Rich and underreported: First integrated assessment of the diversity of mesopelagic fishes in the Southwestern Tropical Atlantic
- 1Universidade Federal Rural de Pernambuco (UFRPE), Departamento de Pesca e Aquicultura, Recife, PE, Brazil
- 2MARBEC, Univ. Montpellier, CNRS, Ifremer, IRD, Sète, France
- 3Universidade Federal de Pernambuco (UFPE), Departamento de Oceanografia, Recife, PE, Brazil
- 4Programa de Pós-Graduação em Ciências Ambientais e Conservação, Universidade Federal do Rio de Janeiro (UFRJ), Macaé, RJ, Brazil
- 5Programa de Pós-Graduação em Biologia Comparada, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo (USP), Ribeirão Preto, SP, Brazil
- 6School of Aquatic and Fishery Sciences and Burke Museum of Natural History and Culture, University of Washington, Seattle, WA, United States
- 7Universidade Federal do Rio de Janeiro (UFRJ), Instituto de Biodiversidade e Sustentabilidade, Macaé, RJ, Brazil
Mesopelagic fishes play critical ecological roles by sequestering carbon, recycling nutrients, and acting as a key trophic link between primary consumers and higher trophic levels. They are also an important food source for harvestable economically valuable fish stocks and a key link between shallow and deep-sea ecosystems. Despite their relevance, mesopelagic ecosystems are increasingly threatened by direct and indirect human activities while representing some of the largest and least understood environments on Earth. The composition, diversity, and other aspects of the most basic biological features of numerous mesopelagic groups of fishes are still poorly known. Here, we provide the first integrative study of the biodiversity of mesopelagic fishes of the southwestern Tropical Atlantic (SWTA), based on two expeditions in northeastern Brazil in 2015 and 2017. A full list of mesopelagic fishes of the region is provided, including rare species and new records for the Brazilian Exclusive Economic Zone and the indication of potentially new species in groups such as the Stomiiformes and Beryciformes. Key aspects of the diversity of mesopelagic fishes of the region were also assessed, considering different depth strata and diel periods. At least 200 species, 130 genera, 56 families, and 22 orders of the Teleostei and one shark (Isistius brasiliensis, Dalatiidae, Squaliformes) were recorded, including potentially eight new species (4%) and 50 (25%) new records for Brazilian waters. Five families accounted for 52% of the diversity, 88% of specimens collected, and 66% of the total biomass: Stomiidae (38 spp., 8% of specimens, 21% of biomass), Myctophidae (34 spp., 36%, 24%), Melamphaidae (11 spp., 2%, 7%), Sternoptychidae (9 spp., 26%, 10%), and Gonostomatidae (7 spp., 16%, 4%). During the day, richness and diversity were higher at lower mesopelagic depths (500–1000 m), with contributions of typically bathypelagic species likely associated with seamounts and oceanic islands. At night, richness and diversity increased at epipelagic depths, indicating the diel ascension of several species (e.g., myctophids and sternoptychids) that can endure temperature ranges of up to 25°C. Information on the geographic distribution of several rare species worldwide is also provided.
Introduction
Mesopelagic fishes (200–1,000 m depth) are among the most abundant vertebrates in the biosphere (Gjøsaeter and Kawaguchi, 1980; Irigoien et al., 2014; Nelson et al., 2016). They often have a global distribution, vertical migratory behavior, and several adaptations to overcome challenges imposed by the deep-sea environment (Gjøsaeter and Kawaguchi, 1980; Sutton, 2013; Priede, 2017). Some of these adaptations include low metabolic rates, high tolerance to environmental changes, and complex visual and bioluminescence systems (Priede, 2017). Consequently, the mesopelagic zone holds one of the most diverse fish communities of the ocean, contributing to several ecosystem processes (Gjøsaeter and Kawaguchi, 1980; St. John et al., 2016). Mesopelagic fishes play critical roles by sequestering carbon, recycling nutrients, and acting as key trophic links between primary consumers and higher trophic levels (e.g., larger fishes, mammals, and seabirds) (e.g., Ariza et al., 2015; Cavan et al., 2019; Eduardo et al., 2020a; Eduardo et al., 2020b; Eduardo et al., 2021). They are also an important food source for harvestable fish stocks and a key link between shallow and deep-sea ecosystems (e.g., Cherel et al., 2010; Eduardo et al., 2020b; Eduardo et al., 2021).
Despite their importance, mesopelagic communities are increasingly threatened by climate change (Levin et al., 2019), plastic pollution (Ferreira et al., 2022; Justino et al., 2022), and exploitation of deep-sea resources (Hidalgo and Browman, 2019; Drazen et al., 2020). There is also a major lack of knowledge of the biology, ecology, distribution, and diversity of mesopelagic species, which are typically under-sampled and sparse in data (Glover et al., 2018; Hidalgo and Browman, 2019; Martin et al., 2020).
The southwestern Tropical Atlantic (SWTA) encompasses oceanic islands, underwater canyons, and several seamounts (Travassos et al., 1999; Tchamabi et al., 2017). This region holds distinct biodiversity and includes several Marine Protected Areas and Ecologically or Biologically Significant Marine Areas (EBSAs) that, by definition, are special places of fundamental importance for biodiversity and life cycles of marine species (CBD, 2014). Moreover, the SWTA includes different biogeographic provinces with contrasting thermodynamic features, current systems, and water-mass properties, leading to shifts in biodiversity and ecosystems (Bourlès et al., 1999; Pinheiro et al., 2018; Assunção et al., 2020; Costa da Silva et al., 2021; Dossa et al., 2021; Tosetto et al., 2021; Silva et al., 2022).
The first collection of deep-sea fishes in the SWTA was carried out by the HMS Challenger (1872–1876; Günther, 1887). Since then, mesopelagic fishes have been sporadically collected by different vessels, such as the RV Akademik Kurchatov (1971–1972; Parin et al., 1974), RV Walther Herwig (1966–1971; many authors), RV Marion Dufresne (1987; Séret and Andreata, 1992), RV Atlântico Sul (1996–1999; Figueiredo et al., 2002; Bernardes et al., 2005), RV Thalassa (1999–2000; Costa et al., 2007), RV Astro Garoupa (2003; Costa and Mincarone, 2010), RV Gyre (2008; Costa et al., 2015; Mincarone et al., 2017), and the RV Luke Thomas and RV Seward Johnson (2009, 2011; Lins Oliveira et al., 2015). Although these expeditions substantially contributed to understanding the diversity and ecology of several groups, they were sparse and focused mostly on demersal species (Melo et al., 2020). Only a few studies focused on the mesopelagic communities of the SWTA, with most of them being restricted to the composition and taxonomy of specific groups (e.g., Lima et al., 2011; Mincarone et al., 2014). Consequently, an integrative overview of the mesopelagic fish community of the region is still lacking, leaving a “dark hole” in our understanding of their diversity, ecology, and function in marine ecosystems.
Two recent expeditions focused on mesopelagic fauna were made aboard the RV Antea, as part of the project ABRACOS (Acoustics along the BRAzilian COaSt; Bertrand, 2015; Bertrand, 2017). For the first time, the mesopelagic zone of the SWTA was extensively surveyed, resulting in collections of thousands of deep-sea invertebrates and fishes. Based on these collections, various studies have been published addressing the diversity and ecology of several fish groups, such as Argentiniformes (Mincarone et al., 2021a), Aulopiformes (Mincarone et al., 2022), Myctophiformes (Eduardo et al., 2021), Beryciformes (Afonso et al., 2021), Stomiiformes (Eduardo et al., 2020a; Eduardo et al., 2020b; Villarins et al., 2022), Ceratioidei (Mincarone et al., 2021a), Caristiidae (Mincarone et al., 2019), Howelidae (Eduardo et al., 2019), and Trichiuridae (Eduardo et al., 2018). However, most of the results of these cruises remains unpublished. Here, we present an integrative study of the biodiversity of mesopelagic fishes from the SWTA. A full list of mesopelagic species collected during the ABRACOS expeditions, including a compilation of published new records and the indication of potentially new species, is provided. Key aspects of the mesopelagic fish diversity of the region were also addressed, considering different depth strata and diel periods.
Methodology
Study area
The study area comprised the northeastern Brazilian coast, from Rio Grande do Norte to Alagoas states (5°–9°S), and the seamounts and oceanic islands of the Fernando de Noronha Ridge, including the Rocas Atoll (3°52′S, 33°49′W) and the Fernando de Noronha Archipelago (3°50′S, 32°25′W) (Figure 1). The main oceanographic physico-chemical features of the region were recently described by Assunção et al. (2020); Costa da Silva et al. (2021), and Dossa et al. (2021). Overall, the SWTA is considered oligotrophic. However, locally the banks and islands act as topographic obstacles to currents, driving subsurface enriched waters to the surface (Travassos et al., 1999; Tchamabi et al., 2017; Costa da Silva et al., 2021; Silva et al., 2022). This process increases primary production and enhances the mass and energy fluxes throughout the food web (Travassos et al., 1999; Tchamabi et al., 2017).
