Alvania scabra ( Philippi, 1844 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4767.3.3 |
|
publication LSID |
urn:lsid:zoobank.org:pub:D599C7BA-CFB5-43D2-BF18-C051A2621DBA |
|
DOI |
https://doi.org/10.5281/zenodo.3797076 |
|
persistent identifier |
https://treatment.plazi.org/id/03AC6921-C331-3769-FF05-46E0FC1C7465 |
|
treatment provided by |
Plazi |
|
scientific name |
Alvania scabra ( Philippi, 1844 ) |
| status |
|
Alvania scabra ( Philippi, 1844) View in CoL
( Figs 1 View FIGURE 1 A–R; 2A–J; 3; 14A–C; 15A–C; 18A–D; Table II)
Rissoa scabra Philippi, 1844: 126 View in CoL , pl. 23, fig. 8
Rissoa oranica Pallary, 1900: 322 View in CoL , pl. VII, fig. 4
Other references.
Rissoa oranica View in CoL ; Pallary, 1920: 50, fig. 20 and 30.
Alvania oranica View in CoL ; van Aartsen, 1982: 5, 6, 12 unnumbered figs; Giannuzzi Savelli et al., 2002: 109, figs 446–447; Perna, 2013: 63, 2 unnumbered figs, 64, 2 unnumbered figs.
