- 1Department of Rheumatology and Immunology, Peking University People’s Hospital, Beijing, China
- 2Beijing Key Laboratory for Rheumatism and Immune Diagnosis (BZ0135), Peking University People’s Hospital, Beijing, China
- 3Department of Rheumatology and Immunology, Gangkou Hospital of Hebei Port Group Company Limited, Qinhuangdao, Hebei, China
Antiphospholipid syndrome (APS) is a systemic autoimmune disease characterized by thrombosis and pregnancy morbidity with the persistent presence of antiphospholipid antibodies (aPLs). Although anticoagulation is the primary treatment for APS, it fails in approximately 20-30% of obstetric APS cases and more than 30% of thrombotic APS cases. Therefore, there is a need for new, targeted treatments beyond anticoagulants. Biologics, such as rituximab and eculizumab, have been recommended for refractory catastrophic APS. This review focuses on the recent advancements in the pathogenesis of APS and explores the potential of targeted treatments, including eculizumab, rituximab, belimumab, daratumumab, obinutuzumab, and anti-TNF-α antibodies, for APS management.
Introduction
Antiphospholipid syndrome (APS) is a systemic autoimmune disease, characterized by thrombosis or recurrent pregnancy morbidity, with the persistent presence of antiphospholipid antibodies (aPLs) (1, 2). APS can occur as an isolated condition (primary APS, PAPS) or secondary to systemic lupus erythematosus (SLE) or other rheumatic diseases (SAPS) (3, 4).
The clinical manifestations of APS include thrombosis, obstetrical complications and “noncriteria” manifestations (2). Thrombosis can cause occlusive events in venous, arterial, or microvascular systems (5). Catastrophic APS (CAPS) affects approximately 1% of APS patients and can cause multiple thromboses of medium and small arteries, leading to fulminant multiple vital organ dysfunction (6–9). Obstetrical complications often involve unexplained, consecutive spontaneous abortions, fetal death, or premature birth due to eclampsia, severe preeclampsia, and intrauterine growth restriction (3, 10, 11). In addition, other clinical manifestations, known as “noncriteria” manifestations, include thrombocytopenia (2), hemolytic anemia (5), livedo reticularis (12), accelerated atherosclerosis and cardiac valve disease (13), nephropathy (14), neurological impairment (2), and bone necrosis (12).
Anticoagulation therapy is considered a fundamental cornerstone of APS management (15). However, conventional prevention and treatment strategies fail in approximately 20-30% of obstetric APS and more than 30% of thrombotic APS cases (16, 17). Furthermore, traditional medications are often ineffective in treating CAPS, refractory APS, and noncriteria manifestations (1, 2, 18–20).
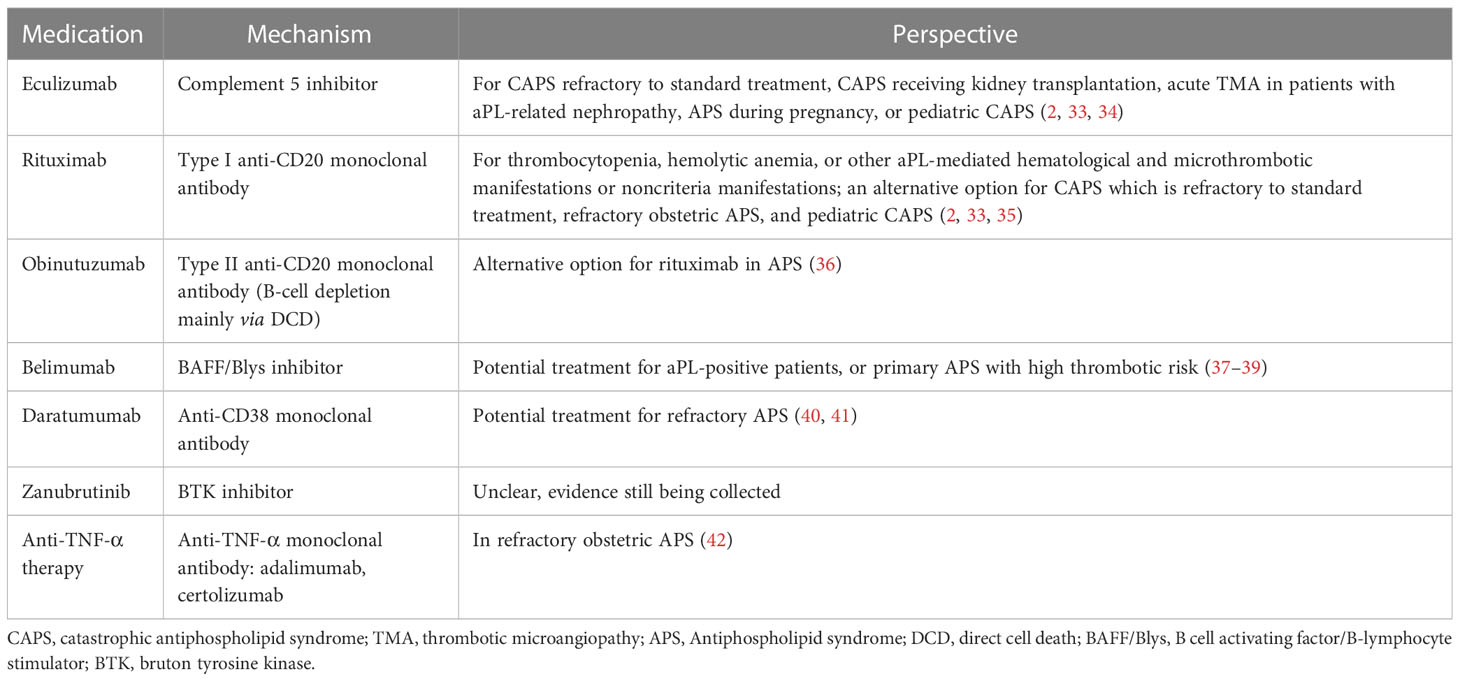
The understanding of APS pathogenesis has grown in recent years, prompting investigation into new targeted therapies (2, 7). Multiple mechanisms have been proposed, such as B cell-mediated production of aPLs, particularly anti-β2-glycoprotein I antibody (21, 22); activation of complement (23–26); and stimulation of endothelial cells (27), platelets (28, 29), neutrophils (30, 31), and monocytes (32). This review focuses on biologics as potential targeted therapies for APS based on its underlying mechanisms. We will discuss possible biologics beyond antithrombotic agents (Table 1).
Pathogenesis of APS
The pathogenesis of APS supports the use of biologics as a targeted treatment approach. A brief overview is presented in Figure 1. In APS, aPLs primarily target β2-glycoprotein I (β2GPI), a plasma protein that binds to phospholipids. Binding of aPLs to β2GPI on the surface of endothelial cells upregulates the expression of prothrombotic cellular adhesion molecules, such as E-selectin and tissue factor (TF) (43). Notably, aPLs against β2GPI disrupt the binding of annexin A5 to phospholipid bilayers, which accelerates coagulation reactions. Annexin A5 is an anticoagulant that binds to phospholipid bilayers, impeding coagulation reactions. It does so by forming an anticoagulant shield that hinders the accessibility of anionic phospholipids (44, 45). In addition, aPLs binding to β2GPI suppress the inhibitors of tissue factor pathway (46), reduce the activity of protein C (2), and activate complement (2).
