An Updated Review of Recent Advances in Neurosyphilis
- 1Department of Dermatology, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
- 2State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
Neurosyphilis is caused by Treponema pallidum invading the central nervous system, of which the incidence is increasing worldwide. Due to its variable clinical manifestations, diagnosis of neurosyphilis remains challenging, especially the asymptomatic form. This review focuses on recent advances in neurosyphilis, including epidemiology, clinical manifestations, laboratory findings, comorbidities, diagnosis, treatment, prognosis, and basic research. The expansion of men who have sex with men and the infection of human immunodeficiency virus mainly accounted for the increasing incidence of neurosyphilis. The rate of some historically described forms of neurosyphilis in the pre-antibiotic era declined significantly; atypical features are more prevalent. Neurosyphilis, regarded as a great mimicker for neuro-ophthalmic, audio-vestibular, and psychiatric disorders, often presents concomitantly with other diseases, including metabolic disorders. Studies on long non-coding RNAs, miRNAs, chemokines, and metabolites in peripheral blood and cerebrospinal fluid may facilitate exploring the pathogenesis and identifying novel biomarkers of neurosyphilis. The drug resistance of Treponema pallidum to penicillin has not been reported; ceftriaxone was proposed to be more effective than penicillin, whereas few randomized controlled trials supported this view. This study may pave the way for further research, especially the diagnosis and treatment of neurosyphilis.
Introduction
Syphilis, caused by the infection of Treponema pallidum (T. pallidum), can progress to neurosyphilis at any time after the initial infection. Due to the widespread use of antibiotics, the reported cases of neurosyphilis are less than those in the pre-antibiotic era. However, there has been a resurgence in recent years (1). Supporting evidence is anticipated for the diagnosis and treatment of neurosyphilis, and new research progress has been made recently (2, 3). Therefore, this article reviews recent advances in neurosyphilis, including epidemiology, clinical manifestations, laboratory findings, comorbidities, diagnosis, treatment, prognosis, and basic research.
Epidemiology
In 2012, 18 million cases of syphilis were reported worldwide, among which 3,50,000 cases caused adverse pregnancy outcomes (4). The incidence continues to rise due to the expansion of susceptible populations, including men who have sex with men (MSM) and people living with HIV (PLWH) (5). The rate of neurosyphilis in PLWH is about twofold as in the immunocompetent population (6). Although the incidence of syphilis is low among women in the USA, it increased by 30.0% in 2018–2019; as for congenital syphilis, the incidence has also been increasing since 2013. In China, from 1990 to 2017, the incidence of syphilis increased from 0.9 to 34.49 per million, second only to viral hepatitis and tuberculosis among infectious diseases. The increasing trend aligned with that of neurosyphilis (7). Male, MSM, advanced age (≥45 years), PLWH without combined antiretroviral therapy, drug use disorder, lack of antisyphilitic therapy, reinfection after a previous syphilis infection, and patients in serofast state are risk factors for neurosyphilis, especially the late forms (8–11). Elevated serum and cerebrospinal fluid (CSF) rapid plasma reagin (RPR) titer, CSF protein concentration, and increased burden of the cerebral small vessel diseases may indicate the aggravation of neurological symptoms (10, 12, 13). Specific subtypes of T. pallidum are more likely to induce neurosyphilis; for instance, subtype 14d is the most common neurosyphilis-related genotype in China (14). Specific genes the hosts carry also determine their susceptibility of neurosyphilis, including some single nucleotide polymorphisms on Toll-like receptor (TLR)1, TLR2, TLR6, and the interleukin 10 (IL-10) promoter (15, 16).
Some novel characteristics of neurosyphilis have also been observed nowadays. Currently, the most common form of neurosyphilis is asymptomatic neurosyphilis and meningoencephalitis, although meningovascular syphilis had the highest incidence in some cohort studies (17). The incidence of prevalent symptoms in the pre-antibiotic era, such as tabes dorsalis, dementia paralytica, and gummatous neurosyphilis declined dramatically, as the widespread use of antibiotics in other diseases might indirectly treat neurosyphilis, and timely diagnosis and treatment shortened the course of the disease (18). Recent studies also reported an increase in the incidence rate of ocular syphilis and otosyphilis (19). However, molecular typing showed little evidence of a type specifically causing ocular syphilis. Thus, this increase might just reflect a resurgence in total syphilis cases (20).
Clinical Manifestations and Laboratory Findings
Based on the disease progression, neurosyphilis can be divided into early and late stages. Manifestations that do not fall into either of the two stages are classified as atypical neurosyphilis. Early neurosyphilis can be classified as asymptomatic and symptomatic forms, and the symptomatic form includes symptomatic meningitis and meningo-vasculitis. The manifestations of late neurosyphilis include dementia paralytica and tabes dorsalis. Patients with early neurosyphilis are more likely to have ocular involvement than those of late stage (18). Cerebral small vessel disease, one of the major causes of cognitive impairment, is more prevalent in patients of late stage (12). These forms were detailed in the following sections.
Asymptomatic neurosyphilis is defined as a neurologically asymptomatic form with serological and CSF abnormalities, typically occurring in the early months of infection. Patients can be diagnosed with the following features, CSF lymphocytosis (<100 cells/μL), elevated protein concentration (<100 mg/dL), a reactive result of cerebrospinal fluid-Venereal Disease Research Laboratory (CSF-VDRL) assay, or a combination of these abnormalities. However, these existing benchmarks cannot be directly used in PLWH, for HIV itself may trigger CSF lymphocytosis and elevated protein concentration.
As a common manifestation of early neurosyphilis, symptomatic meningitis often appears within the first year of infection, in the form of headache, nausea, vomiting, and neck pain, accompanied by ocular involvement like inflammation and blurry vision. It can further lead to cranial neuropathy, meningo-vasculitis, and damage to the brain parenchyma. CSF abnormalities in early symptomatic meningitis are more severe than those in asymptomatic forms, with lymphocyte count around 200–400 cells/μL, protein concentration around 100–200 mg/dL, and CSF-VDRL tests almost always reactive (6). Neuroimaging profile usually demonstrates enhancement of CSF, meninges, or cranial nerves in this stage. Meningo-vasculitis can cause thrombosis, ischemia, and infarction. For young patients with strokes, syphilitic meningo-vasculitis should be considered, since there was one study showing that 14.09% of patients with neurosyphilis had an ischemic stroke as a primary symptom (21). In southern Brazil, the reactive result of serological tests for syphilis is common in patients with acute stroke (22). As in cases of meningo-vasculitis, CSF white blood cell (WBC) count is usually around 5–74 cells/μL, with protein concentration around 22–101 mg/dL, while CSF-VDRL tests are not always reactive (23). In patients with meningo-vasculitis, focal narrowing, dilatation, and occlusion may be seen in angiography, while neuroimaging may show evidence of infarction. It was reported that neuroimaging progression could continue in symptomatic patients even after standardized antisyphilitic therapy, which was confirmed by a study showing infarction lesions in 42.1%, mild to severe brain atrophy in 47.4%, and white matter demyelination in 15.8% of treated patients in the follow-up (24).
Dementia paralytica and tabes dorsalis are two major forms of late neurosyphilis, usually appearing 15–20 years after the initial infection. The CSF of patients with late neurosyphilis shows only mild lymphocytosis or slightly elevated protein concentration, compared to those with early neurosyphilis. Dementia paralytica presents with neurasthenic syndromes and personality disorders in the early stage, which is difficult to distinguish from Alzheimer's disease (25); however, in the middle and late stages, patients will show physical symptoms, such as Argyll Robertson pupils, optic atrophy, sensory ataxia, tremors, and dysarthria. In those paretic patients, CSF protein concentration ranged from 20 to 186 mg/dL, and CSF WBC count ranged from 0 to 98 cells/μL, with 94.9% of CSF-VDRL tests turned to be reactive (26). Signs of tabes dorsalis include areflexia, urinary retention, sexual dysfunction; 60% of patients in that stage present with Argyll Robertson pupils. In tabetic patients in a cohort study, CSF protein concentration ranged from 14 to 64 mg/dL, and CSF WBC count ranged from 2 to 145 cells/μL (26).
