Introduction
The principal components of the lichen symbiosis are fungus and alga. Their intimate trophic relationship remains central to the lichen concept, despite our growing appreciation that other micro-organisms harboured within the thallus might also play significant roles (Lakatos et al. Reference Lakatos, Lange-Bertalot and Büdel2004; Grube & Berg Reference Grube and Berg2009; Bates et al. Reference Bates, Cropsey, Caporaso, Knight and Fierer2011; Grube et al. Reference Grube, Cernava, Soh, Fuchs, Aschenbrenner, Lassek, Wegner, Becher, Riedel and Sensen2015; Spribille et al. Reference Spribille, Tuovinen, Resl, Vanderpool, Wolinski, Aime, Schneider, Stabentheiner, Toome-Heller and Thor2016; Muggia & Grube Reference Muggia and Grube2018; Mark et al. Reference Mark, Laanisto, Bueno, Niinemets, Keller and Scheidegger2020; Smith et al. Reference Smith, Dal Grande, Muggia, Keuler, Divakar, Grewe, Schmitt, Lumbsch and Leavitt2020; Tzovaras et al. Reference Tzovaras, Segers, Bicker, Dal Grande, Otte, Anvar, Hankeln, Schmitt and Ebersberger2020). The lichen-forming fungi typically build distinctive vegetative tissues and characteristic sexual structures, providing numerous biological features for study and significant clues about phylogenetic relationships, which are now relatively well delimited at broader taxonomic levels (Jaklitsch et al. Reference Jaklitsch, Baral, Lücking and Lumbsch2016; Lücking et al. Reference Lücking, Hodkinson and Leavitt2017a). Lichen algae, by contrast, have proved much more elusive. Most are unicells or simple filaments, with sexual structures unknown or seldom reported. The paucity of phenotypic characters is often aggravated by their plasticity. Lichen algae may look and behave quite differently in symbiosis with different lichen-forming fungi, in the free-living condition in nature and in aposymbiotic laboratory culture (Fig. 1; Ahmadjian Reference Ahmadjian1967; Bubrick Reference Bubrick and Galun1988). All this has hindered progress in clarifying their identities, phylogenies and life histories. Schwendener (Reference Schwendener1869) was the first to survey lichen ‘gonidia’ in a phycological context, recognizing them as organisms distinct from the surrounding fungus that correspond to known taxa of free-living algae. In the last half-century, the diversity of lichen-forming algae has been reviewed by various authors (Ahmadjian Reference Ahmadjian1967; Létrouit-Galinou Reference Letrouit-Galinou1968; Henssen & Jahns Reference Henssen and Jahns1974; Friedl & Büdel Reference Friedl, Büdel and Nash2008), with a particularly thorough literature summary compiled and annotated by Tschermak-Woess (Reference Tschermak-Woess and Galun1988a).
In recent decades, our understanding of algal diversity and biosystematics has advanced substantially with the accumulation, analysis and integration of DNA sequence data. Systematic schemes for the eukaryotic algae have changed considerably, as the broad contours of consensus emerge concerning phylogenies and their reconstruction. Recent works have reviewed the current status of some principal algal groups with lichen-forming taxa, such as the genus Trebouxia (Muggia et al. Reference Muggia, Candotto-Carniel, Grube, Grube, Seckbach and Muggia2017), the class Trebouxiophyceae (Muggia et al. Reference Muggia, Leavitt and Barreno2018), the Coccomyxa-Elliptochloris clade (Gustavs et al. Reference Gustavs, Schiefelbein, Darienko, Grube, Seckbach and Muggia2017), the Trentepohliaceae (Grube et al. Reference Grube, Muggia, Baloch, Hametner, Stocker-Wörgötter, Grube, Seckbach and Muggia2017a), and the cyanobacteria (Rikkinen Reference Rikkinen, Grube, Seckbach and Muggia2017). Yet most taxa remain insufficiently understood. Even the most intensively studied genera, such as Trebouxia, are still unresolved with respect to species delimitation, and much new diversity continues to be uncovered (Muggia et al. Reference Muggia, Nelsen, Kirika, Barreno, Beck, Lindgren, Lumbsch and Leavitt2020). A great many algal symbionts, identified phenotypically (often without isolation into culture) or recorded merely as ‘trebouxioid’ or ‘chlorococcalean’, have yet to be revisited with DNA sequence analyses. Identities and relationships remain especially problematic among the cyanobacteria (blue-green algae), where sexual reproduction is absent, diversification is ancient (Garcia-Pichel Reference Garcia-Pichel and Schaechter2009) and horizontal gene transfer events may obscure the vertical components of phylogenies (Zhaxybayeva et al. Reference Zhaxybayeva, Gogrten, Charlebois, Doolittle and Papke2006). The aposymbiotic lives of lichen algae also remain largely unknown, despite their potential importance in active genetic mixing. Here an attempt is made to focus more attention on the algal side of the lichen partnership, still relatively neglected compared to that of the fungus. We include a synopsis of the relevant genera and list citations of algal taxa in lichen symbiosis (Table 1), emphasizing those published since Tschermak-Woess's (Reference Tschermak-Woess and Galun1988a) landmark review, and particularly those accompanied by genetic sequence data.
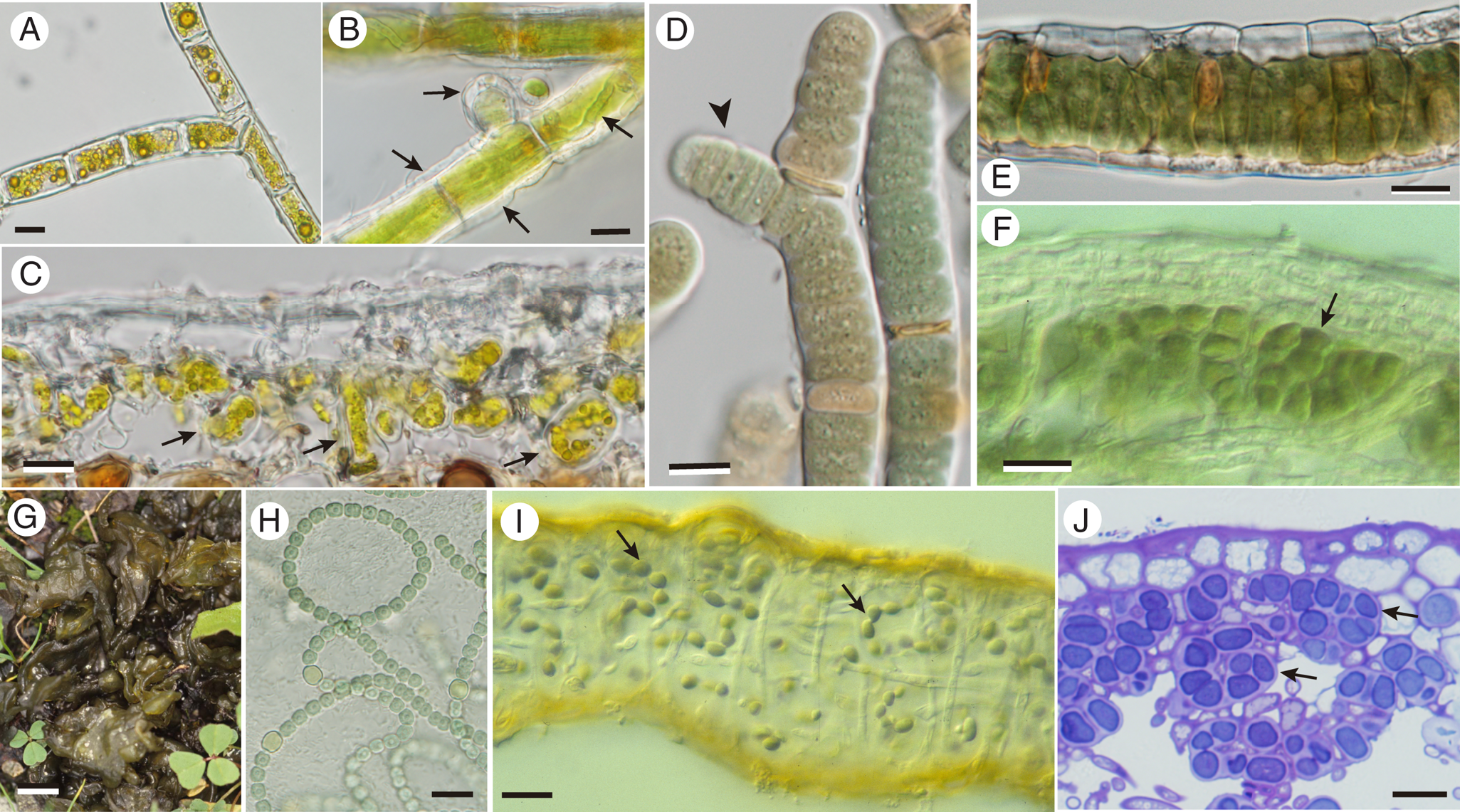
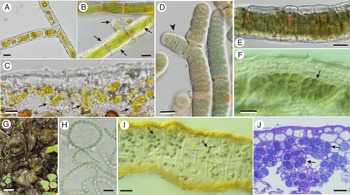
Fig. 1. Three filamentous lichen photobiont genera in aposymbiotic and symbiotic states. A–C, Trentepohlia. A, branching filament free-living on bark. B, lichenized by Coenogonium hyphae (arrows) growing over morphologically unchanged algal filament and its new branches (horizontal arrow). C, lichenized by Arthonia rubrocincta; the alga is largely broken up into individual cells or short segments. D–F, Rhizonema. D, cultured isolate from Dictyonema; note false branching (arrowhead). E, trichome ensheathed by cells of mycobiont Dictyonema. F, contorted or broken filaments (arrow) within thallus of Coccocarpia palmicola. G–J. Nostoc. G, free-living thallus-like macrocolony on soil. H, cultured strain. I, more or less intact filaments (arrows) within thallus of Collema furfuraceum. J, contorted or broken up into cell groups (arrows) within cyanomorph of Sticta canariensis. Scales: A–F, H–J = 10 μm; G = 1 cm.
Table 1. Taxonomically grouped list of photobiont genera and mycobionts reported in association with them. The family names of the mycobionts are included in places where emphasis might be useful. id = procedures used in the study to identify the photobiont. LM = light microscopy, TEM = transmission electron microscopy. See table 1 in Tschermak-Woess (Reference Tschermak-Woess and Galun1988a) for a comprehensive list of photobiont reports prior to 1988. Taxon names follow those used in the original articles.
The Major Algal Groups Involved
Lichen algae are diverse. This may contribute to the distinct distributions and climatic preferences of the symbiotic thalli that enclose them (Marini et al. Reference Marini, Nascimbene and Nimis2011). Most are green algae, a paraphyletic grouping of two major clades: the charophytes (Streptophyta), from which embryophytes descend, and the Chlorophyta s. str. (Leliaert et al. Reference Leliaert, Smith, Moreau, Herron, Verbruggen, Delwiche and De Clerck2012). The latter includes nearly all green algae reported as lichen symbionts. Within the Chlorophyta, lichen symbionts are found principally in the classes Trebouxiophyceae and Ulvophyceae. A third class, the Chlorophyceae, is known or suspected to include the partners of several lichens. The prokaryotic blue-green algae (cyanobacteria) encompass most of the remainder, occurring in c. 10% of the nearly 20 000 known lichen associations (Rikkinen Reference Rikkinen, Grube, Seckbach and Muggia2017). Additionally, two stramenopile algae (a xanthophyte and a phaeophyte) are known to enter into lichen symbioses. The full range of phylogenetic disparity among lichen-forming algae is therefore much wider than that found among the lichen-forming fungi, which all fall within the kingdom's Dikarya crown group (mostly Ascomycota, with several genera of Basidiomycota). Just what common features might permit those disparate algal lineages to form comparable symbioses with lichen-forming fungi remain enigmatic. As colonizers of exposed, subaerial substrata, potentially suitable algae may be pre-adapted to coping with hydric stresses and high radiation loads (Lange et al. Reference Lange, Pfanz, Kilian and Meyer1990; Gustavs et al. Reference Gustavs, Eggert, Michalik and Karsten2010; Candotto Carniel et al. Reference Candotto, Zanelli, Bertuzzi and Tretiach2015). It is striking that most lineages of basidiomycete fungi that independently adopted the lichen lifestyle did not domesticate novel algal genera; instead they chose taxa that associate with ascolichens, such as Coccomyxa, Elliptochloris and Rhizonema (Oberwinkler Reference Oberwinkler and Hock2012; Dal Forno et al. Reference Dal Forno, Lawrey, Sikaroodi, Gillevet, Schuettpelz and Lücking2020; Masumoto Reference Masumoto2020; but see Hodkinson et al. (Reference Hodkinson, Moncada and Lücking2014) concerning Lepidostromatales). It is also noteworthy that quite a number of lichen algae belong to genera (e.g. Chlorella s. str., Coccomyxa, Elliptochloris and Nostoc) that include species occurring in symbiosis (often endosymbioses) with diverse protists, plants and animals (Adams et al. Reference Adams, Duggan, Jackson and Whitton2012; Grube et al. Reference Grube, Seckbach and Muggia2017b).
Algal partners in lichen symbioses were termed phycobionts by Scott (Reference Scott1957). Subsequently, Ahmadjian (Reference Ahmadjian1982) proposed that photobiont replace phycobiont where cyanobacteria are meant to be included, because they ‘are not algae per se but actually bacteria’. No further argumentation was provided; it was presumed self-evident that algae and bacteria must denote mutually exclusive concepts. Some contemporary treatments distinguish cyanobacteria from algae (e.g. Friedl & Büdel Reference Friedl, Büdel and Nash2008; Grube et al. Reference Grube, Seckbach and Muggia2017b), while others consider them as algae (e.g. Graham et al. Reference Graham, Graham and Wilcox2009; Büdel & Kauff Reference Büdel, Kauff and Frey2012; Lee Reference Lee2018). Clearly, there are significant differences between prokaryotes and eukaryotes. At issue, however, is whether those differences are relevant to the concept of algae. This term has no biosystematic status and cannot attain any by exclusion of the blue-greens. The emblematic algal trait, oxygen-generating photosynthesis, is ultimately derived from cyanobacteria. It was subsequently acquired in multiple events involving primary, secondary and tertiary endosymbioses (Keeling Reference Keeling2004, Reference Keeling2013), and now characterizes diverse lineages included within most of the major eukaryote clades (Archaeplastida, Alveolata, Excavata, Rhizaria, Stramenopila, Cryptista and Haptista). The one and only unifying thread in this polyphyletic algal tapestry (Delwiche Reference Delwiche1999) is the common photosynthetic apparatus, originating in cyanobacteria and passed on vertically as well as horizontally. The present work therefore uses the term algae to encompass all non-embryophyte lineages that inherited oxygenic photosynthesis. Phycobiont and photobiont are considered synonymous terms.
The Algal Role in Lichen Symbiosis
The algal partner is the primary producer, sustaining the lichen association by supplying the fungal partner with carbohydrate products of photosynthesis (Smith Reference Smith1974). Those with pyrenoids (Fig. 2) possess CO2-concentrating mechanisms that improve the efficiency of carbon fixation (Smith & Griffiths Reference Smith and Griffiths1996). Green algal symbionts (chlorobionts) transfer their photosynthate as polyol sugar alcohols such as ribitol (Richardson et al. Reference Richardson, Hill and Smith1968). Significantly, these compounds also confer desiccation tolerance by providing osmolarity and protecting cell membranes from damage as water is lost (Smith Reference Smith2019). Polyols are likewise produced by non-symbiotic, aeroterrestrial green algae, particularly under osmotic stress conditions (Darienko et al. Reference Darienko, Gustavs, Mudimu, Menendez, Schumann, Karsten, Friedl and Pröschold2010; Gustavs et al. Reference Gustavs, Eggert, Michalik and Karsten2010, Reference Gustavs, Görs and Karsten2011). Blue-green symbionts (cyanobionts) transfer glucose, or glucan, which their fungal partners take up and immediately convert into the sugar alcohol mannitol (Smith & Drew Reference Smith and Drew1965; Hill Reference Hill1972). When lichenized, the algal symbionts are somehow induced to leak large amounts of carbohydrate to the surrounding fungal cells, a process that quickly ceases when the algae are isolated into culture (Drew & Smith Reference Drew and Smith1967). Fungal penetration of photobionts may occur to varying degrees (Geitler Reference Geitler1934; Tschermak Reference Tschermak1941a; Plessl Reference Plessl1963; Galun et al. Reference Galun, Paran and Ben-Shaul1970, Reference Gallun [sic], Ben-Shaul and Paran1971; Honegger Reference Honegger1986; Matthews et al. Reference Matthews, Tucker and Chapman1989), but these so-called haustoria do not appear to be principal conduits of carbohydrate transfer in ascolichens (Jacobs & Ahmadjian Reference Jacobs and Ahmadjian1971; Collins & Farrar Reference Collins and Farrar1978; Hessler & Peveling Reference Hessler and Peveling1978). The intrusive hyphae of certain basidiolichens that deeply penetrate longitudinally through the centre of their cyanobiont trichomes (Roskin Reference Roskin1970; Oberwinkler Reference Oberwinkler, Schwemmler and Schenk1980, Reference Oberwinkler and Hock2012) have not yet been examined with respect to substance transfer. In most foliose and fruticose lichens examined, haustorial penetrations are either absent altogether or do not fully traverse the algal cell wall. To facilitate transfer, the mycobiont secretes a hydrophobic sealant that envelops the cell surfaces of both symbionts at their contact zones, thereby funnelling carbohydrate released by the alga to the fungus (Honegger Reference Honegger1991; Trembley et al. Reference Trembley, Ringli and Honegger2002a). At least that is the case in the selection of taxa examined so far. Where cyanobacterial symbionts are involved, they provide the lichen fungus with fixed nitrogen as well as carbon (Millbank & Kershaw Reference Millbank, Kershaw, Ahmadjian and Hale1974). In those lichens (chiefly Peltigerales) where a chlorobiont constitutes the main algal layer and cyanobionts are localized within nodules known as cephalodia, the cyanobacteria become highly specialized for nitrogen fixation, with an elevated percentage of cells differentiating as heterocytes (Hitch & Millbank Reference Hitch and Millbank1975). In lichens with only cyanobacterial photobionts, heterocyte frequency can be much lower at the growing margins of the thallus (Bergman & Hällbom Reference Bergman and Hällbom1981), where photosynthate may be in higher demand.
