In Vivo and Clinical Studies of Sea Cucumber-Derived Bioactives for Human Health and Nutrition From 2012-2021
- 1Advanced Marine Biomanufacturing Laboratory, Centre for Marine Bioproducts Development, College of Medicine and Public Health, Flinders University, Adelaide, SA, Australia
- 2School of Basic Medicine, Shanxi University of Chinese Medicine, Taiyuan, China
- 3Biology Institute, Qilu University of Technology (Shandong Academy of Sciences), Jinan, China
- 4China-Australia Joint Laboratory for Native Bioresource Industry Innovation, Qilu University of Technology (Shandong Academy of Sciences), Jinan, China
Sea cucumbers are amongst the highest value seafoods available commercially, especially in the south-east Asian region, primarily due to their nutritional and health benefits as applied in Traditional Chinese Medicine. While the majority of studies for nutritional products derived from sea cucumber compounds have been conducted in vitro, the number of in vivo and evidence-based human clinical studies are limited. This review has critically assessed the advances in in vivo and clinical studies of sea cucumber-derived bioactives (both extracts and compounds) via a comprehensive literature research on papers published in the last ten years from 2012-2021. Sea cucumber-derived compounds were reported to have the following activities: anticancer, antihyperlipidemic, antihyperglycemic, anti-inflammatory, anticoagulant/antithrombotic, antioxidant, and antihypertension, immunomodulatory, wound healing and as treatments for Alzheimer’s and Parkinson’s diseases. These active compounds include triterpene glycosides (saponins), fucosylated chondroitin sulfate (FCS), cerebrosides, glycosaminoglycan, fucoidan, phospholipids, polysaccharides, peptides, long-chain bases, Frondanol A5, acid mucopolysaccharide, and phosphatidylcholines. Gaps, challenges and future directions have been identified and discussed separately to progress different areas of research and to further scientific validation, development and application of sea cucumbers for human health and nutritional products.
Introduction
The oceans cover 70% of the world’s surface and contain approximately 75% of all life on earth. Marine organisms have developed unique health promotive properties and potential bioactive compounds due to the wide range of environmental challenges in the regions they inhabit. Foods and natural health products or supplements containing marine bioactives and nutraceuticals are expected to bestow health benefits to human beings (Lobine et al., 2021).
Amongst the various marine resources, Holothuroidea, commonly known as sea cucumbers, or by the term beche-de-mer in Australia, have been widely researched throughout the world for their potential health benefits. The sea cucumber has a long history of utilization as a food source in south-east Asia (Slater and Chen, 2015) and the proximate composition of sea cucumbers varies significantly depending on the species, season, and food regimen (Lobine et al., 2021). On a dry weight basis, sea cucumbers are reported to contain up to 83% protein, comprised primarily of the amino acids glutamic, glycine, aspartic leucine, histidine, lysine, threonine, arginine, valine, and isoleucine (Lobine et al., 2021). Moreover, sea cucumbers contain thiamine, riboflavin, retinol/carotenoid complex, niacin, minerals and trace elements, and very low concentrations of omega-3 fatty acids (Bordbar et al., 2011). In Traditional Chinese Medicine, sea cucumbers have been used as a general tonic, as a treatment for skeletal and joint weakness including that linked with age associated inflexibility, kidney system disorders, impotence, dry-stool constipation, poor lipid digestion, and circulatory ailments (Gopakumar and Gopakumar, 2020). Creams based on the oil extracted from the sea cucumber have been used as treatments for wounds, aches, and skin diseases, particularly in Malaysia (Slater and Chen, 2015).
Sea cucumbers have a leathery skin, a gelatinous body wall and internal organs (Figure 1). Sea cucumber extracts are prepared primarily from the body wall tissue and longitudinal muscle, and the large range of bioactive compounds includes chondroitin sulfates, glycosaminoglycan, saponins/triterpene polysaccharides, oligosaccharides, sulfated polysaccharide sterols, cerebrosides, glucocerebrosides, lectins, peptides, mucopolysaccharides, phenols, and flavonoids (Bordbar et al., 2011). The presence of these active compounds in sea cucumbers indicates their suitability for various biotechnological applications including pharmaceutical, nutraceutical and cosmeceutical products (Slater and Chen, 2015). Details on the bioactive compounds from sea cucumbers and their biological functions are provided in a review by Xu et al. (2018).
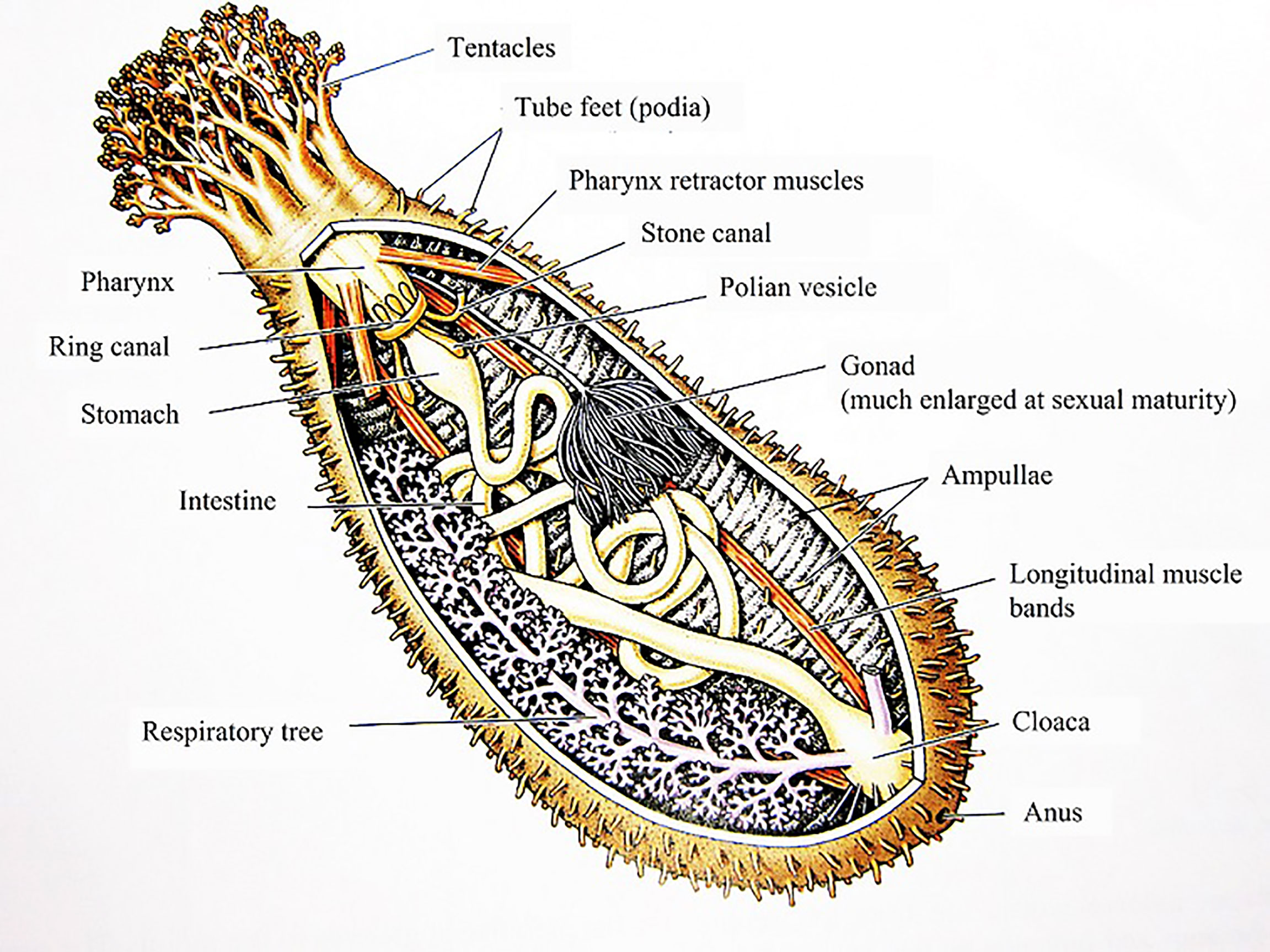
Figure 1 Diagram of the internal anatomy of a generalized Holothuroid. Illustration adapted from Wikimedia commons. (https://www.gbri.org.au/SpeciesList/Actinopygaechinites%7CDaltonBaker?PageContentID=3829).
In vivo and clinical studies are critical steps not only toward drug development but also to confirm the function and bioactivity of compounds for potential application in functional health foods or other health products. These studies develop and validate an understanding of the mechanisms of action of the compounds within the body system. For this reason, these studies are significant within health and nutritional product development for the purpose of disease prevention, treatment and health management (Gur et al., 2018). However, there is no comprehensive review currently available in the public domain despite the high potential value of these studies. Hence, this review includes a critical assessment of in vivo and clinical studies conducted using sea cucumber-derived compounds over the last ten years (2012-2021). A literature search was conducted using the search engines Scopus, PubMed, Web of Science and ScienceDirect. The search terms used were ‘sea cucumber AND in vivo’, and ‘sea cucumber AND clinical trial’. There are 101 papers have been identified and they are separately summarized in Supplementary Table 1(in vivo studies) and Table 1 (clinical studies). The focus of this review is to identify the challenges and research gaps in in vivo and clinical studies pertaining to the health applications of sea cucumber derived compounds to raise the profile and significance of the sea cucumber as a high value marine organism.
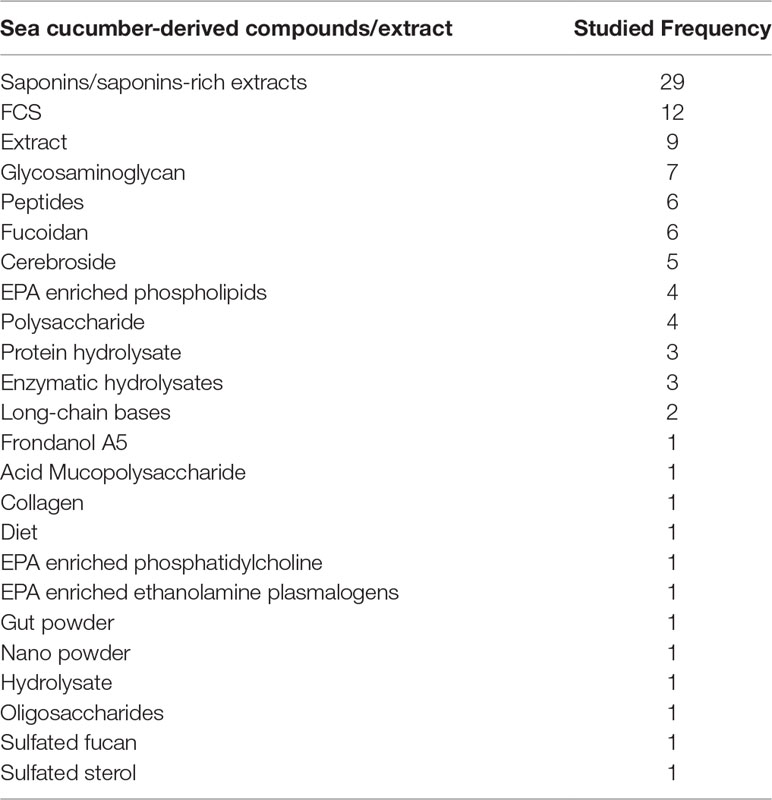
Table 1 The frequency of sea cucumber derived compounds/extracts in in-vivo research and clinical studies over the period from 2012-2021.
2 Research Trends Over the Last Ten Years
Based on our search findings, extracts or compounds derived from a total of 33 different sea cucumber species have been studied using in vivo models. Three species - Cucumaria frondosa, Pearsonothuria graeffei, Acaudina molpadioides - are most researched and account for 17%, 14%, and 11% of in vivo studies, respectively. Altogether 39 diseases were investigated in association with compounds or extracts derived from sea cucumbers using animal models. Studies targeting these diseases represented as a percentage, 27% tumors and cancer, 22% lipid metabolism (hyperlipidemia, obesity, nonalcoholic fatty liver) and 14% glucose metabolism (hyperglycemia, insulin resistance, diabetes). 15 sea cucumber species studied had compounds that were beneficial for the treatment of more than one type of disease (Figure 2). The sea cucumber species used for treating the most types of diseases include C. frondosa and P. graeffei (9 types of diseases), A. molpadioides and A. japonicus (7 types of diseases) and H. leucospilota (6 types of diseases) (Figure 2).
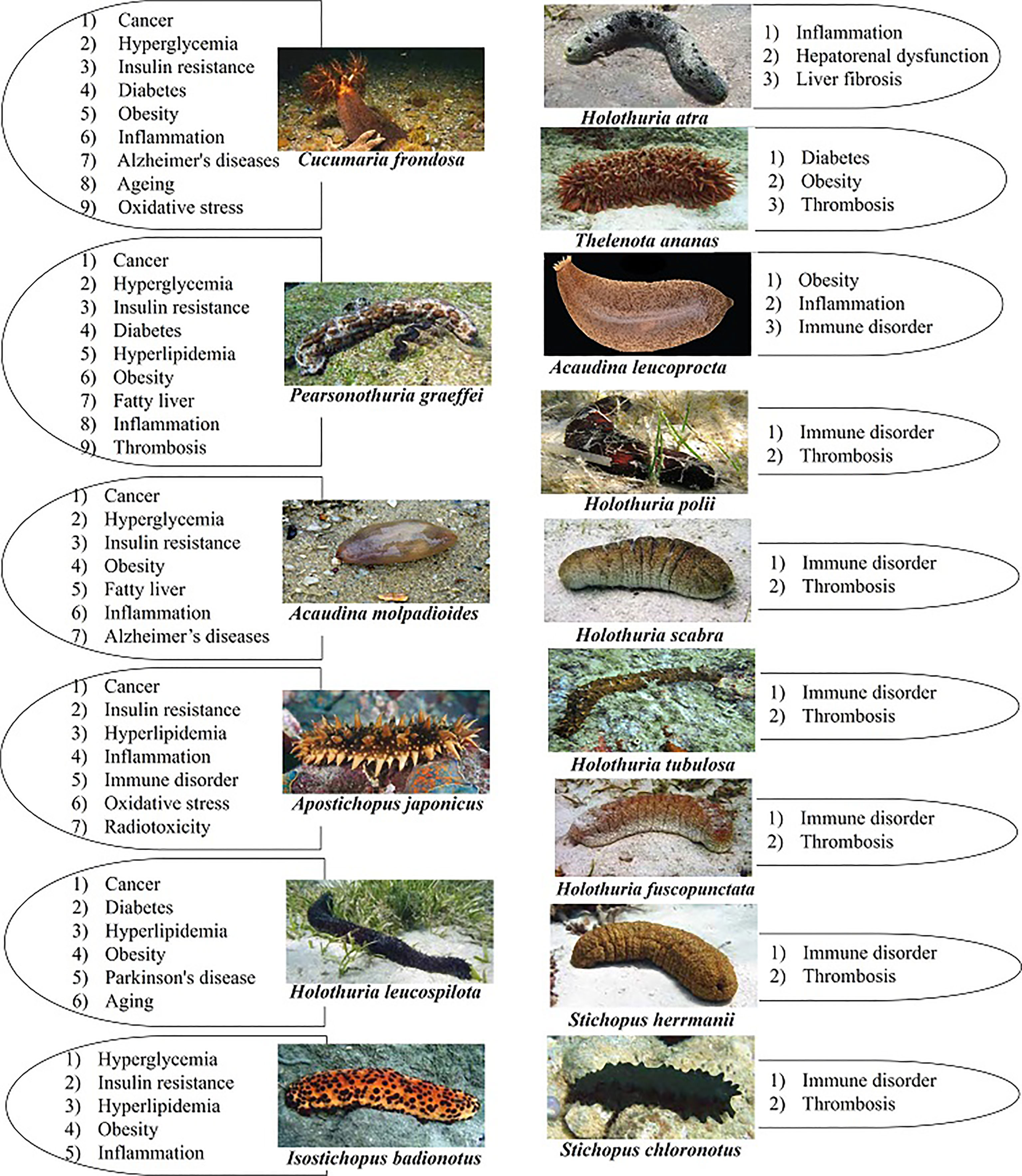
Figure 2 Species of sea cucumber that were studied in vivo for the treatment of more than one type of disease from 2012-2021 with the names of the corresponding targeted diseases. Photograph of live species of P. graeffei, H. leucospilota, H. atra, T. ananas, H. scabra, H. fuscopunctata, S. herrmanii, and S. chloronotus by S.W. Purcell; C. frondose by J.F. Hamel & A. Mercier; A. molpadioides by Chantal Conand (https://commons.wikimedia.org/w/index.php?curid=42992383); A. japonicus by A. Semenov; I. badionotus by E. Ortiz; H. polii by Rpillon; A. leucoprocta by Jose Christopher Escano Mendoza O'Loughlin and Ong (2015); H. tubulosa by Frédéric Ducarme. Photographs from the book of Purcell et al. (2012). Commercially important sea cucumbers of the world. Saponins were the most frequently studied compounds or components of extracts in the last ten years. Major focus was given to the saponin extracts and compounds of Frondoside A, Cucumariosides, and Echinoside (Figure 3). In addition to saponins, the groups of sea cucumber compounds receiving the most attention in the last ten years include fucosylated chondroitin sulfate (FCS), cerebrosides, fucoidan and glycosaminoglycans (Table 1).
