Introduction
Entomopathogenic nematodes (EPNs) are useful biocontrol agents or ‘bio-predators’ that have been successfully used in the management of various soil-inhabiting insect pests worldwide (Nguyen & Smart, Reference Nguyen and Smart1996; Bhat et al., Reference Bhat, Chaubey and Askary2020). The role of EPNs in the development of integrated pest management (IPM) is significant because as natural enemies, EPNs are highly virulent, able to kill their hosts and suppress the population of various insect pests within a short period of time. They also offer an environmentally safe alternative to the use of pesticides, due to their safety to humans and non-target organisms; while retaining their environmental friendliness and relative ease of application. Their function as natural enemies or predators of agricultural pests has made their use as potential alternatives to chemical pesticides a very attractive option in the development of IPM programmes.
The EPNs belonging to the genera Steinernema and Heterorhabditis have been frequently reported as having great potentials for the biological control of insect pests of economic importance worldwide (Kaya & Gaugler, Reference Kaya and Gaugler1993; Lacey & Georgis, Reference Lacey and Georgis2012; Gozel & Gozel, Reference Gozel and Gozel2016; Bhat et al., Reference Bhat, Chaubey and Askary2020). The relevance of these nematodes as biocontrol agents in South Africa is being demonstrated by the extent of research activities that were dedicated to the identification and reporting of new local species (Malan et al., Reference Malan, Nguyen and Addison2006, Reference Malan, Nguyen, De Waal and Tiedt2008, Reference Malan, Knoetze and Moore2011, Reference Malan, Knoetze and Tiedt2014; Malan & Ferreira, Reference Malan, Ferreira, Fourie, Spaull, Jones, Daneel and De Wale2017; Abate et al., Reference Abate, Slippers, Wingfield, Malan and Hurley2018; Ramakuwela et al., Reference Ramakuwela, Hatting, Laing, Thiebaut and Hazir2018; Lephoto & Gray, Reference Lephoto and Gray2019a; Lulamba & Serepa-Dlamini, Reference Lulamba and Serepa-Dlamini2020). These nematodes were shown to exhibit biocontrol potentials against a wide range of insect pests including: codling moth (Cydia pomonella); false codling moth (Thaumatotibia leucotreta); wood wasps (Sirex noctilio); mealybug (Planococcus ficus); the banded fruit weevil (Phlyctinus callosus); fruit flies (Ceratitis capitata and. Ceratitis rosa); sugarcane stalk borer (Eldana saccharina); and leaf miners Holocacista capensis (Lepidoptera: Heliozelidae) (De Waal et al., Reference De Waal, Malan and Addison2011; Odendaal et al., Reference Odendaal, Addison and Malan2016; Malan & Ferreira, Reference Malan, Ferreira, Fourie, Spaull, Jones, Daneel and De Wale2017; Platt et al., Reference Platt, Stokwe and Malan2018; Steyn et al., Reference Steyn, Addison and Malan2019). More recently, attention is being focused on the development of commercial and industrial-scale production of the EPNs (Van Zyl & Malan, Reference Van Zyl and Malan2014; Kagimu & Malan, Reference Kagimu and Malan2019; Dunn et al., Reference Dunn, Belur and Malan2020).
The life cycle of EPNs is similar to other nematodes comprising egg, four juvenile stages, and the adults. These nematodes have a special non-feeding infective 3rd stage dauer larvae which emerges at the depletion of the food resource within the host cadaver and migrates in search of a new host. The dauer larvae carry their symbiotic bacteria in the gut and can survive several months in the soil until a suitable host is found. The duration of the life cycle is dependent on the temperature and can vary between species and locality; it however usually completes its life cycle within 6–18 days at a temperature range of 18–28°C (Subramanian & Muthulakshmi, Reference Subramanian, Muthulakshmi and Omkar2016).
