Introduction
The geographical area of Central America between Mexico and Costa Rica, including the five countries Guatemala, Belize, Honduras, El Salvador, and Nicaragua, is still largely a blank map with regard to lichenological studies. Apart from a number of broader monographic treatments and some miscellaneous works that cite occasional records from these countries, only nine studies have been explicitly dedicated to this area (Resnick & Weberling Reference Resnick and Weberling1963; Nowak & Winkler Reference Nowak and Winkler1972; Barclay-Estrup Reference Barclay-Estrup1992; Barillas & Lücking Reference Barillas and Lücking1992; Barillas et al. Reference Barillas, Lücking and Winkler1993; Sipman Reference Sipman2001; Breuss Reference Breuss2002, Reference Breuss2011; van den Boom et al. Reference van den Boom, Elix and Sipman2007). This is a far cry from the literally hundreds of works available for Mexico, Costa Rica, and Panama, making the central part of the Central American land bridge the least studied area in all of the Neotropics, in terms of lichens.
In this paper, we continue studies initiated by the first author in Nicaragua (Breuss Reference Breuss2002, Reference Breuss2011) and describe three species from that country as new to science, in the genera Eugeniella, Graphis, and Malmidea. We also take the opportunity to provide updated world keys for Eugeniella and Malmidea.
Material and Methods
The specimens treated were collected in Nicaragua in 2001 and examined at the first author's laboratory and at The Field Museum with WILD-M7A, LEICA MS5, and OLYMPUS SZX12 dissecting microscopes and ZEISS Axiolab, ZEISS Axioscop 2, OLYMPUS BH-2, and VISTA VISION VWR