Introduction
Goodpasture syndrome (GS), also known as anti-glomerular basement membrane disease (anti-GBM), is an autoimmune condition that affects the kidneys and lungs by causing the development of autoantibodies that target their basement membranes.
- Ernest Goodpasture, who originally identified this condition in 1919, is honored by having his name attached to the eponym Goodpasture syndrome. The pathophysiology of Goodpasture syndrome was later better understood as a result of the finding of anti-GBM antibodies.
- GS is a rare and organ-specific autoimmune disease characterized by crescentic glomerulonephritis with linear immunofluorescent staining for IgG on the GBM and caused by anti-GBM antibodies.
- The non-collagenous (NC-1) domain of the alpha3 chain of type IV collagen, which is widely distributed in the basement membranes of renal and pulmonary capillaries, is the target of anti-GBM antibodies.
- It often manifests as an acute renal failure caused by quickly developing glomerulonephritis, followed by potentially fatal pulmonary bleeding.
- Early diagnosis and treatment of this disease are crucial since the prognosis for renal function recovery is dependent on the initial amount of damage.
Incidence
- In Asian and European populations, the incidence of the anti-GBM disease varies from 0.5 to 1.8 cases per million per year.
- It causes 1-5% of all glomerulonephritis.
- Moreover, it is responsible for 10%–20% of cases of crescentic glomerulonephritis.
- The age ranges for the condition are bimodal, falling between 20-30 years and 60-70 years.
- The condition tends to affect older women and younger males more frequently.
Causes of Goodpasture Syndrome
Goodpasture syndrome appears to be caused by environmental insults in those who have a genetic susceptibility. HLA-DR15 human leukocyte antigen (HLA) serotype has been highly linked to anti-GBM disease. Anti-GBM antibodies must be exposed to the alveolar capillaries after an initial injury to the pulmonary vasculature.
Risk Factors of Goodpasture Syndrome
Environment stimuli
- Medicines that induce lymphocyte depletion, such as alemtuzumab
- Cocaine inhalation
- Diseases like influenza A2
- Smoking
- Metal dust, organic solvents, or hydrocarbons exposure
- Extracorporeal shock wave lithotripsy
- COVID-19 and COVID-19 vaccinations have been linked to both new and recurring anti-GBM illness cases.
- Immunosuppressive medications
- Radiation therapy
Other stimuli
- Upper respiratory infections
- Septicemia
- Volatile hydrocarbons
- Increased capillary hydrostatic pressure
- High concentrations of FiO2 (fraction of inspired oxygen)
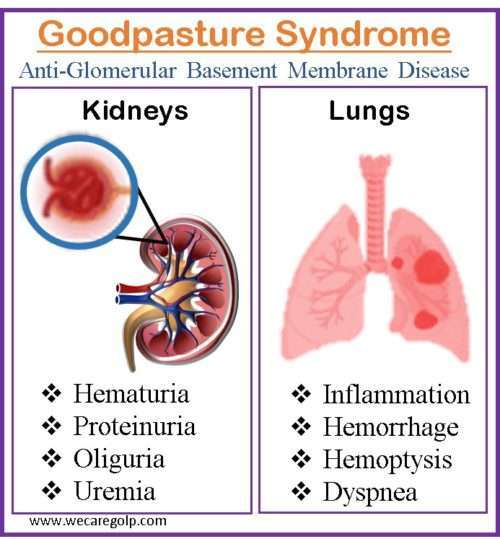
Signs and Symptoms of Goodpasture Syndrome
Pulmonary symptoms
- Dry cough
- Hemoptysis: For pulmonary involvement, hemoptysis of varied degrees is usual, ranging from major, life-threatening bleeding to more subtly diffuse hemorrhage that is only detectable upon closer inspection
- Exertional shortness of breath
- Inspiratory crackles over lung bases
- Tachypnea
- Cyanosis
- Chest pain
- Dyspnea
Renal and other symptoms
- Painful micturition
- Burning micturition
- Hematuria
- Oliguria
- Subnephrotic proteinuria
- Edema of hands and feet
- Glomerulonephritis
- Uremia
- Hypertension
- Easy bruising/rash
- Back pain below the ribs
- Hepatosplenomegaly
- Hearing loss
- Vertigo
Pathophysiology of Goodpasture Syndrome
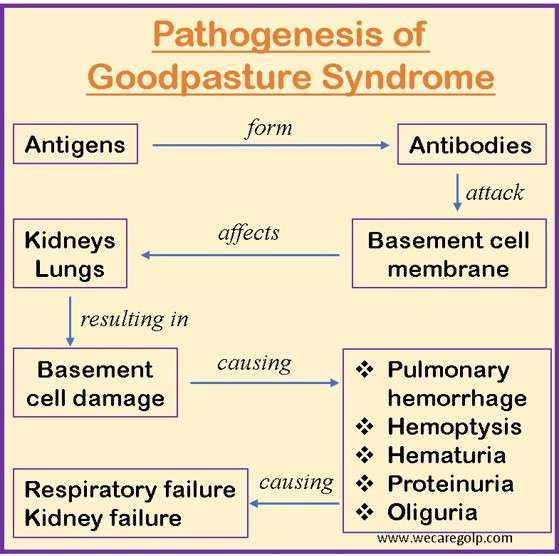
- The presence of autoantibodies directed against the glomerular and alveolar basement membrane contributes to the pathophysiology of Goodpasture syndrome. Like with many autoimmune diseases, the exact etiology of Goodpasture’s Syndrome is unknown.