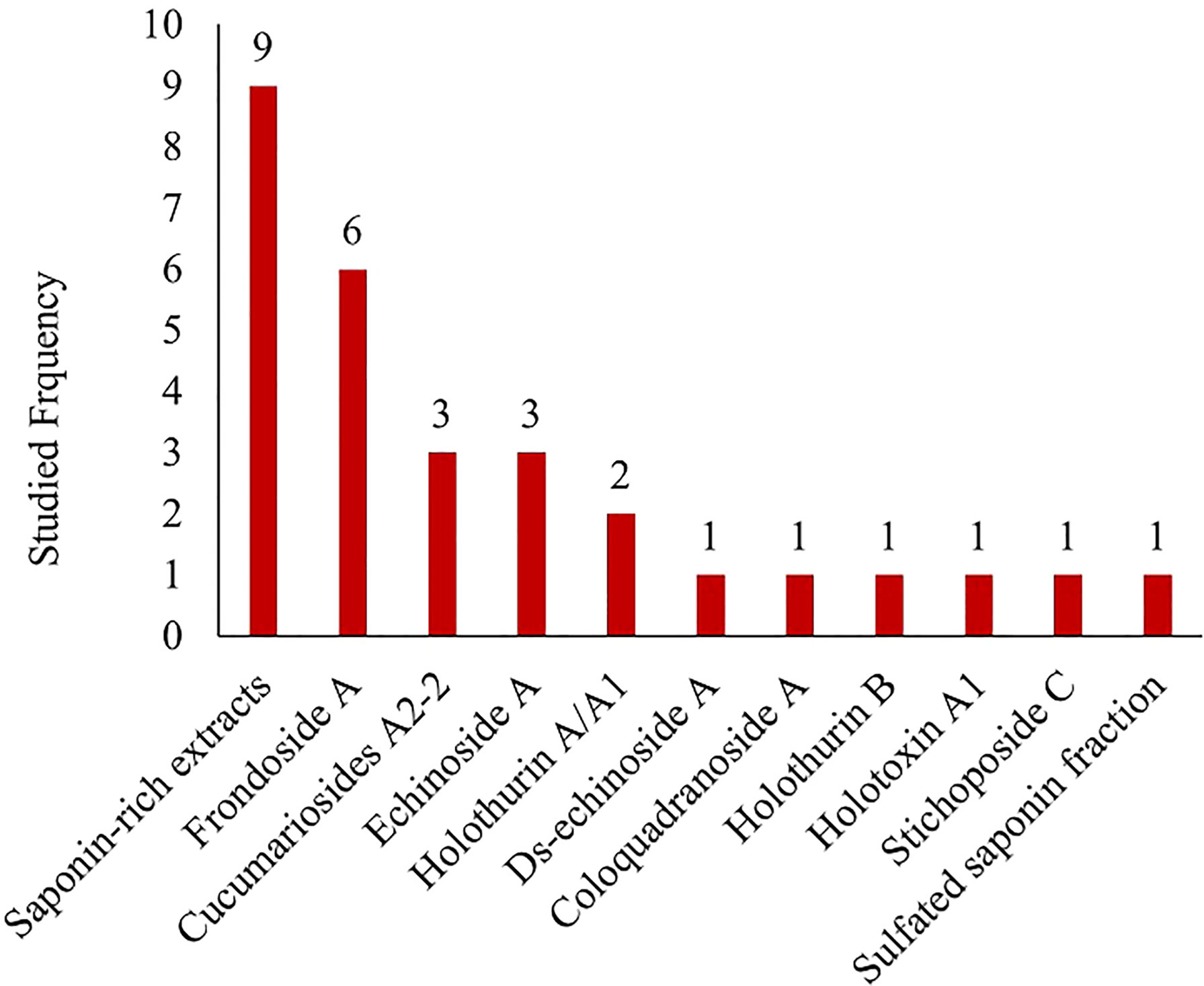
Figure 3 The frequency of sea cucumber derived saponins and saponin-rich extracts researched in-vivo over the period from 2012-2021.
3 In Vivo and Clinical Studies Involving Sea Cucumber Derived Extracts and Compounds
3.1 Cancer
Foods play an important and medicinal role in the prevention and management of cancer (Zhang et al., 2015; Kapinova et al., 2018). The sea cucumber is a representative medicinal food that has been widely studied for its anticancer effects (Wargasetia and Permana, 2018; Eso et al., 2020). In the last 10 years, there have been 23 in vivo studies and 1 clinical trial that focus on the anticancer properties of sea cucumber-derived bioactives. Saponin, cerebrosides, acid mucopolysaccharide, peptides, and FCS are five most-studied compounds derived from the sea cucumber that have proven anticancer activities in animal models.
3.1.1 Saponins
Saponins accounted for 72% of sea cucumber studies in the tumor and cancer management field. In vivo studies of saponins included Frondoside A, Echinoside A, Cucumarioside А2-2, Holotoxin A1, Stichoposide C and their chemical structures are shown in Figure 4.
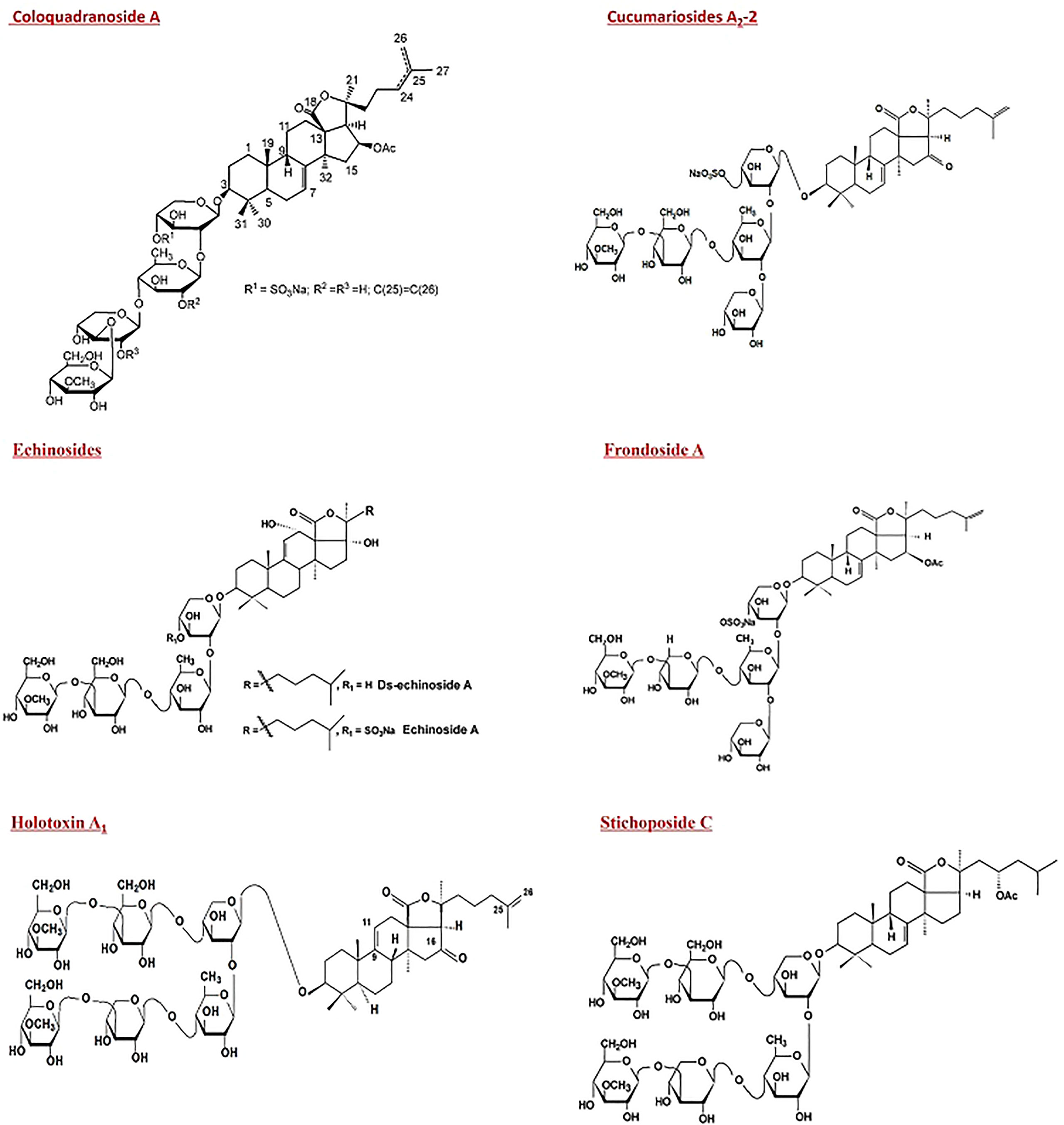
Figure 4 Chemical structure of saponins derived from sea cucumbers that exhibit in vivo activities against cancer.
3.1.2 Frondoside A
The anticancer activities of Frondoside A have been investigated in breast cancer (Ma et al., 2012), lung cancer (Attoub et al., 2013), pancreatic cancer (Li et al., 2008; Al Shemaili et al., 2014; Al Shemaili et al., 2016) and prostate cancer (Dyshlovoy et al., 2016) in mouse models. Intraperitoneal injection (i.p.) of Frondoside A isolated from the Cucumaria frondosa (Li et al., 2008; Ma et al., 2012; Attoub et al., 2013; Al Shemaili et al., 2014; Al Shemaili et al., 2016) and Cucumaria okhotensis (Dyshlovoy et al., 2016) were reported to decrease tumor xenograft growth (Attoub et al., 2013; Al Shemaili et al., 2014; Al Shemaili et al., 2016; Dyshlovoy et al., 2016), reduce lung metastasis formation (Ma et al., 2012; Attoub et al., 2013; Dyshlovoy et al., 2016), and enhance the anticancer properties of chemotherapy medications (Attoub et al., 2013; Al Shemaili et al., 2014), with no toxic side-effects reported (Attoub et al., 2013; Janakiram et al., 2015; Al Shemaili et al., 2016; Dyshlovoy et al., 2016). However, no dose-response relationship could be summarized based on these studies.
Tumor xenograft growth could be inhibited by Frondoside A but no dose- and time-dependent correlation could be concluded thus far. At 10ug/kg/day, Frondoside A i.p. for 25 days was reported to inhibit the volume of tumor xenografts by 41-47% (Li et al., 2008; Attoub et al., 2013). 50 μg/kg/day for 10 days showed no impact on the size of the implanted tumor (Ma et al., 2012). At 100ug/kg/day of Frondoside A, a 40% inhibition rate was reported on day 18 (Dyshlovoy et al., 2016). When the treatment period was continued to 24 -30 days, the inhibition rate varied from 44% to 87% (Al Shemaili et al., 2014; Al Shemaili et al., 2016; Dyshlovoy et al., 2016). A dose of 800ug/kg/day resulted in a 53% inhibition rate of xenografts on the sixth day of the treatment. However, when the administration time was continued to 20 days, the inhibition rate was 55% (Dyshlovoy et al., 2016). A dose administered at 1mg/kg/day for the treatment of LNM35 xenografts for 25 days, had the inhibition rate at 43.9% (Attoub et al., 2013). A different administration mode to i.p., oral gavage, at 100ug/kg/day of Frondoside A for 30 days, was found to have no effect on the growth of AsPC-1 xenografts (Al Shemaili et al., 2016).
Frondoside A, it was found, could significantly reduce tumor metastasis at 10μg/kg/day (Attoub et al., 2013), 50μg/kg/day (Ma et al., 2012), 100ug/kg/day (Dyshlovoy et al., 2016), and 1mg/kg/day (Attoub et al., 2013) via the alternate mode of administration. Treatment with 10ug/kg/day and 1mg/kg/day for 25 days was reported to decrease lymph node metastasis of lung cancer by reducing the weight of lymph nodes by 53% and 51%, respectively (Attoub et al., 2013). At 50 μg/kg/day for 10 days it inhibited breast cancer metastasis to the lung by reducing the number of lung tumor colonies (Ma et al., 2012), whereas at 100ug/kg/day for 30 days it was found to reduce prostate cancer metastasis to the lung by reducing the number of sections from 7.11 to 2.11 (Dyshlovoy et al., 2016).
Frondoside A has also been reported to enhance the anticancer activity of selected chemotherapy medications. When 10μg/kg/day was combined with cisplatin (1mg/kg/day) for 10 days it showed more inhibition in LNM35 tumor xenograft growth than cisplatin alone (67.6% vs 46.9%) (Attoub et al., 2013). Frondoside A at 100ug/kg/day combined with gemcitabine (4mg/kg) for 30 days was found to be more effective than gemcitabine alone in terms of area under the curve and tumor weight (Al Shemaili et al., 2014).
No manifest undesirable effects of Frondoside A treatment on mice behavior and body weight (Li et al., 2008; Al Shemaili et al., 2014) and no visible abnormalities at necropsy or any other obvious signs of toxicity were found at 10ug/kg/day (Attoub et al., 2013) and 1mg/kg/day (Attoub et al., 2013) for 25 days. No significant alterations of the weight of heart, lung, liver, kidney, and no significant changes of thrombocytes and hemoglobin were reported when using Frondoside A at either 100ug/kg/day for 30 days or at 800ug/kg/day for 20 days (Dyshlovoy et al., 2016). Furthermore, the increase of spleen weight (21% increase at 100ug/kg/day and 50% increase at 800ug/kg/day) and the increase of leukocytes due to lymphocytosis was observed in both dose groups and indicated an immunomodulatory effect of Frondoside A (Dyshlovoy et al., 2016).
3.1.3 Echinoside A
Echinoside A isolated from Pearsonothuria graeffei inhibited the rate of tumor growth in a dose dependent manner. Echinoside A administered i.p. at 0.5 mg/kg/day or 2.5 mg/kg/day for 10 days could significantly inhibit tumor growth by 33.8% and 49.8% in a murine-bearing hepatoma model (Zhao et al., 2012). Another related saponin, ds-Echinoside, was reported to inhibit tumor growth by 41.1% and 55% at 0.5 and 2.5 mg/kg/day for 10 days respectively in the hepatoma murine model (Zhao et al., 2012).
3.1.4 Cucumarioside А²-2
Cucumarioside А2-2 alone and complexed with cholesterol were both reported to increase the average life span (ALS) of mice bearing Ehrlich carcinoma. Mice pre-inoculated with Ehrlich ascites carcinoma were then treated with i.p. Cucumarioside A2 -2 at 10 ug/kg for 5 days, then isolated. Subsequently, fresh mice were inoculated with tumor cells from the treated mice and even without any further treatment, the ALS of the mice was 28.9 days, while the ALS of mice inoculated with untreated cancer cells was 21.5 days. Furthermore, another group of mice received tumor cells from mice previously exposed to Cucumarioside A2-2 in vivo and then subsequently were treated with Doxorubicin (i.p. 2mg/kg/3days), the survival rate was 100% for the observation period of 70 days (Menchinskaya et al., 2019). Cucumarioside A2-2 complexed with cholesterol at 0.2ug/mouse i.p. at 4 and 1 days after Ehrlich ascites carcinoma cell inoculation increased the ALS of mice by 17.3% (Menchinskaya et al., 2013).
Cucumarioside A2-2 complexed with cholesterol showed antitumor activities and more pronounced effects occurred when combined prophylactic (treatment before incubation) and curing (treatment after incubation) treatments were administered to the Ehrlich carcinoma mouse model. Daily administration of 0.2ug/mouse, i.p. at 4 and 1 days before and 1 and 4 days after tumor cell inoculation, was reported to inhibit 25-30% tumor development (Menchinskaya et al., 2013).
3.1.5 Stichoposide C
Stichoposide C was isolated from the Thelenota anax and could rapidly (1 day after the first treatment) and markedly (P<0.01 or 0.001) reduce the growth of HL-60 xenograft and CT-26 subcutaneous tumors at 7.19μg/kg/3days via the tail vein and increase ceramide generation. After 21 days of treatment, the mean volume of tumors in mice treated with Stichoposide C was more than 70%, or 60% smaller than the tumors in mice were treated with vehicle control (Yun et al., 2012).
