Population Structure of the Red Macroalga Botryocladia occidentalis (Børgesen) Kylin (Rhodymeniaceae, Rhodymeniales) in the Gulf of Mexico Before the Deepwater Horizon Oil Spill
- Department of Biology, University of Louisiana at Lafayette, Lafayette, LA, United States
Studies on the population structure of common widespread macroalgae in the Gulf of Mexico (GoMx) are scarce, and this knowledge gap limits our understanding on how disturbances affect the genetic diversity of macroalgae in this basin. The latter is due to the lack of a baseline that can be compared with allele frequency surveys conducted after a major disturbance such as the 2010 Deepwater Horizon oil spill (DWH), which leaked 780,000 m3 of crude oil in the vicinity of highly diverse macroalgal communities. Fortunately, quantitative assessments of the population structure pre-DWH can be accomplished for several macroalgae with dried specimens collected from research cruises conducted before 2010 in the offshore GoMx. Based on three markers (cytochrome c oxidase subunit I, COX II-III intergenic spacer, and the RuBisCO large subunit), this study reconstructed the allele frequencies pre-DWH for a GoMx-widespread macroalga, Botryocladia occidentalis, and revealed the existence of distinct populations in each of three distant regions of the GoMx: Florida Middle Grounds (FL), Campeche Banks (CB), and offshore Louisiana (LA). Population structure was assessed with exact tests of population differentiation and Analyses of Molecular Variance. FL harbored the most differentiated and genetically diverse population due to the presence and abundance of unique haplotypes. Interestingly, FL haplotypes were not closely phylogenetically related to each other and included the most divergent lineages of the entire GoMx; this phylogeographic pattern suggests a strong influence of migrants from the Caribbean on the FL population. Additionally, likelihood ratio tests with a small sample collected post-DWH indicated that the LA population underwent strong changes, showing statistically significant differences before (LA) vs. after (L2) the disaster. Whereas the LA population had affinity to CB, L2 showed a FL haplotype that, before the disaster, had never been reported in LA or CB. Such changes may not be permanent but rather a temporary response to disturbance; also, they may not necessarily be caused by the spilled oil but by other factors associated with the DWH.
Introduction
Researching the population structure of marine macroalgae is a challenging endeavor because many species are nearly indistinguishable from each other morphologically and, thus, are virtually impossible to identify in situ (e.g., Krayesky et al., 2009; Balata et al., 2011). Since population genetics studies normally require the collection of numerous conspecific individuals for each location of interest (Nei, 1978; Baverstock and Moritz, 1996; Ruzzante, 1998; Excoffier, 2007), population samples of macroalgae are often found to be multispecies mixtures from which multiple individuals must be discarded, reducing sample sizes. This is especially true for highly biodiverse metacommunities like the offshore hard banks of the Gulf of Mexico (GoMx) (Felder and Camp, 2009) which harbor nearly 700 species and over 70 families as recorded in the most recent review of GoMx macroalgae (Fredericq et al., 2009).
Despite this problem, researching the population structure of marine macroalgae is highly valuable due to their economic and ecological importance. For instance, macroalgae provide food and shelter for numerous marine animals, including invertebrates and fishes of economic interest, at different stages of their life (e.g., larvae, juveniles, and adults) (Chemello and Milazzo, 2002; Epifanio et al., 2003; Hasan and Chakrabarti, 2009; Thomsen, 2010; Vergés et al., 2012; Chaves et al., 2013; Milne and Griffiths, 2014; Shaal et al., 2016). Some macroalgae also contribute largely to the building of reef structures that shelter not only a few species but whole ecosystems (Littler and Littler, 1984; Adey, 1998; Hurd et al., 2014). Therefore, changes in the dominant macroalgal populations of marine ecosystems can impact their associated animal communities as well as the human activities (e.g., fishing). Communities largely dominated by marine macroalgae such as the offshore hard banks of the GoMx (e.g., Gavio and Fredericq, 2003, 2005; Gurgel et al., 2004a; Gavio et al., 2005; Mateo-Cid et al., 2013; Arakaki et al., 2014; Fredericq et al., 2014; Richards et al., 2014, 2016; Schmidt et al., 2016, 2017; Leliaert et al., 2016; Camacho et al., 2018; Richards and Fredericq, 2018), may greatly benefit from studies on the population structure of their most common and widespread macroalgal species.
Unfortunately, few studies have been done on the population structure of dominant macroalgae in the GoMx and this knowledge gap limits our understanding of the impacts of human and natural disturbances in this marine basin. For instance, there is no baseline that can be compared with allele frequency surveys performed after a disturbance event. In April 2010, the NW GoMx was affected by the Deepwater Horizon oil spill (DWH) (Felder et al., 2014), regarded as the largest accidental marine oil spill in U.S. territories (Atlas and Hazen, 2011; Liu et al., 2011; Barron, 2012; Paris et al., 2012; Rabalais, 2014; Beyer et al., 2016). This disaster lasted 87 days, during which 780,000 m3 of crude oil were leaked in the GoMx along with 7,000 m3 of Corexit oil dispersant (Lehr et al., 2010; OSAT, 2010, 2011; Kujawinski et al., 2011). Since the typical genetic diversity and population structure of the dominant GoMx macroalgae were unknown, evaluating the impact of the DWH disaster on macroalgal populations has been unfeasible. There are a few studies (e.g., Gurgel et al., 2004b; Núñez-Resendiz et al., 2017) examining the phylogeography of particular GoMx species using non-quantitative approaches; one study (Gurgel et al., 2004b) showed the presence of two haplotype lineages characteristic of the eastern and western GoMx. Quantitative assessments of the population structure before the DWH can be accomplished for several macroalgae with specimens from research cruises conducted prior to 2010 in the offshore GoMx. Most of these specimens are deposited in the University of Louisiana at Lafayette Herbarium (LAF).
This study reconstructed the allele frequencies before the DWH for a common widespread deepwater red alga in the GoMx, Botryocladia occidentalis (Børgesen) Kylin (Gavio and Fredericq, 2003; Fredericq et al., 2009; Figure 1), and evaluated its population structure and phylogeography across three distant regions of the GoMx. The main goal of the study was to determine whether the B. occidentalis population exposed to the DWH is distinct or belongs to a larger uniform population widespread throughout the GoMx. Additionally, as a secondary analysis, this study evaluated a small sample collected post-DWH for changes in population structure that may be associated with the disaster.

Figure 1. Herbarium specimen of Botryocladia occidentalis (scale bar: 2 cm) collected in the Florida Middle Grounds (28° 5.20′ N, 83° 46.16′ W) during the research cruise NSFIII (collect date: July 05, 2006) of the University of Louisiana at Lafayette (LAF) Seaweeds Lab. This specimen, referenced NSFIII-7-5-06-8-2 in the LAF Herbarium, is part of the biological material used in this study.
Finally, this study also compared the efficacy of three genetic markers in elucidating the genetic structure of B. occidentalis. The value of the mitochondrial cytochrome c oxidase subunit I (Cox1) and the COX II-III intergenic spacer region (Cox2-3) for evaluating population structure and intraspecific genetic diversity in red macroalgae has been validated in multiple studies (e.g., Zuccarello et al., 1999, 2005, 2006; Yang et al., 2008; Kim et al., 2010, 2012; Hernández-Kantún et al., 2014), whereas the plastid-encoded RuBisCO large subunit (rbcL) is generally regarded as a slow-evolving gene with poor resolution at the intra-species level (Yang et al., 2008; Geraldino et al., 2009; Tan et al., 2012). With that said, there are a number of cases in which Cox2-3 has failed to detect important intraspecific variation detected by other markers (e.g., O’Doherty and Sherwood, 2007), and also, there are specific studies in which rbcL has effectively elucidated phylogeographic patterns of Rhodophyta species. One important example for the Western Atlantic and GoMx was studied by Gurgel et al. (2004b) who found 10 haplotypes for Gracilaria tikvahiae spread in four lineages, including two associated with the Western and Eastern GoMx, respectively. As a final point, Cox1 has been a reliable marker for red macroalgae (Geraldino et al., 2009; Kim et al., 2010, 2012).