V036 compound microscopes, in part connected to JENOPTIC ProgRes C3 and C5 digital microscope cameras, using standard techniques of light microscopy. Apothecial sections were mounted in tap water. Chemical tests with K, C, and P were applied to the thallus and apothecial sections. MERCK and FLUKA 62650 Lugol's solution were used to test for hymenium amyloidity. Thin-layer chromatography (TLC) was performed following standard methods for lichens, using solvent C (Arup et al. Reference Arup, Ekman, Lindblom and Mattsson1993; Orange et al. Reference Orange, James and White2010). The specimens are preserved in LI.
The Species
Eugeniella palleola Breuss & Lücking sp. nov.
MycoBank No.: MB 807098
Similar to Eugeniella leucocheila, from which it differs in having pale discs and larger apothecia, in addition to producing norstictic acid in the excipulum and stictic acid and an unknown substance in the thallus.
Type: Nicaragua, Granada, Volcán Mombacho, montane rainforest, c. 1100 m, 8 July 2001, O. Breuss 18.920 (LI—holotype).
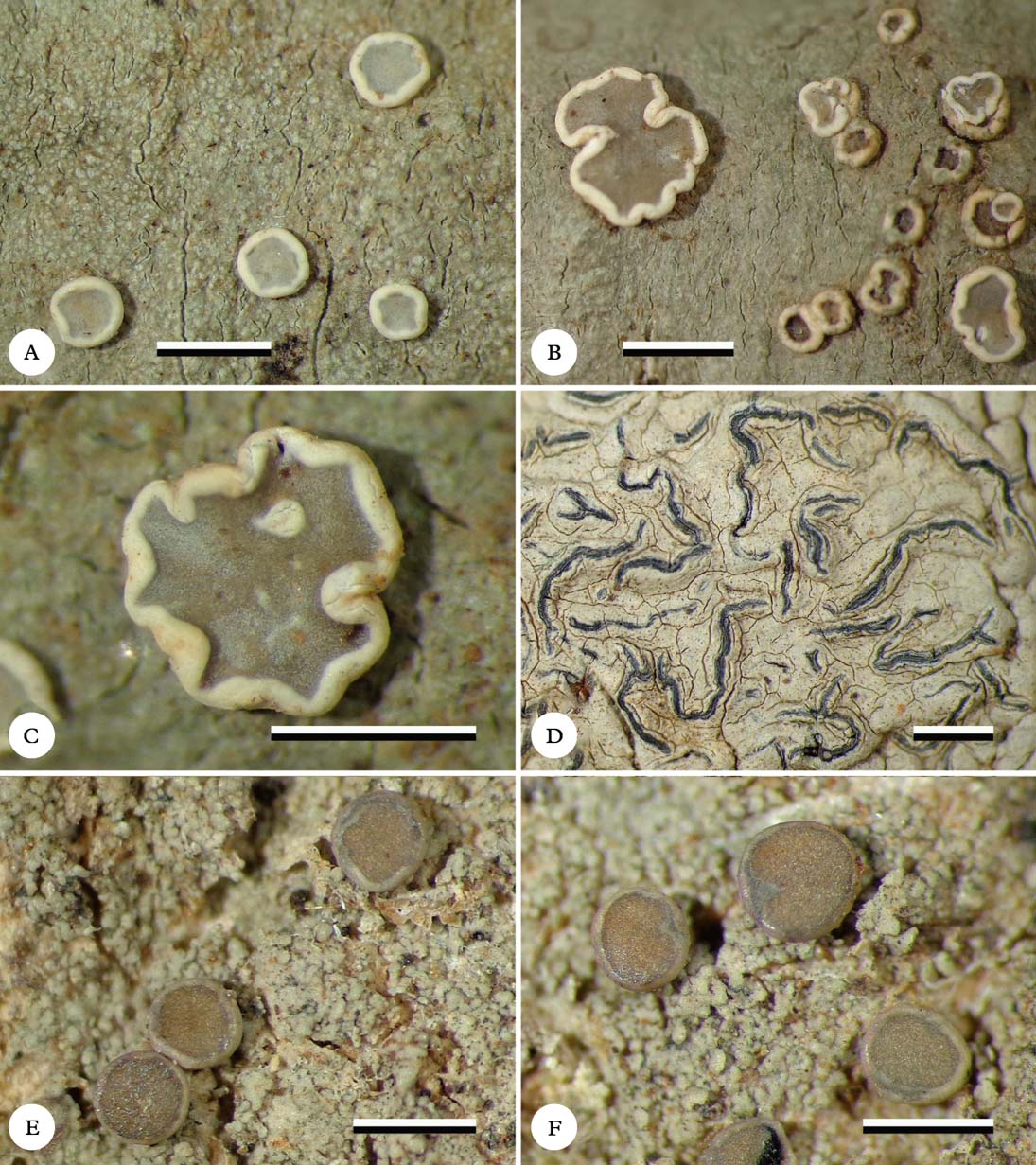
Fig. 1. Habitus of new species (holotypes). A & B, Eugeniella palleola, different parts of the thallus with apothecia; C, Eugeniella palleola, enlarged apothecium; D, Graphis paraschiffneri, thallus with lirellae; E & F, Malmidea cineracea, thallus and apothecia. Scale=1 mm. In colour online.
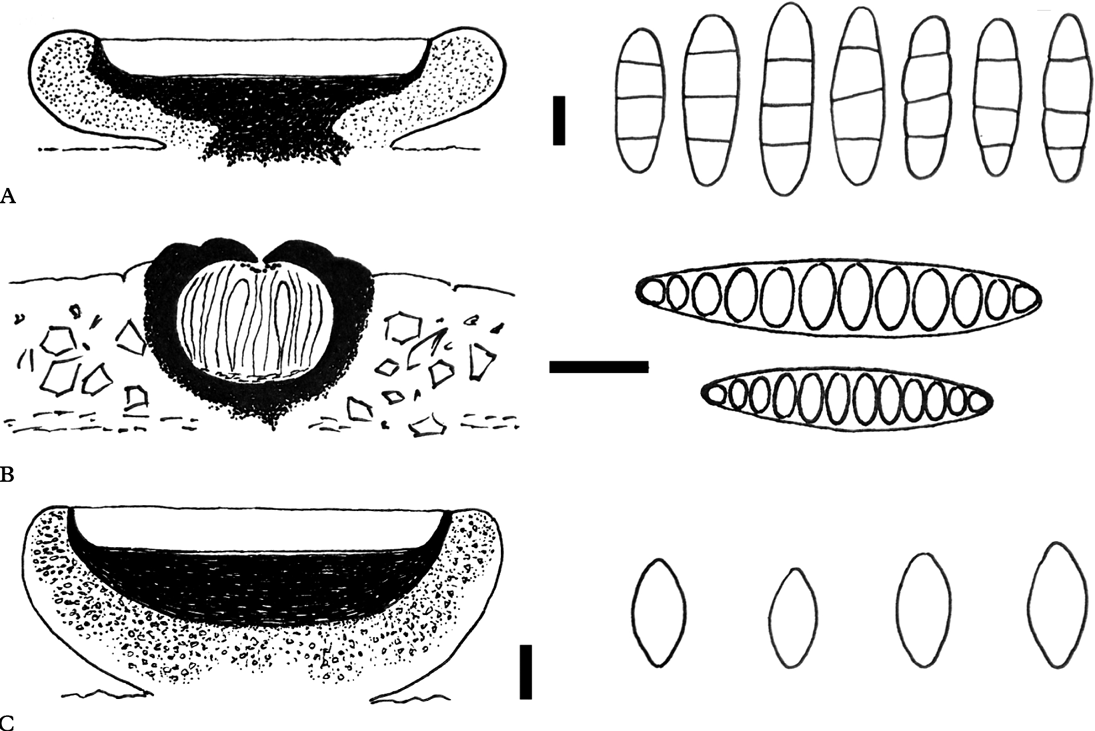
Fig. 2. Anatomies of the new species (holotypes). Schematic views of vertical sections through apothecia and ascospore structures for A, Eugeniella palleola; B, Graphis paraschiffneri, and C, Malmidea cineracea. Scales (sections): A–C=100 μm. Scales (ascospores): A & C=5 μm; B=10 μm.
