- 1Department of Internal Medicine, Saint Peter’s University Hospital, New Brunswick, NJ, United States
- 2Department of Pulmonary, Critical Care and Sleep Medicine, University of Buffalo, Buffalo, NY, United States
- 3Sylvester Comprehensive Cancer Center, University of Miami Health System, Miami, FL, United States
Lung cancer is the leading cause of cancer related deaths. Among the two broad types of lung cancer, non-small cell lung cancer accounts for 85% of the cases. The study of the genetic alteration has facilitated the development of targeted therapeutic interventions. Some of the molecular alterations which are important targets for drug therapy include Kirsten rat sarcoma (KRAS), Epidermal Growth Factor Receptor (EGFR), V-RAF murine sarcoma viral oncogene homolog B (BRAF), anaplastic lymphoma kinase gene (ALK). In the setting of extensive on-going clinical trials, it is imperative to periodically review the advancements and the newer drug therapies being available. Among all mutations, BRAF mutation is common with incidence being 8% overall and 1.5 – 4% in NSCLC. Here, we have summarized the BRAF mutation types and reviewed the various drug therapy available - for both V600 and nonV600 group; the mechanism of resistance to BRAF inhibitors and strategies to overcome it; the significance of comprehensive profiling of concurrent mutations, and the role of immune checkpoint inhibitor in BRAF mutated NSCLC. We have also included the currently ongoing clinical trials and recent advancements including combination therapy that would play a role in improving the overall survival and outcome of NSCLC.
Introduction
Lung cancer is the second most common cancer worldwide. It is the leading cause of cancer-related deaths accounting for 18% of all cancer related deaths (1, 2). In United states alone, it will account for estimated 127,070 deaths in the year 2023 (3). Among the two major subtypes, Non-small cell lung cancer (NSCLC) accounts for 85% of the cases (4). NSCLC is significantly more common than small cell lung cancer (SCLC) and is further subdivided into squamous and non-squamous histological types (5). Although the classification between small cell and non-small cell is still widely used, molecular classification demonstrates that this histological classification is no longer appropriate to guide therapy. NSCLC comprises a group of heterogenous tumors having varied genetic alterations.
The most common genetic alteration associated with NSCLC is Kirsten rat sarcoma (KRAS) and Epidermal Growth Factor Receptor (EGFR) gene. They are involved in tumor initiation and are important targets for drug therapy. Other important mutations observed are anaplastic lymphoma kinase gene (ALK) rearrangement, C-ROS oncogene 1 (ROS1). Certain molecular alterations identified involve hepatocyte growth factor receptor (MET) and human epidermal growth factor receptor 2 (HER2) genes, rearranged during transfection (RET) gene, V-RAF murine sarcoma viral oncogene homolog B (BRAF) and neurotrophic tropomyosin receptor kinase (NTRK) gene (6). Mutations in tumor protein 53 (TP53) have been observed in advanced stages of NSCLC and is poor prognostic marker (7). The understanding of the pathogenic alterations has led to advancement in the therapeutic intervention available especially combination drug therapy and immune checkpoint inhibitors (1, 8).
BRAF mutation
BRAF belongs to rapidly accelerated fibrosarcoma (RAF) group of serine threonine group of kinases and is significantly involved in cell proliferation and differentiation through the mitogen activated protein kinase (MAPK) signaling pathway. The rat sarcoma (RAS)/RAF-MAPK extracellular signal regulated kinase (ERK)-MAPK pathway can get activated by mutation at various levels in the pathway. The various levels of mutation have been seen in multiple cancers including melanoma, NSCLC, papillary thyroid cancer and colorectal cancer and around 300 distinct BRAF mutations have been identified (9–11). The incidence of BRAF mutation in all human cancers is around 8% with the incidence in NSCLC being 1.5 - 4% (12–15).
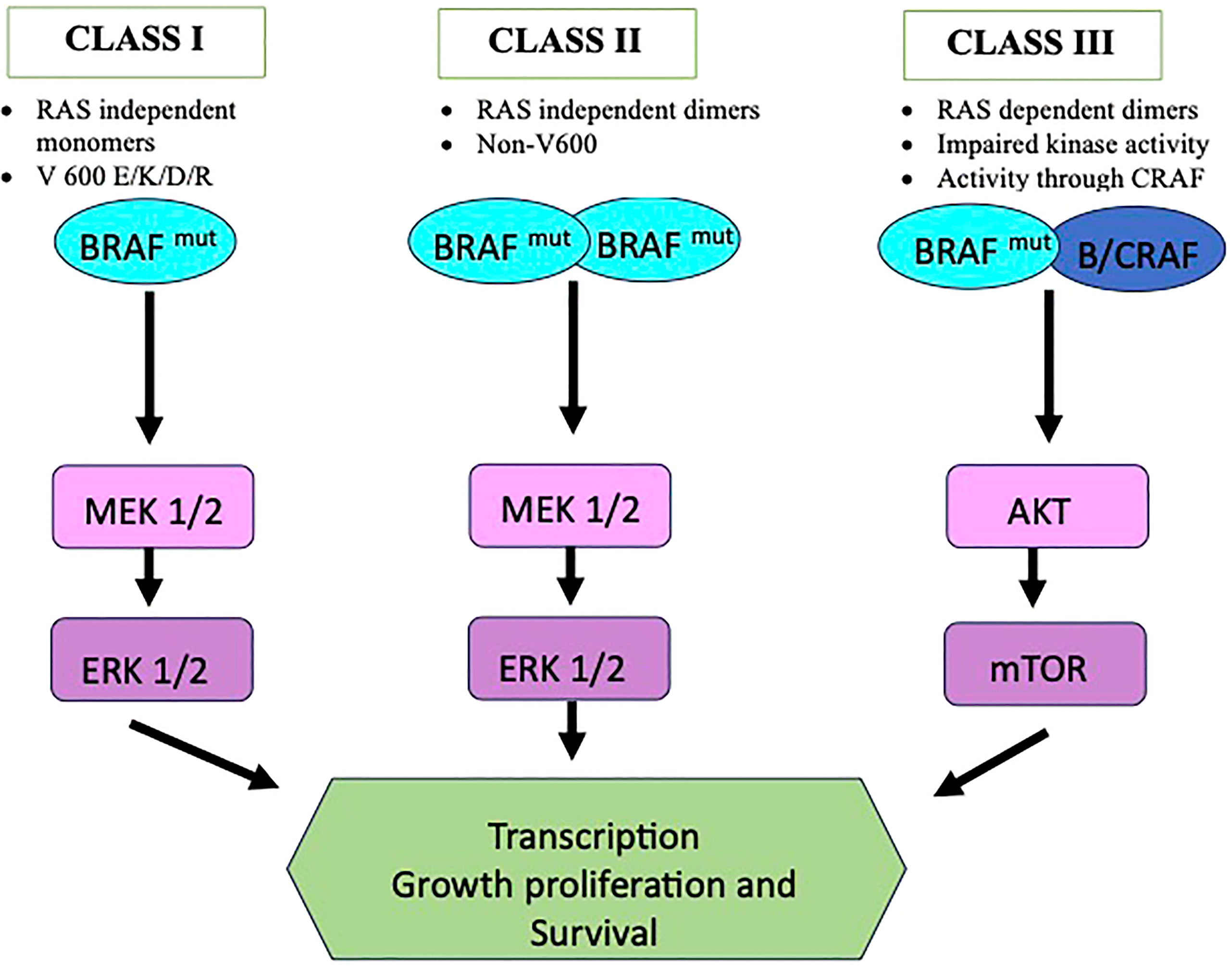
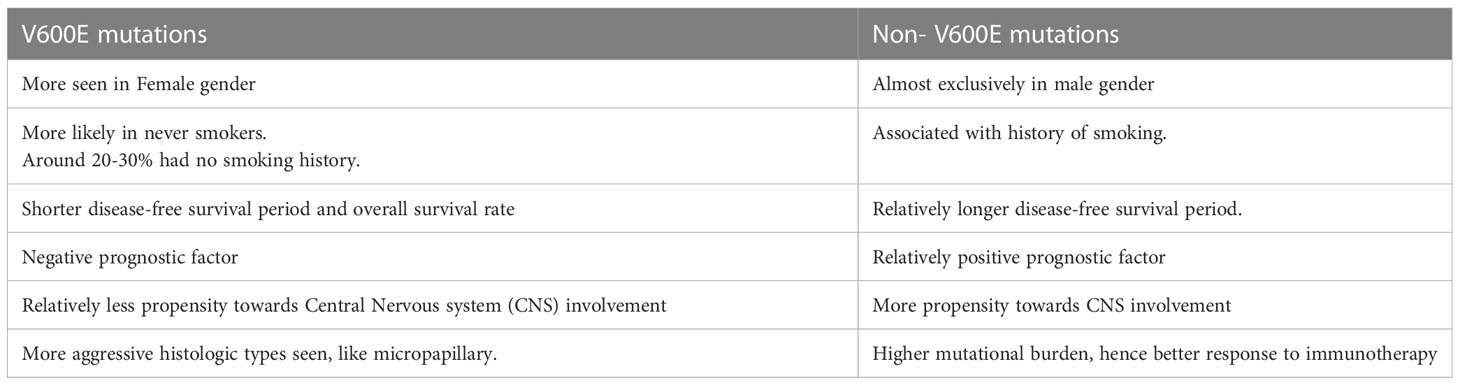
The mutations are broadly named as V600 codon and non-V600 codon mutations and are divided into 3 categories as shown in Figure 1. Class I mutant are constitutive active RAS independent monomers that involves codon 600 (including V600 E/K/D/R) causing strong activation of BRAF kinase. Class II comprises constitutive active RAS independent dimers outside codon 600 (including K601, L597, G464, and G469 mutations) and are located in the P loop segment. Class III includes RAS dependent dimers and have impaired kinase activity. In this case the activity of MAPK pathway is enhanced via raf-1 protooncogene CRAF activation (16, 17). Class II and III are more prevalent for certain tumor types (18). In clinical practice BRAF mutations are commonly classified as V600 and non-V600 mutations. V600 have been seen more in female gender and has been seen as a negative prognostic factor and non-V600 mutations are more seen in male gender (19, 20). BRAF mutations, especially nonV600 have been associated with history of smoking and have more propensity towards central nervous system (CNS) involvement. Class I mutations, however, have shown lower incidence of brain metastasis at the time of diagnosis. Among the V600 codon mutations, around 20-30% have no smoking history. V600 mutations is also associated with shorter disease-free survival period (21, 22). The differences observed between the V600 mutation, mainly V600E, and Non-V600 have been summarized in Table 1.