Although the occurrence of EPNs on many conventional agricultural lands, orchards, vinelands and forest plantations in South Africa has been well documented (Hatting et al., Reference Hatting, Stock and Hazir2009; Malan & Ferreira, Reference Malan, Ferreira, Fourie, Spaull, Jones, Daneel and De Wale2017; Abate et al., Reference Abate, Slippers, Wingfield, Malan and Hurley2018) in many cases, low percentage frequency of occurrence of the EPNs were obtained from the sampling expenditures. Malan et al. (Reference Malan, Nguyen and Addison2006) reported that EPNs were recovered from only about 7% of sampled fields. Hatting et al., Reference Hatting, Stock and Hazir2009 also reported a 5% recovery rate, while a recovery rate of 11% was documented from citrus orchards by Malan et al. (Reference Malan, Knoetze and Moore2011). Elsewhere in Africa, a 6.5% positive record for EPNs was documented from soil samples obtained from a survey of EPNs in Northern Nigeria (Akyazi et al., Reference Akyazi, Ansari, Ahmed, Crow and Mekete2012). According to Abate et al. (Reference Abate, Slippers, Wingfield, Malan and Hurley2018), about 20 species of the EPN have been recorded from the African continent, with South Africa accounting for most of the reported species within the continent. In a survey that cuts across five provinces in South Africa, Hatting et al. (Reference Hatting, Stock and Hazir2009) reported EPNs from different habitats, such as undisturbed natural grasslands, vineyards, fruit and vegetable orchards, wheat, sugarcane and rooibos tea orchards. There are strong indications from the literature (Freckman & Ettema, Reference Freckman and Ettema1993; Birkhofer et al., Reference Birkhofer, Bezemer and Bloem2008) that suggest that the type of habitat and agricultural systems could determine the fauna diversity in the soil and this is especially true of nematodes, which have proven to be useful indicators of soil and environmental health.
The need for more indigenous species of EPNs to provide biological control against endemic insect pests in Africa is increasing in a bid to reduce harvest losses due to pests and diseases. This is crucial in the fight against impending food crisis, combating hunger and promoting food security in the continent. For instance, the biocontrol efficacy of a native EPN species was demonstrated against the devastating tomato leaf miner, Tuta absoluta Meyrick Lepidoptera: Gelechiidae, which was reported as a serious pest against tomato production in many African countries (Ndereyimana et al., Reference Ndereyimana, Nyalala and Murerwa2019; Dlamini et al., Reference Dlamini, Dlamini, Masarirambi and A2020). It is therefore important to identify species that are adaptable to existing IPM systems, well suited to native environmental conditions, and which could be mass-produced for industrial and commercial purposes.
Honeybush, Cyclopia Vent. belongs to the leguminous family Fabaceae. The genus comprises 23 recognized species (Schutte, Reference Schutte1997; Joubert et al., Reference Joubert, Joubert, Bester, de Beer and De Lange2011). Honeybush is an important herbal tea plant that is locally renowned as possessing great medicinal values for controlling type 2 diabetes among other health benefits. It is caffeine-free and rich in anti-inflammatory and antioxidant properties. Organic honeybush tea production is common in the Bredasdorp area of South Africa and is being practised by some farmers due to customer preference for improved quality and health benefits. These, coupled with the relative ease of cultivation have made organic cultivation of this indigenous tea plant an attractive option to some farmers. The organic honeybush farms in Bredasdorp are semi-natural cultivated plantation with a moderate level of disturbance. The farms are more than twenty years old with harvesting regime of 2–3 years. The occurrence of EPNs on organic tea orchards is yet to be documented in South Africa. High susceptibly of honeybush to some plant-parasitic nematodes has been reported; however, this was associated with the conventional system of cultivation of the tea plants (Daramola et al., Reference Daramola, Malan and Lewu2021). Furthermore, there are indications that organic cultivation could support a high diversity of EPNs. In the current study, the diversity and distribution of EPNs that are associated with organic honeybush tea plants in the Western Cape province of South Africa were investigated.
Materials and methods
Soil sampling and isolation of EPNs
Soil samples were collected from four organic honeybush farmlands that are located around Bredasdorp, Western Cape province of South Africa in October 2018. The farmlands comprised a blend of four different species of Cyclopia including: Cyclopia genistoides; Cyclopia longifolia, Cyclopia macula; and Cyclopia subternata. Soil samples were collected from the base of the honeybush plants up to a depth of about 20 cm with the aid of a hand trowel and/or spade. The farmlands were divided into different plots from which 20 soil sub-samples/hectare were collected, bulked and transported inside plastic bags to the laboratory (with ice in cooler boxes) for further processing. A total of 18 bulked soil samples were collected from nine plots on the organic honeybush farms.