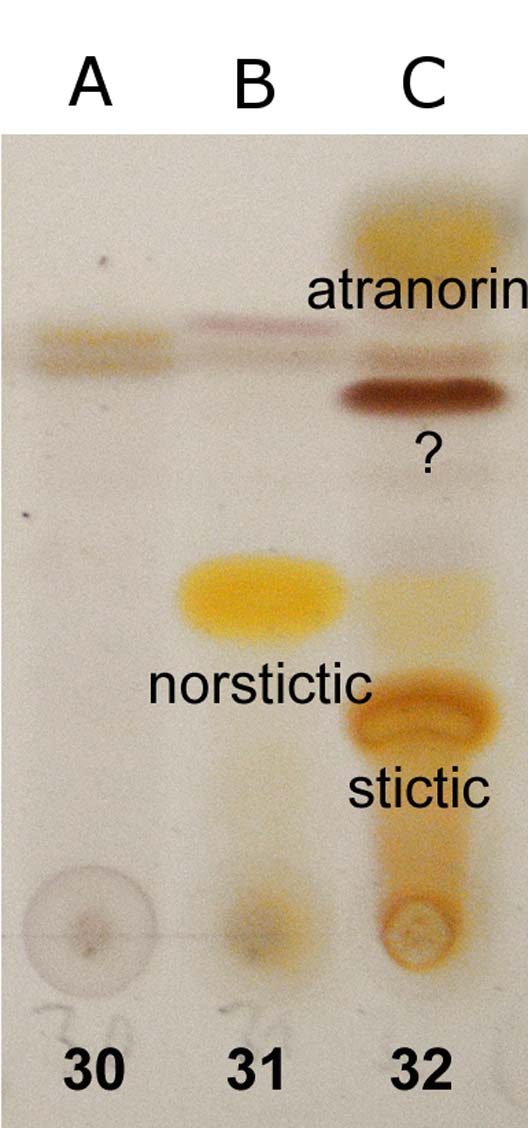
Fig. 3. TLC chromatograms of the holotype specimens. A (30), Malmidea cineracea; B (31), Graphis paraschiffneri; C (32), Eugeniella palleola. In colour online.
Thallus corticolous, 70–100 µm thick, continuous, slightly rimose, dull, uneven to minutely rough, greyish green, K+ yellow (atranorin, stictic acid), C−, P−, with a thin, indistinct, whitish prothallus, ecorticate; photobiont chlorococcoid, algal cells 4–8 µm diam., in irregular clusters 30–50 µm diam.
Apothecia 0·6–1·5 mm diam., sessile, rounded to irregular in outline when older; disc plane, grey to pale brownish, slightly pruinose; margin distinct, prominent, 0·1–0·2 mm thick, whitish, biatorine. Excipulum white under the dissecting microscope, semi-opaque in microscopical section, except for a thin outer, clear zone strongly incrusted with minute, hyaline crystals, with K+ yellow efflux forming red crystals (norstictic acid). Hypothecium 100–150 µm deep, brown-black, K−, fusing with the dark apothecial base. Epihymenium indistinct, with small, hyaline crystals. Hymenium colourless, c. 80 µm tall, I+ blue. Paraphyses unbranched or slightly branched in upper part, not or slightly thickened at apices. Asci narrowly clavate, 60–75×11–15 µm, 8-spored, of Byssoloma-type sensu Hafellner (Reference Hafellner1984). Ascospores 3-septate, oblong-ellipsoidal, colourless, 13–17(–20)×4·5–5·5 µm.
Conidiomata not observed.
Chemistry
Atranorin (major), unknown substance forming red-brown spot with Rf=c. 45 in solvent C (major), stictic acid (major), and norstictic acid (minor, in excipulum) detected with TLC. Spot tests: thallus K+ yellow, C−, P−; excipulum K+ yellow then red, forming red crystals in microscopic sections.
Etymology
The epithet (‘rather pale’) refers to the colour of the discs, which are paler than in the other species of Eugeniella.
Ecology and distribution
The species was found growing on smooth bark of thin stems in a montane rainforest. Known only from Nicaragua.
Notes
Eugeniella Lücking, Sérus. & Kalb is a recently established genus (Lücking Reference Lücking2008) that is recognized by its characteristic excipulum anatomy (composed of moniliform hyphae and strongly incrusted with crystals), in combination with mostly unbranched paraphyses, ‘Byssoloma’ ascus type (tholus with amyloid tubular structure), and septate to muriform ascospores. Nine species are currently known (Lücking Reference Lücking2008; Cáceres et al. Reference Cáceres, Andrade, Océa and Aptroot2013a ; this paper), six of which are predominantly foliicolous. All species with the exception of E. micrommata are neotropical. Further species are likely to be found in the large artificial genus Bacidia. The Nicaraguan material could not be keyed using Santesson's (Reference Santesson1952), Malme's (Reference Malme1935) or Vainio's (Reference Vainio1890) treatments of neotropical Bacidia s. lat.
