Seasonal Photosynthesis, Respiration, and Calcification of a Temperate Maërl Bed in Southern Portugal
- 1CCMAR—Centre of Marine Sciences, University of Algarve, Faro, Portugal
- 2Portuguese Institute for the Ocean and Atmosphere (IPMA), DivRP, Olhão, Portugal
Rhodolith (maërl) beds are biodiversity hotspots with a worldwide distribution. Maërl is the general term for free-living non-geniculate rhodoliths or coralline red algae. In southern Portugal, maërl beds are mainly composed of Phymatolithon lusitanicum, recently identified as a new species and commonly misidentified as Phymatolithon calcareum. Photosynthesis, respiration, and growth rates of the algae were measured seasonally, as well as the photosynthetic pigment composition. To characterize the seasonal and interannual patterns of key abiotic conditions in the largest described maërl bed of the Portuguese coast, temperature, irradiance, and dissolved oxygen were continuously monitored over a 2-year period. At the bed depth (22 m), temperature ranged between 14°C in winter and 24°C in summer, irradiance varied from 5 to 75 μmol m–2 s–1, and dissolved oxygen from 5.8 to 7.25 mg O2 L–1. We found a strong linear relationship (r2 = 0.95) between gross primary production (GPP) and relative electron transport rates (rETRs). Both methods led to similar results and an average molar ratio of 0.24. Photosynthesis and respiration increased in summer and decreased in autumn and winter. In the summer of 2013, the growth rates were twofold higher (1.34 μmol CaCO3 g–1 day–1) than in the other seasons. In winter and spring, to compensate for light deprivation and low temperature, algae increased their chlorophyll a and carotenoid concentrations while also decreasing their phycobilin concentration, in this case probably due to nutrient limitation. To isolate the role of temperature on the algae’s metabolism, the photosynthetic and respiration rates of individual thalli were measured at eight different temperatures in the laboratory (from 12°C to 26°C). Phymatolithon lusitanicum photosynthesis increased twofold after a threshold of 18°C (from 2.2 at 18°C to 3.87 μmol O2 m–2 s–1 at 20°C), whereas respiration increased fourfold with temperature after a threshold of 22°C (from −0.38 at 18°C to −1.81 μmol O2 m–2 s–1 at 24°C). The significant increases on respiration, photosynthetic rates, and maximum growth with temperature reveal that the metabolic rates of P. lusitanicum are highly sensitive to ocean warming.
Introduction
Rhodolith beds are biodiversity spots, globally distributed from the poles to the tropics (Foster et al., 2013). Maërl is a type of rhodolith and the general term used for free-living non-geniculate coralline algae (Bosence, 1976). Rhodolith beds support complex trophic chains and are important carbonate deposits (Steller and Cáceres-Martinez, 2009; van der Heijden and Kamenos, 2015; Hernandez-Kantun et al., 2017; Schoenrock K. et al., 2018). However, research on maërl ecosystems is globally scarce, and the full range of maërl forming species is yet to be listed, which hampers the protection of these habitats (Barberá et al., 2003). In the northeast Atlantic, a few maërl beds are protected and, over the next decade, declines due to fishing activities, mariculture, and commercial extraction will increase (Hall-Spencer et al., 2010). While information on the metabolism of northern beds already exists for Canada (Halfar et al., 2013), Greenland (Schoenrock K. et al., 2018), and France (e.g., Martin et al., 2006, 2007), very little is known about Portuguese maërl beds (Hall-Spencer et al., 2010; Peña and Bárbara, 2013; Brodie et al., 2014; Carro et al., 2014), and only one publication reports the effects of temperature and irradiance on the maërl community (Peña and Bárbara, 2010).
Temperature and irradiance exert determinant effects on the primary production and calcification of maërl (Martin et al., 2006; Peña and Bárbara, 2010; Halfar et al., 2013). Studies on these effects have been conducted elsewhere (e.g., Freiwald and Henrich, 1994 in Norway; Blake and Maggs, 2003 in Ireland; Martin et al., 2006, 2007 in France; Steller et al., 2007 in Baja California; Amado-Filho et al., 2012 in Brazil; Burdett et al., 2012 in Scotland; Adey et al., 2015 in Canada; Schoenrock K.M. et al., 2018 in Greenland), but not yet in southern Europe. The most common methods to assess the metabolism and physiology of maërl beds are in situ benthic incubations (e.g., Martin et al., 2007) and PAM fluorescence (e.g., Burdett et al., 2012; Schoenrock K.M. et al., 2018). In addition, Attard et al. (2015) reported one case where the eddy covariance technique was used to measure the benthic oxygen fluxes in a maërl bed. Seasonal changes in temperature and irradiance regulate the photosynthesis, respiration, and calcification of coralline algae (Martin et al., 2006, 2007, 2013a; Halfar et al., 2013; Egilsdottir et al., 2016; Schoenrock K.M. et al., 2018), but there is scarce information on the interannual variability of environmental factors or on that of biological indicators such as photosynthetic pigments. Because of their annual growth increments and cycling of Mg content with temperature, coralline algae hold a potential to become a high-resolution climate archive that will enhance our understanding of climatic variation (Adey et al., 2015).
Under increasing acidity and temperature, maërl beds are expected to suffer severe impacts worldwide (Hall-Spencer et al., 2010; Foster et al., 2013; Martin and Hall−Spencer, 2017). However, the diversity in results among different experimental studies with positive, negative, and parabolic metabolic responses to ocean acidification (OA) and warming (Martin et al., 2013b) makes it difficult to predict the future outcome of these habitats. Divergences in results may be species specific or related to the interaction with other environmental variables such as temperature (Vásquez-Elizondo and Enríquez, 2016) and irradiance (Gao and Zheng, 2010) that modify the effect of OA on coralline algae (see Martin et al., 2013b for a review). Even if it is essential to apply realistic environmental conditions on OA assays, most experiments are not accompanied by a monitoring of the natural communities on the field, and there is an important gap of information on community-scale studies (McCoy and Kamenos, 2015).
Ocean acidification and warming will negatively affect the maërl beds from the northeastern Atlantic first, as aragonite saturation decreases, while Lusitanian maërl beds are expected to persist (Brodie et al., 2014). In Algarve, maërl beds are primarily composed of the unattached and non-geniculate coralline red algae Phymatolithon lusitanicum, recently described as a new species and commonly misidentified as Phymatolithon calcareum (Peña et al., 2015). Lithothamnion corallioides and P. calcareum are also present in the maërl beds from Algarve, but their occurrence is uncommon (Carro et al., 2014; Peña et al., 2015). Both species are abundant in Brittany and the British Isles, but they are being replaced by P. lusitanicum in Galicia (NW Spain) (Carro et al., 2014). In the Iberian Peninsula, P. lusitanicum is abundant in Galicia (4–13 m) and Algarve (15–25 m). The largest P. lusitanicum individuals with the thickest morphology in the Iberian Peninsula have been found in Algarve (Carro et al., 2014; P. lusitanicum is referred to as Phymatolithon sp3 in Peña et al., 2015). This species has also been identified in Northern Ireland (from the intertidal to 4 m), the Alborán Sea (40–48 m), and in the Balearic Islands (54–64 m) (Peña et al., 2015).
The information currently available on P. lusitanicum is restricted to descriptive studies of its morphology and the composition of the beds (Carro et al., 2014; Peña et al., 2015), associated flora (Peña and Bárbara, 2010, 2013), and the effect of high CO2 and warming on the photosynthesis, calcification, and respiration (Sordo et al., 2016, 2018, 2019). Despite the recognized importance of the habitat, there is no information on the environmental conditions under which these algae live or on the key abiotic variables that regulate the metabolic rates of P. lusitanicum under natural conditions or increasing temperatures. The objectives of this study are 1) to determine the seasonal variations in the photosynthesis, calcification, respiration, and pigment concentration of P. lusitanicum and also 2) to investigate the effects of increasing temperature on the photosynthetic performance and respiration of the algae. This information is deemed essential to understand how climate change might impact maërl beds in southern Europe, and to establish a baseline for a proper management and protection of this poorly understood ecosystem.
Materials and Methods
Environmental Description
The southwest area off the Iberian Peninsula (SWIP, NE Atlantic) is classified as a vulnerable area to climate change because it is a transition zone between temperate and subtropical waters. Phytoplankton abundance in all SWIP regions presents a significant seasonal pattern, increasing during the late winter/early spring period (Krug et al., 2017). However, some strong coastal–oceanic differences in phytoplankton seasonality (chlorophyll-a) were found and especially accentuated in the coastal zone. These differences are probably related to an increase in nutrient concentration due to riverine discharges, mostly during winter, and upwelling induced by zonal westerly winds, stronger during summer (Krug et al., 2017).
The studied maërl bed is located at 4.7 nautical miles offshore in Armação de Pêra, in the region of Algarve, Southern Portugal (N 37°011′.650″/W −8°19′.034″) (Figure 1). This maërl bed has an estimated area of 3 km2 and extends from 13- to 25-m depth. The study site has a sandy bottom where maërl thalli accumulate on the depressions of the ripple marks. The thickness of the maërl bed can reach 5 cm, and most thalli present a discoidal shape. In the summer of 2013, an area of the maërl bed at 22-m depth was delimited to install environmental sensors and a translucent box with tagged algae for growth measurements.
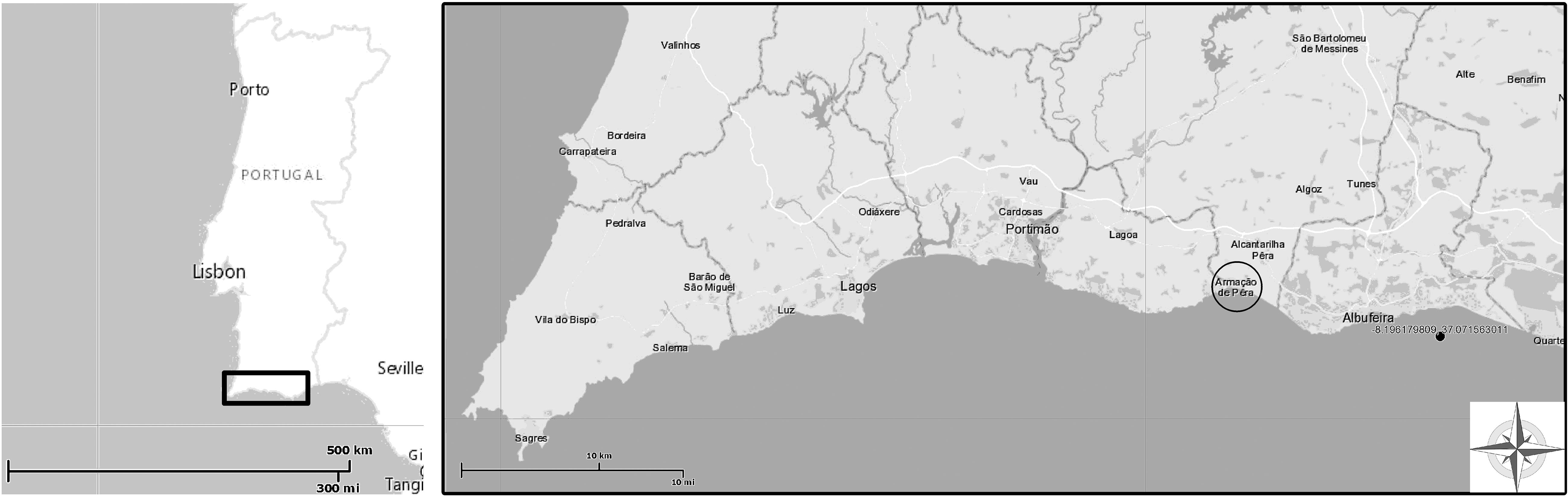
Figure 1. Study site location (SW Iberian Peninsula) in Armação de Pêra Bay, southern Portugal. The maërl bed (20- to 23-m depth) is located 4.7 nautical miles offshore (black dot). Figure modified from source: http://geoportal.lneg.pt/.