Nematodes were recovered from the sub-samples, which were taken from the bulked soil samples from the sampled plots. About 250 ml of soil were placed inside 500 ml plastic containers and 10 greater wax moths, Galleria mellonella (Lepidoptera: Pyralidae) larvae were placed in each container. The containers were covered with lids and placed in a dark room at 25°C for 7 days, after which the Galleria larvae were examined for infection with EPNs.
The EPNs were recovered from the infected cadavers of Galleria larvae that were collected from the containers after incubation for 7 days. The dead larvae were picked individually and placed on top of moist filter papers, which were placed inside a Petri dish using a modified White's trap method (White, Reference White1927). Infective juveniles (IJs) were harvested from the set-up after emergence were further tested for pathogenicity by infecting 10 mealworm, Tenebrio molitor L. (Coleoptera: Tenebrionidae) larvae with about 200 IJs each in a 5-cm-diameter Petri dish which was lined with Whatman No. 1 filter papers. The isolates that satisfied Koch's postulates were stored in sterilized distilled water in 500 ml flat tissue culture flasks with vented lids at 14°C. The EPNs were recycled on Galleria larvae after consecutive three months.
Nematode identification
The EPNs that were recovered from the soil samples were examined under the microscope and identified to their genus level, based on their morphological features. The females, males and hermaphrodites of the first generation were observed and identified microscopically (Nguyen & Smart, Reference Nguyen and Smart1996). However, the species of the EPNs were further confirmed, using molecular tools.
Molecular identification of the EPNs was done by extracting DNA from single young adult females using a modified method of Nguyen (Reference Nguyen, Nguyen, Hunt, Hunt and Perry2007). The nematodes were cut into 2–3 parts with the sharp side of a sterile needle in 30 ml lysis buffer (500 mm magnesium chloride,10 mm dithiothreitol, 4.5% Tween20®, 0.1% gelatine and 3 μl proteinase K at 600 μg ml-1) which was placed on the side of an Eppendorf tube. The tubes were refrigerated at −80°C for 15 min and then incubated in a thermocycler at 65°C for 1 h followed by 95°C for 10 min and then centrifuged at 12,000 rpm for 2 min.
Polymerase chain reaction (PCR) for the amplification of the internal transcribed spacer (ITS) regions of the ribosomal DNA gene was done in a thermocycler using the forward primer TW81 (5′-GTTTCCGTAGGTGAACCTGC-3′) and the reverse primer AB28 (5′ATATGCTTAAGTTCAGCGGGT-3′). The PCR was done in a thermocycler with the cycling profile comprising 1 cycle of initial denaturation at 94°C for 3 min followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 45 s, and a final incubation at 72°C for 7 min (Chaerani et al., Reference Chaerani, Prabowo and Indrayan2019). The PCR products were electrophoresed and separated using 1.5% agarose gel in 0.5× TBE buffer stained with ethidium bromide. Images were taken under ultraviolet light with a transilluminator. Sequencing of the amplicons was done in both directions at the DNA Sequencing Unit (Central Analytical Facility of Stellenbosch University) using the Big Dye Terminator VI sequencing kit.
Sequence and phylogenetic analyses
The sequence data, including the forward and reverse DNA strands, were manually assembled and edited using BioEdit version 7.2 (Hall, Reference Hall1999). Newly obtained sequences were deposited in the GenBank with accession numbers obtained. The sequences were compared with published sequences downloaded from the National Center for Biotechnology Information (NCBI) using the BLASTn sequence nucleotide search (Altschul et al., Reference Altschul, Madden, Schaffer, Zhang, Zhang, Miller and Lipman1997) and the sequences were aligned with multiple alignment programmes for amino acid or nucleotide sequences (MAFFT version 7).
Phylogenetic analysis of the ITS regions of the EPN isolates was done using the software for molecular evolutionary genetics analysis, MEGA X version 10.0.5 (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018). The evolutionary history among species was inferred using the maximum parsimony (MP) method and the confidence intervals for the various branching patterns were measured using bootstraps (Felsenstein, Reference Felsenstein1985) with 1000 replicates. Caenorhabditis elegans accession FJ589008 was used as the outgroup in both analyses.
