Introduction
Acid-base imbalances are disorders that result from disease in organs like the kidney, lungs, and brain. Usually, the buffer system in the body maintains and controls the acid and base balance via the kidney and lungs.
- Since enzyme action is pH dependent, maintaining acid-base balance is essential for the proper operation of the biological systems.
- The human body uses many physiological adaptations to preserve homeostasis.
- Maintaining an acid-base balance is one of them.
- The human body works to keep its acid-base balance within carefully regulated boundaries, just as temperature, blood pressure, osmolality, and many other physiological factors.
Buffer System
- A buffer is a chemical solution that can withstand pH changes.
- There are several buffer systems in the body, but the bicarbonate system found in extracellular fluid is crucial for understanding most acid-base problems.
- The bicarbonate buffer, like every buffer, consists of a weak acid (in this case, carbonic acid, H2CO3) and its conjugate base (the bicarbonate ion, HCO3–), which exist in a dynamic equilibrium.
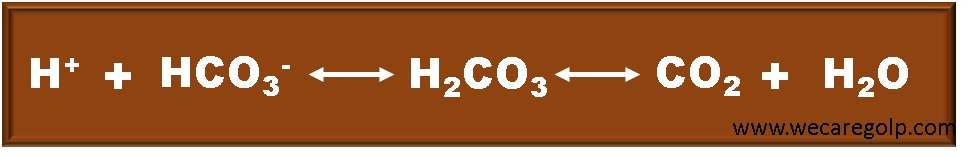
- The concentration of hydrogen ions (H+) in a solution determines its acidity. If a disease condition causes an increase in the concentration of H+, the body should become more acidic.
- The bicarbonate buffer system, on the other hand, resists this shift because an abundance of H+ forces the reaction in given above equation to the right: hydrogen ions react with bicarbonate ions, minimizing any change in acidity.
- This mechanism needs a steady supply of bicarbonate ions.
- The kidneys are influential organs in acid-base balance because they may manufacture new bicarbonate buffer as well as recover filtered bicarbonate in the proximal tubules. The body’s dilemma is that regular metabolism is coupled with the continual creation of hydrogen ions (H+) and carbon dioxide (CO2), both of which tend to lower pH.
- Even minor deviations from normal can be fatal. Thus, it is critical to understand normal acid-base levels, as well as their causes and how to manage them.
Definitions of Acid-Base Imbalances
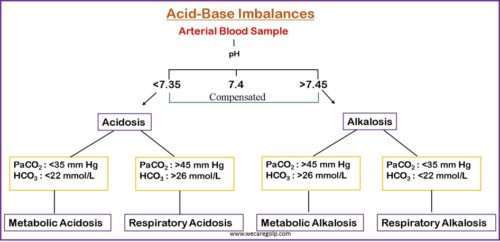
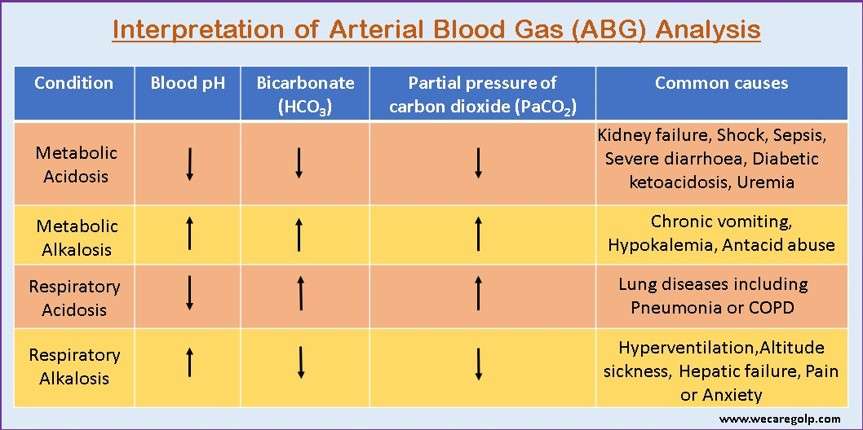
Acid-base imbalances are pathologic variations in the partial pressure of arterial carbon dioxide (PaCO2) or serum bicarbonate (HCO3–) that result in aberrant arterial pH values.