Eugeniella palleola is characterized by large apothecia with pale discs and very prominent white margins, whereas the other species known have darker discs, and it is the only species known thus far to produce norstictic acid (in the excipulum). The diverse thallus chemistry is also unusual.
Key to the presently known species of Eugeniella
-
1 Ascospores submuriform, 7–10 µm broad ... E. newtoniana (Henriques) Lücking et al.
Ascospores transversely septate, 2·5–5·5 µm broad ... 2
-
2(1) Thallus verrucose ... 3
Thallus smooth to minutely farinose ... 4
-
3(2) Paraphyses unbranched; thallus greenish, verrucae white ... E. psychotriae (Müll.Arg.) Lücking et al.
Paraphyses branched and anastomosing; thallus with a bluish tinge, verrucae of same colour as thallus or paler ... E. micrommata (Kremp.) Lücking et al.
-
4(2) Ascospores (3–)5–7-septate, 17–42 µm long ... 5
Ascospores 3-septate, 10–18 µm long ... 6
-
5(4) Ascospores predominantly 5-septate, 17–27 µm long ... E. ortizii (Lücking) Lücking et al.
Ascospores consistently 7-septate, 25–42 µm long ... E. nigrodiscus M. Cáceres et al.
-
6(4) Apothecial discs pale grey; excipulum with norstictic acid ... E. palleola Breuss & Lücking
Apothecial discs dark brownish grey to brown-black; excipulum lacking substances ... 7
-
7(6) Apothecial margins evanescent; tubular pycnidia usually present ... E. corallifera (Lücking) Lücking et al.
Apothecial margins persistent and distinct; pycnidia absent ... 8
-
8(7) Apothecial discs dark greyish brown, margins thin, pale grey to brownish grey ... E. atrichoides (Malme) Lücking et al.
Apothecial discs dark brown to black, margins very distinct, chamois-coloured to white, sharply delimited from disc ... E. leucocheila (Tuck.) Lücking et al.
Graphis paraschiffneri Lücking & Breuss sp. nov.
MycoBank No.: MB 807099
Similar to Graphis schiffneri, from which it differs in having longer ascospores with more numerous septa and much longer lirellae.
Type: Nicaragua, Rivas, Pacific coast, Playa El Coco c. 18 km S of San Juan del Sur, 19 July 2001, O. Breuss 19.338 (LI—holotype).
Thallus epiperidermal, 100–150 µm thick, greyish white, smooth, slightly rimose, thinly corticate, with irregular algal layer and calcium oxalate crystals; prothallus absent. Lirellae immersed to erumpent, (1·0–)1·5–3·0 mm long, c. 0·25 mm wide, simple or sparsely branched, with lateral thalline margin. Labia entire to slightly furrowed apically, apices free and black, non-pruinose; disc concealed. Excipulum completely carbonized, 230–280 µm wide and 170–200 µm high. Hypothecium c. 20 µm tall, colourless. Hymenium clear, 90–120 µm tall, I−; paraphyses hyaline, unbranched. Epihymenium granulose, grey-brown. Ascospores 8 per ascus, colourless, I+ violet-blue, elongate, 30–42(–50)×7·5–8·5 µm, transversely septate, 10–14-locular.
Chemistry
Norstictic acid detected by TLC. Spot tests: thallus K+ yellow then slowly orange-red, C−, P−, in section with K+ yellow efflux slowly forming red crystals.
Ecology and distribution
The species was found growing on tree bark at the Pacific coast. Known only from Nicaragua.
Notes
Lücking (Reference Lücking2009) revised the species concept of Graphis and Lücking et al. (Reference Lücking, Archer and Aptroot2009) accepted 330 species out of the c. 550–600 published taxa in a world key to the genus. More than 40 additional species have been described since then (Barcenas Peña et al. Reference Barcenas Peña, Lücking, Miranda-González and Herrera-Campos2014).
Graphis paraschiffneri is characterized by the following combination of characters: thallus with norstictic acid, slightly striate labia, completely carbonized excipulum, and transversely septate ascospores. It is apparently close to G. schiffneri Zahlbr., which agrees in most features but has much shorter lirellae and smaller ascospores (Lücking et al. Reference Lücking, Archer and Aptroot2009). Graphis caesiocarpa Redinger is similar, but differs in its richly branched lirellae and entire (non-furrowed), white-pruinose labia with a complete thalline cover; its spores are of the same size as those in G. paraschiffneri but have fewer (7–10) septa (Lücking et al. Reference Lücking, Chaves, Sipman, Umaña and Aptroot2008).
Malmidea cineracea Breuss & Lücking sp. nov.
MycoBank No.: MB 807100
Similar to Malmidea furfurosa, from which it differs in having pale, rather than black apothecial margins, an excipulum with crystals but no medullary tissue, and a yellowish medulla.