Alvania scabra View in CoL ; Bogi et al., 1983: 6, fig. 11; van Aartsen et al., 1984: 24, fig. 102 (two figures); Oliver & Templado, 2009: 61 and 63, figs 17–19 and 26–27; Gofas et al., 2011: 183, 3 unnumbered figs; Scaperrotta et al., 2012: 53, 5 unnumbered figs; Bitlis & Öztürk, 2017: 404, fig. 5I, J; Villari & Scuderi, 2017: 197, fig. 16–19; Villari, 2018: 940, 941, figs 10–16; 20; Scaperrotta et al., 2019: 143, pl. IV, fig. H.
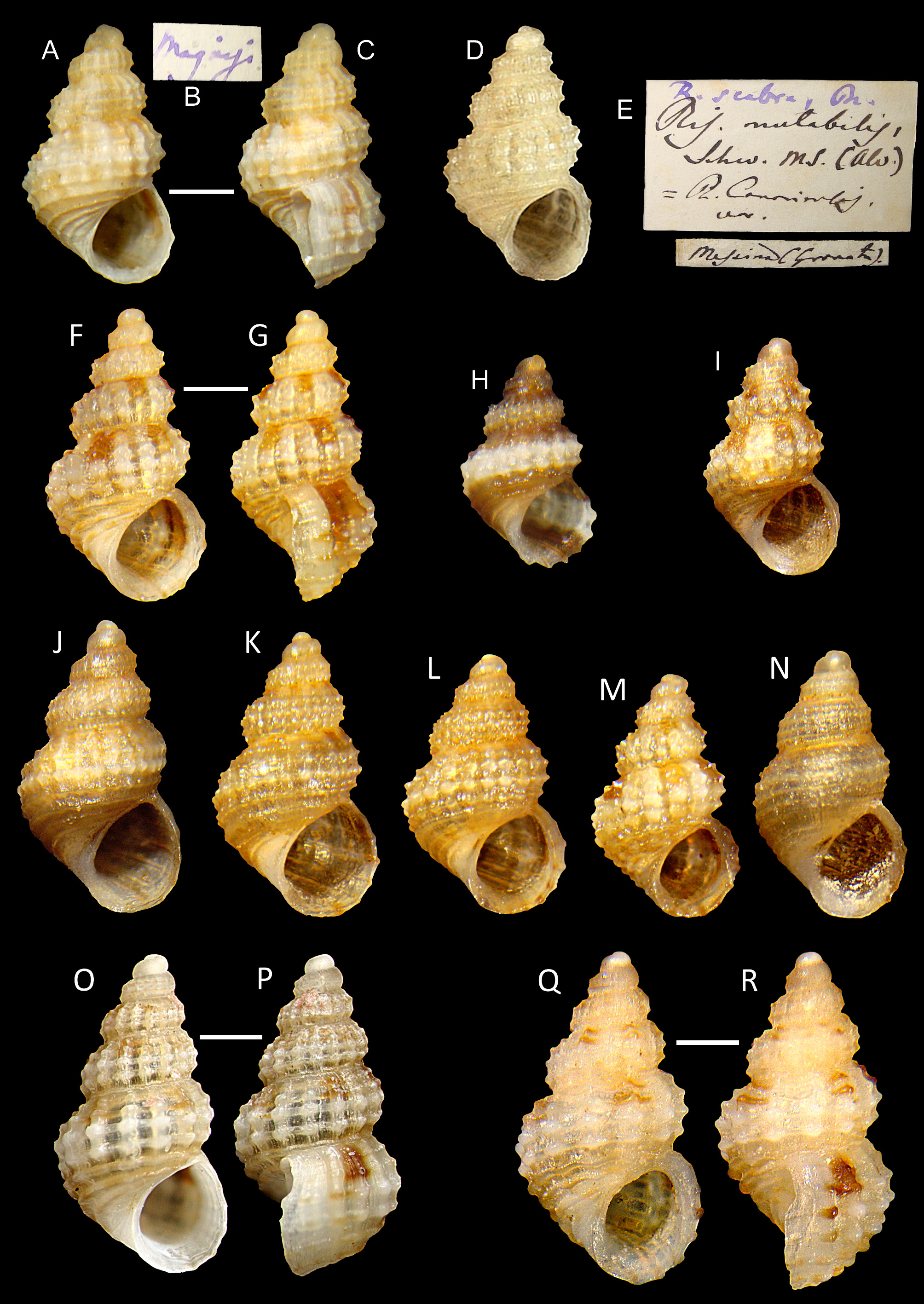
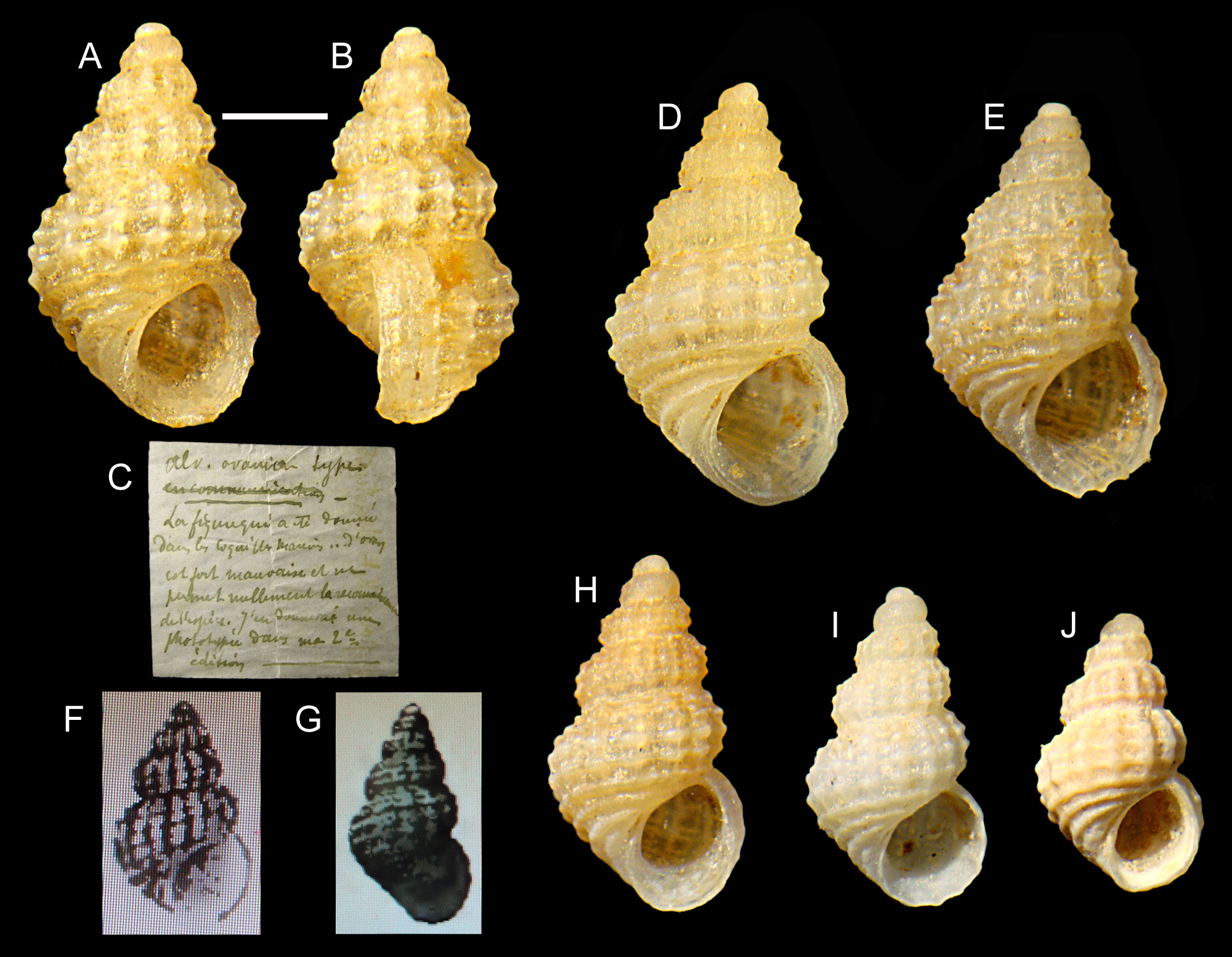
Type material. Rissoa scabra Philippi : Neotype (MCZR-M-22162/N, Monterosato coll.) ( Figs 1 View FIGURE 1 A–C; 15A–C) here designated (H 1.9 mm, W 1.15 mm), type locality: Magnisi ( Italy). Rissoa oranica Pallary : Lectotype (MCZR- M-22059/L) here designated (H 2.25 mm, W 1.35 mm) ( Figs 2 View FIGURE 2 A–C) and 41 paralectotypes (MCZR-M-22059/P, Monterosato coll. ex Pallary coll.) (including one shell of A. sculptilis ), type locality: Arzew, Oran ( Algeria).