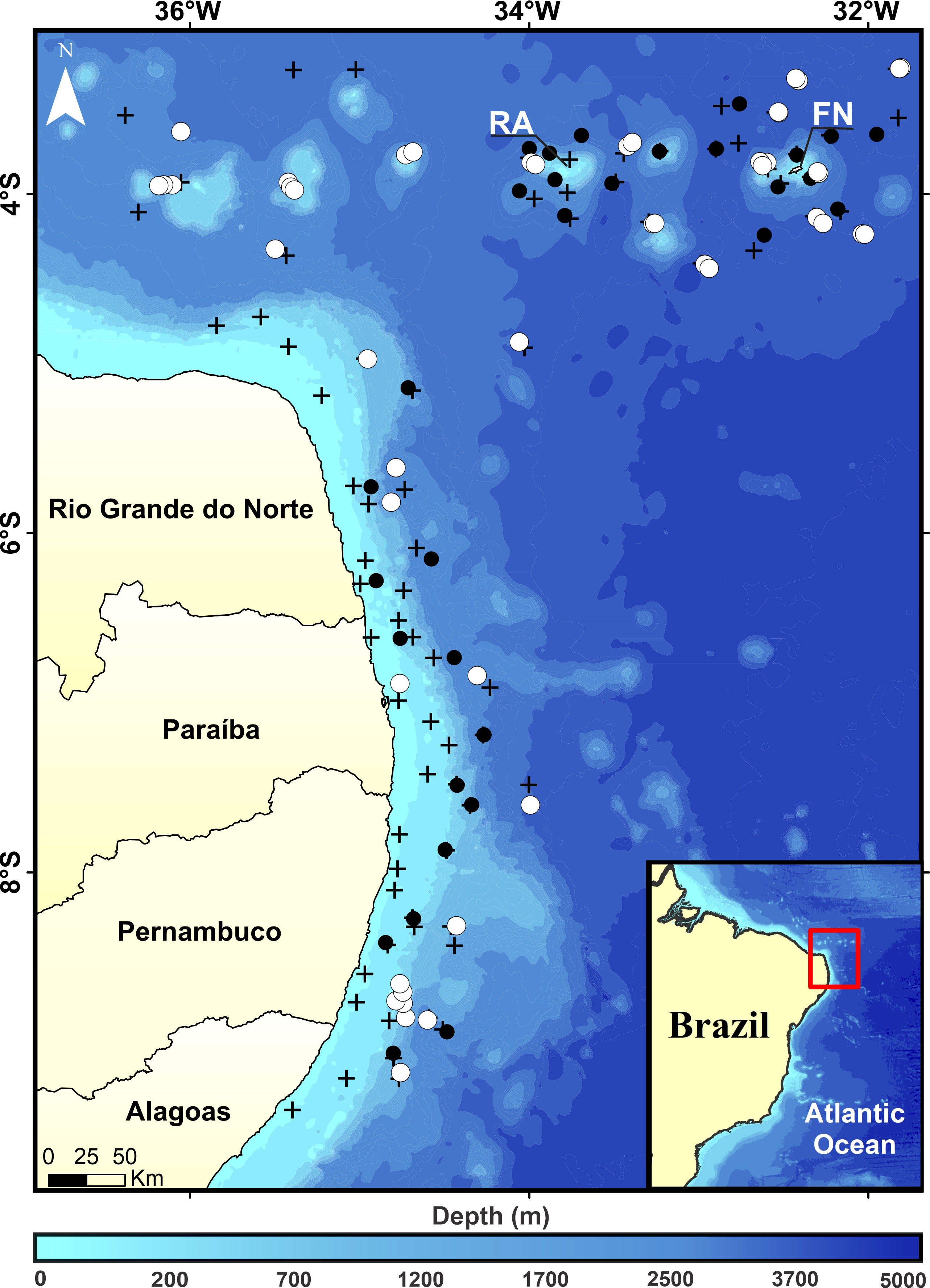
Figure 1 Study area with CTDO profile (cross) and trawl samples (dots). Black and white symbols for ABRACOS 1 and ABRACOS 2, respectively.
Data and specimen collection
Data and specimens were collected during the Acoustics along the BRAzilian COaSt (ABRACOS) surveys, carried out from 29 August to 21 September 2015 (AB1) and from 9 April to 10 May 2017 (AB2), aboard the French RV Antea (Bertrand, 2015; Bertrand, 2017). Temperature profiles were collected using a CTDO SeaBird911+. Mesopelagic fishes were collected day and night at 80 trawl stations by using mesopelagic (AB1; body mesh 30 mm, cod-end mesh 4 mm, size of the net mouth: 16.6 x 8.4 m; Bertrand, 2015) and micronekton (AB2; body mesh 40 mm, cod-end mesh 10 mm, size of the net mouth: 24 x 24 m; Bertrand, 2017) nets (Figure 1; Supplementary Material 1 and 2). Targeted depth ranged from 10 to 1,113 m and was defined by the presence of acoustic scattered layers or patches detected by a Simrad EK60 (Kongsberg Simrad AS) split-beam scientific echosounder, operating at 38, 70, 120, and 200 kHz. Except for the layers 200–300 and 700–800 at night, where no aggregation of organisms were observed through acoustics, all depth strata were sampled at least once (Supplementary Material 1). The net geometry was monitored using SCANMAR sensors, to give headline height, depth, and distance of wings and doors to ensure the net was fishing correctly. Based on SCANMAR the estimated opening area of the micronekton trawl was 120 m2. For the mesopelagic trawl, however, the opening resembled an ellipse of 65 m2. As the trawl was not fitted with an opening and closing mechanism, the collection of specimens during the lowering or hoisting of the net was reduced as much as possible by decreasing ship velocity and increasing winch speed. At the target depths, trawling activity lasted for about 30 minutes at 2–3 kt. Therefore, collection of specimens most likely occurred at target depths, which are indicated as capture depths in the species accounts.
Specimens were sorted to the lowest taxonomic level and frozen or, in the case of rare species or taxonomic uncertainty, fixed in 4% formalin and then preserved in a 70% alcohol solution (Eduardo et al., 2020a). In the laboratory, specimens were identified, measured (nearest 0.1 cm of standard length, SL), and weighed (nearest 0.01 g of total weight, TW). Excluding a few specimens of the Stomiidae, Sternoptychidae, and Myctophidae used for biological analyses (Eduardo et al., 2020a; Eduardo et al., 2020b; Eduardo et al., 2021), all specimens were deposited in the NPM – Fish Collection of the “Instituto de Biodiversidade e Sustentabilidade, Universidade Federal do Rio de Janeiro” (NUPEM/UFRJ). Taxonomic classification follows Nelson et al. (2016), with exceptions noted in Villarins et al. (2022) for the Stomiiformes.
Richness estimators and biodiversity indexes
We first computed a randomised species accumulation curve to assess whether the fish community was exhaustively sampled with the gears employed (Gotelli and Colwell, 2001). This enables calculating a mean number of species for a given number of samples within a 95% confidence interval. The Chao1 index, which extrapolates the total expected number of species in the area for a given sampling gear, was subsequently calculated (Magurran, 2004).
Other aspects of the biodiversity were assessed based on the sample-size-based rarefaction and extrapolation sampling curves, calculated for the species richness, Shannon diversity, and Simpson dominance, the three most widely used species diversity indexes (Magurran, 2004). For that, we used Hill’s numbers, which integrate species richness and relative abundance to propose a more intuitive and statistically rigorous alternative to calculate diversity measures (Chao et al., 2014). Statistical significance was evaluated based on the confidence interval overlapping of the curves.
Sample-size-based rarefaction and extrapolation sampling curves (Hsieh et al., 2016) were also constructed to test for differences in diversity indexes when considering depth strata (epipelagic 0–200 m; upper mesopelagic 200–500 m; lower mesopelagic 500–1000 m) and the diel period (day and night). As the sampling strategy employed in the AB2 expedition was much more efficient in collecting mesopelagic fishes (see Discussion), comparisons using diversity indexes were only made for this survey. Statistical analyses and the calculation of diversity indices were performed using the software R version 4.0.3 through the packages “iNext” (Hsieh et al., 2016) and “vegan” (Oksanen et al., 2017). Fish larvae and species traditionally classified as epipelagic were excluded from the species list, and they were not considered for the diversity assessments. Specimens identified at the genus level only (small-sized and/or damaged specimens), which might represent more than one species, were also excluded from the analyses (Supplementary Material 3).
Results
Biodiversity
Considering our two surveys, 7,119 specimens of mesopelagic fishes, representing 200 species in 130 genera, 56 families, and 22 orders of the Teleostei and one shark (Isistius brasiliensis: Dalatiidae, Squaliformes), were collected and identified (Table 1). The species accumulation curve was steep, indicating that more species would be recorded with additional sampling using the same gears (Figure 2). Indeed, richness estimators indicated that about 100 (50%) additional mesopelagic species of fishes are expected to occur in the area (Figure 2). Additionally, 759 specimens representing about 40 fish taxa were sampled. However, they could not be identified to species level given their small size and/or poor condition. As it was not possible to determine whether these specimens belong to species not listed in Table 1, they were placed in a separate list to ensure a more robust assessment of species diversity (Supplementary Material 3).
