et al. (2019), and Ziegler et al. (2019).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.