Other material examined. Morocco: Al Hoceima, 2 sh ( CS-PM). Algeria: Oran, 40 sh (MCZR-M-22161). Spain: Columbretes Islands, 15 m depth, 15 sh ( JT, JDO); Portichol, Javea, Alicante, 2 m depth, 15 sh ( JT, JDO); Cabo de Palos, 1 sh ( BA); Cala de los Canuelos (Maro), 36°44.5’N 03°47.3’W, rocks, photophile algae 8–10 m depth vii.1999, 6 sh ( SG); Los Escullos, San José, Almería, 36°47.8’N 02°03.6’W, rocks, photophile algae 1–5 m depth, 12 sh ( SG); Playa del Embarcadero, Los Escullos, Almería, infralittoral rocks i.1990 49 sh ( MNHN), 5 sh ( MNHN.AN29); Playazo de Rodalquilar, Almería, infralittoral rocks i.1990, 1 sh ( MNHN); Calahonda, Malága, 36°29.4’N 04°41.8’W, rocks, photophylous algae, low tide, 6 sh ( SG); Benalmadena-Costa, beached 36°35.3’N 04°31.7’W 3 lv ( SG); Getares, Punta Carnero, beached 40 sh ( CS-PM); 1 sh ( BA). Balearic Islands: Colonian Sant’Jordi (Majorca Is.), 0.50 m depth, Caulerpa sp., 15 lv ( CS-PM), 1 sh ( BA); Punta de na Gall ( Menorca Is.), 10 m depth, 20 sh ( JT, JDO). France: Port de St-Jean-Cap-Ferrat, 1 sh. (Gilles Devauchelle coll.). Corsica: Cap Corse, 600 m depth, 2 sh ( AP). Sardinia: Palau, Portu Mannu, 1.5 m depth, 6 sh ( BA); Carloforte, 7 m depth 7.viii.1981, 1 sh ( BA). Sicily: Ustica Is. 35 m depth, 5 sh ( BA); Salina Is., ‘Grotta dei gamberetti’ 35 m depth, 2002,>300 sh ( BA), 40–45 m depth, 6 sh ( CS-PM); Magnisi, 14 sh (MCZR-M-22162); Messina, 1 sh as R. mutabilis (MCZR-M- 22227, Monterosato coll. ex Granata-Grillo coll.); Messina 4 sh (MCZR-M-22227, Monterosato coll. ex Seguenza coll.); Cannizzaro, 43 m depth, 1 sh ( BA); Cannizzaro 35 m depth 2 sh ( BA), 30 m depth 10 sh ( BA); Acitrezza 40 m depth, 4 sh ( BA); Ognina beached, 3 sh ( BA); Siracusa 4–7 m depth viii.1982, 1 sh ( BA); Marettimo Is., 25–45 m depth, 8 sh ( CS-PM); Marettimo Is., Cattedrale Cave, 37°56’45”N 12°4’42”E, 28 m, 150 sh ( BA, MO); Pantelleria Is., Scauri, 13 m depth 12.vi.1991, 9 sh ( BA); Pantelleria Is., Scauri, 15–20 m depth 2.vii.2006, 7 sh ( BA); Pantelleria Is., 50 m depth, 18 sh ( CS-PM); Linosa Is., Balata Piatta, 20 m depth, 100 sh ( CS-PM); Linosa Is., Secchitella 40 m, 1 sh ( CS-PM); Linosa Is., Secchitella 30 m depth, 70 sh ( CS-PM); Linosa Is., Secchitella, 20 m depth, 30 sh ( CS-PM); Linosa Is., Punta Calcarello, 36 m depth, 10 sh ( CS-PM); Linosa Is., Tre Ceppi, 28 m depth, 2 sh ( CS-PM); Lampedusa Is., 30 m depth, 1991, 13 sh ( BA), 1 sh ( MO); Lampedusa Is., Punta Madonna, 5 m depth on Cystoseira sauvageauana (Hamel, 1939) , 3 lv, 11.viii.1990 ( RC); Lampedusa Is., Scoglio Pignalta, 10 m depth on Cystoseira sauvageauana (Hamel, 1939) , 7 lv, 6.viii.1990 ( RC); Lampedusa Is., Punta Galera, 10 m depth on Cystoseira sauvageauana (Hamel, 1939) , 164 lv, 1.viii.1990 ( RC); Lampedusa Is., Punta Sottile, 10 m depth on Halopteris scoparia (Linnaeus) Sauvageau, 1904 , 9 lv, 31.vii.1990 ( RC); Lampedusa Is., Capo Ponente, 10 m depth on Halopteris scoparia (Linnaeus) Sauvageau, 1904 , 3 lv, 3.viii.1990 ( RC); Lampedusa Is., Punta Galera, 3 m depth on Cystoseira barbatula Kützing, 1860 , 19 lv, 1.viii.1990 ( RC); Lampedusa Is., Isola dei Conigli, 10 m depth on Cystoseira sauvageauana (Hamel, 1939) ; Lampedusa Is., 7 lv, 4.viii.1990 ( RC), Punta Galera, 5 m depth on Cystoseira barbatula Kützing, 1860 , 8 lv, 1.viii.1990 ( RC); Lampedusa Is., Punta Galera, 10 m depth on Cystoseira sauvageauana (Hamel, 1939) , 10 lv, 1.viii.1990 ( RC); fossil of Milazzo (Saharian, Pliocene?) 2 sh ( MGUF, vertical showcase 153, Seguenza G. coll.); fossil of Milazzo (Saharian, Pliocene?) 5 sh as Alvania mutabilis Schw. ( MGUF vertical showcase 153, Seguenza G. coll.). Italy: Giannutri Is., 45 m depth 2 sh ( CS-PM); Zannone Is., 25 m depth, ix.2009, 100 sh ( MO); Ventotene Is., Secca di S. Stefano, 70 m depth, 2015, 29 sh ( CS-PM); Ponza Is., 60 m depth 23 sh ( CS-PM); Napoli, 3 sh (MCZR-M-22227, Monterosato coll. ex Tiberi coll.); Maratea, Santo Janni Is., 24 m depth, 2 sh ( BA); Le Castella, Isola di Capo Rizzuto, 0.50 m depth, Zostera sp., 3 lv ( CS-PM); Scilla, 42 m depth 9 sh ( CS-PM), 43–44 m depth vii.2015, 1 sh ( MO); Strait of Messina, 1 lv ( BA); Otranto, 5 sh ( MO); Gallipoli, beached, 1 sh ( BA). Croatia: Lastovo Is., 38 m depth 1 sh ( BA); Split, 50 m depth, 1 sh ( AP). Greece: Kefallonia Is., 1 sh ( BA). Turkey: Aydincik, 34 sh ( BA).