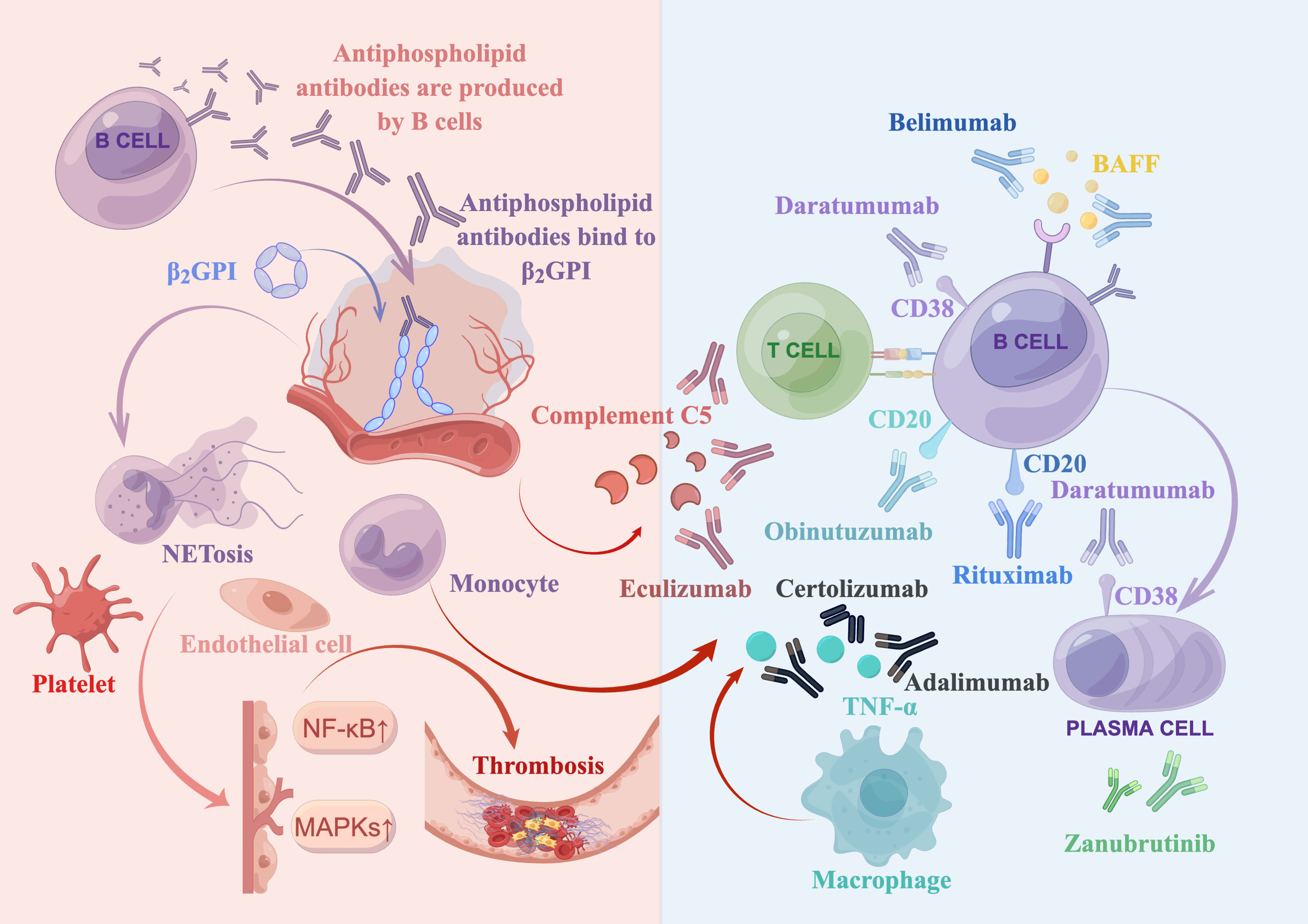
Figure 1 Summary of antiphospholipid syndrome pathogenesis and biologics treatments. Antiphospholipid antibodies, produced by B cells, bind to open and immunogenic β2-glycoprotein I (β2GPI) on the surface of endothelial cells. This leads to the activation of various target cells, such as complement cells, platelets, monocytes (including macrophages that secrete TNF-α), and neutrophils (which release neutrophil extracellular traps [NETosis]). Moreover, it upregulates the mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-κB) pathways, ultimately resulting in thrombosis. Several biologics have been developed to target various factors involved in this process. These include a complement 5 inhibitor (eculizumab), a type I anti-CD20 monoclonal antibody (rituximab), a type II anti-CD20 monoclonal antibody (obinutuzumab), a B cell activating factor (BAFF) inhibitor (belimumab), an anti-CD38 monoclonal antibody (daratumumab), anti-TNF-α monoclonal antibodies (adalimumab and certolizumab), and a bruton tyrosine kinase (BTK) inhibitor (zanubrutinib, which is currently being investigated in clinical trials).
The translocation of aPLs into late endosomes, aided by annexin A2 and multiple toll-like receptors, may contribute to various pathogenic effects (47). These include the activation of multiple target cells through mitogen-activated protein kinases (MAPKs) and the nuclear factor kappa B (NF-kB) (48, 49). Platelets also play a critical role in the prothrombotic interactions between aPLs and endothelial cells (50). Additionally, active neutrophils release tissue factor, neutrophil extracellular traps (NETosis) and interleukin-8 (IL-8) that are involved in thrombosis (2, 30, 31). Furthermore, monocytes in patients with APS can express high levels of tissue factor (2). Besides, aPLs may induce upregulation of the mechanistic target of rapamycin (mTOR) complex on endothelial cells, which is associated with vasculopathy (2).
To summarize, aPLs produced by B cells bind to anionic surfaces of cell membranes and convert the closed β2GPI to the open, immunogenic conformation. Via translocation or other interaction mechanism, aPLs induce a proinflammatory or prothrombotic phenotype of endothelial cells, complement, and other cells (such as platelets, neutrophils, and monocytes). This leads to inflammation, vascular thrombosis, pregnancy complications, and multiple organ dysfunction through multiple mechanisms, such as reduction of protein C activity and suppression of tissue factor inhibitor (2, 33).
Rituximab
Mechanisms of rituximab
Rituximab is a chimeric monoclonal antibody that specifically targets CD20 on B cells. Its use in APS is based on the crucial role of B cells in the pathogenesis of the disease (51). Studies have indicated that rituximab may lower aPL titers, associated with B-cell regulatory effects (as rituximab cannot deplete plasma cell directly) (51, 52). In addition, rituximab may inhibit the expression of inducible co-stimulator (ICOS), which can suppress the activation of T helper cells in the development of APS (53, 54).
Application of rituximab in APS
Rituximab is recommended for use in APS (12), particularly in cases of refractory CAPS (18), or CAPS with noncriteria manifestations such as acute kidney injury and severe thrombocytopenia (55–57). A real-world study of 22 APS patients who received either standard (≥1000 mg overall) or low (<1000 mg overall) dose rituximab showed that after a 6-month follow up, 19 patients achieved varying degrees of remission, with significant decreases in anticardiolipin antibody (aCL) titer and erythrocyte sedimentation rate level (58). Additionally, a recent case report suggested that rituximab may improve renal function and result in a negative aPLs profile in patients with refractory CAPS (59).
Antiphospholipid antibody levels have been shown to be significantly reduced in refractory cases following treatment with rituximab (5). In a systematic review of 20 patients with CAPS who received rituximab in combination with a first-line treatment for aggressive clinical presentation, vascular disease, or noncriteria manifestations, fifteen of them recovered from the acute episode, and 4 patients achieved a negative aPLs profile (60). Even without anticoagulation, rituximab has been shown to improve kidney function and cerebral edema in CAPS patients (61). In a retrospective study of 63 patients with SLE-associated APS, 6 patients who received rituximab therapy showed no relapse of thrombosis and a reduction in lupus activity (62). A study of 24 PAPS patients receiving rituximab found a 75% response rate (63). Furthermore, rituximab has been shown to improve multiple vascular or noncriteria manifestations in APS, including venous thrombosis (52, 64–67), arterial thrombosis (68), thrombocytopenia (66, 69), hemolytic anemia (70), pulmonary hemorrhage (71, 72), CAPS (71, 73), and overlap syndrome (74). Additionally, rituximab may benefit APS patients with concurrent malignancy (75). Notably, in a pregnant woman with primary APS who had severe thrombocytopenia and did not respond conventional therapy, weekly rituximab administration from 12 to 15 weeks of gestation increased platelet count while decreasing aCL titer, resulting in a successful pregnancy without preeclampsia or fetal growth restriction (76). Therefore, rituximab may be able to control disease activity and reduce pregnancy complications, particularly for refractory APS cases.
Limitations
After receiving rituximab retreatment, two patients reported a thrombotic exacerbation. In both cases, it is believed that the immune complex, composed of rituximab and human anti-chimeric antibody (HACA), activated the complement process, resulting in a prothrombotic state in APS. Additionally, it is possible that infusion reactions contributed to the development of transverse myelitis in one of the patients (77). It is important to note that placental transfer of rituximab can affect the development of fetal and neonatal B cells, which could increase susceptibility to infections. Therefore, rituximab should be discontinued at least 6 months before conception (78). Other potential complications of rituximab treatment include neurological injury, infections, recurrence of arterial thromboembolic events, and rarely severe bone pain (79). However, long-term investigations have shown that adverse effects were consistent between the placebo and rituximab cohort, and serious infections or infusion reactions did not increase over time (80).