Materials and Methods
Study Area
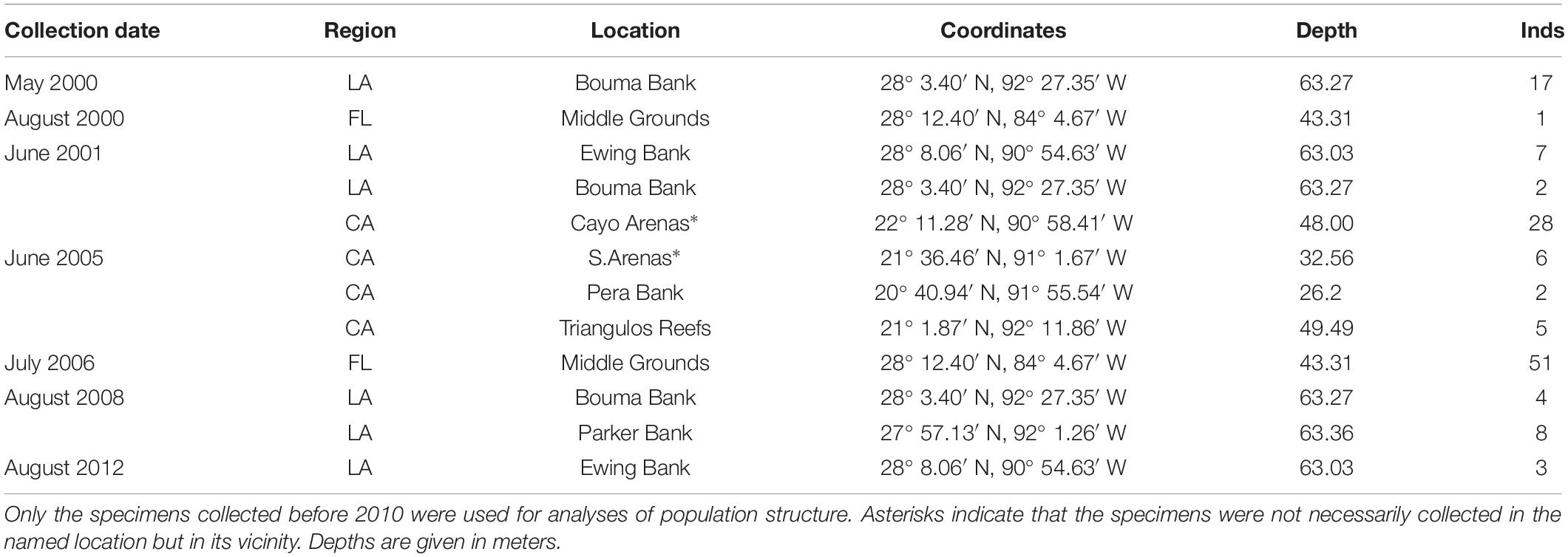
The population structure of Botryocladia occidentalis in the GoMx was inferred from DNA samples of 131 individuals collected during multiple research cruises between 2000 and 2008 (Table 1). The specimens were collected from offshore banks in three geographically distant regions (Felder and Camp, 2009): (1) offshore Louisiana in the NW Gulf comprising a system of hard banks, including salt domes (Rezak et al., 1985); (2) Florida Middle Grounds in the NE Gulf, a system of vermetid reefs formed by discontinuous limestone outcroppings that extend along the West Florida Shelf and are covered with shells, rocks, and corals (Cheney and Dyer, 1974; Reich et al., 2013); and the Campeche Banks, Mexico, in the SW Gulf, a system of tropical biogenic reefs raised in the Southern GoMx from a submerged limestone plateau (the Yucatan Shelf) with virtually no sedimentation from land runoff (Liddell, 2007; Mateo-Cid et al., 2013; Figure 2). These regions are ∼650 km away from each other and include most of the offshore banks and reefs surveyed across the GoMx (Fredericq et al., 2009).
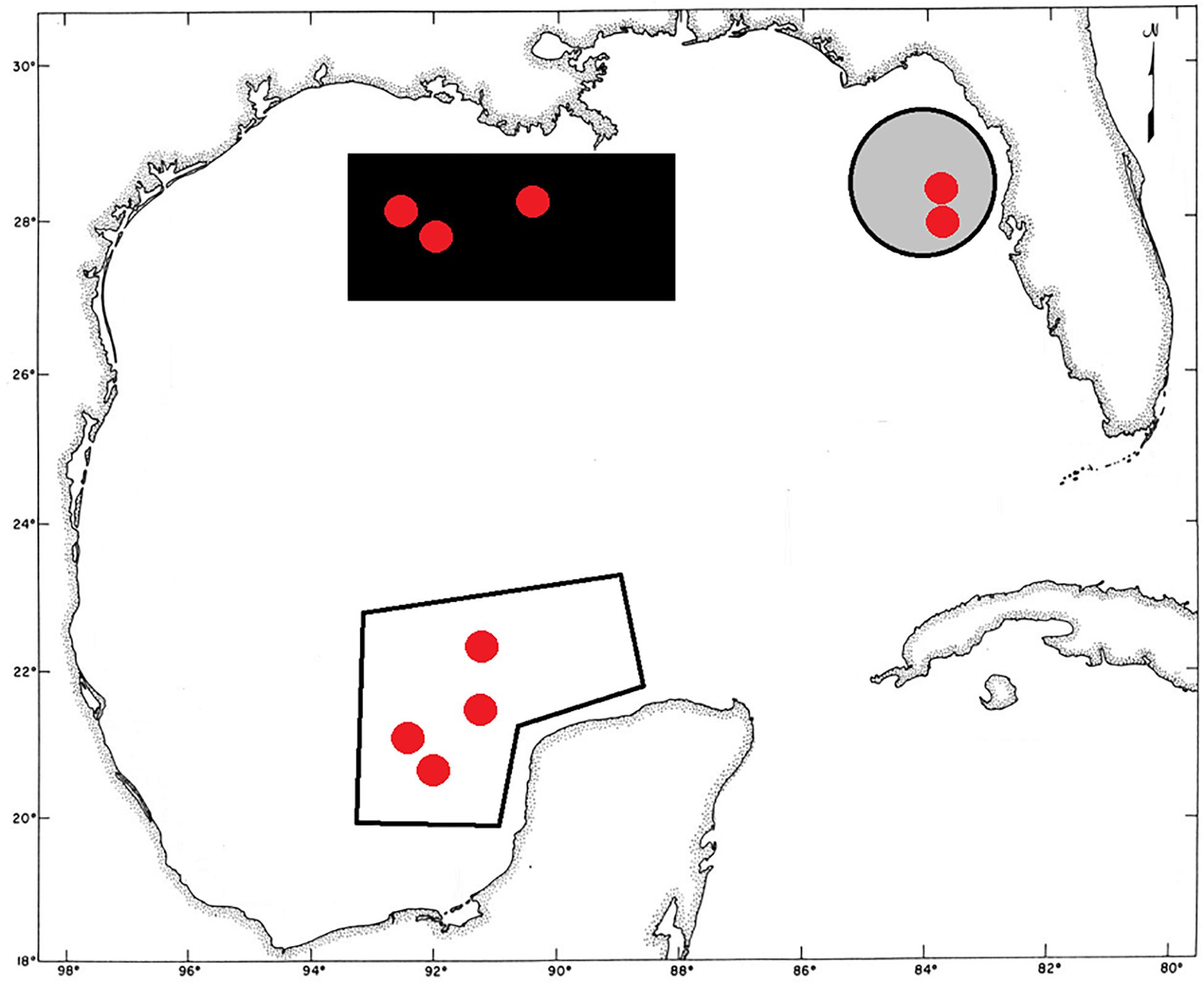
Figure 2. Map of the Gulf of Mexico (GoMx) indicating the three regions evaluated in this study which include most of the offshore banks and reefs surveyed across the basin. The southernmost region (white) corresponds to the Campeche Banks; the easternmost region corresponds to the Florida Middle Grounds (gray); and the NW region (black) corresponds to the offshore banks of Louisiana (black). The collection sites are presented as red points. The coordinate grid shows the position for even degrees of longitude and latitude; two degrees of longitude cover a distance of ∼201.5 km at the GoMx latitudes.
Sample Selection and DNA Extraction
Thirty-eight to fifty-one individuals were sequenced for each region, totaling 131 individuals. Three B. occidentalis specimens collected from offshore Louisiana during August 2012 were also available in the University of Louisiana at Lafayette Herbarium (LAF) but they were assigned to a different group (L2) in the population structure analyses since they came from a community exposed to the 2010 DWH (Ewing Bank, see Fredericq et al., 2014; Venera-Pontón et al., 2019) and may not represent the typical population structure of offshore Louisiana. These three individuals were the only B. occidentalis specimens collected during seven post-DWH research cruises conducted between 2010 and 2014 (personal observation during field trips by Venera-Pontón et al., 2019) despite their sampling effort (number of dredges launched) was comparable to other sampling campaigns conducted pre-DWH (see Venera-Pontón et al., 2019). These observations suggest a strong decrease in the B. occidentalis abundance offshore Louisiana post-DWH.
DNA samples were extracted from dried specimens of B. occidentalis in the University of Louisiana at Lafayette Herbarium (LAF). Specimens were selected with help of collection notes to prevent the DNA sequencing of individuals that were collected together in a same dredge; nevertheless, due to the limited number of specimens, this was not always feasible. DNA extractions followed a modified version of the protocol by Dellaporta et al. (1983). A ∼20 mg sample from each individual was ground to a fine powder with mortar and pestle, and treated with 700 μl of extraction buffer [100 mM Tris (pH 8.0), 50 mM EDTA, 500 mM NaCl, 10 mM 2-Mercaptoethanol], 50 μl of 20% SDS, 10 μl of 0.1 M DTT, and 4 mg of Proteinase K. During this treatment, the samples were incubated overnight at 65°C. To remove polysaccharides, samples were treated with 250 μl of potassium acetate (5 M), incubated on ice during 30 min., and centrifuged at 12,000 g for 30 min. 750 μl of supernatant were then mixed with 750 μl of chloroform and centrifuged at 12,000 g for 15 min, afterward, the supernatant was mixed again with an equal volume of chloroform and centrifuged for 15 min. again. The latter procedure was repeated once more and then, to precipitate the DNA, the supernatant was treated with isopropanol (two-thirds of the supernatant volume), incubated at −20°C overnight, and centrifuged the next day at 12,000 g for 30 min. Afterward, the pellet (which contains the precipitated DNA) was washed twice with 500 μl of 70% ethanol, dried in a Speed-Vac, re-suspended in 50 μl of elution buffer (10 mM Tris-Cl, pH 8.5), and stored at −20°C (Dellaporta et al., 1983).
