Incidence and risk factors of deep vein thrombosis in patients with spinal cord injury: a systematic review with meta-analysis
- 1The First Clinical Medical College of Lanzhou University, Lanzhou, China
- 2Department of Pathology and Pathophysiology, Gansu University of Chinese Medicine, Lanzhou, China
- 3Department of Nephrology, The Second Hospital of Lanzhou University, Lanzhou, China
- 4Chengren Institute of Traditional Chinese Medicine, Lanzhou, China
- 5Department of Spine, Changzheng Hospital, Naval Medical University, Shanghai, China
Background: Spinal cord injury (SCI) is a highly disabling disease with huge public health burden. The complications associated with it, especially deep vein thrombosis (DVT), further aggravate the disability.
Objective: To explore the incidence and risk factors of DVT after SCI, in order to provide guidance for disease prevention in the future.
Methods: A search was performed on PubMed, Web of Science, Embase, and Cochrane database up to November 9, 2022. Literature screening, information extraction and quality evaluation were performed by two researchers. The data was later combined by metaprop and metan commands in STATA 16.0.
Results: A total of 101 articles were included, including 223,221 patients. Meta-analysis showed that the overall incidence of DVT was 9.3% (95% CI: 8.2%–10.6%), and the incidence of DVT in patients with acute and chronic SCI was 10.9% (95% CI: 8.7%–13.2%) and 5.3% (95% CI: 2.2%–9.7%), respectively. The incidence of DVT decreased gradually with the accumulation of publication years and sample size. However, the annual incidence of DVT has increased since 2017. There are 24 kinds of risk factors that may contribute to the formation of DVT, involving multiple aspects of the baseline characteristics of the patient, biochemical indicators, severity of SCI, and comorbidities.
Conclusions: The incidence of DVT after SCI is high and has been gradually increasing in recent years. Moreover, there are numerous risk factors associated with DVT. Comprehensive preventive measures need to be taken as early as possible in the future.
Systematic Review Registration: www.crd.york.ac.uk/prospero, identifier CRD42022377466.
1. Introduction
Spinal cord injury (SCI) is a potentially fatal central nervous system disorder, which can lead to permanent loss of motor, sensory and autonomic nervous system function. There are more than 27 million patients with SCI worldwide, with about 250,000–500,000 new cases each year, with a lifetime cost of $750,000–$3 million per patient (1–4). Due to complex pathophysiological changes, there is no effective treatment available. In addition to the burden of SCI itself, the related complications can also cause serious consequences and burden to patients (5, 6).
The main complications of SCI are pressure sores, urinary complications, respiratory complications, deep venous thrombosis (DVT), spasm, pain, osteoporosis, among which DVT is one of the most serious complications (5, 7, 8). DVT refers to the abnormal coagulation of blood in the deep venous lumen, blocking the venous lumen, resulting in venous reflux obstruction, more common in the lower extremities. Patients with SCI have the highest risk of DVT among all hospitalized patients, up to 100% (9). After the formation of DVT in patients with SCI, only a few of the thromboses are confined to the site of occurrence or disappear on their own, and most of them extend to the trunk of the deep vein of the whole limb, which can cause a variety of complications, including post-thrombotic syndrome, edema, compression ulcer, recurrence of DVT and even pulmonary thromboembolism (10–12). Pulmonary thromboembolism caused by DVT can cause arrhythmia, hypoxia and rapid death, which are the third leading cause of death in SCI (13, 14). As a result, the prognosis for DVT is poor, with mortality rates of 20% at 2 years and up to 31% at 8 years (15).
Although there are obvious clinical manifestations such as muscle pain in the early stage of DVT, the chief complaint of patients is not obvious due to the partial or complete loss of superficial sensation after SCI. 50%–80% of patients with DVT had no obvious clinical symptoms or lack of specificity in the early stage, and more than 50% of the patients had normal physical examination, resulting in a high rate of missed diagnoses (16). The reported incidence of DVT after SCI ranges from 9% to 100% (9, 17). There are significant differences in the epidemiological results of DVT in patients with SCI reported in different studies, indicating that there is a lack of multicenter, large sample epidemiological data, and the risk factors for DVT are not clear (18, 19). In this pioneer study, we will systematically search for studies on DVT after SCI, comprehensively explore the incidence of DVT after SCI, and further explore its risk factors. It is very important for clinicians and patients to improve their awareness of DVT and to take preventive strategies as soon as possible.
2. Methods
This study complies with the PRISMA 2020 statement, the protocol was designed based on population, interventions, comparisons, outcomes, and study design (PICOS) criteria, and the protocol is registered (CRD42022377466) on PROSPERO (www.crd.york.ac.uk/prospero).
2.1. Inclusion and exclusion criteria
2.1.1. Patients (P)
Spinal cord injury.
2.1.2. Interventions (I)
Not applicable.
2.1.3. Control (C)
Not applicable.
2.1.4. Outcome (O)
Incidence and risk factors of DVT.
2.1.5. Type of study (S)
Observational and experimental studies.
2.1.6. Exclusion criteria
(1) Studies not meeting the criteria for PICOS. (2) Studies with small sample sizes (<30 for a single group). (3) Studies with incomplete data or obvious errors. (4) Patients with other serious diseases that may lead to DVT.
2.2. Databases search
PubMed, Web of science, Embase, and Cochrane database was searched by computer from the establishment of the database to November 9, 2022. The retrieval adopts the combination of subject words and free words. At the same time, the references included in the study were searched to supplement and obtain the relevant data. The search terms include: (thrombosis OR thrombus OR blood clots OR thromboembolism OR phlebothrombosis venous thrombosis OR phlebothrombos OR venous thrombos OR vein thrombosis OR deep vein thrombosis OR deep venous thrombosis OR lower extremity deep vein thrombosis OR deep venous thrombosis of lower limb OR venous thromboembolism OR deep vein thrombos OR deep venous thrombos) AND (Spinal cord injury OR Spinal injury OR Spinal Cord Trauma OR Spinal Cord Transection OR Spinal Cord Laceration OR Post-Traumatic Myelopathy OR Spinal Cord Contusion). For more search details about each database, see Table 1.
