- Department of Urology, Morsani College of Medicine, University of South Florida, Tampa, FL, United States
Background: Spindle cell lipomas are uncommon variants of typical lipomas, and presentation within the spermatic cord is exceedingly rare. Only three other cases have been reported in medical literature.
Case Presentation: We present the fourth case of spindle cell lipoma of the spermatic cord, which in this particular case had imaging findings suggestive of a malignant liposarcoma prompting radical orchiectomy. Histological investigation later concluded that this represented a benign, spindle cell lipoma of the spermatic cord as evidence by positive CD34 staining and microscopic analysis.
Conclusion: Although this particular variant of lipoma is rare, it is the hope that these findings contribute to the continued study of this disease process and can potentially prompt further evaluation prior to radical orchiectomy to limit patient morbidity.
Background
Spindle cell lipoma (SCL) is a soft tissue neoplasm first described in 1975 that is typically found on the back, shoulders, and neck of adult males around 40–70 years of age (1). The incidence of spindle cell lipoma in any location is rare, accounting for only 1.5% of all lipomas and even more rare as presentation in the spermatic cord with a literature search demonstrating only a handful of case reports (1). Here, we present a case of spermatic cord spindle cell lipoma to add to the current literature and discuss the current diagnostic paradigm.
Case Report
We present a 65-year-old male with a past medical history significant for benign prostatic hyperplasia status-postgreen light laser ablation, Peyronie’s disease, and bilateral inguinal hernia repair that initially presented for evaluation of recurrent inguinal hernia and found to have a scrotal cord mass on cross-sectional imaging.
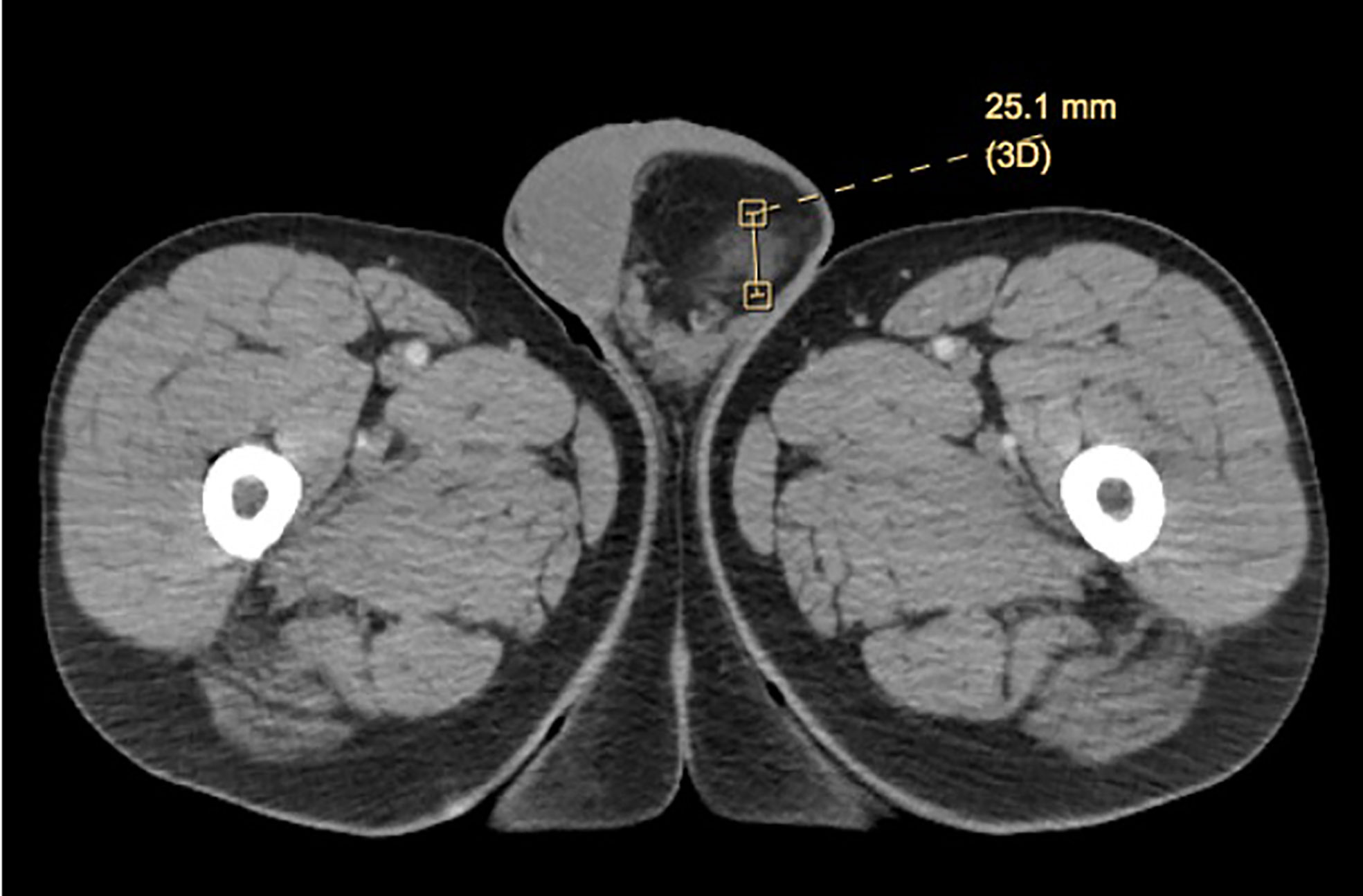
Patient initially presented in the community with left groin bulge in the setting of prior inguinal hernia repair. On initial examination, no discrete testicular mass was described. His initial work-up included a scrotal ultrasound, which was significant for fat containing left inguinal hernia with extension into the scrotal sac and a small left varicocele. A subsequent computed tomography (CT) scan to further characterize the left inguinal hernia showed a 6.7 × 6.1 × 5.8-cm mass suggestive of possible scrotal lipomatosis or spermatic cord lipoma on the left, containing a 2.5-cm ill-defined area of soft tissue attenuation and possible low-grade liposarcoma (Figure 1). No inguinal hernia was noted on the CT scan.