Atypical neurosyphilis is more frequently observed nowadays. The symptoms of early neurosyphilis can mimic most neuro-ophthalmic diseases, while the neurological involvement in the late stage can mimic psychiatric or autoimmune disorders. Rapidly progressive psychosis, such as dementia (27), cognitive impairment (28), and subacute confusion (29), was reported in some cases of late neurosyphilis, some of which were initial clinical presentations. Thus, it is essential to ask for consultation from psychiatrists. Additionally, herpesviral encephalitis, polyradiculoneuropathy, mania, extrapyramidal syndrome, amyotrophic lateral sclerosis, and cauda equina syndrome were also reported in recent cases (28). For patients without other common risk factors but with neuropsychiatric symptoms, screening for syphilis should be recommended.
Comorbidities
Patients with neurosyphilis may have other diseases, such as HIV infection, posing great diagnostic and therapeutic challenges to clinicians. Recent studies focused on the current status and underlying mechanisms of the comorbidities of neurosyphilis and HIV infection. A study from Missouri reported that 7.4% of HIV-positive MSM patients were diagnosed with primary or secondary syphilis in 2016, compared with 3.1% of immunocompetent MSM patients (30). Syphilis can exacerbate HIV infection and inflammation in the central nervous system, increasing HIV viral load and decreasing CD4 + T lymphocyte count. For HIV-uninfected individuals, syphilitic skin lesions may also provide access to HIV acquisition (31). Meanwhile, HIV-associated immunosuppression can increase susceptibility to neurosyphilis. The diagnosis of neurosyphilis is also more difficult in PLWH. On the one hand, PLWH may have false positive reactions to serological tests for syphilis (32). On the other hand, HIV itself can lead to a slight increase in CSF WBC count and protein concentration (9). Besides, cases of gonorrhea, viral hepatitis, and chlamydia comorbid with syphilis were reported (33). Weathers et al. also showed that comorbid hepatitis and herpes simplex would increase the risk of syphilis (34).
Ocular syphilis and otosyphilis were discussed as comorbidities of neurosyphilis here. Ocular syphilis, occurring at any stage of syphilis, is becoming an important cause of uveitis. Furtado et al. found that the most common form of intraocular inflammation induced by ocular syphilis was posterior uveitis, followed by panuveitis (35). Diminished vision, interstitial keratitis, optic neuropathy, and retinal vasculitis are typical manifestations, and ocular hypertension and cataract were also reported (36). Mathew et al. found that 37% of patients with ocular syphilis had neurosyphilis (37). The frequency of neurosyphilis comorbid with ocular syphilis was significantly higher in PLWH than in immunocompetent patients (37). In one study on PLWH with comorbid neurosyphilis, those with ocular syphilis were more likely to have elevated CSF WBC count than those without ocular syphilis (38). Due to these possible misleading symptoms, ocular syphilis was sometimes misdiagnosed as, for example, retinal detachment or vitreous hemorrhage. Delayed management of ocular syphilis can cause poor vision in the follow-up (39). Otosyphilis is also a mimicker for many audio-vestibular disorders, occurring at any stage of syphilis. The main manifestation is sensorineural hearing loss, which will develop into conductive hearing loss. Other common manifestations include vertigo, tinnitus, and gait instability (40); accompanying meningitis and ocular involvement were also reported (41). Some studies suggest that T. pallidum may impair the eighth cranial nerve, temporal bone, and cochleovestibular apparatus (42). Regardless of the CSF profile, otosyphilis and ocular syphilis should be treated with the same protocols for neurosyphilis (43).
Patients with neurosyphilis might have specific metabolic characteristics. The incidence of hyperlipidemia and hypertension in patients with neurosyphilis was 21.4% and 45.9%, respectively, both significantly higher than that in patients with other neurological infections. Patients with neurosyphilis also presented with significantly higher levels of low-density lipoprotein, total cholesterol, systolic and diastolic blood pressure (44). Glycated hemoglobin A1c (HbA1c) is an indicator of diabetes and neurosyphilis, playing an unclear role in blood-brain barrier (BBB) disruption, an essential step in the progression of both diseases (45).
Diagnosis
The diagnosis of neurosyphilis is based on serum and CSF tests, which can be further classified as treponemal and nontreponemal tests. Nontreponemal serum tests mainly include rapid plasma reagin (RPR) and venereal disease research laboratory (VDRL); treponemal serum tests mainly include T. pallidum particle agglutination assay (TPPA), T. pallidum enzyme immunoassay (TP-EIA), chemiluminescence immunoassay, and fluorescent treponemal antibody absorption (FTA-ABS). For patients with ocular or neurological symptoms, features of tertiary syphilis, or serofast status, lumbar puncture and CSF tests are needed for the diagnosis of neurosyphilis. CSF tests mainly include CSF WBC count, CSF protein concentration, CSF-RPR, CSF-TPPA, and CSF-FTA-ABS. As for nontreponemal tests, the sensitivity varies by the progress of neurosyphilis; for patients with late neurosyphilis, nontreponemal tests are of a high false-negative rate (46).
Conventional diagnostic methods of neurosyphilis face some challenges. The diagnosis relies heavily on CSF-VDRL, of which the specificity is high (89–96%), but the sensitivity is relatively low (12–48%) (47). Alternative methods, such as CSF toluidine red unheated serum test (CSF-TRUST), might not perfectly remedy the low sensitivity; CSF chemiluminescence immunoassay was preferred, with the sensitivity of 99.57–100%, and the specificity of 99.81–99.88% (38). Some studies suggested chemokine (C-X-C motif) ligand (CXCL) 13 as a promising indicator, with specificity from 76 to 81% and sensitivity from 78 to 83% (47–49). However, the optimal benchmark for CXCL13 remains controversial. CSF samples from cohorts are needed to establish an appropriate cut-off for the diagnosis of neurosyphilis (47). The combination of CXCL8, CXCL10, and CXCL13 might be a better indicator with specificity of 92.9% and sensitivity of 90.4% (50). CSF WBC count, protein concentration, and clinical manifestations should all be considered along with CSF-TPPA; the sensitivity of this combined diagnostic method was up to 98% (51). Adjusting the cut-off of tests was also proposed as a solution to low specificity and sensitivity. Serum TPPA, commonly used in patients with unknown prior history of syphilis, was recommended to enhance the specificity of CSF test, when the titer cut-off was adjusted to >1:320. For those with high suspicion of neurosyphilis but without a positive result of CSF VDRL test, the CSF treponemal assay like CSF TPPA was a reasonable recommendation to reduce the false-negative rate (52). Polymerase chain reaction (PCR) and immunoblot were recommended as new automated treponemal tests. A study showed that the specificity and sensitivity of PCR ranged from 60 to 100% and 40 to 70%, respectively; as for immunoblot, the specificity varied from 91.7 to 92.0%, and the sensitivity varied from 93.8 to 100% (48). Nowadays, automated treponemal tests are often accompanied by a nontreponemal test as a following confirmatory step, which is called the reverse-sequence algorithm and has shown ideal effects in the low-prevalence populations (53). Consequently, the diagnosis should make full use of serum and CSF samples by a combination of several tests in order to obtain a convincing result.
Conventional benchmarks may not be convincing for neurosyphilis patients coinfected with HIV, because HIV may cause CSF lymphocytosis and elevated protein concentration. Due to the difficulty in diagnosing asymptomatic neurosyphilis in PLWH, lumbar puncture was recommended by some experts for all syphilis patients coinfected HIV, which might also cause unnecessary costs and risks. CDC suggested that lumbar puncture should be given to PLWH with neurological signs or symptoms, and/or serofast status, and/or tertiary syphilis, which was supported by another cohort analysis (30).