2.3. Article selection and information extraction, and quality assessment
Two reviewers conducted the initial screening of the literature according to the title and abstract, and then obtained the full text for re-screening to determine the studies that met the inclusion criteria. In case of disagreement, a third reviewer is requested to assist in the judgment. Finally, the author, year of publication, country, type of study, age, sample size, male/female ratio, site of SCI, American Spinal Injury Association Impairment Scale (AIS), injury time, treatment measures, diagnostic methods, incidence, or number of DVT and influencing factors were extracted from the final included literature. In addition, methodological index for non-randomized study (MINORS) was used to assess the quality of evidence included in the study (20). MINORS consists of 12 items, each with a score of 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). The full score is 24.
2.4. Statistical analysis
The incidence of DVT was calculated by using the metaprop command of STATA16.0 software, and the results were expressed in terms of incidence and 95% confidence interval (CI). The metan command was used to calculate the risk factors of DVT, and the results were expressed by adjusted odds ratio (AOR) and its 95% CI. If there was no statistical heterogeneity among the studies (P < 0.05 and I2 < 50%), the fixed effect model was used for meta-analysis. Otherwise, the source of heterogeneity was further analyzed, and the random effects model was used for meta-analysis. Funnel chart was drawn to detect publication bias.
3. Results
3.1. Literature search and screening
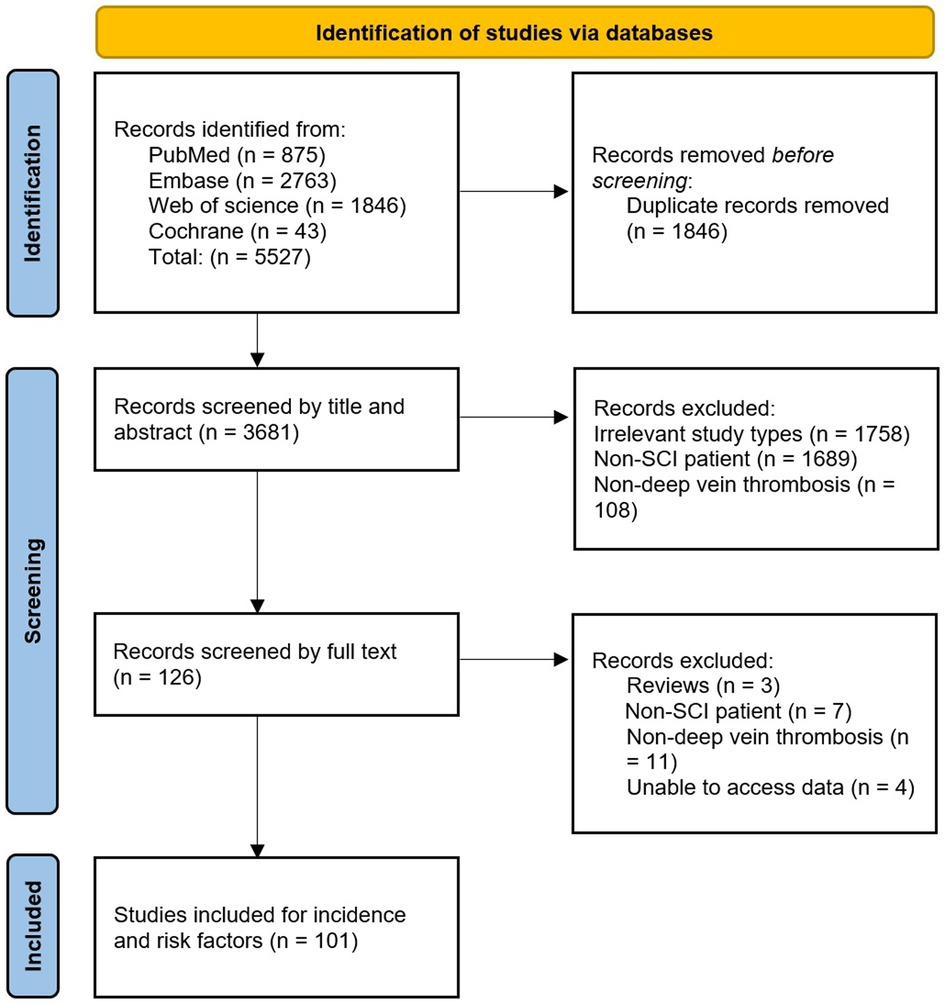
A total of 5,527 records were obtained from four databases. After excluding duplicate records that did not meet the inclusion criteria, 101 studies were included. These studies all reported the incidence of DVT, of which 12 reported risk factors for DVT (Figure 1).