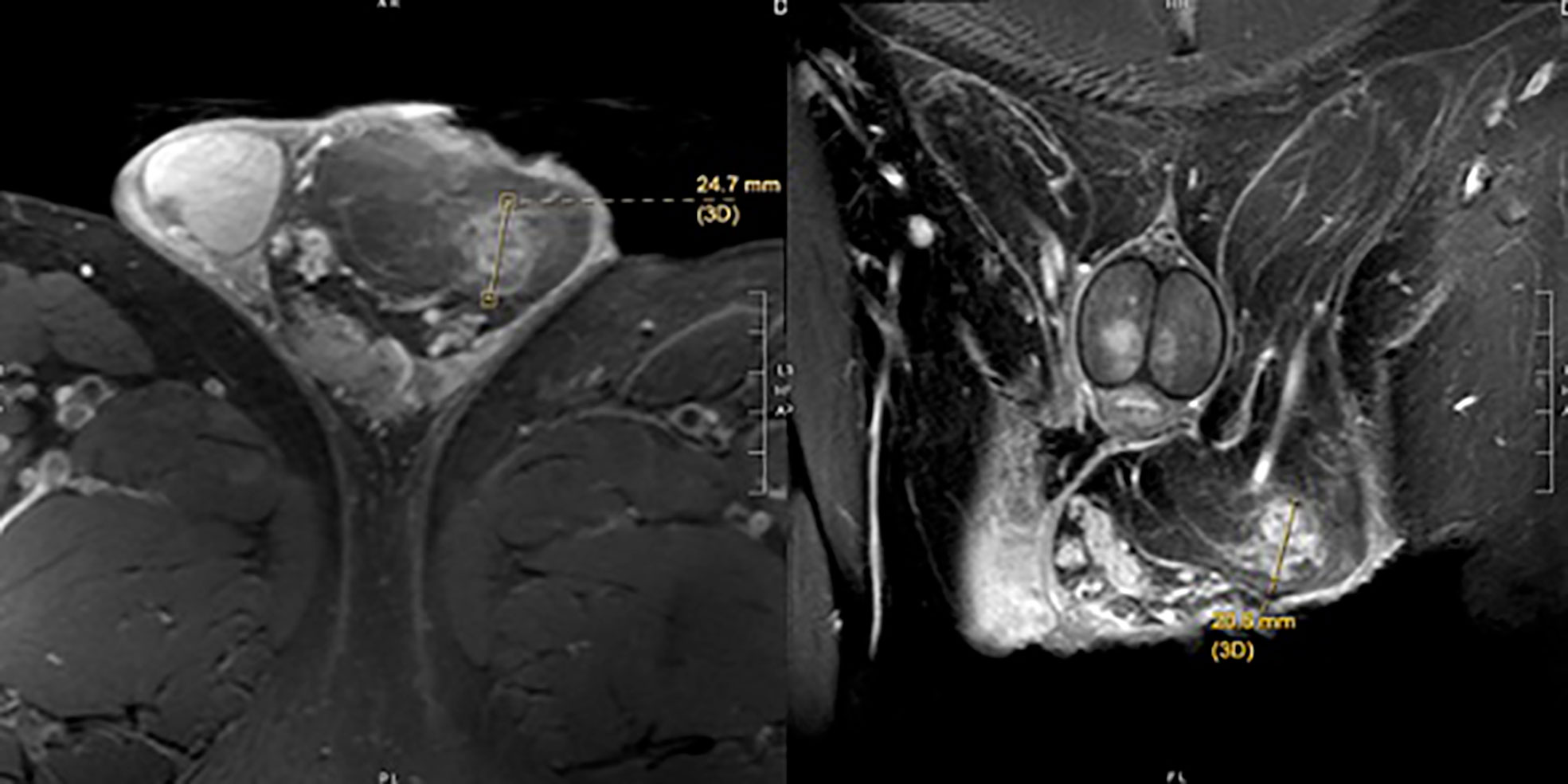
He was thus referred to a tertiary care center for further evaluation and management. On physical examination, there was left-sided scrotal fullness consistent with possible cord or paratesticular lipoma, but no discrete intratesticular mass. Testicular serum tumor markers and a pelvic magnetic resonance imaging (MRI) were obtained for further evaluation. Serum tumor markers resulted as LDH 180 IU/L, AFP 6.5 ng/ml, and beta-HCG <1 mIU/ml. Pelvic MRI was significant for an 8 × 6 × 3.5-cm left spermatic cord lipoma within the scrotum and a 2.5-cm region of the left spermatic cord concerning for liposarcoma, confirming the findings on CT scan (Figure 2).
The patient underwent an uneventful left inguinal radical orchiectomy with high ligation of the spermatic cord in the usual manner. The specimen was examined on the back table and a discrete mass was palpated in the left mid/distal spermatic cord. Final pathology of the mass was spindle cell/pleomorphic lipoma with positive immunoperoxidase stain for cluster of differentiation (CD)34 and negative S100 stain, supporting the diagnosis.
Discussion and Conclusions
Spindle cell lipoma is a distinct histological variant of lipoma, characterized by the replacement of mature fat by collagen forming spindle cells (2). It is generally described clinically as a solid, benign neoplasm that often presents as a solitary, subcutaneous, well circumcised lesion that can appear at various locations in the body. The incidence of spindle cell lipoma occurring in the scrotum or spermatic cord is extraordinarily rare, and based on an extensive review of the literature, with only a few cases reported.
On magnetic resonance imaging (MRI), lipomas typically demonstrate high fat signal, but can demonstrate varying amounts of fat, which can cause concern as lower levels of fat are indicative of liposarcoma (3). These ambiguous findings preclude the diagnostic certainty necessary to make a diagnosis on imaging alone, and thus pathologic diagnosis is still recommended. The ultimate outcome of this, often, as in prior case reports and ours, involves a radical orchiectomy for an ultimately benign disease process. Consideration can be given to frozen section analysis at time of orchiectomy, though it is unclear how precise this may be in providing a definitive diagnosis given the rare nature of the lesion.