Recently, the point-of-care syphilis tests were gradually popularized, due to the epidemic of syphilis in low-and middle- income countries. Low-and middle-income countries also showed high prevalence rates, long diagnostic delays, low follow-up rates, and inaccessibility to trained clinicians and necessary facilities (54). Optimized commercial immunochromatographic strip tests, such as the CSF-Syphicheck and the DPP Chembio syphilis assay, showed satisfying diagnostic specificity and sensitivity compared to CSF-VDRL (62–64% sensitivity, 79–81% specificity in CSF-Syphicheck; 63–92% sensitivity, 85–100% specificity in DPP Chembio syphilis assay) (55, 56). Though further evaluation is needed, point-of-care tests may be widely applied in resource-limited regions and contribute to the control of sexually transmitted diseases, especially for neurosyphilis and congenital syphilis.
Treatment and Prognosis
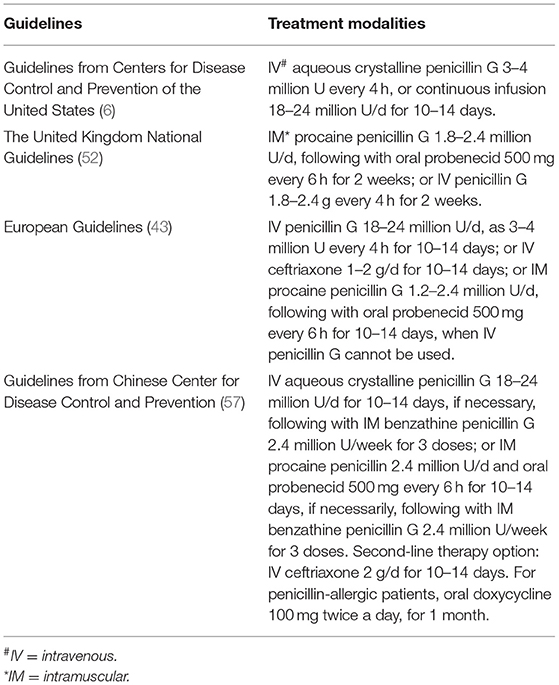
Treatment guidelines for neurosyphilis released by the United States, the United Kingdom, Europe, and China are outlined in Table 1. They all highlighted that PLWH should be treated as immunocompetent patients, and ceftriaxone might be an alternative treatment to penicillin. A divergence of views lay on the adoption of steroids in patients with neurosyphilis, which was only recommended in some of the guidelines. Efficacy of different therapies is always a major concern, whereas it is hard to draw a definite conclusion due to those low-quality, small-size, and highly-biased trials (58, 59). A cohort study including 208 patients with neurosyphilis during 1997–2017 showed serological cure rates of 88% in the ceftriaxone group and 82% in the penicillin G group (2), while another study showed clinical cure rates of only 44% in the ceftriaxone group and 18% in the penicillin G group (59). Meanwhile, neurological sequelae were reported in 41.8% of the neurosyphilis patients in a retrospective study (60). PLWH coinfected with neurosyphilis were more likely to fail the intravenous penicillin G therapy (61, 62).
Penicillin is preferred worldwide as front-line therapy for neurosyphilis, and the drug resistance of T. pallidum to penicillin has not been reported (63). Some studies found that ceftriaxone was more effective than penicillin, but few large randomized controlled trials supported this view (2, 59, 60). Tetracycline, erythromycin, and chloramphenicol have all been applied to treat syphilis, whereas erythromycin and chloramphenicol were reported to induce intestinal dysbiosis and irreversible aplastic anemia, respectively (59). The treatment effects of penicillin and doxycycline were similar in some series (64); doxycycline is cheaper yet forbidden in pregnant women. The only treatment modality certified for pregnant women with syphilis is parenteral penicillin G. The concurrent administration of probenecid is widely used in antibiotic therapy for neurosyphilis. A recent study proved that oral amoxicillin with probenecid could elevate the drug level in CSF (43). However, oral probenecid with intramuscular (IM) procaine penicillin should be carefully used in HIV-positive MSM (65).
The Jarisch-Herxheimer reaction (JHR) may occur within the first 24 h of neurosyphilis treatment, presenting as headache, chills, fever, rashes, and myalgias, of which the mechanism is unclear (66). Commonly used antibiotics, including penicillin, erythromycin, azithromycin, tetracyclines, clarithromycin, and levofloxacin, could all cause JHR (67). JHR usually occurs among patients with early syphilis, as the risk of JHR increases by 19% for every twofold increase in RPR titer, suggesting that higher spirochete load may cause a significantly higher risk of JHR (68). For PLWH, particularly those with encephalitis, JHR may be more severe (28). Steroids, like prednisolone, are routinely used to prevent JHR; TNF-α antibodies and acetaminophen were also used in some cases (69).
Serofast, a lack of serological response to antisyphilitic therapy, is currently a major concern for clinicians. This clinical scenario occurred in over one-third of patients, especially those with lower test titers or late latent syphilis (70). A successful response to antisyphilitic therapy is defined as a fourfold dilution decline in CSF-VDRL and the normalization of CSF WBC count, except for PLWH. Patients with serofast only show a decrease less than fourfold in non-treponemal antibody titers after a specific period of time (6 months for early-stage syphilis; 12 months for late-stage syphilis), or show low titers consistently after treatment (6). The cause of serofast is still unclear. Possible explanations of the lack of serological response to antisyphilitic therapy include that the patients have undiagnosed neurosyphilis and fail to respond to routine therapy; patients have comorbid HIV, malignancies, or immune diseases; and patients are re-infected by having sex with others (71, 72). Prediction of the serofast occurrence is still difficult, and retreatment to serofast patients can not significantly improve the cure rates (73, 74).
Several factors influence the prognosis of patients with neurosyphilis, which can also be used as indicators to assess the therapeutic effect. Although HIV coinfection may confound the diagnosis of neurosyphilis, the prognosis of patients does not differ by the HIV infection status (75). Diplopia and severe atrophy of brain parenchyma were proved to be associated with poor prognosis. Headache often contributes to an early diagnosis of disorders in the central nervous system, which is a favorable prognostic factor in patients with neurosyphilis (60); the changes in electroencephalogram and metabolites in CSF may be auxiliary indicators of the prognosis. Jiang et al. found that the electroencephalogram Lempel-Ziv complexity (EEG-LZC) value after treatment was significantly higher than that before treatment, suggesting that neuron reconnection would improve the brain function (76). In addition, CXCL13, tau, neurogranin, and metabolites in CSF could also be used to evaluate the cognitive function of patients with neurosyphilis and the effectiveness of antisyphilitic therapy (25, 77).
Reinfection of syphilis, which can start with the latent or tertiary stage (78), is more prevalent in MSM and PLWH (79, 80). A retrospective study on MSM in California from 2002 to 2006 showed that 5.9% of MSM had repeat infection of syphilis within 2 years after the first infection (80). Another 8-year surveillance in Brazil showed that 13.6% of patients had reinfection with T. pallidum (81). A four-fold drop in serum RPR titer of patients within 6–12 months is defined as the serological cure. However, some patients have a rise in RPR titer within the first 12 months, for which, the main reason is usually reinfection, rather than recurrence (82). Molecular studies are necessary when the corroborative history of patients is inaccessible to determine whether the rise in RPR titer is due to reinfection or recurrence (83). The mechanisms of reinfection are related to the destruction of adaptive immunity by T. pallidum (84). An immunodominant-evasion model further suggested that the TprK, an outer membrane protein with prolific antigenic variation, explained the ability of T. pallidum to escape the adaptive immunity (84).
Basic Research
A major obstacle for the research on T. pallidum is that it can rarely be cultured continuously in vitro. Recent basic research on neurosyphilis mainly focuses on the pathogenic mechanisms of neurosyphilis, and the potential biomarkers for diagnosis or prognosis monitoring.