DNA Sequencing and Data Analyses
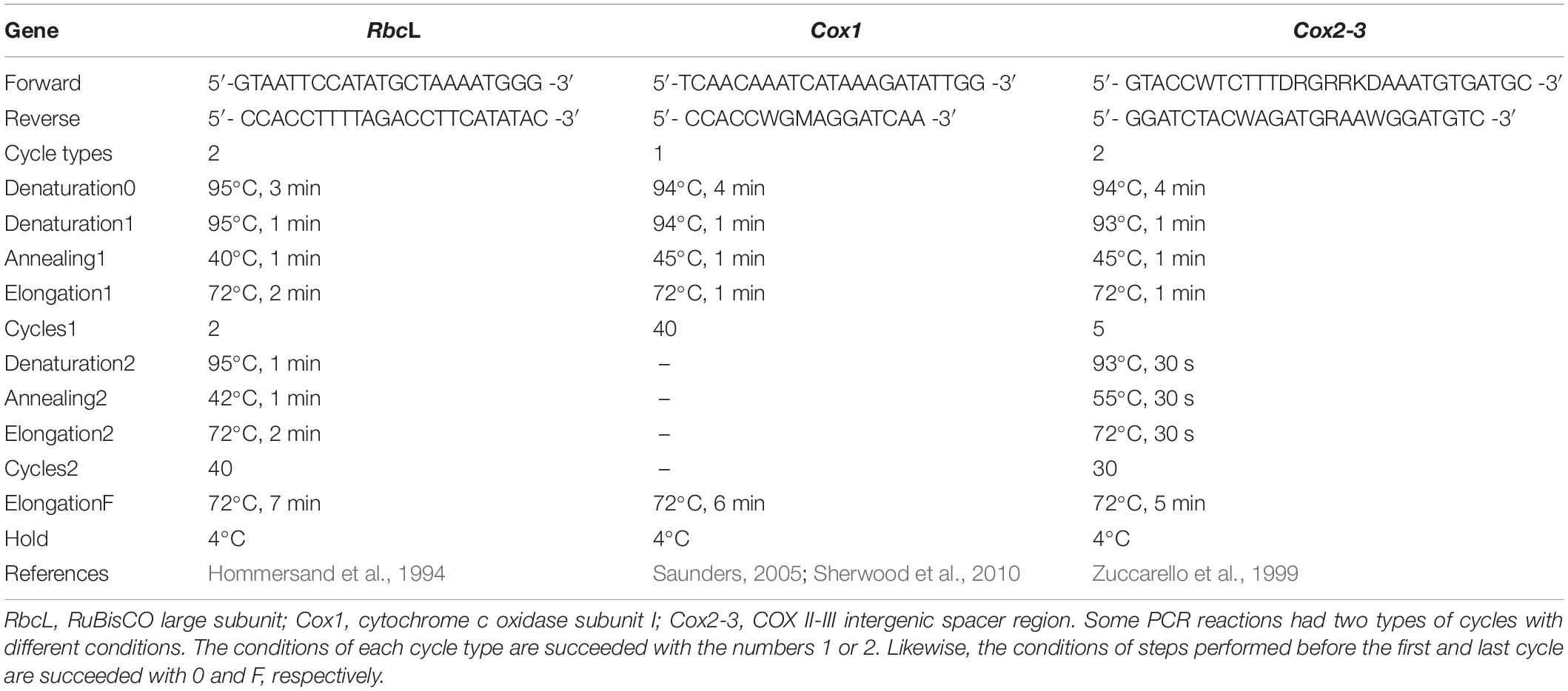
Three polymorphic regions were PCR-amplified from each DNA sample using the Mango-Taq DNA Polymerase Kit (BioLine, Taunton, MA, United States): the RuBisCO large subunit (rbcL, Gurgel et al., 2004a, b; Yang et al., 2008), cytochrome c oxidase subunit I (Cox1, Saunders, 2005; Sherwood et al., 2010), and the COX II-III intergenic spacer region (Cox2-3, Zuccarello et al., 1999). Cox1 and Cox2-3 are mitochondrial while rbcL is a chloroplast gene; all of them, except Cox2-3, are protein-coding regions. The primers and PCR conditions associated with each marker are described in Table 2. Preliminary analyses of rbcL sequences in the first 27 B. occidentalis individuals found three polymorphic sites in a region of ∼500 bp between the F57 and R577 primers, whereas no polymorphic sites were found outside this region. Consequently, rbcL amplifications of the remaining individuals only included the polymorphic region. PCR products were sequenced using the BigDye Terminator v3.1 Cycle Kit (Thermo Fisher Scientific, Grand Island, NY, United States). DNA sequences were aligned with CLUSTALX2 and used to define haplotypes (alleles) as well as their frequencies.
The best model of sequence evolution based on the Bayesian and Akaike information criteria (Posada and Buckley, 2004) and Maximum Likelihood (ML) phylogeny were inferred for each gene using MrAIC (Nylander, 2004) and MEGA6 (Tamura et al., 2013). Moreover, to determine whether each region represents a different sub-population or is part of a uniform meta-population, the allele frequencies and sequence divergences were compared among regions with exact tests of population differentiation (Raymond and Rousset, 1995) and Analyses of Molecular Variance (AMOVA; Excoffier et al., 1992) using Arlequin 3.5 (Excoffier et al., 2005). The AMOVA considers both the frequency and sequence divergence of alleles (Φst), while the exact test index is solely based on allele frequencies (Fst) (Balding et al., 2007). The latter used a 100,000 steps Markov Chain and 10,000 dememorization steps. The gene diversity (Nei) of each region was measured as the probability of obtaining different haplotypes in two randomly drawn individuals, i.e., heterozygosity (Balding et al., 2007).
The phylogeographic relationships of the alleles were evaluated with haplotype networks using Haplotype Viewer (Center for Integrative Bioinformatics Vienna1) which combines their micro-evolutionary history (ML phylogeny) with their regional frequencies. The statistical genetics analyses were separately performed on individual gene sequences as well as on the multigene alleles resulting from concatenating individual genes into single sequences. The distance between multigene haplotypes considered the best model of sequence evolution for each gene as well as the single model that best applied to every region (HKY); both approaches produced the same results and thus only one is presented.
To evaluate potential changes in population structure associated with the 2010 DWH, three individuals collected from Ewing Bank post-DWH were compared with specimens collected pre-DWH in offshore Louisiana using Likelihood Ratio tests (Hernández and Weir, 1989; Weir, 1992a, b) that included the three genes (multi-loci). This test was also applied to evaluate affinities between individuals collected from Louisiana post-DWH (L2) and other GoMx regions.
Results
Among the three genes evaluated, cytochrome c oxidase subunit I (Cox1) showed the highest intraspecific variability for Botryocladia occidentalis in the GoMx, with eight polymorphic sites and five haplotypes. Conversely, the COX II-III intergenic spacer region (Cox2-3) and the RuBisCO large subunit (rbcL) each showed three haplotypes based on two polymorphic sites. When individual genes were concatenated into multigene sequences, the number of haplotypes increased to 11, based on a total of 12 polymorphic sites. Details on the gene haplotypes and their geographical distribution in the GoMx regions are provided in Table 3.
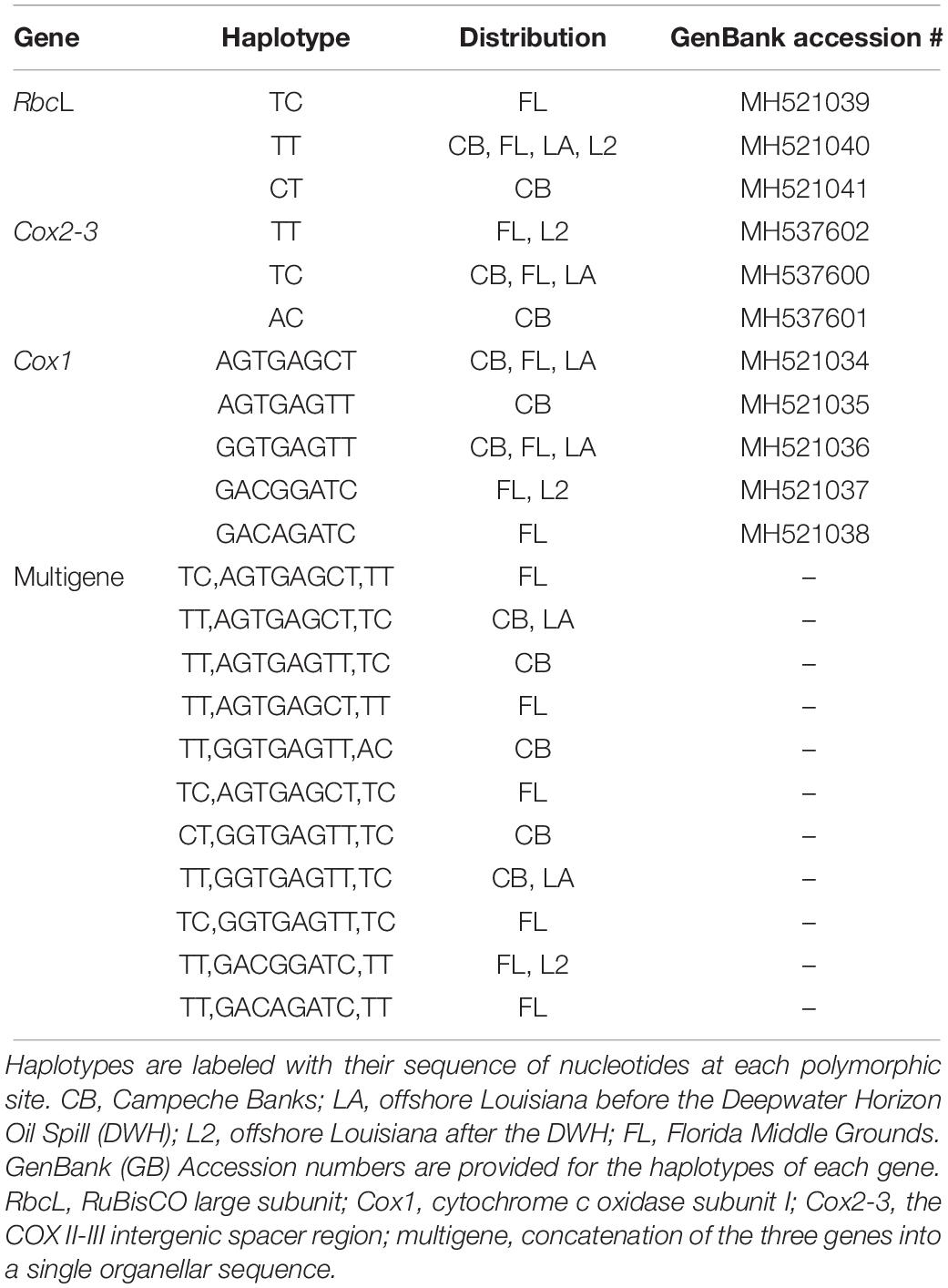
Table 3. Haplotypes of Botryocladia occidentalis and their regional distribution in the Gulf of Mexico.