Sensors for temperature (HOBO temperature loggers Onset Company, United States), irradiance (Odyssey Integrating cosine corrected PAR Sensor; Dataflow Systems Limited, New Zealand), and dissolved oxygen (miniDOT oxygen logger; Precision Measurement Engineering, Inc., PME, United States) were attached to a concrete block at 22-m depth, ∼30 cm above the seabed, and left to record in a continuous basis (every 15 min) from July 2013 to May 2015. Every 3 to 4 months, the sensors were removed from the field, the data were downloaded, and then the sensors were brought back to the field. It was not possible to deploy sensors in the field from December 2013 to February 2014, or from October to November of 2014, due to adverse weather conditions. The mean monthly values (± SE) of the maximum and minimum values recorded daily are represented in Figure 2.
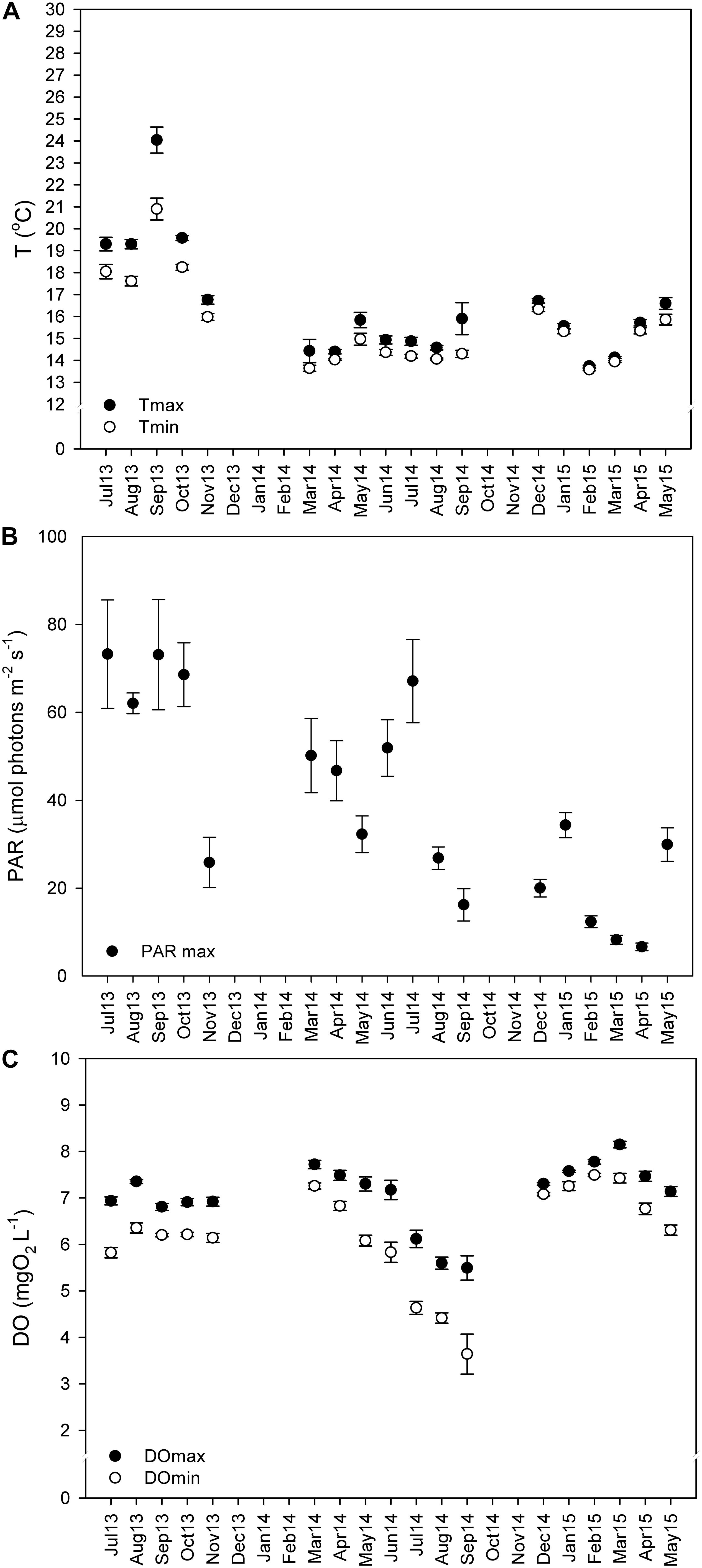
Figure 2. Monthly average of daily maximum and minimum temperatures (A,°C), photosynthetically active radiation (PAR) (B, μmol m–2 s–1), and dissolved oxygen (DO) concentrations (C, mg L–1). Values from summer 2013 (Jul 13–Aug 13), autumn 2013 (Sep 13–Nov 13), winter 2013–2014 (Dec 13–Feb 14), spring 2014 (Mar 14–May 14), summer 2014 (Jun 14–Aug 14), autumn 2014 (Sep 14–Nov 14), winter 2014–2015 (Dec 14–Feb 15), and spring of 2015 (Mar 15–May 15) depicted as mean ± standard error.
Photosynthesis and Respiration
Using a pulse-amplitude-modulated (PAM) fluorometer (Diving-PAM, Heinz Walz, Effeltrich, Germany), effective quantum yield (YII) and relative electron transport rates (rETR) (Schreiber et al., 1995) were seasonally determined in situ as:
where F’m is the maximum chlorophyll fluorescence in the light-adapted thalli, and Ft is the steady-state level of fluorescence under non-saturating illumination (Genty et al., 1989) and
where EPAR is the irradiance, and 0.5 is the fraction of chlorophyll associated to PSII (FII), assuming that PSI and PSII absorb equal amounts of incoming photons (Beer et al., 1998). Several authors (e.g., Grzymski et al., 1997) have pointed out that specific Chl-a absorption coefficients should be used for red algae (see Discussion section), but in the absence of a consensual value, we used the theoretical standard 0.5.
Rapid light curves (RLCs) were also generated in situ (n = 9) using 8 to 12 different light levels ranging from 10 to 655 μmol m–2 s–1. The saturating light pulse applied after 20-s exposure at each light level lasted for 0.8 s.
Seasonal P–E and dark-respiration curves were generated using a Clark-type oxygen electrode following the methodology described in Silva and Santos (2004). Rhodoliths were collected once every season by SCUBA diving (n = 10). Thalli were gently cleaned of epiphytes and biofilm with a soft brush and transferred to a walk-in chamber. Algae were kept under the same light and temperature conditions than those found in the field for 1 day prior to measurements. For each measurement, an individual was held vertically inside an incubation chamber (15 ml volume) coupled to a Clark-type oxygen electrode (DW3/CB1; Hansatech, Norfolk, United Kingdom) and filled with GF/F filtered seawater, which was changed after every three measurements. Actinic light was provided by a slide projector (Pradovit 150; Leica, Germany) equipped with a halogen lamp (Osram Xenophot 150 W). P–E curves were obtained by sequentially applying light levels ranging from 8 to 1,460 μmol m–2 s–1. Neutral density filters mounted on slide frames were used to obtain the different light intensities. Dark respiration was measured after turning off the light source and covering the incubation chambers with a black opaque plastic. Each light level (including darkness) was applied for ca. 10 min, the necessary time to reach steady-state oxygen evolution. A magnetic stirrer was used for water homogenization (Model A1/2; Hansatech Instruments, Norfolk, United Kingdom) (see Silva and Santos, 2004).
A thermostatic bath with a recirculation system (Julabo HC, Julabo LabortechnIk, Seelbach, Germany) was used to maintain the desired temperatures in the incubation chambers. Photosynthetic and dark-respiration rates (oxygen evolution/consumption) were calculated on a dry-weight (seasonal P–E curves) or area basis (P–E curves at different temperatures) using the following formula:
where A is the oxygen evolution rate per unit of area or weight (mg O2 s–1 cm–2 or g–1); [O2] is the O2 saturation concentration at the given temperature and salinity conditions (mg L–1); C1 is the corresponding calibration height (cm) of O2-saturated water in the plotter’s paper; tgα is the angle of the slope of the oxygen evolution/consumption line; p is the plotter speed (cm s−1); U is the area (cm2) or weight (g) of the sample, and Exp. is the signal amplification. At the end of the measurements, the fresh weight or the maximum length and width of the thalli were recorded. For the calculations of the seasonal P–E curves, each individual was placed in an open aluminum foil envelope (to avoid water condensation) and left to dry in the oven at 60°C and weighted 1 week later (dry weight). In order to enable a correlation between these results and the fluorescence data, gross photosynthesis was expressed in μmol O2 m–2 s–1.
In a separate experiment, P–E curves and dark-respiration rates of P. lusitanicum were also determined at eight different temperatures (n = 6): 12°C, 14°C, 16°C, 18°C, 20°C, 22°C, 24°C, and 26°C using a Clark-type oxygen electrode. Algae were collected and maintained under the conditions found in the field (16°C) for 2 weeks. Before the measurements, thalli were left to acclimate for 48 h, at each tested temperature in a walk-in chamber. All the measurements were carried out during 4 weeks as previously described.
To investigate the relationship between the relative electron transport rate (rETR) (μmol e– m–2 s–1) and the gross photosynthesis (μmol O2 m–2 s–1), chlorophyll fluorescence measurements were carried out using a pulse–amplitude-modulated (PAM) fluorometer (Diving-PAM; red light; Heinz Walz, Effeltrich, Germany) coupled to the oxygen electrode (n = 8) (see Silva and Santos, 2004 for further details). The relative electron-transport rate (rETR), the maximum photosynthetic rate (Pmax), the ascending slopes at limiting irradiances (α), and the half-saturation irradiances (Ek) were calculated. Both light-response curves (O2 and fluorescence derived) were fitted with the models of Smith (1936) and Talling (1957):
in which Pm is the maximum photosynthetic rate (or the maximum electron transport rate), α is the ascending slope at limiting irradiances, and EPAR is the irradiance.
Curve fitting was done using the software package SigmaPlot (Systat Software 2008, Inc., Germany). The simultaneous use of both methods allowed us to establish the coupling degree between oxygen production and rETR, expressed by the molar ratio rETR/O2 (Silva and Santos, 2004). Measurements were done with algae incubated under experimental conditions at a temperature of ∼16°C.
Photosynthetic Pigments
Photosynthetic pigment concentrations were analyzed in autumn and summer 2013, winter and spring 2014, and summer 2015 (n = 5). Maërl thalli were sampled seasonally in the field and kept under −80°C until analyses. The thalli were ground in liquid nitrogen, before extraction in acetone (chlorophylls and total carotenoids) or in phosphate buffer (phycoerythrin and phycocyanin), and the extracts were then centrifuged. The absorbances of all pigment extracts were measured with a spectrophotometer (Beckman Coulter; DU-650; United States) in a 1-cm glass cuvette. The pigment concentrations were expressed in mg gFW–1.