Results
Entomopathogenic nematodes associated with Cyclopia species
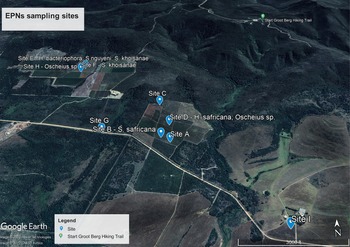
A total of nine EPN isolates were recovered from the eighteen soil samples that were examined for the presence of EPNs. This amounts to 50% of the sampled fields. EPNs belonging to the genera Heterorhabditis, Steinernema and Oscheius were isolated from organic honeybush farms and identified using molecular tools. They include: Heterorhabditis bacteriophora; Heterorhabditis safricana;. Steinernema khoisanae; Steinernema nguyeni; Oscheius sp.; and an unidentified species (table 1). The EPNs were identified from Cyclopia longifolia, C. genistoides, C. maculata and C. subternata, which were the four dominant Cyclopia species on the sampled honeybush farmlands in fig. 1.

Fig. 1. Sampling sites on honeybush orchards around Bredasdorp in the Western Cape province of South Africa (image generated from Google Maps).
Table 1. Heterorhabditis and Steinernema species, obtained from organic honeybush orchards in Western Cape, South Africa.

Pathogenic effect and reproduction of EPNs on G. mellonella larvae
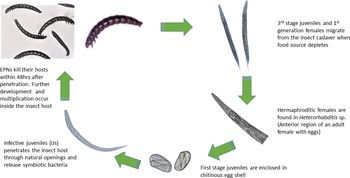
The pathogenic effect of the isolated EPNs was confirmed on G. mellonella larvae. All nine newly obtained isolates showed good potential as biocontrol agents by producing 100% mortality of infected larvae within 48 h of exposure to the IJs. Both sexual and asexual generations (amphimitic and hermaphroditic forms) were observed in the heterorhabtids (fig. 2) while only amphimictic populations of the steinernematids were observed inside their insect hosts. Second generation IJs were seen migrating from the insect cadaver after 7 days. The lifecycle of the EPNs were completed within 18 days at an average temperature of 25°C.
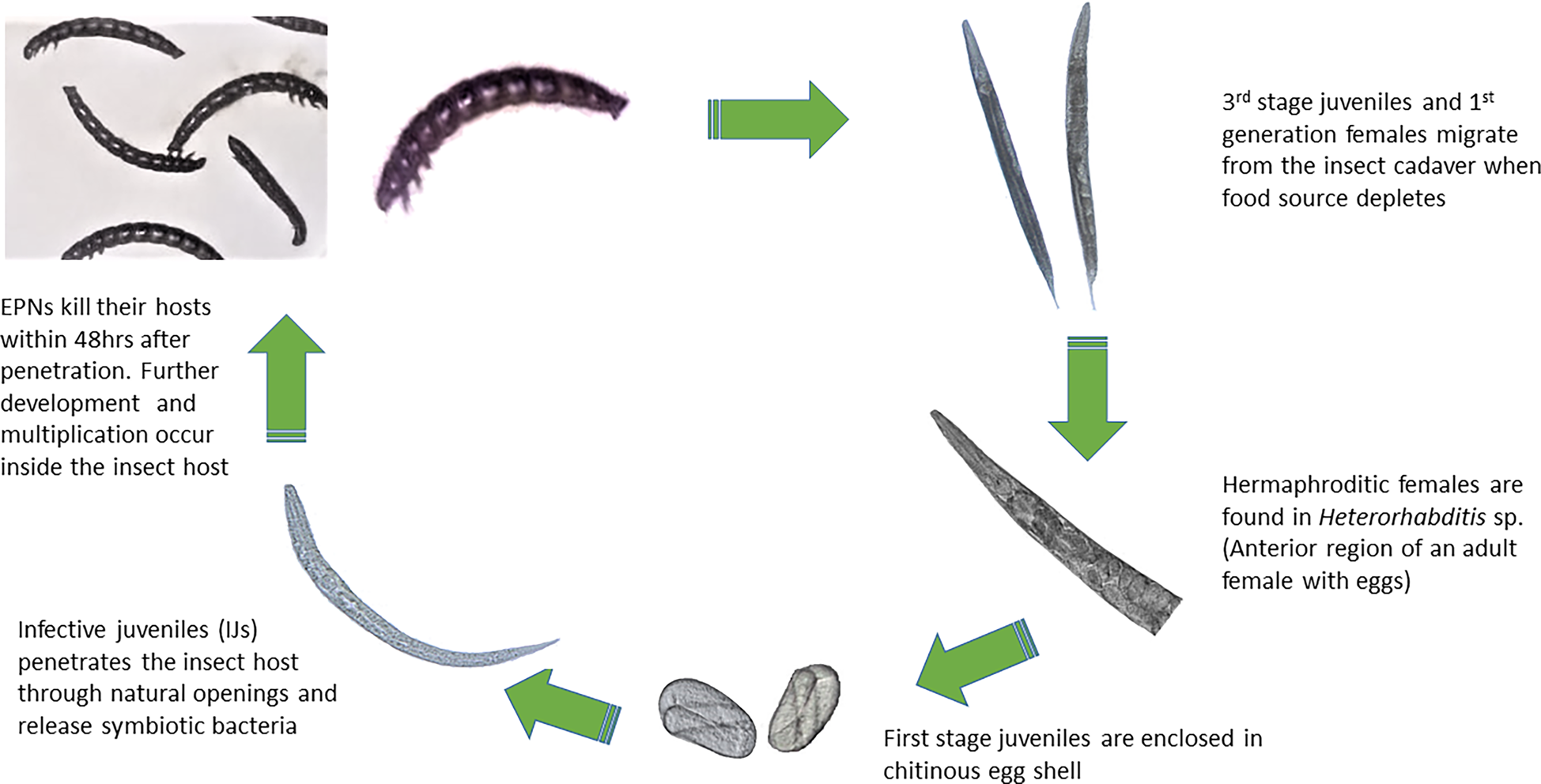
Fig. 2. Life cycle of the entomopathogenic nematode.
Molecular identification and phylogenetic analysis