3.1.6 Holotoxin A1
Holotoxin A1 as a cancer management treatment was reported to reduce the adverse effects of radiation therapy. Malyarenko et al. (2020) investigated Apostichopus japonicas derived Holotoxin A1 and its activities toward the recovery of peripheral blood leukocyte number, mass, and cellularity of the lymphoid organs of irradiated mice. The results suggested that the spleen endogenous colony number and the peripheral blood leukocyte number were increased and the weight and cellularity of the lymphoid organs of the irradiated mice improved due to the holotoxin A1 injection. Furthermore, the growth of Ehrlich solid carcinoma was inhibited by 10% after X-ray radiation was combined with holotoxin A1 injection into the mice (Malyarenko et al., 2020). These results thus showed promise for enhancing radiotherapy efficiency with sea cucumber-derived holotoxin A1.
3.1.7 Coloquadranoside A
A new sulfated saponin, Coloquadranoside A, derived from the sea cucumber Colochirus quadrangularis was found an excellent antitumor activity against both homograft (S-180 and H22) and xenograft (BEL7402) mice models in vivo. Intragastrical administration of coloquadranoside A at the dose of 5 mg/kg and 50mg/kg inhibited (~35%) the weight gaining of tumors in the homograft mouse models, which was same ability as the positive drug, 5-FU. For the xenograft model of human hepatocarcinoma BEL7402, all three doses (5, 50 and 500mg/kg) intragastrically administrated for 25 days significantly lower the average tumor volume weight with the tumor growth inhibition rate > 50% and the relative tumor proliferation rate < 60% (Yang et al., 2021b).
3.1.8 Sulfated Saponins Fraction
Sulfated saponins fraction (7.48% Holothurin A, 7.53% Moebioside A, 69.29% Holothurin B, 6.92% 24-dehydroechinoside B) from Holothuria moebii at 30 and 60 mg/kg by daily i.p. for 12 days into the colorectal of CT-26 tumor mouse model, showed no significant statistical effects of the inhibiting actions. Only the dose of 120mg/kg showed a significant inhibition rate of 55.08%. This dose also induced side effects including reduced liver index and spleen index in the mouse model (Yu et al., 2015).
3.1.9 FCS
As a polysaccharide of the sea cucumber, FCS has been researched and reported to potentially inhibit tumor growth and reduce lung metastasis. When administered at 1,5, and 20 mg/kg/day i.p. for 20 days, FCS inhibited Lewis lung carcinoma growth by 25.8%, 33.1%, and 47.2% respectively, which was related to p53/p21-induced cell cycle arrest and caspase-3-induced apoptosis (Liu et al., 2016). Besides the low molecular weight, FCS demonstrated inhibition of Lewis lung carcinoma metastasis of LLC cells in a dose-dependent manner. The inhibition rates of 1, 5, and 20 mg/kg/day for 20 days were 32.4%, 52.2%, and 69.1%, respectively. These activities related VEGF-mediated angiogenesis and TIMP/MMPs-mediated metastasis by the ERK1/2/p38 MAPK/NF-kB pathway (Liu et al., 2016).
3.1.10 Cerebrosides
Cerebrosides from Acaudina molpadioides are glycosphingolipids that have shown antitumor activities in the S180 tumor mouse model. An intragastric dose at 50mg/kg/day for 10 days decreased the tumor weight by 45.24%, inhibited the ascites fluid growth by 31.23%, reduced the ascites tumor cell viability ratio by 50.89% and increased ALS by 55.28% (Lei et al., 2012). When administered for 14 days the dose could ameliorate cancer-associated cachexia by increasing body weight (via preventing adipose atrophy), increasing white adipose tissue mass, prolonging life span, with no effects on anorexia (Du et al., 2015). The long-chain base of cerebrosides could also prolong ALS (~40%) of the mouse model by both intragastric administration (i.g.) and i.p. cerebrosides by intragastric administration method could only increase the life span by ~31% (Du et al., 2015).
3.1.11 Acid Mucopolysaccharide
Antitumor study of Acid mucopolysaccharide highlighted its anti-metastasis and immunomodulatory effect. Acid mucopolysaccharide from the Stichopus japonicus at 17.5 mg/kg, 35 mg/kg, and 70 mg/kg for 5 days per week (for a period of 15 weeks) via oral gavage in the hepatocellular carcinoma rat model could reduce both the numbers and mean volumes of nodules. Moreover, the mean thymus indices and spleen index increased, and CD3+ and CD4+ T lymphocyte levels recovered significantly and the CD4+/CD8+ T cells ratio normalized in a dose-dependent manner. Stimulating immune organs, increasing tumor suppressor protein P21 and inhibiting the marker of cell proliferation PCNA are the possible anti-cancer mechanisms of Acid mucopolysaccharide (Song et al., 2013).
3.1.12 Oligosaccharides
Zhou et al. (2021) reported a native fucosylated glycosaminoglycan (nHG) extracted from sea cucumber Holothuria fuscopunctata and a depolymerized nHG (dHG) and its contained oligosaccharides (hs17, hs14, hs11, hs8 and hs5), acting as heparinase inhibitors. The research team investigated the antimetastatic effects of oligosaccharides hs17, hs11, and hs8 on a Luc-4T1 mammary carcinoma metastasis experimental mouse model. At the dose of 0.2mg/kg, the groups treated with hs17 (0.2 mg/kg) had almost no tumor cells metastasized to the lungs. Oligosaccharides hs11 and hs8 significantly reduced the size of metastasized lung tumors (Zhou et al., 2021).
3.1.13 Peptides
Latest studies focused on sea cucumber peptides and their anti-cancer properties (Wei et al., 2021; Yu et al., 2021). The human breast cancer cells of MCF-7 were transplanted into the zebrafish yolk sac and three different concentrations of sea cucumber intestinal peptide (SCIP) (27.8, 83.3, and 250μg/ml) were administrated to the model. After the incubation of MCF-7 with SCIP for 24 hours, 48 hours, and 72 hours, the activities of MCF-7 cells showed a time-dependent downward in all the SCIP groups. However, when compared with the low dose SCIP group (27.8μg/ml), only the cells in high dose SCIP group (250μg/ml) showed significant decreased activities after incubation for 24 hour; when compared among three different incubation time points, only the cell of high dose SCIP group showed significantly lower activities after incubation of 72 h than that in the 24 h of SCIP stimulation. Promoting the expression of apoptosis-related proteins and inhibiting the PI3K/AKT signal transduction pathway could be the mechanism (Wei et al., 2021). Yu et al. (2021) using BEL-7402 cells induced Hepatocellular carcinoma nude mouse model to investigate the antitumor effects of a novel recombinant peptide from Apostichopus japonicus, rAj-Tspin. Results showed that 60μg/kg, 120μg/kg, and 240μg/kg of rAj-Tspin intraperitoneal injections twice daily for 21 days dose-dependently decreased the tumor volume and weight, and damaged tumor tissues and destroyed the tumor structure. Moreover, no effects have been found on the body weight of mice (Yu et al., 2021).
3.1.14 Sea Cucumber Extracts
Frondanol A5, alcohol/water extract of the enzymatically hydrolyzed epithelia of the Cucumaria frondosa, has been found to exhibit chemopreventive potential against colorectal cancer (Janakiram et al., 2015). Dietary administration of 250 and 500 ppm for 14 weeks, has identified suppression of the formation and size of small intestinal polyp and colon tumor multiplicities. Simultaneously increased innate immune responses is one of the reasons gender differences has been reported in this research. Female mice are more dose sensitive to Frondanol A5 in the diet than male mice (Janakiram et al., 2015).
A daily oral administration of 50, 100, and 400 mg/kg of aqueous extract of Holothuria arenicola for 2 weeks reduced the tumor volume and size in a colon cancer mouse model in a dose dependent manner. No significant change of body weight was reported in the experimental period. Furthermore, the dose of 100 and 400 mg/kg prolonged survival rate in the mouse model (Baharara et al., 2016).
Only one clinical research source focused on sea cucumber extracts thus far but does not match with the types of cancers investigated in in vivo animal studies. A phase II clinical trial of TBL-12, a product containing sea cucumber extracts, investigated the safety and efficacy of the extract in 20 patients with asymptotic multiple myeloma (Chari et al., 2018). Participants were given TBL-12 in the form of liquid gel at 80mL/day, and the administration was continued until disease progression (Chari et al., 2018). Standard to high-risk patients in the study reported good tolerance of TBL-12. Lack of death during the study (asymptotic for 6 years since the beginning of the trial) indicated significant potential for the extract to reach the next phase of trials.
3.1.15 Synergistic Activity
As a class of compound given the most attention by researchers, saponins showed the most comprehensive anticancer properties. Saponin decreased weight and volume of tumor to inhibit tumor growth. Saponins as a treatment can be beneficial when combined with conventional chemotherapy drugs or radiation therapy. Cucumarioside А2-2 and Frondoside A in combination were more effective than the chemotherapy drugs alone, and Holotoxin A1 enhanced the cancer inhibiting effect of X-ray radiation therapy. Of high importance was that case studies showed no significant side effects in mouse models except that the liver and spleen index was decreased by the Sulfated saponins fraction.
Research supports that the sea cucumber offers great potential to be developed into anticancer functional foods or drugs. Studying administration modes, however, is crucial for the further application of sea cucumber in the cancer management area. The most widely studied and promising compound, Frondoside A, as concluded from the studies, appears to be inactive through the oral route. Except for the sea cucumber extracts, cerebrosides and the mixture of Cucumarioside A2-2 and cholesterol, most of the sea cucumber compounds were i.p. administered. Thus, the method is invasive and might be associated with procedural risks making clinical experiments difficult. Some of the doses were i.v. administrated and therefore bioavailability is fundamentally needed.
3.2 Hyperglycemia, Insulin Resistance, and Diabetes
Sustained hyperglycemia impairs insulin-stimulated glucose utilization and glycogen synthesis in humans and is highly correlated with the onset of insulin resistance. Insulin resistance is a powerful predictor of the future development of type 2 diabetes and is a therapeutic target once hyperglycemia is present. Active compounds including Sulfated polysaccharides, Saponins, EPA-enriched phospholipids, sterol sulfate, and long chain bases from sea cucumber were all reported to play an important role in the management of glucose metabolism in in-vivo studies.
3.2.1 Hyperglycemia
Sulfated polysaccharides including fucoidan and FCS from sea cucumber were reported to exhibit anti-hyperglycemic effect and enhance the activities of the positive drug rosiglitazone (RSG) in the mouse model.
3.2.1.1 Fucoidan
Fucoidan at 80mg/kg/day from Isostichopus badionotus (Wang et al., 2016a) and Cucumaria frondosa (Wang et al., 2016b) dietary was administered for 19 weeks. Together both could reduce body weight gain (15.6%-25.7%), fasting blood glucose (17.6%), and increase glucose tolerance in a high-fat high-sucrose diet (HFSD) induced mouse model (Wang et al., 2016a; Wang et al., 2016b). With the fucoidan supplement a significant reduction in body weight of 13.3% was exhibited after 12 weeks, while the positive control RSG did not decrease in body weight (Wang et al., 2016a). The glucose tolerance level was identified using the area under curve (AUC) and the hemoglobin A1c (HbA1c) level. Fucoidan decreased the AUC by 18.87% (Wang et al., 2016b) and the HbA1c level by 21.9% (Wang et al., 2016a) when at the dose of 80mg/kg/day. Furthermore, regulating glucose metabolism-related enzymes by increasing HK and PK activities and decreasing GP and G6Pase activities in mice liver (Wang et al., 2016a), and stimulating the glucose transporter 4 (GLUT4) protein translocation to the plasma membrane and combination (Wang et al., 2016b) could be mechanisms of fucoidan to maintain glucose homeostasis. Later study also found that 20mg and 80mg/kg/day of fucoidan from Pearsonothuria graeffei gavaged for 6 weeks could dose-dependently attenuate the increased fasting blood-glucose, insulin, and the homeostatic model assessment for insulin resistance (HOMA-IR) levels in the high-fat-diet (HFD) fed mouse model (Li et al., 2018b).
3.2.1.2 FCS
When FCS from Acaudina molpadioides was fed to HFSD mouse model at 80mg/kg/day for 19 weeks, a decrease in the fasting blood glucose level (19.59%) and AUC (10.88%) was recorded as compared to the HFSD mouse model (Hu et al., 2013; Hu et al., 2014a). FCS from Isostichopus badionotus gavaged for 6 weeks at 40mg/kg/day was reported to significantly inhibit fasting blood-glucose level (4.9 mmol/L vs 6.7mmol/L) in the HFSD fed mouse model (Li et al., 2019b).
At the dose of 80mg/kg/day, FCS from Acaudina molpadioides (Hu et al., 2013; Hu et al., 2014a) and fucoidan from Cucumaria frondose (Wang et al., 2016b) administered together were reported to synergistically enhance the effects of RSG on HFSD induced mouse model while 20mg/kg/day had no enhanced effects on the treatment of RSG (Hu et al., 2013; Hu et al., 2014a; Wang et al., 2016b).
3.2.2 Insulin Resistance
3.2.2.1 Saponins
Saponins (mainly Holothurin A and Echinoside A) from Pearsonothuria graeffei enhanced insulin sensitivity in an HFD induced insulin resistance mouse model but the dose-response relationship is not clear. HFD plus 0.07%, 0.03%, and 0.1% of saponins for 8 weeks; all decreased serum glucose and lowered the HOMA-IR index (Hu et al., 2012b; Han et al., 2019). However, only saponins at 0.03% and 0.1% were reported to decrease the AUC of OGTT, and 0.1% of dietary saponins decreased the insulin level (Hu et al., 2012b).
3.2.2.2 FCS
FCS was reported to improve hyperglycemia and active insulin signaling in an HFSD induced insulin resistant mouse model. 80mg/kg/day of FCS from Acaudina molpadioides fed for 19 weeks was reported to significantly reduce mouse model serum insulin levels and HOMA-IR, improve glucose metabolism (Hu et al., 2013; Hu et al., 2014a), and increase the glucose metabolism-related genes mRNA expressions of IR, IRS, PI3K, PKB in skeletal muscle (Hu et al., 2013) and liver (Hu et al., 2014a) of the mouse model. Moreover, the dose could significantly improve the treatment effects of RSG on insulin resistance, while 20mg/kg/day had no promotion effects on the RSG (Hu et al., 2013; Hu et al., 2014a).
3.2.2.3 Fucoidan
Fucoidan from Isostichopus badionotus (Wang et al., 2016a) and Cucumaria frondosa (Wang et al., 2016b) supplied in the diet at the dose of 80mg/kg/day for 19 weeks was reported to ameliorate insulin resistance and enhance insulin sensitivity. Fucoidan treatment showed a decrease of up to 10.2% in the fasting insulin level, a 7.91% decrease in the AUC (Wang et al., 2016b), a 30.8% decrease in HOMA-IR scores and a 1.16-fold increase in QUICKI value in a HFSD induced insulin resistant mouse model (Wang et al., 2016a). Moreover, both treatments increased the mRNA expressions of insulin receptors (IR), insulin receptor substrate (IRS), phosphatidylinositol 3 kinase (PI3K), protein kinase B (PKB) in the liver (Wang et al., 2016a), muscle and adipose tissues (Wang et al., 2016b) of the mouse model. Alleviating the inflammatory response could be a mechanism of the fucoidan from Isostichopus badionotus responsible for the beneficial effects against hepatic insulin resistance (Wang et al., 2016a), while the fucoidan from Cucumaria frondosa was considered to activate the PI3K/PKB pathway and GLUT4 in skeletal muscle and adipose tissue to exhibit significant anti-hyperglycemic effects in insulin resistant mice (Wang et al., 2016b).
3.2.2.4 Glycosaminoglycan
Glycosaminoglycan from the Apostichopus japonicus was considered a potential antihyperglycemic agent. Oral administration of 50mg, and 100mg/kg/day for 8 weeks could reduce body weight, blood glucose level and serum insulin content in a dose-dependent manner and the suppression of gluconeogenesis was reported to be its mechanism of action. Glycosaminoglycan suppressed the protein levels and gene expression of gluconeogenesis rate-limiting enzymes G6Pase and PEPCK. Furthermore, glycosaminoglycan could elevate the phosphorylation of IRS1, Akt, and AMPK in the liver of insulin resistant mice (Chen et al., 2020).
3.2.2.5 EPA-Enriched Phospholipids
2.4% EPA-enriched phospholipids (EPA-PL) from the Cucumaria frondosa were reported to decrease the serum glucose, HOMA-IR index, inhibit the accumulation of white adipose tissues, and elevate the level of hepatic lipolysis in a HFD induced insulin resistance mouse model (Han et al., 2019). Researchers also investigated the synergistic effect of saponins and EPA-PL. 0.035% saponins and 1.2% EPA-PL augmented both systematic and peripheral insulin sensitivity with a notable synergistic effect and exhibited a significant improvement in glucose intolerance and systematic insulin sensitivity than saponin or EPA-PL alone (Han et al., 2019).