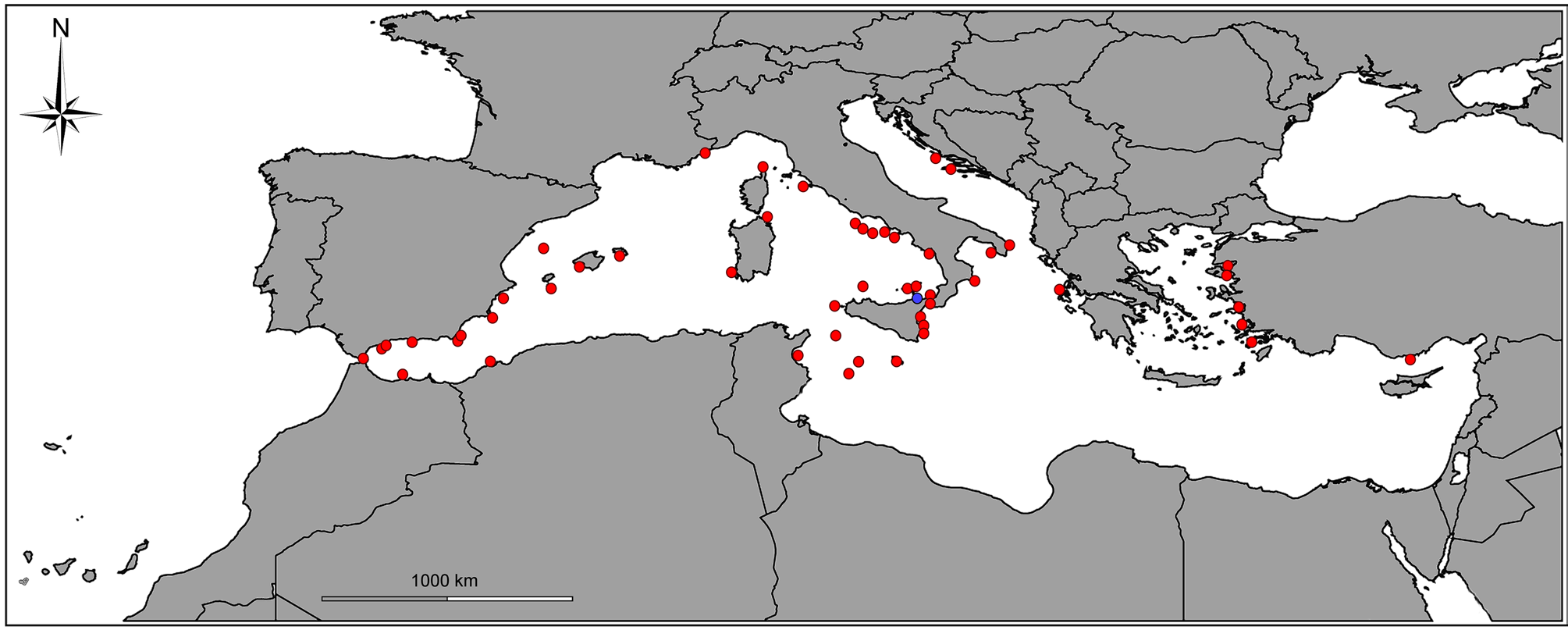
Distribution. Checked data indicate a range from the westernmost Mediterranean Sea to southern Turkey in the Levant Basin (see Remarks and Fig. 3 View FIGURE 3 ). It is common in shallow waters on hard bottoms with photophylous algae ( Dantart et al. 1990; Giacobbe 2002; Gofas et al. 2011; Scaperrotta et al. 2012; Öztürk et al. 2014; Villari & Scuderi 2017). Live specimens are present in 0–50 m depth. Shells from deeper bottoms (e.g. from Cap Corse, France 600 m depth) probably drifted from shallower habitat (Bonfitto et al. 1994a; 1994b; Smriglio & Mariottini 1996). Found sympatric with A. sculptilis , A. sororcula , A. josefoi , A. scuderii , A. lucinae and A. pizzinii n. sp.
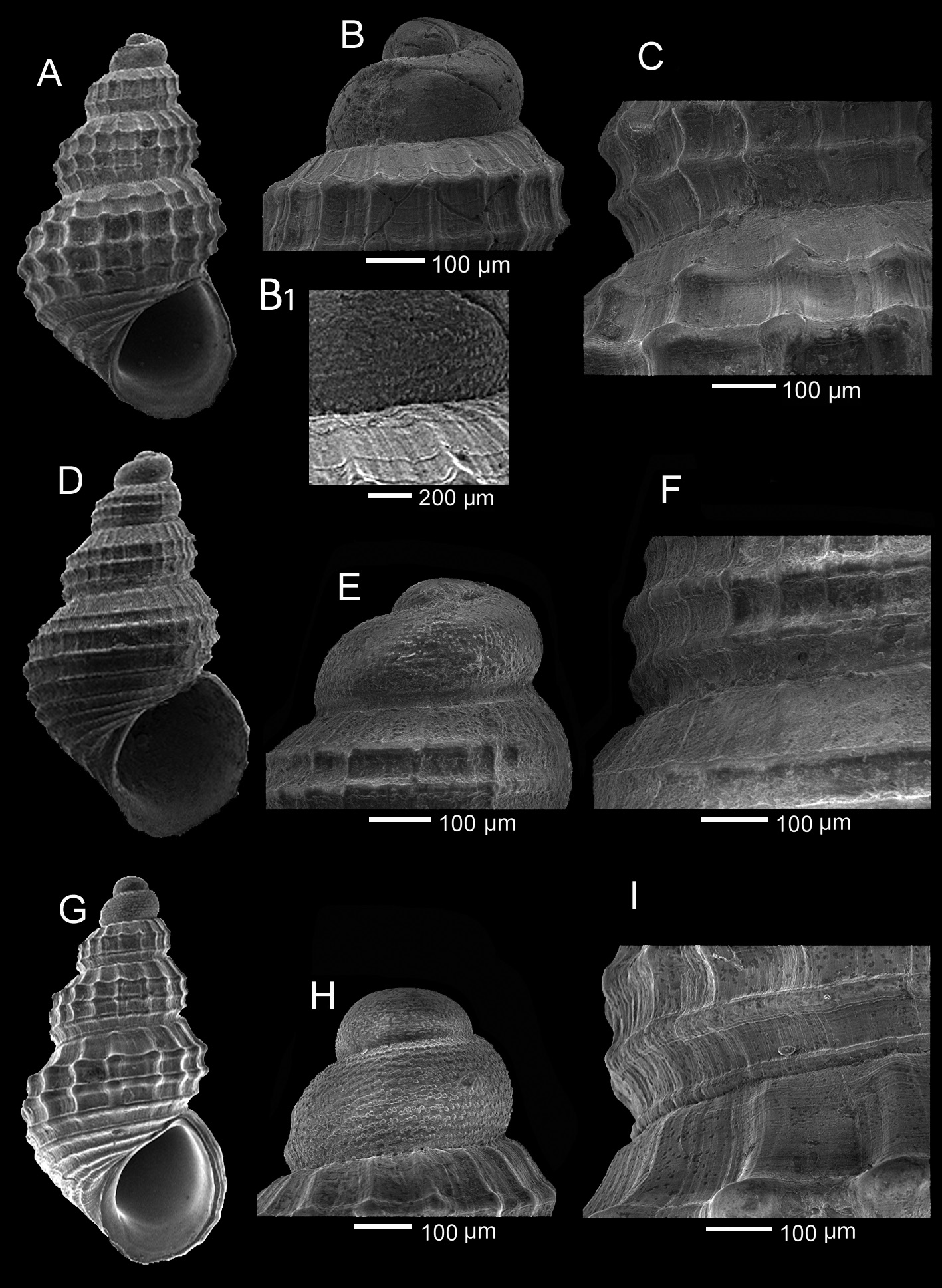
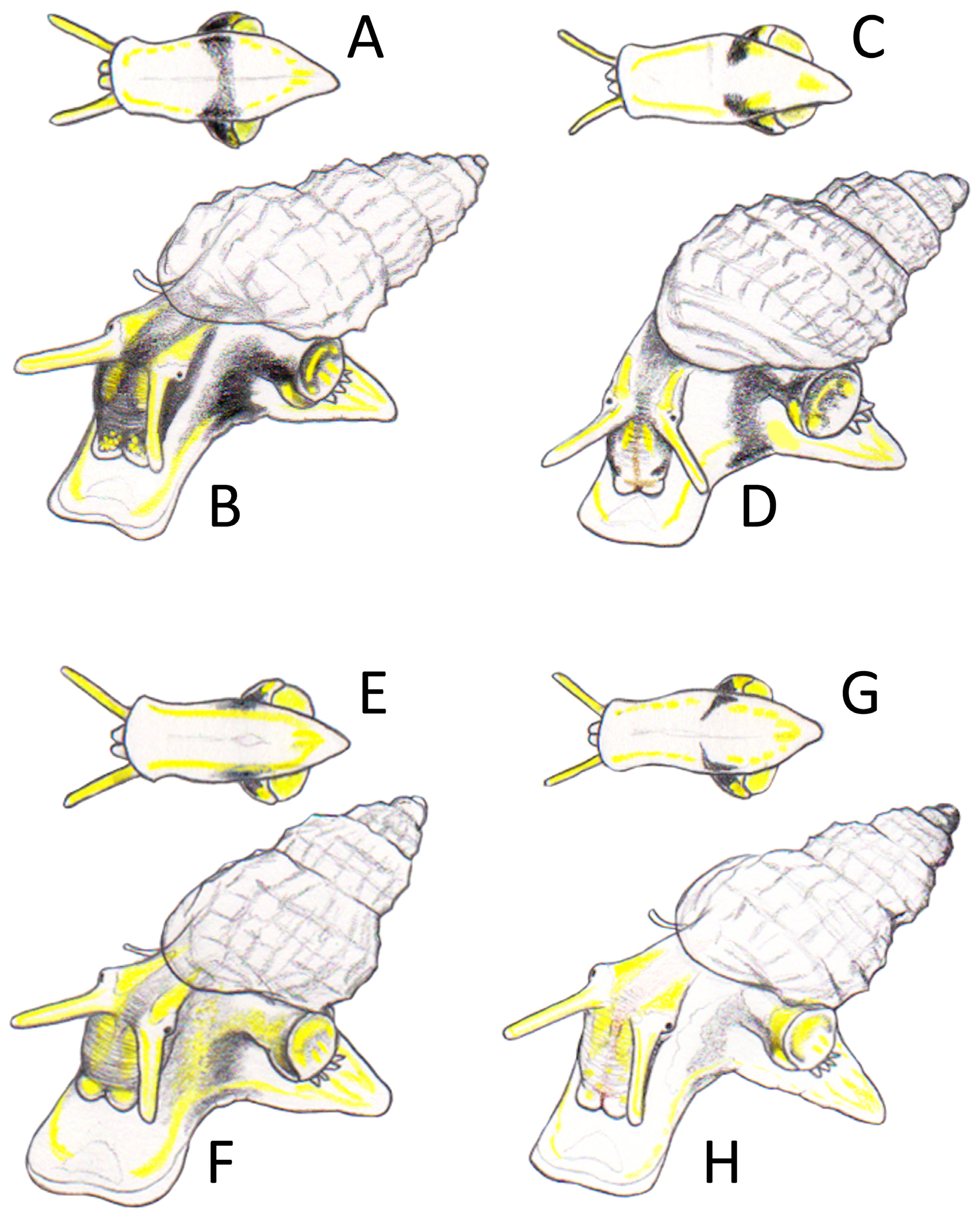
Description (data on the neotype in parentheses). Shell ( Figs 1 View FIGURE 1 A–R; 2A–J; 14A–C; 15A–C) small for the genus, height 1.41–2.55 (1.9) mm, width 0.92–1.37 (1.15) mm, H/W ratio 1.553 –1.951 (1.652), ovate-conical. Protoconch ( Fig. 15B View FIGURE 15 ) paucispiral with nucleus moderately intorted, of 1.2–1.5 (1.2) whorls, height 0.250 –0.320 (0.275) mm, nucleus diameter (d) 0.090 –0.112 (0.100) mm, first half whorl diameter (Do) 0.200 –0.220 (0.200) mm, maximum diameter (DM) 0.312 –0.350 (0.325) mm; sculpture of a dozen parallel rows of rounded to broadly triangular microtubercles, sometimes fused, and occasional spiral threads in the interspaces ( Fig. 15B View FIGURE 15 ). Protoconch-teleoconch boundary well marked. Teleoconch of 2.6–4.1 (3.2) convex whorls, with suture impressed. Axial sculpture on the last whorl of orthocline 11–20 ribs (16), plus the labial varix, narrower than the interspaces, gradually