Chlorophyll d (Chl d) was extracted from 1.5 g of fresh thalli in 5 ml of 90% acetone. The extract was then centrifuged (Heraeus Megafuge 16R; Thermo scientific, MA, United States) for 5 min at 2,000 × g and 4°C. The absorbance of the supernatant was read at 630, 647, 664, and 691 nm. Chl d concentration was calculated according to Ritchie (2008):
Total carotenoids and chlorophyll a (Chl a) were extracted from 1.5 g of fresh thalli in 5 ml of 100% acetone. The extract was then centrifuged (Heraeus Megafuge 16R; Thermo scientific, MA, United States) for 5 min at 2,000 × g and 4°C, and the supernatant absorbance was read at 470 and 661.6 nm. The pigment concentrations were calculated using the equations of Torres et al. (2014) adapted from Lichtenthaler and Buschmann (2001) for red algae:
The phycobilins phycoerythrin (PE) and phycocyanin (PC) were extracted from 1 g of fresh weight in 2 ml of phosphate buffer (pH 6.5) at 4°C. The extract was centrifuged (Heraeus Megafuge 16R; Thermo Scientific, United States) at 4,696 × g for 40 min, and absorbance was read at 564, 618, and 730 nm. The concentrations of phycobilins were determined using the equations of Sampath-Wiley and Neefus (2007):
Growth
The growth of the thalli was followed in situ under natural conditions for 695 days (from summer 2013 to spring 2016). A total of 160 individuals were tagged with small plastic labels attached with nylon wire and placed in a translucent hard plastic box covered with a plastic mesh with uniform holes of about ∼3 cm in all of its surface. Because of this, the water flow and irradiance conditions inside the box were identical to its surroundings (previously assessed in laboratory). The box was fixed with chains and weights to a concrete block at 22-m depth.
Every 2 months, the tagged algae were removed from the box and taken to the laboratory. The excess of epiphytes was gently cleaned, and the rhodoliths’ individual growth was measured using the buoyant weight (BW) technique. Once measured, the tagged individuals were returned to the field. According to Steller et al. (2007), the buoyant weight technique assumes that the skeleton of the algae is mainly composed of calcium carbonate. Because the density of the organic tissue equals that of seawater, any increment on the buoyant weight is assumed to be an increment of CaCO3 deposition or calcification. Prior to the experiment, the CaCO3 composition of P. lusitanicum was determined by eliminating CaCO3 through acidification. After drying and weighing, 24 thalli were placed individually in 25 ml of 5% HCl. The thalli were submerged into the acid solution until all CaCO3 was dissolved. The HCl solution was changed every 24 h. After total decalcification, samples were rinsed in distilled water and allowed to dry to constant weight in an oven at 60°C. Finally, the CaCO3 content was determined by subtracting the dry weight from the pre-acidified weight (Steller et al., 2007).
To determine the individual weight of the thalli, these were suspended by a nylon string attached to an electronic balance (Sartorius 0.1 mg, Germany) in a beaker filled with filtered seawater. The algae were weighted and the water temperature (Roth digital thermometer, Hanna, EU) and salinity (CO310 conductivity meter; VWR, United States) measured to calculate the seawater density. Then, the buoyant weight of the algae was calculated using the equation described in Steller et al. (2007):
where Wcc is the dry weight of the CaCO3, Wb is the buoyant weight of the thalli, Dcc is the density of CaCO3 (2.71 g cm–3), and Dw is the density of seawater displaced by the sample (1.03 g cm–3). The following equation is obtained after substituting the density of seawater and CaCO3:
In order to be able to compare the growth of tagged algae of different sizes, we use the relative growth rate (RGR) as it allows more equitable comparisons (Hunt, 1990) than the absolute growth rate. The mean value of the RGR is the increase in weight per unit of pre-existing weight (W) over the interval t1 to t2:
Statistical Analyses
The software package SigmaPlot version 11.0 (Systat Software 2008, Inc., Germany) was used to perform all statistical analyses. Monthly or seasonal differences among the abiotic variables (temperature, dissolved oxygen, and PAR), respiration, calcification (repeated growth measures of tagged algae), photosynthesis, pigment concentration and, the comparison between the relative electron transport rate (rETR) and gross photosynthesis were analyzed using one-way ANOVA tests. A post hoc test (Student–Newman–Keuls, SNK) was applied to explore differences between treatments when significant differences were found. When data presented equal variance, but did not meet the assumption of normal distribution (Kolmogorov–Smirnov test) even after square root or log transformation, an ANOVA on ranks (Kruskal–Wallis analyses of variance) was applied.
Results
Environmental Variables
Maërl beds in southern Portugal are exposed to low light and temperature conditions all year round (Figure 2). Still, an important inter-annual temperature variability (one-way ANOVA on ranks; H = 392.509; P ≤ 0.001) was observed, with maximum temperatures in summer 2013 (24°C) being 8° higher than those observed in summer of 2014 (16°C) at 22-m depth.
The maximum and minimum mean daily values for each month were calculated (mean ± SE) (see Figure 2). In autumn and summer of 2013 were recorded the highest temperatures (17 ± 0.21 to 24 ± 0.59°C) and PAR irradiances levels (62 ± 2.36 to 73 ± 12.31 μmol m–2 s–1), and high dissolved oxygen concentrations (6.8 ± 0.40 to 7.3 ± 0.23 mg O2 L–1). In spring and early summer of 2014, the PAR irradiances were high (32 ± 4.17 to 67 ± 9.47 μmol m–2 s–1) and so was the dissolved oxygen (7.5 ± 0.16 to 7.7 ± 0.09 mg O2 L–1). By the end of the summer of 2014, irradiance decreased by about 41 μmol m–2 s–1, to 26.8 ± 2.54 μmol m–2 s–1, and dissolved oxygen reached the minimum values (3.64 ± 1.29 mg O2 L–1), while temperature remained unaltered (∼15.9 ± 2.18°C). The minimum temperatures (13.6 ± 0.04°C) and irradiances (6.6 ± 0.87 μmol m–2 s–1) were recorded from winter to spring of 2015, as did the maximum DO concentrations (8.1 ± 0.07 mg O2 L–1). The low PAR and temperature values and the increase in DO during this period were related to agitated seas due to continued rough weather conditions (personal observations) (see Figures 2A–C).
Photosynthesis and Respiration
The highest relative electron transport rates (rETR) were recorded in summer and the lowest in winter (Figures 3B,D and Supplementary Table S1). There were significant differences among seasons (one-way ANOVA; F = 183.928; P ≤ 0.001) but no differences between spring and autumn (P = 0.797) (Figures 3A,C). The ascending slope at limiting irradiance (α) also changed seasonally (one-way ANOVA; F = 13.710; P ≤ 0.001), being higher in winter and autumn and lower in spring and summer, but with no differences between autumn and summer (P = 0.507). The Ek was lower in winter (one-way ANOVA; F = 13.889; P ≤ 0.001), and did not differ between summer and autumn (P = 0.180) or spring (P = 0.187).
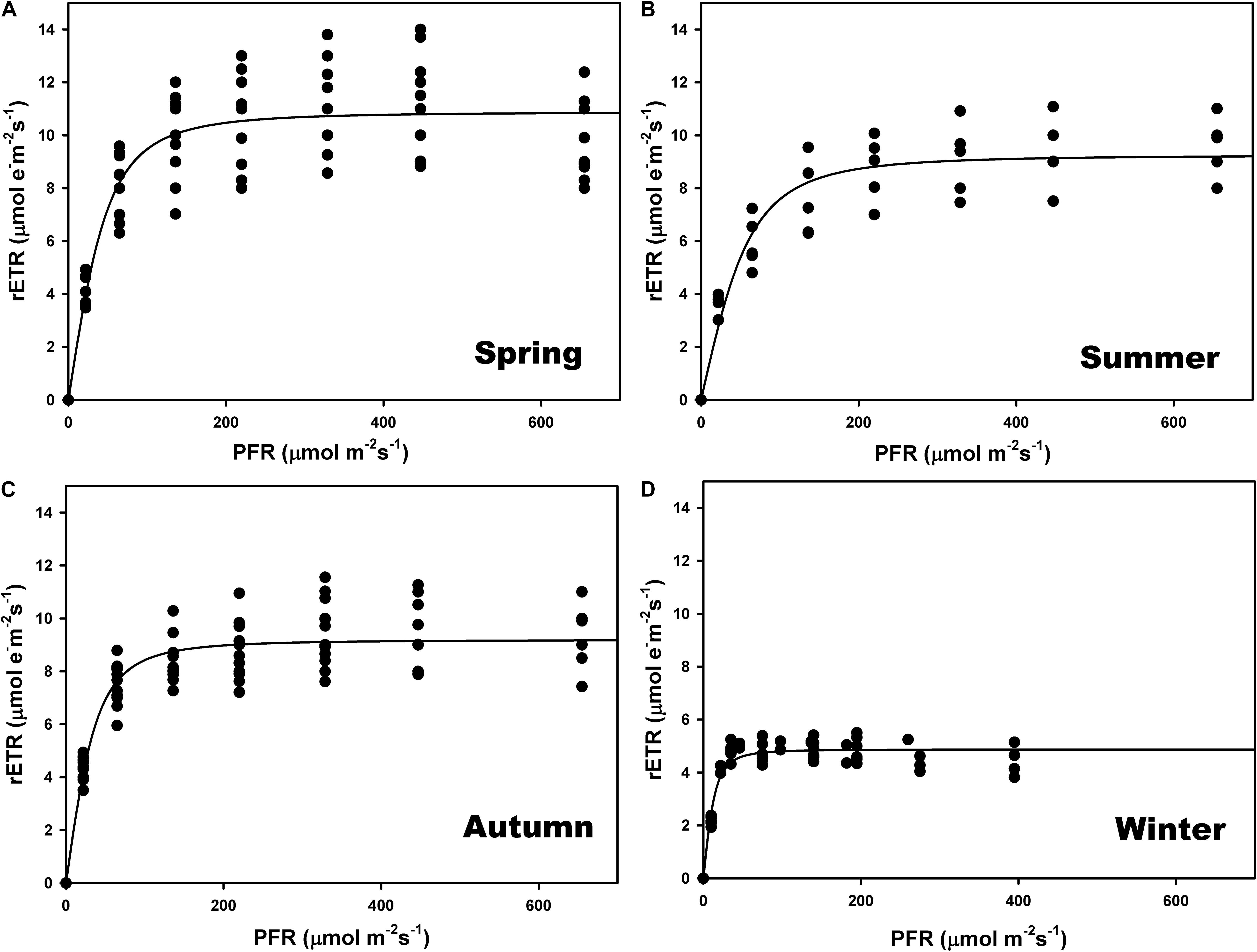
Figure 3. Seasonal rapid light curves (RLC) during spring (A), summer (B), autumn (C), and winter (D). Data generated in situ at a maërl bed of Phymatolithon lusitanicum (∼22-m depth) using a Diving PAM fluorometer. Curves represent the adjustment of the model equation of Smith (1936) and Talling (1957) to the observed values (n = 9).
The maximum photosynthetic rates (Pmax) (one-way ANOVA; F = 98.293; P ≤ 0.001), and the ascending slope at limiting irradiance (α) of the oxygen evolution curve also changed seasonally (one-way ANOVA; F = 17.87; P ≤ 0.001) reaching the highest values in summer and spring (Figures 4B,A) and the lowest in autumn and winter (Figures 4C,D; see Supplementary Table S2). In contrast, there were no significant seasonal differences in Ek (one-way ANOVA; F = 0.509; P = 0.681). We found a linear relationship (r2 = 0.95) between gross primary production (GPP) and relative electron transport rates (rETRs), with an average molar ratio of 0.24 (∼1/4) (Figures 5A,B).