3.2.2.6 Sterol Sulfate
0.4% sterol sulfate derived from Cucumaria frondosa reduced body weight gain, adipocyte hypertrophy, circulating glucose and insulin levels, and the glycogen content of tissues in high-fat-high-fructose diet (HFFD) induced insulin resistance mouse model. This was mainly due to the inhibition of gluconeogenesis, the promotion of glycogen synthesis and GLUT4 translocation by activating PI3K/Akt signaling pathway (Zhang et al., 2020a).
3.2.3 Diabetes
3.2.3.1 Saponins
Saponins, mainly Holothurin A and Echinoside A, could not attenuate postprandial blood glucose, especially within a short time frame. Oral gavage of Saponins, isolated from Pearsonothuria graeffei, at 150mg/kg could not attenuate the with or without starch induced postprandial blood glucose levels at 1 and 2 hours (Fu et al., 2016).
3.2.3.2 Polysaccharides
Polysaccharide from sea cucumber Holothuria leucospilota at 100 and 200mg/kg/day by oral administration for 4 weeks could significantly improve glucose intolerance and regulate blood lipid and hormone levels, repair the impairments of the pancreas and colon, increase the short-chain fatty acid (SCFA)-producing bacteria, and decrease the number of opportunistic bacterial pathogens (Anaerobiospirillum, Colinsella and Treponema) in Goto-Kakizaki (GK) rats. However, only the high dose of 200 mg/kg significantly upregulated the gene expression of peroxisome proliferator-activated receptor-α (PPAR-α), peroxisome proliferator-activated receptor-γ (PPAR-γ), phosphoinositide 3-kinase (PI3K), protein kinase B (PKB/AKT), GLUT4 and anti-apoptotic (Bcl-2), and downregulate the mRNA levels of pro-apoptotic (Bax) and cluster of differentiation 36 (CD36) in GK rats (Zhao et al., 2020).
A fucoidan-dominated polysaccharide fraction from Thelenota ananas (TAPF) and a FCS dominated polysaccharide fraction from Cucumaria frondosa (CFPF) by oral gavage into a HFD and streptozotocin (STZ) induced T2DM rat model for 8 weeks could ameliorate hyperglycemia, restore hypertriglyceridemia and hypercholesterolemia, decrease inflammatory status and oxidative stress, protect against liver injury, as well as improve insulin resistance and promote accumulation of hepatic glycogen by activating IRS/PI3K/AKT signaling and regulating GSK-3β gene expression in streptozotocin induced diabetes rats. However, these activities relied on different dose levels. For example, CFPF at 200mg/kg/d/day was shown to alleviate the decline in β-cell function and improve impaired glucose tolerance, while 400mg/kg/day of CFPF and TAPF could enhance insulin sensitivity and improve glucose tolerance in T2DM rat (Zhu et al., 2020).
3.2.3.3 EPA-Enriched Phosphatidylcholine
A dose of 75 mg/Kg/day of EPA-enriched phosphatidylcholine (isolated from the sea cucumber Cucumaria frondosa) via intragastrical administration on a STZ induced hyperglycemic rat model for 60 days exhibited significant anti-hyperglycemic activities by up-regulating the PI3K/PKB signal pathway mediated by insulin. The blood glucose level was decreased and the fasting glucose levels, AUC, HbA1c, and GSP were lowered by 23.59%, 25.81%, 41.66%, and 21.46%, respectively. Besides, EPA-enriched phosphatidylcholine also caused an increase in (53.47%) serum insulin level, (18.51%) glycogen contents in liver and (141.56%) gastrocnemius, and thereby enhanced glycogen anabolism (Hu et al., 2014b).
3.2.3.4 Long Chain Bases
Long chain bases, the simplest members in the family glycosphingolipids, from sea cucumber Cucumaria frondose, via intragastrical administration at 10mg/kg/day were reported to act against type 2 diabetic nephropathy by declining the progression of renal fibrosis and apoptosis in a type 2 diabetic mouse model (Hu et al., 2018).
3.2.3.5 Sea Cucumber Extracts
The ethanol extract of the golden sea cucumber Stichopus hermanii was reported to improve glucose level and muscle mass. At a dose of 125mg per mouse, 70% ethanol extract administered by gavage twice a day for 5 consecutive days could attenuate decreased body weight, decreased muscle mass, and glucose level in muscle in the STZ induced mouse model. Improvement of the muscle GLUT-4 and interleukin-6 (IL-6) protein levels, and reduction of the protein carbonyl level were the underlying mechanisms of action (Purwanto et al., 2019).
3.2.3.6 Synergistic Activity
More studies are required to provide evidence of the effects of saponins alone or in synergy with other compounds in the glucose metabolism field. Dietary saponins of the sea cucumber were reported to enhance insulin sensitivity but not to attenuate nor increase the postprandial blood glucose level. It is also worth noting, that when combined, saponin with EPA-enriched phospholipids exhibit a strong improvement in glucose intolerance and insulin sensitivity rather than saponin or EPA-enriched phospholipids alone.
EPA-enriched phospholipids/phosphatidylcholine, sterol sulfate, long chain bases, and the ethanol extract of sea cucumber also exhibit promising efficacy as potential agents to manage glucose metabolism, though more in-vivo studies are required for robust evidence.
3.3 Hyperlipidemia, Obesity, and Fatty Liver Disease
Obesity, a prominent global public health problem is associated with chronic diseases such as coronary heart disease, diabetes, and cancer. Hyperlipidemia and non-alcoholic fatty liver disease are predominantly associated with obesity caused by rapid changes in contemporary lifestyle and dietary habits. Polysaccharides, saponins and EPA-enriched phospholipids derived from sea cucumber have exhibited effect on hyperlipidemia, obesity, and non-alcoholic fatty liver disease via different mechanisms of action in the animal models.
3.3.1 Hyperlipidemia
3.3.1.1 Polysaccharides
Polysaccharides from sea cucumber Apostichopus japonicus (Liu et al., 2012) and Holothuria leucospilota (Yuan et al., 2019) orally administrated to an HFD induced rat model for 28 days significantly decreased serum total cholesterol (TC), triglyceride (TG) and low-density lipoprotein (LDL-C) at 400mg and 800mg/kg/day (Liu et al., 2012) and at 100mg and 200mg/kg/day (Yuan et al., 2019). Liu et al. (2012) reported 200 mg of polysaccharides (molecular weight was 36.2kDa) did not affect the TG level after 4 weeks of administration (Liu et al., 2012) while the same dose of polysaccharides (molecular weight was 52.8kDa) in another research was reported to reduce serum TG (40%) after 2 weeks and 4 weeks of administration (Yuan et al., 2019). Furthermore, high-density lipoprotein cholesterol (HDL-C) was significantly increased at 200mg, 400mg and 800mg/kg/day (Liu et al., 2012) but did not result in significant change at 100mg and 200mg in the study of Yuan et al. (Yuan et al., 2019). Polysaccharides at 100mg and 200mg/kg/day could also effectively alleviate liver histological abnormalities, regulate abnormal mRNA and levels of lipogenesis-related hormones and inflammatory cytokines (tumor necrosis factor IL-6 and IL-12). Moreover, they improved the ability of gut microbiota to produce SCFAs which have been found to ameliorate liver lesions (Yuan et al., 2019).
3.3.1.2 Fucoidan/FCS
Fucoidan from Pearsonothuria graeffei (Li et al., 2018b) at 80mg/kg/day and FCS from Isostichopus badionotus at 40mg/kg/day (Li et al., 2019b) gavaged for 6 weeks was reported to inhibit serum TC by 25.9% in an HFD-fed mouse model (Li et al., 2018b) and by 47.8% in an HFSD fed mouse model (Li et al., 2019b). At a dose of 20mg/kg/day, the TC level decreased by 34.8% in the HFSD fed mouse model (Li et al., 2019b), but no significant effects were noted in the HFD-fed mouse model (Li et al., 2018b).
3.3.2 Obesity
Sea cucumbers and their active compounds which include FCS, saponins, EPA-enriched phospholipids, and long-chain bases, exhibit anti-obesity properties by inhibiting hyperglycemia or lipid accumulation, regulating gene or protein expression, and modulating gut microbiota.
3.3.2.1 FCS
Dietary administration of FCS from Acaudina molpadioides at the dose of 80mg/kg/day exhibited anti-obesity activity by decreasing body weight gain and adipose weights (Hu et al., 2014a; Xu et al., 2015). The underlying mechanisms included reduction of protein expression and positive regulators and enhanced negative regulator of adipogenesis (Xu et al., 2015); attenuation of inflammation and modulation of gut microbiota in an obesity mouse model (Hu et al., 2019a). FCS derived from Acaudina molpadioides at 80mg/kg/day intragastrical fed in HFD-induced obesity mouse model for 10 weeks, reduced body weight gain (by 26.38%) and attenuated inflammation by reducing serum TNF-α, IL-1β, IL-6, MMP-9, and iNOS and COX-2 levels, as well as the mRNA expressions, and increased serum level of IL-10 and its mRNA expression. Furthermore, FCS modulation of gut microbiota was one of the anti-obesity mechanisms. It attenuated the F/B ratio, elevated fecal SCFA generated microbiota such as Bacteroides, Lactobacillus, Bifidobacterium, Lachnospiraceae NK4A136 group, and prevented obesity-induced inflammation by decreasing the fecal lipopolysaccharide (LPS) producer, Escherichia coli (Hu et al., 2019a). FCS at 80mg/kg/day fed to a HFSD-induced obesity mouse model for 19 weeks significantly decreased body weight gain and subcutaneous, perirenal and epididymal fat content (Xu et al., 2015).
FCS from Isostichopus badionotus gavaged at the doses of 20mg and 40mg/kg/day for 6 weeks; both doses could significantly decrease the weight gain of mice fed on HFSD, and the lower dosage of FCS (20mg/kg) could significantly inhibit abnormal weight gain. They could also attenuate increase of the F/B ratio caused by the HFSD (Li et al., 2019b).
Although 20mg, 40mg, and 80mg of FCS all showed anti-obesity activity in the mouse model, only the dose of 80mg was reported to have a synergistic effect with RSG. For example, the improved reduction of body weight gain and epididymal adipose weight (Hu et al., 2014a).
3.3.2.2 Fucoidan
Fucoidan from the sea cucumber Acaudina molpadioides (Xu et al., 2014) and Isostichopus badionotus (Wang et al., 2016a) administered at 80mg/kg/day reduced the subcutaneous, perirenal and epididymal fat content in an HFFD induced obesity mouse model when fed for 13 weeks (Xu et al., 2014) and reduced body weight gain in a HFSD-induced mouse model when fed for 19 weeks (Wang et al., 2016a). Fucoidan is also associated with the reduction of inflammation and the improvement of insulin resistance in obesity mice (Wang et al., 2016a).
Xu et al. (2014) reported that the dose of 20mg/kg/day did not show significant effects on the content of subcutaneous, perirenal and epididymal fat in the obesity mouse model (Xu et al., 2014). However, later research reported that 20mg and 80mg/kg/day of fucoidan from the Pearsonothuria graeffei gavaged for 6 weeks could dose-dependently decrease body weight gain, liver, and fat tissue weight. Moreover, the adipocyte sizes in the fucoidan groups were smaller than those in the HFD group, which indicated that fucoidan could reduce lipid accumulation in adipose tissues and thus reduce body weight gain. In fucoidan administered groups the results of histomorphology of the liver showed a relatively normal structure of the hepatic lobules and significantly improved fatty degeneration of liver cells. The revised ratio of F/B was also reported by Li S. et al. (Li et al., 2018b).
Hu et al. (2012b) compared the different effects of the whole sea cucumber, saponins, polysaccharides, collagen peptides, dregs (The remaining mixture after enzymatic hydrolysis) and non-saponin residues on improving lipid metabolism in a rat model. The results showed that saponins were the most effective substance in the sea cucumber Cucumaria frondosa and that it could suppress adipose accumulation and reduce serum and hepatic lipids by suppressing and delaying TC and TC absorption in a basal diet rat model (Hu et al., 2012a). The same research team also reported that dietary saponins from the Pearsonothuria graeffei at 0.03% and 0.1% dose-dependently exhibited a weight-loss effect and significantly decreased visceral and subcutaneous adipose tissue weight in an HFD fed mouse model (Hu et al., 2012b).
Saponin-enriched sea cucumber extracts (Echinoside A is a major component) at 0.1% and 0.2% added to an HFD diet could reduce body weight, TG in serum and liver, and hepatic TC when compared with mice that took HFD alone. The dose of 0.2% could significantly suppress the adipocyte size and inhibit lipid absorption in the intestines of the mouse model (Guo et al., 2016).Saponin-enriched extracts from Holothuria leucospilota significantly reduced the fat deposition and triglyceride levels in a high glucose fed Caenorhabditis elegans model by downregulating the messenger RNA expressions of genes involved in fat storage and metabolism, including sbp-1, cebp, and daf-16 and upregulating the expression of nhr-49 gene (Chumphoochai et al., 2019).
Further study found that sea cucumber (Pearsonothuria graeffei) saponin liposomes exhibited better effects on anti-obesity and anti-hyperlipidemia activities than the common form of sea cucumber saponins (Holothurin A and Echinoside A are main compounds) in the mouse model (Chen et al., 2018).
3.3.2.3 EPA-Enriched Phospholipids
2% of EPA-enriched phospholipids from the sea cucumber Cucumaria frondosa suppressed lipid accumulation and lipid droplets (LDs) in liver and white adipose tissue (WAT) by inhibiting the LD associated protein FSP27 in a high-sucrose diet (HSD) fed mouse model (Zhang et al., 2020b).
3.3.2.4 Sea Cucumber Extracts
Different from those of single compounds are the effects of sea cucumber extracts and the associated physicochemical treatment methods and dose levels. Sea cucumber body wall from Isostichopus badionotus was ground and incorporated into cholesterol-containing diets to ascertain if it could improve the lipid profile, and significantly reduce serum or hepatic TC, low density lipoproteins and TG concentration in a cholesterol rat model. However, the effects were dependent on the preparation methods. When compared to lyophilized extracts, cooked or heat-treated extracts had lowered effects but the preparation method did not abolish hypolipidemic potency (Olivera-Castillo et al., 2013). An HFD enriched with 2.5%, 5% or 7.5% sea cucumber gut powder (SCGP) resulted in the significant reduction of body weight gain and fat weight, compared to the HFD mouse model. However, 7.5% SCGP induced an increase in liver weight, liver TG and TC, and the lesser treatment 2.5% and 5% SCGP significantly reduced plasma glucose, TG and TC. Furthermore, 2.5% SCGP appeared to be more effective at improving the metabolic profile than 5% (Gangadaran and Cheema, 2017).
3.3.2.5 Long-Chain Bases
Long-chain bases from Acaudina molpadioides exerted anti-obesity effects by modulating gut microbiota (Hu et al., 2019b). They were reported to alleviate obesity, inhibit hyperglycemia, reduce lipid accumulation and regulate serum adipokines in an HFD fed mouse model. Alteration of the gut microbiota could be the underlying mechanism of action. The long-chain bases could attenuate the F/B ratio, elevate fecal SCFA generated microbiota such as Bacteroides, Lactobacillus, Bifidobacterium and Lachnospiraceae NK4A136 group, and decrease obesity-related bacteria such as the genera Desulfovibrio, Bifidobacterium and, Romboutsia.
3.3.3 Nonalcoholic Fatty Liver
3.3.3.1 Saponins
Recent research (Zhang et al., 2020c) focused on the structure of the saponin, Echinoside A (EA), and its efficacy on fatty liver. A diet supplemented with 0.04% of EA, DsEA, or aglycone could reduce the hepatic TG and TC, and AST levels in the rat model. Further to this, aglycone exhibited a distinct advantage toward stimulating hepatic lipolysis compared with EA and DsEA by attenuating the serum TC, hepatic PL, and the liver function indexes including ALT, and γ -GT.
3.3.3.2 Cerebroside
The dietary supplements of 0.006% and 0.03% cerebroside, isolated from the sea cucumber Acaudina molpadioides, could significantly reduce hepatic TG and TC, elevate serum TG level, alanine aminotransferase (ALT) aspartate aminotransferase (AST) activities, and attenuate hepatic steatosis in an OA induced fatty liver rat model. The possible mechanisms of action were the inhibition of hepatic lipogenic gene expression and enzymes activities, impairment of the biosynthesis of monounsaturated fatty acid in the liver and the enhancement of TG secretion from the liver (Zhang et al., 2012).
3.4 Inflammation
The mouse model of peritoneal inflammation, obesity-induced inflammation, ear inflammation, and acute intestinal inflammation has been used to evaluate the anti-inflammation properties of sea cucumber bioactives.