3.2. Basic information of included studies
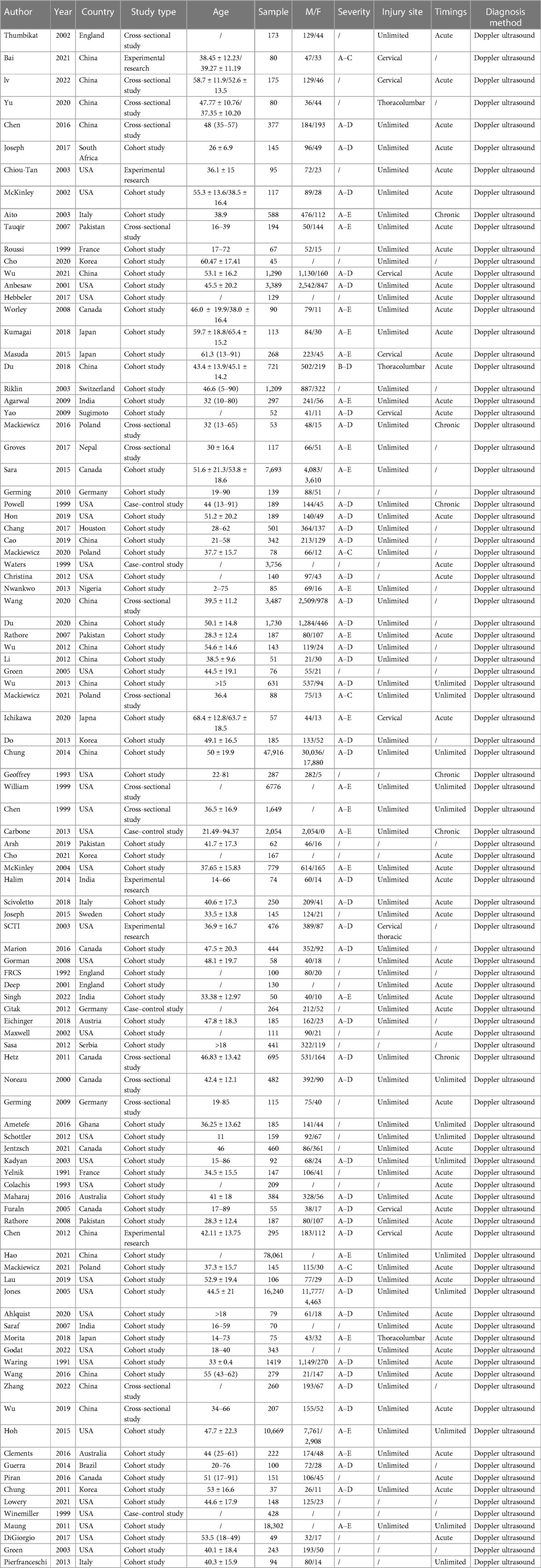
The 101 studies included 75 cohort studies, 16 cross-sectional studies, 5 case-control studies and 5 experimental studies. The age of the patients varied widely, most of them were between 30 and 60 years old, and 16 studies did not report the age of the patients. The sample size of a single study is between 37 and 78,016, and the total sample size is 223,221. The severity of SCI varied from AIS grades A–E, and no AIS score was reported in 35 studies. Sites of injury included the cervical, thoracic, and lumbar cord, and 16 studies did not report sites of injury. 53 studies included patients with acute SCI, 6 studies included patients with chronic SCI, 14 studies did not limit the timing of SCI, and 28 studies did not report the timing of SCI. DVT was diagnosed by doppler ultrasound. For more details about basic information, see Table 2.
3.3. Methodological quality assessment
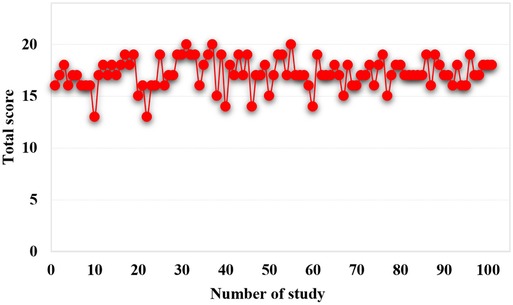
The 101 studies included clearly reported the purpose of the study, 94 studies clearly reported the evaluation criteria of the results, 92 studies had appropriate control groups, 63 studies were balanced and comparable in baseline characteristics, and 97 studies conducted adequate data analysis. However, no study developed a protocol in advance, and no study prospectively calculated the required sample size. Only 4 studies blinded the evaluators of the results, only 21 studies had adequate follow-up of patients, and 57 studies had a loss of follow-up. Overall, out of a total of 24, only 41 studies scored 18 or more (Figure 2).
3.4. Meta-analysis results
3.4.1. Incidence of DVT
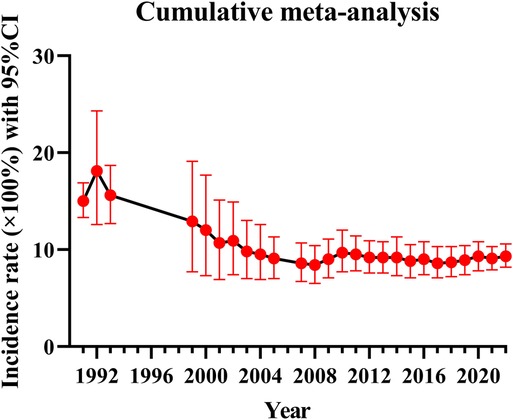
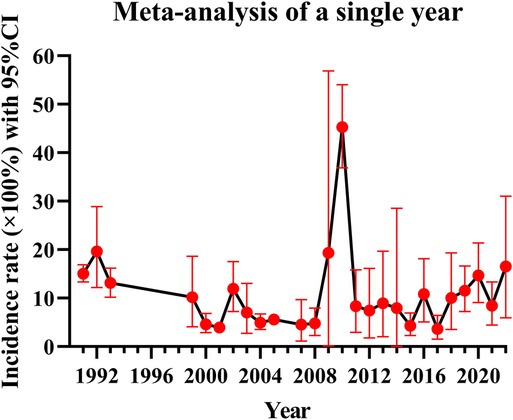
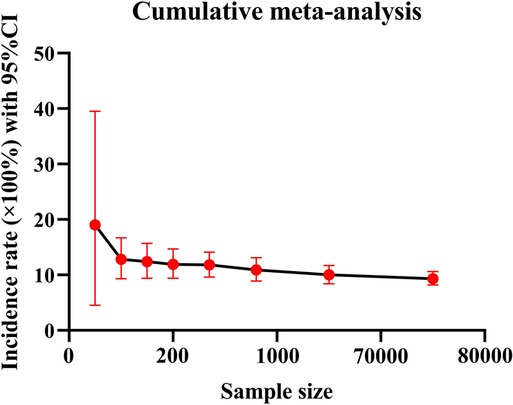
Because of the great heterogeneity among different studies (I2 > 90%), the random effect model was used for Meta-analysis. The results showed that the overall incidence of DVT after SCI was 9.3% (95% CI: 8.2%–10.6%). The incidence of DVT in patients with acute and chronic SCI was 10.9% (95% CI: 8.7%–13.2%) and 5.3% (95% CI: 2.2%–9.7%). Since the incidence of DVT in patients with SCI was first reported in 1991, the overall incidence of DVT gradually decreased and tended to be stable with the accumulation of publication years (Figure 3). However, the incidence of DVT still varies greatly from year to year, and has a gradually increasing trend since 2017 (Figure 4). In addition, when the sample size of a single study gradually increased from 37 to 78,016, the cumulative incidence of DVT decreased with the increase of sample size (Figure 5).