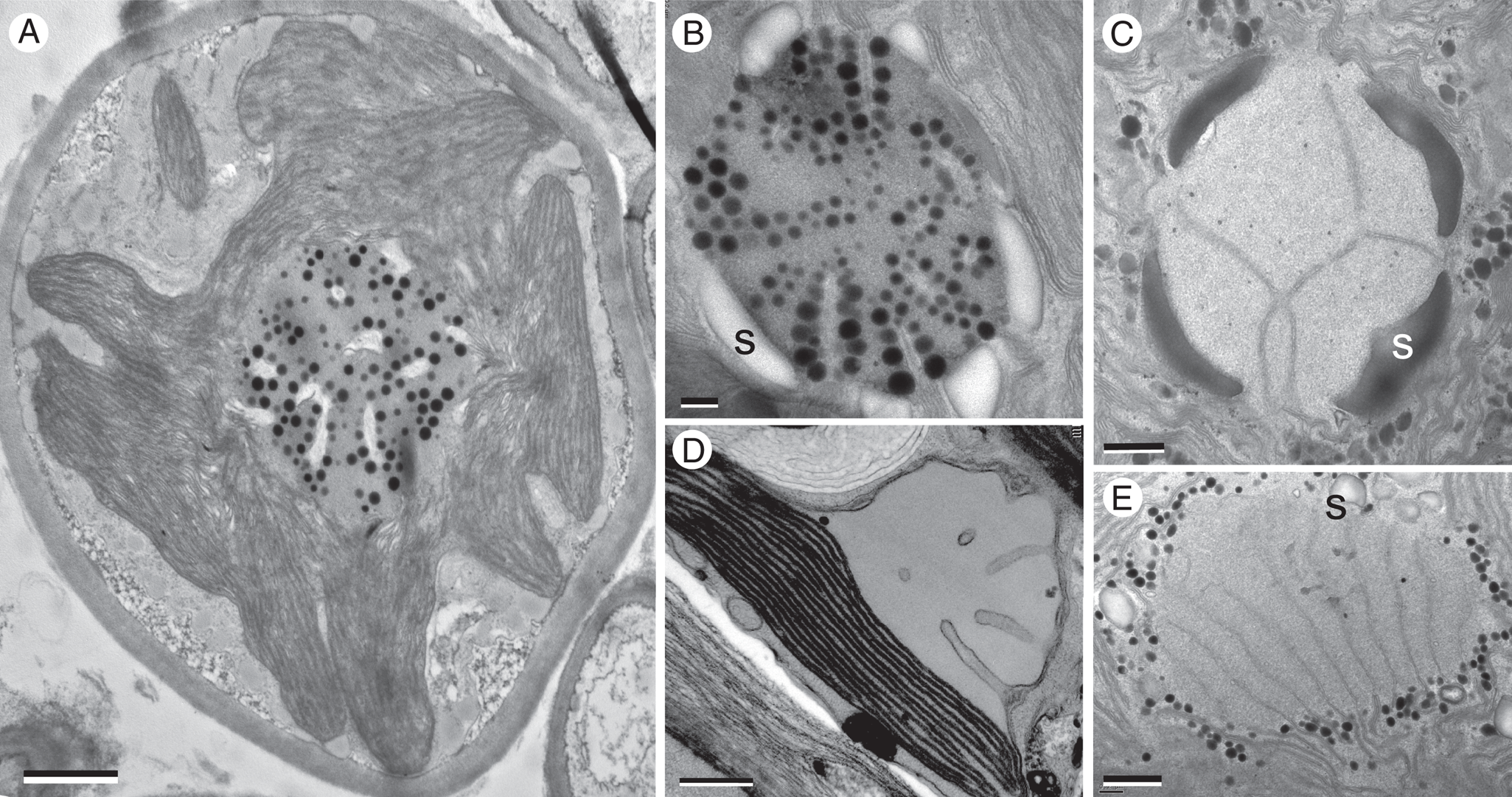
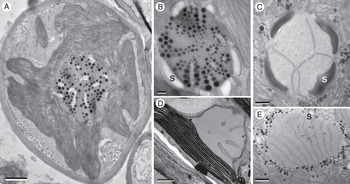
Fig. 2. TEM micrographs of some photobiont pyrenoids, with plastoglobuli (round black dots) and penetrating membranes in various positions and orientations. A, Trebouxia, within thallus of Lasallia pustulata. Note pyrenoid structure here more closely resembles that of distantly related Heveochlorella (B) than that of another species (C) of Trebouxia. B, Heveochlorella, within thallus of Calopadia. C, Trebouxia, within thallus of Ramalina usnea. D, bulging exserted pyrenoid of Petroderma maculiforme. E, Diplosphaera, within thallus of Endocarpon pusillum. S = starch grain or plates. Scales: A = 1 μm; B = 200 nm; C–E = 500 nm.
Whether any substance is transferred from fungus to alga in exchange has yet to be demonstrated. At least some genes relevant to such metabolic transfers appear to be differentially expressed in symbiosis (Kono et al. Reference Kono, Kon, Ohmura, Satta and Terai2020). Certainly, there has been speculation that the fungal partner might apportion carbohydrate, nitrogen, or other substances back to the algal symbiont to regulate its growth (Ahmadjian Reference Ahmadjian1995) in coordination with that of the mycobiont (Greenhalgh & Anglesea Reference Greenhalgh and Anglesea1979; Hill Reference Hill and Brown1985, Reference Hill1989; Honegger Reference Honegger1987). The heterotrophic tendencies shown by many lichen algae (Trebouxia, Asterochloris, Elliptochloris, Coccomyxa, Apatococcus) when cultured in the laboratory (Ahmadjian Reference Ahmadjian1993; Gustavs et al. Reference Gustavs, Schumann and Karsten2016, Reference Gustavs, Schiefelbein, Darienko, Grube, Seckbach and Muggia2017) suggest the possibility that they could be susceptible to such control. Indeed, Ahmadjian (Reference Ahmadjian and Seckbach2001) proposed that Trebouxia is fully dependent upon its mycobiont for nutrition and is therefore unable to survive in the free-living state (Ahmadjian Reference Ahmadjian1988). However, he also promoted the seemingly contradictory viewpoint that Trebouxia is a victim of fungal parasitism rather than a mutualist partner (Ahmadjian Reference Ahmadjian1993, Reference Ahmadjian1995, Reference Ahmadjian2002). This would make Trebouxia a host that cannot survive without its parasite.
In any event, proof of fungus-to-alga nutrient transfer is not required to make the case that lichen symbiosis offers advantages to the algal partner. There is considerable evidence that the surrounding fungal tissues and their secondary metabolites may help protect the lichenized alga from desiccation, photoinhibition, temperature extremes and herbivory (e.g. Solhaug & Gauslaa Reference Solhaug and Gauslaa1996; Kranner et al. Reference Kranner, Beckett, Hochman and Nash2008; Kosugi et al. Reference Kosugi, Arita, Shizuma, Moryama, Kashino, Koike and Satoh2009; Asplund & Wardle Reference Asplund and Wardle2013; Gauslaa et al. Reference Gauslaa, Alam, Lucas, Chowdhury and Solhaug2017; Míguez et al. Reference Míguez, Schiefelbein, Karsten, García-Plazaola and Gustavs2017; Sadowsky & Ott Reference Sadowsky and Ott2016; Beckett et al. Reference Beckett, Solhaug, Gauslaa and Minibayeva2019; Fernández-Marín et al. Reference Fernández-Marín, López-Pozo, Perera-Castro, Irati Arzac, Sáenz-Ceniceros, Colesie, de los Ríos, Sancho, Pintado and Laza2019). Symbiosis may significantly improve the alga's ability to avoid cellular damage caused by highly reactive forms of oxygen (ROS) generated under stress conditions (Kranner et al. Reference Kranner, Cram, Zorn, Wornik, Yoshimura, Stabentheiner and Pfeifhofer2005). With these protections, and the facilitated display for light capture afforded by a supportive mycobiont structure, lichen algae may greatly expand their ecological range and abundance via symbiosis (Honegger Reference Honegger and Hock2012). On the other hand, lichen symbioses are diverse and it is likely that the parameters of the relationship vary among taxa, along environmental gradients, and perhaps also during the course of a single lichen's development. The long history of attempts to maintain or resynthesize lichens in the laboratory has provided a key insight into the nature of this seemingly well-integrated association: it is very much a relationship of contingency. That the partners can often be cultured separately on appropriate media in the laboratory (Ahmadjian Reference Ahmadjian1993; Crittenden et al. Reference Crittenden, David, Hawksworth and Campbell1995; Stocker-Wörgötter & Hager Reference Stocker-Wörgötter, Hager and Nash2008) shows there is no strict physiological impediment to growth without symbiosis. To initiate and support lichen formation, a fluctuating balance of conditions suboptimal for separate fungal or algal growth appears to be necessary. Any combination of culture conditions (light, moisture, nutrient availability) that continuously favours either fungal or algal growth results in the breakdown of symbiotic structures, and the dissociated proliferation of the micro-organisms separately (Thomas Reference Thomas1939; Scott Reference Scott1960; Ahmadjian Reference Ahmadjian1962; Stocker-Wörgötter Reference Stocker-Wörgötter2001; but see Marton & Galun Reference Marton and Galun1976). It therefore seems logical to view the lichen symbiosis as a more or less mutualistic response to conditions that permit neither partner to thrive independently.
Although both partners may derive benefits, the lichen symbiosis is clearly not symmetrical (Hill Reference Hill2009). The heterotrophic mycobionts, with their elaborate structural adaptations for algal cultivation, are more fully committed to symbiosis than their trophically autonomous photobionts. The mycobiont frees itself of symbiosis only in spore dispersal, seeking algal partners again immediately upon germination. To carry out sexual reproduction, it must be in symbiosis, whereas its photobiont appears to need aposymbiotic freedom to do so. From the alga's point of view, whenever unfavourable conditions reduce its possibilities of aposymbiotic success, the benefits of lichenization may begin to outweigh any disadvantages. Photobionts may rely on lichen symbioses for long-term persistence in habitats periodically subject to adverse conditions, while needing intervals of independence under favourable conditions to complete their life cycles. Thus, mycobiont and photobiont life histories do not fully coincide, but produce a lichen where they intersect compatibly. To varying degrees, natural selection has optimized the mycobiont principally for symbiosis, the photobiont for autonomy as well as symbiosis. The trade-off is that greater adaptation to symbiotic compatibility is likely to constrain the possibilities for competitive success in the aposymbiotic state. However, the lingering notion that certain photobionts may not ever occur free-living is probably attributable to insufficient sampling, and the conflation of invisibility with absence. Unsurprisingly, those photobionts that are macroscopically visible (Nostoc, Cephaleuros, Phycopeltis, Trentepohlia, Prasiola, Petroderma) have not had their aposymbiotic occurrence disputed.
Both fungus and alga must adapt, at least to some extent, to be compatible symbionts. For some authors, such mutual adaptation constitutes coevolution (Ahmadjian Reference Ahmadjian1987; Saini et al. Reference Saini, Nayaka, Bast, Satyanarayana, Johri and Das2019); for others, coevolution supposes parallel cladogenesis in partners’ phylogenies, a criterion not generally met by lichen symbioses analyzed in this regard (Piercey-Normore & DePriest Reference Piercey-Normore and DePriest2001; Stenroos et al. Reference Stenroos, Högnabba, Myllys, Hyvönen and Thell2006). However, it has been argued that focusing exclusively on this fine scale ignores broader patterns of co-adaptation, whereby ‘guilds’ of different mycobionts share common pools of photobionts to mutual advantage (Rikkinen Reference Rikkinen2003, Reference Rikkinen2013). According to Hill (Reference Hill2009), photobionts cannot coevolve with their mycobionts because they lack sexual reproduction in the thallus, are not subject to natural selection from one lichen to the next, and are not perpetuated when a lichen thallus dies. Yet photobionts are continually escaping from lichen thalli by means of soredia, isidia, thallus fragments, co-dispersed hymenial, epithecial or conidiomal algae (Fig. 3), and the excreta of lichenivorous invertebrates (Fröberg et al. Reference Fröberg, Björn, Baur and Baur2001; Meier et al. Reference Meier, Scherrer and Honegger2002; Boch et al. Reference Boch, Prati, Werth, Rüetschi and Fischer2011). Such diaspores afford many chances of finding microconditions where independent algal growth is favoured; aposymbiotic, potentially sexual populations may then develop, be they brief or enduring. Selection among genotypes for compatibility (or resistance) will occur when the opportunity for relichenization next presents itself. Compatible genotypes incorporated into a developing lichen may then be subject to further winnowing selection in the course of thallus growth.

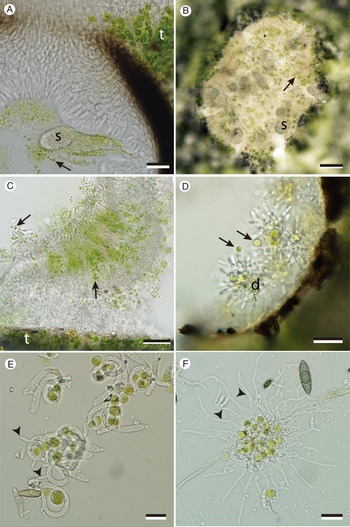
Fig. 3. Liberation and potential co-dispersal of photobionts from the spore-producing structures of certain mycobionts. A, Diplosphaera photobiont (arrows) within perithecium of Endocarpon pusillum; note much smaller size compared to photobiont cells within thalline tissue (t); s = ascospore. B, apothecial surface of foliicolous lichen colonizing plastic cover slip; note epithecial algal cells (arrows) among emerging ascospores (s). C, Heveochlorella photobionts (vertical arrow) within conidiogenous tissue of campylidia and intermixed among filiform macroconidia (oblique arrow). D, hyphophore of Gyalectidium paolae showing diahyphal propagules (bundles of conidial chains dispersed as a unit) with adhering or intermixed Heveochlorella photobionts (arrows). E, campylidial macroconidia, with co-dispersed Heveochlorella photobionts loosely encircled, germinating (arrowheads) on a plastic cover slip. F, diahyphal propagules of Gyalectidium germinating (arrowheads) on a plastic cover slip, with co-dispersed Heveochlorella photobionts. Scales: A, C & D = 20 μm; B = 50 μm; E & F = 10 μm.
Patterns of Symbiont Pairing
The asymmetrical needs of the lichen symbionts are reflected in the non-reciprocal patterns of pairing that have evolved between mycobionts and photobionts. Photobiont genera frequently associate with multiple, phylogenetically disparate lineages of lichen-forming fungi. The converse, however, is much less common; mycobiont genera, and often families and even orders, generally tend to lichenize a single algal genus (Rambold et al. Reference Rambold, Friedl and Beck1998; Peršoh et al. Reference Peršoh, Beck and Rambold2004). There are a number of notable exceptions. Lichen-forming fungi of the family Verrucariaceae partner with an extremely diverse array of eukaryotic algae, including the only reported cases of stramenopile phycobionts (Thüs et al. Reference Thüs, Muggia, Pérez-Ortega, Favero-Longo, Joneson, O'Brien, Nelsen, Duque-Thüs, Grube and Friedl2011). The pin-lichen genus Chaenotheca (Coniocybomycetes) includes species associating with Trebouxia, Trentepohlia, Symbiochloris or Tritostichococcus (Tibell Reference Tibell2001; Škaloud et al. Reference Škaloud, Friedl, Hallmann, Beck and Dal Grande2016; Pröschold & Darienko Reference Pröschold and Darienko2020). The fruticose lichen genus Stereocaulon may harbour thallus photobionts of either Asterochloris, Vulcanochloris or Chloroidium (Vančurová et al. Reference Vančurová, Muggia, Peksa, Řídká and Škaloud2018). Species of Sticta may partner with chlorobionts of Symbiochloris, Coccomyxa, Elliptochloris, Heveochlorella or Chloroidium (Lindgren et al. Reference Lindgren, Moncada, Lücking, Magain, Simon, Goffinet, Sérusiaux, Nelsen, Mercado-Díaz and Widhelm2020). Squamulose Psora decipiens is reported to partner with either Asterochloris, Trebouxia, Chloroidium (Ruprecht et al. Reference Ruprecht, Brunauer and Türk2014) or Myrmecia photobionts (Williams et al. Reference Williams, Colesie, Ullmann, Westberg, Wedin and Büdel2017; Moya et al. Reference Moya, Chiva, Molins, Jadrná, Škaloud, Peksa and Barreno2018). In addition, it is well known that many individual mycobionts, particularly in the Peltigerales, may associate with both green and blue-green algae simultaneously, giving rise to cyanobacterial cephalodia within or upon a chlorophyte-containing thallus, or distinct cyanomorph and chloromorph thalli separately or conjoined (Fig. 4) via a common fungal individual (e.g. James & Henssen Reference James, Henssen, Brown, Hawksworth and Bailey1976). Association with both a chlorobiont and a cyanobiont in separate thallus components has also been reported for certain basidiolichen species in Cyphellostereum (Oberwinkler Reference Oberwinkler and Hock2012) and Lichenomphalia (Gasulla et al. Reference Gasulla, Barrasa, Casano and del Campo2020). In a small number of lichens, green and blue-green photobionts are known to occur intermixed within the same thallus structure (Büdel & Henssen Reference Büdel and Henssen1987; Henskens et al. Reference Henskens, Green and Wilkins2012). There are distinct physiological advantages to each of these two kinds of photobionts. Cyanobionts can fix nitrogen as well as carbon but require liquid water to rehydrate and resume physiological activity, whereas chlorobionts can rehydrate from vapour, although their CO2 fixation rates may be more adversely affected by high thallus water contents (Lange et al. Reference Lange, Kilian and Ziegler1986, Reference Lange, Büdel, Meyer and Kilian1993; Green et al. Reference Green, Büdel, Heber, Meyer, Zellner and Lange1993, Reference Green, Schlensog, Sancho, Winkler, Broom and Schroeter2002). Less obvious are the implications of choosing Trentepohlia (Ulvophyceae) versus Trebouxia (Trebouxiophyceae) photobionts; neither fix nitrogen, although they may differ in their tolerance of freezing temperatures (Nash et al. Reference Nash, Kappen, Lösch, Larson and Matthes-Sears1987). Interestingly, mycobiont genera Ionaspis and Hymenelia (Lecanoromycetes) include trentepohliophilic and trebouxiophilic taxa, and the single species H. epulotica can apparently associate with photobionts of either of these two very different genera (Lutzoni & Brodo Reference Lutzoni and Brodo1995; McCune et al. Reference McCune, Arup, Breuss, Di Meglio, Di Meglio, Esslinger, Magain, Miadlikowska, Miller and Muggia2018). Recently, Ertz et al. (Reference Ertz, Guzow-Krzemińska, Thor, Łubek and Kukwa2018) demonstrated that the lichen fungus Lecanographa amylacea can form morphologically distinct sexual and asexual thalli with Trentepohlia and Trebouxia photobionts, respectively. While the above examples show that significant divergences in photobiont selection have arisen in a number of mycobiont lineages, far more conservative tendencies appear to predominate in the majority of lichen-forming fungal groups.

Fig. 4. Dichotomously lobed chloromorphs of Sticta canariensis emerging from lower surfaces of cyanomorph thalli (arrows). Scale = 5 mm.
