Abnormal Default Mode Network Homogeneity in Major Depressive Disorder With Gastrointestinal Symptoms at Rest
- 1Department of Psychiatry, National Clinical Research Center for Mental Disorders, The Second Xiangya Hospital of Central South University, Changsha, China
- 2Department of Radiology, Tianjin Medical University General Hospital, Tianjin, China
- 3Department of Radiology, The Second Xiangya Hospital of Central South University, Changsha, China
- 4Department of Psychiatry, The Third People’s Hospital of Foshan, Foshan, China
Background: Gastrointestinal (GI) symptoms are prominent in many patients with major depressive disorder (MDD). However, it remains unclear whether MDD patients with GI symptoms have brain imaging alterations in the default mode network (DMN) regions.
Methods: A total of 35 MDD patients with GI symptoms, 17 MDD patients without GI symptoms, and 28 healthy controls (HCs) were recruited. All participants underwent resting-state functional magnetic resonance imaging scans. Network homogeneity (NH) and support vector machine (SVM) methods were used to analyze the imaging data.
Results: Gastrointestinal group showed higher 17-item Hamilton Rating Scale for Depression total scores and factor scores than the non-GI group. Compared with the non-GI group and HCs, the GI group showed decreased NH in the right middle temporal gyrus (MTG) and increased NH in the right precuneus (PCu). The SVM results showed that a combination of NH values of the right PCu and the right MTG exhibited the highest accuracy of 88.46% (46/52) to discriminate MDD patients with GI symptoms from those without GI symptoms.
Conclusion: Major depressive disorder patients with GI symptoms have more severe depressive symptoms than those without GI symptoms. Distinctive NH patterns in the DMN exist in MDD patients with GI symptoms, which can be applied as a potential brain imaging marker to discriminate MDD patients with GI symptoms from those without GI symptoms.
Introduction
Major depressive disorder (MDD) is a globally common, severe, and recurrent mental disorder (Smith, 2014). The World Health Organization (WHO) predicted that MDD will be the leading cause of the global burden of disease by 2030 (Malhi and Mann, 2018). However, the resources of professional psychiatric medical staff are scarce. Almost half of the whole world’s population lives in countries with only two professional psychiatrists per 100,000 people (Smith, 2014). A previous study has indicated that more healthcare resources were required during the episodes of MDD, resulting in a higher economic burden (Painchault et al., 2014). Comorbidity was reported as an important cause of the increasing economic burden of MDD (Greenberg et al., 2015). Many patients with MDD often have some non-specific somatic symptoms as their chief complaints [such as medically unexplained pain, insomnia, blurred vision, chest tightness, tachycardia, and gastrointestinal (GI) symptoms], with the prominent GI symptoms (such as loss of appetite, gastralgia, gastric distention, heartburn, acid reflux, nausea, vomiting, constipation, and diarrhea). In a large study of the general population, 54% of people with depressive symptoms suffered from frequent abdominal pain, constipation, diarrhea, dyspepsia, or irritable bowel syndrome (IBS), whereas only 29% of people with non-depressive symptoms (Hillilä et al., 2008). It has been indicated that somatic symptoms in MDD patients are associated with more severe clinical symptoms, lower remission rates (Novick et al., 2013), and worse prognosis (Bekhuis et al., 2016). Somatic symptoms also led to more utilization of health resources in patients with MDD (García-Campayo et al., 2008). In addition, patients with depressive-anxiety disorder comorbidities were reported to exhibit more several chronic physical conditions than patients without depression and anxiety comorbidities, and having depression and anxiety at the same time further increased the risk of a number of physical conditions co-occurring (Scott et al., 2007). Unfortunately, due to the scarcity of mental health resources, coupled with the social stigma of mental illness/disorders, MDD patients with somatic symptoms as chief complaints tend to seek treatment at general hospitals for the first time, even over and over again, leading to a lack of correct diagnosis and effective treatment (Kirmayer et al., 1993; García-Campayo et al., 2008), which is often called as “masked depression” (Verster and Gagiano, 1995). Thus, correct identifications and diagnoses of MDD patients with GI symptoms as chief complaints and effective treatments for them are urgent. It is not a one-way story that only patients with MDD can have GI symptoms. In clinical practice, patients with the digestive disease can also have psychological problems, such as anxiety, self-reported depressive mood, and even depression. A previous study has reported that most patients with GI motility disorders, IBS, and functional dyspepsia had a number of mental and psychological problems. When patients with IBS were exposed to stressors, their GI symptoms seem to be greatly increased (Whitehead, 1996). In another study, 44.4% of patients with inflammatory bowel disease (IBD) suffered from anxiety or depression or both, resulting in more therapies and healthcare resources (Navabi et al., 2018). IBD patients with baseline depression were reported to show more aggressive symptoms and poorer long-term progress than IBD patients without baseline depression (Kochar et al., 2018). Therefore, it is very important to find out the etiology of GI symptoms in patients with MDD.
But to date, the definite cause of MDD remains unknown. In recent years, the gut-brain axis has become a hot research topic, which is believed as one possible critical mechanism of affective disorders (Mayer et al., 2015). It has previously been reported that the GI microbiota can activate neural pathways and the central nervous system signaling systems, thereby affecting MDD-related symptoms (Foster and McVey Neufeld, 2013). However, it still remains unclear whether MDD patients with GI symptoms have brain imaging alterations. Structural (Bell-McGinty et al., 2002; Depping et al., 2016; Hyett et al., 2018) and functional (Bluhm et al., 2009; Guo et al., 2013b,Chen et al., 2015; Guo et al., 2018a) brain changes have already been reported in many previous studies of MDD. Meanwhile, some previous studies have reported structural and functional brain image changes in digestive system diseases (Kwan et al., 2005; Song et al., 2006; Blankstein et al., 2010; Skrobisz et al., 2020). Thus, some researchers tried to study whether MDD patients with GI symptoms exhibited abnormal brain imaging data and observed altered gray matter volume (GMV) and regional homogeneity (ReHo) (Yan et al., 2019; Liu et al., 2020). However, there are still few functional imaging studies conducted on MDD patients with GI symptoms, and the researchers did not further examine whether these abnormal GMV or ReHo could be used as good neuroimaging markers to discriminate the GI group from the non-GI group in both abovementioned studies.
The default mode network (DMN) is mainly composed of three subdivisions, namely, the ventral medial prefrontal cortex, the dorsal medial prefrontal cortex, the posterior cingulate cortex, and adjacent precuneus (PCu) plus the lateral parietal cortex (Raichle, 2015). Prior to this study, there have been many studies on the DMN of mental disorders, such as schizophrenia (Guo et al., 2014, 2015b; Hu et al., 2017; Hare et al., 2019; Fan et al., 2020; Martin-Subero et al., 2021), MDD (Guo et al., 2015a; Fang et al., 2016; Posner et al., 2016; Yan et al., 2019; Zhou et al., 2020), post-traumatic stress disorder (King et al., 2016; Miller et al., 2017; Viard et al., 2019), and attention deficit hyperactivity disorder (Sidlauskaite et al., 2016; Bozhilova et al., 2018). The increased amplitude of low-frequency fluctuation and functional connectivity (FC) in the DMN have been reported in patients with ulcerative colitis (Fan et al., 2019). Visceral sensory abnormalities are very common in IBS (Song et al., 2006). The DMN plays a critical role in gastric sensations (Skrobisz et al., 2020). A previous study has suggested that somatic symptom disorders may be associated with altered processing of sensory discrimination of pain and other somatic symptoms (Kim et al., 2019). It was indicated that brain areas involved in the process of pain sensory shifted to those involved in the subjective states of emotion and motivation in the majority of chronic pain diseases (Apkarian et al., 2011). Moreover, chronic visceral pain might lead to functional reorganization in the DMN (Farmer et al., 2012; Qi et al., 2016; Kano et al., 2018). Therefore, the abovementioned studies indicated that the DMN may change with the onset and development of GI symptoms. Thus, we were curious about whether there were special brain imaging changes of the DMN in these MDD patients with GI symptoms.