3.4.1 FCS
FCS is the most researched anti-inflammatory compound of the sea cucumber. Panagos et al. (2014) found that FCS oligosaccharides extracted from the sea cucumber Holothuria forskali could cause a reduction in neutrophil infiltrations in a mouse peritoneal inflammation model (Panagos et al., 2014). 20mg and 40mg/kg/day of FCS from Isostichopus badionotus gavaged for six weeks, significantly supressed the inflammatory cytokine level of serum TNF-α and also dampened macrophages infiltrating into adipose tissue in a HFSD fed mouse model (Li et al., 2019b). FCS from Acaudina molpadioides delivered via intragastrically at 80mg/kg/day for ten weeks significantly reduced serum proinflammatory cytokines including TNF-α, IL-1β, IL-6, MMP-9 and their intestinal mRNA expression, and increased interleukin-10. The researchers further revealed that gut microbiota modulation could be one of the mechanisms which results in the decrease of faecal lipopolysaccharide (LPS) producer Escherichia coli and LPS levels. Gut microbiota modulation could elevate faecal SCFA-generated microbiota including Lactobacillus, Bifidobacterium, and Lachnospiraceae NK4A136 group, and increase the SCFA concentrations (Hu et al., 2019a). Olivera-Castillo et al. (2020) also tested the anti-inflammatory effects of FCS in a mouse ear inflammation model, and it was found to modulate the expression of critical genes, including NF-kB, TNF, iNOS, and COX-2, and also attenuate inflammation and tissue damage. Furthermore, they found that 4.5% (w/w) of sulphate may be adequate to maximize its anti-inflammatory actions (Olivera-Castillo et al., 2020).
3.4.2 Fucoidan
Fucoidan derived from Isostichopus badionotus, Pearsonothuria graeffei, and Holothuria tubulosa all exhibited anti-inflammation activities in the obese mouse model. Fucoidan supplement from Isostichopus badionotus at the dose of 80mg/kg/day for 19 weeks could regulate serum inflammatory cytokines TNF-α, CRP, MIP-1, IL-1β, IL-6, and IL-10, and their mRNA expression in the liver of an HFSD induced mouse model (Wang et al., 2016a). The same dose (80mg/kg/day) of fucoidan from Pearsonothuria graeffei gavaged for six weeks was reported to significantly reduce the higher TNF-α level and decrease the expression of CD68 in an HFD-fed mouse model (Hu et al., 2015; Li et al., 2018b). Fucoidan of Holothuria tubulosa at the dose of 60mg/kg/d for 16 weeks reduced serum level of saturated free fatty acids (FFA) and inflammatory cytokines (MCP-1 and TNF-α), increased anti-inflammatory cytokines including IL-10 and ADPN, attenuated M1/M2 polarization of liver Kupffer cells, and attenuated inflammatory infiltration of epididymal adipose tissue in HFD induced obese mouse model. Moreover, the research team highlighted the chain-conformation Fucoidan-3 with molecular weight 195.7 ± 12.5 kDa exhibited the best activities. (Zhu et al., 2021).
3.4.3 Glycosaminoglycan
Oral administration of 80mg/kg/day glycosaminoglycan, purified from the sea cucumber Isostichopus badionotus, for one day prior to and during the six-day experimental period was also reported to mitigate colonic colitis by decreasing the TNF-α gene expression and increasing colon and small intestine lengths in the dextran sodium sulphate-induced mouse model (Olivera-Castillo et al., 2020).
3.4.4 Sea Cucumber Extracts
Methanol extracts of sea cucumber Holothuria atra were reported to inhibit the production of P. aeruginosa virulence factors (elastase, protease, pyocyanin) and restore the expression level of host lys-7 in a Pseudomonas aeruginosa-infected Caenorhabditis elegans model (Lee et al., 2019).
3.4.5 Enzymatic Hydrolysates
Enzymatic hydrolysates of the sea cucumber Apostichopus japonicus (EH-JAP) and Acaudina leucoprocta (EH-LEU) down-regulated the transcription of proinflammatory cytokines including IL-1β, TNF-α, IL-6, up-regulated the transcription of anti-inflammatory cytokines transforming growth factor β and IL-10, and inhibited the activation of the Toll-like receptor 4/myeloid differentiation factor 88/NF-kappaB signalling pathway, leading to the alleviation of renal inflammation in a diet-induced hyperuricemic mouse model. Furthermore, the EH-JAP had better effects than EH-LEU in terms of the release of anti-inflammatory cytokines (Wan et al., 2020).
3.4.6 Sterol Sulphate
0.4% sterol sulphate derived from the sea cucumber Cucumaria frondosa exhibited a significant effect in alleviating HFFD induced inflammation by increasing serum adiponectin (63.5%) and reducing proinflammatory cytokine release (MCP-1 and TNF-α levels were decreased by 26.8% and 35.5% respectively) (Zhang et al., 2020a).
3.5 Wound and Bone Healing
Gamat oil (sea cucumber; Holothuroidea) has been used as positive control for wound healing products in recent research (Rapi et al., 2020). Research revealed that sea cucumber-derived compounds not only showed general wound healing properties but also demonstrated the promotion of wound healing in a diabetic model.
3.5.1 Saponins
Saponins with a purity of 80% were extracted from Filipino sea cucumber was reported could promote new osteoid formation in neonatal mouse calvarias ex vivo. Combined with in vitro studies, the research team found that SCS promoted osteogenic differentiation of pre-osteoblasts by activating the BMP2/Smads molecular pathway (Li et al., 2021).
3.5.2 Water Extracts
Compared to the concentrations 0.1% and 1%, daily use of 0.5% aqueous extract of Stichopus chloronotus was the best dose for wound healing with a significantly higher woutnd reduction rate than other groups (Mazliadiyana et al., 2017). Sunmugam et al. (2021) reported that daily used 0.1 g of sea cucumber extract (Stichopus horrens) based creams (15% w/w) on full thickness excision wounds of rat for 14 days significantly enhanced the wound contraction (Sunmugam et al., 2021). In another study, a significant increase in the number of lymphocytes was reported as a result of treatment with 40% Stichopus hermanii water extracts in Wistar rats’ oral mucous (Arundina et al., 2015). Such increase in the lymphocyte number was an indication of the acceleration of wound healing of wounds sustained by traumatic ulcer. The study results have proven the suitability of sea cucumber extracts for the escalation of wound healing (Arundina et al., 2015).
3.5.3 Peptides
Yang et al. (2021a) first investigated the effects of sea cucumber peptides on bone mineral density regulations. Peptides of Stichopus japonicus at 30 mg/kg, 100mg/kg, and 300mg/kg were administrated intragastrically to the ovariectomized mouse model for 14 weeks. The results showed that sea cucumber peptides protected mice from OVX-induced bone loss by increasing bone mineral density, and prevented the bone loss induced by ovariectomy through the increasing of bone volume, bone surface density, trabecular thickness, and trabecular number values (Yang et al., 2021a).
Sea cucumber-derived compounds not only showed general wound healing properties but also demonstrated the promotion of wound healing in a diabetic mouse model. Small molecule oligopeptides, isolated from sea cucumber, at 0.25g, 0.5g. and 1g/kg dose groups all demonstrated wound sites with less bruising and swelling, and higher vascularization, collagen deposition and epithelialization than the diabetic model control group (Li et al., 2018a).
3.5.4 Collagen
In terms of bone healing, a combination of hydroxyapatite obtained from gypsum powder and Stichopus hermanii-derived collagen was prepared as a bone graft substitute and was studied for bone healing properties in male Sprague-Dawley rats by Wahyuningtyas et al. (2019). The study reported good biocompatibility of the composite material. High osteoblast formation was also demonstrated in the rats after 14 days of healing, indicating that the prepared composite material could be useful for promoting bone healing and regeneration (Wahyuningtyas et al., 2019).
3.5.5 Sea Cucumber Nanopowder
Nanopowder Stichopus hermanii contains bioactive compounds and because of their small size, they enter the human body easily and may disrupt the normal biochemical environment within the cell. A recent study using a Cavia cobaya model to evaluate the safety of the nanopowder solution for therapy in dentistry and reported it did not cause toxicity. A 3.5% Nanopowder solution with a dosage of 0.09ml/kg and 0.36ml/kg body weight for 1 day did not cause increase levels of SGOT, SGPT and decrease levels BUN, Creatinine (Sucahyo et al., 2021).
3.6 Immunological Disorders
3.6.1 Saponins
Coloquadranoside A of sea cucumber Colochirus quadrangularis showed effective immunomodulation activity. Coloquadranoside A at the dose of 50 and 500 mg/kg intragastrically administrated for 14 days increased the clearance index, adjusted phagocytosis index, reversed the lower thymus and spleen index and hemolysin level in the immunosuppressed mouse model which was induced by cyclophosphamide (Yang et al., 2021b)
3.6.2 FCS
In a study by Niu et al. (2020), the immune-enhancing capability of two FCSs from Holothuria polii and Holothuria tubulosa were investigated. The study reported the recovery of immune activity after suppression by cyclophosphamide in mice through significant improvement in blood counts (of WBCs, RBCs, neutrophils, lymphocytes, and platelets), blocking of overproduction of inflammatory cytokines (TNF-α and IL-6), and improvement of histopathological characteristics of spleen damage as a result of the treatments with the FCSs (Niu et al., 2020).
The FCS from Apostichopus japonicus were considered as stimulators of hematopoiesis; when administered subcutaneously at 0.5 mg/mL for 3 days on a cyclophosphamide-induced immunosuppression mouse model it could stimulate the release of white and red blood cells, as well as platelets from bone marrow and restore them to the control levels; and increase the number of neutrophils, monocytes and lymphocytes to balance the populations of leucocytes. The normalization of the level of the pro-inflammatory cytokine IL-6 in the serum and the recovery of cell populations in the spleen have also been observed in the mouse model following treatment with FCS (Ustyuzhanina et al., 2021).
3.6.3 Sulfated Fucan
A water soluble sulfated fucan from the sea cucumber Acaudina leucoprocta was reported to have a significant effect on enhancing the immune response. Intragastrical administered Sulfated fucan at concentrations of 50 mg/kg, 100 mg/kg, 200 mg/kg for 7 days promoted intestinal immunity by increasing secretory immunoglobulins A. It also exhibited antioxidant activity by increasing the antioxidant enzyme levels of T-AOC, SOD, GSH-Px and CAT and decreasing the enzyme amount of MDA on the cyclophosphamide triggered immunosuppression mouse model (Feng et al., 2021).
3.6.4 Glycosaminoglycan
Wang et al. (2017) studied the immunomodulatory activities of Apostichopus japonicas-derived glycosaminoglycan in cyclophosphamide-induced immunosuppressed mice and reported a significant increase in splenic lymphocyte proliferation, splenocytes Ca2+ concentration, total antioxidant capacity, superoxidase dismutase, catalase and glutathione peroxidase activities while reducing malondialdehyde levels in mouse heart, kidney, and liver. The results thus provided evidence of immunostimulatory, immunosuppressive and oxidative damage to the protective roles of glycosaminoglycan (Wang et al., 2017). In a different study, the same research group (Wang et al., 2019), reported on the application of glycosaminoglycan from A. japonicas toward improvement of adaptive immunity through cellular immunity and humoral immunity. Restoration of the immune level, both innate and adaptive, was reported in immunosuppressed mice.
3.7 Neurological Diseases
3.7.1 Saponins
In another study by Tangrodchanapong et al. (2020), Frondoside A derived from Cucumaria frondosa was used to treat transgenic Caenorhabditis elegans model of Alzheimer’s disease and investigated its effects on Amyloid-β (Aβ) aggregation and proteotoxicity. The study reported delayed paralysis, protection from oxidative stress and recovery from chemotaxis dysfunction due to Frondoside A treatments at 1uM dosage. Furthermore, Frondoside A reduced Aβ depositions especially small oligomers and other high molecular weight Aβ species which were the most toxic for the disease (Tangrodchanapong et al., 2020).
3.7.2 Cerebrosides
The first in vivo study for assessing bioactivity against Alzheimer’s disease by sea cucumber Acaudina molpadioides derived glycolipids called cerebrosides were reported by Che et al. (2017). The study reported attenuation of learning and memory deficits, reduction in Aβ accumulation, higher superoxide dismutase and lower malondialdehyde, 8-oxo-7,8-dihydro-20-deoxyguanosine (8-OHdG), 8-hydroxy-20-deoxyguanosine (8-oxo-G), and nitric oxide (NO) activities in the SAMP8 mouse model as a result of the treatments (Che et al., 2017). Cerebrosides from the same sea cucumber species were also employed by Li et al. (2019a) on male SD rats (induced to have Alzheimer’s through ventricle injection of Aβ1–42 peptide). This study also reported attenuation of cognitive deficiency in the male rats after 27 days of oral administration (at a low dosage of 40 mg/kg/day) or dietary supplementation of cerebrosides. Furthermore, the treatments activated BDNF/TrkB/CREB and PI3K/Akt/GSK3β signaling pathways leading to improvement of synaptic function through the higher expression of PSD-95 and synaptophysin and reduction in Aβ1–42-induced tau hyperphosphorylation, respectively (Li et al., 2019a). Cerebroside treatment also caused reduced production of Bax/Bcl-2 resulting in lowered neuronal damage and apoptosis (Li et al., 2019a)
3.7.3 Sea Cucumber Extracts
The neurological illnesses reportedly studied in vivo include Parkinson’s and Alzheimer’s diseases. Chalorak et al. (2018) studied the anti-Parkinson’s effects of whole body-ethyl acetate (WBEA), body wall-ethyl acetate (BWEA), viscera-ethyl acetate (VIEA), whole body-butanol (WBBU), body wall-butanol (BWBU), and viscera-butanol (VIBU) extracts of sea cucumber Holothuria scabra in the nematode Caenorhabditis elegans BZ555 and NL5901 strains. They reported that WBEA, BWEA, and WBBU extracts at 500 ug/ml showed potential anti-Parkinson’s activity through the reduction of selective catecholamine neurotoxin 6-hydroxydopamine (6-OHDA) induced dopaminergic neuron degeneration. Furthermore, lowered α-synuclein aggregation, restoration of lipid content, increased life span and better food-sensing behaviour were reported in these strains as a result of the treatments (Chalorak et al., 2018). In another study by Malaiwong et al. (2019), similar anti-Parkinson’s activities were reported as a result of treatments by ethanol, ethyl acetate, butanol, and aqueous extracts of Holothuria leucospilota in the same nematode strains. The study also reported that the bioactive sea cucumber extracts contained terpenoids, steroids, saponins, and glycosides based on findings from NMR analysis (Malaiwong et al., 2019).
Patients diagnosed with mild to severe forms of Alzheimer’s disease (158) were treated with the capsule named RSC combined with the Alzheimer’s medication Memantine. Two major ingredients of RSC are Reinhardt (Chinese sea snake, Laticauda semifasciata) and sea cucumber (Yu et al., 2017). The combination of RSC and Memantine were reported to improve agitation and mental behavior symptoms in the patients. Only mild skin allergies were reported during the treatment. The combination was reported to be a safe treatment strategy for mild to severe forms of Alzheimer’s disease.
3.7.4 EPA Enriched Ethanolamine Plasmalogens
Another study conducted by Che et al. (2018), reported that ethanolamine plasmalogens enriched with EPA (extracted from Cucumaria frondosa) ameliorated memory, learning and cognitive functionalities of SAMP8 mice as evidenced by the Morris maze test. Furthermore, treatments were reported to prevent oxidative stress, hyper-phosphorylated tau, neuro-inflammation, and apoptosis, resulting in reduced generation of β-amyloid (Che et al., 2018).
3.7.5 Peptides
Sea cucumber peptides (SCP) were reported to improve memory activity in a scopolamine-induced amnestic mouse model. SCP were gavaged at 650 mg, 1300 mg, and 2600 mg/kg. All the doses could significantly reduce the escape latency and shock time and increase the acetylcholine content and acetylcholinesterase activity. Moreover, the number of neurons in the hippocampus of SCP groups were larger, arranged neatly and intact (Xu et al., 2020).
3.7.6 Synergistic Activity
Sea cucumber organic solvent extracts WBEA, BWEA and WBBU showed potential anti-Parkinson’s disease activity in nematodes BZ555 and NLL2 because they reduced dopaminergic neuronal degeneration induced by selective aminopurine neurotoxin 6-hydroxydopamine (6-OHDA). These extracts contain a mixture of terpenoids, steroids, saponins and glycosides that may act synergistically.
3.8 Thrombosis
The main treatment methods to thrombosis are medicines that thin out the blood and/or operations in which stents or catheters are used to open blocked vessels. Some foods and substances can act as natural blood thinners and help to reduce the risk of clots. Sea cucumber derived compounds including FCS, (Fucosylated) glycosaminoglycan, and Fucoidan have been investigated and have shown antithrombotic activities, and their depolymerized products exhibited superior activities.