Photobiont choice and the range of compatible pairings for a given mycobiont were first explored experimentally in classic laboratory resynthesis studies using Cladonia cristatella and Lecanora chrysoleuca (Ahmadjian et al. Reference Ahmadjian, Russell and Hildreth1980; Ahmadjian & Jacobs Reference Ahmadjian and Jacobs1981). Varying degrees of compatibility were observed, with thallus formation reaching different developmental stages depending on the photobiont strain introduced. Nonetheless, overall results generally reflected patterns observable in natural lichens: Cladonia successfully lichenized strains of Asterochloris but not those of Trebouxia (as currently defined), while Lecanora did just the opposite. In the last two decades, genetic markers have been used to characterize the range of photobiont diversity chosen by individual lichen-forming fungal species in nature, and to assess the parameters that might affect their choices. This complex topic has attracted much attention and merits a review of its own, but some general findings can be summarized here. Most mycobiont species appear to be fairly selective; they tend to partner with a limited range of strains or species within a single photobiont genus, but to differing degrees. Some mycobionts accept a substantially broader range of taxa within the photobiont partner genus; this relative liberality is often characteristic of lichen-forming fungi that have attained wider, more cosmopolitan distributions (Blaha et al. Reference Blaha, Baloch and Grube2006; Guzow-Krzemińska Reference Guzow-Krzemińska2006; Leavitt et al. Reference Leavitt, Nelsen, Lumbsch, Johnson and St Clair2013; Muggia et al. Reference Muggia, Pérez-Ortega, Kopun, Zellnig and Grube2014; Magain et al. Reference Magain, Miadlikowska, Goffinet, Sérusiaux and Lutzoni2017; Vančurová et al. Reference Vančurová, Muggia, Peksa, Řídká and Škaloud2018), or those capable of colonizing extreme environments with probably fewer photobiont options available (Romeike et al. Reference Romeike, Friedl, Helm and Ott2002; Wirtz et al. Reference Wirtz, Lumbsch, Green, Türk, Pintado, Sancho and Schroeter2003; Engelen et al. Reference Engelen, Convey and Ott2010; Pérez-Ortega et al. Reference Pérez-Ortega, Ortiz-Álvarez, Green and de los Ríos2012; Osyczka et al. Reference Osyczka, Lenart-Borón, Borón and Rola2021; Rola et al. Reference Rola, Lenart-Borón, Borón and Osyczka2021). Such mycobionts may be closely related to species that accept a much narrower range of photobiont partners (Piercey-Normore Reference Piercey-Normore2004; Yahr et al. Reference Yahr, Vilgalys and DePriest2004; Otálora et al. Reference Otálora, Martínez, O'Brien, Molina, Aragón and Lutzoni2010; Onuţ-Brännström et al. Reference Onuţ-Brännström, Tibell and Johannesson2017). Some studies have correlated symbiont selection patterns with environmental parameters, such as latitude (Singh et al. Reference Singh, Dal Grande, Divakar, Otte, Crespo and Schmitt2017), climate (Řidká et al. Reference Řidká, Peksa, Rai, Upreti, Škaloud, Rai and Upreti2014) and ecological conditions that influence the distribution and availability of photobionts (Yahr et al. Reference Yahr, Vilgalys and DePriest2006; Fernández-Mendoza et al. Reference Fernández-Mendoza, Domaschke, García, Jordan, Martín and Printzen2011; Peksa & Škaloud Reference Peksa and Škaloud2011; Vargas Castillo & Beck Reference Vargas Castillo and Beck2012; Werth & Sork Reference Werth and Sork2014). Photobiont tolerance of heavy metals appears to influence their selection by mycobionts in some lichen communities colonizing metal-rich substrata (Vančurová et al. Reference Vančurová, Muggia, Peksa, Řídká and Škaloud2018; Rola et al. Reference Rola, Lenart-Borón, Borón and Osyczka2021) but not others (Beck Reference Beck2002; Hauck et al. Reference Hauck, Helms and Friedl2007; Bačkor et al. Reference Bačkor, Peksa, Škaloud and Bačkorová2010). Many studies stress the intrinsic compatibility requirements of individual fungal taxa as primary determinants of pairing patterns (Yahr et al. Reference Yahr, Vilgalys and DePriest2004; Stenroos et al. Reference Stenroos, Högnabba, Myllys, Hyvönen and Thell2006; Myllys et al. Reference Myllys, Stenroos, Thell and Kuusinen2007; Leavitt et al. Reference Leavitt, Kraichak, Vondrak, Nelsen, Altermann, Divakar, Alors, Esslinger, Crespo and Lumbsch2015; Joneson & O'Brien Reference Joneson and O'Brien2017), often in conjunction with ecological factors (Elvebakk et al. Reference Elvebakk, Papaefthimiou, Robertsen and Liaimer2008; O'Brien et al. Reference O'Brien, Miadlikowsa and Lutzoni2013; Dal Grande et al. Reference Dal Grande, Rolshausen, Divakar, Crespo, Otte, Schleuning and Schmitt2018; Jüriado et al. Reference Jüriado, Kaasalainen, Jylhä and Rikkinen2019; Pino-Bodas & Stenroos Reference Pino-Bodas and Stenroos2020). In some communities, mycobionts may have adapted to utilize a common pool or pools of photobionts, whose local availability might thereby be sustained for all users (Beck et al. Reference Beck2002; Rikkinen et al. Reference Rikkinen, Oksanen and Lohtander2002; Rikkinen Reference Rikkinen2003; Sanders et al. Reference Sanders, Pérez-Ortega, Nelsen, Lücking and de los Ríos2016; Onuţ-Brännström et al. Reference Onuţ-Brännström, Benjamin, Scofield, Heiđmarsson, Andersson, Lindström and Johannesson2018; Cardós et al. Reference Cardós, Prieto, Jylhä, Aragón, Molina, Martínez and Rikkinen2019). Thallus growth form may also affect photobiont selection patterns. Some authors have suggested that crustose lichens may associate with a broader range of photobionts than do related foliose and fruticose taxa (Helms et al. Reference Helms, Friedl, Rambold and Mayrhofer2001), perhaps because their more extensive and intimate contact with the substratum offers more opportunity to take up additional algae in the course of development. Lichen reproductive mode can also be superimposed upon these factors. Some studies have found that lichens reproducing primarily by vegetative propagules, such as soredia or isidia, associate with a narrower range of photobiont genotypes, presumably due to chiefly vertical transmission of both symbionts together (Dal Grande et al. Reference Dal Grande, Widmer, Wagner and Scheidegger2012; Werth & Scheidegger Reference Werth and Scheidegger2012; Otálora et al. Reference Otálora, Salvador, Martínez and Aragón2013; Cao et al. Reference Cao, Zhang, Liu, Hao, Tian, Zhu and Zhou2015; Hestmark et al. Reference Hestmark, Lutzoni and Miadlikowska2016; Steinová et al. Reference Steinová, Škaloud, Yahr, Bestová and Muggia2019). However, other vegetatively reproducing lichens accept a much broader range of photobionts, suggesting that the fungus does not necessarily maintain partnership with its co-dispersed photobiont throughout development (Ohmura et al. Reference Ohmura, Kawachi, Kasai, Watanabe and Takeshita2006, Reference Ohmura, Takeshita and Kawachi2019; Nelsen & Gargas Reference Nelsen and Gargas2008, Reference Nelsen and Gargas2009; Wornik & Grube Reference Wornik and Grube2010).
Acquisition of New Algal Symbionts
Acquisition of new and different photobionts, ‘photobiont switching’, has clearly been significant in the evolution of lichen relationships. However, this phrase may refer variably to events occurring at different levels of organization. A single mycobiont individual might acquire new photobionts at different times in the course of its development (Friedl Reference Friedl1987; Wedin et al. Reference Wedin, Maier, Fernández-Brime, Cronholm, Westberg and Grube2016), or at separate places along its somatic extension (Létrouit-Galinou & Asta Reference Letrouit-Galinou and Asta1994). The degree to which the newly lichenized alga may differ genetically from algal strain(s) already in possession will be limited by the innate compatibility range of that mycobiont individual. In contrast, a new fungal individual developing from a meiospore may encounter and select a photobiont strain different from the one its parental genotypes associated with. In this case, a generational change in photobiont partner could be enabled by a generational change in mycobiont genotype. At a phylogenetic level, a cladogram may provide evidence that a fungal lineage has changed its association from one photobiont to another in the course of evolution. But at a finer scale, a great many photobiont switches, perhaps back and forth, might have taken place over many generations; comparing taxa will indicate only the overall result.
New photobionts may be acquired in multiple ways. Contact and capture of free-living photobionts in nature by hyphae emerging from germinated spores (Fig. 5), once thought to be unlikely (Lamb Reference Lamb1959), has been documented in a number of studies (Ward Reference Ward1884; Werner Reference Werner1931; Bubrick et al. Reference Bubrick, Galun and Frensdorff1984; Garty & Delarea Reference Garty and Delarea1988; Scheidegger Reference Scheidegger1995; Sanders & Lücking Reference Sanders and Lücking2002; Sanders Reference Sanders2014). In theory, a single compatible algal individual might be sufficient to generate the entire population within a developing thallus. However, there appear to be many opportunities for additional photobionts to be incorporated from exterior sources. Particularly in early developmental stages, prothallic hyphae extending outward along the substratum from the lichenized portions of the organizing thallus can incorporate additional algal cells (Sanders & Lücking Reference Sanders and Lücking2002; Sanders Reference Sanders2014). Vegetative propagules, such as soredia or isidia, also begin development with the emergence and proliferation of such hyphae (Jahns et al. Reference Jahns, Mollenhauer, Jenniger and Schönborn1979; Schuster et al. Reference Schuster, Ott and Jahns1985), anchoring the structure and greatly expanding the available surfaces for potential contact with other compatible photobionts as the thallus is organized. In many crustose lichens, a prothallus remains active at the growing margins of the lichen and may continue to incorporate compatible photobionts falling upon it or encountered on the substratum (Fig. 6; see also Galløe Reference Galløe1927: p. 40, Reference Galløe1932: p. 78; Letrouit-Galinou & Asta Reference Letrouit-Galinou and Asta1994). The multitude of discrete, lichenized units that comprise the thallus of squamulose lichens probably also arise from repeated algal capture by a network of prothallic hyphae interconnecting the squamules. Certain soil- and rock-colonizing squamulose lichens produce hyphal aggregates (cords or rhizomorphs) of indeterminate growth that penetrate the substratum extensively (Poelt & Baumgärtner Reference Poelt and Baumgärtner1964; Sanders et al. Reference Sanders, Wierzchos and Ascaso1994), giving rise to new thallus squamules where compatible algal symbionts are encountered and lichenized (Wagner & Letrouit-Galinou Reference Wagner and Létrouit-Galinou1988; Sanders & Rico Reference Sanders and Rico1992; Sanders Reference Sanders1994). The structurally similar rhizinomorphs of certain umbilicate lichens also appear to have this capability (Schuster Reference Schuster1992). In some foliose and fruticose lichens, organized thallus surfaces may themselves be capable of incorporating compatible algal cells that make external contact (Bitter Reference Bitter1904). Lichens that form cephalodia and/or joined chloromorph and cyanomorph thalli clearly retain this ability (see discussion under Nostoc below). Additionally, certain lichen-forming fungi appear capable of obtaining photobionts from other lichens, upon which their spores may germinate (Hawksworth et al. Reference Hawksworth, Coppins and James1979). The host thallus is eventually destroyed as its photobionts are taken over by the invading hyphae of the aggressor, giving rise to a new lichen (Poelt Reference Poelt1958; Friedl Reference Friedl1987; Feige et al. Reference Feige, Lumbsch and Mies1993; Lücking & Grube Reference Lücking and Grube2002; Wedin et al. Reference Wedin, Maier, Fernández-Brime, Cronholm, Westberg and Grube2016). Thus, capture of free-living algae by spore germlings is clearly not the only opportunity for a mycobiont to acquire new photobionts. On the other hand, some interesting transplant experiments with Psora decipiens suggest that lichens may not always be able to switch to more favourable photobionts when needed (Williams et al. Reference Williams, Colesie, Ullmann, Westberg, Wedin and Büdel2017).

Fig. 5. Muriform ascospore (a), probably of Calopadia, germinating on a plastic cover slip placed in a south-west Florida oak hammock, and lichenizing a group of algal cells (arrow), most likely Heveochlorella. Scale = 20 μm.
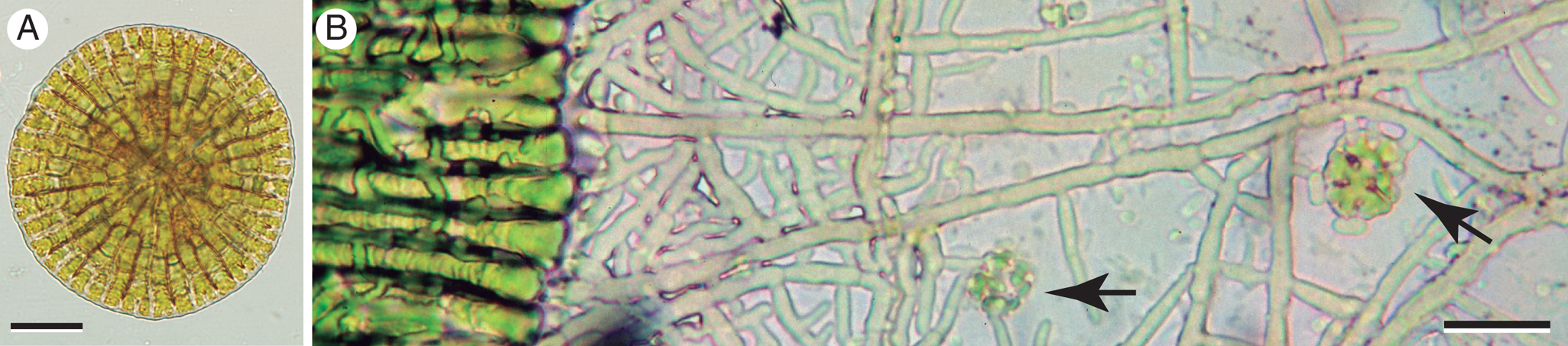
Fig. 6. Phycopeltis free-living and in stages of lichenization. A, free-living. B, edge of developed Phycopeltis thallus (left) lichenized by a network of hyphae (probably foliicolous Porina sp.) that extend over substratum and capture additional young Phycopeltis germlings (arrows). Scales: A = 20 μm; B = 10 μm.
If acquisition of additional photobionts is indeed a common occurrence in the course of lichen development, lichen thalli may be expected to contain a heterogeneous photobiont population, at least at certain stages. Some authors have observed and illustrated quite different chlorobionts occurring together within single thalli (Voytsekhovich et al. Reference Voytsekhovich, Dymytrova and Nadyeina2011). Data from molecular markers have also addressed this question. Some authors found no evidence of multiple photobiont genotypes in single thalli examined (Paulsrud & Lindblad Reference Paulsrud and Lindblad1998; Beck & Koop Reference Beck and Koop2001; Singh et al. Reference Singh, Dal Grande, Divakar, Otte, Crespo and Schmitt2017; Škaloud et al. Reference Škaloud, Moya, Molins, Peksa, Santos-Guerra and Barreno2018); others found occasional occurrences (Guzow-Krzemińska Reference Guzow-Krzemińska2006; Bačkor et al. Reference Bačkor, Peksa, Škaloud and Bačkorová2010; Muggia et al. Reference Muggia, Vancurova, Škaloud, Peksa, Wedin and Grube2013; Nyati et al. Reference Nyati, Bhattacharya, Werth and Honegger2013; Řidka et al. Reference Nyati, Scherrer, Werth and Honegger2014; Onuţ-Brännström et al. Reference Onuţ-Brännström, Benjamin, Scofield, Heiđmarsson, Andersson, Lindström and Johannesson2018; Vančurová et al. Reference Vančurová, Muggia, Peksa, Řídká and Škaloud2018; Molins et al. Reference Molins, Chiva, Calatayud, Marco, García-Breijo, Reig-Armiñana, Carrasco and Moya2020), or frequent presence (Piercey-Normore Reference Piercey-Normore2006; Muggia et al. Reference Muggia, Pérez-Ortega, Kopun, Zellnig and Grube2014; Park et al. Reference Park, Kim, Elvebakk, Kim, Jeong and Hong2015; Dal Grande et al. Reference Dal Grande, Rolshausen, Divakar, Crespo, Otte, Schleuning and Schmitt2018; Osyczka et al. Reference Osyczka, Lenart-Borón, Borón and Rola2021). Intrathalline populations of Trebouxia can also vary in simple sequence DNA regions, which may result from clonal replication errors (Mansournia et al. Reference Mansournia, Wu, Matsushita and Hogetsu2012; Dal Grande et al. Reference Dal Grande, Alors, Divakar, Bálint, Crespo and Schmitt2014a). Individual thalli of Parmotrema pseudotinctorum from the Canary Islands were reported to encompass distinct lineages of Trebouxia as well as Asterochloris (Molins et al. Reference Molins, García-Breijo, Reig-Armiñana, del Campo, Casano and Barreno2013). According to Casano et al. (Reference Casano, del Campo, García-Breijo, Reig-Armiñana, Gasulla, del Hoyo, Guéra and Barreno2011), two genetically distinct strains of Trebouxia are always present together in thalli of Ramalina farinacea, and high-throughput sequencing results suggest that a number of other, minority algae might also be present in this lichen (Moya et al. Reference Moya, Molins, Martínez-Alberola, Muggia and Barreno2017). One constant challenge in assessing photobiont identities is that lichen thallus surfaces are colonized by epibiontic algae (including possible photobionts of other lichens) that are not intimate symbionts of the lichen in question, yet may figure prominently in cultures established or samples obtained from thallus fragments (Warén Reference Warén1920; Muggia et al. Reference Muggia, Vancurova, Škaloud, Peksa, Wedin and Grube2013). Confidence that sampled algae are indeed the thallus photobionts can be improved by establishing cultures from single algal cells extracted from within the thallus using a micromanipulator (Beck & Koop Reference Beck and Koop2001), although the procedure is time-consuming. Additional evidence may be sought in TEM micrographs of photobionts within the same thallus (e.g. Catalá et al. Reference Catalá, del Campo, Barreno, García-Breijo, Reig-Armiñana and Casano2016; Molins et al. Reference Molins, Moya, García-Breijo, Reig-Armiñana and Barreno2018), particularly where more than one pyrenoid type (Friedl Reference Friedl1989) is present. However, variability should first be assessed among individuals of the same genetic strain because chloroplast structure may vary from cell to cell and often looks substantially different according to the plane of ultrathin section examined. In sequencing, conventional dideoxy chain termination (Sanger) technology will reliably identify a predominant photobiont and ignore any others present in low abundance, while the procedure fails if there are secondary photobionts in sufficient abundance (c. 30%; Paul et al. Reference Paul, Otte, Schmitt and Dal Grande2018). High-throughput sequencing will detect minority photobionts but will also be more sensitive to epibiontic algae. A recent comparison of the two sequencing approaches concluded that in most lichens there is a single dominant photobiont genotype, representative of most of the thallus population (Paul et al. Reference Paul, Otte, Schmitt and Dal Grande2018).
The Genera of Lichen Algae
Approximately 50 algal genera are currently said to include lichen photobionts. Some may represent identifications that are erroneous or based on outdated circumscriptions of taxa. Others may spin off new genera as their cryptic genetic diversity is further elucidated. It is evident that a small number of very prominent photobiont genera (Asterochloris, Nostoc, Rhizonema, Trebouxia, Trentepohlia) each partner with many hundreds or thousands of lichen-forming fungal species; a number of others (e.g. Coccomyxa, Elliptochloris, Heveochlorella, Symbiochloris) are lichenized by many dozens or hundreds of different mycobiont species, while much of the remainder participate in only a small number of known lichen associations. It seems probable that further surveys will uncover more photobiont genera in the latter category. While it is widely agreed that the diversity of lichen-forming algae remains considerably less well known than that of lichen-forming fungi, this fact alone is unlikely to account for the enormous disparity between the currently recognized number of photobiont genera (c. 50) and that of mycobiont genera (c. 1000; Lücking et al. Reference Lücking, Hodkinson and Leavitt2017a). The number of photobiont species described, estimated at c. 100 not long ago (Škaloud & Peksa Reference Škaloud and Peksa2010), shows a similar disparity with the number of lichen-forming fungal species (20 000). Indeed, both the generic and species estimates differ between mycobiont and phycobiont by the same factor of 20. Thus, the imbalance is not likely due to differences in genus/species concepts between algae and fungi. Of course, much of the genetic diversity discovered within photobiont genera in the last few years has been reported as clades that still lack taxonomic recognition; species numbers will surely increase substantially in the near future as such diversity becomes formalized biosystematically. However, this still seems unlikely to close the enormous gap with mycobiont species numbers. Rather, the disparities probably indicate a real ecological asymmetry: the large number of lichen-forming fungal taxa may be partnering with a substantially smaller pool of photobiont taxa, many of which are shared among mycobionts. Such was the conclusion reached recently by Dal Forno et al. (Reference Dal Forno, Lawrey, Sikaroodi, Gillevet, Schuettpelz and Lücking2020) in their detailed comparison of genetic diversity in Dictyonema and its Rhizonema photobionts.
A synopsis of algal genera to which lichen photobionts are currently attributed is given below.
Cyanobacteria
Anabaena Bory ex É. Bornet & C. Flahault — See Nostoc. Strains of Anabaena versus Nostoc are resolved in some analyses (Henson et al. Reference Henson, Watson and Barnum2002; Rajaniemi et al. Reference Rajaniemi, Hrouzek, Kaštovská, Willame, Rantala, Hoffmann, Komárek and Sivonen2005; Liu et al. Reference Liu, Zhu, Lu and Song2013; Elshobary et al. Reference Elshobary, Osman, Abushady and Piercey-Normore2015) but formal distinction of the two genera remains controversial (Makra et al. Reference Makra, Gell, Juhász, Soós, Kiss, Molnár, Ördög, Vörös and Balázs2019). Tschermak-Woess (Reference Tschermak-Woess and Galun1988a) recommended re-examination of earlier reports that Anabaena occurs as cephalodial photobiont of Stereocaulon.