BRAF target drug therapy
BRAF inhibitors available are namely Sorafenib, Vemurafenib, Dabrafenib and Encarafenib. MEK inhibitors namely, Trametinib, Cobimetinib, Bimetinib block the ERK signaling in the MAPK pathway and delays the emergence of resistance due to MAPK pathway reactivation (23).
Sofrafenib has weak activity for mutant BRAF and also has significant toxic effects. Vemurafenib (PLX4032) is a inhibitor of mutated V600-BRAF (24). The side effects are dose related and most common observed are rash, arthralgia, nausea, fatigue, photosensitivity, pruritus, palmar-plantar dysesthesia and cutaneous squamous cell carcinoma. A dose of 960 mg twice daily has been determined to be tolerable (25). Dabrafenib is ATP-competitive BRAF kinase inhibitor. The most common adverse effects observed are fatigue, pyrexia and cutaneous squamous cell carcinoma (26). Trametinib (GSK1120212) is a non-ATP competitive inhibitor of both MEK1 and MEK2 (27). The common toxicity observed are diarrhea, peripheral edema, skin related toxicity. The cardiac and hepatic events observed have been reported to be reversible on discontinuation of trametinib (28).
Very few studies are available in the context of Sofrafenib (29). Carter et al (30) used Sorafenib in patients who had received chemotherapy. The idea behind it was the combination could delay tumor growth without increasing toxicity. Certain other trials have been done but did not test for BRAF mutation status (31, 32). It remains largely unexplored.
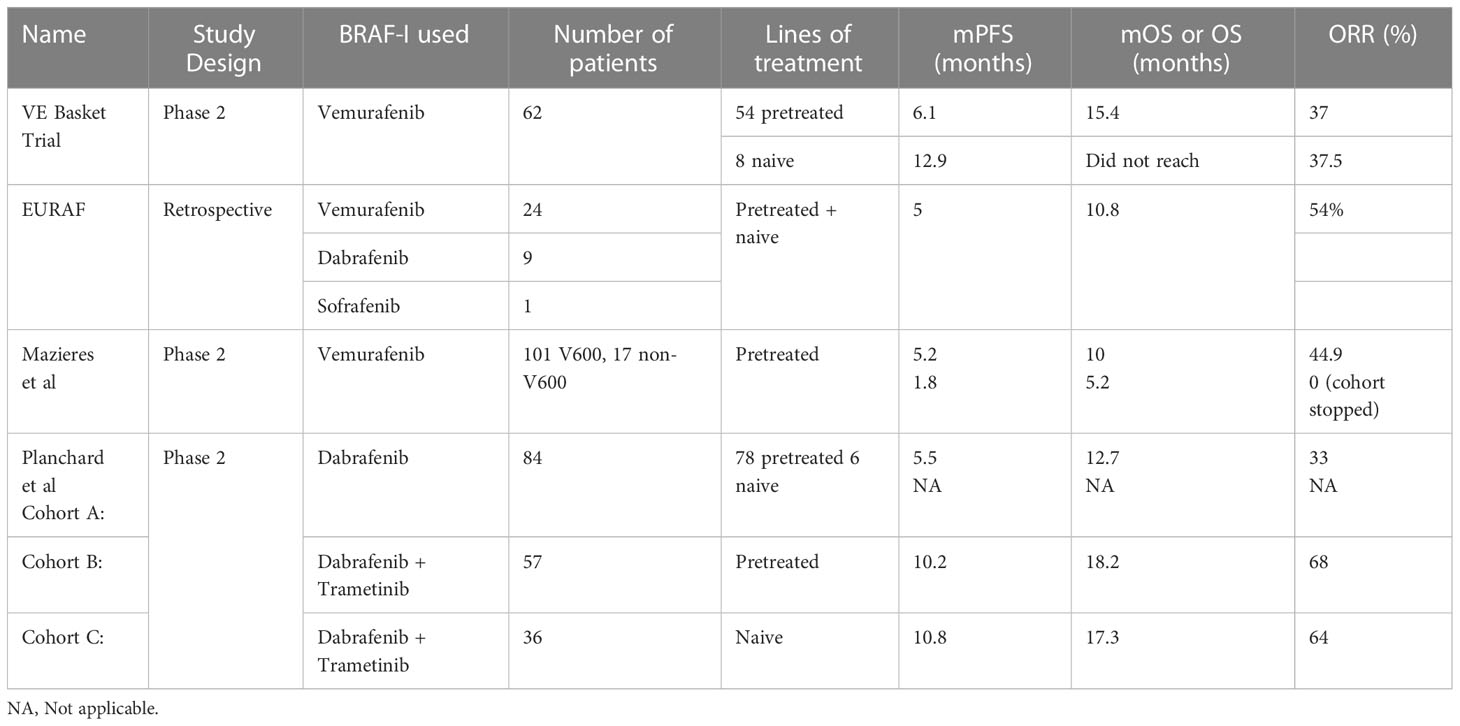
In the VE Basket trial, Vemurafenib 960 mg twice a day was given to 62 patients with NSCLC having V600 mutations. It was a phase II study. Out of the 62 patients, 54 were pretreated and 8 were naïve. It was found that objective response rate (ORR) was similar in both the groups – 37.5 in naïve and 37% in pretreated group. The median progression-free survival (mPFS) was 6.1 and 12.9 respectively in pretreated and naïve groups whereas median overall survival (mOS) was 15.4 in the pretreated group and not reached in the naïve group (33).
The EURAF study done by Gautschi et al. comprised of 35 patients. Out of these, 29 harbored V600 mutation and 6 were nonV600. 5 patients were given BRAF inhibitor (BRAF-i) as first line and 30 were given as subsequent line. So total of 39 lines of BRAF-i were counted in the trial. 29 of the patients received Vemurafenib, 9 received Dabrafenib and 1 received Sofrafenib. The ORR in the V600 group treated with Vemurafenib (n=24) was 54% and disease control rate (DCR) 96%. Overall, the study showed mPFS of 5 months and OS of 10.8 months. In the nonV600 group, only a partial response and poor outcome was seen (34).
Another research by Mazieres et al. used Vemurafenib and all the patients had more than one line of treatment. In the V600 mutation group the mean ORR was 44.9, mPFS 5.2 months and mOS 10 months. In the nonV600 mutation group, no tumor response was seen and the mPFS was 1.8 months (35).
Planchard et al. conducted a phase II non-randomized controlled trial with Dabrafenib in advanced V600 positive NSCLC. They conducted the trial under 3 separated arms. Group A has 84 patients and used Dabrafenib monotherapy. Out of these, 6 were T/t naïve and 78 had received prior systemic therapy. The ORR and DCR for pretreated group was 33% and 58% respectively. Of the T/t naïve, 4 out of 6 had treatment response. Those treated with 1-3 previous treatment lines had mPFS of 5.5 months and mOS of 12.7 months. The Group B used combination Dabrafenib and Trametinib in 57 pretreated V600 positive patients. The median follow up was 16.6 months. The ORR was 68%, mPFS 10.2 months, OS 18.2 months, DCR 81% and duration of response (DoR) 9.8 months. Five-year survival rate was 19%. The Group C used the combination Dabrafenib-Trametinib therapy in 36 treatment naïve patients. The median follow up was 16.3 months. The ORR was found to be 64%, mFS 10.8 months, OS 17.3 months, DoR 10.2 months and the 5-year survival rate 22% (36–39).
