The first definition of Acids and Bases was given by Arrhenius and according to his theory, acids dissociate in water to form protons (H+) and bases dissociate in water to form (–OH):

The limitation of this theory became evident mostly when organic bases were studied since they do react with acids, but are not hydroxide ions.
Therefore, the Brønsted-Lowry acids and base theory took over and is used in qualifying compounds as acids or bases. This theory is more universal and describes bases as species that are proton acceptors which include the hydroxide ions but are not limited to them:

Notice that we are generally talking about a proton transfer in acid-base reactions but the curved arrows, like for any other reaction, show the movement of electrons.
In a Brønsted-Lowry acid-base reactions, the base turns into its conjugate acid and the acid turns into its conjugate base.
Acid-Base role changes depending on the pKa
If you do not know about the pKa, don’t worry too much about it now, but later on, we will see that based on the pKa values any compound can serve as an acid or a base depending on what it is reacted with.
For example, water can be a base when forming the hydronium ion in the reaction with inorganic acids, but it can also serve as an acid when reacted with stronger bases:

The same goes for organic compounds. For example, diisopropylamine or pyridine are common organic bases used in different reactions to neutralize protonated species:

However, diisopropylamine is often converted into a stronger base called Lithium diisopropylamide (LDA) by reacting it with Organo-Lithium compounds:
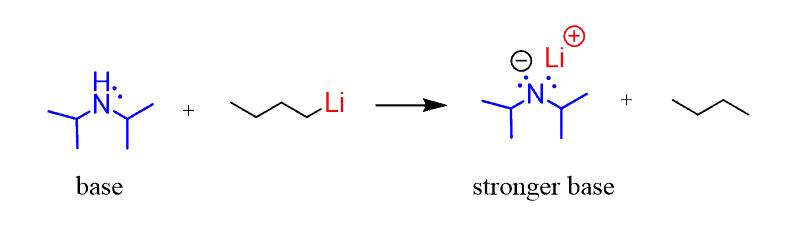
Classifying a compound as an acid or a base can vary, however, there are some common definitions of organic acids and bases.
Carboxylic acids are known as organic acids since they have lower pKa values than most other functional groups.
The pKa values of some common organic compounds are listed below and for a more detailed list you can download this PDF document:

Amines, on the other hand, are known as the organic bases since the lone pair on the nitrogen can be protonated forming relatively stable conjugate acids with pKa ~ 11. There is a separate post about the basicity of amine which covers a lot more details on this topic.
Keeping this in mind, you can quickly recognize the acidic and basic sites of organic molecules.
For example, to identify the organic acidic and basic sites in the following compounds, you simply need to locate the carboxylic acids and amines:

The molecules that contain only carboxylic acids are going to have acidic properties and the ones containing only amino groups will show basic properties.
Let’s also look at one important biological molecule that we all know:
DNA
It is an abbreviation for Deoxyribonucleic Acid.
So, take a look for a moment at the structure of a DNA fragment and figure why is it an acid:
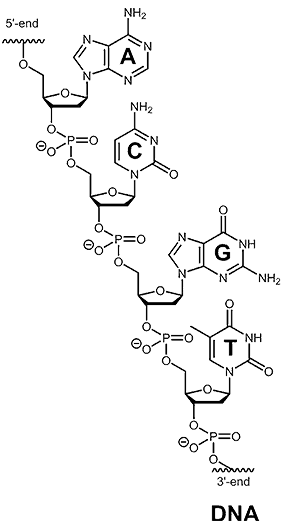
You might have recognized the phosphate group in the DNA backbone. It is a derivative of phosphoric acid – H3PO4 and that is where the name is coming from.
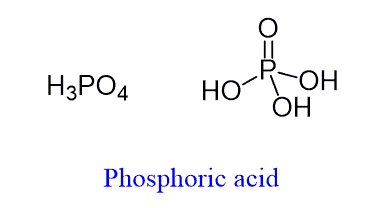
Although under physiological conditions (pH 7.4), it is deprotonated and exists as a phosphate group since the pKa of phosphoric acid is about 2.12.
DNA also contains basic sites known as nucleobases or simply bases. And the reason for classifying them as bases is the presence of nitrogen atoms with lone pairs.
These are the species that form the complementary hydrogen bonding (A:T, G:C) by which the genetic information is passed on:

Practice
Draw a mechanism for the following acid-base reaction. Label the acid, base, conjugate acid, and conjugate base:

This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
Draw a mechanism for the following acid-base reaction. Label the acid, base, conjugate acid, and conjugate base:
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
Draw the conjugate base for each of the following acids:
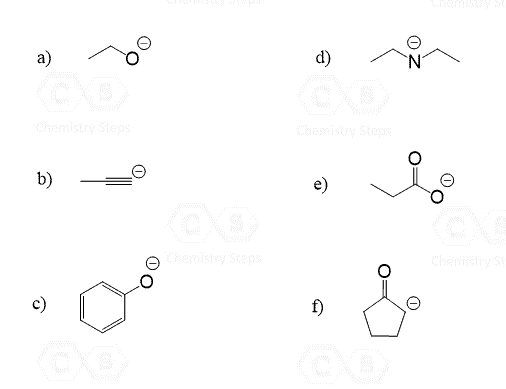
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
Draw the conjugate acid for each of the following bases:

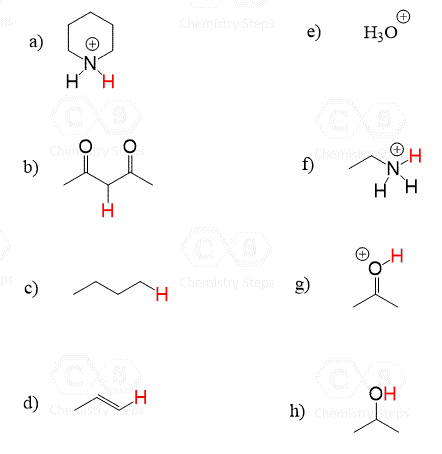
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.


Nice work, thank you!
Thank you, Mia.