Because rituximab does not directly eliminate plasma cells, its use may lead to insufficient suppression of aPL-producing plasma cells in patients with APS (81). In combination therapy, other treatments targeting plasma cells may be considered. Generally, rituximab is not recommended as a first-line treatment for CAPS patients due to uncertainty regarding long-term efficacy or adverse effects, as well as high cost (12, 82). The widespread utility of rituximab in aPL-positive patients still requires further investigation.
Obinutuzumab
Mechanisms of obinutuzumab
Obinutuzumab is a type II anti-CD20 monoclonal antibody that can induce potent direct cell death (DCD) by rupturing lysosomes (83). Due to its ability to avoid rapid internalization like rituximab, this typical type II anti-CD20 antibody may induce more effective antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) (84).
Alternative option for rituximab
Previous studies have shown that obinutuzumab was more effective than rituximab at inducing B cell depletion during in vitro whole blood assays, even in the presence of excess B cell activating factor (BAFF) (84, 85). This suggests that obinutuzumab may be a viable treatment option for APS cases that are resistant to rituximab. In fact, a patient with APS and SLE who was resistant to rituximab showed a remarkable positive response to obinutuzumab treatment (36).
Limitations
Obinutuzumab has shown promise as an alternative to rituximab in patients with refractory APS; however, larger clinical trials and additional data are necessary to confirm potential adverse effects. It is important to note that the impact of obinutuzumab on pregnancy or conception is currently unknown.
Belimumab
Mechanisms of belimumab
Belimumab is a monoclonal antibody targeting the soluble circulating BAFF (7). As previously mentioned, B cells play a crucial role in the pathogenesis of APS. It is noteworthy that APS patients have increased levels of BAFF (86, 87). Additionally, research on murine models has shown that inhibiting BAFF can lead to B cell depletion and an improvement in clinical manifestations (51, 88).
Reducing aPL titers and ameliorating vascular symptoms
Belimumab has recently been found to induce negativity for aPLs in SAPS patients (37). Although initially used in 2 patients with microthrombotic APS manifestations with incomplete response, both patients experienced clinical improvement and stopped taking corticosteroids (38). A post hoc analysis of two randomized placebo-controlled trials in SLE confirmed its effectiveness in reducing aPLs in APS (39). Belimumab may be beneficial in managing APS patients with high thrombotic risk or aPL-positive patients with microthrombotic manifestations (87). Moreover, belimumab has been shown to significantly improve thrombocytopenia in an APS patient who was unresponsive to corticosteroids and rituximab (89).
Limitations
Limited evidence has demonstrated that belimumab is not teratogenic (90), however, current evidence is insufficient to be completely confident that it is compatible with pregnancy; therefore, the use of belimumab should be stopped at conception (78). Currently, belimumab may be considered as a management strategy for severe maternal disease in pregnancy if no other pregnancy-compatible drugs are suitable (78, 90).
Eculizumab
Mechanisms of eculizumab
Unrestricted activation of the complement cascade is involved in CAPS (91–93). The formation of membrane attack complex (MAC) triggers endothelial cell apoptosis, expression of inflammatory cytokines, vascular basement membrane collagen exposure, and platelet aggregation (6, 7, 94). During this process, eculizumab can interrupt formation of MAC by preventing the cleavage of complement C5 (19, 95).
Efficacy in CAPS treatment
Eculizumab can improve outcomes of refractory CAPS described in previous case reports (18, 96–102). When rituximab and immunoglobulins fail to improve platelet count and renal function, eculizumab may be an effective alternative supplement (103, 104). It has been reported that thrombocytopenia or kidney injury in CAPS patients with thrombotic microangiopathy (TMA) may respond to eculizumab (91, 105). A recent systematic review of the efficacy of eculizumab in treatment of TMA confirmed that it resulted in 100% hematological response and 85% kidney recovery (106). In patients with CAPS, eculizumab has resulted in prevention of recurrent APS and rescuing renal allografts after kidney transplantation (96, 98, 107–111). Interestingly, eculizumab may prevent TMA associated with CAPS caused by COVID-19 infection (112). Therefore, eculizumab may be considered effective in APS patients with CAPS to prevent complications.
Application in obstetric APS
Previous case reports have shown no obvious fetal or maternal complications when eculizumab is used during pregnancy (113–115). Recently, two APS patients were reported to have received eculizumab treatment at the end of their pregnancies, demonstrating successful prevention of potentially fatal APS-related complications (116, 117). There was no thrombosis, detectable organ damage, and infectious complications during the postpartum period, with negligible levels of eculizimab detected in the infant (116). The aforementioned case reports revealed that eculizumab may be a viable option for APS treatment during pregnancy and delivery. However, more research is required to ensure its safety and efficacy.
Limitations
Currently, clinical data consists mainly of case reports or series, which may contain significant bias. The 16th International Congress on aPL Task Force Report on APS Treatment Trends highlighted the importance of the addition of eculizumab for the treatment of CAPS, refractory APS with TMA, and obstetric APS (42). However, it remains to be proved whether general aPL-positive patients will respond effectively to eculizumab (92).
In addition, there are safety considerations in the administration of eculizumab, including Neisseria meningitis infections (118) and other encapsulated organisms (e.g., Streptococcus pneumoniae and Haemophilus influenza). Therefore, patients should be vaccinated prior to eculizumab treatment (119). Another concern is the extremely high cost, which exceeds $500,000 per year (120). Due to the high economic burden, the use of eculizumab in prevention of CASP should only be available when standard treatments have failed.
Daratumumab
Mechanisms of daratumumab
CD38 is a glycoprotein which is highly expressed on transmembrane region of plasma cells, functioning as an adhesion molecule, ectoenzyme, and receptor for activation or proliferation signals (121, 122). CD38 antibodies can attack plasma cells directly (through disruption of calcium influx and signal transduction) as well as through Fc-dependent immune-effector mechanisms (complement-dependent cytotoxicity [CDC], ADCC, and ADCP) (122–124).
Application in autoimmune-mediated disease
In recent years, daratumumab has been employed in management of autoimmune-mediated diseases, including APS, SLE, and rheumatoid arthritis (RA) (125). Daratumumab has been effectively used to deplete plasma cells and plasmablasts of patients with SLE and RA in a dose-dependent manner ex vivo (125). A clinically significant improvement was observed in a patient with APS who had recurrent venous thromboembolic events despite anticoagulant therapy. The aPL levels declined significantly and continued to decrease over the next three months (40).
Combination therapy with rituximab
A new drug-free macromolecular therapy has been developed to simultaneously target CD20 and CD38. This therapy consists of crosslinked rituximab and daratumumab using biorecognition of the morpholino oligonucleotide-modified antibody Fab’ fragment and a multivalent effector motif. The goal of this therapy is to address the limitations of standard monoclonal antibodies and induce better ADCC and CDC effects. The complementary effects have been observed in terms of apoptosis induction and degree of synergism (126).
Recommendations and limitations
Daratumumab may be suitable for APS patients who are unresponsive to anticoagulant therapy and standard immunosuppression (40, 41). Due to daratumumab’s ability to only suppress long-lived plasma cells, sustainable responses will rely on disallowing the regeneration of autoreactive plasma cells. Therefore, long-term maintenance therapies, such as belimumab may be required (41). While only a few case reports have provided rationales for targeting of CD38 in autoimmune diseases, large clinical trials, as well as appropriate treatment schedules and populations are still needed (41). It is worth noting that the adverse effects of daratumumab on pregnancy or conception remain unknown.
Anti-TNF-α therapy
Mechanisms of anti-TNF-α therapy
APLs may increase the expression of TNF-α by stimulating monocytes (127), which can lead to an increase in tissue factor production (128). In vitro studies have shown that adalimumab, a TNF-α blocker, completely inhibits anti-β2GPI-induced TF expression in monocytes (129). Studies conducted on mouse models have demonstrated that elevated levels of TNF-α levels in placental tissues are linked with abnormal placenta and pregnancy loss, and that blocking TNF-α can improve endothelial dysfunction and prevent pregnancy loss (130–132).
Application in obstetric APS
In a recent case series, eighteen aPL-positive women with obstetric APS which were refractory to low molecular weight heparin, aspirin, and hydroxychloroquine were treated with adalimumab or certolizumab. Positive obstetric results were obtained in 70% of patients. TNF-α blockers were all well tolerated without adverse effects (133).