Current guidelines recommend the combination of Dabrafenib with Trametinib for BRAF V600 positive NSCLC (23). The studies have been summarized in Table 2.
Resistance to BRAF kinase inhibitors
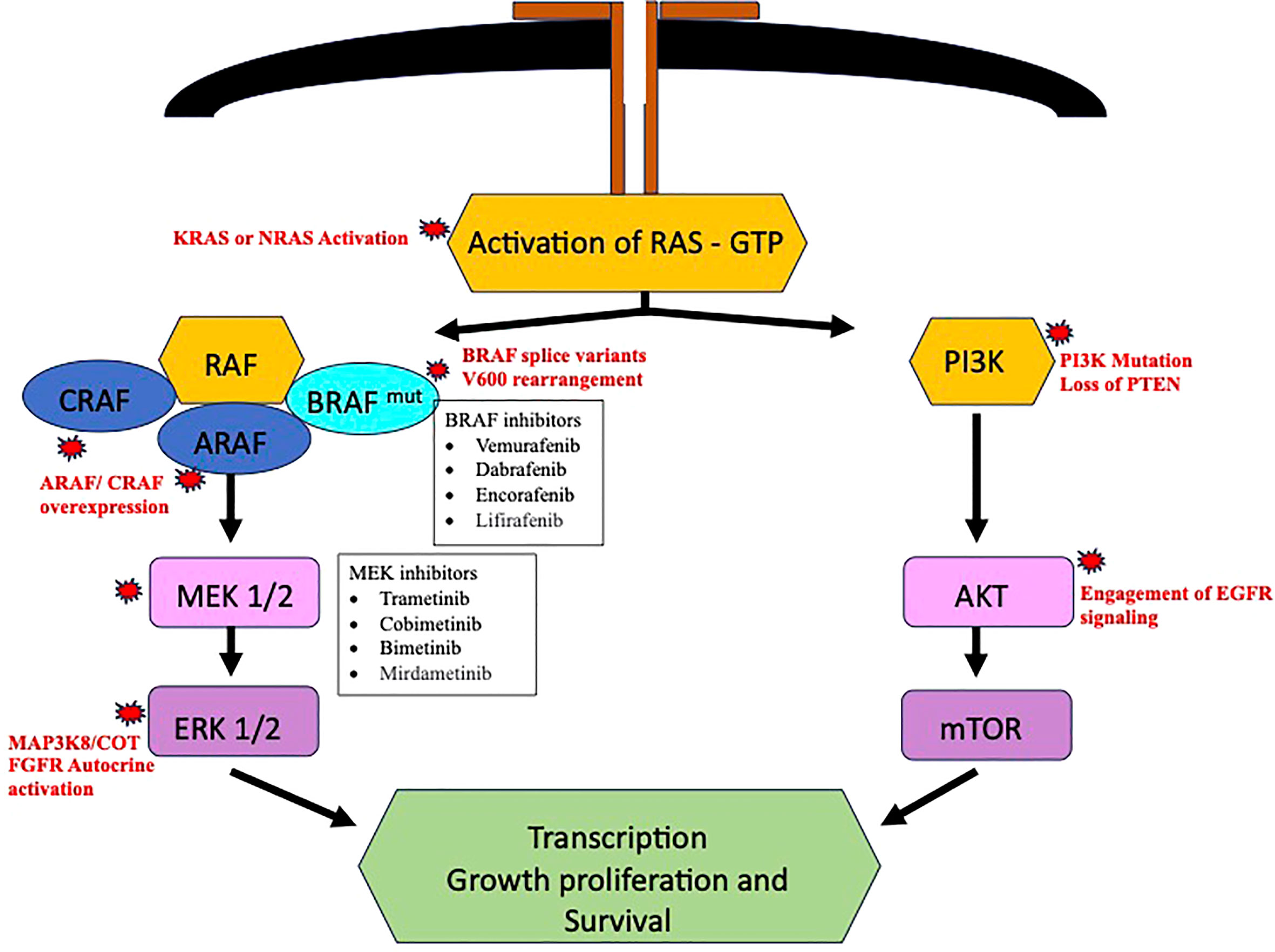
Despite the advances in the available BRAF inhibitors, the disease progression will eventually occur with development of either de novo or acquired BRAF pathway inhibitor resistance. Delineation of resistance mechanism to elucidate alternative drug targets could assist in formulation alternate drug strategies. The bypass activation mutation seems to be the main cause of resistance, out of which the most common are found in central MAPK nodes and lead to MAPK reactivation (40). CRAF and A- raf (ARAF) protooncogene isoforms have been observed in melanoma, which could cause MAPK pathway activation once BRAF is inhibited and cause BRAF-i resistance (41). The COT/TPL2 (MAP3K8) expression has also been observed to cause de novo resistance in melanoma cell lines (42). In BRAF V600 NSCLC, the activating mutations like KRAS or neuroblastoma RAS oncogene (NRAS) after use of BRAF-i or dual blockade with dabrafenib and trametinib often have been observed as causing resistance. These mutation bypasses the BRAF-V600 inhibition and leads to activation of downstream MAPK pathway (43–45).
Resistance mechanism could also involve MAPK pathway through reactivation of ERK signaling through BRAF splice variants or BRAF gene amplification (12, 46). An institutional prospective trial MATCH-R (“Matching Resistance”) revealed potential mutation responsible for resistance, namely, MEK1, NRAS Q61K, KRAS Q61R, and K57N (47). Sheikine et al. identified new post-treatment mutations, that could corelated to acquired resistance. They reported mutations involving KRAS (G12R, K61H, G12D, V141), NRAS (Q61K), a rearrangement in the setting of V600E, biallelic inactivation of SMARCA4 and a homozygous deletion of MAPK2K4 (48). Some less common mechanism could involve other pathways, like activation of PI3K/mTOR through mutations in AKT activation and loss of function of PTEN (12).
The expression of a ligand dependent stimulation of RTKs and p61(aberrant BRAF V600 splice form) are other potential mechanism of MAPK pathway reactivation. Activation of signaling pathway, like phosphatidylinositol-3-kinase – protein kinase B – mammalian target of rapamycin (PI3K/AKT/mTOR) have also been described (49, 50). A case with protein kinase B (AKT) mutation was reported in BRAF-V600 mutant NSCLC. The resistance occurs through engagement of EGFR signaling. The resistance might be overcome by using combined BRAF-i and EGFR inhibitors (49). Autocrine activation of fibroblast growth factor receptor (FGFR) leads to sustained extracellular signal-regulated kinases (ERK) activation and has been more commonly seen in dual BRAF/MEK inhibitor resistance. The potential utility of FGFR inhibitors in such cases could be a promising strategy (50). Planchard et al. had reported presence of co-mutations and alteration of phosphatidylinositol-3-kinase (PI3K) pathway as a negative prognostic factor (39).
Rudin et al. reported a patient to have developed KRAS (G12D) mutation after use of Dabrafenib. Secondary mutations in TP53 and cyclin dependent kinase inhibitor 2A (CDKN2A) were also found. They are not directly related to RAF-dependent pathway and role to the resistance attained is not clear (43). A case of metastatic lung adenocarcinoma was reported by Abravanel et al. wherein the patient received a combination therapy with dabrafenib and trametinib. Initially significant response to therapy was seen, however, within 21 weeks of therapy the patient’s disease progressed and was found to have acquired NRAS-Q61K mutation (44). This patient also has remote history of breast cancer, could the presence/history of another malignancy influence the acquisition of the resistance mutations could be further studied. The RAF activation pathway with potential resistance mechanisms is summarized in Figure 2.
It is imperative to do a comprehensive profiling of the possible co-occurring mutations, to detect the presence of acquired resistance. It would play a significant role in choosing the currently available targeted therapies and guide the scientific community in designing clinical trials targeting the specific resistance. A multi-gene testing panel rather than a single gene panel is more suitable to detect the mutations. Some of the methods to detect this resistance mechanism include analyses of circulating tumor DNA (ctDNA) (51). Liquid biopsy is a non-invasive analysis using either ctDNA or plasma circulating tumor cells. Analytes could also include circulating cell-free DNA or RNA, tumor educated platelets and circulating extracellular vesicles. This method resolves the issues of tissue scarcity and damage associated with the standard tissue biopsy (52, 53). Currently, real time polymerase chain reaction (PCR) and Next generation sequencing (NGS) are the most used methods. It can facilitate testing of multiple biomarkers with use of small amount of tumor tissue. However, the use of NGS has its own drawbacks. It has a high turnaround time and requires use of expensive and high-end equipment and reagents; hence it is not yet accessible all round the world. Cohen et al. reported that combination of sequential DNA NGS and RNA NGS is the most efficient strategy for mutation detection in smoking associated NSCLC and recommended a parallel approach for never smokers (54). In current practice, all metastatic or locally advanced NSCLC should be tested for driver mutations including BRAF, MET, RET, NTRK, KRAS, HER2 if common mutations EGFR, ALK and ROS protooncogene are negative (55, 56).