3.4.2. Risk factors for DVT
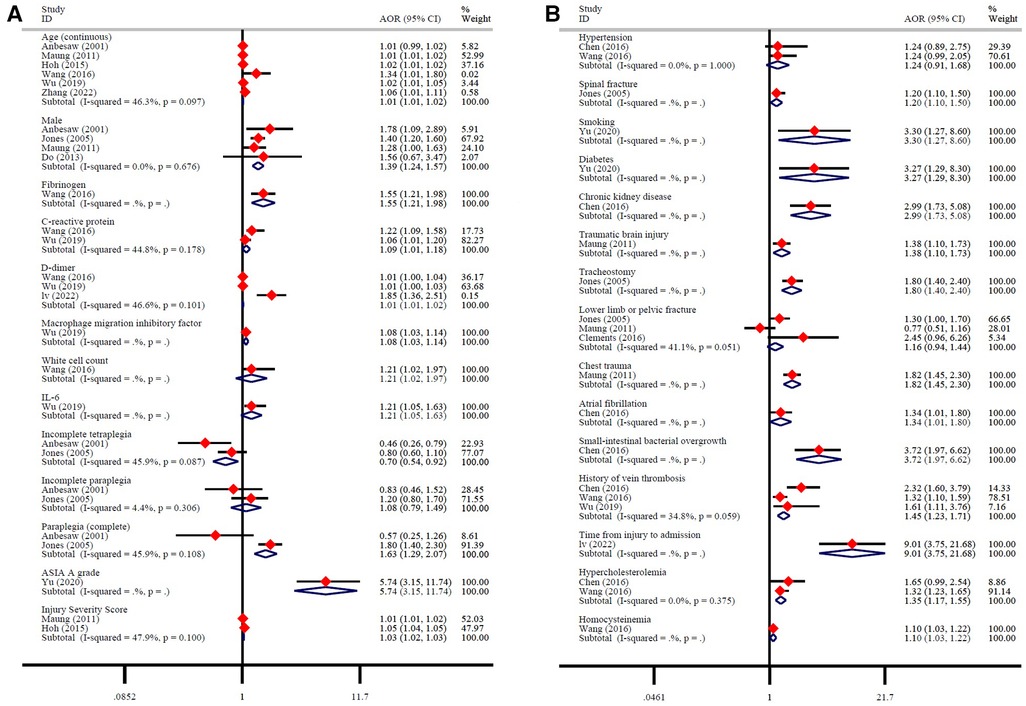
A total of 12 studies reported 28 possible DVT-related factors. These influencing factors can be divided into three aspects. ① Baseline characteristics: Age is a risk factor for DVT. Men with SCI have a higher risk of developing DVT than women (Figure 6A). ② Biochemical indicators: Fibrinogen, C-reactive protein, D-dimer, macrophage migration inhibitory factor, white cell count, IL-6 are risk factors for DVT (Figure 6A). ③ Severity of SCI: AIS grade A, and injury severity score are risk factors for DVT. More specific, compared with tetraplegia, incomplete tetraplegia is the protective factor of DVT, there is no significant correlation between incomplete paraplegia and DVT, while complete paraplegia is the risk factor of DVT (Figure 6A). ④ Comorbidities: spinal fracture, smoking, diabetes, chronic kidney disease, traumatic brain injury, tracheostomy, chest trauma, atrial fibrillation, small-intestinal bacterial overgrowth, history of vein thrombosis, time from injury to admission, hypercholesterolemia, homocysteine are risk factors for DVT. There was no significant correlation between hypertension, lower limb or pelvic fracture and DVT (Figure 6B).
3.4.3. Publication bias detection
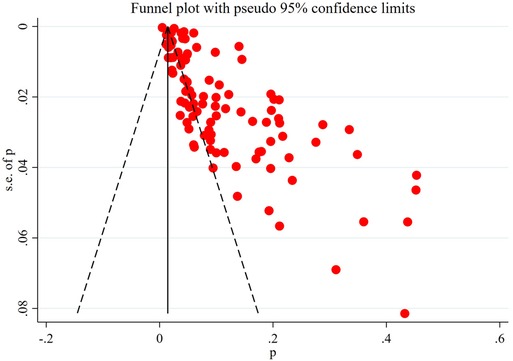
The obviously asymmetric funnel plot showed that there was a greater possibility of publication bias in the current research field (Figure 7).
4. Discussion
4.1. Research overview
After SCI, the dysfunction of the autonomic nervous system leads to the dilatation of venous vessels of lower extremities and the increase of venous blood flow resistance (21, 22), the decrease of platelet inhibition of thrombin production and release of prostaglandins, the decrease of endothelial function, the enhancement of coagulation function and the relative weakening of fibrinolytic function, so that the blood is in a state of hypercoagulability (23, 24). In addition, due to various incentives such as SCI itself, limb paralysis after SCI, limb immobilization, long-term bed rest, surgery and anesthesia for anatomical reduction and maintaining spinal stability, it can favor the formation of DVT. Most studies do not pay enough attention to DVT after SCI, or do not comprehensively and systematically study the incidence and risk factors of DVT. For example, studies by Schottler et al. showed that DVT occurred in 3 of the 138 patients with acute SCI, with an incidence of only 2.2% (25). This is highly misleading, suggesting that the incidence of DVT is low and decreasing the attention of clinicians and patients. Although these data are based on realistic clinical data, the results from these small-sample, single-center studies are difficult to apply to the broader SCI population due to a variety of factors, including the health and disease status of the patients, the diagnostic criteria for DVT, and the design and sample size of the studies. Therefore, a summary analysis of all currently published studies to explore the incidence and risk factors of DVT in a comprehensive manner is essential for future clinical practice.