- It is thought to be a type II hypersensitivity reaction to Goodpasture’s antigens on kidney glomeruli and pulmonary alveoli cells. In Goodpasture syndrome patients, the basement membrane (which includes triple chain type IV collagen) lining the alveoli and glomeruli is severely affected.
- Autoantibodies cause tissue damage by activating the complement system in the basement membrane. A linear deposition of immunoglobulins along the basement membrane can be visible due to autoantibody binding.
- The inflammatory reaction in that location produces the classic glomerulonephritis appearance.
- The non-collagenous 1 domain of alpha-3 type IV collagen is the target antigen associated with the most detrimental impact in anti-GBM disease. There is a substantial link between anti-GBM disease and the HLA DRB1-1501 allele. This allele is linked to renal damage.
- A disruption between the filtration barrier and the Bowman’s capsule results from the capacity of anti-GBM antibodies to bind and activate the complement system and proteases, which is the underlying cause of renal damage.
- The damage to Bowman’s capsule and filtration barrier causes the development of crescent-shaped antigen-antibody complexes in the renal corpuscles. These complexes cause proteinuria and activate neutrophils, macrophages, and CD4+ and CD8+ T cells.
- T cells are crucial in the onset and development of Goodpasture syndrome, even though it is thought to be an autoantibody-mediated disease. T cells have a direct involvement in renal and pulmonary damage by causing B-cells to produce more antibodies.
- The glomerulus and the alveolar basement membrane both have the same collagen target. Type 4 collagen, which may be represented as six distinct chains ranging from alpha 1 to alpha 6, is the major substance of the basement membrane.
- Even though anti-GBM disease has circulating antibodies, pulmonary symptoms are not always present. The likelihood of pulmonary symptoms appears to increase with initiating lung damage.
- The alveolar endothelium in a healthy person serves as a defense against anti-basement membrane antibodies. Yet, if an injury causes the alveolar capillaries to become more permeable, it causes autoantibodies to trespass and bind to the basement membrane.
- Alveolar-capillary permeability may be enhanced as a result of several causes like bacteremia, tobacco smoking, increased capillary hydrostatic pressure, endotoxemia, and upper respiratory tract infections.
Diagnosis of Goodpasture Syndrome
Urine test
- RBC casts
- Gross or microscopic hematuria
- Low-grade proteinuria
Blood test
- Leukocytosis is typically present, and a complete blood count may indicate anemia brought on by intrapulmonary hemorrhages.
- Renal function tests: It may be abnormal as a result of kidney dysfunction.
- Pulmonary function test: Since carbon monoxide binds to intra-alveolar hemoglobin, pulmonary function tests reveal increased carbon monoxide diffusing capacity.
- Anti-GBM antibody titer
- Arterial blood gas
Chest radiograph
- A chestradiograph reveals patchy parenchymal opacifications that are generally bilateral and bibasilar.
- The costophrenic angles and apices are often unaffected.
Biopsy
- The gold standard for diagnosis is a kidney biopsy, however, it is not necessary to start treatment or continue it if a biopsy is not possible.
- When conducted, a biopsy offers crucial details about the level of activity and chronicity of renal involvement that may affect treatment.
- The suggested approach to confirm a diagnosis of Goodpasture syndrome is a percutaneous kidney biopsy.
- Although a kidney biopsy has a significantly better yield than a lung biopsy, a lung biopsy can still be done if a renal biopsy is not an option for whatever reason.
Immunofluorescence stains
- It is a more precise and accurate diagnostic test.
- They show immunoglobulin G (IgG) and complement (C3) deposits on the glomerular basement membrane.