Comprehensive profiling of concurrent mutations
The rate of presence of concurrent mutations in BRAF mutated NSCLC has been reported to have a wide range from 14.3 to 30.2%. It is much higher than concurrent mutation rate seen in other driver mutations (5%) (57–59). Qu et al. reported TP53 to be the most common occurring co-mutation, 6 out of 53 (11.3%) patients included in the study (59). Mayall et al. also reported TP53 to be the most common concurrent mutation (5 out of 8 patients) in the cohort studied. There was no similarity observed in any of the observed five mutations, each of the mutation was unique. The alterations noted were C343F (725 G>T), R248L (743 G>T), E298X (892 G>T), I195T, and splice site 559 + 1 G>C (60). In a retrospective analysis by Krohn et al. in 174 patients, co-occurring mutations were found in 70% (121) BRAF mutated patients. TP53 was found as the most frequent (74%, 89 patients) co-alteration (61). The co-occurrence of other mutations seen with BRAF have been EGFR, PI3KA, KRAS, ALK translocation, c-MET amplification, MSH2 mutation, AXIN2 mutation (15, 19, 59). BRAF V600 and non-V600 mutated NSCLC, both have been reported to have concurrent mutations. Cardarella et al (15) reported presence of KRAS in nonV600 NSCLC and Kinno et al (62) reported presence of EGFR along with non-V600 BRAF mutated NSCLC. It would be interesting to explore whether BRAF are the primary or secondary oncogenic driver mutations in these cases.
Patients with double mutations have been found to have inferior overall survival compared with single BRAF mutation. TP53 and PI3KA co-mutation carries a negative prognosis (39, 58, 59). Hence, important is the role of PCR or NGS in multiplex genotyping for comprehensive profiling of the NSCLC patients to decipher the presence of concurrent mutations. To target co-mutations associated with BRAF, double or triple targeted therapy is used. EGFR targeting tyrosine kinase inhibitors (TKI) are used NSCLC having BRAF plus EGFR mutations. KRAS co-mutation was given Dabrafenib-Trametinib therapy (59). The use of immunotherapy, specifically immune checkpoint inhibitors (ICIs), is more and more being used as a solution to BRAF co-mutations (63). More research is needed to study the clinical implications of the use of multi-targeted chemotherapy drugs and immune therapy in concurrent mutations.
Immune checkpoint inhibitor therapy - monotherapy vs combination therapy
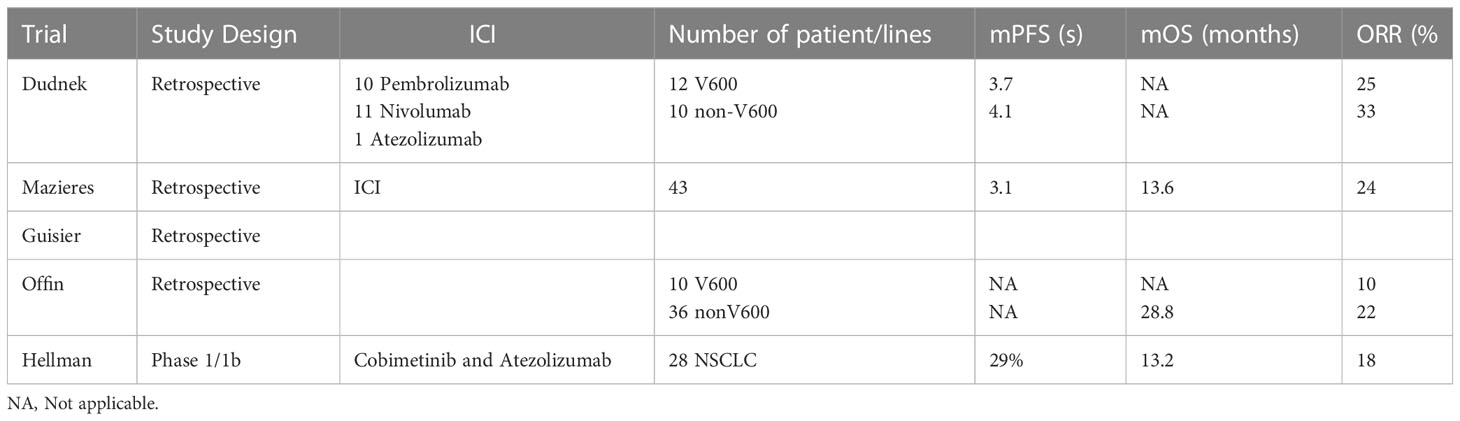
Very limited studies in the form of retrospective studies demonstrated Programmed cell death ligand 1 (PD-L1) positivity in NSCLC with BRAF mutations (64–66). Dudnik et al. demonstrated that use immune checkpoint inhibitors (ICIs) in BRAF mutated patients had a limited response and is similar to the unselected population. They included 39 BRAF mutated patients treated with ICIs. Out which 22 (12 V600 and 10 nonV600) - 10 with Pembrolizumab, 11 with Nivolumab and 1 with atezolizumab. The ORR 25% and 33% and mPFS was 3.7% and 4.1% respectively for the two groups. mOS was not achieved in either of the groups. A low/intermediate tumor mutation burden (TMB) and microsatellite-stable status was found in BRAF mutated NSCLC patients (67). Several other studies also observed a similar response. One study showed ORR, mPFS and mOS of 28.1%, 3.0 and 13.1 months, respectively treated with ICIs in second line of treatment (68). The ORR of PDL1 inhibitor as the only therapy in BRAF-mutant patients is about 10%–30%, with a mPFS of 2–4 months. Guisier et al. supported that the PDL1 inhibitor being used as a second-line ICI monotherapy is similar in to wild-type NSCLC (69). Similar results were obtained by Rihawi et al (70). Offin et al. demonstrated improved ORR of 22% and OS 2.4 years in non-V600 mutations when compared to V600 mutations (71). The non-V600 mutated NSCLC are seen more in non-smoker and have been found to have increased TMB and hence, a better response to ICIs (71–73). In summary, these data indicated limited efficacy of ICIs in BRAF mutant NSCLC. However, the efficacy of PDL1 inhibitors in patients of advanced NSCLC has been noted. This could be attributed to an increased expression of PDL1 in BRAF mutant NSCLC in comparison to wild type (67, 72).
Some of the ongoing trials are exploring a combination regimen of ICIs and targeted BRAF-I and MEK-inhibitor (MEK-i) therapies. The concept is based on the fact that PDL1 expression has been found in BRAF mutants and BRAF-i and MEK-i improve T cell mediated immune activation. The preclinical data suggested that inhibition of BRAF and MEK pathway leads to increased activity of CD4 and CD8 T cells and thereby ability to destroy tumor cells. It also leads to increased granzyme B and perforin levels along with increased expression of cytotoxic T-lymphocyte-associated protein 4 (CTLA4) (74–77). The use of anti-CTLA-4 and MEK-i like Selumetinib and Trametinib has shown to provide survival benefit in murine K-ras bearing tumors (78, 79). A stage IV V600 BRAF mutant NSCLC was reported to have achieve a longer period of response when combination of Atezolizumab and chemotherapy was used (80). Hellmann et al. investigated the combination cobimetinib and atezolizumab in patients with solid tumors covering colorectal cancer, melanoma and NSCLC. Out of the total 152 participants, 28 were NSCLC patients and were found to have ORR of 18%, mOS of 13.2 months. The 12-month PFS and OS for NSCLC was 29% and 57% respectively (81). The enrollment for an ongoing randomized phase II/II study, the B-FAST trial (NCT03178552), is currently underway for patients with advanced or metastatic NSCLC that are found to harbor somatic mutations of have TMB using blood based NGS ctDNA assay. In the cohort E of this trial, the V600 mutant patients are being given the triplet of Vemurafenib, cobimetinib and atezolizumab after a run-in period of BRAF-i/MEK-i combination. This preliminary data about combination ICI-BRAF-i/MEK-i targeted therapy warrants further studies to establish an appropriate drug combination and safety and clinical efficacy (82). Some of the ICIs have been summarized in Table 3.