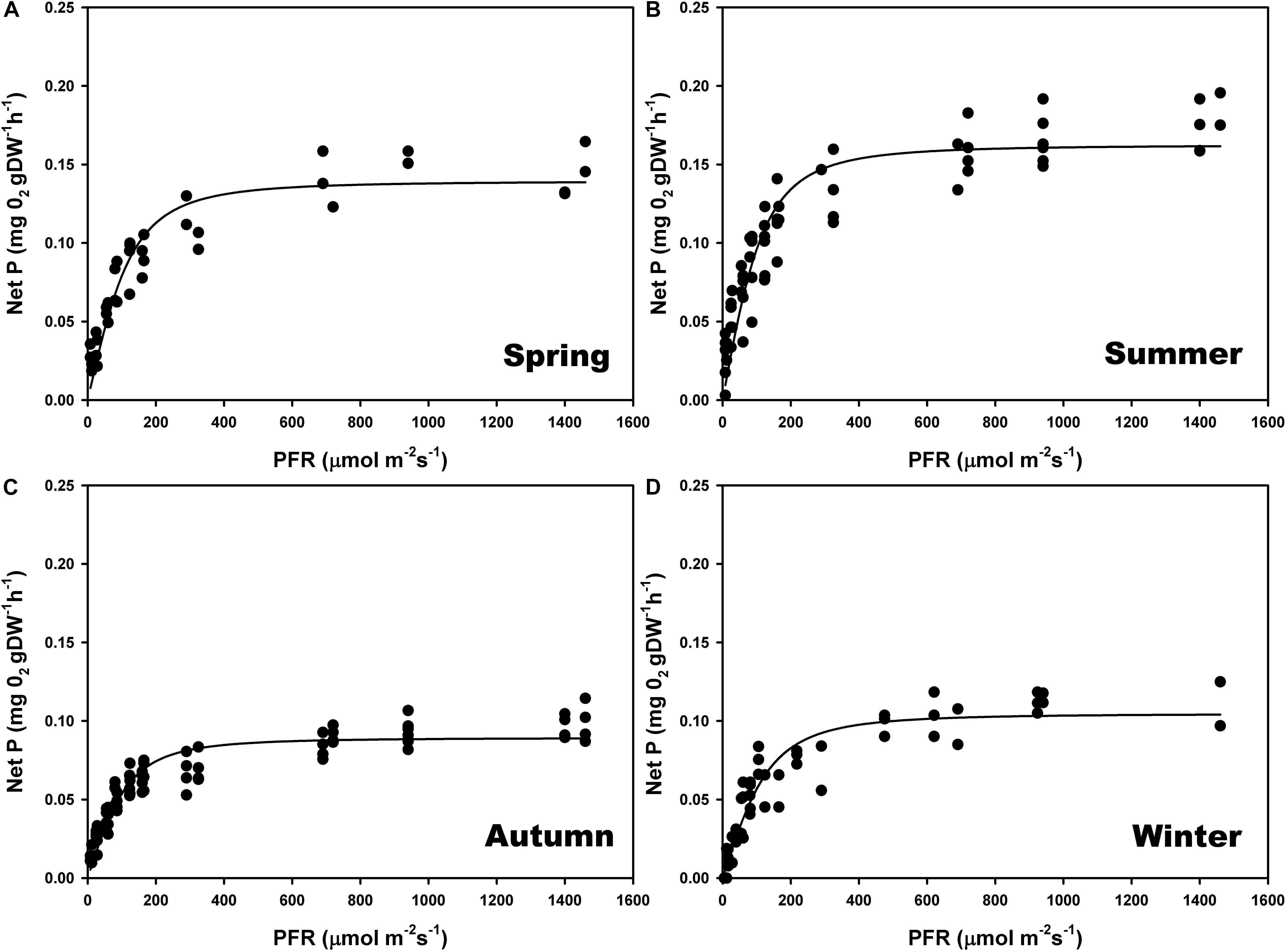
Figure 4. Seasonal photosynthesis–irradiance (P–E) curves, determined on individual thalli of P. lusitanicum in the laboratory using an oxygen Clark-type electrode (n = 5) during spring (A), summer (B), autumn (C), and winter (D). Curves represent the adjustment of the model equation of Smith (1936) and Talling (1957) to the observed values.
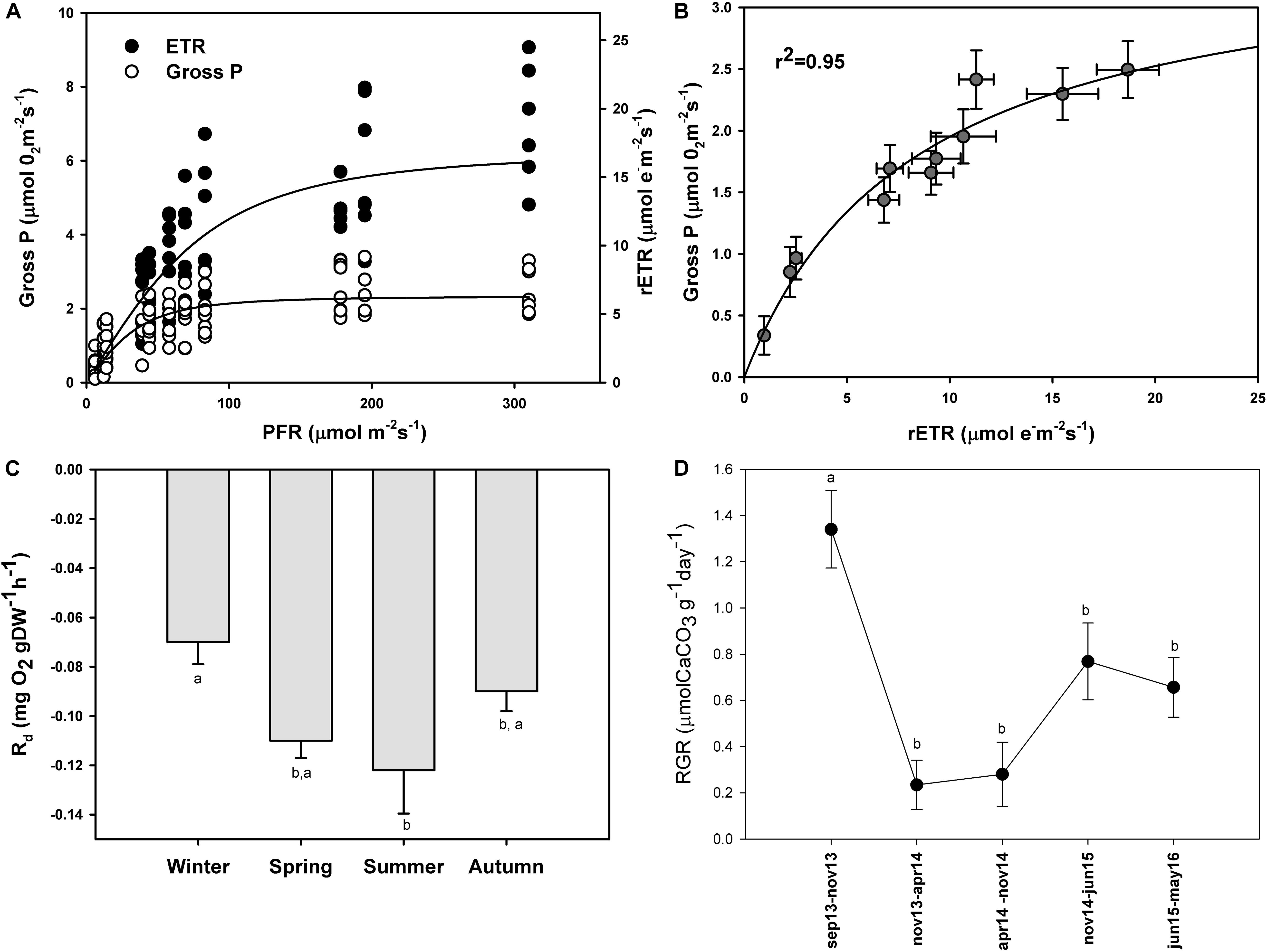
Figure 5. Simultaneous measurements of oxygen evolution (Gross P.; μmol O2 m–2 s–1) and relative electron transport rates (rETR; μmol e– m–2 s–1) in P. lusitanicum (A). Gross photosynthesis vs. rETR relationship (B) (n = 8). Seasonal variation of dark respiration rates of P. lusitanicum (Rd, mg O2 gDW–1 h–1) measured in the laboratory (n = 10). (C) Relative growth rate of P. lusitanicum (RGR) from September 2013 (Sep 13) to May 2016 (May 16) determined on marked individuals on the field (n = 160) (D). Mean values ± standard error. Different letters indicate significant differences.
Dark respiration also changed seasonally with the highest respiration rates in summer and spring and the lowest in winter and autumn (Figure 5C). Significant differences were observed only between winter and summer (one-way ANOVA; F = 4.166; P = 0.023).
Relative Growth Rate (RGR) in situ (Buoyant Weight Technique)
In this study, we estimated through HCl acidification that P. lusitanicum is composed of ∼96% CaCO3. After more than 695 days in the field, the relative growth rate changed with time (ANOVA on ranks; H = 34.011; P ≤ 0.001) (Figure 5D). These differences were due to the high RGR observed from September to November of 2013 (1.34 μmol CaCO3 g–1 day–1). In the rest of the periods, the growth rates were constant, and from November 2014 to May 2016, they went from 0.77 to 0.66 μmol CaCO3 g–1 day–1. The lowest RGR was observed from November 2013 to November of 2014 (0.23–0.28 μmol CaCO3 g–1day–1).
Photosynthetic Pigments
Chlorophyll a concentration changed seasonally (one-way ANOVA; F = 8.230; P ≤ 0.001), being higher in winter and spring of 2014 and lower in autumn and summer of 2013 (Figure 6A). These differences were mostly due to the high values observed in winter 2014 with respect to summer 2013 (P < 0.001), summer 2015 (P < 0.004), autumn 2013 (P < 0.001), and spring 2014 (P = 0.021). Carotenoid concentration also varied seasonally (One-way ANOVA; F = 5.623; P = 0.003), being higher in winter and spring and lower in autumn and summer (Figure 6A). Once again, these differences were due to the high values observed in winter of 2014 with respect to autumn 2013 (P = 0.003), summer 2013 (P = 0.008), summer 2015 (P = 0.013), and spring 2014 (P = 0.043).