All the genes supported the existence of two or more B. occidentalis populations in the GoMx (Table 4). All three genes supported the existence of a unique population in the Florida Middle Grounds (FL) that was greatly differentiated from the rest of the GoMx (rbcL Fst > 0.15; Cox1 and Cox2-3 Fst > 0.25). Cox1 and the multigene haplotypes supported the existence of distinct, highly differentiated populations (Fst > 0.25) in Campeche Banks (CB) and offshore Louisiana (LA) (Table 4).
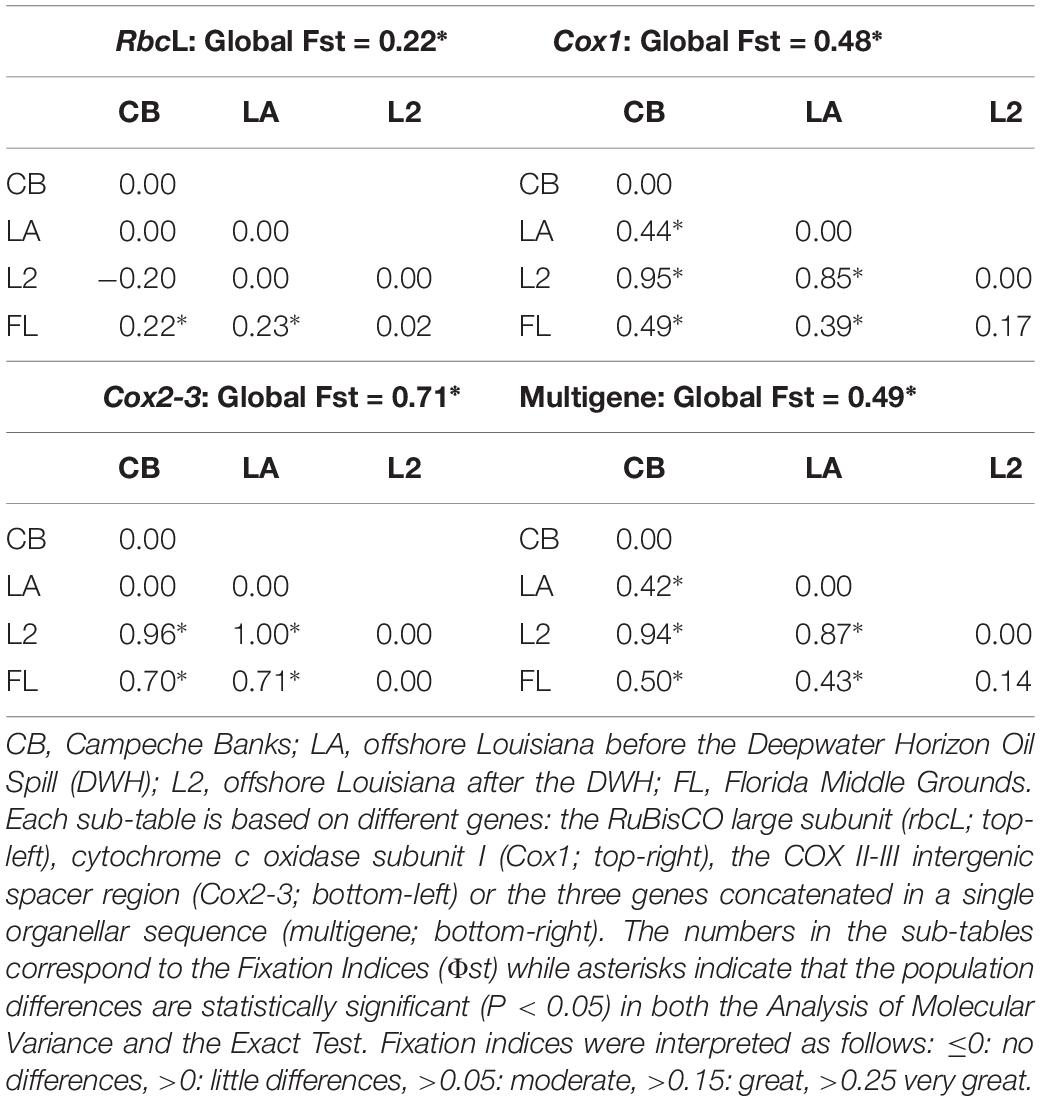
Table 4. Population differentiation of Botryocladia occidentalis in three distant regions of the Gulf of Mexico and before vs. after the Deepwater Horizon Oil Spill (DWH).
Three individuals collected after the DWH from offshore Louisiana (L2) showed statistically significant differences with individuals collected before the DWH in the same region (LA) (Table 4). For every gene, the three L2 individuals shared the same haplotype, which was typically absent in CB and LA but common in FL individuals (Table 3). The only exception to this pattern was rbcL, whose L2 haplotype was common across all the GoMx and thus did not show differences between L2 and any GoMx region. Nonetheless, rbcL showed statistically significant differences between FL and all the groups except L2. The affinity between FL and L2 was further supported by the Likelihood Ratio tests, which showed a P-value of 0.1563 for FL vs. L2 and a P-value < 0.001 for LA vs. L2.
Amongst all the GoMx regions, FL showed the largest gene diversity, regardless of the gene evaluated (Table 5). LA showed the second largest gene diversity with Cox1 and multigene sequences but presented a single haplotype (gene diversity = 0) with Cox2-3 and rbcL, respectively. Interestingly, CB showed more haplotypes than LA in all the genes but its gene diversity was low due to extreme unevenness in its haplotype frequencies. RbcL and Cox2-3 showed an equal number of haplotypes in FL and CB whereas Cox1 and multigene sequences showed more haplotypes in FL, followed by CB; finally, LA showed the lowest number of haplotypes in all the evaluated genes. All the LA haplotypes were present in other GoMx regions whereas some haplotypes from FL and CB were restricted to one region, especially FL before the DWH. Remarkably, none of the six multigene haplotypes of FL were found elsewhere in the GoMx before the DWH (Table 3 and Figure 3).
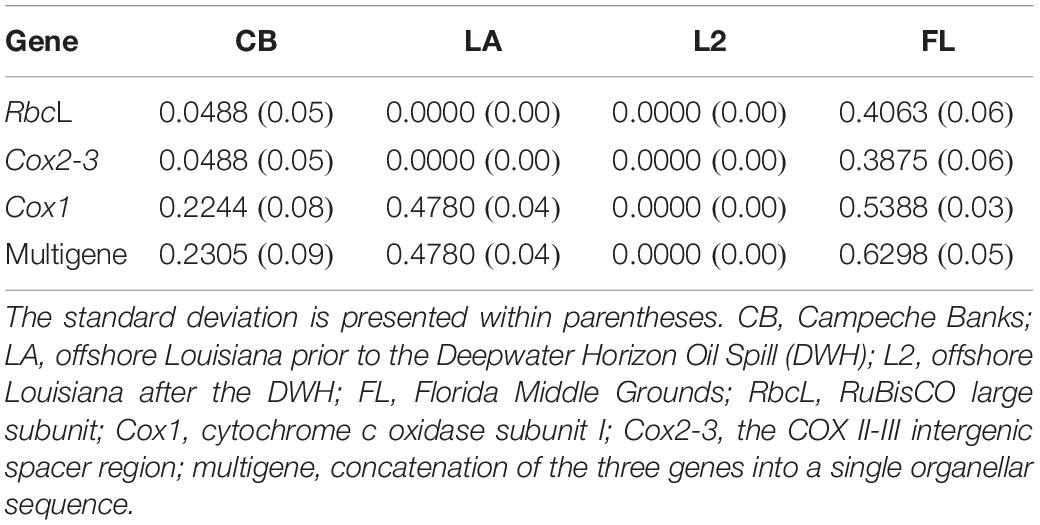
Table 5. Gene diversity (Nei) of Botryocladia occidentalis in three distant regions in the Gulf of Mexico, measured as the probability of obtaining different haplotypes in two randomly drawn individuals (heterozygosity).
