- 1Department of Endocrinology, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China
- 2Department of Intensive Care Unit, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China
- 3Department of Orthopedics, Guangdong Provincial People's Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, China
- 4Department of Respiratory and Critical Care Medicine, Jinling Hospital, Nanjing University School of Medicine, Nanjing, China
Background: Protein-energy malnutrition (PEM) has been recognized as a poor prognostic factor in many clinical issues. However, nationwide population studies concerning the impact of PEM on outcomes after major cancer surgery (MCS) are lacking. We aimed to evaluate the postoperative outcomes associated with PEM following MCS.
Methods: By using the Nationwide Inpatient Sample database, data of patients undergoing MCS including colectomy, cystectomy, esophagectomy, gastrectomy, hysterectomy, lung resection, pancreatectomy, or prostatectomy were analyzed retrospectively from 2009 to 2015, resulting in a weighted estimate of 1,335,681 patients. The prevalence trend of PEM, as well as mortality and major complications after MCS were calculated. Multivariable regression analysis was applied to estimate the impact of PEM on postoperative outcomes after MCS.
Results: PEM showed an estimated annual percentage increase of 7.17% (95% confidence interval (CI): 4-10.44%) from 2009 to 2015, which contrasts with a 4.52% (95% CI: -6.58–2.41%) and 1.21% (95% CI: -1.85–0.56%) annual decrease in mortality and major complications in patients with PEM after MCS. PEM was associated with increased risk of mortality (odds ratio (OR)=2.26; 95% CI: 2.08-2.44; P < 0.0001), major complications (OR=2.46; 95% CI: 2.36-2.56; P < 0.0001), higher total cost ($35814 [$22292, $59579] vs. $16825 [$11393, $24164], P < 0.0001), and longer length of stay (14 [9-21] days vs. 4 [2-7] days, P < 0.0001), especially in patients underwent prostatectomy, hysterectomy and lung resection.
Conclusions: PEM was associated with increased worse outcomes after major cancer surgery. Early identification and timely medical treatment of PEM for patients with cancer are crucial for improving postoperative outcomes.
1. Introduction
Protein-energy malnutrition (PEM), caused by depleted energy and nutrient stores, often leads to alterations in body weight and composition and compromised functioning (1). PEM has been recognized as a poor prognostic factor in many clinical issues, such as acute myocardial infarction, sepsis, and heart failure (1–3). Consequently, the importance of identification and management of PEM has been highlighted in recent years.
Cancer is a major public health problem worldwide and has been the second leading cause of death in the United States (4). In China, cancer death accounted for 24% of all-cause of death during 2014 to 2018 and is the leading cause of death in the population less than 65 years old (5). Metabolic diseases, such as obesity and diabetes, are vital risk factors for cancers, which may resulted from energy imbalance and inflammation (6, 7). Patients with cancer are at a particularly high risk of malnutrition. The etiology is complicated, including impaired food intake due to host and therapeutic factors, increased energy and protein demands, and metabolic abnormalities (8). Although there is a relatively high prevalence of malnutrition ranging from 20% to more than 70% in patients with cancer, only 30-60% of those at risk of malnutrition received nutritional support (8).
Surgery, one of the major cancer treatments, can negatively regulate nutrition status due to the catabolic impact of the surgery itself, inflammation induction, and enhanced metabolic stress response (9). Malnutrition is associated with negative clinical outcomes following certain cancer surgeries such as esophagectomy, gastrectomy, colectomy, hepatectomy, pancreatectomy, lung resection, cystectomy, and hysterectomy (10–16). However, nationwide population studies on the impact of PEM on outcomes after major cancer surgery (MCS) are lacking.
Therefore, we used National Inpatient Sample (NIS) database to explore: 1) prevalence and temporal trends of PEM who underwent MCS; 2) the impact of PEM on mortality, major complications, total cost, and length of stay (LOS) after MCS; 3) the influence of surgical type on the perioperative outcomes of PEM patients.
2. Methods
2.1. Data source and study population
It is a retrospective cohort study investigating the influence of PEM on perioperative outcomes in patients undergoing MCS. Patients aged 18-90 years old who were admitted between January 1st, 2009 to December 31st, 2015 and primarily for MCS were included from NIS database. The NIS database is the largest all-payer administrative database that includes a 20% stratified sample of United States inpatient hospitalizations from nonfederal community hospitals (17). The NIS database provides information including patient features, primary diagnosis, up to 29 secondary diagnoses, up to 15 inpatient procedures, hospitalization costs, and LOS (1). We selected a total of eight major surgical oncological procedures (colectomy, cystectomy, esophagectomy, gastrectomy, hysterectomy, lung resection, pancreatectomy, and prostatectomy) as MCS and evaluated their perioperative outcomes. Relying on specific International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes, each surgical procedure was assessed independently, and analyses were restricted to cancer diagnoses only (Supplementary Table 1) (18).