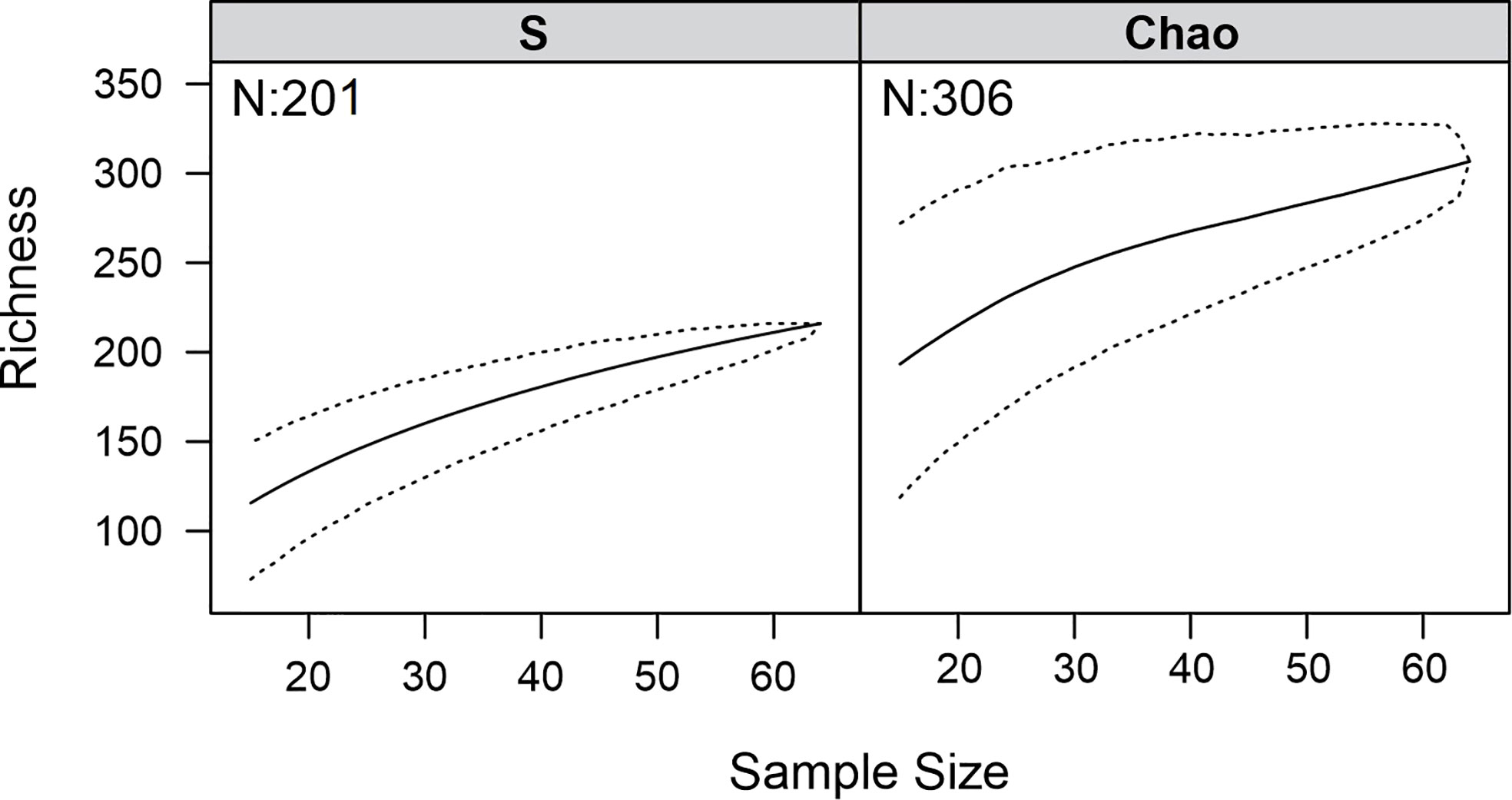
Figure 2 Species accumulation (S) and Chao1 estimator for ABRACOS 1 and 2 together. Dashed lines represent the confidence interval of 95%.
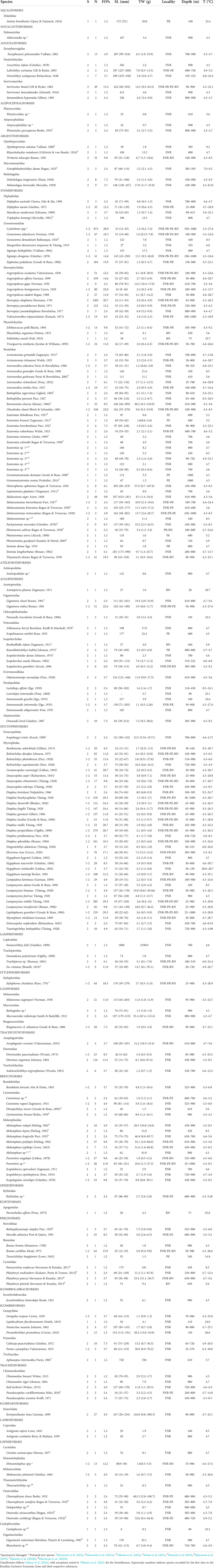
Table 1 Species recorded, survey (S) (1: ABRACOS 1; 2: ABRACOS 2), number of specimens (N), frequency of occurrence to overall samples (FO%), standard length (SL, mean and range), total wet weight (TW, mean and range), collection locality (PE, Pernambuco; PB, Paraíba; RN, Rio Grande do Norte; FNR, Fernando de Noronha Ridge), depth range (based on the target depth of each trawl), temperature range (T), and new records in the Brazilian Exclusive Economic Zone (EEZ).
Ranges of standard length (SL) and wet weight for all species collected on the two surveys are provided in Table 1. Overall, a wide size range was sampled, from 4 mm (Diretmoides pauciradiatus) to 1,880 mm SL (Eumecichthys fiski, Lophotidae). However, 90% of the specimens measured between 30 and 200 mm SL (Supplementary Material 4)
The five orders with the highest number of species were the Stomiiformes (at least 62 species, four families), Myctophiformes (35 spp., two families), Aulopiformes (18 spp., seven families), Beryciformes (16 spp., three families), and Lophiiformes (12 spp., seven families), accounting for 70% of the total number of species recorded on the two surveys. Thirteen orders included less than five species each. Considering families, the most representative were the Stomiidae (38 spp.), Myctophidae (34 spp.), Melamphaidae (11 spp.), Sternoptychidae (10 spp.), and Gonostomatidae (7 spp.) (Figure 3). Half of the families (28) were represented by a single species.
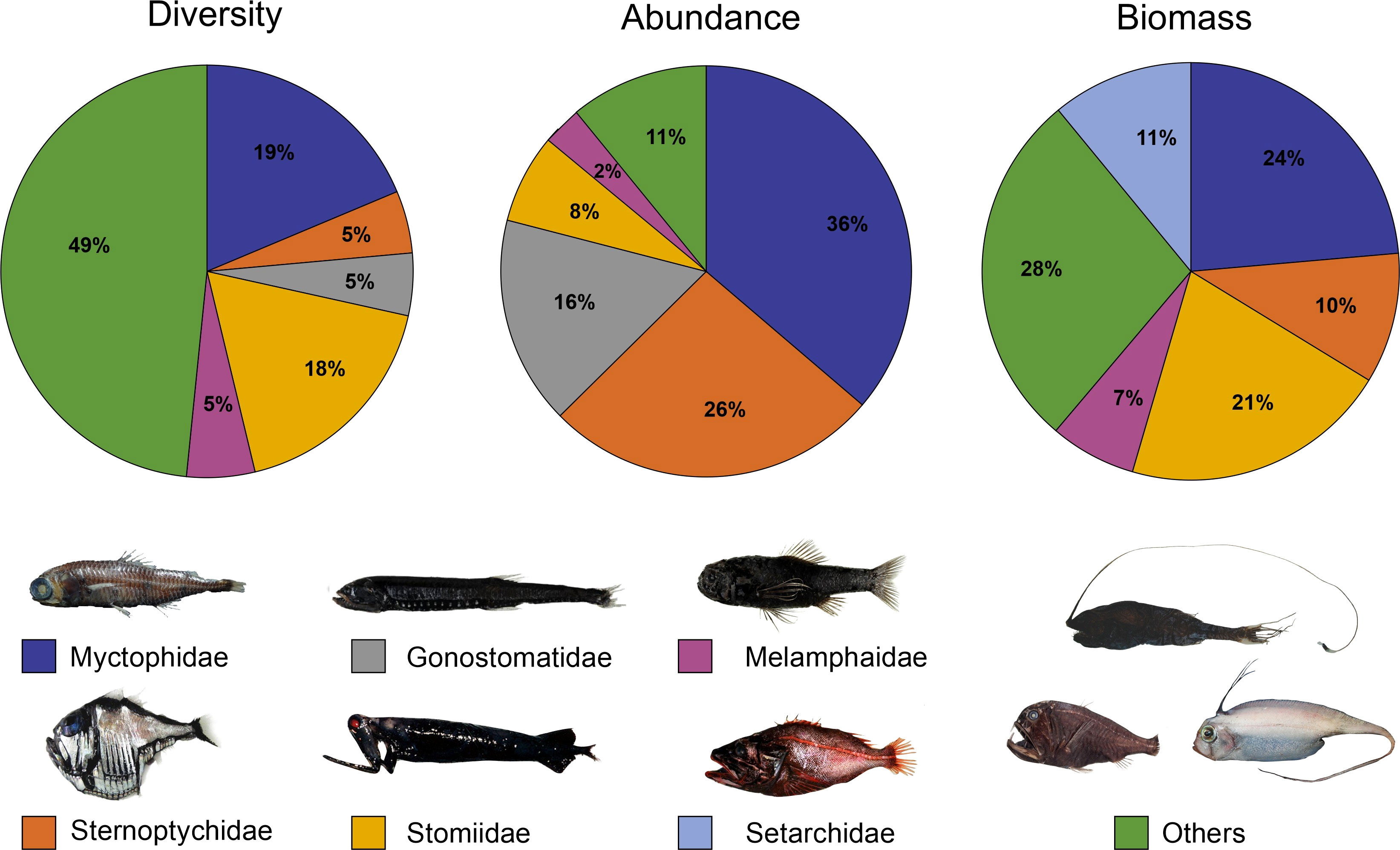
Figure 3 Main mesopelagic fish families collected on the surveys ABRACOS 1 and ABRACOS 2 when considering diversity, abundance, and biomass. Fish images represent only examples of species included in the group.