Serologic testing for ELISA
- On suspicion of the disease, serologic testing for ELISA assay for circulating anti-GBM antibodies should be performed immediately.
- It is critical that the alpha3 NC-1 domain region of collagen 4 be utilized as the target in this assay, as less specific testing may result in false positives.
- Quantitative titers are used to guide treatment decisions during the therapy phases.
Treatment of Goodpasture Syndrome
- Patient with Goodpasture syndrome usually appears in the emergency department in critical condition, therefore the standard indications frequently necessitate
- Urgent hemodialysis, and
- Intubation may be required in cases of respiratory failure.
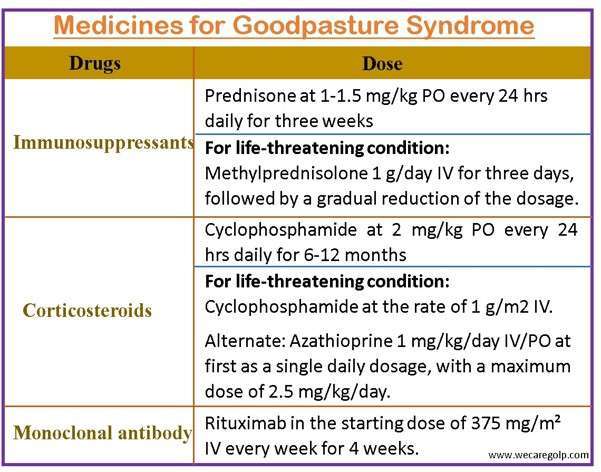
- The cornerstones of treatment for Goodpasture syndrome are
- Plasmapheresis
- Cyclophosphamide
- Corticosteroids
- If this therapy can begin before the patient requires renal replacement therapy, the patient’s overall renal prognosis improves. Regardless of renal state, all patients with pulmonary symptoms should begin combination treatment.
- For patients who do not have pulmonary symptoms and whose renal recovery is highly doubtful (i.e., those who came with an urgent need for dialysis within the first 72 hours), the risks of plasmapheresis and immunosuppressive therapy may exceed the benefits. Because the condition has not been thoroughly investigated, the decision to withhold treatment for these individuals remains controversial.
Plasmapheresis
- Plasmapheresis (plasma exchange) is a procedure that removes unwanted antibodies from the blood.
- Plasmapheresis is done for a total of 10 to 14 sessions.
- Daily plasmapheresis is typically carried out until anti-glomerular basement membrane antibodies are undetectable, followed by 3 to 6 months of steroid and cyclophosphamide treatment until complete remission is obtained.
- This may be assessed by running further titers after plasmapheresis and at any time new symptoms emerge that can be a sign of recurrence.
Immunosuppressant drugs
- Cyclophosphamide is first administered orally at a dosage of 2 mg/kg that is modified to maintain a white cell count of about 5000.
Steroids
- Corticosteroid is used for at least 6 months, followed by immunosuppressive medication in decreasing dosages for 2 to 3 months.
- Acute, life-threatening alveolar hemorrhage is treated with 1g/day IV pulse treatment of methylprednisolone for 3 days, then a tapering dosage of 1 to 1.5 mg/kg orally.
Monoclonal antibody
- Rituximab is an alternate therapy for Goodpasture’s disease.
- Rituximab is useful in the recovery of renal function in individuals receiving renal dialysis for 2 to 4 weeks.
- Because of its lengthy activation period, rituximab is not an appropriate induction treatment for Goodpasture.
Supportive Management
- To restore as much renal function as possible, antihypertensive drugs are required. Angiotensin-converting enzyme (ACE) inhibitors and angiotensinogen receptor blockers (ARBs) are most frequently utilized.
- In case of acute renal failure, the fluid and electrolyte imbalance must be avoided by restricting the total fluids and salt intake. If fluid overload is unavoidable, dialysis may be necessary.
- Till renal recovery, protein-rich foods must be reduced in the diet.
Complications of Goodpasture Syndrome
- Alveolar hemorrhage
- End-stage renal disease: Although renal failure is a typical consequence ofs Goodpasture syndrome patients, only around 30% of those who survive require long-term dialysis. In severe case, a kidney transplant is required.
- Pulmonary failure
- Anemia
- Hypoxemia
Prevention of Goodpasture Syndrome
- Patient education is critical in the early detection and management of Goodpasture syndrome.
- Avoiding exposure to environmental stimuli may prevent GS.
- To prevent repeated pulmonary bleeding, patients with lung involvement should be strongly counseled not to smoke.