Type: Nicaragua, Río San Juan, Indio-Maíz nature reserve, Caribbean lowland rainforest, 13 July 2001, O. Breuss 19.049 (LI—holotypus).
(Figs 1E & F, 2C, 3A)
Thallus corticolous, granulose-isidiate, greenish grey, dull, on a whitish fibrous hypothallus, 150–200 µm thick; granules when discrete 70–150 µm diam., cortex 10–15 µm thick, colourless; photobiont chlorococcoid, cells 6–8 µm diam., in globular or flattened packages 30–60 µm diam., medulla yellowish, K−.
Apothecia sessile, rounded to slightly irregular, 0·5–1·1 mm diam. and 0·20–0·35 mm high; disc plane, grey-brown to brown; margin entire, smooth, pale brownish grey, paler than disc, distinct but hardly prominent, c. 0·1 mm broad. Excipulum compact, hyaline at periphery (10–30 µm), inner part densely encrusted with yellowish brown granules, K+ pale greenish yellow. Subhymenium 10–20 µm high, brownish. Hypothecium dark brown to brownish black, 100–150 µm deep, K−. Hymenium c. 80 µm high, hyaline, I+ blue; paraphyses simple, not thickened at the tips. Epihymenium inapparent. Asci narrowly clavate, 60–70×12–16 µm, apical wall thickening without visible internal structure. Ascospores (6–)8 per ascus, 12–15×6–8 µm, ellipsoidal with somewhat pointed ends, halonate, halo 0·5–1·0 µm thick.
Conidiomata not observed.
Chemistry
No substances detected by TLC except thin bands of terpenoids that possibly originate from the bark. Spot tests: thallus K−, C−, P−; excipulum K+ greenish yellow.
Etymology
The epithet (‘ash-greyish’) refers to the colour of the apothecial margins.
Ecology and distribution
The species was found growing on tree bark in a lowland rainforest. It is known only from Nicaragua.
Notes
The genus Malmidea Kalb et al. in the separate family Malmideaceae was only recently established for tropical, lecideoid lichens characterized by biatorine excipula often incrusted with hydrophobic granules, asci supposedly without a tubular structure in the tholus, and simple ascospores (Kalb et al. Reference Kalb, Rivas Plata, Lücking and Lumbsch2011). Prior to that study, representatives of this genus were treated under the name Malcolmiella Vězda (Lücking & Kalb Reference Lücking and Kalb2000; Kalb Reference Kalb2004; Aptroot et al. Reference Aptroot, Saipunkaew, Sipman, Sparrius and Wolseley2007; Cáceres Reference Cáceres2007; Lücking Reference Lücking2008; Kalb et al. Reference Kalb, Buaruang, Papong and Boonpragob2009). Since the introduction of the genus, many additional species have been newly described or transferred to Malmidea (Kalb et al. Reference Kalb, Rivas Plata, Lücking and Lumbsch2011, Reference Kalb, Buaruang, Mongkolsuk and Boonpragob2012; Spribille et al. Reference Spribille, Goffinet, Klug, Muggia, Obermayer and Mayrhofer2011; Cáceres et al. Reference Cáceres, Santos Vieira, de Jesus and Lücking2012, Reference Cáceres, dos Santos, de Góes, Mota and Aptroot2013b ; Kalb et al. Reference Kalb, Buaruang, Mongkolsuk and Boonpragob2012; Schumm & Aptroot Reference Schumm and Aptroot2012; Weerakoon & Aptroot Reference Weerakoon and Aptroot2013, Reference Weerakoon and Aptroot2014).
Malmidea species occur chiefly in tropical rainforests. They are mainly corticolous, but several species are found also on leaves (Lücking Reference Lücking2008 sub Malcolmiella). Malmidea trailiana is so far known only from foliicolous occurrences (Santesson Reference Santesson1952; Lücking Reference Lücking2008). Currently, 50 species are assigned to Malmidea, but no comprehensive study of the genus has yet been published. The key presented below is preliminary and aims to provide an overview of the presently known species. Additional species are expected to be described or found among tropical reports of Lecidea s. lat. None of the possible candidates in the large artificial genus that have been checked (e.g. in Malme Reference Malme1936) have the combination of characters that is shown by the species described in the present paper. Malmidea cineracea is characterized by a granulose-isidiate thallus with a yellowish medulla and apothecia with a compact but crystal-incrusted excipulum, grey-brown to brown discs and grey margins. It was collected from smooth bark in a lowland rainforest.
Eugeniella (see above) is similar to Malmidea, but differs in having septate ascospores and tholi with a tubular structure. These genera are placed in separate families: Pilocarpaceae and Malmideaceae, respectively (Kalb et al. Reference Kalb, Rivas Plata, Lücking and Lumbsch2011). However, illustrations in Schumm & Aptroot (Reference Schumm and Aptroot2012) demonstrate a tubular structure in two Malmidea species (very weak in M. chrysostigma, clearly visible in M. bakeri) and Spribille et al. (Reference Spribille, Goffinet, Klug, Muggia, Obermayer and Mayrhofer2011) report a dark apical amyloid cylinder in Malmidea indica. This character therefore has to be studied further.