Anacystis Meneghini — According to Bold & Wynne (Reference Bold and Wynne1985), this generic name has been applied to ellipsoid to cylindrical cyanobacteria that often accumulate in a common gelatinous matrix, with some authors also including spheriodal-celled taxa such as Gloeocapsa and Chroococcus. The much-studied ‘Anacystis nidulans’ is usually treated now under Synecococcus; other taxa are currently placed in Microcystis. Photobionts attributed to Anacystis in the past include the partners of a small number of Peltula species and the cephalodial symbionts of a Stereocaulon (see Tschermak-Woess Reference Tschermak-Woess and Galun1988a); determining their identities with confidence will require further study.
Brasilonema Fiore et al. — This cyanobacterial genus, forming a distinct clade in molecular analyses (Fiore et al. Reference Fiore, Sant'Anna, Azevedo, Komárek, Kaštovský, Sulek and Lorenzi2007), has aggregated filaments morphologically similar to Scytonema but only rarely showing false branching. A recent paper reported new species of both Brasilonema and Chroococcidiopsis as co-occurring photobionts of an unidentified lichen growing on gravestones in a northern Florida cemetery (Villanueva et al. Reference Villanueva, Hašler, Dvořák, Poulíčková and Casamatta2018). However, as no description or evidence of this association has yet been published, the status of Brasilonema as lichen photobiont awaits corroboration.
Calothrix C. Agardh ex É. Bornet & C. Flahault and Dichothrix G. Zanardini ex É. Bornet & C. Flahault — These filamentous cyanobacteria are members of the Rivulariaceae; their trichomes have a basal heterocyte and gradually narrow towards the apex. The two genera are morphologically similar and both have been reported as lichen photobionts, particularly in association with certain species of Lichina (see Tschermak-Woess Reference Tschermak-Woess and Galun1988a). However, DNA sequences obtained from two such examples instead placed the algae in question in the genus Rivularia (Ortiz-Álvarez et al. Reference Ortiz-Álvarez, de los Ríos, Fernández-Mendoza, Torralba-Burrial and Pérez-Ortega2015). The photobiont of Placynthium nigrum isolated into culture also shows the distinctive Rivulariaceae morphology (apically tapering filaments with basal heterocytes) while the lichenized filaments rather resemble those now placed in Rhizonema (see Geitler Reference Geitler1934). The circumscription of Calothrix and Dichothrix with respect to lichen photobionts currently remains unresolved.
Chroococcidiopsis Geitler (and Myxosarcina H. Printz) — These unicellular cyanobacteria are found in a great diversity of habitats and include extremophiles. Cells divide in sequence by binary fission, often in alternating planes to produce more or less cubical packages of cells. Cells can also undergo multiple fission to produce four or more autospore-like products known as baeocytes, initially contained within the sheath-like, fibrous outer wall layer of the mother cell (Waterbury & Stanier Reference Waterbury and Stanier1978). The baeocytes of Myxosarcina, unlike those of Chroococcidiopsis, have a brief stage of gliding motility; the genera are said to be otherwise indistinguishable morphologically. The baeocyte-forming cyanobacteria were formerly grouped together in the order Pleurocapsales (Waterbury & Stanier Reference Waterbury and Stanier1978), but SSU sequence analysis has shown this trait to be a convergence shared by a number of lineages of quite different origin (Fewer et al. Reference Fewer, Friedl and Büdel2002). In that study, several photobionts isolated from Lichinaceae appear within the same clade as Chroococcidiopsis thermalis, sister to the heterocyte-forming Stigonematales and Nostocales, and distant from Myxosarcina as well as other morphologically similar taxa formerly attributed to Chroococcidiopsis (Fewer et al. Reference Fewer, Friedl and Büdel2002). Sequences obtained from photobionts of several Peltula species collected in Vietnam also suggested affinities within a broad ‘Chroococcidiopsidales’ clade (Võ Reference Võ2016). Other algal partners of Lichinaceae have been attributed to Chroococcidiopsis based on morphology and the production of baeocytes observed in cultured isolates (Büdel & Henssen Reference Büdel and Henssen1983). Tschermak-Woess (Reference Tschermak-Woess and Galun1988a) suggested that some taxa identified as Chroococcidiopsis might actually belong to Gloeocapsa and require study in culture. Most photobiont isolates attributed to Chroococcidiopsis and Myxosarcina await more detailed molecular scrutiny.
Chroococcus Nägeli — A morphologically distinctive cyanobacterial genus, Chroococcus has relatively large, spherical cells that divide at consecutive right angles to produce small packets of cells, often within concentric, gelatinous sheath layers. A number of reports, compiled by Tschermak-Woess (Reference Tschermak-Woess and Galun1988a), attribute thallus and cephalodial photobionts of various lichens to this genus or merely to Chroococcaceae, or Chroococcales. Many are anecdotal and most await reinvestigation with molecular sequence comparisons. The photobionts of certain Dictyonema species, once attributed to Chroococcus, have been shown to belong instead to Rhizonema, a usually filamentous taxon that may be greatly altered morphologically in certain lichen associations (Lücking et al. Reference Lücking, Lawrey, Sikaroodi, Gilleve, Chaves, Sipman and Bungartz2009). The circumscription of Chroococcus and its status as a lichen photobiont genus remain uncertain at present.
Gloeocapsa Kützing — This colonial cyanobacterium has roundish to oblong cells surrounded individually and communally by successive layers of dense mucilage, reflecting the sequence of cell divisions. Morphologically defined at present, Gloeocapsa commonly occurs free-living in moist terrestrial habitats and is also reported as thallus photobiont in several genera of Lichinaceae, and as cephalodial symbiont in certain species of Stereocaulon and Amygdalaria (Tschermak-Woess Reference Tschermak-Woess and Galun1988a). In the lichen Gonohymenia, contacting mycobiont hyphae broadly invaginate the cells of its photobiont, identified as Gloeocapsa (Paran et al. Reference Paran, Ben-Shaul and Galun1971). Geitler (Reference Geitler1933) described appressorial hyphae in the lichen Synalissa that branch in synchrony with the binary fission of its Gloeocapsa photobiont.
Molecular sequence data are much needed to understand the relationship among taxa currently assigned to Gloeocapsa.
Hyella É. Bornet & C. Flahault — The filamentous cyanobacterium Hyella is a widespread inhabitant of the marine intertidal zone, where it colonizes calcareous substrata such as mollusc shells. The substratum is penetrated by threads arising from a basal system at the surface; endospore-like baeocytes may be formed (Fritsch Reference Fritsch1945). Genomic analysis shows Hyella phylogenetically nearest to the genus Chroococcidiopsis (Brito et al. Reference Brito, Vieira, Vieira, Zhu, Leão, Ramos, Lu, Vasconcelos, Gugger and Tamagnini2020). Hyella is reported to be the photobiont of some species of fungi now assigned to Collemopsidium (Mohr et al. Reference Mohr, Ekman and Heegaard2004). However, details of the symbiotic interaction are few; other genera of cyanobacteria, such as Gloeocapsa and Nostoc, are also said to be photobionts for Collemopsidium [=Pyrenocollema] (Purvis et al. Reference Purvis, Coppins, Hawksworth, James and Moore1992).
Hyphomorpha A. Borzi — These seldom encountered cyanobacteria occur as epiphytes upon tropical liverworts and tree bark, where they form a prostrate filament system. The filaments have an apical cell producing derivatives that may later divide periclinally to become pluriseriate, as do structurally similar species of Stigonema. Cells of these older portions tend to fall out of alignment and become jumbled into a ‘chroococcoid stage’ (Fritsch Reference Fritsch1945). Hyphomorpha was first identified as photobiont in two species of Spilonema lichens by Henssen (Reference Henssen1981), who reported confirmation of the alga's identity by eminent phycologist Lothar Geitler. One of these mycobiont species has been recently reclassified as Erinacellus dendroides (Spribille et al. Reference Spribille, Tønsberg, Stabentheiner and Muggia2014). At present, the algal genus Hyphomorpha is phenotypically defined; it is currently placed in Fischerellaceae (Büdel & Kauff Reference Büdel, Kauff and Frey2012) or included under Hapalosiphonaceae (Komárek et al. Reference Komárek, Kaštovský, Mareš and Johansen2014) within the Nostocales.
Nostoc Vaucher ex É. Bornet & C. Flahault — This genus accommodates cyanobacteria occurring worldwide in fresh water and upon soil, bark and low-growing plants, with some strains highly desiccation-tolerant (Dodds et al. Reference Dodds, Gudder and Mollenhauer1995). Phenotypically defined at present, taxa attributed to Nostoc fall within several distinct clades of the Nostocales, making the genus polyphyletic (Rajaniemi et al. Reference Rajaniemi, Hrouzek, Kaštovská, Willame, Rantala, Hoffmann, Komárek and Sivonen2005; Gagunashvili & Andrésson Reference Gagunashvili and Andrésson2018). These algae typically form darkly pigmented, mucilaginous macrocolonies of highly variable size and shape, ranging from spheres to irregularly pustulose mats to tangles of cord-like axes. Embedded within the gelatinous matrix are uniseriate trichomes markedly constricted at the cross walls, giving individual cells an almost spherical to barrel-shaped form and the filaments a characteristic string-of-beads appearance. Cell division is diffuse, without apical cells or directional polarity. At intervals along the chain of vegetative cells are slightly larger, thicker-walled, lighter-coloured heterocytes (heterocysts) that specialize as centres of nitrogen fixation. Since the enzyme involved in this process is inhibited by the presence of oxygen, heterocytes lack oxygen-generating Photosystem II (Wolk et al. Reference Wolk, Ernst, Elhai and Bryant1994); electron donors are imported and fixed nitrogen is exported via microplasmodesmatal connections with neighbouring vegetative cells (Giddings & Staehelin Reference Giddings TH and Staehelin1981; Kumar et al. Reference Kumar, Mella-Herrera and Golden2010). Thus, prokaryotic Nostoc and its heterocytic relatives show degrees of cell specialization and intercellular transport characteristic of true multicellular organization (Garcia-Pichel Reference Garcia-Pichel and Schaechter2009).
Nostoc, like many filamentous cyanobacteria, has a motile phase. Short filament segments known as hormogonia are produced by multiple divisions of the vegetative cells between two heterocytes, then break free (Boissière et al. Reference Boissière, Boissière, Champion-Arnaud, Lallmant and Wagner1987; Paulsrud Reference Paulsrud2001). The segments disperse or migrate directionally by a gliding motion that involves secretion of polysaccharide, against which proteinaceous pili appear to push or pull the trichome (Khayatan et al. Reference Khayatan, Meeks and Risser2015). Under favourable conditions, the hormogonia lose motility and differentiate heterocytes as they transition to vegetative filaments (Paulsrud Reference Paulsrud2001). It is conceivable that motile hormogonia might facilitate symbiont encounters in the formation of cyanolichens, as also suspected of flagellate stages in eukaryotic photobionts, but direct evidence is lacking. In the establishment of plant-Nostoc symbioses, the role of hormogonia as infective agents is well known (Adams et al. Reference Adams, Duggan, Jackson and Whitton2012), and genes related to hormogonial function have been identified in lichen-symbiotic strains (Gagunashvili & Andrésson Reference Gagunashvili and Andrésson2018). Nostoc may also disperse temporally by forming akinetes, a kind of resistant spore that develops from a vegetative cell and endures adverse conditions.
Nostoc is photobiont in the majority of cyanophilic lichens. In the Peltigerales, Nostoc serves as principal thallus photobiont, or as secondary photobiont specialized for nitrogen fixation within discrete structures known as cephalodia; these are formed upon or within a thallus that has a green alga as principal photobiont. In a number of cases, Nostoc may serve as both principal and secondary photobiont of a single mycobiont species or individual; this results in cyanomorph and cephalodiate chloromorph thalli that may be either separate or conjoined (James & Henssen Reference James, Henssen, Brown, Hawksworth and Bailey1976; Brodo & Richardson Reference Brodo and Richardson1978; Tønsberg & Holtan-Hartwig Reference Tønsberg and Holtan-Hartwig1983; Armaleo & Clerc Reference Armaleo and Clerc1991; Stenroos et al. Reference Stenroos, Stocker-Wörgötter, Yoshimura, Myllys, Thell and Hyvönen2003; Moncada et al. Reference Moncada, Coca and Lücking2013; Simon et al. Reference Simon, Goffinet, Magain and Sérusiaux2018). The same strain of Nostoc may occur in both morphs (Paulsrud et al. Reference Paulsrud, Rikkinen and Lindblad1998, Reference Paulsrud, Rikkinen and Lindblad2001). In many such instances, chloromorph and cyanomorph are both foliose, but in some species of Lobaria and Sticta, the Nostoc-containing cyanomorph is a branching, fruticose growth that bears no resemblance to the foliose chloromorph (Jordan Reference Jordan1972; James & Henssen Reference James, Henssen, Brown, Hawksworth and Bailey1976; Tønsberg & Goward Reference Tønsberg and Goward2001; Magain et al. Reference Magain, Goffinet and Sérusiaux2012); when growing separately, the two morphs were long presumed to represent very different taxa. Thallus morphology would appear to be influenced by the distinct photobionts in such cases. In certain species of Pseudocyphellaria on the other hand, the independently growing ‘cyanomorphs’ include numerous clusters of the green algal symbiont (probably Symbiochloris) spread among the Nostoc within the algal layer (Henskens et al. Reference Henskens, Green and Wilkins2012), with no visible alterations to thallus morphology. Even when Nostoc serves as a secondary (cephalodial) photobiont in a mature lichen, it may be acquired at a very early stage of lichen formation through contact and capture by the developing mycobiont prothallus (Ott Reference Ott1988; de los Ríos et al. Reference de los Ríos, Raggio, Pérez-Ortega, Vivas, Pintado, Green, Ascaso and Sancho2011). Once organized, thallus lobes containing green algae may secondarily encounter and incorporate compatible Nostoc on the lower surface (Jordan Reference Jordan1970; Jordan & Rickson Reference Jordan and Rickson1971), or either the upper or lower surface (Cornejo & Scheidegger Reference Cornejo and Scheidegger2013). Mycobiont selectivity for particular strains of Nostoc can be very high (Paulsrud et al. Reference Paulsrud, Rikkinen and Lindblad2001). The Nostoc-containing cyanomorph may in turn capture compatible green algal symbionts that contact the tomentum hyphae of the lower cortex, from which chloromorph lobes arise (Sanders Reference Sanders2001).
In most lichens where it is primary photobiont, Nostoc is confined to a discrete algal layer; its filaments are often broken up or contorted into cell clusters with little secretion of mucilaginous sheath material (Fig. 1J). When isolated into culture, it reverts to the morphology and growth pattern typical of its free-living state (Kardish et al. Reference Kardish, Kessel and Galun1989). However, in many of the so-called gelatinous lichens, the form of the Nostoc is not fundamentally altered in lichenization; it maintains the necklace-like filaments and extensive surrounding gelatinous sheath, through which the mycobiont hyphae penetrate (Fig. 1I). In such cases, the photobiont constitutes the main structural component of the lichen, which may maintain an appearance and texture rather similar to that of free-living Nostoc macrocolonies. A recent study suggests that these differences in phenotypic expression, leading to stratified versus gelatinous lichens, may be associated with different genetic strains of Nostoc (Magain & Sérusiaux Reference Magain and Sérusiaux2014). This would appear to be another example where major differences in thallus structure may be correlated with photobiont identity.
Cyanophilic mycobionts can be highly selective of their Nostoc partner strains, often overriding geographical factors (Paulsrud et al. Reference Paulsrud, Rikkinen and Lindblad1998, Reference Paulsrud, Rikkinen and Lindblad2000; Stenroos et al. Reference Stenroos, Högnabba, Myllys, Hyvönen and Thell2006; Myllys et al. Reference Myllys, Stenroos, Thell and Kuusinen2007), although a considerably lower selectivity was observed in lichen communities in maritime Antarctica (Wirtz et al. Reference Wirtz, Lumbsch, Green, Türk, Pintado, Sancho and Schroeter2003). Within a single clade of Peltigera, both highly selective and less discriminating generalist species can be recognized (Magain et al. Reference Magain, Miadlikowska, Goffinet, Sérusiaux and Lutzoni2017, Reference Magain, Truong, Goward, Niu, Goffinet, Sérusiaux, Vitikainen, Lutzoni and Miadlikowska2018). A study of temperate and boreal communities reported genetically distinct terricolous and epiphytic pools of Nostoc, from which Peltigera and Nephroma spp. colonizing those respective substrata select their photobionts (Rikkinen et al. Reference Rikkinen, Oksanen and Lohtander2002). Using a larger data set, Stenroos et al. (Reference Stenroos, Högnabba, Myllys, Hyvönen and Thell2006) found Nostoc photobiont strains to be correlated with mycobiont identity rather than ecological guild. However, fungal preference for the Nostoc photobiont strains of other community members over those sampled from the substratum has been reported in other lichen communities (Cardós et al. Reference Cardós, Prieto, Jylhä, Aragón, Molina, Martínez and Rikkinen2019). In other studies, involving Pannaria and other cyanophilic lichens, both corticolous and saxicolous species sometimes chose closely related strains of Nostoc, and more complex combinations of variable mycobiont selectivity and ecological factors were observed (Elvebakk et al. Reference Elvebakk, Papaefthimiou, Robertsen and Liaimer2008).
Nostoc participates in a range of symbioses besides those it forms with lichen-forming fungi (Adams et al. Reference Adams, Duggan, Jackson and Whitton2012). It is taken up by the locally emergent protoplast of the coenocytic, glomeromycete fungus Geosiphon pyriformis, which then produces a swollen bladder within which the endosymbiotic (endocytobiotic) Nostoc is housed. The intracellular location of the algal symbiont and the close affinities of the fungal component to arbuscular mycorrhizal fungi make the Geosiphon-Nostoc symbiosis quite distinct from fungal-algal symbioses treated under the lichen concept (Kluge et al. Reference Kluge, Mollenhauer, Wolf, Schüßler, Rai, Bergman and Rasmussen2002; Schüßler Reference Schüßler and Hock2012). Nostoc also includes obligatory partners of plants representing several major clades of embryophytes; motile hormogonia are the usual infective agent, and fixed nitrogen, usually in the form of ammonium, is supplied to the host from the numerous heterocytes that differentiate in the symbiotic state (Meeks Reference Meeks1998). In hornworts and the liverwort Blasia, hormogonia enter and inhabit specialized, mucilage-secreting chambers within the gametophytes (Adams & Duggan Reference Adams and Duggan2002). Branched filamentous outgrowths from the inner surfaces of these chambers then develop and increase surface contact between the host and the cyanobacterial colonies (Rodgers & Stewart Reference Rodgers and Stewart1977). In cycad gymnosperms, Nostoc colonizes radial cavities in the cortex of specialized, upward-growing coralloid roots (Costa & Lindblad Reference Costa, Lindblad, Rai, Bergman and Rasmussen2002). Symbiosis with the floating aquatic fern Azolla is unique in that the Nostoc (or Anabaena; Svenning et al. Reference Svenning, Eriksson and Rasmussen2005) is vertically inherited through plant generations, obviating the need for new symbiont capture; the principal cyanobacterium involved cannot be cultivated separately, since its genome shows considerable gene degradation (Ran et al. Reference Ran, Larsson, Vigil-Stenman, Nylander, Ininbergs, Zheng, Lapidus, Lowry, Haselkorn and Bergman2010). In the angiosperm Gunnera, symbiotic Nostoc occurs intracellularly in leaf base tissue (Bergman et al. Reference Bergman, Johanssen and Söderbäck1992). Some of these symbiotic strains, as well as free-living isolates, appear to be similar or closely related to those occurring within lichen thalli or cephalodia, whereas certain other Nostoc strains might be more specialized as lichen photobionts (O'Brien et al. Reference O'Brien, Miadlikowsa and Lutzoni2005; Stenroos et al. Reference Stenroos, Högnabba, Myllys, Hyvönen and Thell2006). Recent genomic comparisons identified certain genes of potential relevance to symbiosis in Nostoc, suggesting also that symbiotic strains may have larger genomes than non-symbiotic ones (Gagunashvili & Andrésson Reference Gagunashvili and Andrésson2018).