Molecular identification using the amplification of ITS region of the ribonucleic RNA of the EPN isolates showed the presence of the two families (Heterorhabditidae and Steinernematidae) on the honeybush farms with accession numbers assigned to the newly obtained sequences (MW969648, MW915609, MW915610, MW915805, MW915655, MW970232 and MW970233). Their percentage similarities as compared with closely related sequences on the GenBank are indicated in table 1.
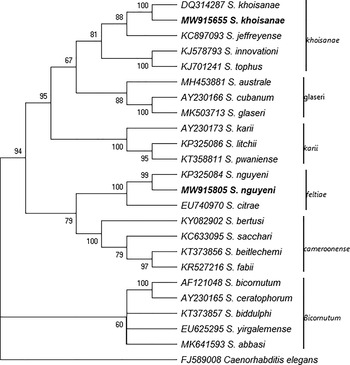
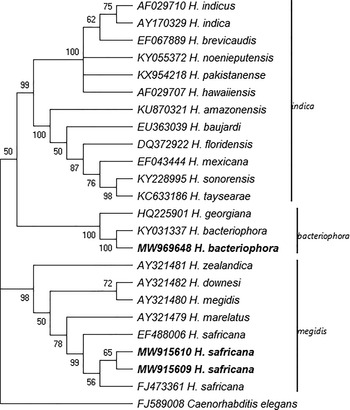
The phylogenetic analyses of the Steinernema and Heterorhabditis species as inferred by the MP method based on the analysis of the ITS rDNA regions are shown in figs. 3 and 4. The analysis involved 23 Steinernema nucleotide sequences that have been reported in South Africa and the type specimen of some closely related species from GenBank using C. elegans as the outgroup. The most parsimonious tree with length 2207 is shown in fig. 3. There were a total of 1013 positions in the final dataset. Figure 4 shows the phylogenetic relationship of the newly obtained sequences of H. bacteriophora and H. safricana with their closely related species. The analysis involved 25 nucleotide sequences and the most parsimonious tree with length 393 is shown. The result of the analysis of the ITS rDNA region shows that the S. nguyeni and S. khoisanae that were isolated in this study clustered with other published S. nguyeni and S. khoisanae sequences. The former (S. nguyeni) falls within the Steinernema feltiae-group along with Steinernema citrae while S. khoisanae falls within the Steinernema glaseri-group. The phylogenetic analysis of the species within the genus Heterorhabditis indicates that the newly obtained sequences of H. bacteriophora and H. safricana obtained from the honeybush samples both clustered with previously published sequences of H. bacteriophora and H. safricana, respectively, and were both placed in the ‘bacteriophora’ and ‘megidis’ clades. The sequence of the unknown EPN species was not included in the analysis.