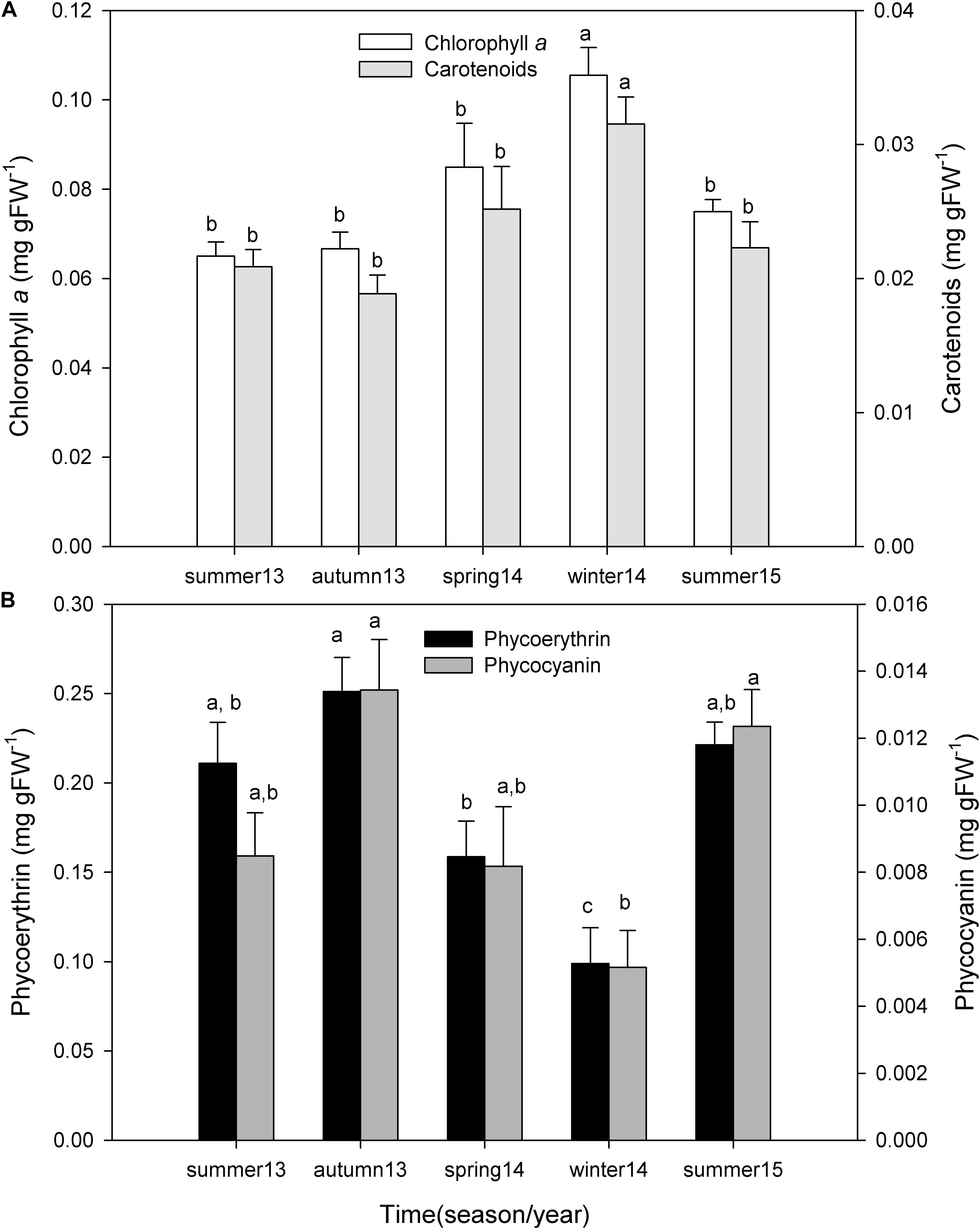
Figure 6. Seasonal variation of chlorophyll a and carotenoid pigment concentration (A), and phycoerythrin and phycocyanin pigment concentration (B) of P. lusitanicum (mg gFW–1). Different letters indicate significant differences between seasons for each photosynthetic pigment. Mean values ± standard error (n = 5).
During all periods, P. lusitanicum presented higher concentrations (mg gFW–1) of phycoerythrin (0.188 ± 0.067) than phycocyanin (0.01 ± 0.004). Phycocyanin (one-way ANOVA; F = 5.959; P = 0.003) and phycoerythrin (one-way ANOVA; F = 9.766; P ≤ 0.001) concentrations changed seasonally, being higher in autumn and summer and lower in winter and spring (Figure 6B). Phycocyanin concentrations were significantly lower in winter 2014 with respect to autumn 2013 (P = 0.003) and summer 2015 (P = 0.007). Phycoerythrin concentrations in winter 2014 were significantly lower with respect to autumn 2013 (P < 0.001), summer 2013 (P = 0.002), and also spring of 2014 (P = 0.014) (post hoc test Student–Newman–Keuls, SNK).
Effects of Temperature on Respiration and Photosynthesis
The dark respiration rates of P. lusitanicum increased with temperature (Figure 7A) and were significantly higher at 24°C and 26°C (one-way ANOVA; F = 9.392; P ≤ 0.001). The maximum photosynthetic rates (Pmax) were observed at 24°C (Figure 7B) (one-way ANOVA; F = 16.618; P ≤ 0.001). The Ek values (μmol m–2 s–1) tended to increase from 18°C to 20°C and decreased under high temperatures (24°–26°C) but with no statistical differences (one-way ANOVA; F = 0.993; P = 0.450) (Table 1), the same occurring for the ascending slope at limiting irradiances (α) (one-way ANOVA; F = 1.311; P = 0.271).
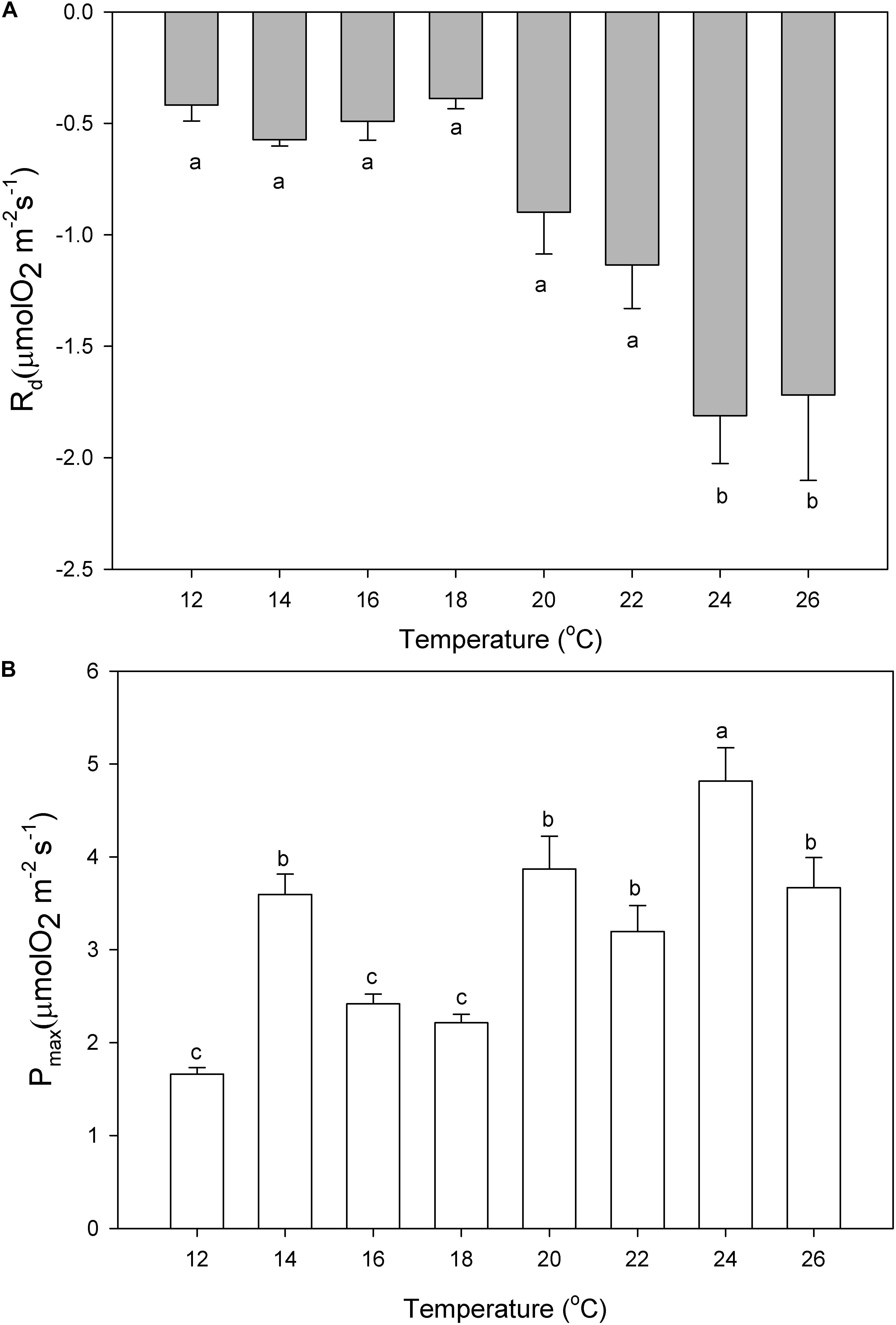
Figure 7. Dark respiration (Rd, A) and maximum photosynthetic rates (Pmax, B) of P. lusitanicum (μmol O2 m–2 s–1) as a function of temperature. Different letters indicate significant differences. Mean values ± standard error (n = 6).
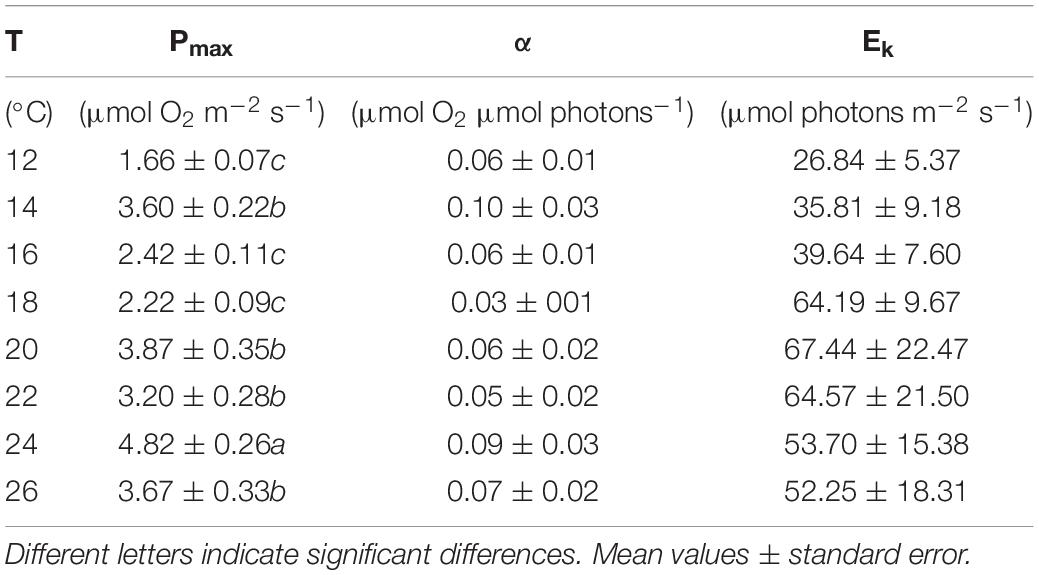
Table 1. Parameters from photosynthesis–irradiance (P–E) curves at different temperatures (12–26°C): maximum photosynthetic rates (Pmax), ascending slope at limiting irradiances (α), and half-saturation irradiation (Ek).
Discussion
Both the maximum electron transport rates measured on the field and the gross photosynthetic rates of P. lusitanicum measured in the laboratory increased in summer and spring and decreased in autumn and winter. This corroborates the results from previous studies where the net production of Lithothamnion corallioides (Martin et al., 2006) and the gross production of Ellisolandia elongata (Egilsdottir et al., 2016) were, respectively, two- and threefold higher in summer than in winter. Martin et al. (2007) also found that the gross community production of maërl beds in the Bay of Brest displayed a seasonal variability, being 3.5-fold higher in summer than in winter.
Previous studies have assessed the photosynthetic activity of non-geniculate coralline algae using the non-invasive chlorophyll-a fluorometry technique (e.g., Wilson et al., 2004; Burdett et al., 2012; Schoenrock K.M. et al., 2018). In this study, the seasonal measurements carried out both in situ (PAM fluorescence) and in the laboratory (O2 Clark electrode) revealed that there is a seasonality in the photosynthetic activity of maërl beds from southern Portugal. We speculate that the lower slope (α) and higher Ek values of the oxygen evolution P–E curves with respect to the values observed for the RLCs was probably related to differences in the light quality and to the acclimation of experimental algae to higher light conditions in the laboratory than those observed under natural conditions in the field. Figueroa et al. (2003) measured the ETR and oxygen evolution of different algal species under different light conditions and also found that the ETR-based α was higher than the O2-based α in all species. The authors pointed out that the quantity and quality of light was a decisive factor on the results and that the absorbed quanta of algae cultivated long term under experimental conditions decreased as a consequence of photoacclimation.