Rhizonema Lücking & Barrie — This cyanobacterial genus was resurrected recently to accommodate filamentous, heterocyte-producing photobionts previously assumed to belong to Scytonema, but distinct from that lineage in their 16S rRNA sequences (Lücking et al. Reference Lücking, Lawrey, Sikaroodi, Gilleve, Chaves, Sipman and Bungartz2009). Rhizonema species may be boreal as well as tropical; they are at present known mainly from lichen symbioses but free-living or liverwort-associated populations have also been reported (Cornejo et al. Reference Cornejo, Nelson, Stepanchikova, Himelbrant, Jørgensen and Scheidegger2016). The filaments may be broken up into cell clusters or remain as discrete trichomes (Fig. 1E & F), with sporadic lateral proliferation that has been interpreted as true branching based on the appearance of a mature branch junction (Lücking et al. Reference Lücking, Barrie and Genney2014). This would presumably distinguish Rhizonema from Scytonema, which shows false branching. Thus, when Võ (Reference Võ2016) observed paired false branching in photobionts of Vietnamese Cyphellostereum and Dictyonema, she concluded that the algae were Scytonema rather than Rhizonema, apparently without corroborating molecular data. However, recent observations of Rhizonema, isolated into culture from Dictyonema and identified with genetic sequence comparisons, show branching that appears distinctly false (Fig. 1D). Interestingly, a 19th century illustration of a Dictyonema sericeum thallus (Bornet Reference Bornet1873: plate 12) depicted the photobiont with both double-false branching and seemingly true branching with a junction similar to that shown in Lücking et al. (Reference Lücking, Barrie and Genney2014). The range of branch development modes possible in Rhizonema strains clearly requires further study in both lichenized and aposymbiotic material.
Major genera of lichen-forming fungal partners known so far include Coccocarpia, Erioderma (Peltigerales), and the basidiomycetes Acantholichen, Dictyonema, Cora, Corella and Cyphellostereum (all Hygrophoraceae). In those basidiolichens, the Rhizonema trichome is usually penetrated longitudinally by a single, central mycobiont haustorium quite unlike anything reported in other lichen groups (Roskin Reference Roskin1970; Oberwinkler Reference Oberwinkler, Schwemmler and Schenk1980, Reference Oberwinkler, Hertel and Oberwinkler1984, Reference Oberwinkler and Hock2012; Slocum Reference Slocum1980; Tschermak-Woess Reference Tschermak-Woess1983). Such elaborate intrusive structures differ dramatically from the very limited penetrations known in other lichenized algae and might represent specialized absorptive structures. Carbon transfer has not yet been studied in basidiolichens.
Rivularia C. Agardh ex É. Bornet & C. Flahault — The trichomes of this cyanobacterial genus occur in clusters, often on submerged rocks; each filament has a heterocyte at the base and tends to taper gradually towards the apex. The genus includes the photobionts of a couple of maritime species of Lichina, whose algal symbionts were previously attributed to the morphologically similar genus Calothrix (Ortiz-Álvarez et al. Reference Ortiz-Álvarez, de los Ríos, Fernández-Mendoza, Torralba-Burrial and Pérez-Ortega2015).
Scytonema C. Agardh ex É. Bornet & C. Flahault — This aquatic or aerophilic genus of cyanobacteria has trichome walls unconstricted at the septa, with vegetative cells usually wider than long, prominent heterocytes, and thick sheaths that are often darkly pigmented. Scytonema is traditionally recognized by the frequently paired (‘double’) false branches, where segments created by a break in the trichome continue linear growth by simply reorienting laterally and emerging from their formerly common sheath. Trichome breaks may arise where intercellular material is deposited as a separation disc, or one or more cells degenerate, or at intercalary heterocyte positions (Bhâradwâja Reference Bhâradwâja1933). Once considered a significant photobiont genus, including both principal and secondary (cephalodial) lichen symbionts, Scytonema in its current sense encompasses an uncertain but much reduced number of lichen algae. Photobionts previously ascribed to Scytonema have been shown by DNA sequence analyses to belong to a quite distinct clade, now designated Rhizonema (Lücking et. al. Reference Lücking, Lawrey, Sikaroodi, Gilleve, Chaves, Sipman and Bungartz2009). Nevertheless, at least one recent photobiont sequence (16s rRNA), from a Heppia thallus, appears to fall within Scytonema in the strict sense (Võ Reference Võ2016). This may provide some corroboration for previous attributions of Heppia photobionts to Scytonema based on morphology of cultured isolates (Wetmore Reference Wetmore1970). The cell shape and division planes of the Heppia photobionts are radically transformed to produce cell clusters in the lichenized state, reverting quickly to typical filamentous growth when cultured aposymbiotically (Marton & Galun Reference Marton and Galun1976). In Pyrenothrix nigra, the lichenized filamentous cyanobiont shows the double-false branching typical of Scytonema (Tschermak-Woess et al. Reference Tschermak-Woess, Bartlett and Peveling1983), although Lücking et al. (Reference Lücking, Lawrey, Sikaroodi, Gilleve, Chaves, Sipman and Bungartz2009) suggested that its photobiont might be Rhizonema. This is quite plausible, since there is some doubt as to whether the two cyanobacterial genera can be reliably distinguished by their mode of branching (see comments under Rhizonema). More sequence data are clearly needed to clarify the extent to which lichen symbioses may involve the genus Scytonema in its current, more restricted sense.
Stigonema C. Agardh ex É. Bornet & C. Flahault — This cyanobacterial genus is recognized by its complex, branching axes with cells dividing in perpendicular planes as in true parenchyma. Filaments are uniseriate at the apex but become locally multiseriate proximally by periclinal divisions, often but not necessarily associated with the formation of true branches laterally. After division, cells retain continuity at the central portion of the septum, where micropores traverse the septal wall (Butler & Allsopp Reference Butler and Allsopp1972). Stigonema has been reported as thallus photobiont in Ephebe and Spilonema, and also as cephalodial partner in numerous species of Stereocaulon (Tschermak-Woess Reference Tschermak-Woess and Galun1988a). The genus awaits molecular treatment, remaining morphologically defined for the time being.
Tolypothrix Kützing ex É. Bornet & C. Flahault — These are filamentous cyanobacteria resembling Scytonema but with usually single- rather than double-false branches emerging from filament breaks; one side of the break grows out as the false branch, the other usually differentiates as a heterocyte. Tolypothrix has been reported as photobiont of the ‘primitively lichenized’ Thermutopsis jamesii based on morphology in collected material (Henssen Reference Henssen1990). Molecular sequences obtained from cephalodia of Placopsis placed the cyanobionts in or near Tolypothrix (Raggio et al. Reference Raggio, Green, Crittenden, Pintado, Vivas, Pérez-Ortega, de los Ríos and Sancho2012).
Green algae (Viridiplantae – Archaeplastida)
Apatococcus F. Brand — Abundant and widely distributed as a free-living organism, Apatococcus has long been known as an omnipresent subaerial unicellular alga, inevitably encountered but not chosen by discriminating germling hyphae of lichen-forming fungi. Now it appears that Apatococcus includes lichen symbionts as well. Light microscopic observations of algal symbionts cultured from several maritime lichen species first implicated Apatococcus as a photobiont (Watanabe et al. Reference Watanabe, Nakano and Deguchi1997); molecular sequence comparisons later identified Apatococcus strains as partners of Scoliciosporum (Beck Reference Beck2002) and Fuscidea species (Zahradníková et al. Reference Zahradníková, Andersen, Tønsberg and Beck2017). Cells are spherical with alternating perpendicular planes of division, producing cuboidal packets of transiently adherent daughter cells. Autospores and biflagellate zoospores are also formed (Ettl & Gärtner Reference Ettl and Gärtner2014). Autospores may be of unequal size within a sporangium (Gärtner & Ingolić Reference Gärtner and Ingolić1989), as also occurs in Watanabalean genera such as Chloroidium and Jaagichlorella. As with Elliptochloris and Trebouxia, Apatococcus is facultatively heterotrophic: it is very slow growing in culture unless carbohydrate is supplied (Gustavs et al. Reference Gustavs, Schumann and Karsten2016). This observation is particularly interesting because the similarly heterotrophic behaviour of Trebouxia in culture was central to Ahmadjian's (Reference Ahmadjian1988, Reference Ahmadjian2002) argument that Trebouxia cannot exist free-living. The seemingly ubiquitous Apatococcus shows quite clearly that a photobiont exhibiting strongly heterotrophic tendencies in culture may nonetheless abound free-living in nature.
Asterochloris Tschermak-Woess — First described to accommodate the trebouxioid photobiont of a single lichen in the Pertusariaceae (Tschermak-Woess Reference Tschermak-Woess1980a), this major photobiont clade now encompasses the former Trebouxia subgenus Eleutherococcus (Tschermak-Woess Reference Tschermak-Woess1989; Škaloud & Peksa Reference Škaloud and Peksa2010). It corresponds roughly to Archibald's (Reference Archibald1975) restricted concept of genus Trebouxia, a source of continual confusion. Asterochloris species produce aplanospores, or zoospores in culture, but most strains do not form the appressed, low-number autospores characteristic of Trebouxia in the current sense. Its deeply-lobed chloroplast becomes flattened and parietal during cell division, while that of Trebouxia remains more or less central (Tschermak-Woess Reference Tschermak-Woess1989). Pyrenoids are present; in TEM they may be distinguished as the irregularis-, erici-, or magna-types of Friedl (Reference Friedl1989). Chloroplast morphology is highly variable and its utility as a marker in species delimitation was emphasized by Škaloud et al. (Reference Škaloud, Steinová, Řidká and Vančurová2015). As with Trebouxia, a considerable amount of genetic diversity is revealed at the molecular level in Asterochloris (Škaloud & Peksa Reference Škaloud and Peksa2010; Peksa & Škaloud Reference Peksa and Škaloud2011).
Sexual fusion of biflagellate isogametes to form a quadriflagellate zygote has been documented in cultures of A. woessiae (Škaloud et al. Reference Škaloud, Steinová, Řidká and Vančurová2015). The detection of genes specific to meiosis in A. glomerata (Armaleo et al. Reference Armaleo, Müller, Lutzoni, Andrésson, Blanc, Bode, Collart, Dal Grande, Dietrich and Grigoriev2019) provides further support for a functioning sexual cycle in Asterochloris.
Asterochloris is associated principally with mycobionts of the Cladoniaceae, Stereocaulaceae, and the genus Lepraria. These fungal partners appear to range from moderately to rather highly selective of their Asterochloris symbionts; there is also some indication that mycobionts of different clades are choosing particular Asterochloris lineages, showing distinct climatic preferences related to rainfall regime (Peksa & Škaloud Reference Peksa and Škaloud2011).
Auxenochlorella (I. Shihira & R. W. Krauss) T. Kalina & M. Puncochárová — Within the Chlorellaceae, Auxenochlorella is related to the fully heterotrophic genus Prototheca, and its type species, A. protothecoides, is also known for its heterotrophic tendencies in culture (Darienko & Pröschold Reference Darienko and Pröschold2015). Auxenochlorella has been implicated in regard to the identity of the photobiont associated with Psoroglaena stigonemoides in the Verrucariaceae (Nyati et al. Reference Nyati, Beck and Honegger2007; Thüs et al. Reference Thüs, Muggia, Pérez-Ortega, Favero-Longo, Joneson, O'Brien, Nelsen, Duque-Thüs, Grube and Friedl2011). Unlike Chlorella, Auxenochlorella lacks a pyrenoid. The genus also includes ‘zoochlorellae’ symbionts of the cnidarian Hydra that are now considered a new species, A. symbiontica (Darienko & Pröschold Reference Darienko and Pröschold2015).
Bracteacoccus Tereg — Bracteacoccus are small, spherical unicells that have a multinucleate stage as they mature, and reproduce by zoospores or aplanospores; chloroplasts lack pyrenoids (Kouwets Reference Kouwets1996). Currently included in the Sphaeropleales (Fučíková et al. Reference Fučíková, Lewis and Lewis2014), Bracteacoccus appears at present to be the only genus of the class Chlorophyceae into which lichen photobionts have been placed with supporting DNA sequence data. The corresponding mycobionts are two species of the basidiomycete Sulzbacheromyces in the Lepidostromatales (Hodkinson et al. Reference Hodkinson, Moncada and Lücking2014; Masumoto Reference Masumoto2020).
Cephaleuros Kunze ex E. M. Fries — These foliicolous relatives of Trentepohlia form macroscopic, multicellular thalli visible as small, fuzzy yellow-orange patches on leaves and fruit in tropical and subtropical climates. Cephaleuros species typically grow beneath the cuticle of the leaf substratum, forming rounded to lobed thalli of more or less integrated horizontal filaments. These give rise to the erect setae and sporangiophores that emerge through the overlying cuticle. Usually, Cephaleuros develops within the space it excavates between the host cuticle and epidermis; in some cases, filaments penetrate deeper among the epidermal or mesophyll cells of the leaf, provoking a localized phellogen wound response. The alga can therefore be mildly pathogenic, but it is more often described as ‘parasitic’, despite an absence of information concerning any nutritional exchange with the plant host. Occasionally, the alga may develop upon the leaf cuticle, like other epiphylls. The behaviour may vary according to the species of Cephaleuros or that of the host plant (Ward Reference Ward1884; Suto & Ohtani Reference Suto and Ohtani2009; Brooks et al. Reference Brooks, Rindi, Suto, Ohtani and Green2015). Lichenization by foliicolous Strigula fungi is said to curb the alga's invasion of host tissue and its localized pathogenic effects (Joubert & Rijkenberg Reference Joubert and Rijkenberg1971).
Morphologically, Cephaleuros can somewhat resemble the related foliicolous genus Phycopeltis, which is not subcuticular and generally lacks vertical hairs and complex, long-stalked sporangiophores. According to molecular sequence analyses, however, the nearest relatives of Cephaleuros lie not within Phycopeltis but rather Stomatochroon (Zhu et al. Reference Zhu, Hu and Liu2017), a microscopic colonizer of the leaf's substomatal cavities. While trentepohliaceous taxa currently ascribed to Phycopeltis and Trentepohlia are phylogenetically intertwined, Cephaleuros appears to be essentially monophyletic (López-Bautista et al. Reference López-Bautista, Rindi and Guiry2006; Rindi et al. Reference Rindi, Lam and López-Bautista2009; Nelsen et al. Reference Nelsen, Rivas Plata, Andrew, Lücking and Lumbsch2011; Zhu et al. Reference Zhu, Hu and Liu2017).
Cephaleuros is one of the few lichen photobiont genera for which life cycle events have been observed in some detail. Thompson & Wujek (Reference Thompson and Wujek1997) describe a haplodiplontic life cycle with heteromorphic multicellular phases. The familiar thallus corresponds to the gametophyte; fusion of gametes produces a zygote that germinates into a short-stalked, dwarf sporophyte bearing a putative meiosporangium. Flagellate meiospores presumably develop into new gametophytic thalli. The Cephaleuros gametophyte is the phase known to serve as phycobiont for the fungus Strigula. Whether or not the sporophytes can also be lichenized is unknown. Perhaps they are too highly reduced or short-lived, but the question does not seem to have been explored. On gametophyte thalli, two distinct structures produce flagellate zoospores or gametes (often called zoïds, or swarmers, when their function is uncertain or polyvalent). Zoosporangia are elevated in groups upon vertical stalks; they produce quadriflagellate zoospores that have been observed to round off, germinate and reproduce the gametophyte thallus asexually (Ward Reference Ward1884; Thompson & Wujek Reference Thompson and Wujek1997). The mature sporangia detach readily as units of dispersal, for which both wind and insects act as vectors. On the horizontal filament system, single, usually terminal cells may enlarge to become what are referred to as gametangia; these produce biflagellate cells that may fuse sexually (Thompson & Wujek Reference Thompson and Wujek1997). However, other authors have been unable to observe any instances of sexual fusions in the taxa they studied, instead reporting that the biflagellate zoïds germinate directly as zoospores to form new gametophyte thalli (Suto & Ohtani Reference Suto and Ohtani2013).
Ward (Reference Ward1884) described in detail the course of lichenization of Cephaleuros by Strigula. Young germlings of Cephaleuros are often quickly overrun by the mycelium of Strigula, suppressing algal reproduction, while individuals contacted at more advanced stages of development may produce abundant sporangia from portions of its thalli remaining relatively free of mycobiont domination. Interestingly, both symbionts grow and sporulate independently upon the leaf substratum, although the fungus Strigula will produce pycnidia and perithecia only after successful lichenization. These observations highlight the flexibility of the symbionts in this particular association.
Chlamydomonas Ehrenberg — This well-known unicellular green algal genus chiefly encompasses aquatic taxa that are flagellate in the vegetative state and unlikely candidates for lichen symbiosis. However, a number of aeroterrestrial species are also known (Ettl & Gärtner Reference Ettl and Gärtner2014). One species of Chlamydomonas (C. augustae) was described in association with the ascomycete Pyronema laetissimum, growing on leaf litter in Latvia (Skuja Reference Skuja1943). It was included in Tschermak-Woess's (Reference Tschermak-Woess and Galun1988a) review of phycobionts as ‘facultatively lichenized’. However, Skuja (Reference Skuja1943) distinguished this association from lichen and lichenoid symbioses, making comparisons instead with green algae known to grow abundantly on the surfaces of perennial basidiocarps. The Chlamydomonas was abundantly present among the dense hyphae below the Pyronema apothecium, but no tissue layer was differentiated, nor were any distinctive contact interfaces noted between symbionts. Skuja also mentioned that other apothecia of the same fungus were fruiting nearby without the alga present. The operculate discomycetes (Pezizales), to which Pyronema belongs, are not otherwise known to include lichen-forming members. The Pyronema-Chlamydomonas association is worthy of further investigation but seems unlikely to fit the criteria usually ascribed to lichen symbioses.
Chlorella Beijerinck — A once-notorious miscellany of indistinguishable ‘little round green things’, this trebouxiophycean genus has been radically deconstructed, particularly with the help of DNA sequence comparisons (Huss et al. Reference Huss, Frank, Hartmann, Hirmer, Kloboucek, Seidel, Wenzeler and Kessler1999). Many formerly included species have been moved to different genera, orders, even classes, while taxa surrounding the type species C. vulgaris, and C. sorokiniana, are retained as true Chlorella. In TEM, they show a distinctive pyrenoid surrounded by a thick sheath of starch and bisected centrally by a single thylakoid (Ikeda & Takeda Reference Ikeda and Takeda1995; Němcová & Kalina Reference Němcová and Kalina2000; Hoshina et al. Reference Hoshina, Iwataki and Imamura2010). Flagellate cells and sexual reproduction are unknown. True Chlorella also includes a number of mucilaginous, colonial forms in its current circumscription (Luo et al. Reference Luo, Pröschold, Bock and Krienitz2010; Bock et al. Reference Bock, Krienitz and Pröschold2011). Many of the lichen photobionts previously attributed to Chlorella s. lat. (e.g. Tschermak-Woess Reference Tschermak-Woess1988b) are among those taxa moved to other genera, especially Chloroidium; others await re-examination. At present, only a couple of lichen-forming fungal species have photobionts of corroborated placement within Chlorella (Porpidia crustulata; Li et al. Reference Li, Feng and Xie2013) or Chlorellales. The genus has also been long identified with endosymbiotic algal symbionts of diverse protists and invertebrates. Molecular sequences confirm that true Chlorella occur as endosymbionts of the ciliate Paramecium bursaria (Hoshina et al. Reference Hoshina, Kamako and Imamura2004; Summerer et al. Reference Summerer, Sonntag and Sommaruga2008) and the cnidarian Hydra (Kovačević et al. Reference Kovačević, Franjević, Jelenčić and Kalafatić2010), which may also utilize Auxenochlorella as its algal symbiont (Pröschold et al. Reference Pröschold, Darienko, Silva, Reisser and Krienitz2011). Chloroplast ultrastructure likewise suggests that the green endosymbiont of the colonial ciliate Ophrydium versatile is a true Chlorella (Forsberg & Lindblad Reference Forsberg and Lindblad1996). However, the phylogenetic affinities of other ‘zoochlorellae’ symbionts appear to fall elsewhere in the Trebouxiophyceae (Lewis & Muller-Parker Reference Lewis and Muller-Parker2004; Kovačević et al. Reference Kovačević, Franjević, Jelenčić and Kalafatić2010; Pröschold et al. Reference Pröschold, Darienko, Silva, Reisser and Krienitz2011), while many have yet to be explored with molecular sequence comparisons.