vanishing at the base. Spiral sculpture finer than axial, of equidistant cords, 7–9 (9) on the last whorl, of which 3–5 (4) above the aperture and 4–5 (5) on the base, 2–4 (3) on the penultimate whorl. Cords II and IV starting immediately after the protoconch-teleoconch boundary; cord III starting as a cordlet after 0.5–2 (0.5) whorl, gradually yet rapidly turning into a cord; cord I appearing at 1.2–2.5 (1.8) whorls (Table II); cord V rarely present (absent), appearing later. Small, rounded tubercles at the intersections, sometimes spinulose, especially on the first whorls; interspaces quadrangular. Microsculpture of growth lines and spiral threads overall ( Fig. 15C View FIGURE 15 ). Umbilical chink absent or barely visible. Aperture pyriform, small, height 0.63–0.95 (0.75) mm, H/Ha ratio 2.312 –2.949 (2.533), peristome continuous, outer varix modest, internally smooth. Colouration translucent yellowish, occasionally whitish, with darker spiral band at the base and quadrangular subsutural brown blotches; periphery and median columellar area whitish. Operculum typical for the genus, thin, corneous, paucispiral with eccentric nucleus. Soft parts ( Figs 18 View FIGURE 18 A–D): semi-transparent whitish background with dark-grey/black areas on head and snout (forming a sort of mask encircling eyes and mouth), opercular area, and on both sides of the body, extending and merging into a median band on the sole of foot. Yellow speckles on snout, merged in short stripes on head behind and in front of eyes, forming lines along cephalic tentacles, on foot, on opercular area and on propodium, and a yellow π figure under the operculum; scattered white speckles on cephalic tentacles; 3–4 metapodial tentacles.