The incidence of DVT in patients with SCI reported in the previous literature varies greatly, ranging from 9% to 100% (9, 17). The reason for this disparity may be due to inconsistencies in the diagnostic methods of DVT, such as venography, doppler ultrasound, clinical manifestations or a combination of multiple examinations, and the small number of cases included in the analysis. Considering the above factors, we only included studies using doppler ultrasound to diagnose DVT according to the unified standard, and collected the data of 223,221 people in 101 studies, which reduced the influence of confounding factors to some extent. We also excluded studies that combined with other diseases that may lead to the formation of DVT and studies with a smaller sample size. Our study found that the overall incidence of DVT after SCI was 9.3% (95% CI: 8.2%–10.6%), and the incidence of DVT in patients with acute SCI was twice as high as that in patients with chronic SCI. This is consistent with the results of most studies. Most cases of DVT occur in the acute stage of SCI (26). When the sample size of a single study gradually increased from 37 to 78,016, the cumulative incidence of DVT decreased with the increase of sample size, indicating that the incidence of DVT was affected by the sample size. When the sample size is too small, the sporadic occurrence of DVT will greatly affect its incidence. This is also the main reason why we exclude small sample studies in advance. Of the 101 studies included, 5 studies had a sample size of more than 10,000 cases. In these five studies, the incidence of DVT ranged from 0.5% to 6%, lower than the overall incidence of 9.3%. Therefore, despite excluding small sample studies, the small sample effect may still affect the incidence of DVT. In addition, since the incidence of DVT in patients with SCI was first reported in 1991, the overall incidence of DVT gradually decreased with the accumulation of publication years and tended to be stable in 2010. In fact, the incidence of DVT still varies greatly from year to year. In particular, the incidence of DVT has been increasing in the past five years after it fell to the lowest point in 2017. The main reason is that in recent years, traffic accidents, falls and sports injuries have been increasing, resulting in an increase in the number of patients with SCI, and the number of DVT will naturally increase accordingly (27). In a nutshell, DVT cannot be ignored after SCI, and more large-scale study is needed in the future.
After defining the incidence of DVT in patients with SCI, it is very important to further explore the risk factors of DVT for its early prevention and treatment. This study shows that men have a higher risk of developing DVT than women. However, it is not clear why gender is an influencing factor of DVT. This may be related to the large difference in the proportion of men and women included in the study (76,715/35,925), or to the different probability of gender-induced exposure to risk factors. We also found that the older the age, the higher the incidence of DVT. With the increase of age, the various organs of the patient are in a state of decline, the blood viscosity increases, the blood flow is slow, the concentration of thrombotic factor in vascular endothelial cells increases, and antithrombin and other substances decrease, resulting in the destruction of coagulation-anticoagulation balance in the blood system, which leads to thrombosis (28). In this process, fibrinogen and D-dimer in blood are closely related to DVT, which are the risk factors of DVT (29). Fibrinogen is a coagulation factor synthesized and secreted by liver cells. When the human fibrinolytic system is activated, fibrinogen is converted into fibrin monomer and crosslinked with factor XIII, which is hydrolyzed into D-dimer induced by plasmin. When fibrinogen increases, the production of fibrinolysis and D-dimer increases, the blood is in a state of hypercoagulability. The blood viscosity increases, and the blood flow slows down, which can easily lead to thrombosis (30). In addition, inflammatory processes plays a key role in the formation of DVT by activating monocytes and endothelial cells, releasing cytokines and chemokines to participate in the activation of blood coagulation system, thus inducing hypercoagulable state (31). van Aken et al. found that the levels of interleukin-6, interleukin-8 and monocytechemotacticprotein1 (MCP-1) were significantly increased in patients with recurrent DVT (31). Ray et al. further found that IL-6 can increase anticoagulant factors such as tissue factor, fibrinogen and coagulation factor VIII, and reduce antithrombin and protein S, thus inducing hypercoagulable state and increasing the incidence of thrombotic diseases (32). Our results showed that inflammatory markers such as IL-6, C-reactive protein, macrophage migration inhibitory factor, and white cell count were significantly elevated in patients who developed DVT after SCI and were risk factors for DVT. In addition to inflammatory and coagulation indexes, hyperhomocysteinemia and hypercholesterolemia are also risk factors for DVT. Homocysteine is the intermediate product of methionine metabolism in vivo, which can directly activate V, X and XII factors, reduce the production of nitric oxide and prostacyclin, tissue plasminogen activator and adenosine diphosphatase, and increase the synthesis and expression of thromboxane A2 and P-selectin, thus promoting platelet adhesion, aggregation and thrombosis (33, 34). Cholesterol can also increase the production of coagulation factors VII, VIII, IX and plasminogen activator inhibitor-1, which can promote thrombosis. Therefore, the above indexes can be used as predictors and auxiliary diagnostic indexes for the formation of DVT (35).
Venous stasis, hypercoagulable state and intimal injury are the three basic factors for the formation of DVT. Any disease or clinical condition that can cause the above abnormalities can induce thrombosis, including primary factors such as genetic variation, as well as secondary factors such as surgery, trauma, underlying diseases, immobilization and so on (36). Diabetes, chronic kidney disease and atrial fibrillation are common diseases in clinic, which often lead to secondary lesions of blood vessels and cause the blood in a hypercoagulable state (37). For example, hyperglycemia, hyperinsulinemia and insulin resistance in patients with diabetes can directly damage endothelial cells and endothelial function, and can cause chronic inflammation and participate in the process of endothelial injury. At the same time, the blood flow in the limbs of patients with diabetes is lower than that of normal people, and severe hyperglycemia can accelerate the state of hypercoagulability (38). In addition, spinal fractures, brain trauma, thoracic trauma, and the severity of SCI will all affect the occurrence of DVT. For example, complete SCI, especially SCI above T6, will seriously affect the sympathetic control of cardiac function, resulting in decreased myocardial contractility and dilatation of capillaries, gastrointestinal vascular beds and coronary arteries, resulting in a reduction of effective blood volume by about 50%. This not only increases the blood viscosity, but also greatly reduces the oxygen supply of muscle and the contractility of muscle, which eventually leads to the decrease of deep venous blood flow velocity of lower extremities and increases the incidence of DVT (39). In addition to the effects of the complications themselves, these complications also prolong the patient's bed rest and braking time. After lying in bed for a long time, the venous blood flow of the lower extremities slows down or even stagnates, and the slow flow leads the valve in a state of hypoxia, causing endothelial injury. Blood stasis can also cause the accumulation of local coagulation factors and the consumption of inhibitory factors, resulting in the formation of thrombus in the vein (40).