Research in the pathogenic mechanisms has proved that T. pallidum crosses the endothelial barriers through interjunctional penetration, disseminating throughout the body and invading various organs (85). This process depends largely on the outer membrane proteins of T. pallidum, such as Tp92, to promote the adhesion and proliferation of spirochetes, enhance the permeability of endothelial cells, and immunomodulate in the inflammatory responses (86). T. pallidum can activate both humoral and cellular immunity by antigens such as T. pallidum repeat protein (Tpr), whereas cellular immunity plays a major role in eliminating pathogens but is also significantly suppressed in the late stage of infection (87). The immune escape mechanism of T. pallidum has been well studied. Due to the lack of exposed lipoproteins on the outer membrane of T. pallidum, antigen-presenting cells are more difficult to contact the pathogen-associated molecular patterns and unable to activate the innate immune system (88). The common targets of humoral immune response are the variable region of membrane proteins such as TprK (89). However, it was reported that the continuous mutation of the TprK gene, especially the variable region, enabled T. pallidum to escape the immune response and caused persistent infection (90). Qin et al. reported that in syphilis patients in serofast state, unbalanced T cell subsets could suppress the cellular immunity with decreased levels of CD4+ T cells, decreased ratio of T helper (Th)1/Th2 cells, and increased levels of CD8+ T cells (91). Another study found increased levels of regulatory T cells and transforming growth factor-β in peripheral blood of the serofast patients with secondary syphilis, which was mainly induced by the antigen TpF1 and might suppress the cellular immunity (92).
Host immune response also plays an important role in the pathogenesis of neurosyphilis, mainly in the form of T cell-mediated delayed-type hypersensitivity (87). Recent studies showed a significantly elevated levels of IL-8, CC chemokine ligand 20 (CCL-20) in serum, elevated levels of CXCL13, interferon(IFN)-γ in CSF, and elevated levels of IL-6, IL-10, IL-17 in both serum and CSF of neurosyphilis patients (49, 93–96). While the imbalance between Th17 and Th1 had been proved to aggravate inflammation in patients with secondary syphilis, how neurosyphilis developed in syphilis patients and the role host immune response played in the development of neurosyphilis remained unclear (97). The latest research proposed that CXCL13/CXCR5 mediated the accumulation of B cells and immunoglobulin G in the CSF of neurosyphilis patients (98); IL-17 not only mediated the inflammatory response, but also activated endothelial contraction and destroyed the tight junctions of the BBB (99); IL-8 and CCL-20 induced by TpF1 could induce vascular inflammation and angiogenesis (100). Liu et al. found that, compared to syphilis/non-neurosyphilis patients, patients with symptomatic neurosyphilis showed a significantly increased expression of CD8+ IFN-γ+ cell but decreased expression of CD8+ IL-17+ cell in the peripheral blood, which might also explain the pathogenesis of symptomatic neurosyphilis (96).
Recent studies also focused on the mechanisms of neurological damage caused by T. pallidum. T. pallidum can invade the central nervous system since the early stage of syphilis; however, the relationship between T. pallidum and the BBB remains unclear. Neurosyphilis, especially in the stage of general paresis, is similar to Alzheimer's disease. Thus, Alzheimer's-associated biomarkers are widely studied in neurosyphilis patients with cognitive impairment. Receptors expressed on myeloid cells 2 (sTREM2), neurofilament light proteins, and phosphorylated neurofilament heavy subunit were elevated in CSF of patients with neurosyphilis, suggesting that activated microglia were associated with neuron damage caused by T. pallidum (101, 102). β-amyloid precursor protein cleaving enzyme (BACE1) and neurogranin also increased in the CSF of patients with neurosyphilis, indicating that the synaptic destruction would appear in the stage of general paresis (25). CXCL13 was also involved in CSF pleocytosis and neurological damage (49, 103).
Studies on RNA, proteins, and metabolites in peripheral blood and CSF have facilitated the discovery of novel indicators for neurosyphilis. Long non-coding RNAs expressed in peripheral blood of patients with neurosyphilis were emphasized in recent studies. IFN-γ production was proved to be mediated by the interaction between lncRNA-ENST00000421645 and PCM1 (104). miRNAs in exosomes released by neuroglial cells could be potential biomarkers in CSF of patients with neurosyphilis for brain parenchymal damage, including miR-570-3p, miR-590-5p, miR-570-5p, and miR-21-5p (105). The elevated level of chemokines was associated with inflammatory disorders in the central nervous system (50); CXCL13, CXCL8, and CXCL10 were suggested as novel biomarkers with high sensitivity (49). Interleukin-10 in CSF was also regarded as a biomarker of neurosyphilis, especially of asymptomatic neurosyphilis (93). In addition, metabolomics studies recently identified representative metabolites in CSF of patients with neurosyphilis as new indicators, such as L-histidine, bilirubin, alpha-kamlolenic acid, prostaglandin E2, palmitoyl-L-carnitine, butyryl-L-carnitine, D-mannose, L-gulono-gamma-lactone, hypoxanthine, and N-acetyl-L-tyrosine (77, 106).
Discussion
As the folk goes, “One night with Venus, a lifetime with Mercury”. The everlasting stigmatization of syphilis has hindered the development of diagnostic and therapeutic management, especially in the follow-up, partner notification, and wide-range screening in susceptible populations (107). As a so-called prevalent disease in celebrities with a libertine lifestyle, syphilis was said to have infected the most influential people like Shakespeare and Columbus, and evoked further panic in Europe by the horrible symptoms of mercury poisoning during treatment (108). Mystified and misread for centuries, syphilis was ultimately controlled by the wide use of antibiotics. However, it has not been eliminated till now, due to the increase in sexual risk behaviors mainly in homosexual communities since the mid-1990s (109). Neurosyphilis, a stage that T. pallidum invades the central nervous system, may progress to severe cardiovascular complications and irreversible neurological damages if treatment is delayed. The alarming resurgence of syphilis also attracts attention to the incidence of neurosyphilis, particularly in MSM and PLWH.
The latest research progress on neurosyphilis in recent years is summarized. In some series, neurosyphilis was diagnosed disproportionately in young patients with ischemic stroke, which argued for the screening in low-risk populations presenting with specific abnormalities, including ischemic stroke, rapidly progressed neuropsychiatric symptoms, and recurrent ocular inflammation (110). However, the implementation of screening for sexually transmitted infections may bring humiliation to the subjects, damage their close relationships and personal image in society (53). Comorbid neurosyphilis with HIV or other sexually transmitted infections has been reported worldwide, of which the comorbidities may cause misdiagnosis and failure of conventional therapy; HIV coinfection does not affect the prognosis of patients with neurosyphilis (75). Most of the basic research focused on the pathogenesis of neurosyphilis, based on the changes of non-coding RNAs, chemokines, proteins, lymphocyte subtypes, and metabolites in CSF of patients with neurosyphilis. These potential biomarkers may be promising for diagnosis and disease monitoring, but whether they can explain the pathogenesis of neurosyphilis remains unclear. Evidence showed similarity between neurodegenerative diseases and neurosyphilis in pathogenesis and clinical manifestations, which suggested that the existing theories of Alzheimer's disease might enlighten the research of neurosyphilis (25). The epidemic of syphilis and neurosyphilis in low- and middle- income countries is difficult to control due to the resource-limited settings there. However, point-of-care syphilis tests developed in recent years may relieve the pressure of disease control for its feasibility and inexpensiveness (56). JHR has also been reported in almost all types of commonly used antibiotics for syphilis, especially in the treatment of PLWH (68).