3.8.1 FCS
A depolymerized fragment (Mw~8.5 kDa) of native FCS (Mw~65.8 kDa) extracted from sea cucumber Thelenota ananas displayed better inhibition of venous thrombus formation at a dose of 14.8mg/kg, which is more potent than FCS at the same dose (thrombosis inhibition of 85% vs 35%) (Wu et al., 2015). The FCS significantly increased blood loss at 53mg and 106mg/kg (approximately 3.5 and 7.5 times the dose required for the inhibition of venous thrombosis) when injected into mice dorsally and subcutaneously (Wu et al., 2015). A highly purified FCS isolated from sea cucumber Pearsonothuria graeffei selectively inhibited intrinsic factor Xase complex and exhibited remarkable antithrombotic activity without hemorrhagic and hypotension side effects (Yan et al., 2019). The research team focused further on the FCS (Mw~3347 Da) (Yan et al., 2021) and found that FCS oligomer-gastro-resistant (GR) microcapsule formulation represents an effective and safe oral anticoagulant. The venous thrombosis rat model demonstrated that FCS oligomers delivered via GR microcapsules produced a potent antithrombotic effect dependent on their anticoagulant properties in the plasma. In contrast, oral administration of unformulated FCS oligomers at the same dose (50mg/kg) exhibited a weaker antithrombotic effect than the formulated version. Oral administration of FCS oligomer-GR microcapsules resulted in no bleeding, while oral administration of native FCS-GR microcapsules resulted in bleeding.
3.8.2 Fucosylated Glycosaminoglycan
Fucosylated Glycosaminoglycan (FG) from sea cucumber Holothuria fuscopunctata, its depolymerized FG (dHG) and a pure octasaccharide have been investigated. dHG 5 (Mw~5.3 kDa) (4.0-9.0mg/kg) and octasaccharide (Mw~2.46 kDa) (3.3-7.5mg/kg) could dose-dependently inhibit deep venous thrombosis in a rat model (up to 92.9% and 86.0%, respectively). The thrombus inhibition rate of FG (Mw~42.6 kDa) at the high dose was markedly reduced (inhibition rate is 51.2% at 12mg/kg) (Zhou et al., 2020). Zhou et al. (2020) did not find, however, that dHG-5 and octasaccharide increase bleeding at doses up to 10-fold of the effectively antithrombotic doses, and only FG significantly increased the bleeding volumes at 60mg/kg in a tail-cut mouse model. Notably, a recent study reported that 4.0 mg/kg (i.v.) of FGs (derived from Thelenota ananas and Holothuria fuscopunctata) could cause significant cardiovascular and respiratory dysfunction in rats which was mainly ascribed to pulmonary microvessel embolism due to platelet aggregation and contact activation-mediated coagulation, while their depolymerized products (dTaFG13 and dHG-5) had no obvious side effects (Lin et al., 2021).
Yin et al. (2021) purified the Compounds 5 from the depolymerized natural Glycosaminoglycans of sea cucumber Thelenota ananas and tested its anticoagulant activity in mice and antithrombotic activity in rat model. Subcutaneously administered the Compound 5 at doses of 4.8, 8.0, and 13.6 mg/kg in tissue thromboplastin-induced venous thrombosis rat model exhibited inhibition of venous thrombus formation with the rates of 83.5%, 92.6%, and 93.1%, respectively. However, no significant effect on blood loss has been reported at a dose of 80 mg/kg in a mouse model (Yin et al., 2021).
3.8.3 Fucoidan
Fucoidan (Mw~ 103.1 ± 2.8 kDa) from sea cucumber Holothuria polii at 8 mg/kg reduced platelet aggregation caused by cyclophosphamide in a mouse model. This might have the effect of reducing the risk of thrombosis (Li et al., 2020).
3.9 Ageing and Memory Impairments
The existence of significant cognitive changes associated with the onset of old age has been very well established. The saponin-rich extract, protein hydrolysates and phospholipids derived from sea cucumber have shown positive anti-ageing effects or prevention of memory impairment in nematodes and mouse models. Further studies are needed to ascertain applications for sea cucumber to delay the ageing process and improve cognition.
3.9.1 Water Extract
Kitisin et al. (2019) focused on the effect of sea cucumber extract on lifespan. In their research, a saponin-rich extract of Holothuria leucospilota increased the lifespan of C. elegans L4 and L1 at 5.92% and 15.76%, respectively, and significantly reduced the accumulation of lipofuscin, which is an ageing-biomarker. However, it could not reduce the intracellular ROS in the model, which implied that the active compounds in the saponin-rich extract might cause the direct modulation of ageing pathways, rather than a scavenging effect upon the free radicals that accumulate in animals (Kitisin et al., 2019).
3.9.2 Protein Hydrolysates
Lim et al. (2019) found that the hydrolysate of sea cucumber Stichopus japonicus could significantly increase the serum testosterone level in the male rat model and could be considered very useful as an andropause-relief agent (Lim et al., 2019). A recent study further reported that protein hydrolysates of Stichopus japonicas could be a bioactive anti-ageing agent. Gavaged 600 mg/kg/day of protein hydrolysates for 28 days on a D-galactose induced ageing mouse model could significantly lower the escape latency times and increase staying times of mice in the target quadrant in Morris water maze, and also improve their memory impairment in shuttle box tests (Amakye et al., 2021).
Anti-ageing properties through antioxidant bioactivity of protein hydrolysates derived from Apostichopus japonicas were studied by Guo et al. (2020) in the nematode model Caenorhabditis elegans. The study reported increased survival rate, reduced ROS level, upregulation of catalase, superoxide dismutase activities and the reduction of malondialdehyde levels in the paraquat-induced oxidative stressed nematodes as a result of the treatments. The protein hydrolysates were reported to scavenge DPPH free radicals, reduce age pigments as well as extend the life span of the nematode while not affecting their food intake, body length and brood size, indicating the capability to delay physiological ageing (Guo et al., 2020).
3.9.3 Phospholipids
Neuroprotective antioxidant activity of EPA-enriched phospholipids from Cucumaria frondosa was investigated in the senescence-accelerated prone mouse model by Wu et al. (2014). The in vivo mouse study reported prevention of the development of learning and memory impairments. The preceding in vitro studies on PC12 cells confirmed antioxidant activity in the H2O2 oxidative-stress induced cells (Wu et al., 2014).
3.10 Other
3.10.1 Fatigue
A pilot study involving the effect of sea cucumber-derived DHA-EPA-enriched phospholipids on aerobic and anaerobic exercise in mice was reported by Wang et al. (2020). The study reported significant promotion of carbohydrate and lipid metabolism and muscular mitochondrial respiratory chain and tricarboxylic acid cycle in the mice during aerobic exercise as a result of the treatments, indicating the suitability of DHA-EPA-enriched phospholipids for reducing physical fatigue (Wang et al., 2020). In another study, antifatigue properties of peptides from S. japonicus were investigated in C57BL/6J mice (Yu et al., 2020). Sea cucumber peptides were reported to improve running time, forelimb grip strength, serum lactic acid and urea nitrogen levels, antioxidant capacity, post-swimming liver and skeletal muscle blood glucose and glycogen levels, and heart and skeletal muscle Na+-K+-ATPase and Ca2+-Mg2+-ATPase activity in the mice (Yu et al., 2020).
3.10.2 Oxidative Stress
Lee et al. (2021) investigated antioxidant properties of enzymatic hydrolysates of Stichopus japonicus using zebrafish models and summarized that the α-chymotrypsin assisted hydrolysate (molecular weight<5 kDa) (α-chy-III) possess strong antioxidant activities. Administrated the α-chy-III with 25, 50, 100, 200 µg/mL at 1 hour before H2O2 treated zebrafish embryo and incubated the model until 24 h post-fertilization could significantly improve both the heart rate and survival rate of zebrafish embryos. When co-treated zebrafish embryos with α-chy-III and H2O2 and incubated until 3 d post-fertilization, 200 µg/mL of α-chy-III showed a protective effect against H2O2 induced cell death, intracellular ROS generation, and lipid peroxidation (Lee et al., 2021).
3.10.3 Hypertension
Oral gavaged Actinopyga lecanora proteolysate at 200, 400, 800 mg/kg into rat model did not affect normal blood pressure. When using proteolysate at 200, 400, 800 mg/kg at 1 hour before rats were injected with angiotension-I to induce hypertension, the dose-dependent treatment significantly suppressed systolic blood pressure (SBP) and diastolic blood pressure (DBP). While using the proteolysate at these doses 1 hour after the model been injected to induce hypertension, only the 800 mg/kg dose significantly reduced DBP and SBP. Furthermore, no significant changes in the heart rate of rats were recorded in both the preventive and treatment groups at all three dose levels, signifying no negative effects on the circulatory system (Sadegh Vishkaei et al., 2016).
3.10.4 Hepatorenal Dysfunction
Dakrory et al. (2015) investigated the antioxidative properties of Holothuria atra extract to treat 7, 12-dimethylbenz[a]anthracene (DMBA)-induced hepatorenal dysfunction in Wistar rats. The study reported reversal of histopathological alteration in the liver caused by DMBA induction in the rats as a result of the treatment. The study thus indicated the extract had curative and protective effects against hepatorenal dysfunction (Dakrory et al., 2015).
3.10.5 Liver Fibrosis
The hepatoprotective activity of the Holothuria atra-derived extracts was studied by Esmat et al. (2013). The study demonstrated that thioacetamide and the extracts co-administration in the rats caused the serum bilirubin, alanine and aspartate aminotransferases, hepatic malondialdehyde and hydroxyproline concentrations and antioxidant enzyme activities to return to a normal level (Esmat et al., 2013). Furthermore, thioacetamide-induced cellular damage was significantly reduced due to the extracts as evidenced by histological examinations (Esmat et al., 2013).
3.10.6 Candidiasis
One clinical trial conducted using sea cucumber derived compounds, targeted candidiasis (Yano et al., 2013). The researchers prepared a jelly containing 50% S. japonicus hydrolysates (the major compound of the hydrolysates was saponin holotoxin). Overall, the ratio of fresh sea cucumber in the jelly was 25%. The researchers fed the jellies to elderly nursing home residents as desserts. Although the sample number was low (only 18 participants), the study demonstrated significant reduction in oral Candida colonies because of the supplementation. The results showed potential suitability of the sea cucumber-based jelly to combat candidiasis in elderly patients although more studies with a high number of participants is needed.
4 Conclusion
A total of 33 sea cucumber species have been researched in animal models and 24 sea cucumber derived compounds/extracts have been reported to have potential biological and pharmacological activities. Cucumaria frondosa is the most researched sea cucumber species, and saponins are the most studied compound. Sea cucumber-derived extracts and compounds possess a range of pharmacological activities. Anti-tumor/anti-cancer activity is the dominant focus of the in-vivo studies, followed by lipid metabolism regulation, and then glucose metabolism management. The protective properties against cancer and Alzheimer’s disease have been evidenced by clinical trials to some extent, though the trial numbers are very limited.
The huge range of potential health benefits of the sea cucumber in vivo and clinical trials are in sharp contrast to the very few (only three) studies in human clinical subjects. The promising results from the in vivo studies warrant greater research and in-depth studies to validate the efficiency of sea cucumber extracts and compounds in clinical trials. Evidence-based research presented in this paper vindicates the longstanding belief in the health benefits of sea cucumber within traditional medicine and we anticipate that further scientific research will increase utilization of this highly valuable but currently underdeveloped resource for human nutritional and medicinal products.
Author Contributions
QL, FA, and MZ drafted the manuscript under the guidance of WZ, CF, and QF. NS completed the language check and polishing. CF and WZ completed proof reading. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are thankful to the CMBD of Flinders University for providing the facilities.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.917857/full#supplementary-material
References
Al Shemaili J., Mensah-Brown E., Parekh K., Thomas S., Attoub S., Hellman B., et al. (2014). Frondoside A Enhances the Antiproliferative Effects of Gemcitabine in Pancreatic Cancer. Eur. J. Cancer 50, 1391–1398. doi: 10.1016/j.ejca.2014.01.002
Al Shemaili J., Parekh K. A., Newman R. A., Hellman B., Woodward C., Adem A., et al. (2016). Pharmacokinetics in Mouse and Comparative Effects of Frondosides in Pancreatic Cancer. Mar. Drugs 14, 115. doi: 10.3390/md14060115
Amakye W. K., Hou C., Xie L., Lin X., Gou N., Yuan E., et al. (2021). Bioactive Anti-Aging Agents and the Identification of New Anti-Oxidant Soybean Peptides. Food Biosci. 42, 101194. doi: 10.1016/j.fbio.2021.101194
Arundina I., Soesilawati P., Damaiyanti D. W., Maharani D. (2015). The Effects of Golden Sea Cucumber Extract (Stichopus Hermanii) on the Number of Lymphocytes During the Healing Process of Traumatic Ulcer on Wistar Rat’s Oral Mucous. Dental J. (Majalah Kedokteran Gigi) 48, 100–103. doi: 10.20473/j.djmkg.v48.i2.p100-103
Attoub S., Arafat K., Gélaude A., Al Sultan M. A., Bracke M., Collin P., et al. (2013). Frondoside a Suppressive Effects on Lung Cancer Survival, Tumor Growth, Angiogenesis, Invasion, and Metastasis. PLoS One 8, e53087. doi: 10.1371/journal.pone.0053087
Baharara J., Amini E., Afzali M., Nikdel N., Mostafapour A., Kerachian M. A. (2016). Apoptosis Inducing Capacity of Holothuria Arenicola in CT26 Colon Carcinoma Cells In Vitro and In Vivo. Iranian J. Basic. Med. Sci. 19, 358.
Bordbar S., Anwar F., Saari N. (2011). High-Value Components and Bioactives From Sea Cucumbers for Functional Foods—A Review. Mar. Drugs 9, 1761–1805. doi: 10.3390/md9101761
Chalorak P., Jattujan P., Nobsathian S., Poomtong T., Sobhon P., Meemon K. (2018). Holothuria Scabra Extracts Exhibit Anti-Parkinson Potential in C. Elegans: A Model for Anti-Parkinson Testing. Nutritional Neurosci. 21, 427–438. doi: 10.1080/1028415X.2017.1299437
Chari A., Mazumder A., Lau K., Catamero D., Galitzeck Z., Jagannath S. (2018). A Phase Ii Trial Of Tbl-12 Sea Cucumber Extract In Patients With Untreated Asymptomatic Myeloma. Br. J. Of Haematol. 180, 296–298. doi: 10.1111/bjh.14314
Che H., Du L., Cong P., Tao S., Ding N., Wu F., et al. (2017). Cerebrosides From Sea Cucumber Protect Against Oxidative Stress In Samp8 Mice And Pc12 Cells. J. Of Medic. Food 20, 392–402. doi: 10.1089/jmf.2016.3789
Chen C., Han X., Dong P., Li Z., Yanagita T., Xue C., et al. (2018). Sea Cucumber Saponin Liposomes Ameliorate Obesity-Induced Inflammation And Insulin Resistance In High-Fat-Diet-Fed Mice. Food Funct. 9, 861–870. doi: 10.1039/C7FO01599B
Chen Y., Wang Y., Yang S., Yu M., Jiang T., Lv Z. (2020). Glycosaminoglycan From Apostichopus Japonicus Improves Glucose Metabolism In The Liver Of Insulin Resistant Mice. Mar. Drugs 18, 1. doi: 10.3390/md18010001
Che H., Zhou M., Zhang T., Zhang L., Ding L., Yanagita T., et al. (2018). Epa Enriched Ethanolamine Plasmalogens Significantly Improve Cognition Of Alzheimer’s Disease Mouse Model By Suppressing β-Amyloid Generation. J. Of Funct. Foods. 41, 9–18. doi: 10.1016/j.jff.2017.12.016
Chumphoochai K., Chalorak P., Suphamungmee W., Sobhon P., Meemon K. (2019). Saponin-Enriched Extracts From Body Wall And Cuvierian Tubule Of Holothuria Leucospilota Reduce Fat Accumulation And Suppress Lipogenesis In Caenorhabditis Elegans. J. Of Sci. Of Food And Agric. 99, 4158–4166. doi: 10.1002/jsfa.9646
Dakrory A. I., Fahmy S. R., Soliman A. M., Mohamed A. S., Amer S. A. (2015). Protective And Curative Effects Of The Sea Cucumber Holothuria Atra Extract Against Dmba-Induced Hepatorenal Diseases In Rats. BioMed. Res. Int. 2015.
