Getting syphilis testing right as case rates rise
Traditional algorithm? Or reverse? Elitza Theel, PhD, D(ABMM), of Mayo Clinic, in an AACC session last year walked through the two primary diagnostic algorithms for syphilis, explaining where the complexities lie and how her laboratory uncovered inappropriate testing for neurosyphilis. Syphilis cases are up in the past decade, and by 11 percent between 2018 and 2020 alone, said Dr. Theel, director of the infectious diseases serology laboratory at Mayo Clinic and Mayo Clinic Laboratories. In women, the increase is 30 percent, and more than 50 percent of all U.S. counties reported cases in women of reproductive age, according to a 2020 CDC report.
Whitepaper
Syphilis testing: Performance Comparisons of Treponemal and Nontreponemal Assays
Summary
Serology testing is important to aid the rule-in or rule-out of syphilis and can include both treponemal and nontreponemal assays. Differences in performance and sensitivity among assays exist and should be considered when implementing a syphilis testing algorithm.
Introduction
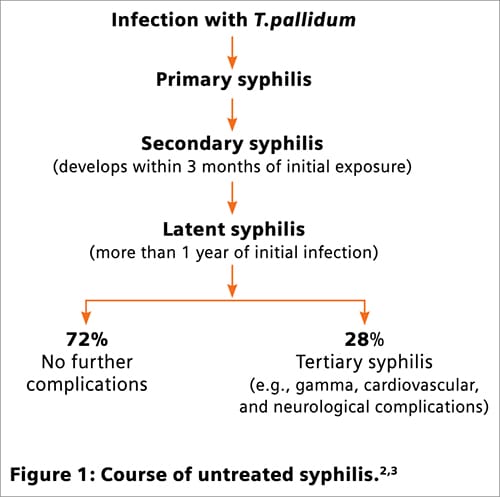
Syphilis is a bacterial disease resulting from infection with Treponema pallidum (subspecies pallidum).1,2 The course of untreated infection is shown in Figure 1.2,3 Testing is typically done for pregnant women and those at risk for, or suspected of, exposure.3,4 The bacterium cannot be cultured using routine microbiology techniques; serology assays provide the primary means of testing and diagnosis.4
Serology testing for syphilis
Serology testing for syphilis involves two different types of antibody-detection assays: treponemal and nontreponemal. Treponemal tests identify antibody to specific bacterial antigens such as Tp15, Tp17, and Tp47.5-7 Studies suggest that the ability to detect Tp47 antibody does not enhance assay sensitivity beyond that achieved by assays designed to detect only Tp17 or both Tp15 and Tp17.7-11 Approximately 85 percent of tested patients will remain seropositive for treponemal antibody even with successful treatment, so previous history must be considered when testing patients with a prior diagnosis of syphilis.12,13
Nontreponemal assays recognize antibody that results from exposure to lipoidal material released from damaged cells and include the rapid plasma reagin (RPR) and venereal disease research laboratory (VDRL) assays.4 Unlike treponemal antibodies, nontreponemal antibodies typically become nondetectable with resolution of infection.13 Importantly, up to 30 percent of untreated late-latent infections may also become nondetectable with nontreponemal assays but remain detectable with treponemal assays.12,13 Other causes of membrane damage such as autoimmune disease can stimulate production of nontreponemal antibody. For this reason, the specificity of nontreponemal assays for syphilis is relatively low compared to that of treponemal assays. Continue reading or download now …
In transplantation, detecting CMV antiviral resistance
Eighteen months after introducing a next-generation sequencing assay to detect CMV antiviral resistance in the transplant population, Matthew Binnicker, PhD, D(ABMM), in an AMP presentation, shared a patient’s case and his laboratory’s broader experience.
The diagnostic advantages of the NGS assay are earlier detection of resistance and better management of cytomegalovirus in transplant patients, said Dr. Binnicker, director of clinical virology and vice chair of practice, Department of Laboratory Medicine and Pathology, Mayo Clinic, in the virtual AMP2020 presentation in November.
As the number of transplants rises in the United States, he said, “we’re going to see an increasing number of individuals who are on high levels of immunosuppressive medications, and this puts them at an increased risk of infectious diseases like CMV.”
Dr. Binnicker, who is also professor of laboratory medicine and pathology, discussed the Mayo Clinic case of a 67-year-old female patient with a history of polycystic kidney disease, who underwent a living, unrelated-donor renal transplant. Her serostatus was determined pre-transplantation to be a mismatch for CMV IgG: “The donor was seropositive, but the recipient was seronegative.” The patient’s Epstein-Barr virus serostatus was donor-positive, recipient-positive.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management