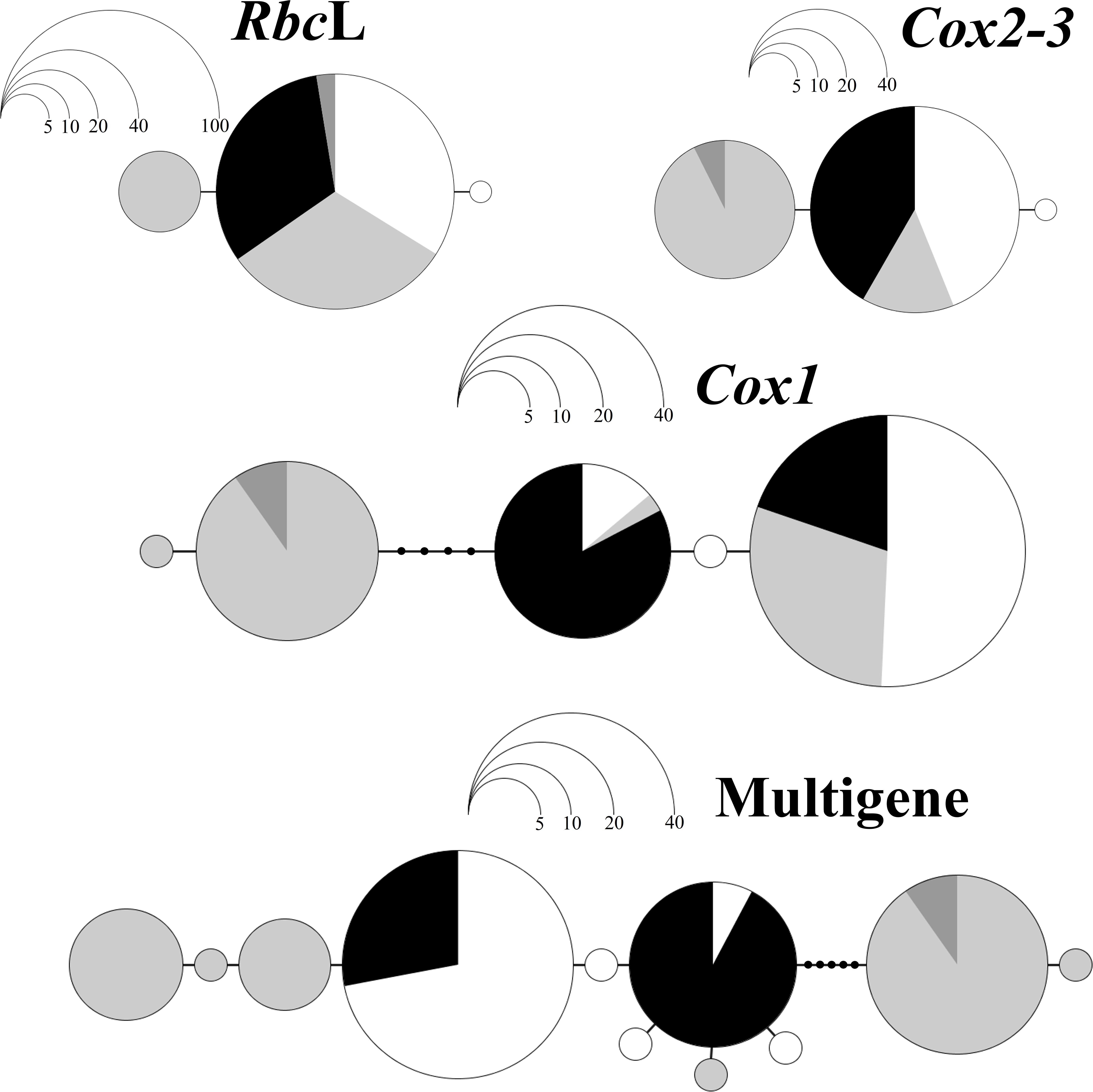
Figure 3. Haplotype Networks of Botryocladia occidentalis in three regions of the Gulf of Mexico. CB, Campeche Banks (white); LA, offshore Louisiana before the Deepwater Horizon Oil Spill (DWH; black); L2, offshore Louisiana after the DWH (dark-gray); FL, Florida Middle Grounds (light-gray). The networks are based on different genes: the RuBisCO large subunit (rbcL), the COX II-III intergenic spacer region (Cox2-3), cytochrome c oxidase subunit I (Cox1), or the three genes combined. Each circle represents an allele while their size indicate their abundance in number of individuals. The position of alleles in the diagram reflects their ML phylogeny: closely related alleles are next to each other while more divergent alleles are farther. The genetic divergence was calculated with the T92 model, except in the multigene network, which used the HKY model.
The haplotype networks of rbcL and Cox2-3 (Figure 3) consisted of simple schemes in which rarer haplotypes, restricted to one region (FL and CB, respectively), are connected to each other, phylogenetically, only by their relationship to the dominant haplotype of the entire GoMx. Conversely, the Cox1 network showed a more complex pattern with two main groups of phylogenetically connected haplotypes: the first group included haplotypes widespread in the entire GoMx and a rare haplotype from CB; the second group included two closely related haplotypes from FL that were relatively genetically divergent from the other group. Finally, the multigene scheme showed that all haplotypes from CB and LA are closely phylogenetically related and appear in the center of the network; the FL haplotypes, on the other hand, came from three separate lineages and include the most divergent alleles of the entire GoMx, which appear in the extremes of the network. Only one FL haplotype was closely related to alleles from LA and CB.
Discussion
Effectiveness of the Genetic Markers
The results of this study further confirm that Cox1 is an effective marker for evaluating population structure and intraspecific genetic diversity in red macroalgae (see Yang et al., 2008; Kim et al., 2010, 2012). The second most effective marker was Cox2-3 which did not separate CB and LA into distinct populations but otherwise showed the same results as Cox1. On the other hand, rbcL only detected the most extreme cases of population differentiation (i.e., FL vs. LA and FL vs. CB). Despite their lower effectiveness, using Cox2-3 and rbcL in the multigene sequences was essential to reveal the divergent origins of FL haplotypes, which was not clearly evident with Cox1 alone. The single-gene approaches found 3–5 haplotypes, depending on the marker, whereas the multigene approach found 11, including six FL haplotypes distributed in three separate lineages with the most divergent alleles of the entire GoMx. The latter was not clearly evident with single-gene approaches, highlighting once more the advantages of a multigene approach.
Population Structure of Botryocladia occidentalis in the Gulf of Mexico
Both the analyses of molecular variance (AMOVAs) and exact tests of population differentiation supported the existence of distinct populations in each of the regions evaluated. The Florida Middle Ground (FL) harbored the most divergent population of the GoMx which was identified even with the less polymorphic genes (rbcL and Cox2-3); conversely, individuals from Campeche Banks (CB) and offshore Louisiana (LA) were more closely related and only the most polymorphic gene (Cox1) and the multigene haplotypes were able to identify them as separated populations. The affinity between LA and CB was due to the fact that, regardless of the gene, all the LA haplotypes occur in CB too and are closely phylogenetically related to every CB haplotype. The latter is clearly evident in the multigene and Cox1 networks which shows CB and LA haplotypes closely and continuously connected toward the center of the plot. Conversely, the population differences between LA and CB are likely due to significant disparities in their allele frequencies.
By contrast, the population divergence of FL, from CB and LA, was due to the presence and relatively high frequencies of unique haplotypes in this region. FL haplotypes are not closely phylogenetically related to each other but instead are spread in the three most divergent lineages of the Cox1 and multigene network. Only one of the FL haplotypes was closely related to LA and CB alleles. This phylogeographic pattern may indicate a strong influence of migrants from outside the GoMx on the FL population. Those migrants would bring haplotypes that considerably diverge from local alleles as well as from each other. Conversely, the populations of CB and LA, represented by local haplotypes, would show the lowest divergence and more phylogenetic relatedness among alleles. Interestingly, the occurrence and relatively high frequencies of unique haplotypes in FL has been also observed in other organisms with planktonic dispersal such as corals (Studivan and Voss, 2018); in their study, one FL population (Pulley Ridge) was identified as a potential sink population with an alternate source population not shared by the other GoMx sites.
It is possible that FL haplotypes occur in other GoMx regions in their early life-stages (e.g., propagules) but are excluded from the substratum by regionally local haplotypes (intraspecific competition) and so their adults would not typically occur in the benthos. This is consistent with previously reported observations at the species level (not haplotypes) by Fredericq et al. (2014, 2019), Sauvage et al. (2016), and Krayesky-Self et al. (2017), who demonstrated the presence of early-life stages (e.g., propagules) inside the interior of rhodoliths of LA for macroalgal species whose adult stages (e.g., sporophytes) had never been reported in the in situ benthos. It is possible that under certain environmental conditions, propagules of FL haplotypes can succeed and reach “adulthood” in other GoMx regions.
The above hypothesis does not necessarily assume that CB and LA haplotypes have higher competitive abilities than FL alleles. CB and LA haplotypes may outcompete FL alleles, locally, simply by outnumbering them so that their frequency remains too low to be detected with the relatively small sample sizes of this study; the same may occur in FL if haplotypes from CB and LA are outnumbered by migrant haplotypes. Such pattern of haplotype disparities appears consistent with the activity of three important oceanographic forces: (1) the Louisiana Coastal Current (Wiseman et al., 2004; Jarosz and Murray, 2005), (2) the GoMx Loop (Sturges and Leben, 2000; Oey et al., 2005), and the Westward Yucatan Current (Martínez-López and Parés-Sierra, 1998; Ochoa et al., 2001).
The Louisiana Coastal Current (LCC) flows westwards from the Mississippi mouth toward Texas and Mexico but away from the Florida Continental Shelf (Wiseman et al., 2004; Jarosz and Murray, 2005) and has the potential to reduce the movement of propagules from LA to FL. Moreover, the LCC may be a key step in the flow of propagules (and their haplotypes) from LA toward CA. Conversely, the GoMx Loop flows northwards from the Yucatan Channel, moving most of the inflow from the Caribbean toward the Florida Continental Shelf and then loops east and south to finally leave the GoMx via the Florida straits. The GoMx loop is the strongest surface current of the NE Gulf and has the potential of moving a vast number of migrant propagules (with their haplotypes) from the Caribbean toward FL. Then, a relatively lower inflow of propagules from LA and CB would facilitate the dominance of migrant haplotypes in FL.