Limitations and adverse effects
There have been reports of APS induction during anti-TNF therapy (134–136). While recent guidelines indicate that anti-TNF-α drugs can be used safely throughout pregnancy due to their very low level of transplacental transit (78), it is important to note that TNF-α blockers may have potential adverse effects (42, 137). Therefore, the rational use of these drugs should be limited to refractory cases of APS.
Clinical trials for various monoclonal antibodies
We have compiled information on clinical trials for various biologics that are either completed or still recruiting. This is due to the high-level of evidence classification and recommendation in clinical trials. Some of these biologics have already been thoroughly introduced above, while others may be simply included due to their presently unknown effects.
A completed phase II clinical trial focused on eculizumab enabling renal transplantation in patients with a history of APS or CAPS, but no results were posted (clinicaltrials.gov#: NCT01029587). In an open-label, prospective pilot study of rituximab in 19 patients with primary APS, although the decrease of aPL titers could not be observed, the rituximab was found to be effective in controlling noncriteria manifestations such as skin ulcers, nephropathy, cognitive dysfunction, and thrombocytopenia. Twelve serious adverse events involving hospitalization in 7 patients were recorded (79). An ongoing open-label, prospective, phase II descriptive pilot trial is evaluating belimumab therapy for refractory or noncriteria manifestations of APS (clinicaltrials.gov#: NCT05020782). Additionally, a phase II clinical trial is currently evaluating the addition of certolizumab to usual treatment (a heparin agent and low-dose aspirin) in pregnant women with APS (clinicaltrials.gov#: NCT03152058).
Bruton tyrosine kinase (BTK) plays a significant role in regulating B cell proliferation, survival, differentiation, and cytokine expression (138), as well as influencing platelet activation (139). As such, zanubrutinib, a BTK inhibitor, is being studied in a prospective, single-arm, open-label clinical trial for the treatment of APS with secondary thrombocytopenia (clinicaltrials.gov#: NCT05199909). Furthermore, an open-label, phase II trial investigating the use of the complement C5 inhibitor ALXN1007 for the treatment of noncriteria manifestations of APS was terminated due to low patient enrollment (clinicaltrials.gov#: NCT02128269).
Conclusions
Although anticoagulation remains the cornerstone of APS treatment, significant attention is being drawn towards studying a number of biologics. It is important to note that biologics are primarily reserved for patients with refractory APS or CAPS. However, the accuracy of biologics make them a promising option for the development of optimal therapies for personalized medicine. Innovative biologic-focused therapeutic approaches are being investigated to treat APS, which may ultimately reduce mortality rates among individuals with APS.
Author contributions
ZY and LD: reviewing of literature, performing analysis, writing of the original draft and editing. QC and XL: reviewing of literature. CL: conceptualization, methodology, supervision, manuscript editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in part by the China International Medical Foundation (No. Z-2018-40-2101) and the Beijing Municipal Science and Technology Projects (No. Z191100006619110).
Conflict of interest
LD is employed by Hebei Port Group Company Limited.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Radin M, Cecchi I, Rubini E, Foddai SG, Barinotti A, Menegatti E, et al. Treatment of antiphospholipid syndrome. Clin Immunol (2020) 221:108597. doi: 10.1016/j.clim.2020.108597
2. Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med (2018) 378(21):2010–21. doi: 10.1056/NEJMra1705454
3. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost (2006) 4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x
4. Ünlü O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol (2016) 3(2):75–84. doi: 10.5152/eurjrheum.2015.0085
5. Chaturvedi S, McCrae KR. Diagnosis and management of the antiphospholipid syndrome. Blood Rev (2017) 31(6):406–17. doi: 10.1016/j.blre.2017.07.006
6. Cervera R, Rodriguez-Pinto I, Espinosa G. The diagnosis and clinical management of the catastrophic antiphospholipid syndrome: a comprehensive review. J Autoimmun (2018) 92:1–11. doi: 10.1016/j.jaut.2018.05.007
7. Dobrowolski C, Erkan D. Treatment of antiphospholipid syndrome beyond anticoagulation. Clin Immunol (2019) 206:53–62. doi: 10.1016/j.clim.2018.03.001
8. Chaturvedi S, Braunstein EM, Brodsky RA. Antiphospholipid syndrome: complement activation, complement gene mutations, and therapeutic implications. J Thromb Haemost (2021) 19(3):607–16. doi: 10.1111/jth.15082
9. Rodríguez-Pintó I, Espinosa G, Erkan D, Shoenfeld Y, Cervera R. The effect of triple therapy on the mortality of catastrophic anti-phospholipid syndrome patients. Rheumatol (Oxford) (2018) 57(7):1264–70. doi: 10.1093/rheumatology/key082
10. Schreiber K, Hunt BJ. Managing antiphospholipid syndrome in pregnancy. Thromb Res (2019) 181:S41–S6. doi: 10.1016/S0049-3848(19)30366-4
11. Schreiber K, Sciascia S, de Groot PG, Devreese K, Jacobsen S, Ruiz-Irastorza G, et al. Antiphospholipid syndrome. Nat Rev Dis Primers (2018) 4:17103. doi: 10.1038/nrdp.2017.103
12. Uthman I, Noureldine MHA, Ruiz-Irastorza G, Khamashta M. Management of antiphospholipid syndrome. Ann Rheum Dis (2019) 78(2):155–61. doi: 10.1136/annrheumdis-2018-213846
13. Denas G, Jose SP, Bracco A, Zoppellaro G, Pengo V. Antiphospholipid syndrome and the heart: a case series and literature review. Autoimmun Rev (2015) 14(3):214–22. doi: 10.1016/j.autrev.2014.11.003
14. Sciascia S, Cuadrado MJ, Khamashta M, Roccatello D. Renal involvement in antiphospholipid syndrome. Nat Rev Nephrol (2014) 10(5):279–89. doi: 10.1038/nrneph.2014.38
15. Cohen H, Efthymiou M, Devreese KMJ. Monitoring of anticoagulation in thrombotic antiphospholipid syndrome. J Thromb Haemost (2021) 19(4):892–908. doi: 10.1111/jth.15217
16. Giacomelli R, Afeltra A, Bartoloni E, Berardicurti O, Bombardieri M, Bortoluzzi A, et al. The growing role of precision medicine for the treatment of autoimmune diseases; results of a systematic review of literature and experts' consensus. Autoimmun Rev (2021) 20(2):102738. doi: 10.1016/j.autrev.2020.102738
17. Cervera R, Serrano R, Pons-Estel GJ, Ceberio-Hualde L, Shoenfeld Y, de Ramón E, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis (2015) 74(6):1011–8. doi: 10.1136/annrheumdis-2013-204838
18. Erkan D. Expert perspective: management of microvascular and catastrophic antiphospholipid syndrome. Arthritis Rheumatol (2021) 73(10):1780–90. doi: 10.1002/art.41891
19. Erton ZB, Erkan D. Treatment advances in antiphospholipid syndrome: 2022 update. Curr Opin Pharmacol (2022) 65:102212. doi: 10.1016/j.coph.2022.102212
20. Scoble T, Wijetilleka S, Khamashta MA. Management of refractory anti-phospholipid syndrome. Autoimmun Rev (2011) 10(11):669–73. doi: 10.1016/j.autrev.2011.04.030
21. Dieudonné Y, Guffroy A, Poindron V, Sprauel PS, Martin T, Korganow AS, et al. B cells in primary antiphospholipid syndrome: review and remaining challenges. Autoimmun Rev (2021) 20(5):102798. doi: 10.1016/j.autrev.2021.102798