Key to the currently known species of Malmidea
-
1 Thallus with isidia or soredia ... 2
Thallus without isidia or soredia, but sometimes farinose to granulose due to formation of goniocysts, often verrucose ... 13
-
2(1) Thallus with coralloid, polysidiangia-like clumps that eventually burst open producing soredia-like corticate granules and exposing the medulla; medulla lemon yellow, K+ orange; ascospores 16–22×9–12 µm ... M. piae (Kalb) Kalb
Thallus not with polysidiangia-like clumps; medulla white or yellowish, K− ... 3
-
3(2) Thallus isidiate ... 4
Thallus sorediate ... 9
-
4(3) Ascospores 2 per ascus, 50–65×20–30 µm; isidia verruciform to cylindrical, internally yellow and K+ orange-red ... M. polisensis (Vain.) Kalb et al.
Ascospores 4–8 per ascus, up to 30×15 µm ... 5
-
5(4) Ascospores c. 30×15 µm; isidia cylindrical or coralloid, thin, dense; apothecial margin with medullary chambers; disc brown, margin whitish or cream ... M. corallophora Aptroot & Schumm
Ascospores smaller (up to 25 µm); apothecial margin compact ... 6
-
6(5) Isidia verruciform to shortly cylindrical, simple, internally white and K+ yellow; apothecial margin verrucose to isidiate; ascospores 14–25×10–15 µm ... M. taytayensis (Vain.) Kalb et al.
Isidia granular to coralloid or thallus irregularly granulose-isidiate ... 7
-
7(6) Isidia granular to coralloid; ascospores 14–22×9–13 µm ... M. perisidiata (Malme) Kalb & Lücking
Thallus irregularly granulose-isidiate; ascospores 11–15×5–8 µm ... 8
-
8(7) Medulla white; apothecial disc beige, margin black; excipulum without crystals ... M. furfurosa (Tuck. ex Nyl.) Kalb & Lücking
Medulla yellowish; apothecial disc brown, margin grey; excipulum with crystals ... M. cineracea Breuss & Lücking
-
9(3) Thallus verrucose, verrucae becoming sorediate ... 10
Thallus not verrucose; soralia patchy or diffuse ... 11
-
10(9) Medulla and soralia yellow-orange, K+ red; apothecial disc grey-brown, margin cream to yellow, thick; ascospores 10–16×6–8 µm ... M. atlantica (Cáceres & Lücking) Cáceres & Kalb
Medulla pale yellow, K+ yellow-orange; apothecial disc brown, margin grey, thin; ascospores 10–14×5–8 µm ... M. flavopustulosa (Cáceres & Lücking) Cáceres & Kalb
-
11(9) Medulla and soralia bright golden yellow; soralia pulverulent, confluent; thallus partly with small, grey, corticate dots (apothecia unknown) ... M. sulphureosorediata Cáceres et al.
Medulla of thallus white ... 12
-
12(11) Thallus and soralia grey-white; soralia roundish to irregularly patchy; excipulum compact; apothecial disc shades of brown, margin brown; ascospores 14–17×8– 10 µm ... M. polycampia (Tuck.) Kalb & Lücking
Thallus and soralia yellowish white; soralia extensive, farinose; excipulum with medullary tissue, granules yellowish, K+ intense yellow; apothecial disc brown, margin yellowish white; ascospores 15–21×7–12 µm ... M. ceylanica (Zahlbr.) Kalb et al.
-
13(1) Ascospores 30–40×12–18 µm, 2–4 per ascus; thallus verrucose, white to grey; apothecial disc dark brown to blackish, excipulum paler ... M. indica (Awasthi & Agarwal) Hafellner & T. Sprib.
Ascospores <30 µm ... 14
-
14(13) Ascospores with distinct terminal wall thickenings ... 15
Ascospores with evenly thickened walls ... 16
-
15(14) Thallus without verrucae, medulla white, K−; excipulum lacking medullary layer, compact, without granules; ascospores 12–17×6–10 µm ... M. incrassata Kalb
Thallus verrucose, medulla cream to yellowish, K+ orange to pale purple; excipulum with medullary layer, with granules (K+ lemon yellow); ascospores 22–30×12– 15 µm; medulla cream to yellowish ... M. reunionis Kalb
-
16(14) Ascospores 22–30×11–15 µm, 4 per ascus; medulla yellowish, K+ intense yellow-orange ... M. sorsogona (Vain.) Kalb et al.