In this study, we applied a network homogeneity (NH) approach to examine whether the NH of the DMN in MDD patients with GI symptoms was abnormal and whether abnormal NH of the DMN could be used as brain imaging markers to separate MDD patients with GI symptoms from MDD patients without GI symptoms. We hypothesized that MDD patients with GI symptoms exhibited altered NH in certain areas of the DMN, which might be used to discriminate the MDD patients with GI symptoms from those without GI symptoms.
Materials and Methods
Participants
A total of 35 patients with at least one GI symptom were recruited as the GI group, and 17 patients without GI symptoms were recruited and assigned to the non-GI group. Main GI symptoms included medically unexplained gastralgia, gastric distention, heartburn, acid reflux, nausea, vomiting, constipation, and diarrhea. All patients were aged from 18 to 55 years and were from the outpatients of the Second Xiangya Hospital of Central South University, China. Their final diagnoses were independently confirmed by two psychiatrists, using the DSM-5 criteria for MDD. The inclusion criteria for all patients were as follows: (1) first major depressive episode; (2) 17-item Hamilton Rating Scale for Depression (HRSD-17) (Hamilton, 1967) total scores ≥18; (3) with no history of antipsychotics and physical therapy (such as electroconvulsive therapy, ECT); and (4) with no confirmed digestive diseases.
A total of 28 healthy controls (HCs) were recruited from the community; the patient groups were matched in age, gender, and education. They would not be recruited if they had a suspicious or explicit family history of mental illness/disorders, especially their first-degree relatives. Besides, HCs with any history of digestive diseases, neurological diseases, substance abuse, or psychotic symptoms would not be enrolled.
All participants were Han Chinese and right-handed. The exclusion criteria for all participants were as follows: (a) other mental disorders meeting DSM-5 diagnostic criteria; (b) any history of neurological diseases, severe physical illnesses, and substance abuse; (c) being pregnant; (d) structural abnormalities of the brain after the initial magnetic resonance imaging (MRI) scan; and (e) any contraindications to MRI scans.
The severity of MDD was evaluated by using the HRSD-17. It can be divided into five types of factors: (1) anxiety/somatization (six items, namely, psychic anxiety, somatic anxiety, GI symptoms, hypochondriasis, insight, and general symptoms); (2) weight loss (one item); (3) cognitive disturbances (three items, namely, self-guilt, suicide, and agitation); (4) retardation symptoms (four items, namely, depression, work and interests, retardation, and sexual symptoms); and (5) sleep disturbances (three items, namely, difficulty in falling asleep, superficial sleep, and early awakening).
The study was approved by the Medical Research Ethics Committee of the Second Xiangya Hospital of Central South University, China. It was conducted in accordance with the Helsinki Declaration. Each participant has submitted a written informed consent before enrollment.
Image Acquisition
By using a 3.0 T Siemens scanner (Germany) at the Second Xiangya Hospital of Central South University, resting-state fMRI data were obtained. All subjects were instructed to close their eyes, stay still, and awake throughout the scan. The resting-state functional images were acquired using the echo-planar imaging sequence. Specific parameters were as follows: 2,000/30 ms of repetition time/echo time, 30 slices, 64 × 64 matrix, 90° flip angle, 24 cm field of view, 4 mm slice thickness, 0.4 mm gap, and 250 volumes lasting for 500 s.
Data Preprocessing
A Data Processing Assistant for Resting-State fMRI was used for data preprocessing in MATLAB (MathWorks) (Chao-Gan and Yu-Feng, 2010). Considering that data errors may increase due to initial signal instability and subjects’ early adaptation time, we deleted the first 10 original images to minimize these potential impacts. Specific processes were as follows: (a) correction for slice timing and head motion: all data were with a maximum displacement of x-, y-, or z-axis less than 2 mm and maximum angular rotation less than 2°; (b) normalization: the corrected imaging data then got spatially normalized to the MNI space with 3 mm × 3mm × 3 mm; and (c) band-pass filtering and detrending: after normalization, the imaging data were temporally band-pass filtered at 0.01–0.08 Hz and got linearly detrended. Several spurious covariates (such as the signal from the ventricular seed-based region of interest, white matter-centered region, and 24-head motion parameters acquired by rigid body correction) were removed from the imaging data. The global signal was not regressed out during data preprocessing according to a previous study (Hahamy et al., 2014).
Default Mode Network Identification
After being preprocessed, all groups were subjected to construct the DMN mask by using Group Independent Component Analysis (ICA) of fMRI toolbox (GIFT).1 Three main steps were included in the analysis as follows: data reduction, independent component separation, and back reconstruction. The DMN components were selected based on the templates provided by GIFT. The specific calculation process of generating the final DMN mask was similar to our previous study (Guo et al., 2018b). The generated DMN mask was applied in the following NH analysis.
Network Homogeneity Analysis
The NH analysis was conducted using MATLAB (MathWorks). Correlation coefficients between each voxel and all other voxels within the DMN mask were calculated for each subject. The average correlation coefficient was defined as the homogeneity of a given voxel. The NH of each voxel in the DMN mask was generated. The NH maps were smoothed with a Gaussian kernel of 4 mm full width at half maximum and were used for group comparisons.
Statistical Analyses
Group differences (such as age, years of education, HRSD-17 total, and the five-factor scores across the three groups) were compared by performing an analysis of variance (ANOVA) using SPSS19.0 (LSD between two group comparisons). A Chi-square test was applied to describe gender distribution. And we applied a two-sample t-test to analyze whether there were differences in the illness duration between the GI and non-GI groups. The p-value of <0.05 was considered statistically significant.
The NH analysis was performed using an analysis of covariance (ANCOVA) across the three groups, followed by post hoc t-tests. The significance threshold for multiple comparisons was set at p < 0.05 by using Gaussian random field (GRF) theory (voxel significance at p < 0.001 and cluster significance at p < 0.05). Frame-wise displacement (FD) values were calculated for every subject, and the average FD was used as one of the covariates according to the previous study (Power et al., 2012). Age, sex, and years of education were other covariates.
Correlation Analyses
The NH values were extracted from the brain clusters with abnormal NH values. Pearson’s correlation analysis was used to determine the correlations between NH abnormality and HRSD-17 total scores as well as the five-factor scores with the Benjamini–Hochberg correction threshold of p < 0.05.
Classification Analyses
The support vector machine (SVM) approach is a popular supervised machine-learning model, which has gotten many applications for classifications in the researches of psychiatry in recent years (Steardo et al., 2020). It can map training examples to points in space and construct a hyperplane with the maximum distance from the nearest training data point of any one of the two predefined categories, known as the maximum-margin hyperplane (Shan et al., 2020). These nearest points are support vectors (Du et al., 2015). For more details about SVM, a previous study (Shan et al., 2020) is available. In this study, SVM was applied to use NH values extracted from the brain regions with abnormal NH values to discriminate MDD patients with GI symptoms from those without GI symptoms by employing the library for SVM (LIBSVM) software package2 in MATLAB. In this study, the method of “leave-one-out” was applied.
Results
Demographic Characteristics and Clinical Information
The three groups did not show any significant group differences in age, years of education, and gender distribution. No significant illness-duration difference was observed between the two patient groups. Both patient groups showed higher HRSD-17 total and factor scores (except for scores of weight loss) than HCs. The weight loss scores of the GI group were higher than those of both the non-GI group and HCs, but there was no significant difference in weight loss scores between the non-GI group and HCs. Besides, the GI group showed higher HRSD-17 total scores, factor scores of anxiety/somatization, and sleep disturbances than those of the non-GI group (Table 1).
Network Homogeneity Differences Across Groups
The NH values showed significant differences mainly in the frontal and temporal regions across the three groups (Figure 1).