When directly compared in a separate laboratorial assay, the two methods yielded comparable results between them, revealing a very strong correlation and an average molar ratio close to 1/4 between gross photosynthesis and electron transport rates. Figueroa et al. (2003) also found that the calculated O2/ETR molar ratios were close to 0.25 for the red algae Porphyra leucosticta. However, for this particular species, the Gross P was associated to the estimated ETR only at high irradiances, when algae were pre-incubated under 500–1,000 μmol m–2 s–1. Herein, we found that at lower irradiances, the relation between GP and ETR is close to four electrons per 1 mol of oxygen produced. However, this value increased to 8 at the saturating irradiance of 200 μmol m–2 s–1. At saturating irradiances, the uncoupling between water splitting activity in PSII and PSI strongly reduces the plastoquinone pool and enhancing the ETR (Prasil et al., 1996). In cases where the xanthophyll cycle activity is limited, this mechanism could be essential for protecting shade-adapted species to saturating irradiance levels (see Figueroa et al., 2003). More extensive research is needed to verify if it is possible to make a generalized extrapolation between Gross P and rETR for the species P. lusitanicum. Additionally, there is a controversy on the value of the FII constant used to calculate rETR for red algae. In most studies, the constant commonly used to calculate the fraction of chlorophyll associated with PSII (FII) in algae is 0.5 (e.g., Beer et al., 1998). However, Grzymski et al. (1997) developed specific Chl-a absorption coefficients for brown and red algae. Because of this, it is worth mentioning that the FII of 0.5 may be more adequate for green algae, while for red algae FII, it is probably closer to 0.15, and for brown algae would be around 0.8 (Grzymski et al., 1997; Figueroa et al., 2014). However, in the absence of a consensual value, we used an FII of 0.5 in our calculations of ETR.
The saturating irradiances for P. lusitanicum in the field were high when compared with other subtidal red calcareous species from temperate latitudes. The Ek values derived from the RLCs ranged from 17 to 44 μmol m–2 s–1 in winter and autumn, and from 56 to 70 μmol m–2 s–1 in summer and spring. These values are higher than the Ek values observed in May and March for the low-light adapted maërl species Lithothamnion glaciale (4.45–54.6 μmol m–2 s–1) (Burdett et al., 2012), for the Arctic species Phymatolithon foecundum and also for Phymatolithon tenue (7 and 17 μmol m–2 s–1) (Roberts et al., 2002), but lower than the values recorded for the tropical Porolithon sp. (∼700 μmol m–2 s–1 at 12:00) (Burdett et al., 2014). Under experimental conditions, the high Ek values observed in a wide range of temperatures suggest that P. lusitanicum, like P. calcareum, can tolerate high temperatures, which explains the wide distribution of these two species (Wilson et al., 2004). While other factors, such as hydrodynamics, can determine the presence or absence of maërl species, light and temperature are the main factors that control the depth and geographical distribution of crustose and unattached corallines (Bosence, 1976).
Respiration was almost twofold higher in summer than in winter. This agrees with previous studies where the respiration rates of the non-geniculate Lithothamnion corallioides (Martin et al., 2006) and the geniculate Ellisolandia elongata (Egilsdottir et al., 2016) were, respectively, three- and 10-fold higher in summer than in winter. Also, Martin et al. (2007) found that in the Bay of Brest (France), the maërl community respiration exhibited a strong seasonality with the greatest rates in summer (August) and the lowest in winter (February).
In more northern maërl beds of the Iberian Peninsula, the maërl community is tightly correlated with temperature, and irradiance increases (Peña and Bárbara, 2010), and seasonality in the flora associated was observed during this study but not quantified. Under a global change scenario, the increase in the abundance of the epiflora during high temperature and irradiance periods could favor calcification by increasing the pH as a consequence of the photosynthetic activity of the non-calcareous algae (Short et al., 2015). Algal cover can also protect the maërl beds from the detrimental effect of high irradiance (Figueiredo et al., 2000). Since the community response to global change is complex, to be able predict the future response of marine communities, it is important to understand the effect of environmental changes on species interactions (Short et al., 2015). Therefore, the seasonal flora associated to the maërl bed is an important factor to consider because it plays a major role in the gross community production (Martin et al., 2007). Also, it is important to understand how environmental changes affect species interactions to determine the effect that global change will have on the maërl community (Legrand et al., 2017).
This is the first study to assess how environmental factors affect the concentration of photosynthetic pigments in P. lusitanicum. Chlorophyll a and carotenoids increased in winter of 2014–2015 and decreased in summer of 2013 and 2015. In this study, algae compensated the light deprivation during winter of 2014–2015 by increasing their photosynthetic pigments. In contrast, under experimental conditions, Noisette et al. (2013) found that the chlorophyll a concentration of the temperate rhodolith algae Lithothamnion corallioides was unaffected by temperature, while carotenoid concentration decreased with temperature. The interaction of environmental variables such as irradiance, nutrients (Lin and Stekoll, 2011), and temperature (Kim et al., 2007), has an effect on pigment production. However, previous studies have found that the biochemical response to these variables is highly species specific (see Kim et al., 2007; Lin and Stekoll, 2011; Stengel et al., 2014).
The lowest phycocyanin and phycoerythrin concentrations were also observed in winter of 2014–2015, and in spring of 2014, when the temperatures were low, but these pigments increased during the warmer seasons (summer and autumn of 2013, and summer of 2015). The phycobilins phycoerythrin and phycocyanin are the main light-harvesting pigments in marine red algae (Beer and Eshel, 1985; Lin and Stekoll, 2011). Also, when nitrogen supply becomes limiting, phycobilin pigments may be used as nitrogen reserves for growth and other physiological processes (Lin and Stekoll, 2011). The phycoerythrin concentrations of P. lusitanicum were in the same order of magnitude as the phycobilin concentrations recorded for the temperate coralline red algae Lithothamnion glaciale (see Donohue, 2015). Donohue (2015) found that the phycoerythrin concentration of low-light adapted Lithothamnion glaciale increased in winter to compensate for the decrease in irradiance. However, this author also found that under experimentally increased temperature and CO2 conditions, phycoerythrin concentrations decreased in winter, probably due to a reallocation of resources (i.e., nitrogen) for other physiological processes. During the warmer months, a higher availability of nutrients can be expected at the studied area because of the upwelling induced in the southwestern area of the Iberian Peninsula by zonal westerly winds, stronger during summer (Krug et al., 2017). Because there are no light limitations during summer, the increase in phycobilin concentrations might be related to a high nutrient availability. In contrast, during low light and temperature periods when there is less primary production and less nutrients available, P. lusitanicum might be using the stored nitrogen in their phycobilin pigments.
Previous studies have found that phycobiliproteins also have a nutrient storage function (Kim et al., 2007; Lin and Stekoll, 2011) and might decline due to a lack of sufficient inorganic nitrogen for continued synthesis of new pigments (Kim et al., 2007). In a short-term experiment, Stengel et al. (2014) observed that the phycobilin content of the intertidal red algae Ellisolandia elongata was unaffected by temperature, nutrients, or irradiance (Stengel et al., 2014). In contrast, in a field study, Pereira et al. (2012) observed that the pigment and protein concentrations of the red algae Gracilaria dominguensis increased with decreasing sunlight and increasing nutrients, and gradually decreased with increasing irradiance and decreasing nutrient concentration. The variability of responses among algal species highlights the importance of further long-term field studies to assess the effect that different environmental conditions exert on the acclimation of algae and concentration of their biochemical components (Pereira et al., 2012; Stengel et al., 2014).
Our results show that calcification in P. lusitanicum is more temperature controlled and not as dependent on light as photosynthesis. In a previous experiment where the effect of temperature and high CO2 in the net production and calcification of P. lusitanicum was assessed, we also found that calcification increases with temperature, and this effect is more pronounced under high CO2 (Sordo et al., 2019). The highest relative growth rates were observed from September to November of 2013 when we recorded the highest temperature and irradiance conditions. According to NOAA (2013), the global temperatures across the world’s ocean surfaces in July of 2013 were higher than the average, and this was the sixth warmest July since records started in 1880. In nearby Spain, July 2013 was the warmest July since 1961. The same pattern was corroborated by data from the fixed monitoring station SIMPATICO (integrated system for in situ multiparametric monitoring in coastal areas), moored offshore from the lower Guadiana Estuary (see Garel and Ferreira, 2015) in southern Portugal (37°11.300N, 7°24.670W), which recorded unusually high temperatures during summer of 2013.
These results suggest that during this period, calcification in the field increased with temperature and irradiance. In a long-term study, Halfar et al. (2013) found that the growth and Mg/Ca ratios of crustose coralline algal buildups in the Arctic were sensitive to changes in both temperature and solar radiation. This agrees with previous studies where the calcification of Lithothamnion corallioides (Martin et al., 2006), Lithothamnion glaciale (Schoenrock K.M. et al., 2018), and Ellisolandia elongata (Egilsdottir et al., 2016) increased with temperature and irradiance in summer and decreased in winter. Also, Martin et al. (2007) found that maërl community calcification was sixfold higher in summer than in winter. From November of 2013 to April of 2014, the temperatures went down and also did the calcification rates. In contrast, from November 2014 to June 2015, the temperatures increased, and despite the low light conditions, algae increased their calcification rates. Many coralline algae species are low-light adapted and can even survive sporadic burial events. According to Wilson et al. (2004), the non-geniculate species P. calcareum showed little stress after spending 4 weeks in the dark. The authors suggested that probably this maërl species is able to survive several months in the darkness without showing deleterious effects. In this study, we found that P. lusitanicum is able to maintain relatively constant growth rates despite low light and temperature conditions.
In the lab assays, dark respiration increased with temperature from 20°C to 24°C but started to decrease at 26°C. The measurements at different temperatures confirmed that respiration of P. lusitanicum increased gradually with temperature. It is widely known that respiration of coralline algae increases with temperature until their thermal tolerance limit is exceeded (see Martin et al., 2013b; Martin and Hall−Spencer, 2017 for a review). In a previous experiment with P. lusitanicum, Sordo et al. (2016) found that respiration was unaffected by CO2 but positively affected by temperature. This increase was significant only from 24°C but decreased under 26°C. Even if not significant, this might indicate that the thermal limit for P. lusitanicum is above 26°C, a higher value than the temperatures observed under natural conditions. In contrast, the lowest respiration rates were observed at the lowest temperatures. This might constitute an algae’s mechanism to avoid excessive loss of carbon through the liberation of CO2 (Martin et al., 2006).
Even though there were no statistical differences with temperature, we observed the highest Ek values at 18°C and above (>50 μmol m–2 s–1), with the exception at 14°C, where the maximum net photosynthetic rates were reached at irradiances of about 35.8 μmol m–2 s–1, resulting in an α slightly higher than the α obtained at 24°C. At low light intensities and temperatures (12°C and 16°C), the Pmax observed was inferior to 3 μmol O2 m–2 s–1 with α values of 0.06 μmol O2 m–2 s–1. When irradiance increases, the adaptation of these algae to low light makes them more susceptible to photoinhibition, especially if this increase is accompanied by temperatures lower or higher than the optimum range for the organism (Kalituho et al., 2003). Because of this, the Ek values found in this study (26-67 μmol m–2 s–1) were lower than the Ek registered by Egilsdottir et al. (2016) for the intertidal red algae Ellisolandia elongata (30.5 in summer and 82.6 μmol m–2 s–1 in summer), but higher than the Ek registered by Figueiredo et al. (2012) for deeper rhodolith communities dominated by the species Mesophyllum engelhartii (12 to 30 μmol m–2 s–1).