Ascospores smaller (mostly <20 µm, rarely up to 25 µm) ... 17
-
17(16) Apothecia with a continuous or granular thalline excipulum surrounding the proper excipulum; disc reddish brown; proper excipulum white to cream; ascospores 20– 24×11–15 µm ... M. duplomarginata (Papong & Kalb) Kalb & Papong
Apothecia without thalline excipulum ... 18
-
18(17) Apothecial margin with medullary layer throughout or in papillae or internal chambers ... 19
Apothecial margin lacking a medullary layer, compact, of conglutinated, radiating hyphae ... 36
-
19(18) Medulla of thallus and verrucae white ... 20
Medulla of thallus and/or verrucae yellowish to orange or red ... 26
-
20(19) Apothecial discs orange-brown to brown-red; margin thick, cream to white; hypothecium orange-brown; ascospores 15–20×6–10 µm ... M. badimioides (Cáceres & Lücking) Cáceres & Kalb
Apothecial discs grey-brown to dark brown or black ... 21
-
21(20) Apothecial margin thin, grey to blackish; epihymenium c. 25 µm, dark brown; ascospores 10–14(–17)×6–8 µm ... M. inflata Kalb
Apothecial margin thick, cream to dark grey, entire or papillose; epihymenium indistinct ... 22
-
22(21) Apothecial margin entire; excipulum with greyish granules ... 23
Apothecial margin papillose; excipulum with yellowish or ochraceous-yellow granules ... 25
-
23(22) Thallus verrucae conspicuous (0·1–0·3 mm diam.), often confluent; medulla K+ orange; ascospores 10–17×6–10 µm ... M. coralliformis Kalb
Thallus verrucae finer ... 24
-
24(23) Ascospores mainly 4 per ascus, 10–15×6–9 µm; medulla of thallus verrucae K+ greenish to orange-yellow ... M. psychotrioides (Kalb & Lücking) Kalb et al.
Ascospores 6–8 per ascus, 11–18×7–12 µm; medulla of thallus verrucae K+ yellow ... M. subgranifera (Kalb & Elix) Kalb & Elix
-
25(22) Medulla of thallus and verrucae K+ yellowish; medullary portion of excipulum K+ lemon yellow to greenish; ascospores 11–16×8–10 µm ... M. bakeri (Vain.) Kalb et al.
Medulla of thallus and verrucae K+ deep orange; medullary portion of excipulum K+ orange-yellow; ascospores 9–12×6–8 µm ... M. variabilis Kalb
-
26(19) Hypothecium colourless or yellowish brown. ... 27
Hypothecium orange-brown to brown-black. ... 29
-
27(26) Medulla of thallus verrucae bright red; medulla of excipulum pale yellow; ascospores 10–13×5–7 µm ... M. sanguineostigma Weerak. & Aptroot
Medulla yellow ... 28
-
28(27) Medulla in verrucae and excipulum bright yellow, K+ red; ascospores 9–11×5–6 µm ... M. pallidoatlantica M. Cáceres et al.
Medulla in verrucae pale to bright yellow, K−, medulla in excipulum yellowish, K+ yellow; ascospores 10–15×6–8 µm ... M. trailiana (Müll.Arg.) Kalb et al.
-
29(26) Ascospores 10–14×5–8 µm; medulla K+ orange to orange-red ... 30
Ascospores larger ... 31
-
30(29) Excipulum with a continuous medullary layer; medulla of thallus verrucae sulphur yellow ... M. aurigera (Fée) Kalb et al.
Excipulum with medullary chambers, therefore appearing papillate; medulla of thallus verrucae pale yellowish ... M. piperina (Zahlbr.) Aptroot & Breuss
-
31(29) Ascospores 12–18×7–10 µm, 8 per ascus ... 32
Ascospores 14–25×8–15 µm, mainly less than 8 per ascus ... 33
-
32(31) Apothecial discs dark brown to blackish, margin thickish, cream to whitish; medulla of excipulum K+ intensely yellow to orange; hypothecium dark brown to brown-black; medulla of thallus verrucae yellowish to peach-coloured, K+ orange ... M. granifera (Ach.) Kalb et al.
Apothecial discs light orange-brown, margin thin, cream; medulla of excipulum K−; hypothecium orange-brown; medulla of thallus pale yellow, K+ dark yellow ... M. leucogranifera M. Cáceres & Lücking
-
33(31) Medulla of thallus white, K+ yellow; medulla of verrucae yellow, K+ red; apothecial discs black, margin grey-brown to blackish, thin; ascospores 6–8 per ascus ... M. fenicis (Vain.) Kalb et al.
Medulla of thallus cream to golden yellow; apothecial discs shades of brown to blackish, margin whitish to cream-coloured, thick, entire to granular ... 34
-
34(33) Excipulum granules and medulla of thallus and verrucae K+ blood red to red-violet; ascospores 6(–8) per ascus, 18–25×10–13 µm; medulla golden orange ... M. chrysostigma (Vain.) Kalb et al.
Excipulum granules K+ orange-yellowish to greenish lemon yellow; medulla of thallus cream to orange-yellow, K+ orange to orange-red ... 35
-
35(34) Apothecial discs light brown to tawny; ascospores 4–6(–8) per ascus, 15–20×8–11 µm; hypothecium 50–75 µm high; medulla of thallus cream to sulphur yellow ... M. eeunae Kalb
Apothecial discs orange-brown or dark brown to blackish; ascospores (4–)6(–8) per ascus, (12–)16–21×9–13 µm; hypothecium 100–120 µm high; medulla of thallus and verrucae orange-yellow ... M. subaurigera (Vain.) Kalb et al.