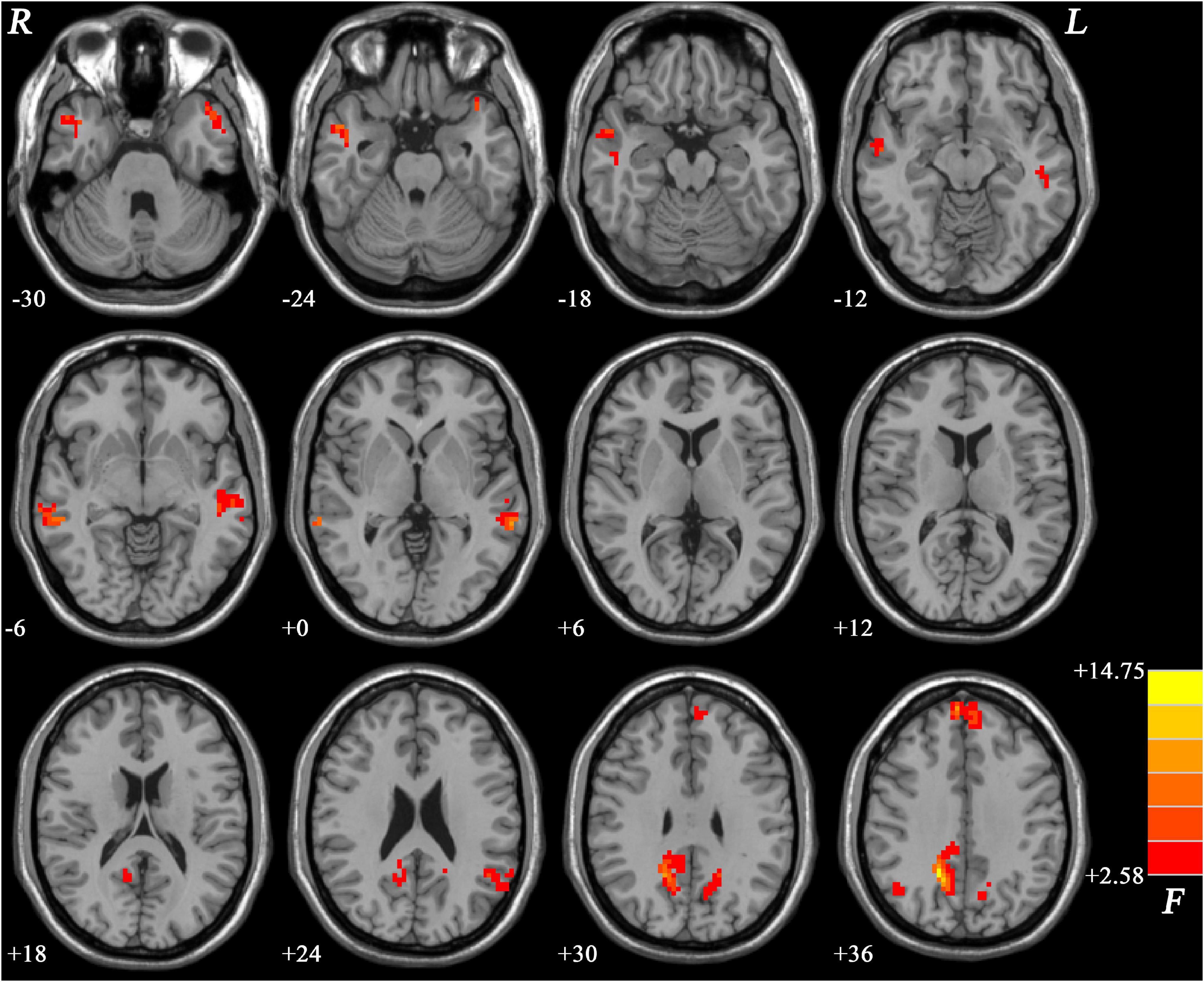
Figure 1. Brain regions within the DMN showing group differences in NH values across the three groups. Color bar indicates F values from ANCOVA (age, sex, years of education, and frame-wise displacement as covariates). NH, network homogeneity; DMN, default mode network; ANCOVA, analysis of covariance.
Compared with MDD patients without GI symptoms, MDD patients with GI symptoms showed decreased NH in the bilateral MTG and increased NH in the right PCu (Figure 2 and Table 2). Compared with HCs, MDD patients with GI symptoms exhibited decreased NH in the bilateral superior medial frontal cortex, left middle temporal pole, right MTG, and increased NH in the right PCu (Figure 3 and Table 2). No abnormal NH in any brain region was found in the non-GI group relative to HCs.
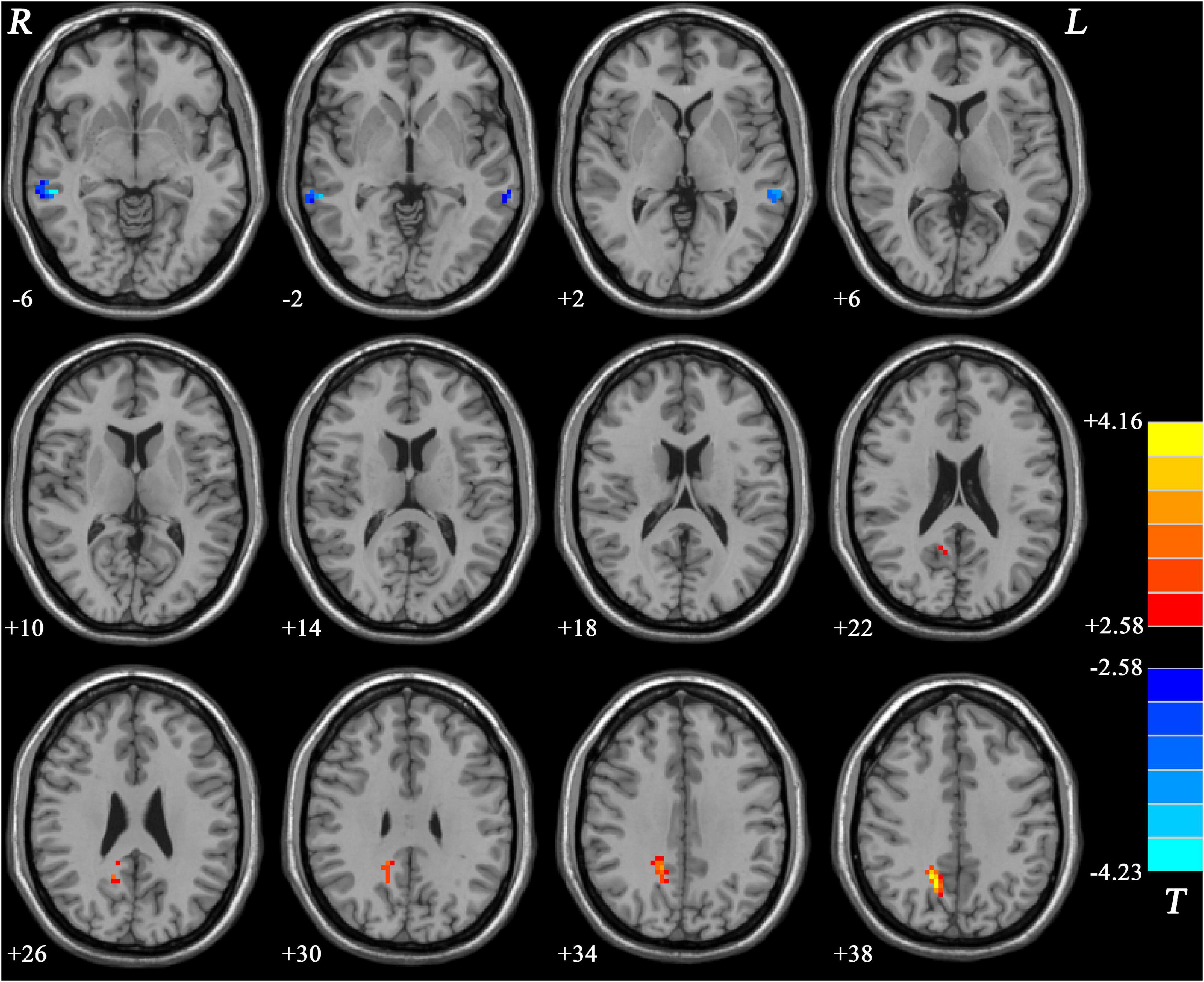
Figure 2. Statistical map depicts higher and lower NH of MDD patients with GI symptoms compared with MDD patients without GI symptoms. The threshold was set at p < 0.05. Blue denotes lower NH, and red denotes higher NH. Color bar indicates T values from the two-sample t-test. NH, network homogeneity; GI, gastrointestinal symptoms.