While the etiology of spindle cell lipoma is unclear, it can be easily identified microscopically. Fine-needle aspiration cytology (FNAC) can be used to accurately diagnose these lipomas by recognizing their histologic features, vascularity, and cytology; however these features may not always be present in the aspiration specimen. Dey et al. report a positive predictive value of 91.5%–95.5% in soft tissue tumors, demonstrating the utility of FNAC in patients with these lesions (4). Open biopsies have demonstrated a diagnostic accuracy of 88%–100%, however are also associated with increased complications (5–7). Optimal diagnostic and management results in patients with these tumors are achieved when examination of these lesions occur in multidisciplinary centers with access to the requisite expertise (5). Histologically, this neoplasm is characterized by mature adipocytes and small uniform spindle cells, interspersed with eosinophilic collagen bundles within a myxoid stroma (2). The spindle cells are positive for CD34 but negative for S-100 protein on immunohistochemistry stains, consistent with what we report. However, CD34 is not specific for spindle cell lipomas and thus is of somewhat limited diagnostic utility (8). Spindle cell lipomas are desmin negative and express losses of chromosomes 12q and/or 16q, which may help the diagnosis (8). On the other hand, well-differentiated liposarcoma, which was considerably high in our differential, does not stain positive for CD34, which can be a helpful method of differentiating the two entities (9). Additionally, because liposarcomas have a propensity for local recurrence, dissemination through lymphatics and direct invasion into surrounding tissue is more common than vascular or hematogenous spreading (10).
Here, we present the fourth case of spindle cell lipoma of the spermatic cord in published literature to date. While spindle cell lipoma is a rare, benign lesion as we outline here, it can mimic malignant liposarcoma of the scrotum in regard to its imaging characteristics. In our case, imaging was suggestive enough to warrant proceeding with radical orchiectomy. Fortunately, given the benign nature of the disease, no further treatment is warranted following excision of the primary lesion.