While several factors such as baseline characteristics, biochemical indicators, and injury severity can contribute to the development of DVT in patients with SCI, it is important to note that not all patients are affected by these factors to the same degree. Furthermore, the impact of various risk factors on DVT can vary among patients. In conclusion, there are significant differences in the risk of DVT among different SCI patients. As reported by Chibbaro et al., patients in different risk strata responded differently to the same thromboprophylaxis (41). Therefore, early identification and classification of patients in different risk strata is crucial. Our study reveals that patients with SCI face 24 risk factors that heighten the risk of DVT. Notably, the severity of SCI, smoking, diabetes, chronic kidney disease, small-intestinal bacterial overgrowth, and time from injury to admission increase the risk of DVT by more than double. These six factors have a significantly greater impact on the occurrence of DVT compared to the remaining 18 risk factors. It is recommended that clinicians and patients give greater consideration to these noteworthy risk factors. Of course, identification and prevention of other risk factors are also important. In addition, our meta-analysis revealed that the occurrence of DVT was twice as high in patients with acute SCI compared to those with chronic SCI, which is in line with previous research studies. Most DVT occurs in the first three months of SCI (acute phase), and its peak occurs from the 7th day to the 14th day after injury (42, 43). The main reason is that as time progresses, the degree of vascular intima damage and blood stasis are gradually reduced, the function of autonomic nerves is also gradually restored, and the regulation of the blood coagulation system is gradually strengthened, resulting in a gradual decrease in the incidence of DVT in the chronic phase (44–46). Therefore, it is very important to take preventive measures as soon as possible to reduce the incidence and severity of DVT after SCI.
It is worth noting that although we get the risk factors of DVT based on the data of multivariate analysis, DVT is not caused by only one factor, and the prevention of DVT should also be a comprehensive prevention in many aspects. At present, the recognized comprehensive prevention strategy requires health education, combined drug therapy and physiotherapy. Health education should include disease education (risk factors, etiology, inducement, symptoms, signs, consequences), diet education (avoid alcohol and tobacco, avoid high-fat and high-salt diet, drink plenty of water appropriately), physical activity education (early physical activity and correct physical activity method) and so on. In the past, anticoagulants such as low molecular weight heparin and warfarin were widely used for early prevention of DVT. Although the effectiveness of these drugs has been proved, the risk of spinal epidural and subdural hemorrhage increases while preventing DVT, and the hematoma may oppress the spinal cord and aggravate the SCI (47). Therefore, patients with SCI are cautiously advised to use anticoagulants, mainly using physical prophylaxis such as drug prophylaxis combined with air pressure therapy and wearing elastic socks, so as to minimize the adverse reactions caused by drugs (9, 48). In short, our study found that the incidence of DVT after SCI is high, there are many risk factors. Clinicians and patients are clearly aware of the current situation of DVT and take preventive measures as soon as possible.
4.2. Limitations
Data sources and analysis methods for the meta-analysis were influenced by the reporting methods of the included studies. ① The included study did not clearly report the severity of SCI (AIS grades A–E), the time of injury (from acute to chronic) and the location of injury (from cervical spinal cord to lumbosacral spinal cord). ② Details such as baseline characteristics, whether blinding was performed, and follow-up of patients were not clearly reported. ③ The treatment of patients with SCI was not reported in detail. It is worth noting that the prevention programs in different studies are significantly different, which may affect the incidence and reporting rate of DVT, thus affecting the reliability of meta-analysis results (49). These problems are the aspects that should be improved and enhanced in future research: to further refine the incidence of DVT in patients with different characteristics of SCI in order to provide more accurate information. The above limitations of the inclusion study also make it impossible for us to conduct further subgroup analysis to provide more information. In addition, even if we collected all the studies on DVT after SCI as much as possible, we did not search the grey database and excluded non-English literature, resulting in a certain publication bias and language bias, which is also proved by the asymmetric funnel plot.
5. Conclusions
DVT increases the disease burden of patients with SCI. However, there is a lack of large sample, multicenter epidemiological evidence. As the first study, having analyzed 101 studies including 223,221 patients, we found that the incidence of DVT in patients with SCI was 9.3% and that the incidence of DVT decreased gradually with the accumulation of publication years and sample size, but the annual incidence of DVT increased gradually from 2017. There are 24 kinds of risk factors that may contribute to the formation of DVT, involving multiple aspects of the baseline characteristics of the patient, biochemical indicators, severity of SCI, and comorbidities. Our results provide effective evidence for taking measures to prevent DVT as early as possible.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Author contributions
XW: undertook the design, guidance and modification of the project and paper. ZS: completed the implementation of project and writing of the paper. PW, MW and BZ: completed the collection and collation of the data. All authors contributed to the article and approved the submitted version.