22. Cervera R. Antiphospholipid syndrome. Thromb Res (2017) 151:S43–S7. doi: 10.1016/S0049-3848(17)30066-X
23. Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest (2003) 112(11):1644–54. doi: 10.1172/JCI200318817
24. Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med (2004) 10(11):1222–6. doi: 10.1038/nm1121
25. Fischetti F, Durigutto P, Pellis V, Debeus A, Macor P, Bulla R, et al. Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood (2005) 106(7):2340–6. doi: 10.1182/blood-2005-03-1319
26. Pierangeli SS, Girardi G, Vega-Ostertag M, Liu X, Espinola RG, Salmon J. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheumatol (2005) 52(7):2120–4. doi: 10.1002/art.21157
27. Zhang J, McCrae KR. Annexin A2 mediates endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood (2005) 105(5):1964–9. doi: 10.1182/blood-2004-05-1708
28. Shi T, Giannakopoulos B, Yan X, Yu P, Berndt MC, Andrews RK, et al. Anti-beta2-glycoprotein I antibodies in complex with beta2-glycoprotein I can activate platelets in a dysregulated manner via glycoprotein ib-IX-V. Arthritis Rheumatol (2006) 54(8):2558–67. doi: 10.1002/art.21968
29. Galli M, Finazzi G, Barbui T. Thrombocytopenia in the antiphospholipid syndrome. Br J Haematol (1996) 93(1):1–5. doi: 10.1046/j.1365-2141.1996.390969.x
30. Meng H, Yalavarthi S, Kanthi Y, Mazza LF, Elfline MA, Luke CE, et al. In vivo role of neutrophil extracellular traps in antiphospholipid antibody-mediated venous thrombosis. Arthritis Rheumatol (2017) 69(3):655–67. doi: 10.1002/art.39938
31. Yalavarthi S, Gould TJ, Rao AN, Mazza LF, Morris AE, Núñez-Álvarez C, et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol (2015) 67(11):2990–3003. doi: 10.1002/art.39247
32. Sorice M, Longo A, Capozzi A, Garofalo T, Misasi R, Alessandri C, et al. Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheumatol (2007) 56(8):2687–97. doi: 10.1002/art.22802
33. Mezhov V, Segan JD, Tran H, Cicuttini FM. Antiphospholipid syndrome: a clinical review. Med J Aust (2019) 211(4):184–8. doi: 10.5694/mja2.50262
34. Oulego-Erroz I, Martínez-Sáenz de Jubera J, Ocaña-Alcober C, Regueras-Santos L, Ferrero-De la Mano L, Martínez-Badás JP. Pediatric catastrophic antiphospholipid syndrome successfully treated with eculizumab. Am J Respir Crit Care Med (2021) 203(5):640–2. doi: 10.1164/rccm.202009-3489LE
35. Groot N, de Graeff N, Avcin T, Bader-Meunier B, Dolezalova P, Feldman B, et al. European Evidence-based recommendations for diagnosis and treatment of paediatric antiphospholipid syndrome: the SHARE initiative. Ann Rheum Dis (2017) 76(10):1637–41. doi: 10.1136/annrheumdis-2016-211001
36. Kvacskay P, Merkt W, Günther J, Blank N, Lorenz HM. Obinutuzumab in connective tissue diseases after former rituximab-non-response: a case series. Ann Rheum Dis (2022) 81(5):744–6. doi: 10.1136/annrheumdis-2021-221756
37. Sciascia S, Rubini E, Radin M, Cecchi I, Rossi D, Roccatello D. Anticardiolipin and anti-beta 2 glycoprotein-I antibodies disappearance in patients with systemic lupus erythematosus and antiphospholipid syndrome while on belimumab. Ann Rheum Dis (2018) 77(11):1694–5. doi: 10.1136/annrheumdis-2018-213496
38. Yazici A, Yazirli B, Erkan D. Belimumab in primary antiphospholipid syndrome. Lupus (2017) 26(10):1123–4. doi: 10.1177/0961203316682102
39. Chatzidionysiou K, Samoli E, Sfikakis PP, Tektonidou MG. Effect of belimumab treatment on antiphospholipid antibody levels: post-hoc analysis based on two randomised placebo-controlled trials in systemic lupus erythematosus. Ann Rheum Dis (2020) 79(2):304–7. doi: 10.1136/annrheumdis-2019-216367
40. Pleguezuelo DE, Díaz-Simón R, Cabrera-Marante O, Lalueza A, Paz-Artal E, Lumbreras C, et al. Case report: resetting the humoral immune response by targeting plasma cells with daratumumab in anti-phospholipid syndrome. Front Immunol (2021) 12:667515. doi: 10.3389/fimmu.2021.667515
41. Ostendorf L, Burns M, Durek P, Heinz GA, Heinrich F, Garantziotis P, et al. Targeting CD38 with daratumumab in refractory systemic lupus erythematosus. N Engl J Med (2020) 383(12):1149–55. doi: 10.1056/NEJMoa2023325
42. Cohen H, Cuadrado MJ, Erkan D, Duarte-Garcia A, Isenberg DA, Knight JS, et al. 16th international congress on antiphospholipid antibodies task force report on antiphospholipid syndrome treatment trends. Lupus (2020) 29(12):1571–93. doi: 10.1177/0961203320950461
43. Vega-Ostertag M, Liu X, Kwan-Ki H, Chen P, Pierangeli S. A human monoclonal antiprothrombin antibody is thrombogenic in vivo and upregulates expression of tissue factor and e-selectin on endothelial cells. Br J Haematol (2006) 135(2):214–9. doi: 10.1111/j.1365-2141.2006.06283.x
44. Rand JH, Wu XX, Quinn AS, Ashton AW, Chen PP, Hathcock JJ, et al. Hydroxychloroquine protects the annexin A5 anticoagulant shield from disruption by antiphospholipid antibodies: evidence for a novel effect for an old antimalarial drug. Blood (2010) 115(11):2292–9. doi: 10.1182/blood-2009-04-213520
45. Wahezi DM, Ilowite NT, Wu XX, Pelkmans L, Laat B, Schanberg LE, et al. Annexin A5 anticoagulant activity in children with systemic lupus erythematosus and the association with antibodies to domain I of β2-glycoprotein I. Lupus (2013) 22(7):702–11. doi: 10.1177/0961203313490241
46. Liestøl S, Sandset PM, Jacobsen EM, Mowinckel MC, Wisløff F. Decreased anticoagulant response to tissue factor pathway inhibitor type 1 in plasmas from patients with lupus anticoagulants. Br J Haematol (2007) 136(1):131–7. doi: 10.1111/j.1365-2141.2006.06385.x
47. Brandt KJ, Kruithof EK, de Moerloose P. Receptors involved in cell activation by antiphospholipid antibodies. Thromb Res (2013) 132(4):408–13. doi: 10.1016/j.thromres.2013.08.015
48. Zhou H, Chen D, Xie H, Xia L, Wang T, Yuan W, et al. Activation of MAPKs in the anti-β2GPI/β2GPI-induced tissue factor expression through TLR4/IRAKs pathway in THP-1 cells. Thromb Res (2012) 130(4):e229–35. doi: 10.1016/j.thromres.2012.08.303
49. Chighizola CB, Raschi E, Borghi MO, Meroni PL. Update on the pathogenesis and treatment of the antiphospholipid syndrome. Curr Opin Rheumatol (2015) 27(5):476–82. doi: 10.1097/BOR.0000000000000200
50. Proulle V, Furie RA, Merrill-Skoloff G, Furie BC, Furie B. Platelets are required for enhanced activation of the endothelium and fibrinogen in a mouse thrombosis model of APS. Blood (2014) 124(4):611–22. doi: 10.1182/blood-2014-02-554980
51. Khattri S, Zandman-Goddard G, Peeva E. B-cell directed therapies in antiphospholipid antibody syndrome–new directions based on murine and human data. Autoimmun Rev (2012) 11(10):717–22. doi: 10.1016/j.autrev.2011.12.011
52. Emmi G, Urban ML, Scalera A, Becatti M, Fiorillo C, Silvestri E, et al. Repeated low-dose courses of rituximab in SLE-associated antiphospholipid syndrome: data from a tertiary dedicated centre. Semin Arthritis Rheumatol (2017) 46(4):e21–e3. doi: 10.1016/j.semarthrit.2016.08.002
53. Iwata S, Saito K, Tokunaga M, Yamaoka K, Nawata M, Yukawa S, et al. Phenotypic changes of lymphocytes in patients with systemic lupus erythematosus who are in longterm remission after b cell depletion therapy with rituximab. J Rheumatol (2011) 38(4):633–41. doi: 10.3899/jrheum.100729