This case presents with a very rare diagnosis of spindle cell/pleomorphic lipoma of the spermatic cord. This case demonstrates only the fourth documented case in medical literature. Additionally, what further emphasizes the importance of this diagnosis is the appearance on imaging that initially suggested a malignant low-grade liposarcoma. In the latter, typical management involves radical orchiectomy, with potential for adjuvant radiation with need for ongoing surveillance, which can be starkly contrasted to the former which is a benign lesion without evidence in the literature to suggest risk of recurrence. Although this particular variant of lipoma is rare, it is the hope that these findings contribute to the continued study of this disease process and can potentially prompt further evaluation prior to radical orchiectomy to limit patient morbidity.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors listed have made substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SCL, spindle-cell lipoma; CT, computed tomography; MRI, magnetic resonance imaging; CD, cluster of differentiation.
References
1. Enzinger FM, Harvey DA. Spindle Cell Lipoma. Cancer (1975) 36(5):1852–9. doi: 10.1002/1097-0142(197511)36:5<1852::AID-CNCR2820360542>3.0.CO;2-U
2. Domanski HA, Carlén B, Jonsson K, Mertens F, Åkerman M. Distinct Cytologic Features of Spindle Cell Lipoma. Cancer (2001) 93(6):381–9. doi: 10.1002/cncr.10142
3. Kirwadi A, Abdul-Halim R, Fernando M, Highland A, Kotnis N. MR Imaging Features of Spindle Cell Lipoma. Skeletal Radiol (2014) 43(2):191–6. doi: 10.1007/s00256-013-1765-6
4. Dey P, Mallik MK, Gupta SK, Vasishta RK. Role of Fine Needle Aspiration Cytology in the Diagnosis of Soft Tissue Tumours and Tumour-Like Lesions. Cytopathology (2004) 15(1):32–7. doi: 10.1046/j.0956-5507.2003.00102.x
5. Skrzynski MC, Biermann JS, Montag A, Simon MA. Diagnostic Accuracy and Charge-Savings of Outpatient Core Needle Biopsy Compared With Open Biopsy of Musculoskeletal Tumors. J Bone Joint Surg Am (1996) 78(5):644–9. doi: 10.2106/00004623-199605000-00002
6. Clayer M. Open Incisional Biopsy Is a Safe and Accurate Technique for Soft Tissue Tumours. ANZ J Surg (2010) 80(11):786–8. doi: 10.1111/j.1445-2197.2010.05340.x
7. Kasraeian S, Allison DC, Ahlmann ER, Fedenko AN, Menendez LR. A Comparison of Fine-Needle Aspiration, Core Biopsy, and Surgical Biopsy in the Diagnosis of Extremity Soft —Tissue Masses. Clin Orthop Relat Res (2010) 468(11):2992–3002. doi: 10.1007/s11999-010-1401-x
8. Templeton SF, Solomon AR. Spindle Cell Lipoma Is Strongly CD34 Positive. An Immunohistochemical Study. J Cutan Pathol (1996) 23(6):546–50. doi: 10.1111/j.1600-0560.1996.tb01447.x
9. Thway K, Flora R, Shah C, Olmos D, Fisher C. Diagnostic Utility of P16, CDK4, and MDM2 as an Immunohistochemical Panel in Distinguishing Well-Differentiated and Dedifferentiated Liposarcomas From Other Adipocytic Tumors. Am J Surg Pathol (2012) 36(3):462–9. doi: 10.1097/PAS.0b013e3182417330
Keywords: Spindle Cell, lipoma, sarcoma, spermatic cord, testicular cancer
Citation: Dahmen A, Juwono T, Russo NW and Patel T (2022) Spindle Cell Lipoma of the Spermatic Cord: A Case Presentation and Literature Review of a Urologic Rarity and Radiologic Mimic of Malignant Liposarcoma. Front. Urol. 2:790522. doi: 10.3389/fruro.2022.790522
Received: 06 October 2021; Accepted: 03 January 2022;
Published: 25 January 2022.
Edited by:
James Eastham, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Richard Matulewicz, Memorial Sloan Kettering Cancer Center, United StatesRui Almeida Pinto, Centro Hospitalar São Joao, Portugal
Copyright © 2022 Dahmen, Juwono, Russo and Patel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicholas W. Russo, nicholasrusso@usf.edu
 Aaron Dahmen
Aaron Dahmen Timothy Juwono
Timothy Juwono Nicholas W. Russo
Nicholas W. Russo Trushar Patel
Trushar Patel