-
36(18) Hypothecium pale ... 37
Hypothecium dark brown to brown-black ... 42
-
37(36) Apothecial discs brown ... 38
Apothecial discs brownish grey, beige or yellowish to orange ... 39
-
38(37) Apothecia 0·25–0·50 mm diam., discs reddish brown, margins pale brown to chamois; thallus strongly granulose; ascospores 9–11×4–6 µm ... M. fellhaneroides (Lücking) Kalb & Lücking
Apothecia 0·4–1·0 mm diam., discs brown, margins pale to dark brown or blackish; thallus smooth to rugulose; ascospores 12–20×6–10 µm ... M. fuscella (Müll.Arg.) Kalb & Lücking
-
39(37) Discs pale orange-yellow to orange, margin pale yellow to chamois, soon disappearing; ascospores 8–14×4–6 µm ... M. bacidinoides (Lücking) Kalb & Lücking
Discs beige or brown-grey, margin persistent ... 40
-
40(39) Apothecial margin thick and prominent when young, paler than disc; ascospores 9– 14×3·5–4·5 µm ... M. gyalectoides (Vain.) Kalb & Lücking
Apothecial margin thin, of same colour as disc or darker; ascospores 9–13×5–7 µm ... 41
-
41(40) Apothecial margin yellowish brown or often apically blackish, disc beige; thallus rugulose ... M. leptoloma (Müll.Arg.) Kalb & Lücking
Apothecial margin whitish grey to dark brownish grey; disc brown-grey; thallus± smooth ... M. perplexa Kalb
-
42(36) Medulla partly or completely pale yellow, orange or red, K+ orange or purple ... 43
Medulla white, K- ... 47
-
43(42) Thallus verrucose ... 44
Thallus smooth ... 45
-
44(43) Medulla of verrucae pale yellow, K+ orange; medulla of thallus white; apothecial discs brown, margin grey; ascospores 15–18×9–11 µm ... M. papillosa Weerak. & Aptroot
Medulla of verrucae yellow-orange and K+ red; medulla of thallus orange-red and K+ purple; apothecial discs brown, margin at least in parts red and K+ purple; ascospores 10–16×6–8 µm ... M. amazonica (Redinger) Kalb et al.
-
45(43) Apothecial margins red or partly reddish; excipulum with red crystals, K+ purple; ascospores 9–15×5–8 µm ... M. rhodopsis (Tuck.) Kalb et al.
Apothecial margins not red; excipulum lacking red crystals, K− ... 46
-
46(45) Apothecial margins brownish grey; ascospores 10–16×6–9 µm ... M. piperis (Spreng.) Kalb et al.
Apothecial margins black; ascospores narrower, 10–14×4–6 µm ... M. nigromarginata (Malme) Lücking & Breuss
-
47(42) Ascospores 20–25 ×10–14 µm; apothecial discs grey-brown to black-brown, margin pale to dark brown ... M. hypomela (Nyl.) Kalb & Lücking
Ascospores smaller ... 48
-
48(47) Thallus granulose (to ± smooth in parts), green to green-grey; apothecial disc dark brown, margin black; ascospores 9–13×5–6 µm ... M. tratiana Kalb & Mongkolsuk
Thallus smooth to wrinkled or slightly verrucose; ascospores 10–17×6–8 µm ... 49
-
49(48) Thallus pale greenish grey; apothecial discs dark reddish to purplish brown; margin rather thick, blackish grey ... M. vinosa (Eschw.) Kalb et al.
Thallus yellowish white; apothecial discs blackish; margin thin, dark grey ... M. cinereonigrella (Vain.) Kalb
New combinations
Malmidea nigromarginata (Malme) Lücking & Breuss comb. nov.
MycoBank No.: MB 807111
Basionym: Lecidea nigromarginata Malme, Ark. Bot. 28A(7): 25 (1936).
Malmidea piperina (Zahlbr.) Aptroot & Breuss comb. nov.
MycoBank No.: MB 807110
Basionym: Lecidea piperina Zahlbr., Fedde Repert. 33: 40 (1933).
Werner Huber (Wien) is thanked for the organization of the field trips in Nicaragua. André Aptroot (Soest), Josef Hafellner (Graz), Klaus Kalb (Neumarkt) and Helmut Mayrhofer (Graz) are thanked for remarks and literature; André Aptroot also checked the draft key on Malmidea. Two anonymous reviewers are thanked for helpful comments on the manuscript. This study was partially supported by a grant from the National Science Foundation: “ATM – Assembling a taxonomic monograph: the lichen family Graphidaceae”(DEB-1025861 to The Field Museum; PI T. Lumbsch, Co-PI R. Lücking).