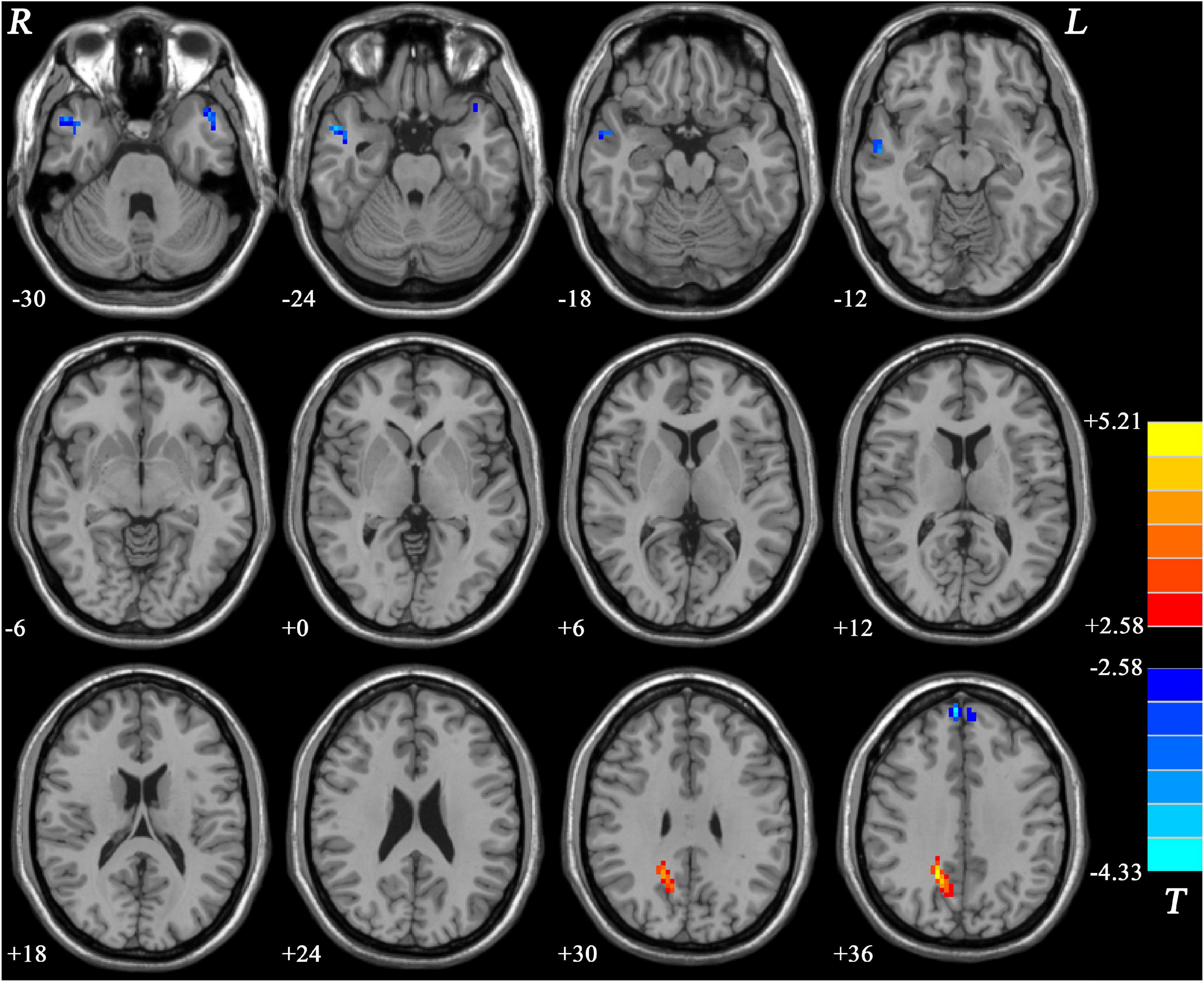
Figure 3. Statistical map depicts higher and lower NH of MDD patients with GI symptoms compared with healthy controls. The threshold was set at p < 0.05. Blue denotes lower NH, and red denotes higher NH. Color bar indicates T values from two-sample t-test. NH, network homogeneity; GI, gastrointestinal symptoms.
Support Vector Machine Analysis in Major Depressive Disorder With and Without Gastrointestinal Symptoms
The SVM results showed that a combination of NH values of the right PCu and the right MTG exhibited the highest accuracy of 88.46% (46/52) to discriminate MDD patients with GI symptoms from those without GI symptoms, with a sensitivity and specificity of 97.14% (34/35) and 70.56% (12/17), respectively. The accuracy of using abnormal NH in different brain regions was 78.85% (41/52) of the right PCu, 76.92% (40/52) of the left MTG, 80.77% (42/52) of the right MTG, 86.54% (45/52) of a combination of the right PCu and the left MTG, and 78.85% (41/52) of a combination of the bilateral MTG (Figure 4 and Figure 5).
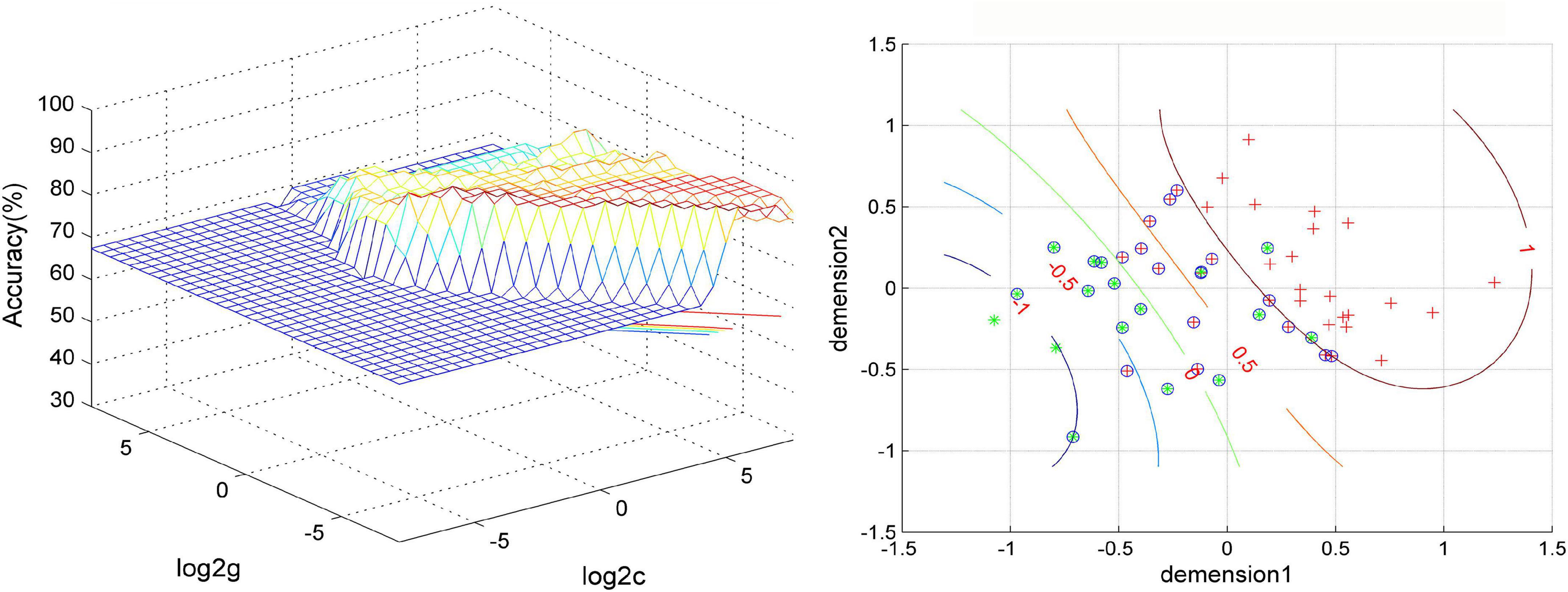
Figure 4. Visualization of classifications through support vector machine (SVM) using a combination of NH values in the right precuneus and right middle temporal gyrus. Left: SVM parameters result of 3D view. Right: dimension 1 and dimension 2 represent the NH values in the right precuneus and right middle temporal gyrus, respectively. Red crosses represent MDD patients with GI symptoms, and green crosses represent MDD patients without GI symptoms. The circles mean support vectors. MDD, major depressive disorder; GI, gastrointestinal symptoms.