Low temperature may decrease the fluidity of the membrane, negatively interfering with the electron transport chain, which, in these conditions, loses electrons for O2 leading to the formation of reactive oxygen species or ROS (Reis et al., 2011; Goh et al., 2012). This could explain the low Pmax values observed in this study for P. lusitanicum at 12°C. Even if temperature was the main factor regulating the photosynthetic activity, irradiance intensified the negative effects on the net production at 26°C. Our results suggest that a decrease in the net production at a high temperature is more evident under high irradiance. Therefore, both temperature and irradiance have a main role on the regulation of the photosynthetic rates in the red algae P. lusitanicum. From 20°C to 26°C, the algae reached the highest Pmax, but also at 14°C (a temperature close to the values observed at the field in winter). Our results suggest that even with lower photosynthetic rates, P. lusitanicum will be able to maintain some growth at low temperatures if the light intensity does not exceed the saturation light intensity for those temperatures (Kalituho et al., 2003).
Conclusion
Despite the relatively stable environmental conditions recorded in the studied area, the photosynthetic rates and pigment concentrations of P. lusitanicum are regulated by temperature and irradiance, while respiration is regulated solely by temperature. Calcification/growth is influenced by irradiance but is more strongly dependent on temperature changes. Both photosynthesis and respiration increased in summer and decreased in autumn and winter, while growth did not change seasonally.
This is the first study to identify and measure the main environmental variables that regulate the metabolism of Lusitanian maërl beds. This information is essential to assess the impacts that global warming and ocean acidification will have on the valuable ecological services these maërl beds provide. Under a global warming scenario, temperature and irradiance will have a positive effect on the photosynthetic rates of maërl beds from southern Portugal, and respiration and calcification will also increase with temperature. In a drastic scenario, above 26°C, respiration and calcification (as CO2 sources) could eventually surpass photosynthetic carbon uptake, rendering the system heterotrophic. In such case, calcium carbonate dissolution could also surpass deposition, with an important negative effect on the maërl community and on the carbon cycle. Cornwall et al. (2020) found that crustose coralline algae (CCA) can gain tolerance to OA after six generations of exposure. However, in shallow areas, the negative effect of OA on the photochemical efficiency of coralline algae is expected to increase with light, constraining CCA species to a narrower range of light environments (Briggs and Carpenter, 2019). Maërl beds from southern Portugal, on the other hand, are located between 13- and 25-m depth, with most of its area below 22 m, where PAR is low and temperature conditions are relatively stable, except for sporadic heat waves. In the projected climate change scenarios, these conditions have the potential to become natural refuges for these algae.
Author Contributions
LS designed the experiments, carried out the research, analyzed the data, and wrote the manuscript. RS and IB contributed to the experimental design, interpretation of data, and revision of the manuscript. CF participated in the research, analysis, and interpretation of data. JS designed the experiments, helped with the research, analysis of data, and revision of the manuscript. The submitted version was approved by all the authors.
Funding
This study received Portuguese national funds from FCT—Foundation for Science and Technology through projects PTDC/MAR/115789/2009 and UIDB/04326/2020. The first author (LS) was funded by the FCT doctoral grant SFRH/BD/76762/2011.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We deeply thank Miguel Rodrigues from Divespot, and Rogerio Nuno Ferreira for their help during the fieldwork. We will like to thank four reviewers for their constructive comments on the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00136/full#supplementary-material
References
Adey, W., Halfar, J., Humphreys, A., Suskiewicz, T., Belanger, D., Gagnon, P., et al. (2015). Subarctic rhodolith beds promote longevity of crustose coralline algal buildups and their climate archiving potential. Palaios 30, 281–293. doi: 10.2110/palo.2014.075
Amado-Filho, G. M., Moura, R. L., Bastos, A. C., Salgado, L. T., Sumida, P. Y., Guth, A. Z., et al. (2012). Rhodolith beds are major CaCO3 bio-factories in the tropical south west atlantic. PLoS One 7:e35171. doi: 10.1371/journal.pone.0035171
Attard, K. M., Stahl, H., Kamenos, N. A., Turner, G., Burdett, H. L., and Glud, R. N. (2015). Benthic oxygen exchange in a live coralline algal bed and an adjacent sandy habitat: an eddy covariance study. Mar. Ecol. Prog. Ser. 535, 99–115. doi: 10.3354/meps11413
Barberá, C., Bordehore, C., Borg, J. A., Glémarec, M., Grall, J., Hall-Spencer, J. M., et al. (2003). Conservation and management of northeast Atlantic and Mediterranean maërl beds. Aquat. Conserv. 13, 65–76. doi: 10.1002/aqc.569
Beer, S., and Eshel, A. (1985). Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Aust. J. Mar. Freshw. Res. 36, 785–792. doi: 10.1071/MF9850785
Beer, S., Vilenkin, B., Weil, A., Veste, M., Susel, L., and Eshel, A. (1998). Measuring photosynthetic rates in seagrass by pulse amplitude modulated (PAM) fluorometry. Mar. Ecol. Prog. Ser. 174, 293–300. doi: 10.3354/meps174293
Blake, C., and Maggs, C. A. (2003). Comparative growth rates and internal banding periodicity of maerl species (Corallinales, Rhodophyta) from northern Europe. Phycologia 42, 606–612. doi: 10.2216/i0031-8884-42-6-606.1
Bosence, D. W. (1976). Ecological studies on two unattached coralline algae from western Ireland. Palaeontology 19, 365–395.
Briggs, A. A., and Carpenter, R. C. (2019). Contrasting responses of photosynthesis and photochemical efficiency to ocean acidification under different light environments in a calcifying alga. Sci. Rep. 9:3986. doi: 10.1038/s41598-019-40620-8
Brodie, J., Williamson, C. J., Smale, D. A., Kamenos, N. A., Mieszkowska, N., Santos, R., et al. (2014). The future of the northeast Atlantic benthic flora in a high CO2 world. Ecol. Evol. 4, 2787–2798. doi: 10.1002/ece3.1105
Burdett, H. L., Hennige, S. J., Francis, F. T. Y., and Kamenos, N. A. (2012). The photosynthetic characteristics of red coralline algae, determined using pulse amplitude modulation (PAM) fluorometry. Bot. Mar. 55, 499–509. doi: 10.1515/bot-2012-0135
Burdett, H. L., Keddie, V., MacArthur, N., McDowall, L., McLeish, J., Spielvogel, E., et al. (2014). Dynamic photoinhibition exhibited by red coralline algae in the red sea. BMC Plant Biol. 14:139. doi: 10.1186/1471-2229-14-139
Carro, B., López, L., Peña, V., Bárbara, I., and Barreiro, R. (2014). DNA barcoding allows the accurate assessment of European maerl diversity : a Proof-of-Concept study. Phytotaxa 190, 176–189. doi: 10.11646/phytotaxa.190.1.12
Cornwall, C. E., Comeau, S., DeCarlo, T. M., Larcombe, E., Moore, B., Giltrow, K., et al. (2020). A coralline alga gains tolerance to ocean acidification over multiple generations of exposure. Nat. Clim. Change 10, 149–146. doi: 10.1038/s41558-019-0681-8
Donohue, P. J. C. (2015). The Effects of Acidification and Warming on Marine Calcifying Biota. Ph.D. thesis, University of Glasgow, Scotland, 256.
Egilsdottir, H., Olafsson, J., and Martin, S. (2016). Photosynthesis and calcification in the articulated coralline alga Ellisolandia elongata (Corallinales, Rhodophyta) from intertidal rock pools. Eur. J. Phycol. 51, 59–70. doi: 10.1080/09670262.2015.1101165
Figueiredo, M. A. O., Coutinho, R., Villas-Boas, A. B., Tâmega, F. T. S., and Mariath, R. (2012). Deep-water rhodolith productivity and growth in the southwestern Atlantic. J. Appl. Phycol. 24, 487–493. doi: 10.1007/s10811-012-9802-8
Figueiredo, M. A. O., Kain, J. M., and Norton, T. A. (2000). Responses of crustose corallines to epiphyte and canopy cover. J. Phycol. 36, 17–24. doi: 10.1046/j.1529-8817.2000.98208.x
Figueroa, F. L., Conde-Álvarez, R., and Gómez, I. (2003). Relations between electron transport rates determined by pulse amplitude modulated chlorophyll fluorescence and oxygen evolution in macroalgae under different light conditions. Photosynth. Res. 75, 259–275. doi: 10.1023/A:1023936313544
Figueroa, F. L., Domínguez-González, B., and Korbee, N. (2014). Vulnerability and acclimation to increased UVB radiation in three intertidal macroalgae of different morpho-functional groups. Mar. Environ. Res. 97, 30–38. doi: 10.1016/j.marenvres.2014.01.009
Foster, M. S., Amado-Filho, G. M., Kamenos, N. A., Riosmena-Rodriguez, R., and Steller, D. L. (2013). “Rhodoliths and rhodolith beds,” in Research and Discoveries: The Revolution of Science Through SCUBA, eds M. A. Lang, R. L. Marinelli, S. J. Roberts, and P. R. Taylor, (Washington, DC: Smithsonian Contributions to the Marine Sciences), 143–155.
Freiwald, A., and Henrich, R. (1994). Reefal coralline algal build-ups within the Arctic Circle: morphology and sedimentary dynamics under extreme environmental seasonality. Sedimentology 41, 963–984. doi: 10.1111/j.1365-3091.1994.tb01435.x
Gao, K., and Zheng, Y. (2010). Combined effects of ocean acidification and solar UV radiation on photosynthesis, growth, pigmentation and calcification of the coralline alga Corallina sessilis (Rhodophyta). Glob. Change Biol. 16, 2388–2398. doi: 10.1111/j.1365-2486.2009.02113.x
Garel, E., and Ferreira, Ó (2015). Multi-year high-frequency physical and environmental observations at the Guadiana Estuary. Earth Syst. Sci. Data 7, 299–309. doi: 10.5194/essd-7-1-2015
Genty, B., Briantais, J., and Baker, N. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990, 87–92. doi: 10.1016/S0304-4165(89)80016-9
Goh, C. H., Ko, S. M., Koh, S., Kim, Y. J., and Bae, H. J. (2012). Photosynthesis and environments: photoinhibition and repair mechanisms in plants. J. Plant Biol. 55, 93–101. doi: 10.1007/s12374-011-9195-2
Grzymski, J., Johnsen, G., and Sakshaug, E. (1997). The significance of intracellular self-shading on the bio optical properties of brown, red and green macroalgae. J. Phycol. 33, 408–414. doi: 10.1111/j.0022-3646.1997.00408.x
Halfar, J., Adey, W. H., Kronz, A., Hetzinger, S., Edinger, E., and Fitzhugh, W. W. (2013). Arctic sea−ice decline archived by multicentury annual−resolution record from crustose coralline algal proxy. Proc. Natl. Acad. Sci. U.S.A. 110, 19737–19741. doi: 10.1073/pnas.1313775110
Hall-Spencer, J. M., Kelly, J., and Maggs, C. A. (2010). Background Document for Maerl. London: OSPAR Commission.
Hernandez-Kantun, J. J., Hall-Spencer, J. M., Grall, J., Adey, W., Rindi, F., Maggs, C. A., et al. (2017). “North Atlantic rhodolith/maërl beds,” in Rhodolith/Maërl Beds: A Global Perspective. Coastal Research Library, eds R. Riosmena-Rodriguez, W. Nelson, and J. Aguirre, (Cham: Springer), 265–279. doi: 10.1007/978-3-319-29315-8_10
Hunt, R. (1990). “Relative growth rates,” in Basic Growth Analysis, eds G. E. Wickens, J. R. Goodin, and D. V. Field, (London: Unwin Hyman), 25–34. doi: 10.1007/978-94-010-9117-6_3
Kalituho, L. N., Pshybytko, N. L., Kabashnikova, L. F., and Jahns, P. (2003). Photosynthetic apparatus and high temperature: role of light. Bulg. J. Plant Physiol. 29, 281–289.