Fig. 3. Phylogenetic relationship of the Steinernema species reported from South Africa and the type specimen of closely related species from GenBank as inferred by maximum parsimony (MP) based on analysis of the internal transcribed spacer region using Cyclopia elegans as the outgroup. The newly obtained sequences are in boldface type.

Fig. 4. Phylogenetic relationship of the species belonging to the genus Heterorhabtid as inferred by maximum parsimony (MP) based on analysis of the internal transcribed spacer region using Cyclopia elegans as the outgroup. The newly obtained sequences are in boldface type.
Discussion
Some of the EPNs that were isolated from organic honeybush orchards in the current study, include those that have been demonstrated as useful biocontrol agents with proven potential against serious agricultural pests such as T. absoluta, C. pomonella and fruit fly species, Ceratitis capitata, and C. rosa (Malan & Manrakhan, Reference Malan and Manrakhan2009; De Waal et al., Reference De Waal, Malan and Addison2011; Ndereyimana et al., Reference Ndereyimana, Nyalala and Murerwa2019). The result of this study also corroborates the reports of previous surveys on the biogeography of these nematodes (Malan et al., Reference Malan, Nguyen and Addison2006; Hatting et al., Reference Hatting, Stock and Hazir2009; Malan et al., Reference Malan, Knoetze and Moore2011), therefore confirming that these EPN species are widely distributed in the Western Cape province of South Africa. Some of these nematodes have also been reported outside the shores of South Africa with H. bacteriophora being the most frequently reported of the EPNs (Nguyen & Hunt, Reference Nguyen, Hunt, Nguyen, Hunt, Hunt and Perry2007; Bhat et al., Reference Bhat, Askary, Ahmad, Suman, Aasha and Chaubey2019).
Most of the EPNs species that act as vectors transporting pathogenic bacteria and causing insect mortality belong to the genera Steinernema and Heterorhabditis (Shapiro-Ilan et al., Reference Shapiro-Ilan, Mbata, Nguyen, Peat, Blackburn and Adams2009). Also, nematodes belonging to genus Oscheius have shown great biocontrol potential against insect species (Torres-Barragan et al., Reference Torres-Barragan, Suazo, Buhler and Cardoza2011; Ghavamabad et al., Reference Ghavamabad, Talebi, Mehrabadi, Farashiani and Pedram2021). However, their entomopathogenic potential is not as widely studied and reported in the literature (Dillman & Sternberg, Reference Dillman and Sternberg2012). In the present investigation, Oscheius species that were recovered from the honeybush orchards caused mortality of Galleria larvae, thereby confirming their biological control potentials against the insect bait. More recently, reports of the biological control activities of Oscheius species on insect pests in South Africa has been documented (Serepa-Dlamini & Gray, Reference Serepa-Dlamini and Gray2018; Lephoto & Gray, Reference Lephoto and Gray2019b).
The biocontrol efficiency of these EPN genera (Steinernema, Heterorhabditis and Oscheius) to cause mortality of their insect hosts is associated with the virulence of their symbiotic bacteria: Xenorhabdus; Photorhabdus and Serratia spp., respectively (Dillman & Sternberg, Reference Dillman and Sternberg2012; Lulamba et al., Reference Lulamba, Green and Serepa-Dlamini2019). However, studies on the relationship of EPNs with their symbiotic bacteria was beyond the scope of the current investigation but will be considered in future studies. An unidentified EPN species was also isolated from the honeybush orchards. The DNA sequence was not similar to any previously known or published EPN sequence on the NCBI. The current finding attests to the fact that there is a whole lot of undiscovered biodiversity of EPNs existing in nature, thereby underscoring the need for a more rigorous and extensive survey to unravel the enormous bio-resource potential within the soil ecosystem.