- The pH value of 7 indicates neutral. A pH value of less than 7 denotes acidity. In contrast, a pH value of greater than 7 indicates a base.
- If the pH increases, the H+ concentration decreases. Conversely, if the pH decreases, the H+ concentration increases. Thus, the pH and H+ have a direct relationship with each other.
- Acidemia is defined as a serum pH of 7.35.
- Serum pH greater than 7.45 indicates alkalemia.
- Acidosis refers to physiologic processes that result in acid buildup or alkali loss.
- Alkalosis is a term used to describe physiologic processes that result in alkali buildup or acid loss.
- Actual pH variations are determined by the degree of physiologic compensation and the presence of numerous mechanisms.
Causes of Acid-Base Imbalances
The majority of Acid-base imbalances are caused by:
- Infection, disease, or damage to organs (kidneys, lungs, brain) whose proper function is required for acid-base homeostasis
- Disease-causing abnormally high generation of metabolic acids to the point that homeostatic systems are overwhelmed
- Medical intervention (e.g., mechanical ventilation, some drugs).
Classification of Acid-Base Imbalances
Acid-base balance disorders are categorized based on their cause and the direction of the pH change.
- Respiratory Acidosis
- Respiratory Alkalosis
- Metabolic Acidosis
- Metabolic Alkalosis
Respiratory Acidosis
- Respiratory acidosis is a condition of acid-base imbalance due to alveolar hypoventilation (i.e., hypercapnia)
- Due to hypoventilation (or failure of ventilation), the production of CO2 increases. In this condition, the level of PaCO2 increases suddenly. This condition is called hypercapnia. The normal value of PaCO2 is 35 – 45 mmHg.
- The principal effect of high PaCO2 reduces the ratio of HCO3– to PaCO2, lowering the pH.
- When ventilation is impaired, and the elimination of carbon dioxide by the respiratory system is less than the creation of carbon dioxide in the tissues, hypercapnia, and respiratory acidosis result.
Causes of Respiratory Acidosis
- Respiratory diseases such as bronchopneumonia, emphysema, asthma, and chronic obstructive pulmonary disease can produce hypoventilation.
- Some medicines, such as morphine and barbiturates, can produce respiratory acidosis by suppressing the brain’s respiratory center.
- Damage or injuries to the chest wall and the muscles involved in breathing mechanics may limit the ventilation rate.
- Obstructive sleep apnea
- Obesity-hypoventilation syndrome
- Sepsis, malignant hyperthermia, thyroid crisis, fever, and overfeeding can all cause an increase in carbon dioxide levels.
- Similarly, the administration of bicarbonate to buffer acidosis or citrate-containing anticoagulants used in dialysis can cause an increase in PaCO2 levels, which can lead to the development of acute respiratory acidosis in critically ill patients.
Clinical Presentation
- Headache
- Hypokalemia
- Dysrhythmias
- Muscle weakness, hyperreflexia, disorientation
- Drowsiness, dizziness
- Skin/mucosa pale to cyanotic
Respiratory Alkalosis
- Respiratory alkalosis is an acid-base imbalance caused by alveolar hyperventilation.
- Alveolar hyperventilation lowers the PaCO2.
- As a result of the drop in PaCO2, the ratio of bicarbonate concentration to PaCO2 rises, raising the pH level; hence the descriptive term respiratory alkalosis.
- When a strong respiratory stimulation induces the respiratory system to eliminate more carbon dioxide than is created metabolically in the tissues, a fall in PaCO2 (hypocapnia) occurs.
Causes of Respiratory Alkalosis
- Anxiety
- Hypoxia
- Lung disease like severe asthma, pneumonia, pulmonary embolus
- Infection: Gram negative sepsis, fever
- Increased intracranial pressure: Head trauma, stroke/hemorrhage, brain tumor
- Excessive mechanical ventilation
- Hormones-Drugs: Salicylates, catecholamines, progesterone
Clinical Presentation
- Tachycardia
- Hypokalemia
- Numbness and tingling of extremities
- Light headedness
- Inability to concentrate
- Convulsions
- Positive chvostek’s sign: nausea and vomiting
- Muscle twitching
Metabolic Acidosis
- Metabolic acidosis is defined by a rise in the concentration of hydrogen ions in the systemic circulation, resulting in blood HCO3 levels of less than 24 mEq/L.