54. Rauch J, Salem D, Subang R, Kuwana M, Levine JS. β2-glycoprotein I-reactive T cells in autoimmune disease. Front Immunol (2018) 9:2836. doi: 10.3389/fimmu.2018.02836
55. Nath K, McCann A. Immunomodulation in the treatment of refractory catastrophic antiphospholipid syndrome. Case Rep Hematol (2018) 2018:1041396. doi: 10.1155/2018/1041396
56. Diószegi Á, Tarr T, Nagy-Vincze M, Nánásy-Vass M, Veisz R, Bidiga L, et al. Microthrombotic renal involvement in an SLE patient with concomitant catastrophic antiphospholipid syndrome: the beneficial effect of rituximab treatment. Lupus (2018) 27(9):1552–8. doi: 10.1177/0961203318768890
57. Stanescu C, Andronesi AG, Jurcut C, Gherghiceanu M, Vornicu A, Burcea FA, et al. Successful treatment of catastrophic antiphospholipid syndrome using rituximab: case report and review of the literature. Med (Kaunas) (2021) 57(9):912. doi: 10.3390/medicina57090912
58. Gan Y, Zhong X, Zhao Y, Li G, Ye H, Li C. Low dose versus standard dose rituximab for the treatment of antiphospholipid syndrome: a pilot study from a tertiary medical center. Front Immunol (2022) 13:971366. doi: 10.3389/fimmu.2022.971366
59. Rymarz A, Niemczyk S. The complex treatment including rituximab in the management of catastrophic antiphospholid syndrome with renal involvement. BMC Nephrol (2018) 19(1):132. doi: 10.1186/s12882-018-0928-z
60. Berman H, Rodríguez-Pintó I, Cervera R, Morel N, Costedoat-Chalumeau N, Erkan D, et al. Rituximab use in the catastrophic antiphospholipid syndrome: descriptive analysis of the CAPS registry patients receiving rituximab. Autoimmun Rev (2013) 12(11):1085–90. doi: 10.1016/j.autrev.2013.05.004
61. Liu LW, Tsai HH, Lafian A, Sadeghi-Najafabadi E. A case of probable catastrophic antiphospholipid syndrome treated with rituximab and without anticoagulation. J Clin Rheumatol (2021) 27(8s):S541–s2. doi: 10.1097/RHU.0000000000001078
62. Wang CR, Liu MF. Rituximab usage in systemic lupus erythematosus-associated antiphospholipid syndrome: a single-center experience. Semin Arthritis Rheumatol (2016) 46(1):102–8. doi: 10.1016/j.semarthrit.2016.02.002
63. Pons I, Espinosa G, Cervera R. [Efficacy and safety of rituximab in the treatment of primary antiphospholipid syndrome: analysis of 24 cases from the bibliography review]. Med Clin (Barc) (2015) 144(3):97–104. doi: 10.1016/j.medcli.2014.01.034
64. Costa R, Fazal S, Kaplan RB, Spero J, Costa R. Successful plasma exchange combined with rituximab therapy in aggressive APS-related cutaneous necrosis. Clin Rheumatol (2013) 32 Suppl 1:S79–82. doi: 10.1007/s10067-010-1506-3
65. Gkogkolou P, Ehrchen J, Goerge T. Severe antiphospholipid antibody syndrome - response to plasmapheresis and rituximab. J Dermatol Treat (2017) 28(6):564–6. doi: 10.1080/09546634.2017.1282599
66. Gamoudi D, Cutajar M, Gamoudi N, Camilleri DJ, Gatt A. Achieving a satisfactory clinical and biochemical response in antiphospholipid syndrome and severe thrombocytopenia with rituximab: two case reports. Clin Case Rep (2017) 5(6):845–8. doi: 10.1002/ccr3.946
67. Wang CR, Weng CT, Liu MF. Monocentric experience of the rituximab therapy in systemic lupus erythematosus-associated antiphospholipid syndrome with warfarin therapy failure. Semin Arthritis Rheumatol (2017) 47(1):e7–8. doi: 10.1016/j.semarthrit.2017.03.012
68. Sciascia S, Naretto C, Rossi D, Bazzan M, Roccatello D. Treatment-induced downregulation of antiphospholipid antibodies: effect of rituximab alone on clinical and laboratory features of antiphospholipid syndrome. Lupus (2011) 20(10):1106–8. doi: 10.1177/0961203311400115
69. Sciascia S, Radin M, Cecchi I, Rubini E, Bazzan M, Roccatello D. Long-term effect of b-cells depletion alone as rescue therapy for severe thrombocytopenia in primary antiphospholipid syndrome. Semin Arthritis Rheumatol (2019) 48(4):741–4. doi: 10.1016/j.semarthrit.2018.04.001
70. Rückert A, Glimm H, Lübbert M, Grüllich C. Successful treatment of life-threatening Evans syndrome due to antiphospholipid antibody syndrome by rituximab-based regimen: a case with long-term follow-up. Lupus (2008) 17(8):757–60. doi: 10.1177/0961203307087876
71. Scheiman Elazary A, Klahr PP, Hershko AY, Dranitzki Z, Rubinow A, Naparstek Y. Rituximab induces resolution of recurrent diffuse alveolar hemorrhage in a patient with primary antiphospholipid antibody syndrome. Lupus (2012) 21(4):438–40. doi: 10.1177/0961203311422713
72. Aakjær S, Bendstrup E, Ivarsen P, Madsen LB. Continous rituximab treatment for recurrent diffuse alveolar hemorrhage in a patient with systemic lupus erythematosus and antiphosholipid syndrome. Respir Med Case Rep (2017) 22:263–5. doi: 10.1016/j.rmcr.2017.09.012
73. Doğru A, Ugan Y, Şahin M, Karahan N, Tunç ŞE. Catastrophic antiphospholipid syndrome treated with rituximab: a case report. Eur J Rheumatol (2017) 4(2):145–7. doi: 10.5152/eurjrheum.2017.160073
74. Guiomar V, Oliveira D, Correia C, Pereira E. Efficacy of rituximab in refractory inflammatory myopathy associated with coexistence of behçet's disease and antiphospholipid syndrome. Eur J Case Rep Intern Med (2019) 6(11):001294. doi: 10.12890/2019_001294
75. Manner H, Jung B, Tonassi L, Hackenberg U, Plum N, Josten KM, et al. Successful treatment of catastrophic antiphospholipid antibody syndrome (CAPS) associated with splenic marginal-zone lymphoma with low-molecular weight heparin, rituximab and bendamustine. Am J Med Sci (2008) 335(5):394–7. doi: 10.1097/MAJ.0b013e31815203ad
76. Nagata M, Kaneko K, Kohno C, Mishima S, Okazaki Y, Murashima A. A case of successful pregnancy following multidrug treatment including rituximab and intravenous immunoglobulin for primary antiphospholipid antibody syndrome refractory to conventional treatment. Mod Rheumatol Case Rep (2020) 4(1):47–50. doi: 10.1080/24725625.2019.1648633
77. Suzuki K, Nagasawa H, Kameda H, Amano K, Kondo T, Itoyama S, et al. Severe acute thrombotic exacerbation in two cases with anti-phospholipid syndrome after retreatment with rituximab in phase I/II clinical trial for refractory systemic lupus erythematosus. Rheumatol (Oxford) (2009) 48(2):198–9. doi: 10.1093/rheumatology/ken421
78. Russell MD, Dey M, Flint J, Davie P, Allen A, Crossley A, et al. British Society for rheumatology guideline on prescribing drugs in pregnancy and breastfeeding: immunomodulatory anti-rheumatic drugs and corticosteroids. Rheumatol (Oxford) (2022) 62(4):e48-e88. doi: 10.1093/rheumatology/keac551
79. Erkan D, Vega J, Ramón G, Kozora E, Lockshin MD. A pilot open-label phase II trial of rituximab for non-criteria manifestations of antiphospholipid syndrome. Arthritis Rheumatol (2013) 65(2):464–71. doi: 10.1002/art.37759
80. van Vollenhoven RF, Emery P, Bingham CO, Keystone EC, Fleischmann R, Furst DE, et al. Longterm safety of patients receiving rituximab in rheumatoid arthritis clinical trials. J Rheumatol (2010) 37(3):558–67. doi: 10.3899/jrheum.090856
81. Hisada R, Kato M, Sugawara E, Kanda M, Fujieda Y, Oku K, et al. Circulating plasmablasts contribute to antiphospholipid antibody production, associated with type I interferon upregulation. J Thromb Haemost (2019) 17(7):1134–43. doi: 10.1111/jth.14427