In terms of abundance, the most representative families when considering the two surveys were the Myctophidae (Myctophiformes; 36%), Sternoptychidae (Stomiiformes; 26%), Gonostomatidae (Stomiiformes; 16%), Stomiidae (Stomiiformes; 8%), and Melamphaidae (Beryciformes; 2%) (Figure 3). These families together accounted for 88% of all specimens collected. The remaining families represented individually no more than 2% of the total number of specimens collected. At the alpha taxonomic level, the following taxa represented almost 50% of all specimens collected: Sternoptyx diaphana (14%), Cyclothone spp. (11%; see Discussion), Diaphus brachycephalus (6%), Argyropelecus affinis (6%), Chauliodus sloani (5%), Lampanyctus nobilis (4%), and Diaphus perspicillatus (4%). About 126 species were represented by five specimens or less, of which 62 were represented by a single specimen.
Considering biomass, the most representative families were the Myctophidae (24%), Stomiidae (21%), Setarchidae (Scorpaeniformes, 11%), Sternoptychidae (10%), and Melamphaidae (7%) (Figure 3). These families together accounted for 73% of the biomass of all fishes collected. The remaining families individually accounted for less than 4% of the total weight. At the specific level, the following species represented 42% of the biomass: Ectreposebastes imus (11%), Chauliodus sloani (9%), Borostomias elucens (6%), Eumecichthys fiski (6%, a single specimen), Sternoptyx diaphana (4%), Melamphaes polylepis (3%), and Argyropelecus affinis (3%).
Distribution, vertical migration, biodiversity indexes, and size
Based on the two campaigns, 60 species (29%) were recorded in a wide longitudinal distribution (Table 1). In contrast, 133 species (64%) were collected only in a few localities, with 116 being restricted to the Fernando de Noronha Ridge area, which aggregates most specimens collected (Table 1). Considering depth and period, the highest diversity, abundance, and biomass were found between depths of 700 and 1,000 m during the day (Figure 4). At night, the highest number of species was recorded at lower mesopelagic depths (500–1,000 m). However, much larger values of number of species, abundance, and biomass were detected in shallow waters (0–200 m), likely reflecting the ascent in the water column of several species at night. At least 50 species seem to have a wide range of depth distribution and tolerance to variations in water temperature (up to 800 m and 25°C; e.g., species of Sternoptychidae and Myctophidae). However, 66 species seem to be restricted to deeper (> 600 m) and colder waters (< 6° C) regardless of the time period (e.g., Lophiiformes and Beryciformes; Table 1).
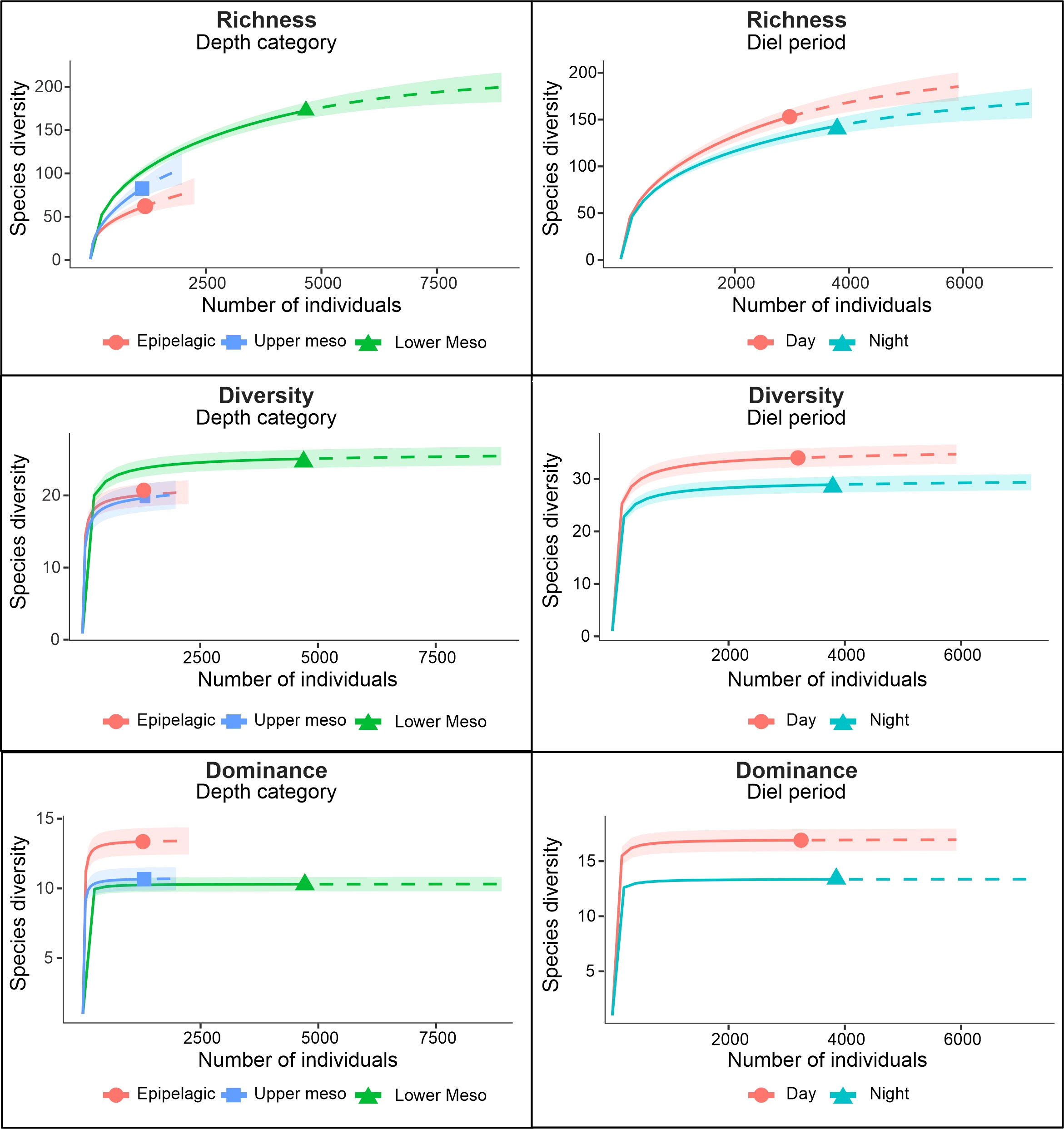
Figure 4 Sample-size-based rarefaction (solid line segment) and extrapolation (dotted line segments) sampling curves for species richness, diversity, and dominance of mesopelagic fish data at different depth categories and diel periods. Curves include the confidence intervals of 95% (shaded areas). For this analysis, only species recorded in the ABRACOS 2 survey were considered.
Significant differences in biodiversity indexes (calculated only for ABS2, see methodology) were found when considering diel periods and depth. Higher values of richness and diversity were found in lower mesopelagic waters and during the daytime. However, dominance values were significantly higher in epipelagic waters and also during daytime (Figure 5). Detailed values for the calculated indexes are given in the Supplementary Material 5.