The ITS region of the ribonucleic RNA provided a good molecular marker for the identification of the EPNs in this study. The use of molecular tools for nematode identification provides a rapid and reliable option for nematode identification and helps to reduce erroneous identification/misidentifications of species due to morphological similarity, overlapping morphometric measurements and variability of some diagnostic features (Van den Berg et al., Reference Van den Berg, Tiedt and Subbotin2014). Molecular identification in combination with morphological characters is often recommended for nematode characterization (Oliveira et al., Reference Oliveira, Monteiro and Blok2011); however in the present study, new species are not being described but we identified EPN species that have been previously described, therefore detailed morphological characterization and morphometric measurements were not required. Phylogenetic analysis of the EPNs isolated in the current study also gave an accurate picture of the isolates and their genetic relationship with other species within the Steinernema and Heterorhabditis genera, as compared to other published isolates that are reported in South Africa and from other countries.
Successful application of EPNs as a biocontrol agent of pests is hinged on appropriate temperature and moisture. According to Kaya (Reference Kaya, Gaugler and Kaya1990), the temperature range for survival and infectivity will depend on the species of EPN and its native habitat and centre of origin. Thus, there is a continuous drive for the identification of more indigenous species that can adapt well to prevailing climatic conditions and be more suited for South African soil. Environmental factors such as soil type, aeration, temperature and moisture contribute significantly to the distribution, survival and infectivity of EPNs (Kaya, Reference Kaya, Gaugler and Kaya1990; Lacey & Georgis, Reference Lacey and Georgis2012). Other factors include the presence of suitable host and soil fauna. Furthermore, agronomic practices such as tillage methods, pest control, fertilizer application and cropping systems could affect the abundance and survival of these nematodes in the soil. There are indications that the type of farming system such as conventional agriculture could contribute to a disturbance in the soil ecosystem, thereby having a consequent negative effect on the diversity and distribution of EPNs in the soil whereas organic agriculture seems to promote conservation of soil fauna diversity.
The recovery rate of 50% in the isolated EPNs from the sampled honeybush fields in the Bredasdorp area of Western Cape, therefore, reflects an abundance of the EPNs in this habitat and geographical location. The prevalence of EPNs in this region might be due to the availability of insect hosts, a suitable soil type along with behavioural and physiological adaptations (Adams et al., Reference Adams, Fodor, Koppenhöfer, Stackebrandt, Stock and Klein2006). Species richness and substantial diversity of the EPNs are also implied from the study. According to Malan et al. (Reference Malan, Knoetze and Moore2011), low recovery of the EPNs is common in many surveys and low detection of EPNs in the soil does not necessarily mean that the EPNs are not present, but might be due to insect aggregation (Campos-Herrera et al., Reference Campos-Herrera, Pathak, El-Borai, Stuart, Gutiérrez, Rodríguez-Martín, Graham and Duncan2013). EPNs tend to aggregate more in the soil rather than being randomly distributed and larger sample sizes have been recommended to increase chances of detection and diversity (Chaerani et al., Reference Chaerani, Prabowo and Indrayan2019). In the present study, however, the 50% recovery rate of the EPNs was recorded from a relatively smaller sample size. Such a high rate of recovery could also be linked to the conservation tillage management that was practised on the organic honeybush orchards which tend to favour more detection of EPNs more than conventional tillage management where fluctuations in temperature and moisture reduce EPN population in the soil (Brust, Reference Brust1991; Campos-Herrera et al., Reference Campos-Herrera, Pathak, El-Borai, Stuart, Gutiérrez, Rodríguez-Martín, Graham and Duncan2013). Further intensive survey that involves a large scale sample size might reveal a higher diversity and increase in the number of native EPNs from this geographical region and that can be useful biocontrol tools against targeted insect pests. In addition, more laboratory investigations will provide a better understanding of the host specificity of the understudied EPNs and give better insight into the processes that can facilitate successful mass production of the EPNs into useful commercial products.
Acknowledgement
The authors are grateful to the management of Stellenbosch University for providing the facility for this study.
Financial support
None.
Conflicts of interest
None.