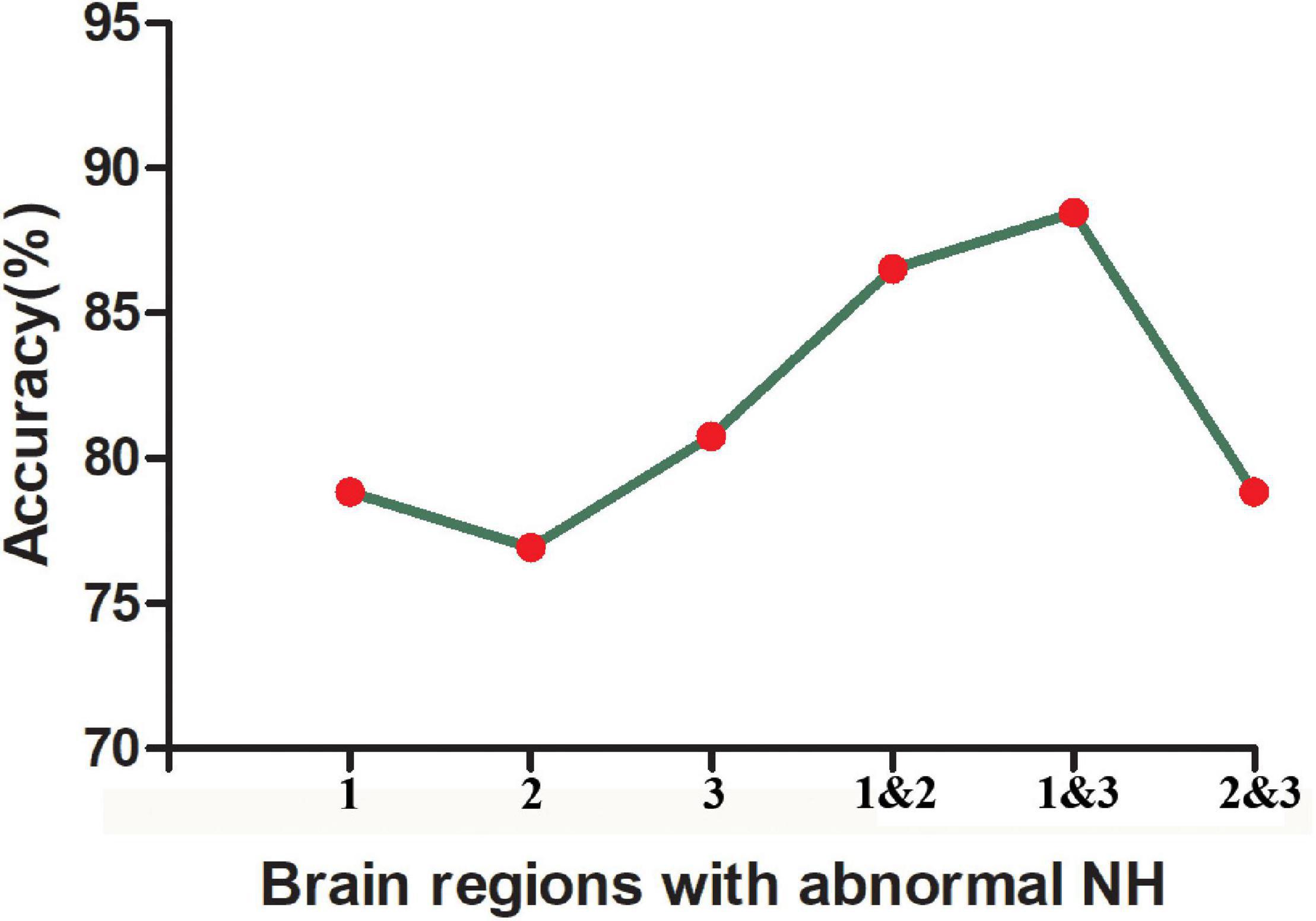
Figure 5. The accuracy of using abnormal NH values of different brain regions to classify two patient groups. 1, right precuneus; 2, left middle temporal gyrus; 3, right middle temporal gyrus; 1&2, right precuneus and left middle temporal gyrus; 1&3, right precuneus and right middle temporal gyrus; 2&3, left middle temporal gyrus and right precuneus and right middle temporal gyrus.
Correlations Between Network Homogeneity and Clinical Characteristics
There was no significant correlation between abnormal NH and the HRSD-17 total scores as well as the five-factor scores for both GI and non-GI symptom groups.
Discussion
Major depressive disorder patients with GI symptoms showed greater severity of depressive symptoms than MDD patients without GI symptoms. Distinctive NH patterns existed in MDD patients with GI symptoms. Compared with the non-GI group and HCs, the GI group showed decreased NH in the right MTG and increased NH in the right PCu. SVM results showed that a combination of NH values of the right PCu and the right MTG might be a potential brain imaging marker to discriminate MDD patients with GI symptoms from those without GI symptoms.
Depression, anxiety, and other psychological factors were considered as one of the important causes or inducements of some digestive system diseases (Drossman et al., 1999; Fond et al., 2014; Lee et al., 2017). A previous study has reported more serious depression in MDD patients with GI symptoms (Liu et al., 2020). Consistent with it, we observed that the GI group showed higher HRSD-17 total scores, factor scores of anxiety/somatization, weight loss, and sleep disturbances than the non-GI group in this study, indicating that the GI symptoms may be related to more severe depression.
The temporal gyrus is involved in attention control with the frontal and parietal lobe together (Sani et al., 2021), in which the MTG participated in cued attention (Corbetta and Shulman, 2002). Our previous study has observed that melancholic MDD showed lower NH in the right MTG than HCs (Cui et al., 2017). Reduced gray matter (Peng et al., 2011; Ma et al., 2012) and abnormal functional network connectivity (Zhi et al., 2018) were also observed in the MTG in MDD compared with HCs. Furthermore, a previous study found that MDD patients with somatic symptoms showed lower ReHo in the right MTG compared with MDD patients without somatic symptoms (Geng et al., 2019). In line with these studies, we observed decreased NH in the right MTG in MDD patients with GI symptoms compared with both MDD patients without GI symptoms and HCs. These issues might explain the phenomenon that MDD patients with GI symptoms pay much more attention to their somatic symptoms. Thus, we suspected that the right MTG might play an important role in the pathophysiology of MDD with GI symptoms. Additionally, we observed increased NH in the right PCu in MDD patients with GI symptoms compared with both MDD patients without GI symptoms and HCs. Some previous studies have reported abnormal PCu in MDD, including higher activity (Sheline et al., 2010) and lower activity (Chen et al., 2012; Guo et al., 2013a; Shi et al., 2020). It was proposed that PCu was involved in consciousness, specifically in the processes of self-reflection and episodic memory retrieval (Cavanna and Trimble, 2006; Cavanna, 2007). PCu would selectively deactivate during sleep (Cavanna and Trimble, 2006; Cavanna, 2007). Thus, we suspected that abnormal NH in the right PCu might be correlated with the phenomenon that the GI group showed more severe sleep disturbance than the non-GI group. Unfortunately, we did not find any statistically significant correlation between abnormal NH in the right PCu and clinical features. It was inconsistent with the results of the abovementioned study since we recruited different types of patients with MDD. Interestingly, a previous study reported that the interregional FC of the DMN between the MTG and PCu was reduced in IBS patients compared with HCs (Qi et al., 2016). Although we did not obtain more interregional FC data here to further test it, it makes us sure that decreased NH in the right MTG and increased NH in the right PCu might be distinctive NH patterns in MDD with GI symptoms.