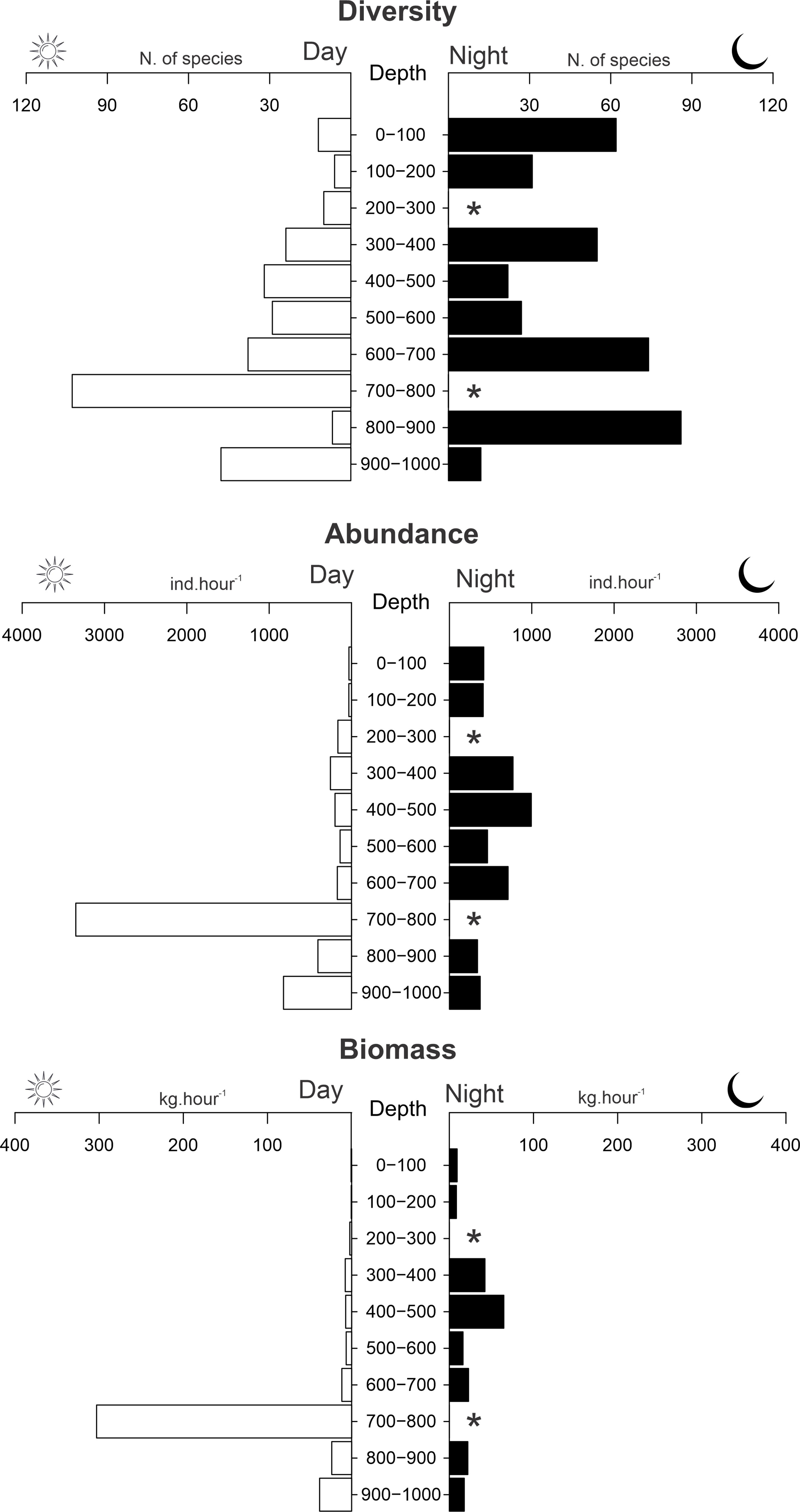
Figure 5 Number of species and average values of abudance (individuals.hour-1) and biomass (kg.hour-1x102) of mesopelagic species of fishes collected on the survey ABRACOS 2. *Depth strata not sampled.
Discussion
Diversity and distribution
Based on our two campaigns, at least 201 species of mesopelagic fishes occur in the SWTA. Results also indicate that about 100 additional species could have been collected if sampling efforts were increased. The taxonomically diverse pool of mesopelagic species recorded in our surveys also reveals a vast array of diversity not only in terms of the number of species but also in terms of size, anatomy, and behaviour. In a recent global biogeographic classification of the mesopelagic zone (Sutton et al., 2017), the Tropical and western Equatorial Atlantic, which is the larger area encompassing the SWTA, was not considered a region particularly diverse in terms of mesopelagic fishes. However, the mesopelagic species richness revealed by our two campaigns is higher than those reported for other parts of the world, such as the Mediterranean (25 spp.; Olivar et al., 2012), central Equatorial Pacific (113 spp.; Barnett, 1984), southwestern Indian Ocean (121 spp.; Cherel et al., 2020), eastern Equatorial Atlantic (132 spp.; Olivar et al., 2017), and South China Sea (169 spp.; Wang et al., 2019). The species richness of mesopelagic fishes in the SWTA is actually more similar to that reported for the North Pacific (228 spp.; Barnett, 1984) and the Gulf of Mexico (approximately 300 spp.; Sutton et al., 2020), which are considered as comprising some of the most speciose deep-sea ichthyofaunas of the world (Sutton et al., 2017). Major factors driving deep-sea biodiversity, such as climate, seabed structure, water masses, and phylogenetic history, are likely responsible for the variation in species richness of different parts of the world. However, an asymmetry in collecting effort is certainly affecting the values recorded so far. In the Gulf of Mexico, a much higher sampling effort has been deployed to assess the deep-sea diversity compared with most regions of the world, with several expeditions conducted only in the last decade (Sutton et al., 2020). That situation is in striking contrast with the SWTA, where just a handful of expeditions to assess deep-sea diversity have been conducted in the last centuries.
The relatively high number of mesopelagic species of fishes recorded in our two campaigns is likely related to the diversity of habitats and the high variability of oceanographic processes of the SWTA. Despite being located in an oligotrophic portion of the ocean, this region is also characterized by the presence of underwater canyons, oceanic islands, and several seamounts that interact with local currents and enhance marine productivity (Travassos et al., 1999; Tchamabi et al., 2017; Costa da Silva et al., 2021). As an example, small uplifting processes have been reported along the shelf-break and oceanic islands of the region (Travassos et al., 1999; MMA, 2006; Tchamabi et al., 2017; Silva et al., 2022), a situation that has been directly associated with the occurrence of hotspots of fish biodiversity (Hazin, 1993; Eduardo et al., 2018; Eduardo et al., 2020a). Distinct biogeographic provinces, with different thermodynamic features, current systems and water mass properties, are also present in the SWTA (Bourlès et al., 1999; Assunção et al., 2020; Costa da Silva et al., 2021; Dossa et al., 2021; Tosetto et al., 2021). This results in a high complexity of habitats and oceanographic conditions that likely contribute to higher levels of species diversity (Levin et al., 2001).
The highest levels of richness and diversity (considering only specimens collected during AB2, see methodology) were found at lower mesopelagic depths (500–1,000 m), with several species collected only at these depths (e.g., species of the Beryciformes and Lophiiformes). Interestingly, many of these species are considered bathypelagic and/or benthopelagic (Priede, 2017; Melo et al., 2020). The collection of those species in mesopelagic waters is likely related to the presence of seamounts and oceanic islands. In addition to being related to an increase in habitat complexity, seamounts may increase the occurrence of pelagic and benthic predators that actively seek these areas to hunt for prey trapped by flow-topographic processes (Cascão et al., 2019). For instance, in the Azorean seamounts plateau, the micronekton community is dominated by non- or weakly migratory benthopelagic fishes (Cascão et al., 2019). In summary, our results also seem to indicate that seamounts play a significant role in the biodiversity structuring and ecology of mesopelagic fishes in the SWTA.
The two surveys conducted during this study resulted in different patterns of species richness. For example, 17 species were exclusively recorded in AB1 (mesopelagic trawl), whereas 136 species were recorded only in AB2 (micronekton trawl). The two campaigns were conducted in different seasons. However, since the study area is located in a tropical region, few oceanographic differences were noted in the mesopelagic zone (for further info refer to Assunção et al., 2020; Costa da Silva et al., 2021; Dossa et al., 2021). Therefore, the significant disparity in species richness between the two expeditions is clearly related to differences in sampling strategies. The use of multiple sampling gears is vital to maximizing the representation of fish diversity (Magurran, 2004), especially in the deep-sea. However, the sampling strategy used in AB2, which included the use of larger gear, with greater mesh sizes, deeper hauls, and broader sampling area, resulted in the collection of a higher number of specimens of different species in a broader size range (Supplementary Material 2 and 3).
In terms of taxonomic composition, five families of the Teleostei accounted for 52% of the species richness, 88% of the specimens, and 66% of the total biomass collected on the two surveys: the Stomiidae (38 spp., 8% of the specimens, 21% of the biomass), Myctophidae (34 spp., 36%, 24%), Melamphaidae (11 spp., 2%, 7%), Sternoptychidae (9 spp., 26%, 10%), and Gonostomatidae (7 spp., 16%, 4%). These families, therefore, seem to be the most represented in the mesopelagic fish fauna of the SWTA. The dominance of these families in mesopelagic waters has also been reported in other parts of the world (e.g., Gjøsaeter and Kawaguchi, 1980; Olivar et al., 2017; Wang et al., 2019; Cherel et al., 2020). A strong pattern of dominance was also observed within these families, with few species accounting for 50% of the total number of specimens: Sternoptyx diaphana (14%), Cyclothone spp. (11%), Argyropelecus affinis (6%), Diaphus brachycephalus (6%), Chauliodus sloani (5%), Diaphus perspicillatus (4%), and Lampanyctus nobilis (4%). The pattern of dominance at the species level detected in the SWTA was, however, distinct from other parts of the world. In the Eastern Tropical Atlantic, for instance, the lanternfishes B. suborbitale, C. warmingii, and H. macrochir were dominant (Olivar et al., 2017), whereas these same species were considered rare in our study. The viperfish C. sloani is usually globally recorded in low abundances (e.g., Olivar et al., 2017; Wang et al., 2019; Cherel et al., 2020), whereas the species is among the most relevant mesopelagic species in the SWTA considering the abundance and total weight (Eduardo et al., 2020c). These differences in the pattern of dominance at the species level in different parts of the world are likely associated with different sampling strategies employed and differences in oceanographic and biogeographic features (e.g., seabed structure, water masses, and hydrographic fronts), which are major factors driving the structure and composition of mesopelagic assemblages (Hulley and Krefft, 1985; Olivar et al., 2017; Cascão et al., 2019). Cyclotone is another seemingly abundant genus of mesopelagic fish in the SWTA (Olivar et al., 2017). Eight species of the genus were reported for the SWTA: C. acclinidens, C. alba, C. braueri, C. microdon, C. obscura, C. pallida, C. parapallida, and C. pseudopallida (Villarins et al., 2022) The sampling gears employed in the study, however, seemed to be only partially adequate to collect specimens of the genus. In several trawls we observed onboard that a substantial number of specimens of Cyclothone escaped back into the sea during the hoisting of the net. Additionally, given their poor condition of preservation, specimens of the genus could not be identified at species level. Therefore, the abundance of species of Cyclothone presented here is underestimated.