Kim, J. K., Kraemer, G. P., Neefus, C. D., Chung, I. K., and Yarish, C. (2007). Effects of temperature and ammonium on growth, pigment production and nitrogen uptake by four species of Porphyra (Bangiales, Rhodophyta) native to the New England coast. J. Appl. Phycol. 19, 431–440. doi: 10.1007/s10811-006-9150-7
Krug, L. A., Platt, T., Sathyendranath, S., and Barbosa, A. B. (2017). Unravelling region-specific environmental drivers of phytoplankton across a complex marine domain (off SW Iberia). Remote Sens. Environ. 203, 162–184. doi: 10.1016/j.rse.2017.05.029
Legrand, E., Riera, P., Lutier, M., Coudret, J., Grall, J., and Martin, S. (2017). Species interactions can shift the response of a maërl bed community to ocean acidification and warming. Biogeosciences 14, 5359–5376. doi: 10.5194/bg-14-5359-2017
Lichtenthaler, H. K., and Buschmann, C. (2001). “Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy,” in Current Protocols in Food Analytical Chemistry (CPFA), eds R. E. Wrolstad, T. E. Acree, H. An, E. A. Decker, M. H. Penner, D. S. Reid, et al. (New York, NY: John Wiley and Sons). F4.3.1–F4.3.8.
Lin, R., and Stekoll, M. S. (2011). Phycobilin content of the conchocelis phase of Alaskan Porphyra (bangiales, rhodophyta) species: responses to environmental variables. J. Phycol. 47, 208–214. doi: 10.1111/j.1529-8817.2010.00933.x
Martin, S., Castets, M.-D., and Clavier, J. (2006). Primary production, respiration and calcification of the temperate free-living coralline alga Lithothamnion corallioides. Aquat. Bot. 85, 121–128. doi: 10.1016/j.aquabot.2006.02.005
Martin, S., Charnoz, A., and Gattuso, J. P. (2013a). Photosynthesis, respiration and calcification in the Mediterranean crustose coralline alga Lithophyllum cabiochae (Corallinales, Rhodophyta). Eur. J. Phycol. 48, 163–172.
Martin, S., Clavier, J., Chauvaud, L., and Thouzeau, G. (2007). Community metabolism in temperate maërl beds. I. Carbon and carbonate fluxes. Mar. Ecol. Prog. Ser. 335, 19–29. doi: 10.3354/meps335019
Martin, S., Cohu, S., Vignot, C., Zimmerman, G., and Gattuso, J.-P. (2013b). One-year experiment on the physiological response of the Mediterranean crustose coralline alga, Lithophyllum cabiochae, to elevated pCO2 and temperature. Ecol. Evol. 3, 676–693. doi: 10.1002/ece3.475
Martin, S., and Hall−Spencer, J. M. (2017). “Effects of ocean warming and acidification on rhodolith/maërl beds,” in Rhodolith/Maërl Beds: A Global Perspective. Coastal Research Library, eds R. Riosmena−Rodríguez, W. Nelson, and J. Aguirre, (Cham: Springer), 55–85. doi: 10.1007/978-3-319-29315-8_3
McCoy, S. J., and Kamenos, N. A. (2015). Coralline algae in a changing world: integrating ecological, physiological, and geochemical responses to global change. J. Phycol. 51, 6–24. doi: 10.1111/jpy.12262
NOAA (2013). National Centers for Environmental Information, State of the Climate: Global Climate Report for July 2013, Published Online August 2013. Available online at: https://www.ncdc.noaa.gov/sotc/global/201307 (accessed February 4, 2019).
Noisette, F., Duong, G., Six, C., Davoult, D., and Martin, S. (2013). Effects of elevated pCO2 on the metabolism of a temperate rhodolith Lithothamnion corallioides grown under different temperatures. J. Phycol. 49, 746–757. doi: 10.1111/jpy.12085
Peña, V., and Bárbara, I. (2010). Seasonal patterns in the maërl community of shallow European Atlantic beds and their use as a baseline for monitoring studies. Eur. J. Phycol. 45, 327–342. doi: 10.1080/09670261003586938
Peña, V., and Bárbara, I. (2013). Non-coralline crustose algae associated with maërl beds in Portugal: a reappraisal of their diversity in the Atlantic Iberian beds. Bot. Mar. 55, 481–493. doi: 10.1515/bot-2013-0083
Peña, V., Pardo, C., López, L., Carro, B., Hernandez-Kantun, J., Adey, W. H., et al. (2015). Phymatolithon lusitanicum sp. nov. (Hapalidiales, Rhodophyta): the third most abundant maërl-forming species in the Atlantic Iberian Peninsula. Crypt. Algol. 36, 429–459. doi: 10.7872/crya/v36.iss4.2015.429
Pereira, D. C., Trigueiro, T. G., Colepicolo, P., and Marinho-Soriano, E. (2012). Seasonal changes in the pigment composition of natural population of Gracilaria domingensis (Gracilariales. Rhodophyta). Rev. Bras. Farmacogn. 22, 874–880. doi: 10.1590/S0102-695X2012005000075
Prasil, O., Kolber, Z., Berry, J. A., and Falkowski, P. G. (1996). Cyclic electron flow around Photosystem II in vivo. Photosynth. Res. 48, 395–410. doi: 10.1007/BF00029472
Reis, M. O., Necchi, O., Pio, C., and Marcelo, B. (2011). Co-stressors chilling and high light increase photooxidative stress in diuron-treated red alga Kappaphycus alvarezii but with lower involvement of H2O2. Pest. Biochem. Physiol. 99, 7–15. doi: 10.1016/j.pestbp.2010.09.003
Ritchie, R. J. (2008). Universal chlorophyll equations for estimating chlorophylls a, b, c and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol or ethanol solvents. Photosynthetica 46, 115–126. doi: 10.1007/s11099-008-0019-7
Roberts, R. D., Kühl, M., Glud, R. N., and Rysgaard, S. (2002). Primary production of crustose coralline red algae in a high arctic fjord. J. Phycol. 38, 273–283. doi: 10.1046/j.1529-8817.2002.01104.x
Sampath-Wiley, P., and Neefus, C. (2007). An improved method for estimating R-phycoerythrin and R-phycocyanin contents from crude aqueous extracts of Porphyra (Bangiales, Rhodophyta). J. Appl. Phycol. 19, 123–129. doi: 10.1007/s10811-006-9118-7
Schoenrock, K., Vad, J., Muth, A., Pearce, D., Rea, B., Schofield, J., et al. (2018). Biodiversity of Kelp Forests and Coralline Algae Habitats in Southwestern Greenland. Diversity 10:117. doi: 10.3390/d10040117
Schoenrock, K. M., Bacquet, M., Pearce, D., Rea, B. R., Schofield, J. E., Lea, J., et al. (2018). Influences of salinity on the physiology and distribution of the arctic coralline algae, Lithothamnion glaciale (Corallinales, Rhodophyta). J. Phycol. 54, 690–702. doi: 10.1111/jpy.12774
Schreiber, U., Bilger, W., and Neubauer, C. (1995). “Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis,” in Ecophysiology of Photosynthesis, eds E. D. Schulze, and M. M. Caldwell, (Berlin: Springer-Verlag), 49–70. doi: 10.1007/978-3-642-79354-7_3
Short, J. A., Pedersen, O., and Kendrick, G. A. (2015). Turf algal epiphytes metabolically induce local pH increase, with implications for underlying coralline algae under ocean acidification. Estuar. Coast. Shelf. Sci. 164, 463–470. doi: 10.1016/j.ecss.2015.08.006
Silva, J., and Santos, R. (2004). Can chlorophyll fluorescence be used to estimate photosynthetic production in the seagrass Zostera noltii? J. Exp. Mar. Biol. Ecol. 307, 207–216. doi: 10.1016/j.jembe.2004.02.009
Smith, E. L. (1936). Photosynthesis in relation to light and carbon dioxide. Proc. Natl. Acad. Sci. U.S.A. 22, 504–511. doi: 10.1073/pnas.22.8.504
Sordo, L., Santos, R., Barrote, I., and Silva, J. (2018). High CO2 decreases the long -term resilience of the free-living coralline algae Phymatolithon lusitanicum. Ecol. Evol. 8, 4781–4792. doi: 10.1002/ece3.4020
Sordo, L., Santos, R., Barrote, I., and Silva, J. (2019). Temperature amplifies the effect of high CO2 on the photosynthesis, respiration, and calcification of the coralline algae Phymatolithon lusitanicum. Ecol. Evol. 9, 11000–11009. doi: 10.1002/ece3.5560
Sordo, L., Santos, R., Reis, J., Shulika, A., and Silva, J. (2016). A direct CO2 control system for ocean acidification experiments: testing effects on the coralline red algae Phymatolithon lusitanicum. PeerJ 4:e2503. doi: 10.7717/peerj.2503
Steller, D. L., and Cáceres-Martinez, C. (2009). Coralline algal rhodoliths enhance larval settlement and early growth of the Pacific calico scallop Argopecten ventricosus. Mar. Ecol. Prog. Ser. 396, 49–60. doi: 10.3354/meps08261
Steller, D. L., Hernandez-Ayon, J. M., Riosmena-Rodriguez, R., and Cabello-Pasini, A. (2007). Effect of temperature on photosynthesis, growth and calcification rates of the free-living coralline alga Lithophyllum margaritae. Cienc. Mar. 33, 441–456. doi: 10.7773/cm.v33i4.1255
Stengel, D. B., Conde-Álvarez, R., Connan, S., Nitschke, U., Arenas, F., Abreu, H., et al. (2014). Short-term effects of CO2, nutrients and temperature on three marine macroalgae under solar radiation. Aquat. Biol. 22, 159–176. doi: 10.3354/ab00576
Talling, J. F. (1957). Photosynthetic characteristics of some freshwater plankton diatoms in relation to underwater radiation. New Phytol. 56, 29–50. doi: 10.1111/j.1469-8137.1957.tb07447.x
Torres, P. B., Chow, F., Furlan, C. M., Mandelli, F., Mercadante, A., and Santos, D. Y. A. C. (2014). Standardization of a protocol to extract and analyze chlorophyll a and carotenoids in Gracilaria tenuistipitata Var. Liui. Zhang and Xia (Rhodophyta). Braz. J. Oceanogr. 62, 57–63. doi: 10.1590/s1679-87592014068106201
van der Heijden, L. H., and Kamenos, N. A. (2015). Calculating the global contribution of coralline algae to carbon burial. Biogeosci. Discuss. 12, 7845–7877. doi: 10.5194/bgd-12-7845-2015
Vásquez-Elizondo, R. M., and Enríquez, S. (2016). Coralline algal physiology is more adversely affected by elevated temperature than reduced pH. Sci. Rep. 6:19030. doi: 10.1038/srep19030
Keywords: coralline algae (maërl), photosynthesis, respiration, calcification, photosynthetic pigments, temperature, irradiance
Citation: Sordo L, Santos R, Barrote I, Freitas C and Silva J (2020) Seasonal Photosynthesis, Respiration, and Calcification of a Temperate Maërl Bed in Southern Portugal. Front. Mar. Sci. 7:136. doi: 10.3389/fmars.2020.00136
Received: 31 October 2018; Accepted: 20 February 2020;
Published: 19 March 2020.
Edited by:
Christos Dimitrios Arvanitidis, Hellenic Centre for Marine Research (HCMR), GreeceReviewed by:
Figueroa L. Felix, University of Málaga, SpainJosé M. Rico, University of Oviedo, Spain
Copyright © 2020 Sordo, Santos, Barrote, Freitas and Silva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Sordo, laura.sordo@ipma.pt; l.sordodelasnieves@hotmail.com; João Silva, jmsilva@ualg.pt
 Laura Sordo
Laura Sordo Rui Santos
Rui Santos Isabel Barrote
Isabel Barrote Cátia Freitas
Cátia Freitas João Silva
João Silva