Remarks (See also Table II and Figs 1 View FIGURE 1 A–R; 2A–J; 3; 14A–C; 15A–C; 18A–D). Alvania scabra is known from most of the Mediterranean Sea (Material examined, and Fig. 3 View FIGURE 3 ), and although major checklists of some countries do not report this species explicitly, it is frequently reported from neighbouring countries. For instance, neither B.D.D. (1884) nor Giribet & Peñas (1997) reported it from France and Catalunia, respectively; however, it is rare, but present in Mediterranean France and at Baleares (see Material examined). It was not reported by Crocetta et al. (2019) for Lebanon, or by Pallary (1912) for Egypt, but it is known from southern Turkey and from Malta ( Cachia et al. 1996). Cecalupo et al. (2008) did not report it from the Gulf of Gabès but Fekih & Gougerot (1974) did report it from the Gulf of Tunis.
A. scabra is strikingly variable in size, proportions and thickness of the shell, and in sculpture, with some degree of variation even within populations ( Figs 1 View FIGURE 1 I–N), and with almost all intermediates. The size of the protoconch microtubercles is variable. Rare specimens have stronger teleoconch spirals than the axials ( Fig. 1N View FIGURE 1 ). In some specimens the tubercles are very small or absent ( Fig. 1N View FIGURE 1 ), or so large that they tend to nearly fuse vertically into a rib ( Fig. 1M View FIGURE 1 ). Colouration varies in the intensity of the background from whitish to yellowish, or yellow, and in the size and shape of the brown blotches, from dark quadrangular subsutural ( Figs 1F, G View FIGURE 1 ), to pairs of smaller ones ( Figs 1Q, R View FIGURE 1 ). Monochrome white ( Fig. 2I View FIGURE 2 ), orange and brownish specimens are occasionally found ( Figs 1L View FIGURE 1 , 2H View FIGURE 2 ), as also are shells with short and fine dashed stripes on the base over the spirals or more rarely banded with two darker brown bands ( Fig. 1H View FIGURE 1 ). The few observations on live animals revealed also some variation; specimens from Sicily (S. Giovanni Li Cuti, Catania, 4 m depth: Figs 18A, B View FIGURE 18 ) showed four metapodial tentacles, and darker and more intense black staining on the animal, with the lateral black stripes continuing almost uninterrupted on the foot-sole. The animal sketched by Serge Gofas (Malaga) from Southern Spain (Los Escullos, Cabo de Palos, H. 2.35 mm, AN29. MNHN: Figs 18C, D View FIGURE 18 ) showed three metapodial tentacles, lighter black staining on the animal, with the lateral black stripes reaching the foot-sole but not merging into continuous band.
This is the type species of Alvaniella Sacco, 1895 ex Monterosato ms, currently regarded as a synonym of Alvania Risso, 1826 . The type material of Rissoa scabra Philippi, 1844 is lost, having not been found in the Philippi collections either at MNHNS (Santiago de Chile) (Oscar Alfredo Galvez Herrera, Santiago de Chile, pers. comm. 2017) or at MNH (Berlin) (Christine Zorn, Berlin, pers. comm. 2017). The report of two syntypes (MNHNS 178) of A. scabra ( Coan & Kabat 2017) was recently refuted by the curator (Oscar Alfredo Galvez Herrera, Santiago de Chile, pers. comm. 2018). These two non-typical specimens, belonging to the Philippi collection, are very worn and lack the protoconch. They are certainly not referable to A. scabra ; the original label is lost and they most probably belong to the extra-Mediterranean fauna. Given the complexity of the present complex, and to stabilize the use of the name, we have selected a neotype, conforming to the original description, from Magnisi, Sicily (Monterosato coll. MCZR-M-22162/N) ( Figs 1 View FIGURE 1 A–C; 15A–C).