The Yucatan current, where the GoMx Loop originates, has a portion that flows westwards toward CB and loops northwards alongside the Mexican and US Gulf coasts. This current has the potential of moving migrant propagules to other regions of the GoMx where, unlike in FL, may be outcompeted by local haplotypes. Eddies originating from the GoMx Loop (Sturges and Leben, 2000; Oey et al., 2005) can also move FL haplotypes westwards. Nevertheless, it is possible that migrant haplotypes increase their abundances temporarily when local haplotypes are affected by disturbances. Importantly, the previous hypotheses on the role of migrant haplotypes should be considered with caution since many of their main assumptions warrant further research; for example, the relative proportion of haplotypes in the early-life stages of B. occidentalis has yet to be explored.
Finally, despite the fact that the three populations evaluated occur at different depths (Table 1), no correlation appears to occur between the pairwise population differentiations (Table 3) and depth differences. For example, the deepest (LA) and shallowest (CB) populations showed the lowest Fst whereas the population with intermediate depth (FL) showed the highest Fsts with LA and CB. Therefore, despite the lack of additional tools or data to further assess this factor, depth does not seem to be a crucial predictor of population structure for B. occidentalis in the GoMx.
Changes in Population Structure After the Deepwater Horizon Oil Spill
Three individuals collected from offshore LA after the DWH (L2) showed a haplotype that, before the disaster, had never been reported in LA or CB (Table 3 and Figure 3) but was found in FL. This finding was consistent for all the genes, except rbcL, in which the L2 haplotype was common in all the GoMx regions before the DWH. The analyses of molecular variance (AMOVAs), exact tests of population differentiation (Fst > 0.8 and P < 0.05), and Likelihood Ratio tests (P < 0.001) indicated a significant shift in haplotype frequencies in LA from before and after the DWH. For example, the Likelihood Ratio test indicated that, if L2 and LA are the same population, the probability of obtaining three FL haplotypes in the L2 individuals is nearly zero. Moreover, the LA vs. L2 Fst were supported by statistically significant P-values. The affinity between L2 and FL was also confirmed in all the analyses. L2 vs. FL Fsts were close to zero for rbcL and Cox2-3, and supported by P < 0.05. Conversely, Cox1 and the multigene, showed relatively large Fsts (∼0.15) for L2 vs. FL, but their P-values were lower than 0.05. The occurrence of FL haplotypes in LA after the DWH is greatly consistent with the hypothesis that early-life stages with FL haplotypes continuously occur in LA and CB but are typically outcompeted or outnumbered by local haplotypes until a disturbance allows for a change in haplotype frequencies.
Additional Considerations and Limitations
Due to the retrospective nature of this study, the research cruise specimen collections were not planned in function of our quantitative assessments of population structure. The pre-DWH specimens were collected during different sampling periods, between 2000 and 2008, at each region (Table 1); to group the specimens into three putative regional populations our study assumed that the allele frequencies within each region has been relatively stable between 2000 and 2008. This assumption is not unrealistic in our study area since no major disturbance appears to have impacted the natural dynamics of the GoMx between 2000 and 2008. Therefore, this study is still highly valuable for understanding the population structure of a common widespread GoMx macroalgal species in the context of the DWH. This type of assumption is necessary when the data are not ideal but can produce valuable information under certain considerations.
Likewise, due to the retrospective nature of this study, the use of herbarium specimens was our best possible approach. Such an approach may lead to pseudoreplication and frequency overestimation of haplotypes if fragments of the same specimen are treated as separate individuals; for that reason, we used the collection notes associated to each specimen to decide which individuals would be included in this study. We avoided specimens collected from the same dredge but, unfortunately, due to the limited number of specimens it was not always feasible. Despite this limitation, individuals sequenced from the same dredge often showed different haplotypes.
Finally, due to the decreased abundance of Botryocladia occidentalis offshore Louisiana after the DWH personal observations during field trips by Venera-Pontón et al. (2019), only three individuals were available for the analyses of the post-DWH population (L2) in spite that the sampling effort (number of dredges launched) of the post-DWH research cruises was comparable to the sampling campaigns conducted pre-DWH (see Venera-Pontón et al., 2019). Even with this extremely low sample size, the fact that none of the L2 individuals showed the Cox1 or Cox2-3 haplotypes collected offshore Louisiana between 2000 and 2008 (LA) is, by itself, strong evidence of a change in allele frequencies. Undersampling typically favors the exclusion of rare haplotypes (which require larger sampling efforts to be detected) and an overestimation of frequencies in the common haplotypes (which require a smaller effort to be detected); consequently, the fact that a previously undetected haplotype (absent in a sample of 41 individuals) occurred only in the three samples collected post-DWH is solid evidence that its frequency significantly increased. Since such change may be temporary, we encourage new studies to test whether the haplotypes found offshore Louisiana post-DWH are still found in the region or whether the pre-DWH haplotypes regained their frequency in the present Louisiana population. Nevertheless, the population changes occurred after the DWH may not necessarily be a consequence of crude oil contamination but could be caused by other DWH factors such as the release of Corexit oil dispersant (OSAT, 2010; Kujawinski et al., 2011) or the closure of fisheries for a prolonged period, temporarily increasing the abundance of fishes that graze on macroalgae. Likewise, it is a possibility too that these population changes were driven by factors unrelated to the DWH. Unfortunately, further assumptions or conclusions on the L2 population are very problematic due to the small sample size.
Data Availability Statement
The datasets generated for this study can be found in GenBank, MH521039, MH521040, and MH521041 (rbcL); MH537602, MH537600, and MH537601 (Cox2-3); MH521034, MH521035, MH521036, MH521037, and MH521038 (Cox1).
Author Contributions
DV-P conceived the study, conducted the laboratory work, and performed the data analyses. DV-P wrote the manuscript with contributions from SF and WS. All authors edited the manuscript before submission.
Funding
This work was funded by NSF grants DEB-0315995, DEB-1455569, DEB-1045690, and DEB-1754504, and the Coastal Water Consortium of the Gulf of Mexico Research Initiative (GoMRI-I), GoMRI-III.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Joseph Neigel, Darryl Felder, Caryl Chlan, and Thomas Sauvage for their valuable comments and suggestions during the planning and execution of this project, and undergraduate students Marcela Trochez, Victoria Marcil, and Adrian Oudomrath for their valuable assistance during the molecular lab work associated with this study. We greatly acknowledge support in part from NSF grants DEB-0315995, DEB-1455569, DEB-1045690, and DEB-1754504, and from the Coastal Water Consortium of the Gulf of Mexico Research Initiative (GoMRI-I), GoMRI-III and NSF RAPID grant DEB-1045690, following the Macondo oil spill. We also thank the crew of the R/V Pelican for their help with sampling protocols aboard ship.
Footnotes
References
Adey, W. H. (1998). Coral reefs: algal structured and mediated ecosystems in shallow, turbulent, alkaline waters. J. Phycol. 34, 393–406. doi: 10.1046/j.1529-8817.1998.340393.x
Arakaki, N., Suzuki, M., and Fredericq, S. (2014). Halarachnion (Furcellariaceae, Rhodophyta), a newly reported genus for the Gulf of Mexico, with the description of H. louisianensis, sp. nov. Phycol. Res. 62, 306–315. doi: 10.1111/pre.12065
Atlas, R. M., and Hazen, T. C. (2011). Oil biodegradation and bioremediation: a tale of the two worst spills in U.S. history. Environ. Sci. Technol. 45, 6709–6715. doi: 10.1021/es2013227
Balata, D., Piazzi, L., and Rindi, F. (2011). Testing a new classification of morphological functional groups of marine macroalgae for the detection of responses to stress. Mar. Biol. 158, 2459–2469. doi: 10.1007/s00227-011-1747-y
Balding, D. J., Bishop, M., and Cannings, C. (2007). Handbook of Statistical Genetics, 3rd Edn. Chichester: John Wiley & Sons.
Barron, M. G. (2012). Ecological impacts of the deepwater horizon oil spill: implications for immunotoxicity. Toxicol. Pathol. 40, 315–320. doi: 10.1177/0192623311428474
Baverstock, P. R., and Moritz, C. (1996). “Project design,” in Molecular Systematics, eds D. M. Hillis, C. Moritz, and B. K. Mable, (Sunderland MA: Sinauer Associates Inc.), 17–27.
Beyer, J., Trannum, H. C., Bakke, T., Hodson, P. V., and Collier, T. K. (2016). Environmental effects of the deepwater horizon oil spill: a review. Mar. Poll. Bull. 110, 28–51. doi: 10.1016/j.marpolbul.2016.06.027
Camacho, O., Sauvage, T., and Fredericq, S. (2018). Taxonomic transfer of Syringoderma to Microzonia (Syringodermataceae, Syringodermatales), including the new record M. floridana (E.C.Henry) comb. nov. in the Gulf of Mexico. Phycologia 57, 413–421. doi: 10.2216/17-51.1
Chaves, L. T. C., Pereira, P. H. C., and Feitosa, J. L. L. (2013). Coral reef fish association with macroalgal beds on a tropical reef system in North-eastern Brazil. Mar. Freshw. Res. 64, 1101–1111.
Chemello, R., and Milazzo, M. (2002). Effect of algal architecture on associated fauna: some evidence from phytal molluscs. Mar. Biol. 140, 981–990. doi: 10.1007/s00227-002-0777-x
Cheney, D. P., and Dyer, J. P. I. I. I. (1974). Deep-water benthic algae of the Florida middle grounds. Mar. Biol. 27, 185–190. doi: 10.1007/bf00391942
Dellaporta, S. L., Wood, J., and Hicks, J. B. (1983). A plant DNA mini-preparation: version II. Plant Mol. Biol. Rep. 1, 19–21. doi: 10.1007/bf02712670
Epifanio, C. E., Ditel, A. I., Rodriguez, R. A., and Target, T. E. (2003). The role of macroalgal beds as nursery habitat for juvenile blue crabs, Callinextes sapidus. J. Shellfish Res. 22, 881–886.