We performed the SVM analysis to test whether abnormal NH in the DMN region could be used as a brain imaging marker to screen MDD patients with GI symptoms from MDD patients without GI symptoms. The results showed that a combination of NH values of the right PCu and right MTG showed the highest accuracy of 88.46% (46/52) to discriminate MDD patients with GI symptoms from those without GI symptoms, with a sensitivity of 97.14% (34/35) and a specificity of 70.56% (12/17). The establishment of good diagnostic indicators requires sensitivity or specificity of at least 60%, preferably greater than 70% (Swets, 1988; Gong et al., 2011). Thus, the combination of NH values of the right PCu and right MTG may be a potential brain imaging marker to screen MDD patients with GI symptoms from MDD patients without GI symptoms.
There were some limitations. First, we did not evaluate the severity of GI symptoms and did not further classify them. Second, we did not know whether changes in NH occurred before or as a result of GI symptoms. A long-term follow-up observation in the non-GI group may help us to understand the cause and effect. If the NH changes are prior, we could use the neuroimaging marker to identify patients who may have GI symptoms in advance in the future, provide appropriate intervention to prevent more serious symptoms, and then save medical resources. On the contrary, if abnormal NH is the result of GI symptoms, it will provide a new research direction for related treatments.
Conclusion
Major depressive disorder with GI symptoms shows more severe depressive symptoms than MDD without GI symptoms. Distinctive NH patterns in DMN exist in MDD with GI symptoms that can be applied as a potential brain imaging marker to discriminate MDD with GI symptoms from those without GI symptoms.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Research Ethics Committee of the Second Xiangya Hospital of Central South University, China. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MY, HL, and WG contributed to the conception and design of the study. HL, JC, and JZ supervised the progress of the study. MY, FL, and WG performed the data analysis. MY wrote the manuscript. All authors contributed to manuscript revision, read, and approved it for publication.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Grant Nos. 82171508 and 81771447), Natural Science Foundation of Hunan (Grant No. 2020JJ4784), Science and Technology Program of Hunan Province (Grant No. 2020SK53413), Key-Area Research and Development Program of Guangdong Province (Grant No. 2018B030334001), and Natural Science Foundation of Tianjin (Grant No. 18JCQNJC10900).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the participants who served as research participants.
Footnotes
References
Apkarian, V. A., Hashmi, J. A., and Baliki, M. N. (2011). Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain 152(3 Suppl.), S49–S64. doi: 10.1016/j.pain.2010.11.010
Bekhuis, E., Boschloo, L., Rosmalen, J. G., de Boer, M. K., and Schoevers, R. A. (2016). The impact of somatic symptoms on the course of major depressive disorder. J. Affect. Disord. 205, 112–118. doi: 10.1016/j.jad.2016.06.030
Bell-McGinty, S., Butters, M. A., Meltzer, C. C., Greer, P. J., Reynolds, C. F. III, and Becker, J. T. (2002). Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. Am. J. Psychiatry 159, 1424–1427.
Blankstein, U., Chen, J., Diamant, N. E., and Davis, K. D. (2010). Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology 138, 1783–1789. doi: 10.1053/j.gastro.2009.12.043
Bluhm, R., Williamson, P., Lanius, R., Théberge, J., Densmore, M., Bartha, R., et al. (2009). Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin. Neurosci. 63, 754–761. doi: 10.1111/j.1440-1819.2009.02030.x
Bozhilova, N. S., Michelini, G., Kuntsi, J., and Asherson, P. (2018). Mind wandering perspective on attention-deficit/hyperactivity disorder. Neurosci. Biobehav. Rev. 92, 464–476. doi: 10.1016/j.neubiorev.2018.07.010
Cavanna, A. E. (2007). The precuneus and consciousness. CNS Spectr. 12, 545–552. doi: 10.1017/s1092852900021295
Cavanna, A. E., and Trimble, M. R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129(Pt 3), 564–583. doi: 10.1093/brain/awl004
Chao-Gan, Y., and Yu-Feng, Z. (2010). DPARSF: a MATLAB toolbox for “Pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 4:13. doi: 10.3389/fnsys.2010.00013
Chen, J. D., Liu, F., Xun, G. L., Chen, H. F., Hu, M. R., Guo, X. F., et al. (2012). Early and late onset, first-episode, treatment-naive depression: same clinical symptoms, different regional neural activities. J. Affect. Disord. 143, 56–63. doi: 10.1016/j.jad.2012.05.025
Chen, Y., Wang, C., Zhu, X., Tan, Y., and Zhong, Y. (2015). Aberrant connectivity within the default mode network in first-episode, treatment-naive major depressive disorder. J. Affect. Disord. 183, 49–56. doi: 10.1016/j.jad.2015.04.052
Corbetta, M., and Shulman, G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215. doi: 10.1038/nrn755
Cui, X., Guo, W., Wang, Y., Yang, T. X., Yang, X. H., Wang, Y., et al. (2017). Aberrant default mode network homogeneity in patients with first-episode treatment-naive melancholic depression. Int. J. Psychophysiol. 112, 46–51. doi: 10.1016/j.ijpsycho.2016.12.005
Depping, M. S., Wolf, N. D., Vasic, N., Sambataro, F., Hirjak, D., Thomann, P. A., et al. (2016). Abnormal cerebellar volume in acute and remitted major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 71, 97–102. doi: 10.1016/j.pnpbp.2016.06.005
Drossman, D. A., Creed, F. H., Olden, K. W., Svedlund, J., Toner, B. B., and Whitehead, W. E. (1999). Psychosocial aspects of the functional gastrointestinal disorders. Gut 45, (Suppl. 2), Ii25–Ii30. doi: 10.1136/gut.45.2008.ii25
Du, Y., Pearlson, G. D., Liu, J., Sui, J., Yu, Q., He, H., et al. (2015). A group ICA based framework for evaluating resting fMRI markers when disease categories are unclear: application to schizophrenia, bipolar, and schizoaffective disorders. Neuroimage 122, 272–280. doi: 10.1016/j.neuroimage.2015.07.054
Fan, F., Tan, S., Huang, J., Chen, S., Fan, H., Wang, Z., et al. (2020). Functional disconnection between subsystems of the default mode network in schizophrenia. Psychol. Med. [Epub ahead of print]. doi: 10.1017/s003329172000416x
Fan, W., Zhang, S., Hu, J., Liu, B., Wen, L., Gong, M., et al. (2019). Aberrant brain function in active-stage ulcerative colitis patients: a resting-state functional MRI study. Front. Hum. Neurosci. 13:107. doi: 10.3389/fnhum.2019.00107
Fang, J., Rong, P., Hong, Y., Fan, Y., Liu, J., Wang, H., et al. (2016). Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol. Psychiatry 79, 266–273. doi: 10.1016/j.biopsych.2015.03.025
Farmer, M. A., Baliki, M. N., and Apkarian, A. V. (2012). A dynamic network perspective of chronic pain. Neurosci. Lett. 520, 197–203. doi: 10.1016/j.neulet.2012.05.001