82. Legault K, Schunemann H, Hillis C, Yeung C, Akl EA, Carrier M, et al. McMaster RARE-bestpractices clinical practice guideline on diagnosis and management of the catastrophic antiphospholipid syndrome. J Thromb Haemostasis (2018) 16(8):1656–64. doi: 10.1111/jth.14192
83. Klein C, Lammens A, Schäfer W, Georges G, Schwaiger M, Mössner E, et al. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. mAbs (2013) 5(1):22–33. doi: 10.4161/mabs.22771
84. Reddy V, Klein C, Isenberg DA, Glennie MJ, Cambridge G, Cragg MS, et al. Obinutuzumab induces superior b-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatol (Oxford) (2017) 56(7):1227–37. doi: 10.1093/rheumatology/kex067
85. Reddy VR, Pepper RJ, Shah K, Cambridge G, Henderson SR, Klein C, et al. Disparity in peripheral and renal b-cell depletion with rituximab in systemic lupus erythematosus: an opportunity for obinutuzumab? Rheumatol (Oxford) (2022) 61(7):2894–904. doi: 10.1093/rheumatology/keab827
86. Alvarez-Rodriguez L, Riancho-Zarrabeitia L, Calvo-Alén J, López-Hoyos M, Martínez-Taboada V. Peripheral b-cell subset distribution in primary antiphospholipid syndrome. Int J Mol Sci (2018) 19(2):589. doi: 10.3390/ijms19020589
87. van den Hoogen LL, Palla G, Bekker CPJ, Fritsch-Stork RDE, Radstake T, van Roon JAG. Increased b-cell activating factor (BAFF)/B-lymphocyte stimulator (BLyS) in primary antiphospholipid syndrome is associated with higher adjusted global antiphospholipid syndrome scores. RMD Open (2018) 4(2):e000693. doi: 10.1136/rmdopen-2018-000693
88. Kahn P, Ramanujam M, Bethunaickan R, Huang W, Tao H, Madaio MP, et al. Prevention of murine antiphospholipid syndrome by BAFF blockade. Arthritis Rheumatol (2008) 58(9):2824–34. doi: 10.1002/art.23764
89. Yoshizuka R, Hasegawa H, Kamiya M, Umezawa N, Yasuda S. Refractory antiphospholipid antibody syndrome-induced thrombocytopaenia successfully treated with belimumab. Lupus (2022) 31(5):624–7. doi: 10.1177/09612033221089138
90. Powell M, Hill D, Eudy A, Landy H, Petri M. OP0041 pregnancy outcomes for systemic lupus erythematosus (SLE) subjects with conception during belimumab intravenous (IV) and subcutaneous (SC) placebo-controlled clinical trials and long term extension trials. Ann Rheumatic Dis (2014) 73(Suppl 2):75–6. doi: 10.1136/annrheumdis-2014-eular.4484
91. Kello N, Khoury LE, Marder G, Furie R, Zapantis E, Horowitz DL. Secondary thrombotic microangiopathy in systemic lupus erythematosus and antiphospholipid syndrome, the role of complement and use of eculizumab: case series and review of literature. Semin Arthritis Rheumatol (2019) 49(1):74–83. doi: 10.1016/j.semarthrit.2018.11.005
92. Erkan D, Salmon JE. The role of complement inhibition in thrombotic angiopathies and antiphospholipid syndrome. Turk J Haematol (2016) 33(1):1–7. doi: 10.4274/tjh.2015.0197
93. Chaturvedi S, Braunstein EM, Yuan X, Yu J, Alexander A, Chen H, et al. Complement activity and complement regulatory gene mutations are associated with thrombosis in APS and CAPS. Blood (2020) 135(4):239–51. doi: 10.1182/blood.2019003863
94. Trouw LA, Pickering MC, Blom AM. The complement system as a potential therapeutic target in rheumatic disease. Nat Rev Rheumatol (2017) 13(9):538–47. doi: 10.1038/nrrheum.2017.125
95. Romay-Penabad Z, Liu XX, Montiel-Manzano G, De Martinez EP, Pierangeli SS. C5a receptor-deficient mice are protected from thrombophilia and endothelial cell activation induced by some antiphospholipid antibodies. Ann New York Acad Sci (2007) 1108(1):554–66. doi: 10.1196/annals.1422.058
96. Shapira I, Andrade D, Allen SL, Salmon JE. Brief report: induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheumatol (2012) 64(8):2719–23. doi: 10.1002/art.34440
97. López-Benjume B, Rodríguez-Pintó I, Amigo MC, Erkan D, Shoenfeld Y, Cervera R, et al. Eculizumab use in catastrophic antiphospholipid syndrome (CAPS): descriptive analysis from the "CAPS registry". Autoimmun Rev (2022) 21(4):103055. doi: 10.1016/j.autrev.2022.103055
98. Kronbichler A, Frank R, Kirschfink M, Szilágyi Á, Csuka D, Prohászka Z, et al. Efficacy of eculizumab in a patient with immunoadsorption-dependent catastrophic antiphospholipid syndrome: a case report. Med (Baltimore) (2014) 93(26):e143. doi: 10.1097/MD.0000000000000143
99. Zikos TA, Sokolove J, Ahuja N, Berube C. Eculizumab induces sustained remission in a patient with refractory primary catastrophic antiphospholipid syndrome. J Clin Rheumatol (2015) 21(6):311–3. doi: 10.1097/RHU.0000000000000290
100. Strakhan M, Hurtado-Sbordoni M, Galeas N, Bakirhan K, Alexis K, Elrafei T. 36-year-old female with catastrophic antiphospholipid syndrome treated with eculizumab: a case report and review of literature. Case Rep Hematol (2014) 2014:704371. doi: 10.1155/2014/704371
101. Guillot M, Rafat C, Buob D, Coppo P, Jamme M, Rondeau E, et al. Eculizumab for catastrophic antiphospholipid syndrome-a case report and literature review. Rheumatol (Oxford) (2018) 57(11):2055–7. doi: 10.1093/rheumatology/key228
102. Tinti MG, Carnevale V, Inglese M, Molinaro F, Bernal M, Migliore A, et al. Eculizumab in refractory catastrophic antiphospholipid syndrome: a case report and systematic review of the literature. Clin Exp Med (2019) 19(3):281–8. doi: 10.1007/s10238-019-00565-8
103. Nauseef JT, Lim HI, DeSancho MT. Successful outcome with eculizumab treatment in a patient with antiphospholipid syndrome presenting with an unusual thrombotic storm. J Thromb Thrombolysis (2021) 52(2):597–600. doi: 10.1007/s11239-020-02343-w
104. Wig S, Chan M, Thachil J, Bruce I, Barnes T. A case of relapsing and refractory catastrophic anti-phospholipid syndrome successfully managed with eculizumab, a complement 5 inhibitor. Rheumatol (Oxford) (2016) 55(2):382–4. doi: 10.1093/rheumatology/kev371
105. Skoczynska M, Crowther MA, Chowaniec M, Ponikowska M, Chaturvedi S, Legault K. Thrombotic microangiopathy in the course of catastrophic antiphospholipid syndrome successfully treated with eculizumab: case report and systematic review of the literature. Lupus (2020) 29(6):631–9. doi: 10.1177/0961203320917460
106. de Holanda MI, Pôrto LC, Wagner T, Christiani LF, Palma LMP. Use of eculizumab in a systemic lupus erythemathosus patient presenting thrombotic microangiopathy and heterozygous deletion in CFHR1-CFHR3. A Case Rep Systematic Rev Clin Rheumatol (2017) 36(12):2859–67. doi: 10.1007/s10067-017-3823-2
107. Lonze BE, Singer AL, Montgomery RA. Eculizumab and renal transplantation in a patient with CAPS. N Engl J Med (2010) 362(18):1744–5. doi: 10.1056/NEJMc0910965
108. Lonze BE, Zachary AA, Magro CM, Desai NM, Orandi BJ, Dagher NN, et al. Eculizumab prevents recurrent antiphospholipid antibody syndrome and enables successful renal transplantation. Am J Transpl (2014) 14(2):459–65. doi: 10.1111/ajt.12540
109. Geethakumari PR, Mille P, Gulati R, Nagalla S. Complement inhibition with eculizumab for thrombotic microangiopathy rescues a living-donor kidney transplant in a patient with antiphospholipid antibody syndrome. Transfus Apher Sci (2017) 56(3):400–3. doi: 10.1016/j.transci.2017.02.007
110. Bakhtar O, Thajudeen B, Braunhut BL, Yost SE, Bracamonte ER, Sussman AN, et al. A case of thrombotic microangiopathy associated with antiphospholipid antibody syndrome successfully treated with eculizumab. Transplantation (2014) 98(3):e17–8. doi: 10.1097/TP.0000000000000267