Notable records
Among the 201 species of mesopelagic fishes recorded during the ABRACOS expeditions, 50 (25%) represent new records for Brazilian waters, all of which have been dealt with in a series of recent papers (Table 1). In addition to these new records, eight species (five Eustomias, one Melanostomias, one Melamphaes, and one Poromitra) are potentially new and will be formally described later. Several species recorded here are also rare worldwide, and their occurrence in the SWTA adds new information on their global distribution. For instance, three specimens of Platyberyx paucus and one of Platyberyx pietschi were collected during the AB2. Before these records, only four specimens of P. paucus were known, from the central North Pacific and western Central Atlantic. Platyberyx pietschi, in turn, was known from just two specimens collected in the western Central Atlantic, one specimen collected in the central Pacific, and another from the western South Pacific (Stevenson and Kenaley, 2013; Mincarone et al., 2019). Other species considered rare worldwide that were collected in the ABRACOS expeditions are Aulotrachichthys argyrophanus, Rhynchohyalus natalensis, Eumecichthys fiski, Macrouroides inflaticeps, Pseudoscopelus cordilluminatus, Melamphaes leprus, and Gigantactis watermani (Pimentel et al., 2020; Afonso et al., 2021; Mincarone et al., 2021b; Mincarone et al., 2022).
Role of international cooperation for the decade of deep ocean science
The high number of new records made during the ABRACOS expeditions reflects not only the high diversity of the SWTA, but also the overall lack of scientific information on deep-sea diversity in the region, as noted previously (e.g., Reis et al., 2016; Mincarone et al., 2022). The United Nations Decade of Ocean Science roadmap recognizes the deep-sea as a frontier of science and discovery (Ryabinin et al., 2019). There is an unequal capacity to conduct science among nations, with developing economies facing substantial barriers to participating in deep-sea research. Consequently, the least-studied parts of the deep-sea are located off the least economically developed countries (Howell et al., 2020). These biases are highlighted by the fact that a French research institution financed the surveys described here, and that those expeditions are among the very few that have addressed the mesopelagic ichthyofauna of Brazil. To achieve sustainability, we need a well-known and predictable ocean. Only by thinking globally and strengthening international cooperation we will develop an ocean research that corrects asymmetry in funding and knowledge among countries, meeting the crucial need for a more encompassing deep-sea knowledge aimed at the conservation and sustainable use of its unique habitats.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
All collecting methods and specimen handling procedures were approved and carried out in accordance with relevant guidelines and regulations of the Brazilian Ministry of Environment (SISBIO; authorization number: 47270–5).
Author contributions
All co-authors declare no competing interests and agree with the submission of this manuscript. All authors significantly contributed to the development of the article.
Acknowledgments
We acknowledge the French oceanographic fleet for funding the at-sea survey ABRACOS, and the officers and crew of the RV Antea for their contribution to the success of the operations. Thanks also to the BIOIMPACT (UFRPE) and LIZ (UFRJ) students for their support. For providing literature we thank Alexei Orlov (P.P. Shirshov Institute of Oceanology), Artem Prokofiev (A.N. Severtsov Institute of Ecology and Evolution), Jørgen Nielsen (Natural History Museum of Denmark), Ron Fricke (State Museum of Natural History Stuttgart), Sergei Evseenko (in memorian, P.P. Shirshov Institute of Oceanology), and Thomas Munroe (National Systematics Laboratory, NOAA). This study is part of the PhD thesis conducted by the first author, who is especially grateful to members of the examination committee, Anne Lebourges-Dhaussy, Emmanuel Paradis, Heino Fock, Juan Carlos Molinero, Luiz A. Rocha, Paulo Travassos, Rosângela Lessa, and Yves Cherel, for their critical reviews and constructive comments. CAPES (Coordination for the Improvement of Higher Education Personnel) provided a student scholarship to Leandro Eduardo, who is also supported by FUNBIO and Humanize under the grant “Programa Bolsas Funbio - Conservando o Futuro 2018” (011/2019). We thank CNPq (Brazilian National Council for Scientific and Technological Development) for providing research grants to Flávia Lucena-Frédou, Thierry Frédou, and Michael Mincarone (grants 308554/2019-1, 307422/2020-8, and 314644/2020-2, respectively). Gabriel Afonso was supported by PIBIC/CNPq, Bárbara Villarins was supported by PIBIC/CNPq and PROTAX/CNPq (443302/2020), and Júlia Martins was supported by CAPES, FUNBIO, and PROTAX/CNPq (443302/2020). This study is a contribution to the LMI TAPIOCA, program CAPES/COFECUB (88881.142689/2017-01), EU H2020 TRIATLAS project (grant agreement 817578). The NPM Fish Collection is supported by the Project MULTIPESCA (FUNBIO) under the grant ‘Pesquisa Marinha e Pesqueira’ and FAPERJ (grant E-26/210.290/2021).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.937154/full#supplementary-material
References
Afonso G. V. F., Di Dario F., Eduardo L. N., Lucena-Frédou F., Bertrand A., Mincarone M. M. (2021). Taxonomy and distribution of deep-sea bigscales and whalefishes (Teleostei: Stephanoberycoidei) collected off northeastern Brazil, including seamounts and oceanic islands. Ichthyol. Herpetol. 109 (2), 467–488. doi: 10.1643/i2020069
Ariza A., Garijo J. C., Landeira J. M., Bordes F., Hernández-León S. (2015). Migrant biomass and respiratory carbon flux by zooplankton and micronekton in the subtropical northeast Atlantic ocean (Canary islands). Prog. Oceanogr. 134, 330–342. doi: 10.1016/j.pocean.2015.03.003
Assunção R. V., Silva A. C., Roy A., Bourlès B., Silva C., Ternon J.-F., et al. (2020). 3D characterisation of the thermohaline structure in the southwestern tropical Atlantic derived from functional data analysis of in situ profiles. Prog. Oceanogr. 187, 102399. doi: 10.1016/j.pocean.2020.102399
Barnett M. A. (1984). Mesopelagic fish zoogeography in the central tropical and subtropical pacific ocean: species composition and structure at representative locations in three ecosystems. Mar. Biol. 82, 199–208. doi: 10.1007/BF00394103
Bernardes R. A., Rossi-Wongtschowski C. L. D. B., Wahrlich R., Vieira R. C. (2005). Prospecção pesqueira de recursos demersais com armadilhas e pargueiras na zona econômica exclusiva da região sudeste-sul do Brasil. Serie Documentos REVIZEE/Score-Sul.
Bourlès B., Gouriou Y., Chuchla R. (1999). On the circulation in the upper layer of the western equatorial Atlantic. J. Geophys. Res. Ocean. 104, 21151–21170. doi: 10.1029/1999jc900058
Cascão I., Domokos R., Lammers M. O., Santos R. S., Silva M. A. (2019). Seamount effects on the diel vertical migration and spatial structure of micronekton. Prog. Oceanogr. 175, 1–13. doi: 10.1016/J.POCEAN.2019.03.008
Cavan E. L., Laurenceau-Cornec E. C., Bressac M., Boyd P. W. (2019). Exploring the ecology of the mesopelagic biological pump. Prog. Oceanogr. 176, 102–125. doi: 10.1016/j.pocean.2019.102125
CBD (2014). “Ecologically or biologically significant marine areas (EBSAs),” in Special places in the world’s oceans, 2nd ed (Recife: Secretariat of the Convention on Biological Diversity).
Chao A., Gotelli N. J., Hsieh T. C., Sander E. L., Ma K. H., Colwell R. K., et al. (2014). Rarefaction and extrapolation with hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 84, 45–67. doi: 10.1890/13-0133.1
Cherel Y., Fontaine C., Richard P., Labat J. P. (2010). Isotopic niches and trophic levels of myctophid fishes and their predators in the southern ocean. Limnol. Oceanogr. 55, 324–332. doi: 10.4319/lo.2010.55.1.0324
Cherel Y., Romanov E. V., Annasawmy P., Thibault D., Ménard F. (2020). Micronektonic fish species over three seamounts in the southwestern Indian ocean. Deep. Res. Part II 176, 102161. doi: 10.1016/j.dsr2.2020.104777
Costa P., Olavo G., Martins A (2007). Biodiversidade da fauna marinha profunda na costa central brasileira. Rio de Janeiro: Museu Nacional
Costa da Silva A., Chaigneau A., Dossa A. N., Eldin G., Araujo M., Bertrand A. (2021). Surface circulation and vertical structure of upper ocean variability around Fernando de Noronha Archipelago and rocas atoll during spring 2015 and fall 2017. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.598101
Costa P. A. S., Mincarone M. M. (2010). Biodiversidade da região oceânica profunda da Bacia de Campos: Megafauna e ictiofauna demersal. SAG Serv Rio Janeiro pp, 295–373.