- It is not a harmless condition; it indicates an underlying illness that must be treated to reduce morbidity and death.
- The many causes of metabolic acidosis are grouped into four major mechanisms: increased acid generation, reduced acid excretion, acid consumption, and renal or gastrointestinal (GI) bicarbonate losses.
- Metabolic acidosis is characterized by a primary decrease in HCO3, often accompanied by a compensatory reduction ofPaCO2; pH may be very low or slightly subnormal.
- The presence or absence of unmeasured anions in serum determines whether a metabolic acidosis has a high or normal anion gap.
Causes of Metabolic Acidosis
- Diabetic ketoacidosis
- Hypermetabolism
- Renal failure
- Dehydration
- Liver failure
- Diarrhea
- Fistulas
Clinical Presentation
- Headache
- Decreased blood pressure
- Hyperkalemia
- Muscle twitching
- Warm, flushed skin
- Nausea, vomiting, diarrhea
- Fatigue, weakness, confusion
- Kussmaul respirations
Metabolic Alkalosis
- Metabolic alkalosis is a primary acid-base imbalance that causes the plasma bicarbonate to rise to a level higher than expected.
- It is characterized as an increase in body pH exceeding 7.45.
- High serum bicarbonate (HCO3–) concentration occurs as a result of a loss of H+ from the body or a gain in HCO3–.
- As a compensatory strategy, metabolic alkalosis causes alveolar hypoventilation and an increase in PaCO2, which reduce the pH change that would otherwise occur.
Causes of Metabolic Alkalosis
- Loss of gastric secretions (vomiting, villous adenoma)
- Congenital chloridorrhoea
- Previous diuretic treatment
- Hypokalemia
- Cushing syndrome
- NG suction, excessive GI suctioning
- Magnesium deficiency
- Cystic fibrosis
Clinical Presentation
- Restlessness followed by lethargy
- Tachycardia
- Confusion, dizziness, irritable
- Tremors, muscle cramps
- Tingling of fingers and toes
- Prolonged spasms
Diagnosis of Acid-Base Imbalance
- Arterial blood gases (ABG)
- Serum electrolytes
- Calculated anion gap
- If metabolic acidosis exists, the delta gap is determined and the Winters formula is used.
- Look for compensating alterations.
Arterial Blood Gas (ABG) Analysis
- An arterial blood gas measurement provides valuable information about the blood pH and the PaCO2 and the partial pressure of arterial oxygen (PaO2).
- Most analyzers calculate serum HCO3 – and the base excess. Some analyzers also measure electrolytes, hemoglobin, glucose, lactate, and other analytes.
Components of ABG
| Parameter | Arterial | Venous |
| pH | 7.35-7.45 | 7.35-7.45 |
| Partial pressure of arterial carbon dioxide (PaCO2) | 35-45mm Hg | 40-45mm Hg |
| Bicarbonate (HCO3 –) | 22-26 mEq/L(Mmol/L) | 22-26 mEq |
| Partial pressure of arterial oxygen (PaO2) | 80-100 mm Hg | 40-50 mm Hg |
| Oxygen saturation (SaO2) | 96-100% | 60-85% |
Interpretation of ABG
Anion Gap
- When excess H+ ions or acids are released, the individual experiences a condition known as metabolic acidosis.
- This raises respiratory rate and lowers plasma levels. The anion gap is used to pinpoint the specific cause of metabolic acidosis. It determines unmeasured anions in the plasma, such as phosphates, sulfates, and proteins. The usual formula makes calculating the anion gap fairly straightforward.
Formula 1: Na+ + K+ – (Cl– + HCO3 –).
Formula 2: Na+ – (Cl– + HCO3 –)
- The normal value is 8 – 12 mEq/l without potassium and 12 – 16 mEq/l with potassium.