Recent advances and future prospectives
On June 22, 2022, the FDA granted accelerated approval to dabrafenib- trametinib combination for the treatment of all patients more than or equal to 6 years of age with unresectable or metastatic BRAF-V600E mutant solid tumors, who have no alternate treatment option and have progressed following prior treatment. It is not indicated for wild-type BRAF solid tumors. It was based on evaluation of 131 adult patients from open-label, cohort trials BRF117019 (NCT02034110) and NCI-MATCH (NCT02465060), 36 pediatric patients from CTMT212X2101 (NCT02124772), and supported by results in COMBI-v, COMBI-d, and BRF113928. From the adult patient group, 54 (41% 95% confidence interval of 33,50) experienced ORR. The most common adverse reactions in the adults were nausea, rash, fever, chills, fatigue, headache, myalgia, constipation, diarrhea, arthralgia and edema. In adults, the dose recommended for dabrafenib is 150 mg oral twice daily with Trametinib 2 mg oral once daily (83).
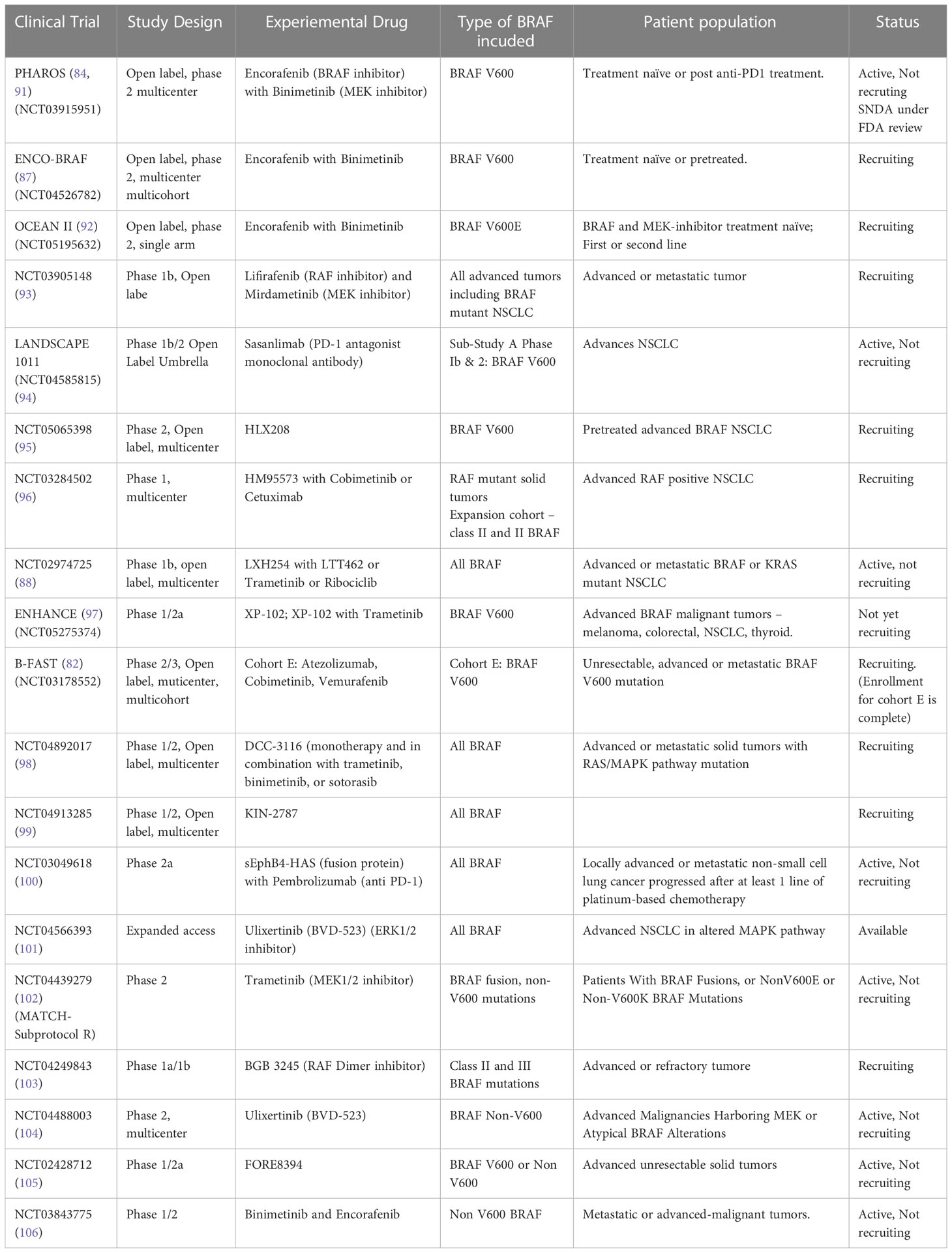
PHAROS trial (84) is an open-label, non-randomized, Phase 2 at a multicenter level is going on to determine the safety and efficacy of Encorafenib in combination with Binimetinib in NSCLC patients with BRAF V600 mutation. The patients who were treatment naïve, or first line treatment with an anti-PD1 given as monotherapy of with a platinum-based chemotherapy were enrolled. A total of 98 patients have been recruited. The doses used were 450 mg once daily of Encorafenib and 45 mg twice daily of Binimetinib for a 28-day cycle. Primary set endpoint of ORR was met. Currently, the combination is approved for use in BRAF -V600 positive melanoma patients. A supplemental new drug application (SNDA) is currently under review by the FDA for patients with metastatic NSCLC w a BRAF V600 mutation (85, 86).
ENCO-BRAF trial is another Phase 2 trial currently recruiting patients to assess the Encorafenib and Binimetinib on the same dosing schedule (87).
LXH254 (BRAF inhibitor) plus LTT462 (ERK ½ inhibitor) is being explored for its efficacy in advanced metastatic K-ras or BRAF-mutant NSCLC under clinical trial NCT02974725 (88).
Lifirafenib (BGB-283) is a novel RAF kinase and EGFR inhibitor and antitumor activity in B-RAF mutated solid tumors and KRAS mutant NSCLC with tolerable adverse effects. Future exploration is warranted to explore lifiranib monotherapy of combination therapy in patient with BRAF-i resistance and harbouring RAS mutations (89).
Another Phase I multicenter study (NCT03284502) is exploring the dose, safety and pharmacokinetics of HM95573 in combination with either Cobimetinib or Cetuximab in locally advanced or metastatic Solid Tumors (90).
Some of the current ongoing trials have been summarized in Table 4.
Conclusion
Precision medicine has revolutionized modern oncology. However, despite the significant progress made in the landscape of NSCLC, treatment for BRAF mutated NSCLC is not satisfactory due to low incidence of this disease. Dabrafenib and Trametinib is the only approved treatment of choice for BRAF-V600E mutated NSCLC and exhibits poor efficacy against non-V600E mutations. As mentioned above, the common mechanisms of resistance for V600E mutant NSCLC involves MAPK reactivation, loss of length BRAF V600E in concert with expression of a truncated form of mutant protein and enhanced EGFR signaling (49). Mechanisms of resistance for BRAF V600E have not been clearly defined. Molecular profiling with next generation sequencing, genomics and single cell sequencing may help in identifying resistance pathways and mutations. Further research is warranted to elucidate and identify mechanisms of resistance in BRAF non-V600E NSCLC and to develop drugs to overcome resistance in BRAF mutations. It is imperative to conduct more clinical trials in future to explore sequencing of therapy and to develop targets targeting resistance of BRAF inhibitors. In future, it will be exciting to see if BRAF inhibitors will have a role in neoadjuvant or adjuvant setting in this group of patients.
Author contributions
MP wrote the draft of the manuscript. KG and RD reviewed and edited the manuscript. All authors contributed to the manuscript revision, read and approved the submitted version. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: A review. JAMA - J Am Med Assoc (2019) 322(8):764–74. doi: 10.1001/jama.2019.11058
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Lung and bronchus Cancer — Cancer Stat Facts. Available at: https://seer.cancer.gov/statfacts/html/lungb.html (Accessed May 25, 2023).
4. Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol (2006) 24(28):4539–44. doi: 10.1200/JCO.2005.04.4859
5. Ruiz-Cordero R, Devine WP. Targeted therapy and checkpoint immunotherapy in lung cancer. Surg Pathol Clin (2020) 13(1):17–33. doi: 10.1016/j.path.2019.11.002
6. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature (2018) 553(7689):446–54. doi: 10.1038/nature25183
7. Ahrendt SA, Hu Y, Buta M, McDermott MP, Benoit N, Yang SC, et al. p53 mutations and survival in stage I non-small-cell lung cancer: results of a prospective study. J Natl Cancer Inst (2003) 95(13):961–70. doi: 10.1093/jnci/95.13.961
8. Relli V, Trerotola M, Guerra E, Alberti S. Abandoning the notion of non-small cell lung cancer. Trends Mol Med (2019) 25(7):585–94. doi: 10.1016/j.molmed.2019.04.012
9. Joneson T, Bar-Sagi D. Ras effectors and their role in mitogenesis and oncogenesis. J Mol Med (Berl). (1997) 75(8):587–93. doi: 10.1007/s001090050143
10. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature (2002) 417(6892):949–54. doi: 10.1038/nature00766
11. Forbes SA, Bindal N, Bamford S, Stephens P, Edkins S, Clegg S, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res (2011) 39(Database issue):D945–50. doi: 10.1093/nar/gkq929
12. Leonetti A, Facchinetti F, Rossi G, Minari R, Conti A, Friboulet L, et al. BRAF in non-small cell lung cancer (NSCLC): Pickaxing another brick in the wall. Cancer Treat Rev (2018) 66:82–94. doi: 10.1016/j.ctrv.2018.04.006
13. Mendiratta G, Ke E, Aziz M, Liarakos D, Tong M, Stites EC. Cancer gene mutation frequencies for the U.S. population. Nat Commun (2021) 12(1):5961. doi: 10.1038/s41467-021-26213-y
14. Paik PK, Arcila ME, Fara M, Sima CS, Miller VA, Kris MG, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol (2011) 29(15):2046–51. doi: 10.1200/JCO.2010.33.1280
15. Cardarella S, Ogino A, Nishino M, Butaney M, Shen J, Lydon C, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res (2013) 19(16):4532–40. doi: 10.1158/1078-0432.CCR-13-0657
16. Yao Z, Yaeger R, Rodrik-Outmezguine VS, Tao A, Torres NM, Chang MT, et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature (2017) 548(7666):234–8. doi: 10.1038/nature23291
17. Nieto P, Ambrogio C, Esteban-Burgos L, Gomez-Lopez G, Blasco MT, Yao Z, et al. A Braf kinase-inactive mutant induces lung adenocarcinoma. Nature (2017) 548(7666):239–43. doi: 10.1038/nature23297
18. Dankner M, Rose AAN, Rajkumar S, Siegel PM, Watson IR. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene (2018) 37(24):3183–99. doi: 10.1038/s41388-018-0171-x
19. Marchetti A, Felicioni L, Malatesta S, Sciarrotta MG, Guetti L, Chella A, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol (2011) 29(26):3574–9. doi: 10.1200/JCO.2011.35.9638
20. Cui G, Liu D, Li W, Fu X, Liang Y, Li Y, et al. A meta-analysis of the association between BRAF mutation and nonsmall cell lung cancer. Medicine (2017) 96(14):e6552. doi: 10.1097/MD.0000000000006552
21. Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland A, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol (2017) 18(10):1307–16. doi: 10.1016/S1470-2045(17)30679-4
22. Dagogo-Jack I, Martinez P, Yeap BY, Ambrogio C, Ferris LA, Lydon C, et al. Impact of BRAF mutation class on disease characteristics and clinical outcomes in BRAF-mutant lung cancer. Clin Cancer Res (2019) 25(1):158–65. doi: 10.1158/1078-0432.CCR-18-2062
23. Tabbò F, Pisano C, Mazieres J, Mezquita L, Nadal E, Planchard D, et al. How far we have come targeting BRAF-mutant non-small cell lung cancer (NSCLC). Cancer Treat Rev (2022) 103. doi: 10.1016/j.ctrv.2021.102335
24. Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature (2010) 467(7315):596–9. doi: 10.1038/nature09454
25. Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. New Engl J Med (2010) 363(9):809–19. doi: 10.1056/NEJMoa1002011
26. Falchook GS, Lo0ng GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet (2012) 379(9829):1893–901. doi: 10.1016/S0140-6736(12)60398-5
27. Gilmartin AG, Bleam MR, Groy A, Moss KG, Minthorn EA, Kulkarni SG, et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res (2011) 17(5):989–1000. doi: 10.1158/1078-0432.CCR-10-2200
28. Kim KB, Kefford R, Pavlick AC, Infante JR, Ribas A, Sosman JA, et al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol (2013) 31(4):482–9. doi: 10.1200/JCO.2012.43.5966
29. Amaravadi RK, Schuchter LM, McDermott DF, Kramer A, Giles L, Gramlich K, et al. Phase II trial of temozolomide and sorafenib in advanced melanoma patients with or without brain metastases. Clin Cancer Res (2009) 15(24):7711–8. doi: 10.1158/1078-0432.CCR-09-2074
30. Carter CA, Chen C, Brink C, Vincent P, Maxuitenko YY, Gilbert KS, et al. Sorafenib is efficacious and tolerated in combination with cytotoxic or cytostatic agents in preclinical models of human non-small cell lung carcinoma. Cancer Chemother Pharmacol (2007) 59(2):183–95. doi: 10.1007/s00280-006-0257-y
31. Blumenschein GR, Gatzemeier U, Fossella F, Stewart DJ, Cupit L, Cihon F, et al. multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J Clin Oncol (2009) 27(26):4274–80. doi: 10.1200/JCO.2009.22.0541
32. Wakelee HA, Lee JW, Hanna NH, Traynor AM, Carbone DP, Schiller JH. A double-blind randomized discontinuation phase-II study of sorafenib (BAY 43-9006) in previously treated non-small-cell lung cancer patients: eastern cooperative oncology group study E2501. J Thorac Oncol (2012) 7(10):1574–82. doi: 10.1097/JTO.0b013e31826149ba
33. Subbiah V, Gervais R, Riely G, Hollebecque A, Blay J-Y, Felip E, et al. Efficacy of vemurafenib in patients with non-small-cell lung cancer with BRAF V600 mutation: an open-label, single-arm cohort of the histology-independent VE-BASKET study. JCO Precis Oncol (2019) 3. doi: 10.1200/PO.18.00266
34. Gautschi O, Milia J, Cabarrou B, Bluthgen MV, Besse B, Smit EF, et al. Targeted therapy for patients with BRAF-mutant lung cancer: results from the european EURAF cohort. J Thorac Oncol (2015) 10(10):1451–7. doi: 10.1097/JTO.0000000000000625
35. Mazieres J, Cropet C, Montané L, Barlesi F, Souquet JP, Quantin X, et al. Vemurafenib in non-small-cell lung cancer patients with BRAFV600 and BRAFnonV600 mutations. Ann Oncol (2020) 31(2):289–94. doi: 10.1016/j.annonc.2019.10.022
36. Planchard D, Kim TM, Mazieres J, Barlesi F, Souquet PJ, Quantin X, et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol (2016) 17(5):642–50. doi: 10.1016/S1470-2045(16)00077-2
37. Planchard D, Besse B, Groen HJM, Souquet PJ, Quoix E, Baik CS, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol (2016) 17(7):984–93. doi: 10.1016/S1470-2045(16)30146-2
38. Planchard D, Besse B, Kim TM, Quoix EA, Souquet PJ, Mazieres J, et al. Updated survival of patients (pts) with previously treated BRAF V600E–mutant advanced non-small cell lung cancer (NSCLC) who received dabrafenib (D) or D + trametinib (T) in the phase II BRF113928 study. J Clin Oncol (2017) 35(15_suppl):9075–5. doi: 10.1200/JCO.2017.35.15_suppl.9075
39. Planchard D, Besse B, Groen HJM, Hashemi SMS, Mazieres J, Kim TM, et al. Phase 2 study of dabrafenib plus trametinib in patients with BRAF V600E-mutant metastatic NSCLC: updated 5-year survival rates and genomic analysis. J Thorac Oncol (2022) 17(1):103–15. doi: 10.1016/j.jtho.2021.08.011
40. Luebker SA, Koepsell SA. Diverse mechanisms of BRAF inhibitor resistance in melanoma identified in clinical and preclinical studies. Front Oncol (2019) 9:268. doi: 10.3389/fonc.2019.00268
41. Villanueva J, Vultur A, Herlyn M. Resistance to BRAF inhibitors: unraveling mechanisms and future treatment options. Cancer Res (2011) 71(23):7137–40. doi: 10.1158/0008-5472.CAN-11-1243
42. Johannessen CM, Boehm JS, Kim SY, Thoma SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature (2010) 468(7326):968–72. doi: 10.1038/nature09627
43. Rudin CM, Hong K, Streit M. Molecular characterization of acquired resistance to the BRAF inhibitor dabrafenib in a patient with BRAF-mutant non-small-cell lung cancer. J Thorac Oncol (2013) 8(5):e41–2. doi: 10.1097/JTO.0b013e31828bb1b3
44. Abravanel DL, Nishino M, Sholl LM, Ambrogio C, Awad MM. An acquired NRAS Q61K mutation in BRAF V600E-mutant lung adenocarcinoma resistant to dabrafenib plus trametinib. J Thorac Oncol (2018) 13(8):e131–3. doi: 10.1016/j.jtho.2018.03.026
45. Niemantsverdriet M, Schuuring E, ter EA, van der Wekken AJ, van Kempen LC, van den Berg A, et al. KRAS mutation as a resistance mechanism to BRAF/MEK inhibition in NSCLC. J Thorac Oncol (2018) 13(12):e249–51. doi: 10.1016/j.jtho.2018.07.103