Excoffier, L. (2007). “Analysis of population subdivision,” in Handbook of Statistical Genetics, 3rd Edn, eds D. J. Balding, M. Bishop, and C. Cannings, (Hoboken, NJ: John Wiley & Sons, Ltd), 980–1020.
Excoffier, L., Laval, G., and Schneider, S. (2005). Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol. Bioinform. Online 1, 47–50.
Excoffier, L., Smouse, P., and Quattro, J. (1992). Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131, 479–491.
Felder, D. L., and Camp, D. K. (2009). Gulf of Mexico origin, Waters, and Biota, Biodiversity, Vol. 1. College Station, TX: Texas A&M University Press, 1393.
Felder, D. L., Thoma, B. P., Schmidt, W. E., Sauvage, T., Self-Krayesky, S., Chistoserdov, A., et al. (2014). Seaweeds and decapod crustaceans on Gulf deep banks after the Macondo Oil Spill. Bioscience 64, 808–819. doi: 10.1093/biosci/biu119
Fredericq, S., Arakaki, N., Camacho, O., Gabriel, D., Krayesky, D., Self-Krayesky, S., et al. (2014). A dynamic approach to the study of rhodoliths: a case study for the Northwestern Gulf of Mexico. Cryptog. Algol. 35, 77–98. doi: 10.7872/crya.v35.iss1.2014.77
Fredericq, S., Cho, T. O., Earle, S. A., Gurgel, C. F., Krayesky, D. M., Mateo-Cid, L. E., et al. (2009). “Seaweeds of the Gulf of Mexico,” in Gulf of Mexico: Origins, Waters, and Biota: Biodiversity, Vol. 1, eds D. L. Felder, and D. K. Camp, (College Station, TX: Texas A&M Press), 187–259.
Fredericq, S., Krayesky-Self, S., Sauvage, T., Richards, J., Kittle, R., Arakaki, N., et al. (2019). The critical importance of rhodoliths in the life cycle completion of both macro- and microalgae, and as holobionts for the establishment and maintenance of marine biodiversity. Front. Mar. Sci. 5:502. doi: 10.3389/fmars.2018.00502
Gavio, B., and Fredericq, S. (2003). Botryocladia caraibicia sp. nov. (Rhodymeniales, Rhodophyta), a new species from the Caribbean. Cryptog. Algol. 24, 93–106.
Gavio, B., and Fredericq, S. (2005). New species and new records of offshore members of the Rhodymeniales (Rhodophyta) in the northern Gulf of Mexico. Gulf Mexico Sci. 23, 58–83.
Gavio, B., Hickerson, E., and Fredericq, S. (2005). Platoma chrysymenioides sp. nov. (Schizymeniaceae), and Sebdenia integra sp. nov. (Sebdeniaceae), two new red algal species from the northwestern Gulf of Mexico, with a phylogenetic assessment of the Cryptonemiales complex (Rhodophyta). Gulf Mexico Sci. 23, 38–57.
Geraldino, P. J., Yang, E. C., Kim, M. S., and Boo, S. M. (2009). Systematics of Hypnea asiatica sp. nov. (Hypneaceae, Rhodophyta) based on morphology and nrDNA SSU, plastid rbcL, and mitochondrial cox1. Taxon 57, 606–616. doi: 10.1002/tax.582023
Gurgel, C. F. D., Fredericq, S., and Norris, J. N. (2004a). “Molecular systematics and taxonomy of flattened species of Gracilaria Greville (Gracilariaceae, Gracilariales, Rhodophyta) from the Western Atlantic,” in Taxonomy of Economic Seaweeds IX (with reference to the Pacific and other locations), eds I. A. Abbott, and K. McDermid, (At Honolulu: University of Hawaii), 159–199.
Gurgel, C. F. D., Fredericq, S., and Norris, J. N. (2004b). Phylogeography of Gracilaria tikvahiae (Gracilariaceae, Rhodophyta): a study of genetic discontinuity in a continuously distributed species based on molecular evidence. J. Phycol. 40, 748–758. doi: 10.1111/j.1529-8817.2004.03070.x
Hasan, M. R., and Chakrabarti, R. (2009). Use of Algae and Aquatic Macrophytes as Feed in Small-Scale Agriculture: a Review. FAO Fisheries and Agriculture Technical Paper 532. Rome: Food and Agriculture Organization of the United Nations.
Hernández, J. L., and Weir, B. S. (1989). A disequilibrium coefficient approach to Hardy-Weinberg testing. Biometrics 45, 53–70.
Hernández-Kantún, J. J., Riosmena-Rodriguez, R., Adey, W. H., and Rindi, F. (2014). Analysis of the cox2-3 spacer region for population diversity and taxonomic implications in rhodolith-forming species (Rhodophyta: Corallinales). Phytotaxa 190, 331–354.
Hommersand, M. H., Fredericq, S., and Freshwater, D. W. (1994). Phylogenetic systematics and biogeography of the Gigartinaceae (Gigartinales, Rhodophyta) based on sequence analysis of rbcL. Bot. Mar. 37, 193–203.
Hurd, C. L., Harrison, P. J., Bischof, K., and Lobban, C. S. (2014). Seaweed Ecology and Physiology, 2nd Edn. Cambridge: Cambridge University Press, 562.
Jarosz, E., and Murray, S. P. (2005). “Velocity and transport characteristics of the Louisiana-Texas coastal current,” in Circulation in the Gulf of Mexico: Observations and Models, eds W. Sturges, and A. Lugo-Fernandez, (Washington DC: American Geophysical Union), doi: 10.1029/161GM11
Kim, K. M., Hoarau, G. G., and Boo, S. M. (2012). Genetic structure and distribution of Gelidium elegans (Gelidiales, Rhodophyta) in Korea based on mitochondrial cox1 sequence data. Aquat. Bot. 98, 27–33. doi: 10.1016/j.aquabot.2011.12.005
Kim, S. Y., Weinberger, F., and Boo, S. M. (2010). Genetic diversity hints at a common donor region of the invasive Atlantic and Pacific populations of Gracilaria vermiculophylla (Rhodophyta). J. Phycol. 46, 1346–1349. doi: 10.1111/j.1529-8817.2010.00905.x
Krayesky, D. M., Norris, J. N., Gabrielson, P. W., Gabriel, D., and Fredericq, S. (2009). A new order of crustose red algae based on the Peyssonneliaceae, with an evaluation of the ordinal classification of the Florideophyceae (Rhodophyta). Proc. Biol. Soc. Wash. 123, 364–391. doi: 10.2988/08-43.1
Krayesky-Self, S., Schmidt, W. E., Phung, D., Henry, C., Sauvage, T., Camacho, O., et al. (2017). Eukaryotic life inhabits rhodolith-forming coralline algae (Hapalidiales, Rhodophyta), remarkable marine benthic microhabitats. Sci. Rep. 7:45850. doi: 10.1038/srep45850
Kujawinski, E. B., Kido Soule, M. C., Valentine, D. L., Boysen, A. K., Longnecker, K., and Redmont, M. C. (2011). Fate of dispersants associated with the deepwater horizon oil spill. Environ. Sci. Technol. 45, 1298–1306. doi: 10.1021/es103838p
Lehr, B., Bristol, S., and Possolo, A. (2010). Oil Budget Calculator: Deepwater Horizon. Washington DC: Federal Interagency Solutions Group, Oil Budget Calculator Science and Engineering Team, 217.
Leliaert, F., Tronholm, A., Lemieux, C., Turmel, M., DePriest, M. S., Fredericq, S., et al. (2016). Chloroplast phylogenomic analyses reveal the deepest-branching lineage of the Chlorophyta, Palmophyllophyceae class. nov. Sci. Rep. 6:25367. doi: 10.1038/srep25367
Liddell, W. D. (2007). “Origin and geology,” in Coral Reefs of the Southern Gulf of Mexico, eds J. W. Tunnell, E. A. Chávez, and K. Withers, (College Station, TX: Texas A&M University Press), 23–33.
Littler, M. M., and Littler, D. S. (1984). Models of tropical reef biogenesis: the contribution of algae. Prog. Phycol. Res. 3, 323–364.
Liu, Y., Weisberg, R. H., Hu, C., and Zheng, L. (2011). Tracking the deepwater horizon oil spill: a modeling perspective. Eos Trans. AGU 92, 45–52.
Martínez-López, B., and Parés-Sierra, A. (1998). Circulation in the Gulf of Mexico induced by tides, wind and the Yucatan Current. Cienc. Mar. 24, 65–93. doi: 10.7773/cm.v24i1.740
Mateo-Cid, L. E., Mendoza-González, A. C., and Fredericq, S. (2013). A checklist of subtidal seaweeds from Campeche Banks, Mexico. Acta Bot. Venez. 36, 92–108.
Milne, R., and Griffiths, C. (2014). Invertebrate biodiversity associated with algal turfs on a coral-dominated reef. Mar. Biodiv. 44, 181–188. doi: 10.1007/s12526-013-0199-7
Nei, M. (1978). Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89, 583–590.
Núñez-Resendiz, M. L., Zuccarello, G. C., Dreckmann, K. M., and Sentíes, A. (2017). Phylogeography of Hydropuntia cornea/Hydropuntia usneoides complex (Gracilariales, Rhodophyta) in the Yucatan Peninsula. Phycologia 56, 14–20. doi: 10.2216/16-46.1
Nylander, J. A. A. (2004). MrAIC.pl. Program Distributed by the Author. Uppsala: Uppsala University.