- Patients should be educated on the signs and symptoms of renal failure since some steroid and immunosuppressive therapies might affect the kidneys.
- The patient’s renal function and drug doses should be checked regularly.
- Individuals with severe renal failure require long-term dialysis and should be recommended to seek medical attention as soon as possible.
- Individuals whose renal function is decreasing despite maximal medical therapy should be placed on a renal transplant waiting list, and patients should be warned that Goodpasture syndrome may reoccur in the donated kidney, though this is extremely unusual.
Prognosis
- The emergence of aggressive therapy such as plasmapheresis, corticosteroids, and immunosuppressive drugs has considerably improved the prognosis of Goodpasture syndrome.
- The 5-year survival rate with these medicines has surpassed 80%, and less than 30% of patients require long-term dialysis.
- Yet, delayed cyclophosphamide therapy has been linked to a catastrophic prognosis.
- Individuals with serum creatinine levels of more than 4 mg/dL, oliguria, and more than 50% crescents on renal biopsy have a low chance of recovery.
- They typically proceed to end-stage renal failure, necessitating long-term dialysis.
Summary
- Goodpasture syndrome, also known as anti-GBM antibody disease, is an autoimmune disease that is characterized by cytotoxic antibody damage to the alveolar and renal glomerular basement membranes. It is classified as a pulmonary-renal condition.
- The antibodies in the lungs attack a protein called collagen found in the basement membrane of the alveoli, causing swelling and bleeding. Antibodies assault the glomerular basement membrane of the kidneys, resulting in kidney damage and failure.
- It is characterized by hemoptysis, respiratory problems, exhaustion, and renal failure. Anti-GBM antibodies are frequently tested for during diagnosis.
- Immunosuppressive drugs to lower the immune response, plasma exchange to eliminate antibodies from the blood, and occasionally kidney dialysis or transplantation make up the standard treatment regimen. The Goodpasture syndrome can be fatal if left untreated.
Other Autoimmune Disorders
- Multiple Sclerosis (MS)
- Guillain-Barré syndrome (GBS)
- Myasthenia Gravis
- Rheumatoid Arthritis
- Vasculitis
- Type 1 Diabetes
- Systemic Lupus Erythematosus (SLE)
- Inflammatory Bowel Disease (IBD)
- Hashimoto’s Thyroiditis
References
- Borza, D. B., Neilson, E. G., & Hudson, B. G. (2003, November). Pathogenesis of Goodpasture syndrome: a molecular perspective. In Seminars in nephrology, 23(6), pp. 522-531). WB Saunders. https://doi.org/10.1053/S0270-9295(03)00131-1
- DeVrieze, B. W., & Hurley, J. A. (2022). Goodpasture syndrome. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK459291/
- Dolkar, M. D., Dhasmana, M. S., & Pant, M. (2021). GOODPASTURE’S SYNDROME: TREATMENT AND PROGNOSIS UPDATE. Webology, 18(1). DOI: 10.29121/WEB/V18I1/33
- Gibson, C.M., (2018, May 2). Goodpasture Syndrome. WikiDoc. Retrieved on 2023, April 7 from https://www.wikidoc.org/index.php/Goodpasture_syndrome
- Greco, A., Rizzo, M. I., De Virgilio, A., Gallo, A., Fusconi, M., Pagliuca, G., … & De Vincentiis, M. (2015). Goodpasture’s syndrome: a clinical update. Autoimmunity reviews, 14(3), 246-253. https://doi.org/10.1016/j.autrev.2014.11.006
- Kathuria, P., (2023, March 22). Goodpasture Syndrome. Medscape. Retrieved on 2023, April 7 from https://emedicine.medscape.com/article/240556-overview#a1
- Kielstein, J. T., Helmchen, U., Netzer, K. O., Weber, M., Haller, H., & Floege, J. (2001). Conversion of Goodpasture’s syndrome into membranous glomerulonephritis. Nephrology Dialysis Transplantation, 16(10), 2082-2085. https://doi.org/10.1093/ndt/16.10.2082
- Lee, J., (2022, September). Goodpasture Syndrome. MSD Manual. Retrieved on 2023, April 6 from https://www.msdmanuals.com/professional/pulmonary-disorders/diffuse-alveolar-hemorrhage-and-pulmonary-renal-syndrome/goodpasture-syndrome
- Reggiani, F., L’Imperio, V., Calatroni, M., Pagni, F., & Sinico, R. A. (2023). Goodpasture syndrome and anti-glomerular basement membrane disease. Clinical and experimental rheumatology. doi: 10.55563/clinexprheumatol/tep3k5