46. Chan XY, Singh A, Osman N, Piva TJ. Molecular sciences role played by signalling pathways in overcoming BRAF inhibitor resistance in melanoma. Int J Mol Sci (2017) 18(7):1527. doi: 10.3390/ijms18071527
47. Facchinetti F, Lacroix L, Mezquita L, Scoazec JY, Loriot Y, Tselikas L, et al. Molecular mechanisms of resistance to BRAF and MEK inhibitors in BRAF V600E nonesmall cell lung cancer. Eur J Cancer (2020) 132:211–23. doi: 10.1016/j.ejca.2020.03.025
48. Sheikine Y, Pavlick D, Klempner SJ, Trabucco SE, Chung JH, Rosenzweig M, et al. BRAF in lung cancers: analysis of patient cases reveals recurrent BRAF mutations, fusions, kinase duplications, and concurrent alterations. JCO Precis Oncol (2018) 2. doi: 10.1200/PO.17.00172
49. Lin L, Asthana S, Chan E, Bandyopadhyay S, Martins MM, Olivas V, et al. Mapping the molecular determinants of BRAF oncogene dependence in human lung cancer. Proc Natl Acad Sci U S A. (2014) 111(7):E748–57. doi: 10.1073/pnas.1320956111
50. Wang VE, Xue JY, Frederick DT, Cao Y, Lin E, Wilson C, et al. Adaptive resistance to dual BRAF/MEK inhibition in BRAF-driven tumors through autocrine FGFR pathway activation. Clin Cancer Res (2019) 25(23):7202–17. doi: 10.1158/1078-0432.CCR-18-2779
51. Ortiz-Cuaran S, Mezquita L, Swalduz A, Aldea M, Mazieres J, Leonce C, et al. Circulating tumor DNA genomics reveal potential mechanisms of resistance to BRAF-targeted therapies in patients with BRAF-mutant metastatic non-small cell lung cancer. Clin Cancer Res (2020) 26(23):6242–53. doi: 10.1158/1078-0432.CCR-20-1037
52. Abdayem P, Planchard D. Update on molecular pathology and role of liquid biopsy in nonsmall cell lung cancer. Eur Respir Review. (2021) 30(161). doi: 10.1183/16000617.0294-2020
53. Abdayem P, Planchard D. Ongoing progress in BRAF-mutated non-small cell lung cancer. Clin Adv Hematol Oncol (2022) 20(11):662–72.
54. Cohen D, Hondelink LM, Solleveld-Westerink N, Uljee SM, Ruano D, Cleton-Jansen Marie A, et al. Optimizing mutation and fusion detection in NSCLC by sequential DNA and RNA sequencing. J Thorac Oncol (2020) 15(6):1000–14. doi: 10.1016/j.jtho.2020.01.019
55. Calles A, Riess JW, Brahmer JR. Checkpoint blockade in lung cancer with driver mutation: choose the road wisely. Am Soc Clin Oncol Educ Book. (2020) 40):372–84. doi: 10.1200/edbk_280795
56. Imyanitov EN, Iyevleva AG, Levchenko EN. Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit Rev Oncol Hematol (2021) 157. doi: 10.1016/j.critrevonc.2020.103194
57. Ding X, Zhang Z, Jiang T, Li X, Zhao C, Su B, et al. Clinicopathologic characteristics and outcomes of Chinese patients with non-small-cell lung cancer and BRAF mutation. Cancer Med (2017) 6(3):555–62. doi: 10.1002/cam4.1014
58. Villaruz LC, Socinski MA, Abberbock S, Berry LD, Johnson BE, Kwiatkowski DJ, et al. Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the Lung Cancer Mutation Consortium. Cancer (2015) 121(3):448–56. doi: 10.1002/cncr.29042
59. Qu J, Shen Q, Li Y, Kalyani FS, Liu L, Zhou J, et al. Clinical characteristics, co-mutations, and treatment outcomes in advanced non-small-cell lung cancer patients with the BRAF-V600E mutation. Front Oncol (2022) 12:911303. doi: 10.3389/fonc.2022.911303
60. Myall NJ, Henry S, Wood D, Neal JW, Han SS, Padda SK, et al. Natural disease history, outcomes, and co-mutations in a series of patients with BRAF-mutated non–small-cell lung cancer. Clin Lung Cancer. (2019) 20(2):e208–17. doi: 10.1016/J.CLLC.2018.10.003
61. Kron A, Riedel R, Michels S, Fassunke J, Merkelbach-Bruse S, Scheffler M, et al. Impact of co-occurring genomic alterations on overall survival of BRAF V600E and non-V600E mutated NSCLC patients: Results of the Network Genomic Medicine. Ann Oncol (2017) 28:v461–2. doi: 10.1093/annonc/mdx380.003
62. Kinno T, Tsuta K, Shiraishi K, Mizukami T, Suzuki M, Yoshida A, et al. Clinicopathological features of nonsmall cell lung carcinomas with BRAF mutations. Ann Oncol (2014) 25(1):138–42. doi: 10.1093/annonc/mdt495
63. Zhang L, Zheng L, Yang Q, Sun J. The evolution of BRAF activation in non-small-cell lung cancer. Front Oncol (2022) 12:882940. doi: 10.3389/fonc.2022.882940
64. Song Z, Yu X, Cheng G, Zhang Y. Programmed death-ligand 1 expression associated with molecular characteristics in surgically resected lung adenocarcinoma. J Transl Med (2016) 14(1):188. doi: 10.1186/s12967-016-0943-4
65. Lan B, Ma C, Zhang C, Chai S, Wang P, Ding L, et al. Association between PD-L1 expression and driver gene status in non-small-cell lung cancer: a meta-analysis. Oncotarget (2018) 9(7):7684–99. doi: 10.18632/oncotarget.23969
66. Tseng JS, Yang TY, Wu CY, Ku WH, Chen KC, Hsu KH, et al. Characteristics and predictive value of PD-L1 status in real-world non–small cell lung cancer patients. J Immunother (2018) 41(6):292–9. doi: 10.1097/CJI.0000000000000226
67. Dudnik E, Peled N, Nechushtan H, Wollner M, Onn A, Agbarya A, et al. BRAF mutant lung cancer: programmed death ligand 1 expression, tumor mutational burden, microsatellite instability status, and response to immune check-point inhibitors. J Thorac Oncol (2018) 13(8):1128–37. doi: 10.1016/j.jtho.2018.04.024
68. Mazieres J, Drilon A, Lusque A, Mhanna L, A, Cortot B, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol (2019) 30(8):1321–8. doi: 10.1093/annonc/mdz167
69. Guisier F, Dubos-Arvis C, Viñas F, Doubre H, Ricordel C, Ropert S, et al. Efficacy and safety of anti–PD-1 immunotherapy in patients with advanced NSCLC with BRAF, HER2, or MET mutations or RET translocation: GFPC 01-2018. J Thorac Oncol (2020) 15(4):628–36. doi: 10.1016/j.jtho.2019.12.129
70. Rihawi K, Giannarelli D, Galetta D, Delmonte A, Giavarra M, Turci D, et al. BRAF mutant NSCLC and immune checkpoint inhibitors: results from a real-world experience. J Thorac Oncol (2019) 14(3):e57–9. doi: 10.1016/j.jtho.2018.11.036
71. Offin M, Pak T, Mondaca S, Montecalvo J, Rekhtman N, Halpenny D, et al. P1.04-39 molecular characteristics, immunophenotype, and immune checkpoint inhibitor response in BRAF non-V600 mutant lung cancers. J Thorac Oncol (2019) 14(10):S455. doi: 10.1016/j.jtho.2019.08.942
72. Hellmann M, Rizvi N, Wolchok JD, Chan TA. Genomic profile, smoking, and response to anti-PD-1 therapy in non-small cell lung carcinoma. Mol Cell Oncol (2016) 3(1):e1048929. doi: 10.1080/23723556.2015.1048929
73. Gainor JF, Rizvi H, Jimenez Aguilar E, Skoulidis F, Yeap BY, Naidoo J, et al. Clinical activity of programmed cell death 1 (PD-1) blockade in never, light, and heavy smokers with non-small-cell lung cancer and PD-L1 expression ≥50%. Ann Oncol (2020) 31(3):404–11. doi: 10.1016/j.annonc.2019.11.015
74. Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med (2006) 203(7):1651–6. doi: 10.1084/jem.20051848
75. Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res (2012) 18(5):1386–94. doi: 10.1158/1078-0432.CCR-11-2479
76. Frederick DT, Piris A, Cogdill AP, Cooper A, Lezcano C, Ferrone R, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res (2013) 19(5):1225–31. doi: 10.1158/1078-0432.CCR-12-1630
77. Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw C-NJ, Sloss CM, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res (2010) 70(13):5213–9. doi: 10.1158/0008-5472.CAN-10-0118
78. Poon E, Mullins S, Watkins A, Williams GS, Koopmann J-O, Genova Di G, et al. The MEK inhibitor selumetinib complements CTLA-4 blockade by reprogramming the tumor immune microenvironment. J Immunother Cancer. (2017) 5(1):63. doi: 10.1186/s40425-017-0268-8
79. Choi H, Deng J, Li S, Silk T, Dong L, Brea EJ, et al. Pulsatile MEK inhibition improves anti-tumor immunity and T cell function in murine kras mutant lung cancer. Cell Rep (2019) 27(3):806–819.e5. doi: 10.1016/j.celrep.2019.03.066
80. Niu X, Sun Y, Planchard D, Chiu L, Bai J, Ai X, et al. Durable response to the combination of atezolizumab with platinum-based chemotherapy in an untreated non-smoking lung adenocarcinoma patient with BRAF V600E mutation: A case report. Front Oncol (2021) 11:634920. doi: 10.3389/fonc.2021.634920
81. Hellmann MD, Kim TW, Lee CB, Goh BC, Miller WH, Oh DY, et al. Phase Ib study of atezolizumab combined with cobimetinib in patients with solid tumors. Ann Oncol (2019) 30(7):1134–42. doi: 10.1093/annonc/mdz113
82. A Study to Evaluate the Efficacy and Safety of Multiple Targeted Therapies as Treatments for Participants With Non-Small Cell Lung Cancer (NSCLC). Available at: https://clinicaltrials.gov/ct2/show/NCT03178552 (Accessed June 7, 2023).