Funding
We acknowledge the financial support from Gansu Natural Science Foundation (grant no. 21JR7RA362), Gansu Youth Doctoral Fund Project of Colleges and Universities (grant no. 2022QB-007), and The First Hospital of Lanzhou University Foundation (grant no. ldyyyn2021-121).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Injury GBDTB, Spinal Cord Injury C. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. (2019) 18(1):56–87. doi: 10.1016/S1474-4422(18)30415-0
2. Anjum A, Yazid MD, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A, et al. Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci. (2020) 21(20):7533. doi: 10.3390/ijms21207533
3. Katoh H, Yokota K, Fehlings MG. Regeneration of spinal cord connectivity through stem cell transplantation and biomaterial scaffolds. Front Cell Neurosci. (2019) 13:248. doi: 10.3389/fncel.2019.00248
4. Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. (2014) 52(2):110–6. doi: 10.1038/sc.2012.158
5. Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. (2017) 3:17018. doi: 10.1038/nrdp.2017.18
6. Jain NB, Ayers GD, Peterson EN, Harris MB, Morse L, O’Connor KC, et al. Traumatic spinal cord injury in the United States, 1993–2012. JAMA. (2015) 313(22):2236–43. doi: 10.1001/jama.2015.6250
7. Paffrath T, Wafaisade A, Lefering R, Simanski C, Bouillon B, Spanholtz T, et al. Venous thromboembolism after severe trauma: incidence, risk factors and outcome. Injury. (2010) 41(1):97–101. doi: 10.1016/j.injury.2009.06.010
8. Azu MC, McCormack JE, Huang EC, Lee TK, Shapiro MJ. Venous thromboembolic events in hospitalized trauma patients. Am Surg. (2007) 73(12):1228–31. doi: 10.1177/000313480707301206
9. Christie S, Thibault-Halman G, Casha S. Acute pharmacological DVT prophylaxis after spinal cord injury. J Neurotrauma. (2011) 28(8):1509–14. doi: 10.1089/neu.2009.1155-A
10. Qiu T, Zhang T, Liu L, Li W, Li Q, Zhang X, et al. The anatomic distribution and pulmonary embolism complications of hospital-acquired lower extremity deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. (2021) 9(6):1391–98 e3. doi: 10.1016/j.jvsv.2021.03.004
11. Huisman MV, Barco S, Cannegieter SC, Le Gal G, Konstantinides SV, Reitsma PH, et al. Pulmonary embolism. Nat Rev Dis Primers. (2018) 4:18028. doi: 10.1038/nrdp.2018.28
12. Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A, et al. American society of hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. (2020) 4(19):4693–738. doi: 10.1182/bloodadvances.2020001830
13. Alabed S, de Heredia LL, Naidoo A, Belci M, Hughes RJ, Meagher TM. Incidence of pulmonary embolism after the first 3 months of spinal cord injury. Spinal Cord. (2015) 53(11):835–7. doi: 10.1038/sc.2015.105
14. Chung WS, Lin CL, Chang SN, Chung HA, Sung FC, Kao CH. Increased risk of deep vein thrombosis and pulmonary thromboembolism in patients with spinal cord injury: a nationwide cohort prospective study. Thromb Res. (2014) 133(4):579–84. doi: 10.1016/j.thromres.2014.01.008
15. Rathore MF, Hanif S, New PW, Butt AW, Aasi MH, Khan SU. The prevalence of deep vein thrombosis in a cohort of patients with spinal cord injury following the Pakistan earthquake of October 2005. Spinal Cord. (2008) 46(7):523–6. doi: 10.1038/sj.sc.3102170
16. Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galie N, Pruszczyk P, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Heart J. (2008) 29(18):2276–315. doi: 10.1093/eurheartj/ehn310
17. Chang R, Scerbo MH, Schmitt KM, Adams SD, Choi TJ, Wade CE, et al. Early chemoprophylaxis is associated with decreased venous thromboembolism risk without concomitant increase in intraspinal hematoma expansion after traumatic spinal cord injury. J Trauma Acute Care Surg. (2017) 83(6):1088–94. doi: 10.1097/TA.0000000000001675
18. Cheng X, Zhang L, Xie NC, Xu HL, Lian YJ. Association between small-intestinal bacterial overgrowth and deep vein thrombosis in patients with spinal cord injuries. J Thromb. Haemost. (2017) 15(2):304–11. doi: 10.1111/jth.13583
19. Selassie AW, Varma A, Saunders LL. Current trends in venous thromboembolism among persons hospitalized with acute traumatic spinal cord injury: does early access to rehabilitation matter? Arch Phys Med Rehabil. (2011) 92(10):1534–41. doi: 10.1016/j.apmr.2011.04.018
20. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73(9):712–6. doi: 10.1046/j.1445-2197.2003.02748.x
21. Hopman MT, Verheijen PH, Binkhorst RA. Volume changes in the legs of paraplegic subjects during arm exercise. J Appl Physiol. (1993) 75(5):2079–83. doi: 10.1152/jappl.1993.75.5.2079
22. Hopman MT, Nommensen E, van Asten WN, Oeseburg B, Binkhorst RA. Properties of the venous vascular system in the lower extremities of individuals with paraplegia. Paraplegia. (1994) 32(12):810–6. doi: 10.1038/sc.1994.128
23. Kahn NN, Bauman WA, Sinha AK. Demonstration of a novel circulating anti-prostacyclin receptor antibody. Proc Natl Acad Sci U S A. (1997) 94(16):8779–82. doi: 10.1073/pnas.94.16.8779
24. Kahn NN, Bauman WA, Sinha AK. Loss of high-affinity prostacyclin receptors in platelets and the lack of prostaglandin-induced inhibition of platelet-stimulated thrombin generation in subjects with spinal cord injury. Proc Natl Acad Sci U S A. (1996) 93(1):245–9. doi: 10.1073/pnas.93.1.245
25. Schottler J, Vogel LC, Sturm P. Spinal cord injuries in young children: a review of children injured at 5 years of age and younger. Dev Med Child Neurol. (2012) 54(12):1138–43. doi: 10.1111/j.1469-8749.2012.04411.x
26. Mackiewicz-Milewska M, Jung S, Kroszczynski AC, Mackiewicz-Nartowicz H, Serafin Z, Cisowska-Adamiak M, et al. Deep venous thrombosis in patients with chronic spinal cord injury. J Spinal Cord Med. (2016) 39(4):400–4. doi: 10.1179/2045772315Y.0000000032