111. Canaud G, Kamar N, Anglicheau D, Esposito L, Rabant M, Noël LH, et al. Eculizumab improves posttransplant thrombotic microangiopathy due to antiphospholipid syndrome recurrence but fails to prevent chronic vascular changes. Am J Transpl (2013) 13(8):2179–85. doi: 10.1111/ajt.12319
112. Chidharla A, Syed SB, Chatterjee T, Tarantino MD. A case report of COVID-associated catastrophic antiphospholipid syndrome successfully treated with eculizumab. J Blood Med (2021) 12:929–33. doi: 10.2147/JBM.S324873
113. Miyasaka N, Miura O, Kawaguchi T, Arima N, Morishita E, Usuki K, et al. Pregnancy outcomes of patients with paroxysmal nocturnal hemoglobinuria treated with eculizumab: a Japanese experience and updated review. Int J Hematol (2016) 103(6):703–12. doi: 10.1007/s12185-016-1946-x
114. Kelly RJ, Höchsmann B, Szer J, Kulasekararaj A, de Guibert S, Röth A, et al. Eculizumab in pregnant patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med (2015) 373(11):1032–9. doi: 10.1056/NEJMoa1502950
115. Stefanovic V. The extended use of eculizumab in pregnancy and complement Activation-Associated diseases affecting maternal, fetal and neonatal kidneys-the future is now? J Clin Med (2019) 8(3):407. doi: 10.3390/jcm8030407
116. Gustavsen A, Skattum L, Bergseth G, Lorentzen B, Floisand Y, Bosnes V, et al. Effect on mother and child of eculizumab given before caesarean section in a patient with severe antiphospholipid syndrome: a case report. Med (Baltimore) (2017) 96(11):e6338. doi: 10.1097/MD.0000000000006338
117. Rovere-Querini P, Canti V, Erra R, Bianchi E, Slaviero G, D'Angelo A, et al. Eculizumab in a pregnant patient with laboratory onset of catastrophic antiphospholipid syndrome: a case report. Med (Baltimore) (2018) 97(40):e12584. doi: 10.1097/MD.0000000000012584
118. Dmytrijuk A, Robie-Suh K, Cohen MH, Rieves D, Weiss K, Pazdur R. FDA Report: eculizumab (Soliris) for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Oncologist (2008) 13(9):993–1000. doi: 10.1634/theoncologist.2008-0086
119. Crum-Cianflone N, Sullivan E. Meningococcal vaccinations. Infect Dis Ther (2016) 5(2):89–112. doi: 10.1007/s40121-016-0107-0
120. Coyle D, Cheung MC, Evans GA. Opportunity cost of funding drugs for rare diseases: the cost-effectiveness of eculizumab in paroxysmal nocturnal hemoglobinuria. Med Decis Making (2014) 34(8):1016–29. doi: 10.1177/0272989X14539731
121. Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev (2008) 88(3):841–86. doi: 10.1152/physrev.00035.2007
122. van de Donk N, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood (2018) 131(1):13–29. doi: 10.1182/blood-2017-06-740944
123. Overdijk MB, Jansen JH, Nederend M, Lammerts van Bueren JJ, Groen RW, Parren PW, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via fcγ receptor-mediated cross-linking. J Immunol (2016) 197(3):807–13. doi: 10.4049/jimmunol.1501351
124. de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol (2011) 186(3):1840–8. doi: 10.4049/jimmunol.1003032
125. Cole S, Walsh A, Yin X, Wechalekar MD, Smith MD, Proudman SM, et al. Integrative analysis reveals CD38 as a therapeutic target for plasma cell-rich pre-disease and established rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther (2018) 20(1):85. doi: 10.1186/s13075-018-1578-z
126. Tommy Gambles M, Li J, Christopher Radford D, Sborov D, Shami P, Yang J, et al. Simultaneous crosslinking of CD20 and CD38 receptors by drug-free macromolecular therapeutics enhances b cell apoptosis in vitro and in vivo. J Control Release (2022) 350:584–99. doi: 10.1016/j.jconrel.2022.08.045
127. Xie H, Zhou H, Wang H, Chen D, Xia L, Wang T, et al. Anti-β(2)GPI/β(2)GPI induced TF and TNF-α expression in monocytes involving both TLR4/MyD88 and TLR4/TRIF signaling pathways. Mol Immunol (2013) 53(3):246–54. doi: 10.1016/j.molimm.2012.08.012
128. Hollerbach A, Müller-Calleja N, Canisius A, Orning C, Lackner KJ. Induction of tissue factor expression by anti-β2-glycoprotein I is mediated by tumor necrosis factor α. J Thromb Thrombolysis (2020) 49(2):228–34. doi: 10.1007/s11239-019-01970-2
129. Müller-Calleja N, Hollerbach A, Häuser F, Canisius A, Orning C, Lackner KJ. Antiphospholipid antibody-induced cellular responses depend on epitope specificity : implications for treatment of antiphospholipid syndrome. J Thromb Haemost (2017) 15(12):2367–76. doi: 10.1111/jth.13865
130. Berman J, Girardi G, Salmon JE. TNF-alpha is a critical effector and a target for therapy in antiphospholipid antibody-induced pregnancy loss. J Immunol (2005) 174(1):485–90. doi: 10.4049/jimmunol.174.1.485
131. Gelber SE, Brent E, Redecha P, Perino G, Tomlinson S, Davisson RL, et al. Prevention of defective placentation and pregnancy loss by blocking innate immune pathways in a syngeneic model of placental insufficiency. J Immunol (2015) 195(3):1129–38. doi: 10.4049/jimmunol.1402220
132. Benhamou Y, Miranda S, Armengol G, Harouki N, Drouot L, Zahr N, et al. Infliximab improves endothelial dysfunction in a mouse model of antiphospholipid syndrome: role of reduced oxidative stress. Vascul Pharmacol (2015) 71:93–101. doi: 10.1016/j.vph.2015.03.014
133. Alijotas-Reig J, Esteve-Valverde E, Llurba E, Gris JM. Treatment of refractory poor aPL-related obstetric outcomes with TNF-alpha blockers: maternal-fetal outcomes in a series of 18 cases. Semin Arthritis Rheumatol (2019) 49(2):314–8. doi: 10.1016/j.semarthrit.2019.02.006
134. Ramos-Casals M, Brito-Zerón P, Soto MJ, Cuadrado MJ, Khamashta MA. Autoimmune diseases induced by TNF-targeted therapies. Best Pract Res Clin Rheumatol (2008) 22(5):847–61. doi: 10.1016/j.berh.2008.09.008
135. Hemmati I, Kur J. Adalimumab-associated antiphospholipid syndrome: a case report and review of the literature. Clin Rheumatol (2013) 32(7):1095–8. doi: 10.1007/s10067-013-2244-0
136. Cheemalavagu S, McCoy SS, Knight JS. Digital ischaemia secondary to adalimumab-induced antiphospholipid syndrome. BMJ Case Rep (2020) 13(2):e232907. doi: 10.1136/bcr-2019-232907
137. Bećarević M. Detrimental roles of TNF-alpha in the antiphospholipid syndrome and de novo synthesis of antiphospholipid antibodies induced by biopharmaceuticals against TNF-alpha. J Thromb Thrombolysis (2017) 44(4):565–70. doi: 10.1007/s11239-017-1571-4
138. Pal Singh S, Dammeijer F, Hendriks RW. Role of bruton's tyrosine kinase in b cells and malignancies. Mol Cancer (2018) 17(1):57. doi: 10.1186/s12943-018-0779-z
Keywords: biologics, antiphospholipid syndrome, eculizumab, rituximab, belimumab, daratumumab, anti-TNF-α antibodies, obinutuzumab
Citation: Yun Z, Duan L, Liu X, Cai Q and Li C (2023) An update on the biologics for the treatment of antiphospholipid syndrome. Front. Immunol. 14:1145145. doi: 10.3389/fimmu.2023.1145145
Received: 15 January 2023; Accepted: 04 May 2023;
Published: 19 May 2023.
Edited by:
Ljudmila Stojanovich, University of Belgrade, SerbiaReviewed by:
Aleksandra Djokovic, University Medical Center, SerbiaGuilherme Ramires De Jesús, Rio de Janeiro State University, Brazil
Copyright © 2023 Yun, Duan, Liu, Cai and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun Li, 13811190098@163.com
†These authors have contributed equally to this work
 Zelin Yun
Zelin Yun Lizhi Duan
Lizhi Duan Xiangjun Liu
Xiangjun Liu Qingmeng Cai
Qingmeng Cai Chun Li
Chun Li