There are still some problems to be solved. (1) Due to the inaccessible in-vitro culture and unknown host response to the infection of T. pallidum, the development of vaccines still has a long way to go (111). (2) The false-negative and false-positive rates remain high in a single treponemal or nontreponemal test, thus combined use of various diagnostic methods is recommended, alongside neuroimaging and individuals' history of sex life. It should be noticed that not only false-negative tests may delay the treatment, but false-positive tests may also cause unnecessary management and stigmatization to patients. (3) Penicillin remains the first choice for the treatment of neurosyphilis, but there are few alternative treatment modalities for patients with cross-allergy of penicillin and ceftriaxone (59). Some conventional therapies also have side effects for PLWH (65). (4) Another challenge in the treatment is serofast. The cause of the serofast is still unclear and retreatment to serofast patients can not significantly improve the cure rates (71, 72). Prediction of the serofast occurrence is also difficult. Moreover, some serofast patients may no longer be threatened by syphilis, but other patients may still be at risk, which is hard to evaluate. (5) Lumbar puncture also remains controversial in the diagnosis of neurosyphilis, due to its difficulty of implementation and potential risks posed to patients. CDC does not recommend lumbar puncture for PLWH with syphilis yet without neurological symptoms, and only one lumbar puncture is allowed every 6 months after treatment to monitor the prognosis. Thus, patients with neurosyphilis are easily lost to follow-up. (6) Additionally, the ignorance of patients about the symptoms of early syphilis or asymptomatic neurosyphilis is common and often results in delayed diagnosis and treatment, which might account for the rising incidence of neurosyphilis. A study from Tanzania found that the average consultation delay for rural patients was almost double that for urban patients (112). Another study demonstrated that only 16.7% patients with symptomatic neurosyphilis visited dermatology department, as patients rarely paid attention to their skin conditions (24). (7) Some essential approaches for controlling neurosyphilis are still time-consuming and difficult to undertake, including public education, partner notification, and screening projects. Whereas, excessive screening and prophylactic treatment of syphilis may bring humiliation to the individuals and waste of resources to the society. Thus, when developing new screening or prevention policies, balance should be maintained carefully. For instance, prophylactic treatment is not recommended for partners of neurosyphilis patients due to the limited infectiousness of neurosyphilis, whereas serological tests are necessary for them to exclude the risk of transmission (54). Further research could concentrate on the pathogenesis, standards and guidelines, treatment modalities, JHR and serofast, follow-up, and the prevention of large-scale coinfection in MSM and PLWH.
Conclusions
This article reviews recent advances in neurosyphilis, including epidemiology, clinical manifestations, laboratory findings, comorbidities, diagnosis, treatment, prognosis, and basic research. The incidence of neurosyphilis has been increasing in recent years, mainly in MSM. The incidence of historically described forms of neurosyphilis in the pre-antibiotic era declined significantly nowadays, with atypical features becoming more common. Neurosyphilis can mimic most neuro-ophthalmic, audio-vestibular, and psychiatric disorders. Comorbidities of neurosyphilis are also prevalent and variable, and patients may present with specific metabolic characteristics. Clinical diagnostic methods remain immature, whereas recent studies on long non-coding RNA, miRNA, chemokines, and metabolites in peripheral blood and CSF may facilitate the research on the pathogenesis and new indicators of neurosyphilis. The resistance of T. pallidum to penicillin has not been discovered. Some studies also found that ceftriaxone was more effective than penicillin, but few large randomized controlled trials supported this view. Attention should also be paid to specific populations, such as PLWH, pregnant women, and those allergic to penicillin.
Author Contributions
JZ, HZ, and KT reviewed the literature and wrote the manuscript. RL reviewed the literature. JL revised the manuscript. All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
The Rapid Service Fee was funded by Peking Union Medical College Hospital. We acknowledge the support from Beijing Municipal Science and Technology Commission (No. Z191100006619011), the Capital's Funds for Health Improvement and Research (2020-2-4016), and CAMS Innovation Fund for Medical Sciences (CIFMS 2020-I2M-C&T-B-048).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Bettuzzi T, Jourdes A, Robineau O, Alcaraz I, Manda V, Molina J, et al. Ceftriaxone compared with benzylpenicillin in the treatment of neurosyphilis in France: a retrospective multicentre study. Lancet Infect Dis. (2021) 21:1441–7. doi: 10.1016/S1473-3099(20)30857-4
3. Zhang K, Chu F, Wang C, Shi M, Yang Y. Progressive stroke caused by neurosyphilis with concentric enhancement in the internal cerebral artery on high-resolution magnetic resonance imaging: a case report. Front Neurol. (2021) 12: 675083. doi: 10.3389/fneur.2021.675083
4. Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS ONE. (2015) 10:e0143304. doi: 10.1371/journal.pone.0143304
5. de Voux A, Kidd S, Torrone EA. Reported cases of neurosyphilis among early syphilis cases-United States, 2009 to 2015. Sex Transm Dis. (2018) 45:39–41. doi: 10.1097/OLQ.0000000000000687
6. Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. (2021) 70:1–187. doi: 10.15585/mmwr.rr7004a1
7. Ting-ting TIAN Y-xH, Yu-qing LI, Hong-jiao QI, Mo CHEN, Mei-xia LÜ. Spatio-temporal analysis of incidence rate of syphilis in China. J Shanghai Jiaotong Univ. (2021) 41:648–52. doi: 10.3969/j.issn.1674-8115.2021.05.015
8. Pintado Maury I, Alves M, Fonseca T. Neurosyphilis prevalence at a Portuguese stroke unit care. Aging Clin Exp Res. (2019) 31:1155–61. doi: 10.1007/s40520-018-1052-4
9. Salado-Rasmussen K, Wessman M, Cowan S, Gerstoft J, Katzenstein T. Syphilitic hepatitis and neurosyphilis: an observational study of Danish HIV-infected individuals during a 13-year period. Sex Transm Infect. (2019) 95:416–8. doi: 10.1136/sextrans-2018-053921
10. Li W, Jiang M, Xu D, Kou C, Zhang L, Gao J, et al. Clinical and laboratory characteristics of symptomatic and asymptomatic neurosyphilis in HIV-negative patients: a retrospective study of 264 cases. Biomed Res Int. (2019) 2019:2426313. doi: 10.1155/2019/2426313
11. Centers for Disease Control and Prevention (CDC). Notes from the field: repeat syphilis infection and HIV coinfection among men who have sex with men–Baltimore, Maryland, 2010-2011. MMWR Morb Mortal Wkly Rep. (2013) 62:649–50.
12. Xiang L, Zhang T, Zhang B, Zhang C, Hou S, Yue W. The associations of increased cerebral small vessel disease with cognitive impairment in neurosyphilis presenting with ischemic stroke. Brain Behav. (2021) 11:e02187. doi: 10.1002/brb3.2187
13. Tan X, Zhang J, Li J, Yue X, Gong X. The prevalence of asymptomatic neurosyphilis among HIV-negative serofast patients in China: a meta-analysis. PLoS ONE. (2020) 15:e0241572. doi: 10.1371/journal.pone.0241572
14. Dai T, Li K, Lu H, Gu X, Wang Q, Zhou P. Molecular typing of Treponema pallidum: a 5-year surveillance in Shanghai, China. J Clin Microbiol. (2012) 50:3674–7. doi: 10.1128/JCM.01195-12
15. Marra CM, Sahi SK, Tantalo LC, Ho EL, Dunaway SB, Jones T, et al. Toll-like receptor polymorphisms are associated with increased neurosyphilis risk. Sex Transm Dis. (2014) 41:440–6. doi: 10.1097/OLQ.0000000000000149