Ochoa, J., Sheinbaum, H., Badan, A., Candela, J., and Wilson, D. (2001). Geostrophy via potential vorticity inversion in the Yucatan Channel. J. Mar. Res. 59, 725–747. doi: 10.1357/002224001762674917
O’Doherty, D. C., and Sherwood, A. R. (2007). Genetic population structure of the Hawaiian alien invasive seaweed Acanthophora spicifera (Rhodophyta) as revealed by DNA sequencing and ISSR analyses. Pac. Sci. 61, 223–233. doi: 10.2984/1534-6188(2007)61%5B223:gpsoth%5D2.0.co;2
Oey, L.-Y., Ezer, T., and Lee, H.-C. (2005). “Loop current, rings and related circulation in the Gulf of Mexico: a review of numerical models and future challenges,” in Geophysical Monograph Ser, Vol. 161, eds W. Sturges, and A. Lugo-Fernandez, (Washington DC: American Geophysical Union), 31–56. doi: 10.1029/161gm04
OSAT, (2010). Summary Report of Sub-Sea And Sub-Surface Oil and Dispersant Detection: Sampling and Monitoring. Operational Science Advisory Team Report 1. New Orleans: OSAT, 131.
OSAT, (2011). Summary Report for Fate and Effects of Remnant Oil in the Beach Environment. Operational Science Advisory Team Report 2. New Orleans: OSAT, 36.
Paris, C. B., Hénaff, M. L., Aman, Z. M., Subramaniam, A., Helgers, J., Wang, D. P., et al. (2012). Evolution of the Macondo well blowout: simulating the effects of the circulation and synthetic dispersants on the subsea oil transport. Environ. Sci. Technol. 46, 13293–13302. doi: 10.1021/es303197h
Posada, D., and Buckley, T. R. (2004). Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst. Biol. 53, 793–808. doi: 10.1080/10635150490522304
Rabalais, N. (2014). Assessing early looks at biological responses to the macondo event. BioScience 64, 757–759. doi: 10.1093/biosci/biu132
Raymond, M., and Rousset, F. (1995). An exact test for population differentiation. Evolution 49, 1280–1283. doi: 10.1111/j.1558-5646.1995.tb04456.x
Reich, C. D., Poore, R. Z., and Hickey, T. D. (2013). The role of vermetid gastropods in the development of the Florida Middle Ground, northeast Gulf of Mexico. J. Coast. Res. 63, 46–57. doi: 10.2112/si63-005.1
Rezak, R., Bright, T. J., and McGrail, D. M. (1985). Reefs and Banks of the Northwestern Gulf of Mexico: Their Geological, Biological, and Physical Dynamics. New York, NY: John Wiley & Sons.
Richards, J., and Fredericq, S. (2018). Sporolithon sinusmexicanum sp. nov. (Sporolithales, Rhodophyta): a new rhodolith-forming coralline species from deepwater rhodolith beds in the Gulf of Mexico. Phytotaxa 350, 135–146.
Richards, J., Vieira-Pinto, T., Schmidt, W. E., Sauvage, T., Gabrielson, P. W., Oliveira, M. C., et al. (2016). Molecular and morphological diversity of Lithothamnion spp. rhodoliths (Hapalidiaceae, Hapalidiales) from deepwater rhodolith beds in the northwestern Gulf of Mexico. Phytotaxa 278, 81–114.
Richards, J. L., Gabrielson, P. W., and Fredericq, S. (2014). New insights into the genus Lithophyllum (Lithophylloideae, Corallinaceae, Corallinales) from offshore the NW Gulf of Mexico. Phytotaxa 190, 162–175.
Ruzzante, D. E. (1998). A comparison of several measures of genetic distance and population structure with microsatellite data: bias and sampling variance. Can. J. Fish. Aquat. Sci. 55, 1–14. doi: 10.1139/cjfas-55-1-1
Saunders, G. W. (2005). Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Philos. Trans. R. Soc. B Biol. Sci. 360, 1879–1888. doi: 10.1098/rstb.2005.1719
Sauvage, T., Schmidt, W. E., Suda, S., and Fredericq, S. (2016). A metabarcoding framework for facilitated survey of endolithic phototrophs with tuf A. BMC Ecol. 16:8. doi: 10.1186/s12898-016-0068-8
Schmidt, W. E., Gurgel, C. F. D., and Fredericq, S. (2016). Taxonomic transfer of the red algal genus Gloiosaccion to Chrysymenia (Rhodymeniaceae, Rhodymeniales), including the description of a new species, C. pseudoventricosa, for the Gulf of Mexico. Phytotaxa 243, 54–70.
Schmidt, W. E., Lozada-Troche, C., Ballantine, D. L., Arakaki, N., Norris, J. N., Gabriel, D., et al. (2017). Taxonomic transfer of Chrysymenia enteromorpha and C. wrightii to Botryocladia (Rhodymeniaceae, Rhodymeniales, Rhodophyta). Phytotaxa 324, 122–138.
Shaal, G., Leclerc, J. C., Droual, G., Leroux, C., and Riera, P. (2016). Biodiversity and trophic structure of invertebrate assemblages associated with understorey red algae in a Laminaria digitata bed. Mar. Biol. Res. 12, 513–523. doi: 10.1080/17451000.2016.1164318
Sherwood, A. R., Sauvage, T., Kurihara, A., Conklin, K. Y., and Presting, G. G. (2010). A comparative analysis of COI, LSU and UPA marker data for the Hawaiian florideophyte Rhodophyta: implications for DNA barcoding of red algae. Crypt. Algol. 31, 451–465.
Studivan, D. S., and Voss, J. D. (2018). Population connectivity among shallow and mesophotic Montastraea cavernosa corals in the Gulf of Mexico identifies potential for refugia. Coral Reefs 37, 1183–1196. doi: 10.1007/s00338-018-1733-7
Sturges, W., and Leben, R. (2000). Frequency of ring separations from the loop current in the Gulf of Mexico: a revised estimate. J. Phys. Oceanogr. 30, 1814–1819. doi: 10.1175/1520-0485(2000)030<1814:forsft>2.0.co;2
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Tan, J., Lim, P.-E., Phang, S.-M., Hong, D. D., Sunarpi, H., and Hurtado, A. Q. (2012). Assessment of four molecular markers as potential DNA barcodes for red algae Kappaphycus Doty and Eucheuma J. Agardh (Solieriaceae, Rhodophyta). PLoS One 7:e52905. doi: 10.1371/journal.pone.0052905
Thomsen, M. S. (2010). Experimental evidence for positive effects of invasive seaweed on native invertebrates via habitat-formation in a seagrass bed. Aquat. Invas. 5, 341–346. doi: 10.3391/ai.2010.5.4.02
Venera-Pontón, D., Schmidt, W. E., and Fredericq, S. (2019). Structure of the mesophotic macroalgal communities from offshore hard banks in Louisiana before and after the 2010 deepwater horizon oil spill. J. Oceanogr. Mar. Res. 7:194. doi: 10.24105/2572-3103.1000194
Vergés, A., Bennett, S., and Bellwood, D. R. (2012). Diversity among macroalgal-consuming fishes on coral reefs: a transcontinental comparison. PLoS One 7:e45543. doi: 10.1371/journal.pone.0045543
Weir, B. S. (1992b). Population genetics in the forensic DNA debate. Proc. Natl. Acad. Sci. U.S.A. 89, 11654–11659. doi: 10.1073/pnas.89.24.11654
Wiseman, W. J., Turner, R. E., Justic, D., and Rabalais, N. N. (2004). “Hypoxia and the physics of the Louisiana coastal current,” in Dying and Dead Seas-Climatic Versus Anthropic Causes. NATO Science Series IV: Earth and Environmental Sciences, Vol. 36, eds J. C. J. Nihoul, P. O. Zavialov, and P. P. Micklin, (Dordrecht: Springer), 384.
Yang, E. C., Kim, M. S., Geraldino, P. J. L., Sahoo, D., Shin, J.-A., and Boo, S. M. (2008). Mitochondrial cox1 and plastid rbcL genes of Gracilaria vermiculophylla (Gracilariaceae, Rhodophyta). J. Appl. Phycol. 20, 161–168.
Zuccarello, G. C., Burger, G., West, J. A., and King, R. J. (1999). A mitochondrial marker for red algal intraspecific relationships. Mol. Ecol. 8, 1443–1447. doi: 10.1046/j.1365-294x.1999.00710.x
Zuccarello, G. C., Critchley, A. T., Smith, J., Sieber, V., Lhonneur, G. B., and West, J. A. (2006). Systematics and genetic variation in commercial Kappaphycus and Euchema (Solieriaceae, Rhodophyta). J. Appl. Phycol. 18, 643–651. doi: 10.1007/s10811-006-9066-2
Keywords: Macondo oil spill, genetic diversity, mesophotic, seaweeds, population genetics, molecular ecology, offshore Louisiana
Citation: Venera-Pontón DE, Schmidt WE and Fredericq S (2019) Population Structure of the Red Macroalga Botryocladia occidentalis (Børgesen) Kylin (Rhodymeniaceae, Rhodymeniales) in the Gulf of Mexico Before the Deepwater Horizon Oil Spill. Front. Mar. Sci. 6:652. doi: 10.3389/fmars.2019.00652
Received: 13 February 2019; Accepted: 04 October 2019;
Published: 18 October 2019.
Edited by:
Daniel M. Holstein, Louisiana State University, United StatesReviewed by:
José M. Rico, Universidad de Oviedo, SpainMichael S. Studivan, Florida Atlantic University, United States
Copyright © 2019 Venera-Pontón, Schmidt and Fredericq. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dagoberto E. Venera-Pontón, dagovenera@gmail.com; Suzanne Fredericq, slf9209@louisiana.edu
 Dagoberto E. Venera-Pontón
Dagoberto E. Venera-Pontón William E. Schmidt
William E. Schmidt Suzanne Fredericq
Suzanne Fredericq