Alvania oranica ( Pallary, 1900) has either been considered a valid species (e.g. Pallary 1900; Giannuzzi Savelli et al. 2002; Tabanelli et al. 2011), or as a synonym of A. scabra ( Philippi, 1844) (e.g. Amati 1985; CLEMAM 2015; WoRMS 2019), or finally as a synonym of A. sculptilis ( Monterosato, 1877) (e.g. Tringali 2001; Gofas et al. 2011). Pallary (1900) stated that his A. oranica differs from A. scabra by the smaller size (H 2 mm), the stouter profile (not so evident in the original drawing), the last whorl larger and the different sculpture. However, the original description and figure of Alvania oranica ( Pallary, 1900: 322, pl. VII, fig. 4) are not concordant with the syntypes found in the Monterosato collection (MCZR), which in turn show a remarkable morphological variation ( Figs 2 View FIGURE 2 A–J). The label, written by Pallary, reads: “ Alv. oranica types—La figure qui a été donneé dans les Coquilles Marins d’Oran est fort mauvaise et ne permet nullement la reconnaissance de l’espéce. J’ai donnerai une photographie dans ma 2° editions. Pour M. de Monterosato” [ Alv. oranica types—The figure provided in the Coquilles Marins d’Oran is bad and does not allow the recognition of the species. I shall provide a photograph in my second edition. For Mr. de Monterosato]. Subsequently, Pallary (1920: 50, figs 20, 30) pictured two specimens of A. oranica from Tétouan and Tangier, Morocco, that are more concordant with the types at MCZR. The shell in his fig. 30 ( Fig. 2G View FIGURE 2 ) is somehow different, possibly being a mere Atlantic variety ( Pallary 1920) or a distinct, still unidentified species. Since there have been difficulties in the past in identifying this species, and the type series available (there is no material at MNHN, Virginie Heros pers. comm. 3.ix.2019) includes at least one specimen of a different species, there is the need for a lectotype designation. To stabilize the name usage, we have selected as lectotype the shell, among the syntypes, best corresponding to the original description ( Figs 2 View FIGURE 2 A–C), also in consideration of Pallary’s subsequent remarks on the label.
The following nomina nuda probably refer to this species: Alvania mutabilis Brusina, 1866: 27 ex Schwartz ms (nomen nudum), from Molat and Zadar (Dalmatia); Rissoa mutabilis Weinkauff, 1868: 311 ex Schwartz ms (nomen nudum), from Algeria; Rissoa mutabilis Monterosato, 1872: 36 ex Schwartz ms (nomen nudum), from Palermo, Ognina, Pantelleria and Algeria; Rissoa (Alvania) mutabilis Seguenza G., 1874: 4 ex Schwartz ms (nomen nudum); Rissoa mutabilis Aradas & Benoit, 1874: 201 ex Schwartz ms (nomen nudum), from?Sicily; Rissoa etnea Monterosato, 1884: 59 ex Aradas ms (nomen nudum), from Catania, Sicily; Alvania mutabilis Carus, 1893: 329 ex Schwartz ms (as synonym of the A. scabra ) (nomen nudum), from several localities for A. scabra ; Alvania sericea Parenzan, 1970: 78 ex Monterosato ms (as synonym of the A. scabra ) (nomen nudum). Rissoa mutabilis Weinkauff, 1868 ex Schwartz ms has been regarded as either available and a synonym of A. scabra ( Bogi, Coppini & Margelli 1983; WoRMS 2019), or a nomen nudum ( van Aartsen 1982; Moolenbeek & H.J. Hoenselaar 1998). Actually, Weinkauff (1868) only reported, without any description, the record by Brusina (1866) from Dalmatia, which is also a nomen nudum, and his own findings in Algeria. Subsequently, this binomen was not made available by other authors (e.g. Monterosato 1872; G. Seguenza 1874; Aradas & Benoit; Carus 1893; all ex Schwartz ms), nor is our intention to make them available in this work. Specimens (see ‘Other material examined’) in the Monterosato collection (ex Granata-Grillo coll. MCZR-M-22227: labelled “ Ris. mutabilis Schw. ms. (Alv.)”, subsequently corrected into “ R. scabra Ph. ”) ( Fig. 1D, E View FIGURE 1 ) and in the G. Seguenza collection (MGUF, vertical showcase 153: labelled “ Rissoa mutabilis Schw. ”), are in fact A. scabra .
Rissoa schwartzii Aradas & Benoit, 1874 was introduced for A. scabra sensu Jeffreys (1856) ; Jeffreys & Capellini (1860); Tapparone Canefri (1869) non Philippi, 1844. It is probably a synonym of R. lanciae Calcara, 1845 (see B.D.D. 1884: 289; Monterosato 1884: 59; Priolo 1953: 72), and not of R. scabra Philippi as suggested by Monterosato (1878: 85; 1884: 59), but is anyway preoccupied by Rissoa schwartzi Hörnes, 1856 (currently Alvania schwartzi ) ( Hörnes 1856: 573, pl. 48, fig. 18; Kowalke & Harzhauser 2004: 121, 122, fig. 7D). No specimens under this name were found in the Aradas collection at MCSNM, although Priolo (1953) mentioned finding some among the material studied therein.
Tabanelli et al. (2011) used the names Alvania cf. scabra ( Philippi, 1844) and Alvania cf. oranica ( Pallary, 1900) for Pliocene specimens that evidently may be referred to another species, Rissoa (Alvaniella) pagodulina Sacco, 1895 sensu Chirli & Linse (2011) . Alvania cf. scabra sensu Tabanelli et al. (2011) differs from A. scabra ( Philippi, 1844) by the protruding spiral cord III, that gives a keeled appearance to the whorls (particularly the last one), never so marked in A. scabra (but similar to what is obsereved in A. lucinae and A. leopardiana Brunetti & Vecchi, 2012 ), and for the presence of denticles on the inner side of the outer lip, never observed in A. scabra (despite their erroneous record in the original description by Philippi).
Ponder (1985) used the name Alvania scabra ( Philippi, 1844) for specimens from Madeira, probably corresponding to Alvania canariensis ( d’Orbigny, 1840) (see also van Aartsen & van der Linden 1986; Moolenbeek & Hoenselaar 1989).
Bitlis Bakir & Öztürk (2016: 446, 448, pl. 2, fig. 20) figured as A. scabra a subadult specimen of A. clarae Nofroni & Pizzini, 1991 , recognizable by its cylindrical outline, the lack of the first subsutural cord producing more stepped whorls, the opisthocline axials (vs orthocline or sligthly prosocline in A. scabra ), the tubercles at the intersections more spinose and directed toward the apex, the 3 spiral cords above the aperture vs 4 in A. scabra , and the 6 spirals on the base vs 5 in A. scabra .