16. Pastuszczak M, Jakiela B, Jaworek AK, Wypasek E, Zeman J, Wojas-Pelc A. Association of Interleukin-10 promoter polymorphisms with neurosyphilis. Hum Immunol. (2015) 76:469–72. doi: 10.1016/j.humimm.2015.06.010
17. Kissani N, Nafia S, Zahlane S, Louhab N. Neurosyphilis: a series of 178 cases at the 3rd-level hospital of Marrakesh (Morocco). Eur J Clin Microbiol Infect Dis. (2021) 40:2129–35. doi: 10.1007/s10096-021-04253-y
18. Landry T, Smyczek P, Cooper R, Gratrix J, Bertholet L, Read R, et al. Retrospective review of tertiary and neurosyphilis cases in Alberta, 1973-2017. BMJ Open. (2019) 9:e025995. doi: 10.1136/bmjopen-2018-025995
19. Singh AE. Ocular and neurosyphilis: epidemiology and approach to management. Curr Opin Infect Dis. (2020) 33:66–72. doi: 10.1097/QCO.0000000000000617
20. Theeuwen H, Whipple M, Litvack JR. Otosyphilis: resurgence of an old disease. Laryngoscope. (2019) 129:1680–4. doi: 10.1002/lary.27635
21. Liu LL, Zheng WH, Tong ML, Liu GL, Zhang HL, Fu ZG, et al. Ischemic stroke as a primary symptom of neurosyphilis among HIV-negative emergency patients. J Neurol Sci. (2012) 317:35–9. 2012/04/10. doi: 10.1016/j.jns.2012.03.003
22. Targa Martins R, Castilhos R, Silva da. Silva P, Costa L. Frequency of screening and prevalence of neurosyphilis in stroke population. Cerebrovasc Dis. (2020) 49:301–6. doi: 10.1159/000508491
23. Gonzalez H, Koralnik I, Marra C. Neurosyphilis. Semin Neurol. (2019) 39:448–55. doi: 10.1055/s-0039-1688942
24. Shang X, He C, Tang B, Chang X, Ci C, Sang H. Neuroimaging features, follow-up analyses, and comparisons between asymptomatic and symptomatic neurosyphilis. Dermatol Ther. (2020) 10:273–83. doi: 10.1007/s13555-020-00361-3
25. Zhang M, Zhong X, Shi H, Vanmechelen E, De Vos A, Liu S, et al. BACE1 and Other Alzheimer's-related biomarkers in cerebrospinal fluid and plasma distinguish Alzheimer's disease patients from cognitively-impaired neurosyphilis patients. J Alzheimer's Dis. (2020) 77:313–22. doi: 10.3233/JAD-200362
26. Wang C, Zhu L, Gao Z, Guan Z, Lu H, Shi M, et al. Increased interleukin-17 in peripheral blood and cerebrospinal fluid of neurosyphilis patients. PLoS Negl Trop Dis. (2014) 8:e3004. doi: 10.1371/journal.pntd.0003004
27. Mukku S, Safal S, Pritam R, Nashi S, Nagarathna C, Pt S, et al. Neurosyphilis presenting as rapidly progressive psychosis & dementia - a forgotten entity. Asian J Psychiatr. (2019) 40:103–6. doi: 10.1016/j.ajp.2019.02.010
28. Spelber D, Lahijani S. Neurosyphilis presenting as mania and psychosis after incidental treatment with cephalexin: a case report and literature review of jarisch-herxheimer reactions. Psychosomatics. (2020) 61:177–80. doi: 10.1016/j.psym.2019.06.001
29. G N, Moore E, Sithole N. The great imitator: neurosyphilis presenting as subacute confusion. Br J Hosp Med. (2021) 82:1–3. doi: 10.12968/hmed.2020.0669
30. Rotman L, Luo X, Thompson A, Mackesy-Amiti M, Young L, Young J. Risk of neurosyphilis in HIV-infected persons with syphilis lacking signs or symptoms of central nervous system infection. HIV Med. (2019) 20:27–32. doi: 10.1111/hiv.12677
31. Wu MY, Gong HZ, Hu KR, Zheng HY, Wan X, Li J. Effect of syphilis infection on HIV acquisition: a systematic review and meta-analysis. Sex Transm Infect. (2021) 97:525–33. doi: 10.1136/sextrans-2020-054706
32. Nandwani R, Evans DT. Are you sure it's syphilis? A review of false positive serology Int J STD AIDS. (1995) 6:241–8. doi: 10.1177/095646249500600404
33. Buder S, Schöfer H, Meyer T, Bremer V, Kohl PK, Skaletz-Rorowski A, et al. Bacterial sexually transmitted infections. J Dtsch Dermatol Ges. (2019) 17:287–315. doi: 10.1111/ddg.13804
34. Weathers EN, Waller JL, Nahman NS Jr, Colombo RE, Kheda MF, Baer SL. Incidence, risk factors and distribution of syphilis in the end-stage renal disease population in the USA. Clin Kidney J. (2020) 13:625–30. doi: 10.1093/ckj/sfz090
35. Furtado JM, Simões M, Vasconcelos-Santos D, Oliver GF, Tyagi M, Nascimento H, et al. Ocular syphilis. Surv Ophthalmol. (2021) 67:440–62. doi: 10.1016/j.survophthal.2021.06.003
36. Oliver GF, Stathis RM, Furtado JM, Arantes TE, McCluskey PJ, Matthews JM, et al. Current ophthalmology practice patterns for syphilitic uveitis. Br J Ophthalmol. (2019) 103:1645–9. doi: 10.1136/bjophthalmol-2018-313207
37. Mathew D, Smit D. Clinical and laboratory characteristics of ocular syphilis and neurosyphilis among individuals with and without HIV infection. Br J Ophthalmol. (2021) 105:70–4. doi: 10.1136/bjophthalmol-2019-315699
38. Bazewicz M, Lhoir S, Makhoul D, Libois A, Van den Wijngaert S, Caspers L, et al. Neurosyphilis cerebrospinal fluid findings in patients with ocular syphilis. Ocul Immunol Inflamm. (2021) 29:95–101. doi: 10.1080/09273948.2019.1672193
39. Uhr JH, Dubovy SR, Gregori NZ. Syphilitic uveitis masquerading as a vitreous hemorrhage and retinal detachment. Ophthalmol Retina. (2021) 5:729. doi: 10.1016/j.oret.2021.04.018
40. Ramchandani MS, Litvack JR, Marra CM. Otosyphilis: a review of the literature. Sex Transm Dis. (2020) 47:296–300. doi: 10.1097/OLQ.0000000000001155
41. Bradshaw D, Pallawela S, Nelson M, Scott C, Day S. Otosyphilis: missed opportunities for early treatment? Sex Transm Infect. (2012) 88:573. doi: 10.1136/sextrans-2012-050792
42. Kivekäs I, Vasama JP, Hakomäki J. Bilateral temporal bone otosyphilis. Otol Neurotol. (2014) 35:e90–1. doi: 10.1097/MAO.0b013e3182a3603f
43. Janier M, Unemo M, Dupin N, Tiplica GS, Potočnik M, Patel R. 2020 European guideline on the management of syphilis. J Eur Acad Dermatol Venereol. (2021) 35:574–88. doi: 10.1111/jdv.16946
44. Xiao Y, Chen MJ, Shen X, Lin LR, Liu LL, Yang TC, et al. Metabolic disorders in patients with central nervous system infections: associations with neurosyphilis. Eur Neurol. (2019) 81:270–7. doi: 10.1159/000503626
45. Wang F, Ge H, Su X, Wang R, Zeng J, Miao J. High HbA(1c) level is correlated with blood-brain barrier disruption in syphilis patients. Neurol Sci. (2020) 41:83–90. doi: 10.1007/s10072-019-04031-x
46. Boog G, Lopes J, Mahler J, Solti M, Kawahara L, Teng A, et al. Diagnostic tools for neurosyphilis: a systematic review. BMC Infect Dis. (2021) 21:568. doi: 10.1186/s12879-021-06264-8
47. Marra C. Alternatives to the cerebrospinal fluid venereal disease research laboratory test for neurosyphilis diagnosis. Sex Transm Dis. (2021) 48:S54–7. doi: 10.1097/OLQ.0000000000001450
48. Smit D, De Graaf M, Meyer D, de Groot-Mijnes J. Immunoblot and polymerase chain reaction to diagnose ocular syphilis and neurosyphilis in HIV-positive and HIV-negative Patients. Ocul Immunol Inflamm. (2020) 28:1049–55. doi: 10.1080/09273948.2019.1698753
49. Gudowska-Sawczuk M, Mroczko B. Chemokine ligand 13 (CXCL13) in neuroborreliosis and neurosyphilis as selected spirochetal neurological diseases: a review of its diagnostic significance. Int J Mol Sci. (2020) 21:2927. doi: 10.3390/ijms21082927
50. Wang C, Wu K, Yu Q, Zhang S, Gao Z, Liu Y, et al. CXCL13, CXCL10 and CXCL8 as potential biomarkers for the diagnosis of neurosyphilis patients. Sci Rep. (2016) 6:33569. doi: 10.1038/srep33569
51. Lu Y, Ke W, Yang L, Wang Z, Lv P, Gu J, et al. Clinical prediction and diagnosis of neurosyphilis in HIV-negative patients: a case-control study. BMC Infect Dis. (2019) 19:1017. doi: 10.1186/s12879-019-4582-2
52. Kingston M, French P, Higgins S, McQuillan O, Sukthankar A, Stott C, et al. UK national guidelines on the management of syphilis 2015. Int J STD AIDS. (2016) 27:421–46. doi: 10.1177/0956462415624059
53. Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention. J Am Acad Dermatol. (2020) 82:17–28. doi: 10.1016/j.jaad.2019.02.074
54. Judith W. WHO Guidelines for the Treatment of Treponema pallidum (Syphilis). Geneva: World Health Organization (2016).