Costa P. A. S., Mincarone M. M., Braga A. C., Martins A. S., Lavrado H. P., Haimovici M., et al. (2015). Megafaunal communities along a depth gradient on the tropical Brazilian continental margin. Mar. Biol. Res. 11, 1053–1064. doi: 10.1080/17451000.2015.1062521
Dossa A. N., Costa da Silva A., Chaigneau A., Eldin G., Araujo M., Bertrand A. (2021). Near-surface western boundary circulation off northeast Brazil. Prog. Oceanogr. 190, 102475. doi: 10.1016/j.pocean.2020.102475
Drazen J. C., Smith C. R., Gjerde K. M., Haddock S. H. D., Carter G. S., Choy C. A., et al. (2020). Midwater ecosystems must be considered when evaluating environmental risks of deep-sea mining. Proc. Natl. Acad. Sci. 117, 17455–17460. doi: 10.1073/pnas.2011914117
Eduardo L., Frédou T., Lira A. S., Ferreira B. P., Bertrand A., Ménard F., et al. (2018). Identifying key habitat and spatial patterns of fish biodiversity in the tropical Brazilian continental shelf. Cont. Shelf Res. 166, 108–118. doi: 10.1016/j.csr.2018.07.002
Eduardo L. N., Villarins B. T., Lucena-Frédou F., Frédou T., Lira A. S., Bertrand A, et al. (2018b). First record of the intermediate scabbardfish Aphanopus intermedius (Scombriformes: Trichiuridae) in the western South Atlantic Ocean. J. Fish Biol. 93, 992–5. doi: 10.1111/jfb.13796
Eduardo L. N., Villarins B. T., Martins J. R., Lucena-Frédou F., Frédou T., Lira A. S., et al. (2019). Deep-sea oceanic basslets (Perciformes, Howellidae) from Brazil: New records and range extensions. Check List 15, 965–971. doi: 10.15560/15.6.965
Eduardo L. N., Bertrand A., Frédou T., Lira A. S., Lima R. S., Ferreira B. P., et al. (2020a). Biodiversity, ecology, fisheries, and use and trade of tetraodontiformes fishes reveal their socio-ecological significance along the tropical Brazilian continental shelf. Aquat. Conserv. Mar. Freshw. Ecosyst. 30, 761–774. doi: 10.1002/aqc.3278
Eduardo L. N., Bertrand A., Mincarone M. M., Santos Silva L. V., Frédou T., Assunção R. V., et al. (2020b). Hatchetfishes (Stomiiformes: Sternoptychidae) biodiversity, trophic ecology, vertical niche partitioning and functional roles in the western tropical Atlantic. Prog. Oceanogr. 186, 102389. doi: 10.1016/j.pocean.2020.102389
Eduardo L. N., Mincarone M. M., Lucena-Frédou F., Martins J. R., Afonso G. V. F., Villarins B. T., et al. (2020c). Length-weight relationship of twelve mesopelagic fishes from the western tropical Atlantic. J. Appl. Ichthyol. 36, 845–848. doi: 10.1111/jai.14084
Eduardo L. N., Bertrand A., Mincarone M. M., Martins J. R., Frédou T., Assunção R. V., et al. (2021). Distribution, vertical migration, and trophic ecology of lanternfishes (Myctophidae) in the southwestern tropical Atlantic. Prog. Oceanogr. 199, 102695. doi: 10.1016/j.pocean.2021.102695
Ferreira G. V. B., Justino A. K. S., Eduardo L. N., Lenoble V., Fauvelle V., Schmidt N., et al. (2022). Plastic in the inferno: Microplastic contamination in deep-sea cephalopods (Vampyroteuthis infernalis and Abralia veranyi) from the southwestern Atlantic. Mar. Pollut. Bull. 174. doi: 10.1016/j.marpolbul.2021.113309
Figueiredo J., Santos A., Yamaguti N., Bernardes R., Rossi-Wongtschowski C. L. D. (2002). Peixes da zona econômica exclusiva da região sudeste-sul do Brasil: levantamento com rede de meia-água (São Paulo: Editora da Universidade de São Paulo).
Gjøsaeter J., Kawaguchi K. (1980). A review of the world resources of mesopelagic fish. FAO Fish. Tech. Pap. 193, 123–134.
Glover A. G., Wiklund H., Chen C., Dahlgren T. G. (2018). Point of view: Managing a sustainable deep-sea ‘blue economy’ requires knowledge of what actually lives there. Elife 7. doi: 10.7554/eLife.41319
Gotelli N. J., Colwell R. K. (2001). Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 4, 379–391. doi: 10.1046/j.1461-0248.2001.00230.x
Günther A. (1887). Report on the deep-sea fishes collected by H.M.S. Challenger during the years 1873-76, in: Thomson C.W. (Ed.), Report on the scientific results of the voyage of H. M. S. Challenger during the years 1873-76. Zoology 22 (57), i–lxv + 1–268, Pls. 1–66.
Hazin F. V. (1993). Fisheries – oceanographical study of tunas, billfishes and sharks in the southwestern equatorial Atlantic Ocean. (Thesis) (Tokyo: Tokyo University of Fisheries).
Hidalgo M., Browman H. I. (2019). Developing the knowledge base needed to sustainably manage mesopelagic resources. ICES J. Mar. Sci. 76, 609–615. doi: 10.1093/icesjms/fsz067
Howell K. L., Hilário A., Allcock A. L., Bailey D., Baker M., Clark M. R., et al. (2020). A decade to study deep-sea life. Nat. Ecol. Evol. 6, 470. doi: 10.1038/s41559-020-01352-5
Hsieh T. C., Ma K. H., Chao A. (2016). iNEXT: an r package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 7, 1451–1456. doi: 10.1111/2041-210X.12613
Hulley P. A., Krefft G. (1985). A zoogeographic analysis of the fishes of the family Myctophidae (Osteichthyes, Myctophiformes) from the 1979-Sargasso Sea expedition of RV Anton Dohrn. Ann. S. Afr. Mus. 96 (2), 19–53.
Irigoien X., Klevjer T. A., Røstad A., Martinez U., Boyra G., Acuña J. L., et al. (2014). Large Mesopelagic fishes biomass and trophic efficiency in the open ocean. Nat. Commun. 5, 3271. doi: 10.1038/ncomms4271
Justino A. K. S., Ferreira G. V. B., Schmidt N., Eduardo L. N., Fauvelle V., Lenoble V., et al. (2022). The role of mesopelagic fishes as microplastics vectors across the deep-sea layers from the southwestern tropical Atlantic. Environ. pollut. 300, 118988. doi: 10.1016/j.envpol.2022.118988
Levin L., Baker M., Thomson A. (2019). Deep-ocean climate change impacts on habitat, fish and fisheries (Rome: FAO).
Levin L. A., Etter R. J., Rex M. A., Gooday A. J., Smith C. R., Pineda J., et al. (2001). Environmental influences on regional deep-sea species diversity. Annu. Rev. Ecol. Syst. 32, 51–93. doi: 10.1146/annurev.ecolsys.32.081501.114002
Lins Oliveira J. E., Nóbrega M. F., Garcia Júnior J., Sampaio C. L. S., Di Dario F., Fischer L. G., et al. (2015). Biodiversidade marinha da bacia Potiguar/RN: Peixes do talude continental (Rio de Janeiro: Museu Nacional).
Lima A. T., Costa P. A. S., Braga A. C., Nunan G. W. A., Mincarone M. M. (2011). Fishes of the family Sternoptychidae (Stomiiformes) collected on the Brazilian continental slope between 11° and 23°S. Zootaxa 2742, 34–48. doi: 10.11646/zootaxa.2742.1.2
Martin A., Boyd P., Buesseler K., Cetinic I., Claustre H., Giering S., et al. (2020). The oceans’ twilight zone must be studied now, before it is too late. Nature 580, 26–28. doi: 10.1038/d41586-020-00915-7
Melo M. R. S., Caires R. A., Sutton T. T. (2020). “The scientific explorations for deep-sea fishes in Brazil: The known knowns, the known unknowns, and the unknown unknowns” in Brazilian Deep-sea biodiversity. Eds. Sumida P. Y. G., Bernardino A. F., Léo F. C. (Cham, Switzerland: Springer Netherlands), 153–216.