Du L., Xu J., Xue Y., Takahashi K., Xue C.-H., Wang J.-F., et al. (2015). Cerebrosides From Sea Cucumber Ameliorates Cancer-Associated Cachexia In Mice By Attenuating Adipose Atrophy. J. Of Funct. Foods. 17, 352–363. doi: 10.1016/j.jff.2015.05.040
Dyshlovoy S. A., Menchinskaya E. S., Venz S., Rast S., Amann K., Hauschild J., et al. (2016). The Marine Triterpene Glycoside Frondoside A Exhibits Activity In Vitro And In Vivo In Prostate Cancer. Int. J. Of Cancer 138, 2450–2465. doi: 10.1002/ijc.29977
Esmat A. Y., Said M. M., Soliman A. A., El-Masry K. S., Badiea E. A. (2013). Bioactive Compounds, Antioxidant Potential, And Hepatoprotective Activity Of Sea Cucumber (Holothuria Atra) Against Thioacetamide Intoxication In Rats. Nutrition 29, 258–267. doi: 10.1016/j.nut.2012.06.004
Eso A., Uinarni H., Tommy T., Sitepu R. K., Effendi I. K., Ariestiyanto Y. C., et al. (2020). A Reviews On Use Of Sea Cucumber As A Treatment For Oral Cancer. System. Rev. In Pharm. 11, 299–307.
Feng G., Laijin S., Chen S., Teng W., Dejian Z., Yin C., et al. (2021). In Vitro And In Vivo Immunoregulatory Activity Of Sulfated Fucan From The Sea Cucumber A. Leucoprocta. Int. J. Of Biol. Macromol. 187, 931–938. doi: 10.1016/j.ijbiomac.2021.08.008
Fu X., Wen M., Han X., Yanagita T., Xue Y., Wang J., et al. (2016). Effect And Potential Mechanism Of Action Of Sea Cucumber Saponins On Postprandial Blood Glucose In Mice. Biosci. Biotechn. And Biochem. 80, 1081–1087. doi: 10.1080/09168451.2016.1153950
Gangadaran S., Cheema S. K. (2017). A High Fat Diet Enriched With Sea Cucumber Gut Powder Provides Cardio-Protective and Anti-Obesity Effects in C57BL/6 Mice. Food Res. Int. 99, 799–806. doi: 10.1016/j.foodres.2017.06.066
Guo L., Gao Z., Zhang L., Guo F., Chen Y., Li Y., et al. (2016). Saponin-Enriched Sea Cucumber Extracts Exhibit an Antiobesity Effect Through Inhibition of Pancreatic Lipase Activity and Upregulation of LXR-β Signaling. Pharm. Biol. 54, 1312–1325. doi: 10.3109/13880209.2015.1075047
Guo K., Su L., Wang Y., Liu H., Lin J., Cheng P., et al. (2020). Antioxidant and Anti-Aging Effects of a Sea Cucumber Protein Hydrolyzate and Bioinformatic Characterization of Its Composing Peptides. Food Funct. 11, 5004–5016. doi: 10.1039/D0FO00560F
Gur J., Mawuntu M., Martirosyan D. (2018). Ffc’s Advancement of Functional Food Definition. Funct. Foods. Health Dis. 8, 385–397. doi: 10.31989/ffhd.v8i7.531
Han X.-Q., Zhang L.-Y., Ding L., Shi H.-H., Xue C.-H., Zhang T.-T., et al. (2019). Synergistic Effect Of Sea Cucumber Saponins And Epa-Enriched Phospholipids On Insulin Resistance In High-Fat Diet-Induced Obese Mice. Food Funct. 10, 3955–3964. doi: 10.1039/C9FO01147A
Hu S., Chang Y., Wang J., Xue C., Shi D., Xu H., et al. (2013). Fucosylated Chondroitin Sulfate From Acaudina Molpadioides Improves Hyperglycemia Via Activation Of Pkb/Glut4 Signaling In Skeletal Muscle Of Insulin Resistant Mice. Food Funct. 4, 1639–1646. doi: 10.1039/c3fo60247h
Hu Y., Li S., Li J., Ye X., Ding T., Liu D., et al. (2015). Identification Of A Highly Sulfated Fucoidan From Sea Cucumber Pearsonothuria Graeffei With Well-Repeated Tetrasaccharides Units. Carbohydr. Polym. 134, 808–816. doi: 10.1016/j.carbpol.2015.06.088
Hu X., Li Z., Xue Y., Xu J., Xue C., Wang J., et al. (2012b). Dietary Saponins Of Sea Cucumber Ameliorate Obesity, Hepatic Steatosis, And Glucose Intolerance In High-Fat Diet–Fed Mice. J. Of Medic. Food 15, 909–916. doi: 10.1089/jmf.2011.2042
Hu S.-W., Tian Y.-Y., Chang Y.-G., Li Z.-J., Xue C.-H., Wang Y.-M. (2014a). Fucosylated Chondroitin Sulfate From Sea Cucumber Improves Glucose Metabolism And Activates Insulin Signaling In The Liver Of Insulin-Resistant Mice. J. Of Medic. Food 17, 749–757. doi: 10.1089/jmf.2013.2924
Hu S., Wang J., Wang J., Yang H., Li S., Jiang W., et al. (2018). Long-Chain Bases From Sea Cucumber Inhibits Renal Fibrosis And Apoptosis In Type 2 Diabetic Mice. J. Of Funct. Foods. 40, 760–768. doi: 10.1016/j.jff.2017.12.013
Hu S., Wang J., Xu Y., Yang H., Wang J., Xue C., et al. (2019a). Anti-Inflammation Effects Of Fucosylated Chondroitin Sulphate From Acaudina Molpadioides By Altering Gut Microbiota In Obese Mice. Food Funct. 10, 1736–1746. doi: 10.1039/C8FO02364F
Hu S., Xu Y., Gao X., Li S., Jiang W., Liu Y., et al. (2019b). Long-Chain Bases From Sea Cucumber Alleviate Obesity By Modulating Gut Microbiota. Mar. Drugs 17, 455. doi: 10.3390/md17080455
Hu S., Xu L., Shi D., Wang J., Wang Y., Lou Q., et al. (2014b). Eicosapentaenoic Acid-Enriched Phosphatidylcholine Isolated From Cucumaria Frondosa Exhibits Anti-Hyperglycemic Effects Via Activating Phosphoinositide 3-Kinase/Protein Kinase B Signal Pathway. J. Of Biosci. Bioengineering 117, 457–463. doi: 10.1016/j.jbiosc.2013.09.005
Hu X.-Q., Xu J., Xue Y., Li Z.-J., Wang J.-F., Wang J.-H., et al. (2012a). Effects Of Bioactive Components Of Sea Cucumber On The Serum, Liver Lipid Profile And Lipid Absorption. Biosci. Biotechn. Biochem. 76, 2214–2218. doi: 10.1271/bbb.120495
Janakiram N. B., Mohammed A., Bryant T., Lightfoot S., Collin P. D., Steele V. E., et al. (2015). Improved Innate Immune Responses By Frondanol A5, A Sea Cucumber Extract, Prevent Intestinal Tumorigenesis. Cancer Prev. Res. 8, 327–337. doi: 10.1158/1940-6207.CAPR-14-0380
Kapinova A., Kubatka P., Golubnitschaja O., Kello M., Zubor P., Solar P., et al. (2018). Dietary Phytochemicals In Breast Cancer Research: Anticancer Effects And Potential Utility For Effective Chemoprevention. Environ. Health Prev. Med. 23, 1–18. doi: 10.1186/s12199-018-0724-1
Kitisin T., Suphamungmee W., Meemon K. (2019). Saponin-Rich Extracts From Holothuria Leucospilota Mediate Lifespan Extension And Stress Resistance In Caenorhabditis Elegans Via Daf-16. J. Food Biochem. 43, E13075. doi: 10.1111/jfbc.13075
Lee H.-G., Kim H.-S., Oh J.-Y., Lee D.-S., Yang H.-W., Kang M.-C., et al. (2021). Potential Antioxidant Properties Of Enzymatic Hydrolysates From Stichopus Japonicus Against Hydrogen Peroxide-Induced Oxidative Stress. Antioxidants 10, 110. doi: 10.3390/antiox10010110
Lee W.-T., Tan B.-K., Eng S.-A., Yuen G. C., Chan K. L., Sim Y. K., et al. (2019). Black Sea Cucumber (Holothuria Atra Jaeger 1833) Rescues Pseudomonas Aeruginosa-Infected Caenorhabditis Elegans Via Reduction Of Pathogen Virulence Factors And Enhancement Of Host Immunity. Food Funct. 10, 5759–5767. doi: 10.1039/C9FO01357A
Lei D., Zhao-Jie L., Jie X., Jing-Feng W., Yong X., Chang-Hu X., et al. (2012). The Anti-Tumor Activities Of Cerebrosides Derived From Sea Cucumber Acaudina Molpadioides And Starfish Asterias Amurensis In Vitro And In Vivo. J. Of Oleo Sci. 61, 321–330. doi: 10.5650/jos.61.321
Li Q., Che H. X., Wang C. C., Zhang L. Y., Ding L., Xue C. H., et al. (2019a). Cerebrosides From Sea Cucumber Improved Aβ1–42-Induced Cognitive Deficiency In A Rat Model Of Alzheimer’s Disease. Mol. Nutr. Food Res. 63, 1800707. doi: 10.1002/mnfr.201800707
Li S., Li J., Mao G., Wu T., Hu Y., Ye X., et al. (2018b). A Fucoidan From Sea Cucumber Pearsonothuria Graeffei With Well-Repeated Structure Alleviates Gut Microbiota Dysbiosis and Metabolic Syndromes in HFD-Fed Mice. Food Funct. 9, 5371–5380. doi: 10.1039/c8fo01174e
Li S., Li J., Mao G., Wu T., Lin D., Hu Y., et al. (2019b). Fucosylated Chondroitin Sulfate From Isostichopus Badionotus Alleviates Metabolic Syndromes And Gut Microbiota Dysbiosis Induced By High-Fat And High-Fructose Diet. Int. J. Biol. Macromol. 124, 377–388. doi: 10.1016/j.ijbiomac.2018.11.167
Li D., Li L., Xu T., Wang T., Ren J., Liu X., et al. (2018a). Effect Of Low Molecular Weight Oligopeptides Isolated From Sea Cucumber On Diabetic Wound Healing In Db/Db Mice. Mar. Drugs 16, 16. doi: 10.3390/md16010016
Lim J.-M., Kim Y. D., Kim Y. S., Cho H.-R., Lee K. H., Yoon I. J., et al. (2019). Sea Cucumber Stichopus Japonicus Hydrolysate Alleviates Late-Onset-Hypogonadism in Aged SD Rats. Toxicol. Environ. Health Sci. 11, 312–319.
Li C., Niu Q., Li S., Zhang X., Liu C., Cai C., et al. (2020). Fucoidan From Sea Cucumber Holothuria Polii: Structural Elucidation And Stimulation Of Hematopoietic Activity. Int. J. Of Biol. Macromol. 154, 1123–1131. doi: 10.1016/j.ijbiomac.2019.11.036
Lin L., Li S., Gao N., Wang W., Zhang T., Yang L., et al. (2021). The Toxicology of Native Fucosylated Glycosaminoglycans and the Safety of Their Depolymerized Products as Anticoagulants. Mar. Drugs 19, 487. doi: 10.3390/md19090487
Li X., Roginsky A., Ding X. Z., Woodward C., Collin P., Newman R., et al. (2008). Review of the Apoptosis Pathways in Pancreatic Cancer and the Anti-Apoptotic Effects of the Novel Sea Cucumber Compound, Frondoside a. Ann. New York Acad. Sci. 1138, 181–198. doi: 10.1196/annals.1414.025
Li Z., Tian Y., Ma H., Wang M., Yan Z., Xue C., et al. (2021). Saponins From the Sea Cucumber Promote the Osteoblast Differentiation in MC3T3-E1 Cells Through the Activation of the BMP2/Smads Pathway. Curr. Pharm. Biotechnol. 22, 1942–1952. doi: 10.2174/1389201021666200519135446
Liu X., Liu Y., Hao J., Zhao X., Lang Y., Fan F., et al. (2016). In Vivo Anti-Cancer Mechanism of Low-Molecular-Weight Fucosylated Chondroitin Sulfate (LFCS) From Sea Cucumber Cucumaria Frondosa. Molecules 21, 625. doi: 10.3390/molecules21050625
Liu X., Sun Z., Zhang M., Meng X., Xia X., Yuan W., et al. (2012). Antioxidant and Antihyperlipidemic Activities of Polysaccharides From Sea Cucumber. Apostichopus japonicus. Carbohydr. Polym. 90, 1664–1670. doi: 10.1016/j.carbpol.2012.07.047
Lobine D., Rengasamy K. R., Mahomoodally M. F. (2021). Functional Foods And Bioactive Ingredients Harnessed From The Ocean: Current Status And Future Perspectives. Crit. Rev. In Food Sci. And Nutr., 1–30. doi: 10.1080/10408398.2021.1893643
Ma X., Kundu N., Collin P. D., Goloubeva O., Fulton A. M. (2012). Frondoside A Inhibits Breast Cancer Metastasis And Antagonizes Prostaglandin E Receptors Ep4 And Ep2. Breast Cancer Res. And Treat 132, 1001–1008. doi: 10.1007/s10549-011-1675-z
Malaiwong N., Chalorak P., Jattujan P., Manohong P., Niamnont N., Suphamungmee W., et al. (2019). Anti-Parkinson Activity Of Bioactive Substances Extracted From. Holothuria Leucospilota. Biomed. Pharmacother. 109, 1967–1977. doi: 10.1016/j.biopha.2018.11.063
Malyarenko O. S., Ivanushko L. A., Chaikina E. L., Kusaykin M. I., Silchenko A. S., Avilov S. A., et al. (2020). In Vitro And In Vivo Effects Of Holotoxin A1 From The Sea Cucumber Apostichopus Japonicus During Ionizing Radiation. Natural Prod. Commun. 15, 1–19.
Mazliadiyana M., Nazrun A., Isa N. (2017). Optimum Dose Of Sea Cucumber (Stichopus Chloronotus) Extract For Wound Healing. Med. Health 12, 83–89. doi: 10.17576/MH.2017.1201.09
Menchinskaya E. S., Aminin D. L., Avilov S. A., Silchenko A. S., Andryjashchenko P. V., Kalinin V. I., et al. (2013). Inhibition Of Tumor Cells Multidrug Resistance By Cucumarioside A2-2, Frondoside A And Their Complexes With Cholesterol. Natural Prod. Commun. 8, 1934578x1300801009. doi: 10.1177/1934578X1300801009
Menchinskaya E., Gorpenchenko T., Silchenko A., Avilov S., Aminin D. (2019). Modulation Of Doxorubicin Intracellular Accumulation And Anticancer Activity By Triterpene Glycoside Cucumarioside A2-2. Mar. Drugs 17, 597. doi: 10.3390/md17110597
Niu Q., Li G., Li C., Li Q., Li J., Liu C., et al. (2020). Two Different Fucosylated Chondroitin Sulfates: Structural Elucidation, Stimulating Hematopoiesis And Immune-Enhancing Effects. Carbohydr. Polym. 230, 115698. doi: 10.1016/j.carbpol.2019.115698
Olivera-Castillo L., Davalos A., Grant G., Valadez-Gonzalez N., Montero J., Barrera-Perez H. A. M., et al. (2013). Diets Containing Sea Cucumber (Isostichopus Badionotus) Meals Are Hypocholesterolemic In Young Rats. PLoS One 8, E79446. doi: 10.1371/journal.pone.0079446
Olivera-Castillo L., Grant G., Kantún-Moreno N., Barrera-Pérez H. A., Montero J., Olvera-Novoa M. A., et al. (2020). A Glycosaminoglycan-Rich Fraction From Sea Cucumber Isostichopus Badionotus Has Potent Anti-Inflammatory Properties In Vitro And In Vivo. Nutrients 12. 1698 doi: 10.3390/nu12061698
Panagos C. G., Thomson D. S., Moss C., Hughes A. D., Kelly M. S., Liu Y., et al. (2014). Fucosylated Chondroitin Sulfates From The Body Wall Of The Sea Cucumber Holothuria Forskali: Conformation, Selectin Binding, And Biological Activity. J. Of Biol. Chem. 289, 28284–28298. doi: 10.1074/jbc.M114.572297
Purcell S. W., Samyn Y., Conand C. (2012). Commercially Important Sea Cucumbers of the World. FAO Species Catalogue for Fishery Purposes. No. 6 (Rome: FAO).