55. Ho EL, Tantalo LC, Jones T, Sahi SK, Marra CM. Point-of-care treponemal tests for neurosyphilis diagnosis. Sex Transm Dis. (2015) 42:48–52. doi: 10.1097/OLQ.0000000000000222
56. Gonzalez H, Koralnik I, Huhn G, Tantalo L, Ritz E, Orban Z, et al. A dual-platform point-of-care test for neurosyphilis diagnosis. Sex Transm Dis. (2021) 48:353–6. doi: 10.1097/OLQ.0000000000001308
57. Chinese expert consensus statement on diagnosis and treatment of syphilis. Chin Med J. (2020) 133:2335–7. doi: 10.1097/CM9.0000000000001035
58. Marra CM, Boutin P, McArthur JC, Hurwitz S, Simpson PA, Haslett JA, et al. A pilot study evaluating ceftriaxone and penicillin G as treatment agents for neurosyphilis in human immunodeficiency virus-infected individuals. Clin Infect Dis. (2000) 30:540–4. doi: 10.1086/313725
59. Buitrago-Garcia D, Martí-Carvajal A, Jimenez A, Conterno L, Pardo R. Antibiotic therapy for adults with neurosyphilis. Cochrane Database Syst Rev. (2019) 5:CD011399. doi: 10.1002/14651858.CD011399.pub2
60. Ozturk-Engin D, Erdem H, Hasbun R, Wang S, Tireli H, Tattevin P, et al. Predictors of unfavorable outcome in neurosyphilis: multicenter ID-IRI study. Eur J Clin Microbiol Infect Dis. (2019) 38:125–34. doi: 10.1007/s10096-018-3403-7
61. Gordon SM, Eaton ME, George R, Larsen S, Lukehart SA, Kuypers J, et al. The response of symptomatic neurosyphilis to high-dose intravenous penicillin G in patients with human immunodeficiency virus infection. N Engl J Med. (1994) 331:1469–73. doi: 10.1056/NEJM199412013312201
62. Marra CM, Longstreth WT Jr, Maxwell CL, Lukehart SA. Resolution of serum and cerebrospinal fluid abnormalities after treatment of neurosyphilis Influence of concomitant human immunodeficiency virus infection. Sex Transm Dis. (1996) 23:184–9. doi: 10.1097/00007435-199605000-00005
63. Peermohamed S, Kogilwaimath S, Sanche S. Neurosyphilis. CMAJ. (2020) 192:E844. doi: 10.1503/cmaj.200189
64. Girometti N, Junejo M, Nugent D, McOwan A, Whitlock G. Clinical and serological outcomes in patients treated with oral doxycycline for early neurosyphilis. J Antimicrob Chemother. (2021) 76:1916–9. doi: 10.1093/jac/dkab100
65. Richardson D, Goldmeier D. Probenecid in the treatment of neurosyphilis in men who have sex with men: a commentary. Sex Transm Infect. (2021). doi: 10.1136/sextrans-2021-055278 [Epub ahead of print].
66. Butler T. The jarisch-herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesis. Am J Trop Med Hyg. (2017) 96:46–52. doi: 10.4269/ajtmh.16-0434
67. Tsai MS, Yang CJ, Lee NY, Hsieh SM, Lin YH, Sun HY, et al. Jarisch-Herxheimer reaction among HIV-positive patients with early syphilis: azithromycin versus benzathine penicillin G therapy. J Int AIDS Soc. (2014) 17:18993. doi: 10.7448/IAS.17.1.18993
68. Kapila R, Schwartz R. Neurosyphilis and the jarisch-herxheimer reaction: a therapy concern with HIV disease. Dermatol Ther. (2021) 34:e14839. doi: 10.1111/dth.14839
69. Cui L, Xu Z, Hou H. Diagnosis and treatment of spinal syphilitic gumma: a case report. Front Neurol. (2019) 10:1352. doi: 10.3389/fneur.2019.01352
70. Marra CM, Ghanem KG. Centers for disease control and prevention syphilis summit: difficult clinical and patient management issues. Sex Transm Dis. (2018) 45(Suppl. 1):S10–2. doi: 10.1097/OLQ.0000000000000851
71. Ren M, Szadkowski L, Walmsley SL. Deciphering the serological response to syphilis treatment in men living with HIV. Aids. (2020) 34:2089–96. doi: 10.1097/QAD.0000000000002656
72. Jia X, Wang Z, Liu X, Zheng H, Li J. Peripheral blood mononuclear cell microRNA profiles in syphilitic patients with serofast status. Mol Biol Rep. (2020) 47:3407–21. doi: 10.1007/s11033-020-05421-7
73. Wang ZS, Liu XK Li J. Serological response to therapy following retreatment of serofast early syphilis patients with benzathine penicillin. J Antimicrob Chemother. (2018) 73:1348–51. doi: 10.1093/jac/dky006
74. Liu XK, Wang ZS Li J. Predictors of serofast state after treatment of patients with syphilis. Chin Med J (Engl). (2020) 133:2874–6. doi: 10.1097/CM9.0000000000001175
75. Dunaway S, Maxwell C, Tantalo L, Sahi S, Marra C. Neurosyphilis treatment outcomes after intravenous penicillin G versus intramuscular procaine penicillin plus oral probenecid. Clin Infect Dis. (2020) 71:267–73. doi: 10.1093/cid/ciz795
76. Jiang M, Zhang H, Li W, Wu W, Huang Y, Xu D, et al. Analysis of EEG Lemple-Ziv complexity and correlative aspects before and after treatment of anti-syphilis therapy for neurosyphilis. Neurol Res. (2019) 41:199–203. doi: 10.1080/01616412.2018.1520438
77. Qi S, Xu Y, Luo R, Li P, Huang Z, Huang S, et al. Novel biochemical insights in the cerebrospinal fluid of patients with neurosyphilis based on a metabonomics study. J Mol Neurosci. (2019) 69:39–48. doi: 10.1007/s12031-019-01320-0
78. Jame R, Al-Saeigh Y, Wang LL, Wang K. Justified suspicion: symptomatic syphilitic alopecia in a patient with well-controlled HIV. Case Rep Infect Dis. (2021) 2021:1124033. doi: 10.1155/2021/1124033
79. Jain J, Santos GM, Scheer S, Gibson S, Crouch PC, Kohn R, et al. Rates and correlates of syphilis reinfection in men who have sex with men. LGBT Health. (2017) 4:232–6. doi: 10.1089/lgbt.2016.0095
80. Cohen SE, Chew Ng RA, Katz KA, Bernstein KT, Samuel MC, Kerndt PR, et al. Repeat syphilis among men who have sex with men in California, 2002-2006: implications for syphilis elimination efforts. Am J Public Health. (2012) 102:e1–8. doi: 10.2105/AJPH.2011.300383
81. Almeida VC, Donalisio MR, Cordeiro R. Factors associated with reinfection of syphilis in reference centers for sexually transmitted infections. Rev Saude Publica. (2017) 51:64. doi: 10.1590/s1518-8787.2017051006432
82. Read PJ, Donovan B. Clinical aspects of adult syphilis. Intern Med J. (2012) 42:614–20. doi: 10.1111/j.1445-5994.2012.02814.x
83. Chang CC, Leslie DE, Spelman D, Chua K, Fairley CK, Street A, et al. Symptomatic and asymptomatic early neurosyphilis in HIV-infected men who have sex with men: a retrospective case series from 2000 to 2007. Sex Health. (2011) 8:207–13. doi: 10.1071/SH10060
84. Addetia A, Tantalo LC, Lin MJ, Xie H, Huang ML, Marra CM, et al. Comparative genomics and full-length Tprk profiling of Treponema pallidum subsp. pallidum reinfection. PLoS Negl Trop Dis. (2020) 14:e0007921. doi: 10.1371/journal.pntd.0007921
85. Thomas DD, Navab M, Haake DA, Fogelman AM, Miller JN, Lovett MA. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc Natl Acad Sci U S A. (1988) 85:3608–12. doi: 10.1073/pnas.85.10.3608
86. Zhang RL, Wang QQ. The Treponema pallidum outer membrane protein Tp92 activates endothelial cells via the chemerin/CMKLR1 pathway. Int J Med Microbiol. (2020) 310:151416. doi: 10.1016/j.ijmm.2020.151416
87. Carlson JA, Dabiri G, Cribier B, Sell S. The immunopathobiology of syphilis: the manifestations and course of syphilis are determined by the level of delayed-type hypersensitivity. Am J Dermatopathol. (2011) 33:433–60. doi: 10.1097/DAD.0b013e3181e8b587
88. Cruz AR, Ramirez LG, Zuluaga AV, Pillay A, Abreu C, Valencia CA, et al. Immune evasion and recognition of the syphilis spirochete in blood and skin of secondary syphilis patients: two immunologically distinct compartments. PLoS Negl Trop Dis. (2012) 6:e1717. doi: 10.1371/journal.pntd.0001717
89. Centurion-Lara A, Giacani L, Godornes C, Molini BJ, Brinck Reid T, Lukehart SA. Fine analysis of genetic diversity of the tpr gene family among treponemal species, subspecies and strains. PLoS Negl Trop Dis. (2013) 7:e2222. doi: 10.1371/journal.pntd.0002222
90. Reid TB, Molini BJ, Fernandez MC, Lukehart SA. Antigenic variation of TprK facilitates development of secondary syphilis. Infect Immun. (2014) 82:4959–67. doi: 10.1128/IAI.02236-14
91. Qin J, Yang T, Wang H, Feng T, Liu X. Potential predictors for serofast state after treatment among HIV-negative persons with syphilis in china: a systematic review and meta-analysis. Iran J Public Health. (2015) 44:155–69.