Fond, G., Loundou, A., Hamdani, N., Boukouaci, W., Dargel, A., Oliveira, J., et al. (2014). Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 264, 651–660. doi: 10.1007/s00406-014-0502-z
Foster, J. A., and McVey Neufeld, K. A. (2013). Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 36, 305–312. doi: 10.1016/j.tins.2013.01.005
García-Campayo, J., Ayuso-Mateos, J. L., Caballero, L., Romera, I., Aragonés, E., Rodríguez-Artalejo, F., et al. (2008). Relationship of somatic symptoms with depression severity, quality of life, and health resources utilization in patients with major depressive disorder seeking primary health care in Spain. Prim. Care Companion J. Clin. Psychiatry 10, 355–362. doi: 10.4088/pcc.v10n0502
Geng, J., Yan, R., Shi, J., Chen, Y., Mo, Z., Shao, J., et al. (2019). Altered regional homogeneity in patients with somatic depression: a resting-state fMRI study. J. Affect. Disord. 246, 498–505. doi: 10.1016/j.jad.2018.12.066
Gong, Q., Wu, Q., Scarpazza, C., Lui, S., Jia, Z., Marquand, A., et al. (2011). Prognostic prediction of therapeutic response in depression using high-field MR imaging. Neuroimage 55, 1497–1503. doi: 10.1016/j.neuroimage.2010.11.079
Greenberg, P. E., Fournier, A. A., Sisitsky, T., Pike, C. T., and Kessler, R. C. (2015). The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J. Clin. Psychiatry 76, 155–162. doi: 10.4088/JCP.14m09298
Guo, W., Cui, X., Liu, F., Chen, J., Xie, G., Wu, R., et al. (2018a). Decreased interhemispheric coordination in the posterior default-mode network and visual regions as trait alterations in first-episode, drug-naive major depressive disorder. Brain Imaging Behav. 12, 1251–1258. doi: 10.1007/s11682-017-9794-8
Guo, W., Cui, X., Liu, F., Chen, J., Xie, G., Wu, R., et al. (2018b). Increased anterior default-mode network homogeneity in first-episode, drug-naive major depressive disorder: a replication study. J. Affect. Disord. 225, 767–772. doi: 10.1016/j.jad.2017.08.089
Guo, W., Liu, F., Xue, Z., Gao, K., Liu, Z., Xiao, C., et al. (2013b). Abnormal resting-state cerebellar-cerebral functional connectivity in treatment-resistant depression and treatment sensitive depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 44, 51–57. doi: 10.1016/j.pnpbp.2013.01.010
Guo, W., Liu, F., Dai, Y., Jiang, M., Zhang, J., Yu, L., et al. (2013a). Decreased interhemispheric resting-state functional connectivity in first-episode, drug-naive major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 41, 24–29. doi: 10.1016/j.pnpbp.2012.11.003
Guo, W., Liu, F., Zhang, Z., Liu, G., Liu, J., Yu, L., et al. (2015b). Increased cerebellar functional connectivity with the default-mode network in unaffected siblings of schizophrenia patients at rest. Schizophr. Bull. 41, 1317–1325. doi: 10.1093/schbul/sbv062
Guo, W., Liu, F., Liu, J., Yu, M., Zhang, Z., Liu, G., et al. (2015a). Increased cerebellar-default-mode-network connectivity in drug-naive major depressive disorder at rest. Medicine 94:e560. doi: 10.1097/md.0000000000000560
Guo, W., Yao, D., Jiang, J., Su, Q., Zhang, Z., Zhang, J., et al. (2014). Abnormal default-mode network homogeneity in first-episode, drug-naive schizophrenia at rest. Prog. Neuropsychopharmacol. Biol. Psychiatry 49, 16–20. doi: 10.1016/j.pnpbp.2013.10.021
Hahamy, A., Calhoun, V., Pearlson, G., Harel, M., Stern, N., Attar, F., et al. (2014). Save the global: global signal connectivity as a tool for studying clinical populations with functional magnetic resonance imaging. Brain Connect. 4, 395–403. doi: 10.1089/brain.2014.0244
Hamilton, M. (1967). Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 6, 278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x
Hare, S. M., Ford, J. M., Mathalon, D. H., Damaraju, E., Bustillo, J., Belger, A., et al. (2019). Salience-default mode functional network connectivity linked to positive and negative symptoms of schizophrenia. Schizophr. Bull. 45, 892–901. doi: 10.1093/schbul/sby112
Hillilä, M. T., Hämäläinen, J., Heikkinen, M. E., and Färkkilä, M. A. (2008). Gastrointestinal complaints among subjects with depressive symptoms in the general population. Aliment. Pharmacol. Ther. 28, 648–654. doi: 10.1111/j.1365-2036.2008.03771.x
Hu, M. L., Zong, X. F., Mann, J. J., Zheng, J. J., Liao, Y. H., Li, Z. C., et al. (2017). A review of the functional and anatomical default mode network in schizophrenia. Neurosci. Bull. 33, 73–84. doi: 10.1007/s12264-016-0090-1
Hyett, M. P., Perry, A., Breakspear, M., Wen, W., and Parker, G. B. (2018). White matter alterations in the internal capsule and psychomotor impairment in melancholic depression. PLoS One 13:e0195672. doi: 10.1371/journal.pone.0195672
Kano, M., Dupont, P., Aziz, Q., and Fukudo, S. (2018). Understanding neurogastroenterology from neuroimaging perspective: a comprehensive review of functional and structural brain imaging in functional gastrointestinal disorders. J. Neurogastroenterol. Motil. 24, 512–527. doi: 10.5056/jnm18072
Kim, S. M., Hong, J. S., Min, K. J., and Han, D. H. (2019). Brain functional connectivity in patients with somatic symptom disorder. Psychosom. Med. 81, 313–318. doi: 10.1097/psy.0000000000000681
King, A. P., Block, S. R., Sripada, R. K., Rauch, S., Giardino, N., Favorite, T., et al. (2016). ALTERED DEFAULT MODE NETWORK (DMN) RESTING STATE FUNCTIONAL CONNECTIVITY FOLLOWING A MINDFULNESS-BASED EXPOSURE THERAPY FOR POSTTRAUMATIC STRESS DISORDER (PTSD) IN COMBAT VETERANS OF AFGHANISTAN AND IRAQ. Depress. Anxiety 33, 289–299. doi: 10.1002/da.22481
Kirmayer, L. J., Robbins, J. M., Dworkind, M., and Yaffe, M. J. (1993). Somatization and the recognition of depression and anxiety in primary care. Am. J. Psychiatry 150, 734–741. doi: 10.1176/ajp.150.5.734
Kochar, B., Barnes, E. L., Long, M. D., Cushing, K. C., Galanko, J., Martin, C. F., et al. (2018). Depression is associated with more aggressive inflammatory bowel disease. Am. J. Gastroenterol. 113, 80–85. doi: 10.1038/ajg.2017.423
Kwan, C. L., Diamant, N. E., Pope, G., Mikula, K., Mikulis, D. J., and Davis, K. D. (2005). Abnormal forebrain activity in functional bowel disorder patients with chronic pain. Neurology 65, 1268–1277. doi: 10.1212/01.wnl.0000180971.95473.cc
Lee, C., Doo, E., Choi, J. M., Jang, S. H., Ryu, H. S., Lee, J. Y., et al. (2017). The increased level of depression and anxiety in irritable bowel syndrome patients compared with healthy controls: systematic review and meta-analysis. J. Neurogastroenterol. Motil. 23, 349–362. doi: 10.5056/jnm16220
Liu, P., Li, G., Zhang, A., Yang, C., Liu, Z., Sun, N., et al. (2020). Brain structural and functional alterations in MDD patient with gastrointestinal symptoms: a resting-state MRI study. J. Affect. Disord. 273, 95–105. doi: 10.1016/j.jad.2020.03.107