83. FDA grants accelerated approval to dabrafenib in combination with trametinib for unresectable or metastatic solid tumors with BRAF V600E mutation. FDA. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid (Accessed September 12, 2022).
84. Riely GJ, Ahn MJ, Felip E, Ramalingam SS, Smit EF, Tsao AS, et al. Encorafenib plus binimetinib in patients with BRAFV600-mutant non-small cell lung cancer: Phase II PHAROS study design. Future Oncol (2022) 18(7):781–91. doi: 10.2217/fon-2021-1250
85. An Open-label Study of Encorafenib + Binimetinib in Patients With BRAFV600-mutant Non-small Cell Lung Cancer. Available at: https://clinicaltrials.gov/ct2/show/record/NCT03915951 (Accessed May 25, 2023).
86. FDA Accepts Pfizer’s Supplemental New Drug Applications for BRAFTOVI + MEKTOVI. Pfizer. Available at: https://www.pfizer.com/news/press-release/press-release-detail/fda-accepts-pfizers-supplemental-new-drug-applications (Accessed May 25, 2023).
87. Study Record. Available at: https://beta.clinicaltrials.gov/study/NCT04526782?tab=table (Accessed May 25, 2023).
88. A Phase Ib Study of LXH254-centric Combinations in NSCLC or Melanoma. Available at: https://clinicaltrials.gov/ct2/show/record/NCT02974725 (Accessed May 26, 2023).
89. Desai J, Gan H, Barrow C, Phase I, Jameson M, Atkinson V, Haydon A, et al. Open-label, Dose-Escalation/dose-expansion study of lifirafenib (BGB-283), an RAF family kinase inhibitor, in patients with solid tumors. J Clin Oncol (2020) 38(19):2140–50. doi: 10.1200/JCO.19.02654
90. HM95573 in combination With Either Cobimetinib or Cetuximab in Patients With Locally Advanced or Metastatic Solid Tumors. Available at: https://clinicaltrials.gov/ct2/show/NCT03284502 (Accessed September 12, 2022).
91. An Open-label Study of Encorafenib + Binimetinib in Patients With BRAFV600-mutant Non-small Cell Lung Cancer. Available at: https://clinicaltrials.gov/ct2/show/record/NCT03915951 (Accessed June 7, 2023).
92. Phase II Study Investigating the Combination of Encorafenib and Binimetinib in BRAF V600E Mutated Chinese Patients With Metastatic Non-Small Cell Lung Cancer. Available at: https://clinicaltrials.gov/ct2/show/record/NCT05195632 (Accessed June 7, 2023).
93. Study of the Safety and Pharmacokinetics of BGB-283 (Lifirafenib) and PD-0325901 (Mirdametinib) in Participants With Advanced or Refractory Solid Tumors. Available at: https://clinicaltrials.gov/ct2/show/record/NCT03905148 (Accessed June 7, 2023).
94. Study of Immunotherapy (Sasanlimab) in Combination With Targeted Therapies in People With Advanced Non-small Cell Lung Cancer (NSCLC) (Landscape 1011 Study). Available at: https://clinicaltrials.gov/ct2/show/record/NCT04585815 (Accessed June 7, 2023).
95. A Phase II Clinical Trial to Evaluate HLX208 in Advanced Non-small Cell Lung Cancer Patients With BRAF V600 Mutation. Available at: https://clinicaltrials.gov/ct2/show/NCT05065398 (Accessed June 7, 2023).
96. HM95573 in Combination With Either Cobimetinib or Cetuximab in Patients With Locally Advanced or Metastatic Solid Tumors. Available at: https://clinicaltrials.gov/ct2/show/NCT03284502 (Accessed June 7, 2023).
97. XP-102 and XP-102 in Combination With Trametinib in Advanced Solid Tumor Patients With a BRAF V600 Mutation. Available at: https://clinicaltrials.gov/ct2/show/NCT05275374 (Accessed June 7, 2023).
98. A Phase 1/2 Study of DCC-3116 in Patients With MAPK Pathway Mutant Solid Tumors. Available at: https://www.clinicaltrials.gov/ct2/show/record/NCT04892017?view=record (Accessed June 9, 2023).
99. A Study to Evaluate KIN-2787 in Participants With BRAF and/or NRAS Mutation Positive Solid Tumors. Available at: https://www.clinicaltrials.gov/ct2/show/record/NCT04913285 (Accessed June 9, 2023).
100. Recombinant EphB4-HSA Fusion Protein and Pembrolizumab, MK-3475. Available at: https://clinicaltrials.gov/ct2/show/record/NCT03049618 (Accessed June 11, 2023).
101. Expanded Access to Ulixertinib (BVD-523) in Patients With Advanced MAPK Pathway-Altered MALIgnancies. Available at: https://www.clinicaltrials.gov/ct2/show/record/NCT04566393?view=record (Accessed June 11, 2023).
102. Testing Trametinib as a Potential Targeted Treatment in Cancers With BRAF Genetic Changes (MATCH-Subprotocol R). Available at: https://www.clinicaltrials.gov/ct2/show/record/NCT04439279?view=record (Accessed June 11, 2023).
103. Study of Safety, Pharmacokinetics, and Antitumor Activity of BGB-3245 in Participants With Advanced or Refractory Tumors. Available at: https://www.clinicaltrials.gov/ct2/show/record/NCT04249843 (Accessed June 11, 2023).
104. Study of Ulixertinib for Patients With Advanced MALIgnancies Harboring MEK or Atypical BRAF Alterations. Available at: https://www.clinicaltrials.gov/ct2/show/record/NCT04488003 (Accessed June 11, 2023).
105. A Study of FORE8394 as a Single Agent in Patients With Advanced Unresectable Solid Tumors. Available at: https://www.clinicaltrials.gov/ct2/show/record/NCT02428712?view=record (Accessed June 12, 2023).
106. A Study of Binimetinib and Encorafenib in Advanced BRAF Mutant Cancers. Available at: https://www.clinicaltrials.gov/ct2/show/record/NCT03843775 (Accessed June 12, 2023).
Keywords: non-small cell lung cancer, BRAF mutation, BRAF mutation V600, non-V600 mutation, lung cancer
Citation: Puri M, Gawri K and Dawar R (2023) Therapeutic strategies for BRAF mutation in non-small cell lung cancer: a review. Front. Oncol. 13:1141876. doi: 10.3389/fonc.2023.1141876
Received: 10 January 2023; Accepted: 24 July 2023;
Published: 14 August 2023.
Edited by:
Sharon R. Pine, University of Colorado, United StatesReviewed by:
Le Son Tran, Medical Genetics Institute, VietnamCopyright © 2023 Puri, Gawri and Dawar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Megha Puri, mpuri@saintpetersuh.com
 Megha Puri
Megha Puri Kunal Gawri
Kunal Gawri Richa Dawar3
Richa Dawar3