Purwanto B., Wiyasihati S. I., Masyitha P. A., Wigati K. W., Irwadi I. (2019). Golden Sea Cucumber Extract Revives Glucose Transporter-4 and Interleukin-6 Protein Level in Diabetic Mouse Muscle. Vet. World 12, 684. doi: 10.14202/vetworld.2019.684-68
Rapi H. S., Che Soh N. A., Mohd Azam N. S., Maulidiani M., Assaw S., Haron M. N., et al. (2020). Effectiveness of Aqueous Extract of Marine Baitworm Marphysa Moribidii Idris, Hutchings and Arshad 2014 (Annelida, Polychaeta), on Acute Wound Healing Using Sprague Dawley Rats. Evidence-Based Complementary Altern. Med. 2020, 1–17. doi: 10.1155/2020/1408926
Sadegh Vishkaei M., Ebrahimpour A., Abdul-Hamid A., Ismail A., Saari N. (2016). Angiotensin-I Converting Enzyme (ACE) Inhibitory and Anti-Hypertensive Effect of Protein Hydrolysate From Actinopyga Lecanora (Sea Cucumber) in Rats. Mar. Drugs 14, 176. doi: 10.3390/md14100176
Slater M., Chen J.. 2015Use and Exploitation of Sea Cucumbers. In (Eds, N.P.Brown and S.D. Eddy) Echinoderm Aquaculture. New Jersey: Wiley-Blackwell, 5773. doi: 10.1002/9781119005810.ch4
Song Y., Jin S.-J., Cui L.-H., Ji X.-J., Yang F.-G. (2013). Immunomodulatory Effect of Stichopus Japonicus Acid Mucopolysaccharide on Experimental Hepatocellular Carcinoma in Rats. Molecules 18, 7179–7193. doi: 10.3390/molecules18067179
Sucahyo B., Prameswari N., Brahmanta A., Tandjung L. (2021). In Vitro And In Vivo Evaluation Of Nanopowder Golden Sea Cucumber (Stichopus Hermanii) For Therapy In Dentistry. Biochem. Cell. Arch. 21, 1839–1845.
Sunmugam T., Azhari H., Ng S.-F., Azmi F. (2021). Influence Of The Oil Phase On The Wound Healing Activity Of Sea Cucumber Extract-Based Cream Formulations. Sains Malaysiana 50, 839–847. doi: 10.17576/jsm-2021-5003-24
Tangrodchanapong T., Sobhon P., Meemon K. (2020). Frondoside A Attenuates Amyloid-β Proteotoxicity In Transgenic Caenorhabditis Elegans By Suppressing Its Formation. Front. In Pharmacol. 1437. doi: 10.3389/fphar.2020.553579
Ustyuzhanina N. E., Anisimova N. Y., Bilan M. I., Donenko F. V., Morozevich G. E., Yashunskiy D. V., et al. (2021). Chondroitin Sulfate And Fucosylated Chondroitin Sulfate As Stimulators Of Hematopoiesis In Cyclophosphamide-Induced Mice. Pharmaceuticals 14, 1074. doi: 10.3390/ph14111074
Wahyuningtyas E., Hsu L.-C., Lan W.-C., Wen S.-C., Ou K.-L., Chou H.-H., et al. (2019). Application Of A Promising Bone Graft Substitute In Bone Tissue Regeneration: Characterization, Biocompatibility, And In Vivo Animal Study. BioMed. Res. Int. 2019.
Wang C.-C., Ding L., Zhang L.-Y., Shi H.-H., Xue C.-H., Chi N.-Q., et al. (2020). A Pilot Study On The Effects Of Dha/Epa-Enriched Phospholipids On Aerobic And Anaerobic Exercises In Mice. Food Funct. 11, 1441–1454. doi: 10.1039/C9FO02489A
Wang J., Hu S., Jiang W., Song W., Cai L., Wang J. (2016a). Fucoidan From Sea Cucumber May Improve Hepatic Inflammatory Response And Insulin Resistance In Mice. Int. Immunopharmacol. 31, 15–23. doi: 10.1016/j.intimp.2015.12.009
Wang Y., Wang J., Zhao Y., Hu S., Shi D., Xue C. (2016b). Fucoidan From Sea Cucumber Cucumaria Frondosa Exhibits Anti-Hyperglycemic Effects in Insulin Resistant Mice via Activating the PI3K/PKB Pathway and GLUT4. J. Biosci. Bioengineering 121, 36–42. doi: 10.1016/j.jbiosc.2015.05.012
Wang H., Xu L., Yu M., Wang Y., Jiang T., Yang S., et al. (2019). Glycosaminoglycan From Apostichopus Japonicus Induces Immunomodulatory Activity In Cyclophosphamide-Treated Mice And In Macrophages. Int. J. Of Biol. Macromol. 130, 229–237. doi: 10.1016/j.ijbiomac.2019.02.093
Wang H., Yang S., Wang Y., Jiang T., Li S., Lv Z. (2017). Immunoenhancement Effects Of Glycosaminoglycan From Apostichopus Japonicus: In Vitro And In Cyclophosphamide-Induced Immunosuppressed Mice Studies. Mar. Drugs 15, 347. doi: 10.3390/md15110347
Wan H., Han J., Tang S., Bao W., Lu C., Zhou J., et al. (2020). Comparisons Of Protective Effects Between Two Sea Cucumber Hydrolysates Against Diet Induced Hyperuricemia And Renal Inflammation In Mice. Food Funct. 11, 1074–1086. doi: 10.1039/C9FO02425E
Wargasetia T. L., Permana S. (2018). The Role of Sea Cucumber Active Compound and its Derivative as an Anti-Cancer Agent. Curr. Pharmacol. Rep. 4, 27–32. doi: 10.1007/s40495-018-0121-x
Wei W., Fan X.-M., Jia S.-H., Zhang X.-P., Zhang Z., Zhang X.-J., et al. (2021). Sea Cucumber Intestinal Peptide Induces the Apoptosis of MCF-7 Cells by Inhibiting PI3K/AKT Pathway. Front. Nutr. 1012. doi: 10.3389/fnut.2021.763692
Wu M., Wen D., Gao N., Xiao C., Yang L., Xu L., et al. (2015). Anticoagulant and Antithrombotic Evaluation of Native Fucosylated Chondroitin Sulfates and Their Derivatives as Selective Inhibitors of Intrinsic Factor Xase. Eur. J. Medic. Chem. 92, 257–269. doi: 10.1016/j.ejmech.2014.12.054
Wu F.-J., Xue Y., Liu X.-F., Xue C.-H., Wang J.-F., Du L., et al. (2014). The Protective Effect of Eicosapentaenoic Acid-Enriched Phospholipids From Sea Cucumber Cucumaria Frondosa on Oxidative Stress in PC12 Cells and SAMP8 Mice. Neurochem. Int. 64, 9–17. doi: 10.1016/j.neuint.2013.10.015
Xu X., Liang R., Li D., Jiang C., Lin S. (2020). Evaluation Of Sea Cucumber Peptides-Assisted Memory Activity And Acetylation Modification In Hippocampus Of Test Mice Based On Scopolamine-Induced Experimental Animal Model Of Memory Disorder. J. Funct. Foods. 68, 103909. doi: 10.1016/j.jff.2020.103909
Xu H., Wang J., Chang Y., Xu J., Wang Y., Long T., et al. (2014). Fucoidan From The Sea Cucumber Acaudina Molpadioides Exhibits Anti-Adipogenic Activity By Modulating The Wnt/β-Catenin Pathway And Down-Regulating The Srebp-1c Expression. Food Funct. 5, 1547–1555. doi: 10.1039/C3FO60716J
Xu H., Wang J., Zhang X., Li Z., Wang Y., Xue C. (2015). Inhibitory Effect Of Fucosylated Chondroitin Sulfate From The Sea Cucumber Acaudina Molpadioides On Adipogenesis Is Dependent On Wnt/β-Catenin Pathway. J. Of Biosci. And Bioengineering 119, 85–91. doi: 10.1016/j.jbiosc.2014.05.026
Xu C., Zhang R., Wen Z. (2018). Bioactive Compounds And Biological Functions Of Sea Cucumbers As Potential Functional Foods. J. Funct. Foods. 49, 73–84. doi: 10.1016/j.jff.2018.08.009
Yang W.-S., Qi X.-R., Xu Q.-Z., Yuan C.-H., Yi Y.-H., Tang H.-F., et al. (2021b). A New Sulfated Triterpene Glycoside From The Sea Cucumber Colochirus Quadrangularis, And Evaluation Of Its Antifungal, Antitumor And Immunomodulatory Activities. Bioorg. Medic. Chem. 41, 116188. doi: 10.1016/j.bmc.2021.116188
Yang M., Xu Z., Wu D., Dong Y., Wang Z., Du M., 15623 (2021a). Characterizations And The Mechanism Underlying Osteogenic Activity Of Peptides From Enzymatic Hydrolysates Of Stichopus Japonicus. J. Agric. Food Chem. 69, 15611–. doi: 10.1021/acs.jafc.1c06028
Yano A., Abe A., Aizawa F., Yamada H., Minami K., Matsui M., et al. (2013). The Effect Of Eating Sea Cucumber Jelly On Candida Load In The Oral Cavity Of Elderly Individuals In A Nursing Home. Mar. Drugs 11, 4993–5007. doi: 10.3390/md11124993
Yan L., Wang D., Zhu M., Yu Y., Zhang F., Ye X., et al. (2019). Highly Purified Fucosylated Chondroitin Sulfate Oligomers With Selective Intrinsic Factor Xase Complex Inhibition. Carbohydr. Polym. 222, 115025. doi: 10.1016/j.carbpol.2019.115025
Yan L., Zhu M., Wang D., Tao W., Liu D., Zhang F., et al. (2021). Oral Administration Of Fucosylated Chondroitin Sulfate Oligomers In Gastro-Resistant Microcapsules Exhibits A Safe Antithrombotic Activity. Thromb. Haemostasis 121, 015–026. doi: 10.1055/s-0040-1714738
Yin R., Zhou L., Gao N., Lin L., Sun H., Chen D., et al. (2021). Unveiling The Disaccharide-Branched Glycosaminoglycan And Anticoagulant Potential Of Its Derivatives. Biomacromolecules 22, 1244–1255. doi: 10.1021/acs.biomac.0c01739
Yuan Y., Liu Q., Zhao F., Cao J., Shen X., Li C. (2019). Holothuria Leucospilota Polysaccharides Ameliorate Hyperlipidemia In High-Fat Diet-Induced Rats Via Short-Chain Fatty Acids Production And Lipid Metabolism Regulation. Int. J. Of Mol. Sci. 20. 4738 doi: 10.3390/ijms20194738
Yun S.-H., Park E.-S., Shin S.-W., Na Y.-W., Han J.-Y., Jeong J.-S., et al. (2012). Stichoposide C Induces Apoptosis Through The Generation Of Ceramide In Leukemia And Colorectal Cancer Cells And Shows In Vivo Antitumor Activity. Clin. Cancer Res. 18, 5934–5948. doi: 10.1158/1078-0432.CCR-12-0655
Yu E., Liao Z., Tan Y., Qiu Y., Zhu J., Chen Y., et al. (2017). Efficacy and Tolerance of Memantine Monotherapy and Combination Therapy With Reinhartdt And Sea Cucumber Capsule on Agitation in Moderate to Severe Alzheimer Disease.Chin. Med. J. 97, 2091–2094. doi: 10.3760/cma.j.issn.0376-2491.2017.27.003
Yu Y., Wu G., Jiang Y., Li B., Feng C., Ge Y., et al. (2020). Sea Cucumber Peptides Improved The Mitochondrial Capacity Of Mice: A Potential Mechanism To Enhance Gluconeogenesis And Fat Catabolism During Exercise For Improved Antifatigue Property. Oxid. Med. Cell. Longevity 1–19. doi: 10.1155/2020/4604387
Yu P., Wu R., Zhou Z., Zhang X., Wang R., Wang X., et al. (2021). Raj-Tspin, A Novel Recombinant Peptide From Apostichopus Japonicus, Suppresses The Proliferation, Migration, And Invasion Of Bel-7402 Cells Via A Mechanism Associated With The Itgb1-Fak-Akt Pathway. Investigational New Drugs 39, 377–385. doi: 10.1007/s10637-020-01008-y
Yu S., Ye X., Chen L., Xie X., Zhou Q., Lian X.-Y., et al. (2015). Cytotoxic And Anti-Colorectal Tumor Effects Of Sulfated Saponins From Sea Cucumber. Holothuria Moebii. Phytomedic. 22, 1112–1119. doi: 10.1016/j.phymed.2015.08.007
Zhang H.-J., Chen C., Ding L., Shi H.-H., Wang C.-C., Xue C.-H., et al. (2020a). Sea Cucumbers-Derived Sterol Sulfate Alleviates Insulin Resistance And Inflammation In High-Fat-High-Fructose Diet-Induced Obese Mice. Pharmacol. Res. 160, 105191. doi: 10.1016/j.phrs.2020.105191
Zhang L., Ding L., Shi H., Wang C., Xue C., Zhang T., et al. (2020b). Eicosapentaenoic Acid-Enriched Phospholipids Suppressed Lipid Accumulation By Specific Inhibition Of Lipid Droplet-Associated Protein Fsp27 In Mice. J. Sci. Food Agric. 100, 2244–2251. doi: 10.1002/jsfa.10250
Zhang T., Li R., Han X., Chu Y., Wang C., Chi N., et al. (2020c). Relationship Between Structure And Efficacy Of Sea Cucumber Saponins Echinoside A And Its Derivatives On Hemolytic Activity And Prevention Of Nonalcoholic Fatty Liver Disease. J. Food Sci. 85, 2198–2206. doi: 10.1111/1750-3841.15126
Zhang B., Xue C., Hu X., Xu J., Li Z., Wang J., et al. (2012). Dietary Sea Cucumber Cerebroside Alleviates Orotic Acid-Induced Excess Hepatic Adipopexis In Rats. Lipids Health Dis. 11, 1–9. doi: 10.1186/1476-511X-11-1
Zhang W., Xu P., Zhang H. (2015). Pectin In Cancer Therapy: A Review. Trends In Food Sci. Technol. 44, 258–271. doi: 10.1016/j.tifs.2015.04.001
Zhao F., Liu Q., Cao J., Xu Y., Pei Z., Fan H., et al. (2020). A Sea Cucumber (Holothuria Leucospilota) Polysaccharide Improves The Gut Microbiome To Alleviate The Symptoms Of Type 2 Diabetes Mellitus In Goto-Kakizaki Rats. Food Chem. Toxicol. 135, 110886. doi: 10.1016/j.fct.2019.110886
Zhao Q., Xue Y., Wang J. F., Li H., Long T. T., Li Z., et al. (2012). In Vitro And In Vivo Anti-Tumour Activities Of Echinoside A And Ds-Echinoside A From Pearsonothuria Graeffei. J. Sci. Food And Agric. 92, 965–974. doi: 10.1002/jsfa.4678
Zhou L., Gao N., Sun H., Xiao C., Yang L., Lin L., et al. (2020). Effects Of Native Fucosylated Glycosaminoglycan, Its Depolymerized Derivatives On Intrinsic Factor Xase, Coagulation, Thrombosis, And Hemorrhagic Risk. Thromb. Haemostasis 120, 607–619. doi: 10.1055/s-0040-1708480
Zhou L., Yin R., Gao N., Sun H., Chen D., Cai Y., et al. (2021). Oligosaccharides From Fucosylated Glycosaminoglycan Prevent Breast Cancer Metastasis In Mice By Inhibiting Heparanase Activity And Angiogenesis. Pharmacol. Res. 166, 105527. doi: 10.1016/j.phrs.2021.105527
Zhu Q., Lin L., Zhao M. (2020). Sulfated Fucan/Fucosylated Chondroitin Sulfate-Dominated Polysaccharide Fraction From Low-Edible-Value Sea Cucumber Ameliorates Type 2 Diabetes In Rats: New Prospects For Sea Cucumber Polysaccharide Based-Hypoglycemic Functional Food. Int. J. Biol. Macromol. 159, 34–45. doi: 10.1016/j.ijbiomac.2020.05.043
Keywords: sea cucumber, bioactives, saponins, in vivo studies, clinical trials, human health
Citation: Liang Q, Ahmed F, Zhang M, Sperou N, Franco CMM, Feng Q and Zhang W (2022) In Vivo and Clinical Studies of Sea Cucumber-Derived Bioactives for Human Health and Nutrition From 2012-2021. Front. Mar. Sci. 9:917857. doi: 10.3389/fmars.2022.917857
Received: 11 April 2022; Accepted: 14 June 2022;
Published: 18 July 2022.
Edited by:
Mubarakali Davoodbasha, B. S. Abdur Rahman Crescent Institute Of Science And Technology, IndiaReviewed by:
Eva Martins, Universidade Católica Portuguesa, PortugalWan Iryani Wan Ismail, University of Malaysia Terengganu, Malaysia
Copyright © 2022 Liang, Ahmed, Zhang, Sperou, Franco, Feng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhang, wei.zhang@flinders.edu.au
 Qi Liang
Qi Liang Faruq Ahmed1
Faruq Ahmed1  Christopher M. M. Franco
Christopher M. M. Franco Wei Zhang
Wei Zhang