92. Guo N, Liu L, Yang X, Song T, Li G, Li L, et al. Immunological changes in monocyte subsets and their association with Foxp3(+) Regulatory T cells in HIV-1-infected individuals with syphilis: a brief research report. Front Immunol. (2019) 10:714. doi: 10.3389/fimmu.2019.00714
93. Li W, Wu W, Chang H, Jiang M, Gao J, Xu Y, et al. Cerebrospinal fluid cytokines in patients with neurosyphilis: the significance of interleukin-10 for the disease. Biomed Res Int. (2020) 2020:3812671. doi: 10.1155/2020/3812671
94. Yan Y, Wang J, Qu B, Zhang Y, Wei Y, Liu H, et al. CXCL13 and TH1/Th2 cytokines in the serum and cerebrospinal fluid of neurosyphilis patients. Medicine (Baltimore). (2017) 96:e8850. doi: 10.1097/MD.0000000000008850
95. Luo X, Zhang X, Gan L, Zhou C, Zhao T, Zeng T, et al. The outer membrane protein Tp92 of Treponema pallidum induces human mononuclear cell death and IL-8 secretion. J Cell Mol Med. (2018) 22:6039–54. doi: 10.1111/jcmm.13879
96. Liu L. Changes of T lymphocyte subsets in patients with HIV-negative symptomatic neurosyphilis. Microb Pathog. (2019) 130:213–8. doi: 10.1016/j.micpath.2019.03.008
97. Zhu A, Han H, Zhao H, Hu J, Jiang C, Xie F, et al. Increased frequencies of Th17 and Th22 cells in the peripheral blood of patients with secondary syphilis. FEMS Immunol Med Microbiol. (2012) 66:299–306. doi: 10.1111/j.1574-695X.2012.01007.x
98. Yu Q, Cheng Y, Wang Y, Wang C, Lu H, Guan Z, et al. Aberrant humoral immune responses in neurosyphilis: CXCL13/CXCR5 play a pivotal role for B-cell recruitment to the cerebrospinal fluid. J Infect Dis. (2017) 216:534–44. doi: 10.1093/infdis/jix233
99. Balasa R, Barcutean L, Balasa A, Motataianu A, Roman-Filip C, Manu D. The action of TH17 cells on blood brain barrier in multiple sclerosis and experimental autoimmune encephalomyelitis. Hum Immunol. (2020) 81:237–43. doi: 10.1016/j.humimm.2020.02.009
100. Guziejko K, Czupryna P, Pancewicz S, Swierzbińska R, Dunaj J, Kruszewska E, et al. Analysis of CCL-4, CCL-17, CCL-20 and IL-8 concentrations in the serum of patients with tick-borne encephalitis and anaplasmosis. Cytokine. (2020) 125:154852. doi: 10.1016/j.cyto.2019.154852
101. Li W, Chang H, Wu W, Xu D, Jiang M, Gao J, et al. Increased CSF soluble TREM2 concentration in patients with neurosyphilis. Front Neurol. (2020) 11:62. doi: 10.3389/fneur.2020.00062
102. Xu D, Cai S, Li R, Wu Y, Liu S, Lun W. Elevation of cerebrospinal fluid light and heavy neurofilament levels in symptomatic neurosyphilis. Sex Transm Dis. (2020) 47:634–8. doi: 10.1097/OLQ.0000000000001236
103. Lämmermann T, Kastenmüller W. Concepts of GPCR-controlled navigation in the immune system. Immunol Rev. (2019) 289:205–31. doi: 10.1111/imr.12752
104. Liu W, Wu K, Wang X, Lin L, Tong M, Liu L. ENST00000421645LncRNA- promotes T cells to secrete IFN-γ by sponging PCM-1 in neurosyphilis. Epigenomics. (2021). doi: 10.2217/epi-2021-0163
105. Chen H, Zhou Y, Wang Z, Yan B, Zhou W, Wang T, et al. Exosomal microRNA profiles from serum and cerebrospinal fluid in neurosyphilis. Sex Transm Infect. (2019) 95:246–50. doi: 10.1136/sextrans-2018-053813
106. Liu L. Metabolite profiles of the cerebrospinal fluid in neurosyphilis patients determined by untargeted metabolomics analysis. Front Neurosci. (2019) 13:150. doi: 10.3389/fnins.2019.00150
108. Heymann WR. The history of syphilis. J Am Acad Dermatol. (2006) 54:322–3. doi: 10.1016/j.jaad.2005.10.003
109. Jin F, Prestage GP, Kippax SC, Pell CM, Donovan BJ, Kaldor JM, et al. Epidemic syphilis among homosexually active men in Sydney. Med J Aust. (2005) 183:179–83. doi: 10.5694/j.1326-5377.2005.tb06989.x
110. Andreae A, Barr A, Fulton Z, Enterline D, Dicks K. Appearance of meningovascular neurosyphilis causing an acute ischemic stroke. Sex Transm Dis. (2021) 48:e109–10. doi: 10.1097/OLQ.0000000000001330
111. Gottlieb SL, Johnston C. Future prospects for new vaccines against sexually transmitted infections. Curr Opin Infect Dis. (2017) 30:77–86. doi: 10.1097/QCO.0000000000000343
Keywords: neurosyphilis, syphilis, Treponema pallidum, cerebrospinal fluid, ocular syphilis, otosyphilis, penicillin, ceftriaxone
Citation: Zhou J, Zhang H, Tang K, Liu R and Li J (2022) An Updated Review of Recent Advances in Neurosyphilis. Front. Med. 9:800383. doi: 10.3389/fmed.2022.800383
Received: 23 October 2021; Accepted: 14 March 2022;
Published: 20 September 2022.
Edited by:
Pingyu Zhou, Tongji University, ChinaReviewed by:
Ahmet Cagkan Inkaya, Hacettepe University, TurkeyMei Shi, Shanghai Dermatology Hospital, China
Copyright © 2022 Zhou, Zhang, Tang, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Li, lijun35@hotmail.com
†These authors have contributed equally to this work
 Jia Zhou
Jia Zhou Hanlin Zhang
Hanlin Zhang Keyun Tang
Keyun Tang Runzhu Liu
Runzhu Liu Jun Li
Jun Li