Management of Acid Base Imbalance
General considerations
- Acid-base imbalances should be treated by addressing the underlying cause.
- Medications used to treat acid-base imbalances (e.g., sodium bicarbonate, acetazolamide) should be started in consultation with a specialist (e.g., nephrologist).
- Mechanical ventilation may be necessary in cases of severe respiratory problems and metabolic acidosis.
- As needed, optimize ventilation in mechanically ventilated patients.
Acid-base imbalances are common in clinical practice, and many have life-threatening consequences. The treatment of an acid-base imbalance is contingent on accurately detecting the condition and, where feasible, addressing the underlying causative mechanism.
- Bicarbonate is the preferred therapy for acute metabolic acidosis. Although the utility of alkali treatment in lactic acidosis and diabetic ketoacidosis is debatable, bicarbonate should unquestionably be delivered in severe acidosis.
- Providing an appropriate dose of chloride salt to most individuals with mild to severe chloride-responsive metabolic alkalosis can return the acid-base balance to normal in a few days. Therapy for chloride-resistant metabolic alkalosis, on the other hand, is best focused on the underlying condition. When alkalemia is severe, hydrochloric acid or a hydrochloric acid precursor may be required.
- The goal of treating respiratory acidosis should be to restore ventilation; alkali should only be used to treat superimposed metabolic acidosis.
- The treatment of respiratory alkalosis is focused on reversing the underlying cause; without this objective, there is no effective treatment of primary hypocapnia.
It is not rare for more than one acid-base imbalance (i.e., a mixed condition) to coexist. Extreme pH changes can occur when plasma bicarbonate concentration and arterial carbon dioxide tension (paCO2) are changed in opposing directions. In such circumstances, it is critical to identify the source of the problem early on and to provide therapy that addresses both illnesses.
Summary
- Acidosis and alkalosis are physiologic processes that produce acid and/or alkali buildup or loss; blood pH may or may not be abnormal.
- Acidemia and alkalemia are conditions in which the serum pH is unusually acidic (pH 7.35) and alkaline (pH > 7.45).
- Acid-base imbalances are characterized as metabolic if the pH change is caused largely by a change in serum bicarbonate (HCO3) and respiratory if the change is caused primarily by a change in PaCO2 (increase or decrease in ventilation).
- The fundamental process (acidosis or alkalosis) is established by the pH, changes in PCO2 indicate the respiratory component and changes in HCO3 reflect the metabolic component.
- All acid-base abnormalities result in compensation, which tends to bring the pH back to normal. Respiratory acid-base disorders result in metabolic compensation (change in HCO3); metabolic acid-base disorders result in respiratory compensation (change in PaCO2).
- There may be more than one major acid-base problem present at the same time. Each fundamental acid-base problem must identify and address.
- The measurement of arterial blood gases and serum electrolytes, as well as the computation of the anion gap, are all part of the first laboratory examination acid-base imbalances. Treatment is based on the underlying cause.
References
- Hamilton, P. K., Morgan, N. A., Connolly, G. M., & Maxwell, A. P. (2017). Understanding acid-base disorders. The Ulster medical journal, 86(3), 161. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5849971/
- Hopkins, E., Sanvictores, T., & Sharma, S. (2021). Physiology, acid base balance. In StatPearls [Internet]. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK507807/
- Jameson, J., & Loscalzo, J. (2013). Harrison’s Nephrology and Acid-Base Disorders, 2e. McGraw-Hill Education.
- Robertson, S. A. (1989). Simple acid-base disorders. Veterinary clinics of north America: Small animal practice, 19(2), 289-306. https://pubmed.ncbi.nlm.nih.gov/2648667/
- Seifter, J. L., & Chang, H. Y. (2016). Disorders of acid-base balance: new perspectives. Kidney Diseases, 2(4), 170-186. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5260542/
- Williamson, J. C. (1995). Acid-base disorders: classification and management strategies. American Family Physician, 52(2), 584-590. https://pubmed.ncbi.nlm.nih.gov/7625331/
- https://emedicine.medscape.com/article/243160-overview#a6
- https://emedicine.medscape.com/article/301574-clinical#b2