Ma, C., Ding, J., Li, J., Guo, W., Long, Z., Liu, F., et al. (2012). Resting-state functional connectivity bias of middle temporal gyrus and caudate with altered gray matter volume in major depression. PLoS One 7:e45263. doi: 10.1371/journal.pone.0045263
Malhi, G. S., and Mann, J. J. (2018). Depression. Lancet 392, 2299–2312. doi: 10.1016/s0140-6736(18)31948-2
Martin-Subero, M., Fuentes-Claramonte, P., Salgado-Pineda, P., Salavert, J., Arevalo, A., Bosque, C., et al. (2021). Autobiographical memory and default mode network function in schizophrenia: an fMRI study. Psychol. Med. 51, 121–128. doi: 10.1017/s0033291719003052
Mayer, E. A., Tillisch, K., and Gupta, A. (2015). Gut/brain axis and the microbiota. J. Clin. Invest. 125, 926–938. doi: 10.1172/jci76304
Miller, D. R., Hayes, S. M., Hayes, J. P., Spielberg, J. M., Lafleche, G., and Verfaellie, M. (2017). Default mode network subsystems are differentially disrupted in posttraumatic stress disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 363–371. doi: 10.1016/j.bpsc.2016.12.006
Navabi, S., Gorrepati, V. S., Yadav, S., Chintanaboina, J., Maher, S., Demuth, P., et al. (2018). Influences and impact of anxiety and depression in the setting of inflammatory bowel disease. Inflamm. Bowel Dis. 24, 2303–2308. doi: 10.1093/ibd/izy143
Novick, D., Montgomery, W., Aguado, J., Kadziola, Z., Peng, X., Brugnoli, R., et al. (2013). Which somatic symptoms are associated with an unfavorable course in Asian patients with major depressive disorder? J. Affect. Disord. 149, 182–188. doi: 10.1016/j.jad.2013.01.020
Painchault, C., Brignone, M., Lamy, F. X., Diamand, F., and Saragoussi, D. (2014). Economic Burden of Major Depressive Disorder (Mdd) in five european countries: description of resource use by health state. Value Health 17:A465. doi: 10.1016/j.jval.2014.08.1300
Peng, J., Liu, J., Nie, B., Li, Y., Shan, B., Wang, G., et al. (2011). Cerebral and cerebellar gray matter reduction in first-episode patients with major depressive disorder: a voxel-based morphometry study. Eur. J. Radiol. 80, 395–399. doi: 10.1016/j.ejrad.2010.04.006
Posner, J., Cha, J., Wang, Z., Talati, A., Warner, V., Gerber, A., et al. (2016). Increased default mode network connectivity in individuals at high familial risk for depression. Neuropsychopharmacology 41, 1759–1767. doi: 10.1038/npp.2015.342
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., and Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154. doi: 10.1016/j.neuroimage.2011.10.018
Qi, R., Ke, J., Schoepf, U. J., Varga-Szemes, A., Milliken, C. M., Liu, C., et al. (2016). Topological reorganization of the default mode network in irritable bowel syndrome. Mol. Neurobiol. 53, 6585–6593. doi: 10.1007/s12035-015-9558-7
Raichle, M. E. (2015). The brain’s default mode network. Annu. Rev. Neurosci. 38, 433–447. doi: 10.1146/annurev-neuro-071013-014030
Sani, I., Stemmann, H., Caron, B., Bullock, D., Stemmler, T., Fahle, M., et al. (2021). The human endogenous attentional control network includes a ventro-temporal cortical node. Nat. Commun. 12:360. doi: 10.1038/s41467-020-20583-5
Scott, K. M., Bruffaerts, R., Tsang, A., Ormel, J., Alonso, J., Angermeyer, M. C., et al. (2007). Depression-anxiety relationships with chronic physical conditions: results from the World Mental Health Surveys. J. Affect. Disord. 103, 113–120. doi: 10.1016/j.jad.2007.01.015
Shan, X., Liao, R., Ou, Y., Ding, Y., Liu, F., Chen, J., et al. (2020). Metacognitive training modulates default-mode network homogeneity during 8-week olanzapine treatment in patients with schizophrenia. Front. Psychiatry 11:234. doi: 10.3389/fpsyt.2020.00234
Sheline, Y. I., Price, J. L., Yan, Z., and Mintun, M. A. (2010). Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. U.S.A. 107, 11020–11025. doi: 10.1073/pnas.1000446107
Shi, Y., Li, J., Feng, Z., Xie, H., Duan, J., Chen, F., et al. (2020). Abnormal functional connectivity strength in first-episode, drug-naive adult patients with major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 97:109759. doi: 10.1016/j.pnpbp.2019.109759
Sidlauskaite, J., Sonuga-Barke, E., Roeyers, H., and Wiersema, J. R. (2016). Default mode network abnormalities during state switching in attention deficit hyperactivity disorder. Psychol. Med. 46, 519–528. doi: 10.1017/s0033291715002019
Skrobisz, K., Piotrowicz, G., Naumczyk, P., Sabisz, A., Markiet, K., Rydzewska, G., et al. (2020). Imaging of morphological background in selected functional and inflammatory gastrointestinal diseases in fMRI. Front. Psychiatry 11:461. doi: 10.3389/fpsyt.2020.00461
Song, G. H., Venkatraman, V., Ho, K. Y., Chee, M. W., Yeoh, K. G., and Wilder-Smith, C. H. (2006). Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain 126, 79–90. doi: 10.1016/j.pain.2006.06.017
Steardo, L. Jr., Carbone, E. A., de Filippis, R., Pisanu, C., Segura-Garcia, C., Squassina, A., et al. (2020). Application of support vector machine on fMRI data as biomarkers in schizophrenia diagnosis: a systematic review. Front. Psychiatry 11:588. doi: 10.3389/fpsyt.2020.00588
Swets, J. A. (1988). Measuring the accuracy of diagnostic systems. Science 240, 1285–1293. doi: 10.1126/science.3287615
Viard, A., Mutlu, J., Chanraud, S., Guenolé, F., Egler, P. J., Gérardin, P., et al. (2019). Altered default mode network connectivity in adolescents with post-traumatic stress disorder. Neuroimage Clin. 22:101731. doi: 10.1016/j.nicl.2019.101731
Whitehead, W. E. (1996). Psychosocial aspects of functional gastrointestinal disorders. Gastroenterol. Clin. North Am. 25, 21–34. doi: 10.1016/s0889-8553(05)70363-0
Yan, C. G., Chen, X., Li, L., Castellanos, F. X., Bai, T. J., Bo, Q. J., et al. (2019). Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc. Natl. Acad. Sci. U.S.A. 116, 9078–9083. doi: 10.1073/pnas.1900390116
Zhi, D., Calhoun, V. D., Lv, L., Ma, X., Ke, Q., Fu, Z., et al. (2018). Aberrant dynamic functional network connectivity and graph properties in major depressive disorder. Front. Psychiatry 9:339. doi: 10.3389/fpsyt.2018.00339
Keywords: major depressive disorder, default mode network, network homogeneity, gastrointestinal symptoms, magnetic resonance imaging
Citation: Yan M, Chen J, Liu F, Li H, Zhao J and Guo W (2022) Abnormal Default Mode Network Homogeneity in Major Depressive Disorder With Gastrointestinal Symptoms at Rest. Front. Aging Neurosci. 14:804621. doi: 10.3389/fnagi.2022.804621
Received: 29 October 2021; Accepted: 01 March 2022;
Published: 30 March 2022.
Edited by:
Wei Deng, Affiliated Mental Health Center and Hangzhou Seventh People’s Hospital, ChinaReviewed by:
Zhiliang Long, Southwest University, ChinaJiajia Zhu, The First Affiliated Hospital of Anhui Medical University, China
Copyright © 2022 Yan, Chen, Liu, Li, Zhao and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenbin Guo, guowenbin76@csu.edu.cn
 Meiqi Yan1
Meiqi Yan1  Feng Liu
Feng Liu Jingping Zhao
Jingping Zhao Wenbin Guo
Wenbin Guo