Chloroidium Nadson — Resurrected to accommodate segregates from Chlorella s. lat. (Darienko et al. Reference Darienko, Gustavs, Mudimu, Menendez, Schumann, Karsten, Friedl and Pröschold2010), Chloroidium falls within the trebouxiophycean assemblage now formalized as Watanabeales (Li et al. Reference Li, Tan, Liu, Zhu, Hu and Liu2021). Cells have a parietal chloroplast with or without a pyrenoid; in C. saccharophilum, a prominent pyrenoid with surrounding plastoglobuli and traversing membranes has been observed (González et al. Reference González, Pröschold, Palacios, Aguayo, Inostroza and Gómez2013). Reproduction is by autospores, often of variable number and different sizes within a single sporangium. The genus encompasses diverse taxa found in a wide variety of habitats (Darienko et al. Reference Darienko, Lukešová and Pröschold2018), including extremophiles capable of using a variety of carbon sources (Nelson et al. Reference Nelson, Khraiwesh, Fu, Alseekh, Jaiswal, Chaiboonchoe, Hazzouri, O'Connor, Butterfoss and Dro2017). Since its recent emendation, Chloroidium includes photobiont partners of a growing number of lichen-forming fungi, including some species of Gomphillaceae, Verrucariaceae, Psora, Stereocaulon and Sticta.
Chlorosarcinopsis Herndon — In the course of her studies on lichen haustoria, Plessl (Reference Plessl1963) identified as Chlorosarcina [=Chlorosarcinopsis] minor the photobionts she isolated from two species of Lecidea, L. plana and L. lapicida. Chlorosarcinopsis has traditionally accommodated spherical unicellular green algae dividing to form cuboidal packets. According to Neustupa (Reference Neustupa and Frey2015), the genus is polyphyletic, with members scattered among the Chlamydomonadales (Chlorophyceae). As this clade is not otherwise known for lichen symbionts (but see Skuja Reference Skuja1943), the photobionts of the Lecidea species in question need further study.
Coccobotrys Chodat (now Uvulifera Molinari-Novoa) — This green alga forms irregular cuboidal cell packages or branched multiseriate filaments in culture (Neustupa Reference Neustupa and Frey2015). The genus Coccobotrys was described by Chodat (Reference Chodat1913) and emended by Vischer (Reference Vischer1960) to accommodate the putative photobiont C. verrucariae isolated from a thallus of Verrucaria nigrescens. Thüs et al. (Reference Thüs, Muggia, Pérez-Ortega, Favero-Longo, Joneson, O'Brien, Nelsen, Duque-Thüs, Grube and Friedl2011), on the other hand, reported Diplosphaera as photobiont of the V. nigrescens thallus they sampled. Coccobotrys verrucariae was also cited among algae isolated from soil crusts (Flechtner et al. Reference Flechtner, Johansen and Belnap2009), and a photobiont identified with microscopy as ‘probably Coccobotrys’ (Canals et al. Reference Canals, Hernández-Mariné, Gómez-Bolea and Llimona1997) was isolated from Botrylepraria lesdainii, another member of the Verrucariales (Kukwa & Pérez-Ortega Reference Kukwa and Pérez-Ortega2010). A second species of Coccobotrys was described by Warén (Reference Warén1920) as the photobiont of Lecidea fuliginosa, but Tschermak-Woess (Reference Tschermak-Woess and Galun1988a) expressed doubt that the alga he described belongs in Coccobotrys. The photobiont status of species in this genus should be corroborated. Genetic sequence analyses place Coccobotrys in the Trebouxiophyceae (e.g. Thüs et al. Reference Thüs, Muggia, Pérez-Ortega, Favero-Longo, Joneson, O'Brien, Nelsen, Duque-Thüs, Grube and Friedl2011; Mikhailyuk et al. Reference Mikhailyuk, Holzinger, Tsarenko, Glaser, Demchenko and Karsten2020), but its affinities among the defined clades within this class remain uncertain. Molinari-Novoa (Reference Molinari-Novoa2016) recently found Coccobotrys Chodat to be a later homonym of a name applied to an anamorphic basidiomycete and renamed the algal genus Uvulifera.
Coccomyxa Schmidle — This trebouxiophycean algal genus is notable for the diversity of habitats and ecological circumstances in which its species are known to occur. Environmental surveys have found Coccomyxa sequences to be among the most widely distributed OTUs, and notably well represented in cold high-latitude climates (Metz et al. Reference Metz, Singer, Domaizon, Unrein and Lara2019). It is commonly reported free-living on terrestrial substrata and in aquatic environments, including those highly polluted with heavy metals and radioactive materials (see Gustavs et al. Reference Gustavs, Schiefelbein, Darienko, Grube, Seckbach and Muggia2017). Coccomyxa species are subspherical to ovoid-ellipsoidal unicells, often embedded colonially in thick gelatinous sheath material with concentric layering that reflects the cell division pattern. The chloroplast is parietal, not markedly lobed, and lacks a pyrenoid. In TEM, thylakoid bands often show a distinctly longitudinal orientation over the length of the chloroplast, with interspersed starch grains (Peveling & Galun Reference Peveling and Galun1976; Palmqvist et al. Reference Palmqvist, de los Ríos, Ascaso and Samuelsson1997). Flagellate cells and sexual reproduction are unknown; cells subdivide into packages of 2–8 autospores (Tschermak-Woess Reference Tschermak-Woess and Galun1988a). Recent assessments of species number within the genus range from seven (Darienko et al. Reference Darienko, Gustavs, Eggert, Wolf and Pröschold2015) to as many as 27 (Malavasi et al. Reference Malavasi, Škaloud, Rindi, Tempesta, Paoletti and Pasqualetti2016). The genus is thought to include the photobionts of diverse lichen-forming fungi, such as species of Icmadophila, Micarea, Nephroma, Peltigera, Solorina, the stalked-apotheciate genera Baeomyces, Dibaeis and Phyllobaeis, and the basidiomycete Lichenomphalia (Table 1). Some of these reports await confirmation with genetic sequence data. The photobionts do not form a single clade but instead represent several distinct lineages within Coccomyxa, intermixed among free-living isolates (Darienko et al. Reference Darienko, Gustavs, Eggert, Wolf and Pröschold2015). In lichen symbiosis, the cells are often more spheroidal, and extensive gelatinous sheath material is not usually produced (Tschermak-Woess Reference Tschermak-Woess and Galun1988a). Interestingly, while the cells of Coccomyxa and Elliptochloris photobionts are tightly enveloped by mycobiont hyphae, their walls are usually not penetrated (Tschermak Reference Tschermak1941a; Geitler Reference Geitler1955; Plessl Reference Plessl1963; but see Coppins Reference Coppins1983: figs 2 & 55). This has been attributed to degradation-resistant polymers resembling sporopollenin in the multilayered cell wall (Honegger & Brunner Reference Honegger and Brunner1981; Brunner & Honegger Reference Brunner and Honegger1985). However, Coccomyxa cells are fully penetrated by Aphelidium collabens, a parasitoid basal within, or sister to, the kingdom Fungi (Seto et al. Reference Seto, Matsuzawa, Kuno and Kagami2020).
Species of the genus Coccomyxa also live in poorly understood symbioses within molluscs (Stevenson & South Reference Stevenson and South1974; Syasina et al. Reference Syasina, Kukhlevsky, Kovaleva and Vaschenko2012) and echinoderms, and endocytotically within ovules and other tissues of the gymnosperm Ginkgo biloba (Trémouillaux-Guiller et al. Reference Trémouillaux-Guiller, Rohr, Rohr and Huss2002; Trémouillaux-Guiller & Huss Reference Trémouillaux-Guiller and Huss2007). Molecular sequence comparisons have shown that some zoochlorellae isolated from certain strains of Paramecium bursaria correspond to Coccomyxa, while most others are true Chlorella (Hoshina & Imamura Reference Hoshina and Imamura2008).
Deuterostichococcus Pröschold & Darienko — A recent segregate of Stichococcus s. lat. (Pröschold & Darienko Reference Pröschold and Darienko2020), this trebouxiophycean genus currently includes, in addition to free-living isolates, the photobiont of two Placopsis species (Beck et al. Reference Beck, Bechteler, Casanova-Katny and Dzhilyanova2019) and Staurothele clopima (Hodač et al. Reference Hodač, Hallmann, Spitzer, Elster, Faßhauer, Brinkmann, Lepka, Diwan and Fried2016); the latter is also known to partner with Diplosphaera algae (Thüs et al. Reference Thüs, Muggia, Pérez-Ortega, Favero-Longo, Joneson, O'Brien, Nelsen, Duque-Thüs, Grube and Friedl2011).
Dictyochloropsis Geitler — See Symbiochloris.
Dilabifilum Tschermak-Woess — Polymorphic, unicellular to sarcinoid to filamentous algae with pyrenoids and quadriflagellate zoospores have been included in this ulvalean genus. They occur free-living, as photobionts, or both. Recently, Darienko & Pröschold (Reference Darienko and Pröschold2017) deconstructed Dilabifilum, recognizing at generic level several distinct clades resolved in their gene-based phylogenies. A number of photobionts previously contained therein are now distributed in Halofilum, Lithotrichon, Paulbroadya and Pseudendoclonium, while others await reassessment.
Diplosphaera Bialosuknia — This prasiolalean genus appears to contain the majority of the unicellular photobiont strains attributed until recently to the related Stichococcus. Apparently, the two morphologically plastic genera are often not distinguishable microscopically, although Diplosphaera may produce distinctive, adherent two-celled clusters in division. Pyrenoids may be absent (Pröschold & Darienko Reference Pröschold and Darienko2020) or weakly visible (Ettl & Gärtner Reference Ettl and Gärtner2014) but some taxa falling within the Diplosphaera clade, including lichen photobionts, show prominent pyrenoids in TEM (Fig. 2E). The main fungal partners of Diplosphaera are members of the Verrucariaceae. Photobiont strains compared so far appear to represent the same species and are similar to free-living collections (Pröschold & Darienko Reference Pröschold and Darienko2020).
In association with certain lichen genera, such as Endocarpon and Staurothele, Diplosphaera photobionts ‘escape’ vegetative hyphal contacts and penetrate into the hymenial layer of developing perithecia, where they freely intermix among the asci (Fig. 3A). These algal cells are typically much smaller than those within the algal layer of the vegetative thallus; they scatter everywhere when a hand-cut section is water-mounted, indicating that unlike the photobionts in the vegetative thallus, those entering the perithecia are not bound in place by lichenizing contacts with the mycobiont. The unassociated photobionts may adhere to the large ascospores as they are ejected, and can be dispersed with them. Many readily detach and divide aposymbiotically; they are available to the mycobiont if the spore germinates successfully (Stahl Reference Stahl1877; Bertsch & Butin Reference Bertsch and Butin1967; Ahmadjian & Heikkilä Reference Ahmadjian and Heikkilä1970) or might otherwise divide to form free-living populations. Potentially co-dispersable photobionts also occur in the conidiomata and ascomal epithecia of many foliicolous lichens of the Gomphillaceae and Pilocarpaceae (see Heveochlorella). Dispersal of liberated photobionts can thereby provide a direct connection between lichenized and free-living populations of the alga.
Elliptochloris Tschermak-Woess — Like its sister genus Coccomyxa, Elliptochloris has subspherical to ellipsoidal unicells with a parietal chloroplast, bearing two opposed indentations in the type species E. bilobata (Tschermak-Woess Reference Tschermak-Woess1980b). Sexual or flagellate stages are unknown; reproduction occurs by autospores, of which there are usually two morphologically distinct types. Autosporangia may contain a low number (usually four in cultured E. bilobata) of spherical spores appressed together at flattened junctions, or more numerous (16–32) cylindrical-ellipsoidal spores (Tschermak-Woess Reference Tschermak-Woess1980b; Darienko et al. Reference Darienko, Gustavs and Pröschold2016). The multilayered cell wall, as in Coccomyxa, is impregnated with degradation-resistant polymers, which are thought to explain the lack of haustorial penetration by their lichen-forming partners (Brunner & Honegger Reference Brunner and Honegger1985). However, haustoria have been noted in certain species of Micarea (Coppins Reference Coppins1983: figs 2 & 55), a lichen-forming genus known to partner with Elliptochloris and Coccomyxa photobionts. Unlike Coccomyxa, at least some species of Elliptochloris possess pyrenoids, and layered mucilaginous sheaths are typically lacking. However, gelatinous extracellular material may be copious in free-living populations, and was observed in association with Protothelenella thalli where the photobiont population grew beyond the reach of mycobiont hyphae (Tschermak-Woess Reference Tschermak-Woess1985).
Elliptochloris is somewhat less often reported than Coccomyxa but is known from a similarly diverse array of habitats. It is said to be quite strongly heterotrophic in culture, where it depends heavily on organic materials to thrive; this might in part account for its less frequent recovery in isolation procedures (Gustavs et al. Reference Gustavs, Schiefelbein, Darienko, Grube, Seckbach and Muggia2017). Species of Elliptochloris are known to partner with mycobionts of diverse genera including Catillaria, Catolechia, Fuscidea, Micarea, Sticta, Stictis, Verrucaria, and the basidiolichen-forming Bryoclavula and Multiclavula (see Table 1). They also occur as endosymbionts of the marine anenome Anthopleura (Letsch et al. Reference Letsch, Muller-Parker, Friedl and Lewis2009).
Gloeocystis Nägeli — Taxa treated under this genus are unicellular green algae that form occasionally macroscopic colonies of ellipsoidal cells with a parietal chloroplast possessing a pyrenoid. Thick, colourless, often layered gelatinous sheaths surround the cells. Reports of Gloeocystis as photobiont of Cryptodiscus [Bryophagus] gloeocapsa and Epigloea bactrospora were cited by Ahmadjian (Reference Ahmadjian1967) and Tschermak-Woess (Reference Tschermak-Woess and Galun1988a). There is doubt as to whether Epigloea is lichenized (Kirk et al. Reference Kirk, Cannon, David and Stalpers2001; not included in Lücking et al. (Reference Lücking, Hodkinson and Leavitt2017a)), although distinctly symbiotic contacts with living, unicellular green algae were illustrated by Jaag & Thomas (Reference Jaag and Thomas1934) and Döbbler (Reference Döbbler1984).
According to Neustupa (Reference Neustupa and Frey2015), the algal genus Gloeocystis is highly polyphyletic, encompassing members of both Chlorophyceae and Trebouxiophyceae. The identities of the photobionts associated with the mycobionts mentioned above will therefore require further study.
Halofilum Darienko & Pröschold — This is another genus that now accommodates taxa previously treated under Dilabifilum (Darienko & Pröschold Reference Darienko and Pröschold2017). These algae consist of branched filaments with parietal chloroplasts containing pyrenoids; flagellated stages are unknown. The species H. ramosum occurs as photobiont of Hydropunctaria maura and Wahlenbergiella striatula (Verrucariaceae), as well as free-living (Darienko & Pröschold Reference Darienko and Pröschold2017).
Heveochlorella J. Zhang et al. — Unicellular algae attributed to Heveochlorella have a prominent, somewhat lobed chloroplast with a central pyrenoid that is readily visible with light microscopy. TEM shows the pyrenoid surrounded by several irregular starch plates and penetrated centripetally by thylakoid-derived tubules that are lined with pyrenoglobuli (Fig. 2B). Cells reproduce by autospores, usually in low number (2–8) and not infrequently of unequal size within sporangia, at least in culture (Zhang et al. Reference Zhang, Huss, Sun, Chang and Pang2008; Ma et al. Reference Ma, Huss, Tan, Sun, Chun, Xie and Zhang2013; Sanders et al. Reference Sanders, Pérez-Ortega, Nelsen, Lücking and de los Ríos2016). Darienko & Pröschold (Reference Darienko and Pröschold2019) recently subsumed both Heveochlorella (Zhang et al. Reference Zhang, Huss, Sun, Chang and Pang2008) and the related Heterochlorella (Neustupa et al. Reference Neustupa, Němcová, Eliáš and Škaloud2009), which has not been reported from lichen symbioses, into the resurrected genus Jaagichlorella. These algae belong to the trebouxiophyceaen clade recently formalized as Watanabeales (Li et al. Reference Li, Tan, Liu, Zhu, Hu and Liu2021).
The first indication that lichen symbionts belonged in this group was the report of Heveochlorella isolated as photobiont from one specimen of Sticta and two of Pseudocyphellaria from Taiwan (Dal Grande et al. Reference Dal Grande, Beck, Cornejo, Singh, Cheenacharoen, Nelsen and Scheidegger2014b). Soon thereafter, the ‘trebouxioid’ photobionts associated with foliicolous Gomphillaceae and Pilocarpaceae were also attributed to this genus (Sanders et al. Reference Sanders, Pérez-Ortega, Nelsen, Lücking and de los Ríos2016). More recently, a study of Sticta lichens worldwide reported Heveochlorella to be the photobiont of numerous specimens from New Zealand and Indian Ocean islands, including six identified species and many undetermined collections (Lindgren et al. Reference Lindgren, Moncada, Lücking, Magain, Simon, Goffinet, Sérusiaux, Nelsen, Mercado-Díaz and Widhelm2020). In the opinion of Darienko & Pröschold (Reference Darienko and Pröschold2019), the algae encompassed by Jaagichlorella, though distributed worldwide, are rare taxa. While more surveys will be necessary to evaluate this view, a number of observations suggest that these algae might be in fact quite common and merely overlooked. We know, for example, that foliicolous lichens of the Gomphillaceae and Pilocarpaceae occur in abundance throughout much of the humid tropics (Santesson Reference Santesson1952; Lücking Reference Lücking2008), although it is not yet clear how consistently they harbour Heveochlorella (Jaagichlorella) photobionts. Recent sampling of the phyllosphere community in Asian tropical forests has revealed a major representation of Heveochlorella genotypes (Zhu et al. Reference Zhu, Li, Hu and Liu2018), as well as several new species in related genera (Li et al. Reference Li, Sun, Hu, Liu, Zhu, Hu and Liu2020, Reference Li, Tan, Liu, Zhu, Hu and Liu2021). Some of the most frequently detected OTUs in environmental surveys of marine habitats (Metz et al. Reference Metz, Singer, Domaizon, Unrein and Lara2019) were also attributed to Heveochlorella.
In many foliicolous lichens, dividing Heveochlorella photobionts may escape the lichenizing vegetative hyphae and proliferate among the spore-generating fungal structures, upon apothecia and within specialized conidiomata such as campylidia and hyphophores (Fig. 3B & C). They can be dispersed from these structures, as are the fungal spores or diahyphae to which the algal cells may adhere (Fig. 3D–F). Once dispersed, they may become lichenized by the germinating fungal propagules, or divide to produce independent populations on the substratum (Sanders Reference Sanders2014; Sanders & de los Ríos Reference Sanders and de los Ríos2015). Co-dispersal and relichenization thereby provide Heveochlorella with abundant opportunities for exchange between lichenized and free-living populations.