27. Du J, Hao D, He B, Yan L, Tang Q, Zhang Z, et al. Epidemiological characteristics of traumatic spinal cord injury in Xi’an, China. Spinal Cord. (2021) 59(7):804–13. doi: 10.1038/s41393-020-00592-3
28. Jang MJ, Bang SM, Oh D. Incidence of venous thromboembolism in Korea: from the health insurance review and assessment service database. J Thromb. Haemost. (2011) 9(1):85–91. doi: 10.1111/j.1538-7836.2010.04108.x
29. Timp JF, Lijfering WM, Flinterman LE, van Hylckama Vlieg A, le Cessie S, Rosendaal FR, et al. Predictive value of factor VIII levels for recurrent venous thrombosis: results from the MEGA follow-up study. J Thromb. Haemost. (2015) 13(10):1823–32. doi: 10.1111/jth.13113
30. Wolberg AS. Fibrinogen and factor XIII: newly recognized roles in venous thrombus formation and composition. Curr Opin Hematol. (2018) 25(5):358–64. doi: 10.1097/MOH.0000000000000445
31. van Aken BE, den Heijer M, Bos GM, van Deventer SJ, Reitsma PH. Recurrent venous thrombosis and markers of inflammation. Thromb Haemost. (2000) 83(4):536–9. doi: 10.1055/s-0037-1613858
32. Ray A. A SAF binding site in the promoter region of human gamma-fibrinogen gene functions as an IL-6 response element. J Immunol. (2000) 165(6):3411–7. doi: 10.4049/jimmunol.165.6.3411
33. Spencer CG, Martin SC, Felmeden DC, Blann AD, Beevers GD, Lip GY. Relationship of homocysteine to markers of platelet and endothelial activation in “high risk” hypertensives: a substudy of the anglo-scandinavian cardiac outcomes trial. Int J Cardiol. (2004) 94(2-3):293–300. doi: 10.1016/j.ijcard.2003.06.002
34. Thambyrajah J, Townend JN. Homocysteine and atherothrombosis–mechanisms for injury. Eur Heart J. (2000) 21(12):967–74. doi: 10.1053/euhj.1999.1914
35. Sartori M, Cosmi B, Legnani C, Favaretto E, Valdre L, Guazzaloca G, et al. The wells rule and D-dimer for the diagnosis of isolated distal deep vein thrombosis. J Thromb. Haemost. (2012) 10(11):2264–9. doi: 10.1111/j.1538-7836.2012.04895.x
36. Lutsey PL, Zakai NA. Epidemiology and prevention of venous thromboembolism. Nat Rev Cardiol. (2023) 20(4):248–62. doi: 10.1038/s41569-022-00787-6
37. Potpara TS, Ferro CJ, Lip GYH. Use of oral anticoagulants in patients with atrial fibrillation and renal dysfunction. Nat Rev Nephrol. (2018) 14(5):337–51. doi: 10.1038/nrneph.2018.19
38. Tripodi A, Branchi A, Chantarangkul V, Clerici M, Merati G, Artoni A, et al. Hypercoagulability in patients with type 2 diabetes mellitus detected by a thrombin generation assay. J Thromb Thrombolysis. (2011) 31(2):165–72. doi: 10.1007/s11239-010-0506-0
39. Kim SW, Park CJ, Kim K, Kim YC. Cardiac arrest attributable to dysfunction of the autonomic nervous system after traumatic cervical spinal cord injury. Chin J Traumatol. (2017) 20(2):118–21. doi: 10.1016/j.cjtee.2016.11.004
40. Martinelli I, Bucciarelli P, Mannucci PM. Thrombotic risk factors: basic pathophysiology. Crit Care Med. (2010) 38(2 Suppl):S3–S9. doi: 10.1097/CCM.0b013e3181c9cbd9
41. Chibbaro S, Cebula H, Todeschi J, Fricia M, Vigouroux D, Abid H, et al. Evolution of prophylaxis protocols for venous thromboembolism in neurosurgery: results from a prospective comparative study on low-molecular-weight heparin, elastic stockings, and intermittent pneumatic compression devices. World Neurosurg. (2018) 109:e510–6. doi: 10.1016/j.wneu.2017.10.012
42. Ploumis A, Ponnappan RK, Bessey JT, Patel R, Vaccaro AR. Thromboprophylaxis in spinal trauma surgery: consensus among spine trauma surgeons. Spine J. (2009) 9(7):530–6. doi: 10.1016/j.spinee.2009.01.008
43. Powell M, Kirshblum S, O'Connor KC. Duplex ultrasound screening for deep vein thrombosis in spinal cord injured patients at rehabilitation admission. Arch Phys Med Rehabil. (1999) 80(9):1044–6. doi: 10.1016/s0003-9993(99)90058-8
44. Iversen PO, Groot PD, Hjeltnes N, Andersen TO, Mowinckel MC, Sandset PM. Impaired circadian variations of haemostatic and fibrinolytic parameters in tetraplegia. Br J Haematol. (2002) 119(4):1011–6. doi: 10.1046/j.1365-2141.2002.03953.x
45. Anderson FA J, Spencer FA. Risk factors for venous thromboembolism. Circulation. (2003) 107(23 Suppl 1):I9–16. doi: 10.1161/01.CIR.0000078469.07362.E6
46. Furlan JC, Fehlings MG. Role of screening tests for deep venous thrombosis in asymptomatic adults with acute spinal cord injury: an evidence-based analysis. Spine. (2007) 32(17):1908–16. doi: 10.1097/BRS.0b013e31811ec26a
47. Zeeshan M, Khan M, O’Keeffe T, Pollack N, Hamidi M, Kulvatunyou N, et al. Optimal timing of initiation of thromboprophylaxis in spine trauma managed operatively: a nationwide propensity-matched analysis of trauma quality improvement program. J Trauma Acute Care Surg. (2018) 85(2):387–92. doi: 10.1097/TA.0000000000001916
48. Teasell RW, Hsieh JT, Aubut JA, Eng JJ, Krassioukov A, Tu L. Spinal cord injury rehabilitation evidence review research T. Venous thromboembolism after spinal cord injury. Arch Phys Med Rehabil. (2009) 90(2):232–45. doi: 10.1016/j.apmr.2008.09.557
Keywords: spinal cord injury, deep vein thrombosis, incidence, risk factor, systematic review
Citation: Shang Z, Wanyan P, Zhang B, Wang M and Wang X (2023) Incidence and risk factors of deep vein thrombosis in patients with spinal cord injury: a systematic review with meta-analysis. Front. Cardiovasc. Med. 10:1153432. doi: 10.3389/fcvm.2023.1153432
Received: 7 February 2023; Accepted: 25 April 2023;
Published: 12 May 2023.
Edited by:
Kohei Tatsumi, Nara Medical University, JapanReviewed by:
Tjokorda Gde Bagus Mahadewa, Udayana University, IndonesiaMario Ganau, Oxford University Hospitals NHS Trust, United Kingdom
© 2023 Shang, Wanyan, Zhang, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Wang wangxinldyy@126.com
 Zhizhong Shang
Zhizhong Shang Pingping Wanyan2,3
Pingping Wanyan2,3  Xin Wang
Xin Wang