Mincarone M. M., Afonso G. V. F., Di Dario F., Eduardo L. N., Frédou T., Lucena-Frédou F., et al. (2021b). Deep-sea anglerfishes (Lophiiformes: Ceratioidei) from off northeastern Brazil, with remarks on the ceratioids reported from the Brazilian Exclusive Economic Zone. Neotrop. Ichthyol. 19 (2), 1–28. doi: 10.1590/1982-0224-2020-0151
Mincarone M. M., Di Dario F., Costa P. A. S. (2014). Deep-sea bigscales, pricklefishes, gibberfishes and whalefishes (Teleostei: Stephanoberycoidei) off Brazil: New records, range extensions for the south-western Atlantic ocean and remarks on the taxonomy of Poromitra. J. Fish Biol. 85, 1546–1570. doi: 10.1111/jfb.12515
Mincarone M. M., Eduardo L. N., Di Dario F., Frédou T., Bertrand A., Lucena-Frédou F. (2022). New records of rare deep-sea fishes (Teleostei) collected from off northeastern Brazil, including seamounts and islands of the Fernando de Noronha Ridge. J. Fish Biol. 100. doi: 10.1111/jfb.15155
Mincarone M. M., Martins A. S., Costa P. A. S., Braga A. C., Haimovici M. (2017). “Peixes marinhos da bacia de Campos: uma revisão da diversidade,” in Comunidades demersais e bioconstrutores: caracterização ambiental regional da bacia de Campos, Atlântico Sudoeste. Eds. Curbelo-Fernandez M. P., Braga A. C. (Rio de Janeiro: Elsevier), 187–216.
Mincarone M. M., Martins J. R., Di Dario F., Eduardo L. N., Frédou T., Lucena-Frédou F., et al. (2021a). Deep-sea smelts, pencil smelts, and barreleyes (Teleostei: Argentiniformes) from oceanic islands and seamounts off northeastern Brazil. Mar. Biol. Res. 16 (10), 762–773. doi: 10.1080/17451000.2021.1891806
Mincarone M. M., Villarins B. T., Eduardo L. N., Caires R. A., Lucena-Frédou F., Frédou T., et al. (2019). Deep-sea manefishes (Perciformes: Caristiidae) from oceanic islands and seamounts off northeastern Brazil, with comments on the caristiids previously reported in Brazilian waters. Mar. Biol. Res. 15 (3), 297–304. doi: 10.1080/17451000.2019.1636281
MMA (2006). Programa REVIZEE: avaliação do potencial sustentável de recursos vivos da Zona Econômica Exclusiva do Brasil - relatório executivo (Brasília: Ministério do Meio Ambiente).
Nelson J. S., Grande T. C., Wilson M. V. H. (2016). Fish of the world, 5th ed (John. Hoboken: Wiley & Sons).
Oksanen J., Guillaume Blanchet F., Kindt R., Legendre P., Dan McGlinn P. R. M., Simpson L., et al. (2017). Vegan: Community ecology package.
Olivar M. P., Bernal A., Molí B., Peña M., Balbín R., Castellón A., et al. (2012). Vertical distribution, diversity and assemblages of mesopelagic fishes in the western Mediterranean. Deep. Res. Part I 62, 53–69. doi: 10.1016/j.dsr.2011.12.014
Olivar M. P., Hulley P. A., Castellón A., Emelianov M., López C., Tuset V. M., et al. (2017). Mesopelagic fishes across the tropical and equatorial Atlantic: Biogeographical and vertical patterns. Prog. Oceanogr. 151, 116–137. doi: 10.1016/j.pocean.2016.12.001
Parin N. V., Andriashev A. P., Borodulina O. D., Tchuvasov V. M. (1974). Midwater fishes of the southwestern Atlantic ocean. Tr. Inst. Okeanol. Akad. Nauk. SSSR 98, 70–140.
Pimentel C. R., Rocha L. A., Shepherd B., Phelps T. A. Y., Joyeux J.-C., Martins A. S., et al. (2020). Mesophotic ecosystems at Fernando de Noronha Archipelago, Brazil (South-western Atlantic), reveal unique ichthyofauna and need for conservation. Neotrop. Ichthyol. 18. doi: 10.1590/1982-0224-2020-0050
Pinheiro H. T., Rocha L. A., Macieira R. M., Carvalho-Filho A., Anderson A. B., Bender M. G., et al. (2018). South-western Atlantic reef fishes: Zoogeographical patterns and ecological drivers reveal a secondary biodiversity centre in the Atlantic ocean. Divers. Distrib. 24, 951–965. doi: 10.1111/ddi.12729
Priede I. G. (2017). Deep-sea fishes: Biology, diversity, ecology and fisheries (New York: Cambridge University Press). doi: 10.1017/9781316018330
Reis R. E., Albert J. S., Di Dario F., Mincarone M. M., Petry P., Rocha L. (2016). Fish biodiversity and conservation in South America. J. Fish Biol. 89, 12–47. doi: 10.1111/jfb.13016
Ryabinin V., Barbière J., Haugan P., Kullenberg G., Smith N., McLean C., et al. (2019). The UN decade of ocean science for sustainable development. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00470
Séret B., Andreata J. (1992). Deep-sea fishes collected during cruise MD-55 off Brazil. Cybium 16, 81–100.
Silva M. V. B., Ferreira B., Maida M., Queiroz S., Silva M., Varona H. L., et al. (2022). Flow-topography interactions in the western tropical Atlantic boundary off northeast Brazil. J. Mar. Syst. 227, 103690. doi: 10.1016/j.jmarsys.2021.103690
Stevenson D. E., Kenaley C. P. (2013). Revision of the manefish genera Caristius and Platyberyx (Teleostei: Percomorpha: Caristiidae), with descriptions of five new species. Copeia 2013(3):415–434. doi: 10.1643/CI-12-086
St. John M. A. S., Borja A., Chust G., Heath M., Grigorov I., Mariani P., et al. (2016). A dark hole in our understanding of marine ecosystems and their services: Perspectives from the mesopelagic community. Front. Mar. Sci. 3. doi: 10.3389/fmars.2016.00031
Sutton T. T. (2013). Vertical ecology of the pelagic ocean: Classical patterns and new perspectives. J. Fish Biol. 83, 1508–1527. doi: 10.1111/jfb.12263
Sutton T. T., Clark M. R., Dunn D. C., Halpin P. N., Rogers A. D., Guinotte J., et al. (2017). A global biogeographic classification of the mesopelagic zone. Deep. Res. Part I 126, 85–102. doi: 10.1016/j.dsr.2017.05.006
Sutton T., Frank T., Judkins H., Romero I. C. (2020). “As gulf oil extraction goes deeper, who is at risk? community structure, distribution, and connectivity of the deep-pelagic fauna,” in Scenarios and responses to future deep oil spills. Eds. Murawski S., Ainsworth C., Gilbert S., Hollander D., Paris C., Schlüter M., Wetzel D. (Cham, Switzerland:Springer Nature Switzerland), 403–418. doi: 10.1007/978-3-030-12963-7
Tchamabi C. C., Araujo M., Silva M., Bourlès B. (2017). A study of the Brazilian Fernando de Noronha island and Rocas Atoll wakes in the tropical Atlantic. Ocean Model. 111, 9–18. doi: 10.1016/j.ocemod.2016.12.009
Tosetto E. G., Bertrand A., Neumann-Leitão S. A. C., Nogueira Júnior M. (2021). Spatial patterns in planktonic cnidarian distribution in the western boundary current system of the tropical South Atlantic Ocean. J. Plankton Res. 43 (2), 270–287. doi: 10.1093/plankt/fbaa066
Travassos P., Hazin F. V., Zagaglia J. R. (1999). Thermohaline structure around seamounts and islands off north-eastern Brazil. Arch. Fish. Mar. Res. 47, 211–222.
Villarins B. T., Di Dario F., Eduardo L. N., Lucena-Frédou F., Bertrand A., Prokofiev A. M., et al. (2022). Deep-sea dragonfishes (Teleostei: Stomiiformes) collected from off northeastern Brazil, with a review of the species reported from the Brazilian Exclusive Economic Zone. Neotrop. Ichthyol. 20 (2). doi: 10.1590/1982-0224-2022-0004
Keywords: deep-sea, oceanic islands, seamounts, biodiversity, Brazil, Fernando de Noronha Ridge
Citation: Eduardo LN, Bertrand A, Lucena-Frédou F, Villarins BT, Martins JR, Afonso GVF, Pietsch TW, Frédou T, Di Dario F and Mincarone MM (2022) Rich and underreported: First integrated assessment of the diversity of mesopelagic fishes in the Southwestern Tropical Atlantic. Front. Mar. Sci. 9:937154. doi: 10.3389/fmars.2022.937154
Received: 05 May 2022; Accepted: 25 July 2022;
Published: 30 August 2022.
Edited by:
Lynne Jane Shannon, University of Cape Town, South AfricaReviewed by:
Cristina López-Pérez, Spanish National Research Council (CSIC), SpainTracey T. Sutton, Nova Southeastern University, United States
Copyright © 2022 Eduardo, Bertrand, Lucena-Frédou, Villarins, Martins, Afonso, Pietsch, Frédou, Di Dario and Mincarone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leandro Nolé Eduardo, leandronole@hotmail.com
 Leandro Nolé Eduardo
Leandro Nolé Eduardo Arnaud Bertrand
Arnaud Bertrand Flávia Lucena-Frédou1
Flávia Lucena-Frédou1  Thierry Frédou
Thierry Frédou Fabio Di Dario
Fabio Di Dario Michael Maia Mincarone
Michael Maia Mincarone