Interfilum Chodat — This genus of aeroterrestrial charophytes (Streptophyta) includes taxa that form single, paired or sarcinoid packets of cells or grow filamentously, often closely resembling unrelated Chlorophyta, such as Desmococcus (Mikhailyuk et al. Reference Mikhailyuk, Sluiman, Massalski, Mudimu, Demchenko, Kondratyuk and Friedl2008). It is sister to clades of the widely distributed Klebsormidium (Rindi et al. Reference Rindi, Mikhailyuk, Sluiman, Friedl and López-Bautista2011). Interfilum was reported by Voytsekhovich et al. (Reference Voytsekhovich, Dymytrova and Nadyeina2011) as a secondary photobiont within the algal layer of Micarea and Placynthiella thalli collected in Ukraine, based on light microscopic examination of thalli and cultured isolates. The principal photobionts in those lichens were reported to be Elliptochloris and Radiococcus, respectively. As the charophytes are not otherwise known as lichen symbionts, and other algal genera were cited as the main photobionts within the thalli in question, further study of the reported associations is warranted.
Jaagichlorella Reisigl — See Heveochlorella.
Leptosira A. Borzì — This photobiont grows as unicells tightly wrapped by mycobiont hyphae or separated by copious sheath material free of the mycobiont; in agar culture, it produces short filaments (Tschermak-Woess Reference Tschermak-Woess1953). Leptosira is a trebouxiophycean of uncertain placement, appearing in the vicinity of the Microthamniales clade in recent gene-based phylogenies (Lemieux et al. Reference Lemieux, Otis and Turmel2014; Neustupa Reference Neustupa and Frey2015; Hallmann et al. Reference Hallmann, Hoppert, Mudimu and Friedl2016). According to Mattox & Stewart (Reference Mattox, Stewart, Irvine and John1984), ‘Pleurastrum terrestre’ (a synonym of Leptosira obovata, now L. terrestris; Friedl Reference Friedl1996) is so similar ultrastructurally to the genus Trebouxia that they could be combined in the same genus. Ahmadjian (Reference Ahmadjian1988) went one step further, opining that Trebouxia was merely the lichenized form of this taxon. However, the aforementioned gene-based cladograms do not show a close relationship between Leptosira and the Trebouxiales.
Leptosira terrestris, in lichen symbiosis with Vezdaea aestivalis, grows subcuticularly (Tschermak-Woess & Poelt Reference Tschermak-Woess, Poelt, Brown, Hawksworth and Bailey1976), a distinction shared with the photobiont Cephaleuros. Leptosira is also among the very few photobiont genera (Phycopeltis and Cephaleuros are others) reported to produce zoospores in the lichenized state (Tschermak-Woess & Poelt Reference Tschermak-Woess, Poelt, Brown, Hawksworth and Bailey1976).
Lithotrichon Darienko & Pröschold — Another genus separated from the Dilabifilum (Ulvales) complex, Lithotrichon forms clustered cell packets as well as branching filaments and is distinguished from similar genera by SSU and ITS sequence data. The species L. pulchrum occurs as photobiont of the freshwater lichen Hydropunctaria rheitrophila (Darienko & Pröschold Reference Darienko and Pröschold2017).
Myrmecia Printz — These spherical to pyriform unicells have a parietal chloroplast, without a pyrenoid, extending around most of the cell, with 2–4 broad lobes defined by deep notches. Cells proliferate via zoospores, aplanospores, or autospores (Ettl & Gärtner Reference Ettl and Gärtner2014). Gene-based phylogenies consistently place Myrmecia in the Trebouxiales, sister to Trebouxia (Muggia et al. Reference Muggia, Nelsen, Kirika, Barreno, Beck, Lindgren, Lumbsch and Leavitt2020) or to the Asterochloris + Vulcanochloris clade (Vančurová et al. Reference Vančurová, Peksa, Němcová and Škaloud2015). Myrmecia occurs free-living as well as in lichen symbiosis. An aerophilic alga, originally described as Friedmannia from Negev Desert rocks, is now recognized as Myrmecia israelensis (Friedl Reference Friedl1995) and was recently reported as lichen photobiont (Thüs et al. Reference Thüs, Muggia, Pérez-Ortega, Favero-Longo, Joneson, O'Brien, Nelsen, Duque-Thüs, Grube and Friedl2011; Moya et al. Reference Moya, Chiva, Molins, Jadrná, Škaloud, Peksa and Barreno2018). Psora decipiens and a number of species in the Verrucariaceae are among the lichen-forming fungi known to partner with Myrmecia.
Nannochloris Naumann — The genus Nannochloris has encompassed simple, extremely tiny (1.5–2 μm) chlorophyte algae that reproduce by binary division or autospores. Circumscription of the genus has been controversial, but molecular data indicate that most of the species belong in Chlorellales (Henley et al. Reference Henley, Hironaka, Gillou, Buchheim, Buchheim, Fawley and Fawley2004). Tschermak-Woess (Reference Tschermak-Woess1981) recognized Nannochloris normandinae as the photobiont partner of lichen-forming Normandina pulchella; in other works, Nannochloris has been mentioned more indirectly in the context of photobionts (e.g. Lohtander et al. Reference Lohtander, Oksanen and Rikkinen2003). However, Thüs et al. (Reference Thüs, Muggia, Pérez-Ortega, Favero-Longo, Joneson, O'Brien, Nelsen, Duque-Thüs, Grube and Friedl2011) found only Diplosphaera as photobiont in the 10 Normandina thalli they examined and, more recently, Pröschold & Darienko (Reference Pröschold and Darienko2020) reduced Nannochloris normandinae to synonymy with Diplosphaera chodatii (Prasiolales). Thus, clear evidence of lichen photobionts belonging in Nannochloris appears to be lacking at present.
Neocystis F. Hindák — Members of this trebouxiophycean genus produce mucilaginous colonies of spherical to ellipsoidal or crescent-shaped cells that reproduce by autospores (Neustupa Reference Neustupa and Frey2015). Cultures assigned to Neocystis as well as other genera were recently reviewed with molecular sequence analyses, revealing considerable taxonomic redundancy assigned to only two closely related, genetically distinct but morphologically plastic species (Eliáš et al. Reference Eliáš, Neustupa, Pažoutová and Škaloud2013). An alga identified as Neocystis sp. was cited as ‘additional photobiont’ of Micarea misella, in thalli having Elliptochloris bilobata as principal photobiont (Voytsekhovich et al. Reference Voytsekhovich, Dymytrova and Nadyeina2011).
Paulbroadya Darienko & Pröschold — This recently recognized clade in the Ulvales is distinguished by SSU-ITS sequences from other taxa previously treated under Dilabifilum (Darienko & Pröschold Reference Darienko and Pröschold2017). The species Paulbroadya petersii occurs as photobiont of the marine intertidal lichen Wahlenbergiella mucosa (Darienko & Pröschold Reference Darienko and Pröschold2017).
Phycopeltis Millardet — Members of this trentepohliaceous genus are most often seen as coppery orange discs a few mm in diameter on leaf surfaces in humid subtropical and tropical regions, with one or two species extending to cooler regions such as oceanic Europe (Rindi et al. Reference Rindi, Menéndez, Guiry and Rico2004). Thallus discs consist of a monostromatic layer of closely appressed, bifurcating filaments (Fig. 6A). Unlike those of Cephaleuros, Phycopeltis thalli are supracuticular, non-pathogenic, and at least some species readily colonize other favourably displayed plant substrata besides leaves. Sporangia are usually borne erect on a very short stalk, and release quadriflagellate zoospores through a pore at the end opposite to the point of attachment. Gametangia are sessile and develop from intercalary compartments of the horizontal filament system in most species; gametes are biflagellate and isomorphic, and their fusion has been observed (Thompson & Wujek Reference Thompson and Wujek1997). The life cycle of Phycopeltis is believed to be haplodiplontic, with alternation of gametophytes and sporophytes that are isomorphic, rather than heteromorphic as in Cephaleuros and Stomatochroon (Thompson & Wujek Reference Thompson and Wujek1997). If this is the case, recognizing meiosporangia by the presence of tetrads might be the only means of distinguishing the phases phenotypically, but there do not appear to be such reports. Whether the gametophytes and sporophytes are equally susceptible to lichenization would be an interesting question to examine.
Although distinguishing Phycopeltis from Trentepohlia under current morphological concepts appears fairly straightforward, DNA sequence data show species of the two genera to be intertwined phylogenetically (Zhu et al. Reference Zhu, Zhao, Xia, Hu and Liu2015, Reference Zhu, Hu and Liu2017; Grube et al. Reference Grube, Muggia, Baloch, Hametner, Stocker-Wörgötter, Grube, Seckbach and Muggia2017a). Phycopeltis is particularly under-sampled at present. The morphological distinction between the two genera may also break down in the lichenized condition. Although Phycopeltis species can retain their placoid thallus characteristics when partnering with certain foliicolous mycobionts (Grube & Lücking Reference Grube and Lücking2002), in other lichens the algal filaments may be broken up into individual cells indistinguishable from those of Trentepohlia (see fig. 9; Lücking Reference Lücking2008). Using TEM, Matthews et al. (Reference Matthews, Tucker and Chapman1989) believed they could differentiate the two genera in such cases by features of the septal wall near plasmodesmata. It would be useful to test how well such traits correlate with molecular markers.
As widespread colonizers of the warm-temperate and tropical phyllosphere, species of Phycopeltis are important photobionts in foliicolous lichen communities, where they partner with diverse leaf-dwelling mycobionts including species of Arthonia, Chroodiscus, Mazosia, Opegrapha, Porina, Trichothelium, and supracuticular taxa of Strigulaceae, among others (Santesson Reference Santesson1952; Lücking Reference Lücking2008). Multiple Phycopeltis thalli may occur edge-to-edge within a single foliicolous lichen, as additional individuals are incorporated by the mycobiont's expanding prothallus (Sanders Reference Sanders2002). There are some reports of algal gametangia or sporangia being produced in the lichenized state, particularly in those taxa where the Phycopeltis thalli are sparsely covered by the mycobiont (Santesson Reference Santesson1952; Lücking Reference Lücking1994; Sanders Reference Sanders2002).
In at least one species of Phycopeltis (P. epiphyton), the highly degradation-resistant biopolymer sporopollenin was detected in the cell wall (Good & Chapman Reference Good and Chapman1978). Its presence in the walls of other photobionts (Coccomyxa and Elliptochloris) has been correlated with the absence of haustorial penetration by mycobionts (Honegger & Brunner Reference Honegger and Brunner1981; Brunner & Honegger Reference Brunner and Honegger1985). However, at least some strains of lichenized Phycopeltis may be deeply penetrated by mycobiont haustoria, such as those of Porina (Matthews et al. Reference Matthews, Tucker and Chapman1989).
Prasiola Meneghini — This trebouxiophycean seaweed of high-latitude supratidal zones is exceptional for its class in having a multicellular, macroscopic blade-like thallus. It is often abundant and readily visible both free-living and in symbiosis with mycobiont Mastodia tessellata (Verrucariaceae). Two or three distinct species of Prasiola appear to serve as photobiont to the bipolarly distributed Mastodia (Garrido-Benavent et al. Reference Garrido-Benavent, Pérez-Ortega and de los Ríos2017, Reference Garrido-Benavent, de los Ríos, Fernández-Mendoza and Pérez-Ortega2018). There has been some discussion, on structural grounds, as to whether this fungal-algal partnership ought to be considered a true lichen (Lud et al. Reference Lud, Huiskes and Ott2001; Kohlmeyer et al. Reference Kohlmeyer, Hawksworth and Volkmann-Kohlmeyer2004; Pérez-Ortega et al. Reference Pérez-Ortega, de los Ríos, Crespo and Sancho2010). There is no fungal cortex, nor does symbiosis substantially change algal thallus morphology; its anatomy, however, is significantly altered, as algal cells become well separated by a proliferation of encircling mycobiont hyphae (Kovačik & Batista Pereira Reference Kovačik and Batista Pereira2001; Lud et al. Reference Lud, Huiskes and Ott2001). The symbiosis therefore entails considerably more structural transformation than that produced by the marine fungus Mycophycias upon its seaweed host Ascophyllum (Xu et al. Reference Xu, Deckert and Garbary2008), or Turgidosculum upon Blidingia (Pérez-Ortega et al. Reference Pérez-Ortega, Miller and de los Ríos2018). From a phylogenetic perspective, it is worth noting that close relatives of both the mycobiont (Verrucariaceae) and the alga (Prasiolales) participate in symbioses that are unambiguously lichenic.
Pseudendoclonium Wille — These ulvalean algae have variably packet-forming to filamentous morphologies and may be differentiated into prostrate and erect filament systems. Darienko & Pröschold (Reference Darienko and Pröschold2017) moved into this genus a couple of photobionts previously treated under Dilabifilum, recognizing the photobiont of Arthopyrenia kelpii as Pseudendoclonium arthopyreniae, and the photobiont of Hydropunctaria maura as P. commune, which is also widespread as a free-living alga on intertidal rocks. Pseudendoclonium arthopyreniae has a pyrenoid surrounded by thick plates of starch and traversed by several narrow, thylakoid-derived membranes lacking pyrenoglobuli (Namba & Nakayama Reference Namba and Nakayama2021).
Pseudochlorella J. W. G. Lund — This unicellular genus of Chlorella-like algae is now placed in the Prasiolales. Molecular data support inclusion of the photobiont of at least one lichen-forming fungus, Trapelia coarctata (Darienko et al. Reference Darienko, Gustavs and Pröschold2016). Other reports attribute to Pseudochlorella the photobionts of certain Micarea, Placynthiella and Stereocaulon species (see Tschermak-Woess Reference Tschermak-Woess and Galun1988a; Voytsekhovich et al. Reference Voytsekhovich, Dymytrova and Nadyeina2011). However, molecular sequence studies have so far identified photobionts from Micarea as Coccomyxa and Elliptochloris, and those from the green algal layer of Stereocaulon as Asterochloris, Chloroidium and Vulcanochloris.
Pseudococcomyxa Korshikov — Isolates identified as Pseudococcomyxa simplex have been reported as photobionts of a maritime Leproloma sp. (Watanabe et al. Reference Watanabe, Nakano and Deguchi1997) and also Micarea prasina (Voytsekhovich et al. Reference Voytsekhovich, Dymytrova and Nadyeina2011), based on light microscopy and culture studies. The genus Pseudococcomyxa has been distinguished morphologically from Coccomyxa by the polarized secretion of mucilage to form a cap at one end of the cell. However, Darienko et al. (Reference Darienko, Gustavs, Eggert, Wolf and Pröschold2015) found this character to be culture-dependent, and the Pseudococcomyxa strains they analyzed phylogenetically appeared intermixed with those of Coccomyxa (see also Yahr et al. Reference Yahr, Florence, Škaloud and Voytsekhovich2015). Isolates attributed to P. simplex in particular occurred in several distinct clades. Darienko et al. (Reference Darienko, Gustavs, Eggert, Wolf and Pröschold2015) reassigned all these strains to Coccomyxa. While lichen photobiont isolates attributed to Pseudococcomyxa remain to be examined, support for distinction of the genus from Coccomyxa now appears to be lacking.
Pseudostichococcus L. Moewus — Morphologically similar to Stichococcus, this genus was recently revised with molecular data (Pröschold & Darienko Reference Pröschold and Darienko2020). It currently includes the photobiont partner of Neocatapyrenium rhizinosum (Hodač et al. Reference Hodač, Hallmann, Spitzer, Elster, Faßhauer, Brinkmann, Lepka, Diwan and Fried2016) in the Verrucariaceae.
Pseudotrebouxia P. A. Archibald — See Trebouxia.
Radiococcus Schmidle — Species of this genus have been reported to occur as principal photobiont in thalli of two species of Placynthiella (P. icmalea and P. uliginosa) from the Ukraine (Voytsekhovich et al. Reference Voytsekhovich, Dymytrova and Nadyeina2011). Corroboration with DNA sequence data is needed, particularly since diverse, unrelated taxa have been repeatedly ascribed to this genus in the past (Wolf et al. Reference Wolf, Hepperle and Krienitz2003). According to a recent taxonomic treatment, Radiococcaceae and Radiococcus belong in the order Sphaeropleales of the Chlorophyceae (Neustupa Reference Neustupa and Frey2015), although these names are still being applied to taxa falling in other groups, such as the Trebouxiophyceae (e.g. Metz et al. Reference Metz, Singer, Domaizon, Unrein and Lara2019).
Stichococcus Nägeli s. lat. — In its broad sense, Stichococcus (Prasiolales) has encompassed smallish unicellular to filamentous algae of notably labile morphology, the most commonly recognized form represented by short-cylindrical cells. The chloroplast is parietal, often extending to no more than half of the cell circumference, not markedly lobed, with or without a pyrenoid. Culture conditions appear to have a significant effect on cell form. The straight or slightly curved, rod-shaped cells may separate or remain together after division to form very short filaments or swell to more spherical shapes, and may or may not produce a surrounding gelatinous sheath (Ettl & Gärtner Reference Ettl and Gärtner2014). Quite a number of lichen photobionts have been ascribed to Stichococcus, but as their diversity is studied at the molecular level, these taxa are being placed in segregate genera or other prasiolalean clades. Some seven to nine clades have now been recognized within Stichococcus s. lat. (Hodač et al. Reference Hodač, Hallmann, Spitzer, Elster, Faßhauer, Brinkmann, Lepka, Diwan and Fried2016; Pröschold & Darienko Reference Pröschold and Darienko2020). All Stichococcus-like photobionts examined in a study of the Verrucariaceae by Thüs et al. (Reference Thüs, Muggia, Pérez-Ortega, Favero-Longo, Joneson, O'Brien, Nelsen, Duque-Thüs, Grube and Friedl2011), were shown to belong in Diplosphaera. Others now appear to fall within Pseudostichococcus, Deuterostichococcus or Tritostichococcus (Pröschold & Darienko Reference Pröschold and Darienko2020). It is not yet clear whether Stichococcus in the restricted sense (near to type species S. bacillaris) includes lichen photobionts.
Symbiochloris Škaloud et al. — Formally described by Škaloud et al. (Reference Škaloud, Friedl, Hallmann, Beck and Dal Grande2016), the genus corresponds to a distinct clade of Dictyochloropsis s. lat. previously recognized by Dal Grande et al. (Reference Dal Grande, Beck, Cornejo, Singh, Cheenacharoen, Nelsen and Scheidegger2014b). Symbiochloris is currently thought to include all lichen photobionts previously included in Dictyochloropsis, as well as some free-living taxa. Principal mycobiont partner genera are Lobaria, Pseudocyphellaria, Sticta and their recent segregates Crocodia, Dendriscosticta and Ricasolia, all members of the Lobariaceae. Other lichens reported harbouring Symbiochloris photobionts include species of Biatora, Brigantiaea, Chaenotheca, Megalospora and Phlyctis.
The net-like chloroplast of Symbiochloris, similar to that of Dictyochloropsis, has reticulations that vary in form, thickness and orientation according to species and developmental stage (Škaloud et al. Reference Škaloud, Neustupa, Radochová and Kubínová2005, Reference Škaloud, Friedl, Hallmann, Beck and Dal Grande2016). Lichenized populations reproduce by aplanospores, but zoospore production may be observed in isolated culture. Cells of free-living populations often attain much larger sizes and their surfaces may be covered with scales (Tschermak-Woess Reference Tschermak-Woess1995).