Rissoa alleryana Monterosato, 1872 was introduced as a synonym of A. scabra , and is thus not available ( Giannuzzi-Savelli & Piani 1990; ICZN 1999: Art. 11.6).
Some species of Alvania are characterized, in adult specimens with a well-formed labial varix, by the consistent presence of denticles or lirae on the inner side of the outer lip, for example Alvania discors (Allan, 1818) , Alvania cancellata (da Costa, 1778) , Alvania cimicoides (Forbes, 1844) and Alvania subcrenulata (B.D.D., 1884). Others are characterised by their inconsistent presence/absence, for example Alvania mamillata Risso, 1826 , Alvania dictyophora ( Philippi, 1844) , Alvania hallgassi Amati & Oliverio, 1985 and Alvania desabatae Amati & Smriglio, 2016 . In over 1500 specimens of A. scabra examined we have never observed any such feature (see also Gofas et al. 2011), similarly to what happens in Alvania datchaensis Amati & Oliverio, 1987 , Alvania lactea (Michaud, 1830) and Alvania punctura (Montagu, 1803) . It therefore seems unlikely that Philippi (1844) observed grooves in the type material: “Labrum extus incrassatum, intus sulcatum est.”. Most likely Philippi was deceived by the transparency of the shell that often allows seeing the external spirals.
A. scabra and A. sculptilis are very similar, share a good deal of morphological variation and often can be very hard to separate in syntopic samples. The great majority (95%) of shells of A. sculptilis lack spiral cord I, which is always present in A. scabra (there is a single, probably anomalous shell lacking it from Kefallonia Is.). Additionally, in A. scabra the axial and spiral sculpture are very often of the same strength, whereas in A. sculptilis the spiral sculpture is usually predominant over the axial (cf. Figs 4A, B, E View FIGURE 4 ). Finally, in a sample of 41 shells of A. sculptilis , 13 (32%) showed the spiral cords II, III and IV starting immediately after the protoconch-teleoconch boundary, and 28 (68%) the cords II and IV, whereas A. scabra always has cords II and IV starting immediately after the protoconch-teleoconch boundary. Under these circumstances, we keep the two taxa separate, following Tringali (2001), but urge genetic analysis on sympatric samples from the Western Mediterranean to test this hypothesis.
A. scabra differs from A. sororcula by the tubercles at the intersections (rounded nodulose, rarely slightly spinose in A. scabra vs smaller in A. sororcula , particularly those on spiral cord I); the outer lip (thickened to very thickened in A. scabra vs sligthly thickened in A. sororcula ); the last whorl (proportionally larger in A. sororcula ); the axials on the last whorl (11–20 in A. scabra vs 25–29 in A. sororcula ); the subsutural blotches (orthocline in A. scabra vs slightly prosocline in A. sororcula ). Villari & Scuderi (2017: 198) also noted that “the external soft body parts are similar, but in A. sororcula the colour pattern is constantly less shiny, with only grayish bands, which are almost black in A. scabra ”.
A. scabra differs from A. lucinae by the tubercles at the intersections (rounded nodulose, rarely slightly spinose in A. scabra vs spinose or almost spinose in A. lucinae ), and the spiral cords (all the same strength in A. scabra vs cord III more protruding in A. lucinae ). A. scabra differs from A. josefoi by the appearance of the teleoconch spiral cords (cord I at 1.2–2.5 whorls (mean 1.72), cord III at 0.5–2 (mean 1.2) whorls in A. scabra vs cord I at 1–1.5 (mean 1.2) whorls, cord III at 0.5–0.8 (mean 0.54) whorls in A. josefoi ). The spiral microsculpture of the teleoconch also differs (spaced threadlets, more closely set only those on the spiral cords in A. scabra vs numerous, dense and fine threadlets in A. josefoi ), as does the colouration (translucent whitish-yellowish, with a broad brown band on the base, and quadrangular subsutural blotches in A. scabra vs monochrome white, with the typical brown subsutural blotch before the varix in A. josefoi ).
A. scabra differs from A. scuderii by the shell colouration (translucent yellowish-whitish, with darker spiral band at the base and quadrangular subsutural brown blotches in A. scabra vs brownish background with dark-brown spirals in A. scuderii ), and by the colouration of the soft parts (white with black blotches in A. scabra vs whitish in A. scuderii ). See under A. pizzinii n. sp. for differences with A. scabra .
| BA |
Museo Argentino de Ciencias Naturales Bernardino Rivadavia |
| SG |
Shanghai Botanical Garden |
| MNHN |
Museum National d'Histoire Naturelle |
| MO |
Missouri Botanical Garden |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Alvania scabra ( Philippi, 1844 )
| Amati, Bruno, Appolloni, Massimo, Giulio, Andrea Di, Scuderi, Danilo, Smriglio, Carlo & Oliverio, Marco 2020 |
Alvania scabra
| Scaperrotta, M. & Bartolini, S. & Bogi, C. 2019: 143 |
| Bitlis Bakyr, B. & Ozturk, B. 2017: 404 |
| Villari, A. & Scuderi, D. 2017: 197 |
| Scaperrotta, M. & Bartolini, S. & Bogi, C. 2012: 53 |
| Gofas, S. & Moreno, D. & Salas, C. 2011: 183 |
| Oliver, J. D. & Templado, J. 2009: 61 |
| van Aartsen, J. J. & Menkhorst, H. P. M. G. & Gittenberger, E. 1984: 24 |
| Bogi, C. & Coppini, M. & Margelli, A. 1983: 6 |
Alvania oranica
| Perna, E. 2013: 63 |
| Giannuzzi Savelli, R. & Pusateri, F. & Palmeri, A. & Ebreo, C. 2002: 109 |
| van Aartsen, J. J. 1982: 5 |
Rissoa oranica
| Pallary, P. 1920: 50 |
Rissoa oranica
| Pallary, P. 1900: 322 |
Rissoa scabra
| Philippi, R. A. 1844: 126 |