Trebouxia Puymaly — The principal crop of the alga-farming fungi, unicellular Trebouxia is thought to include the photobionts chosen by the largest proportion (nearly half) of known mycobiont species. Together with the closely related Asterochloris, Trebouxia is chlorobiont of most Lecanorales and Teloschistales, as well as many other taxa of the other species-rich lecanoromycetid orders (Miadlikowska et al. Reference Miadlikowska, Kauff, Högnabba, Oliver, Molnár, Fraker, Gaya, Hafellner, Hofstetter and Gueidan2014). Species of Trebouxia are spherical or occasionally ellipsoidal unicells with a variously lobed, axial chloroplast that fills much of the cell and bears a prominent pyrenoid (Fig. 2A & C). There is considerable diversity of pyrenoid ultrastructure within the genus, involving differences in the morphology of penetrating membranes and the distribution of starch deposits and pyrenoglobuli, when present (Peveling Reference Peveling1968, Reference Peveling1969; Fisher & Lang Reference Fisher and Lang1971; Friedl Reference Friedl1989). CO2-fixing Rubisco is concentrated in the pyrenoids, which in some instances also comprise additional, smaller, satellite substructures within the chloroplast (Ascaso et al. Reference Ascaso, Valladares and de los Ríos1995). Although pyrenoid types do not correspond precisely to the Trebouxia clades supported in molecular sequence analyses, and several are strikingly convergent with pyrenoids of distantly related algae, they can nonetheless be useful in distinguishing certain groupings of taxa at close range (Friedl Reference Friedl1989; but see also Muggia et al. (Reference Muggia, Zellnig, Rabensteiner and Grube2010)). Some 30 species of Trebouxia, distributed among four major clades, are currently recognized. However, this figure is believed to grossly underestimate the true genetic diversity present in the genus (Leavitt et al. Reference Leavitt, Kraichak, Vondrak, Nelsen, Altermann, Divakar, Alors, Esslinger, Crespo and Lumbsch2015; Muggia et al. Reference Muggia, Nelsen, Kirika, Barreno, Beck, Lindgren, Lumbsch and Leavitt2020). The boundaries among the formally described species remain largely unresolved, since much of the genetic diversity uncovered in recent studies is not fully congruent with the phenotypically defined taxa. Muggia et al. (Reference Muggia, Candotto-Carniel, Grube, Grube, Seckbach and Muggia2017) postulated that the application of a phylogenetic species concept would at least triple the number of species currently recognized in Trebouxia.
Two different groups were long distinguished within Trebouxia s. lat. (Ahmadjian Reference Ahmadjian1960), which was previously known as Cystococcus. Archibald (Reference Archibald1975) recognized two genera, Trebouxia and Pseudotrebouxia, based on differences in cell division which were judged sufficient to separate them into two distinct orders. However, Gärtner (Reference Gärtner1985) and Tschermak-Woess (Reference Tschermak-Woess1989) found Archibald's subdivision untenable and reunited the genus, while acknowledging that differences in cell division were present. Tschermak-Woess (Reference Tschermak-Woess1989) distinguished two subgenera: Trebouxia (corresponding roughly to Pseudotrebouxia), which forms aplanospores (or zoospores in culture) and also autospores, and Eleutherococcus (later Asterochloris), which produces aplanospores/zoospores but not autospores. Autospores are distinguishable from aplanospores in that they are produced in lower numbers and are tightly appressed together within the sporangium such that their walls form angular junctions between them (Tschermak-Woess Reference Tschermak-Woess1989). Another difference is the position of the chloroplast during cell division, which remains more or less central in Trebouxia but becomes parietal and flattened in Asterochloris (Ahmadjian Reference Ahmadjian1960; Tschermak-Woess Reference Tschermak-Woess1989). Molecular data firmly distinguish the two clades, which have been formally recognized as distinct genera for the past decade (Škaloud & Peksa Reference Škaloud and Peksa2010).
As a lichen symbiont, Trebouxia is abundant in a great diversity of habitats worldwide. It is said to be infrequently reported in the free-living state, although researchers who sample substrata with microscopy have often found it (Tschermak-Woess Reference Tschermak-Woess1978; Bubrick et al. Reference Bubrick, Galun and Frensdorff1984; Mukhtar et al. Reference Mukhtar, Garty and Galun1994; Sanders Reference Sanders2005; Handa et al. Reference Handa, Ohmura, Nakano and Nakahara-Tsubota2007; Uher Reference Uher2008; Neustupa & Štifterová Reference Neustupa and Štifterová2013), with the notable exception of Degelius (Reference Degelius1964). Clearly, the germinating spores of trebouxiophilic mycobionts manage to obtain it, often without needing to produce an extensive mycelium (Werner Reference Werner1931; Clayden Reference Clayden1998). Recent environmental sequencing studies have found Trebouxia on a variety of surfaces (Darienko et al. Reference Darienko, Gruber, Pröschold and Schagerl2013; Hallmann et al. Reference Hallmann, Stannek, Fritzlar, Hause-Reitner, Friedl and Hoppert2013, Reference Hallmann, Hoppert, Mudimu and Friedl2016; Yung et al. Reference Yung, Chan, Lacap, Pérez-Ortega, de los Ríos, Lee, Cary and Pointing2014) and well represented in soil, fresh water and even marine environments (Metz et al. Reference Metz, Singer, Domaizon, Unrein and Lara2019), although one cannot be certain that the detected sequences represent free-living individuals. By contrast, two other principal lichen photobiont genera, Trentepohlia and Nostoc, are uncontroversially well known in the free-living state. The comparison may not be fair, however, because Trentepohlia and Nostoc both form easily recognized macrocolonies (bright orange tufts and distinctive gelatinous globs, respectively) whereas Trebouxia cannot be distinguished without a microscope and some degree of effort. In any case, a shadow of doubt still seems to haunt the status of free-living Trebouxia populations, to judge from the cautious wording in even quite recent literature (e.g. Friedl & Büdel Reference Friedl, Büdel and Nash2008). Although he never claimed to have searched for it in nature, Ahmadjian (Reference Ahmadjian1988, Reference Ahmadjian1993, Reference Ahmadjian and Seckbach2001, Reference Ahmadjian2002) repeatedly affirmed that Trebouxia existed only in highly coevolved symbiosis with lichen fungi and did not occur free-living. Yet he acknowledged that aposymbiotic populations of Trebouxia could appear in nature. He even proposed, as have others, that they arose from the breakdown of lichenized propagules, such as soredia and isidia, that reach microhabitats unsuitable for the partners to develop symbiotically (Ahmadjian Reference Ahmadjian1988). Ahmadjian asserted, however, that such populations were not truly free-living, except in a ‘secondary sense’. Apparently, he meant that they were ephemeral rather than stably established, but stable or not, aposymbiotic populations of Trebouxia are likely to be significant. Like other micro-organisms, many algae take advantage of ephemeral resources and transiently favourable microenvironments, then complete their life cycles with sexual reproduction when conditions deteriorate. Some then survive as resistant spores; others may escape adversity by entering into lichen symbioses. Within a lichen thallus, an algal population may be perpetuated for many years, yet continually disperse via soredia, isidia, lichenized fragments and other propagules that can seed new free-living populations. This has been characterized as photobiont ‘escape’ from the lichen fungus (Werth Reference Werth and Fontaneto2010). It may be equally valid to view relichenization as photobiont escape from conditions that aposymbiotic populations might not long endure.
Although stages of flagellar development within a lichen thallus were reported (Slocum et al. Reference Slocum, Ahmadjian and Hildreth1980), authors have expressed scepticism that Trebouxia could produce motile or sexual cells in the symbiotic state (Tschermak-Woess Reference Tschermak-Woess1989), where all algal cells are held by one or more appressorial hyphae (Honegger Reference Honegger, Mendgen and Leseman1990). In aposymbiotic culture, by contrast, the production and release of Trebouxia zoids are well documented (Ahmadjian Reference Ahmadjian1960, Reference Ahmadjian1967; Tschermak-Woess Reference Tschermak-Woess1989; Takeshita Reference Takeshita2001). The huge genetic diversity present (Muggia et al. Reference Muggia, Nelsen, Kirika, Barreno, Beck, Lindgren, Lumbsch and Leavitt2020) and its structure within populations (Kroken & Taylor Reference Kroken and Taylor2000) suggest that Trebouxia is reproducing sexually, but virtually nothing is known about how or when the sexual cycle proceeds in nature. Although it is often said that sexual reproduction has not been observed in this genus, both Warén (Reference Warén1920) and Ahmadjian (Reference Ahmadjian1960) reported and illustrated the fusion of flagellate isogametes in Trebouxia cultures. However, Ahmadjian (Reference Ahmadjian1988, Reference Ahmadjian and Seckbach2001) believed that these features were vestiges of the alga's free-living ancestry that no longer play any role in their present life histories. Further investigation of aposymbiotic populations is needed, since considerable indirect evidence suggests that they may reveal key events in the Trebouxia life cycle.
Trentepohlia C. Martius — The filamentous taxa currently treated under this cosmopolitan genus are among the most familiar of subaerial algae, often forming readily visible yellowish orange tufts on bark, rocks and other substrata in a wide variety of environments. They are also among the phycobionts chosen by the most diverse lichen-forming ascomycetes, including members of the Arthoniomycetes, Coniocybomycetes, Dothidiomycetes, Eurotiomycetes (Pyrenulales) and ostropalean Lecanoromycetes such as the species-rich Graphidaceae. Members of the order Trentepohliales and its sole family Trentepohliaceae present a distinctive combination of features: phragmoplastic cell division with plasmodesmata (otherwise characteristic of charophycean algae), a uniquely structured flagellar apparatus, peculiar sporangiophores, and distinctive orange pigmentation. Consequently, widely divergent interpretations of their phylogenetic affinities have been proposed, with some authors even placing the group in a separate class of its own (van den Hoek et al. Reference van den Hoek, Mann and Jahns1995). However, rDNA sequence data firmly place the subaerial Trentepohliales among orders of mainly marine taxa within the Ulvophyceae (López-Bautista & Chapman Reference López-Bautista and Chapman2003; Leliaert et al. Reference Leliaert, Smith, Moreau, Herron, Verbruggen, Delwiche and De Clerck2012).
Among the taxa currently treated under Trentepohlia, a number of genera were described to accommodate the morphological diversity represented, most recently Printzina (Thompson & Wujek Reference Thompson and Wujek1992). However, DNA sequence analyses have so far shown that the phenotypic similarities recognized are unreliable indicators of phylogenetic affinity (López-Bautista et al. Reference López-Bautista, Rindi and Guiry2006; Rindi et al. Reference Rindi, Lam and López-Bautista2009). This also applies to some of the morphological traits currently used to distinguish Trentepohlia species from those of Phycopeltis. Free-living and lichenized isolates of Trentepohlia occur intermixed in gene-based phylogenies (Nelsen et al. Reference Nelsen, Rivas Plata, Andrew, Lücking and Lumbsch2011; Hametner et al. Reference Hametner, Stocker-Wörgötter and Grube2014a, Reference Hametner, Stocker-Wörgötter, Rindi and Grubeb; Kosecka et al. Reference Kosecka, Jabłońska, Flakus, Rodriguez-Flakus, Kukwa and Guzow-Krzemińska2020). Due to its visible and widespread presence in the free-living state, Trentepohlia is an excellent subject for studying the relationship between lichenized and aposymbiotic populations in nature (Fig. 1A–C). So far, however, the genus has been the focus of relatively few modern phylogenetic studies, despite its visibility and primary importance in lichen symbioses.
The pigments characteristic of the Trentepohliaceae, called ‘haematochrome’ in the older literature, are carotenoids that occur abundantly as lipidic globules in the cytoplasm. Some authors have attributed the colour to astaxanthin (Thompson & Wujek Reference Thompson and Wujek1997; Grube et al. Reference Grube, Muggia, Baloch, Hametner, Stocker-Wörgötter, Grube, Seckbach and Muggia2017a), a deep red carotenoid known from other chlorophytes such as Haematococcus and the snow alga Chlamydomonas nivalis. However, analyses of Trentepohlia haematochrome show principally beta-carotenes (Czeczuga & Maximov Reference Czeczuga and Maximov1996; Mukherjee et al. Reference Mukherjee, Borah and Goswami2010; Kharkongor & Ramanujam Reference Kharkongor and Ramanujam2015; Chen et al. Reference Chen, Zhang and Liu2016). Located outside the plastids, these secondary carotenoids do not participate in photosynthetic light-harvesting but are hypothesized to filter excess light and suppress any damaging reactive oxygen species thereby generated (Solovchenko Reference Solovchenko2013). This may contribute to the visible success of Trentepohlia in colonizing exposed substrata in diverse environments.
The sporangia of Trentepohlia are themselves units of dispersal, easily detached when mature and vectored by wind, rain or insects. They then initiate a second round of shorter-distance dispersal by releasing quadriflagellate zoospores (Thompson & Wujek Reference Thompson and Wujek1997). Trentepohlia also produces putative gametangia that are morphologically distinct from sporangia. However, the biflagellate zoïds released have most often been observed germinating as spores rather than fusing as gametes (Rindi & Guiry Reference Rindi and Guiry2002).
Cellular contacts between mycobionts and their trentepohliaceous photobionts often appear to be superficial. However, most of these lichens, when carefully examined microscopically, reveal haustorial penetration, often deeply into the algal cells (Tschermak Reference Tschermak1941a; Withrow & Ahmadjian Reference Withrow and Ahmadjian1983; Matthews et al. Reference Matthews, Tucker and Chapman1989; but see Meier & Chapman Reference Meier and Chapman1983).
Trentepohlia photobionts have been occasionally reported to grow out from the algal layer and emerge as free filaments projecting from the lichen thallus or thalline apothecial margin (Zahlbruckner Reference Zahlbruckner, Engler and Prantl1907: p. 126; McGee Reference McGee2002). In one case, such a filament was seen bearing a sporangium (Tschermak Reference Tschermak1941a: p. 289). However, some authors have expressed scepticism about this interpretation, suggesting that epiphytic Trentepohlia might instead develop upon, and then grow into, an already formed thallus (Henssen & Jahns Reference Henssen and Jahns1974: p. 196). More detailed observations are clearly required, but either explanation could represent another potentially significant mechanism by which exchange may occur between lichenized and free-living populations.
Tritostichococcus Pröschold & Darienko — This recent segregate of Stichococcus s. lat. (Pröschold & Darienko Reference Pröschold and Darienko2020) includes the Stichococcus-like photobionts that associate with Chaenotheca, a genus of lichen-forming fungi that partner with a remarkably broad spectrum of photobionts (Tibell Reference Tibell2001).
Trochiscia Kützing — This genus of unicellar algae is characterized by an often-thick cell wall with spine- or wart-like projections, an irregularly stellate chloroplast, and two forms of endogenous spore production, resulting in hundreds of small cylindrical autospores or just two rounded endospores (Tschermak Reference Tschermak1941b). Trochiscia currently appears to be placed among the Chlorophyceae, in or near Sphaeropleales (Fučíková et al. Reference Fučíková, Lewis, Neupane, Karol and Lewis2019). It was identified as photobiont of Polyblastia amota and P. hyperborea (Tschermak Reference Tschermak1941b; Ahmadjian Reference Ahmadjian1967) in the Verrucariaceae, but those reports appear to be in doubt (Ettl & Gärtner Reference Ettl and Gärtner2014) and further studies are needed. Trochiscia was not among the photobionts detected in the survey by Thüs et al. (Reference Thüs, Muggia, Pérez-Ortega, Favero-Longo, Joneson, O'Brien, Nelsen, Duque-Thüs, Grube and Friedl2011) of the algal partners of Verrucariaceae.
Vulcanochloris Vancurová et al. — This newest addition to the Trebouxia family encompasses three recently described species with a distinctive, highly dissected chloroplast structure, and molecular sequences that place them as sister to Asterochloris (Vančurová et al. Reference Vančurová, Peksa, Němcová and Škaloud2015). They are known mainly as principal photobionts from some thalli of Stereocaulon vesuvianum in the Canary Islands, although there is also a very recent report of Vulcanochloris from a Stereocaulon thallus collected in highland Bolivia (Kosecka et al. Reference Kosecka, Guzow-Krzemińska, Čemajová, Škaloud, Jabłońska and Kukwa2021). Most other Stereocaulon lineages investigated to date appear to associate with Asterochloris or Chloroidium (Vančurová et al. Reference Vančurová, Muggia, Peksa, Řídká and Škaloud2018). Vulcanochloris has also been recently reported as a minority photobiont in thalli of Ramalina farinacea (Moya et al. Reference Moya, Molins, Martínez-Alberola, Muggia and Barreno2017).
Stramenopila (Heterokontae)
Heterococcus Chodat — The yellow-green (xanthophyte) algae lack fucoxanthin, the golden brownish plastidial carotenoid otherwise characteristic of the photosynthetic stramenopiles. The absence of fucoxanthin makes them rather easy to confuse with green algae. Their zoospores, however, will have the characteristic stramenopilous flagellum bearing stiff, hollow, tripartite appendages (mastigonemes). Heterococcus forms irregular filaments and/or cell clusters when isolated into culture (Zeitler Reference Zeitler1954). Molecular sequences support the light microscope identification of Heterococcus as photobiont in thalli of three species of Verrucariaceae (Hydropunctaria rheitrophila, Verrucaria funckii and V. hydrela) that are each in separate clades and not closely related to one another (Thüs et al. Reference Thüs, Muggia, Pérez-Ortega, Favero-Longo, Joneson, O'Brien, Nelsen, Duque-Thüs, Grube and Friedl2011). Another xanthophyte, Heterothrix (now Xanthonema; Silva Reference Silva1979) was identified via light microscopy as photobiont of Staurothele clopimoides (Pereira Riquelme Reference Pereira Riquelme1992) but that interesting report requires corroboration.
Petroderma Kuckuck — Petroderma maculiforme is a small crustose brown alga (Phaeophyceae) found on rocks in the lower intertidal zone of western North America and Europe. In San Francisco Bay, it is particularly common on discarded plastic (Sanders et al. Reference Sanders, Moe and Ascaso2004). The alga is a disc of tightly branched, radiating horizontal filaments, rather similar in morphology to the chlorophyte Phycopeltis but with a dense carpet of short, erect filaments arising proximally from the horizontal system. In the free-living state, these erect filaments may bear unilocular and/or plurilocular sporangia (zoidangia) terminally (Fritsch Reference Fritsch1945). Chloroplasts typically possess one or several large pyrenoids that are prominent in electron micrographs (Fig. 2D) but not readily visible with light microscopy. The pyrenoids are traversed by branching tubules arising from invagination of the plastidial boundary membranes (rather than thylakoids, as in Trebouxia and Heveochlorella); the position of the pyrenoid may be laminar, protruding to exserted, or enfolded by chloroplast lobes (Sanders et al. Reference Sanders, Moe and Ascaso2005). The alga was first brought to the attention of lichenologists by a footnote in a phycology dissertation (Wynne Reference Wynne1969) that reported it in symbiosis with a Verrucaria species on intertidal rocks in northern California. The lichen was not studied further until Moe (Reference Moe1997) re-collected it and formally described the fungal symbiont as Verrucaria tavaresiae (now Wahlenbergiella tavaresiae; Gueidan et al. Reference Gueidan, Thüs and Pérez-Ortega2011). When lichenized, the Petroderma filaments are separated by fungal tissue, into which they grow and branch downwards rather than upwards as in the free-living condition (Sanders et al. Reference Sanders, Moe and Ascaso2004). Petroderma is the only member of the Phaeophyceae known to enter into lichen symbiosis. However, certain larger brown seaweeds, such as Ascophyllum, have intimate, mutualistic associations with verrucariacean fungi (Garbary & London Reference Garbary and London1995; Garbary & MacDonald Reference Garbary and MacDonald1995) that are generally not considered to be lichens on structural grounds, since the fungus grows within algal tissues as a conventional mycelium (Hawksworth Reference Hawksworth1988).
Acknowledgements
The manuscript benefited from critical review by Dr Richard L. Moe and two anonymous referees, and the expert scrutiny of the journal production staff. Funding for colour in print was kindly provided by the College of Arts and Sciences and the Department of Biological Sciences, Florida Gulf Coast University.
Author ORCID
William B. Sanders, 0000-0001-9572-4244.