2.2. Ethical approval
The data collected in the present study is from an open access database, where the ethics approval and consent to participated had been made when the database setup. Hence, it is not applicable in the present study.
2.3. Outcomes
The primary outcome was perioperative outcomes, which included mortality, major complications, total costs, and LOS. Mortality, total costs, and LOS were directly extracted from NIS database. Major complications were identified through ICD-9-CM diagnosis codes, defined as pneumonia, pulmonary embolism, renal failure, acute ischemic stroke, acute myocardial infarction, cardiac arrest, adult respiratory distress syndrome, sepsis, and septic shock (Supplementary Table 2).
2.4. Predictor
PEM (primary predictor) was identified with ICD-9-CM diagnosis codes (260, 261, 262, 263, 2698, 7994, 7833, 7837, 78321, 78322), which included kwashiorkor, marasmus, cachexia, other severe protein-calorie malnutrition (severe and unspecified), adult failure to thrive, loss of weight, and underweight. These set of diagnosis codes is recommended by the Academy of Nutrition and Dietetics, and the American Society for Parenteral and Enteral Nutrition and have been used by many studies (1–3, 19).
2.5. Patient and hospital characteristics
For all patients, the following independent variables were potential confounders and were available for analyses: patient age at hospitalization, sex, elective status, race (white, black, Hispanic, other (Asian, Pacific Islander, or Native American), or unknown), insurance status, income quartile, hospital type, hospital region, hospital bed size, baseline comorbidities, and type of cancer surgery. All the potential confounders were identified either as already present in NIS database or clinical classification software codes to abstract them from the diagnosis variables (2).
Patient age was regarded as a continuous variable. Insurance categories included Medicare, Medicaid, private insurance, and other insurance types (self-pay). Income was stratified into four quartiles based on the average annual household income of the zip code of residence (0-25th, 26-50th, 51-75th, and 76-100th quartiles). The hospital type was categorized by the hospital’s teaching status (rural, urban non-teaching, and teaching). The Hospital region included the Northeast, Midwest, South, and West regions. Hospitals bed size was stratified as small, medium and large hospital size (20). Baseline comorbidities were quantified using an Elixhauser comorbidity index (ECI) (21). Elixhauser comorbid conditions included: alcohol abuse, acquired immune deficiency syndrome, deficiency anemias, rheumatoid arthritis/collagen vascular diseases, chronic blood loss anemia, congestive heart failure, chronic pulmonary disease, coagulopathy, depression, diabetes without complications, diabetes with chronic complications, drug abuse, hypertension (uncomplicated and complicated), hypothyroidism, liver disease, lymphoma, fluid and electrolyte disorders, obesity, other neurological disorders, paralysis, peripheral vascular disorders, psychoses, pulmonary circulation disorders, renal failure, peptic ulcer disease excluding bleeding, valvular disease, and weight loss (Supplementary Table 3).
2.6. Statistical analysis
All analyses were performed on the provided NIS population (268,595 individuals), and P-values were calculated for the weighted population (1,335,681 individuals). Descriptive statistics were generated on frequencies and proportions of categorical variables (gender, type of admission, race, insurance status, median zip code household income, hospital teaching status, hospital region, hospital bed size, ECI, and type of cancer surgery) and stratified according to PEM occurrence. Means were reported for continuously coded variables (age). Chi-square tests were applied to compare the statistical significance of differences within categorical variables. Temporal trends in rates were analyzed by the estimated annual percent change (EAPC) using linear regression analyses. To further investigate the relationship between PEM and outcomes after MCS, we used multivariable logistic regression models adjusted for age, sex, race, type of insurance, elective status, income quartile, hospital type, hospital region, hospital bed size, ECI, and surgical type. Subgroup analyses stratified by surgical type were applied. Sensitivity analyses were performed to test the robustness of our findings. We reassessed the relationship between PEM and clinical outcomes in patients undergoing MCS based on a double robust inverse probability of treatment weighting method (22). The probability of treatment or propensity score was calculated using multivariable logistic regression models adjusted for the aforementioned variables. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC). Statistical significance was defined as a P-value < 0.05 on two-tailed testing.
3. Results
3.1. Baseline descriptive statistics
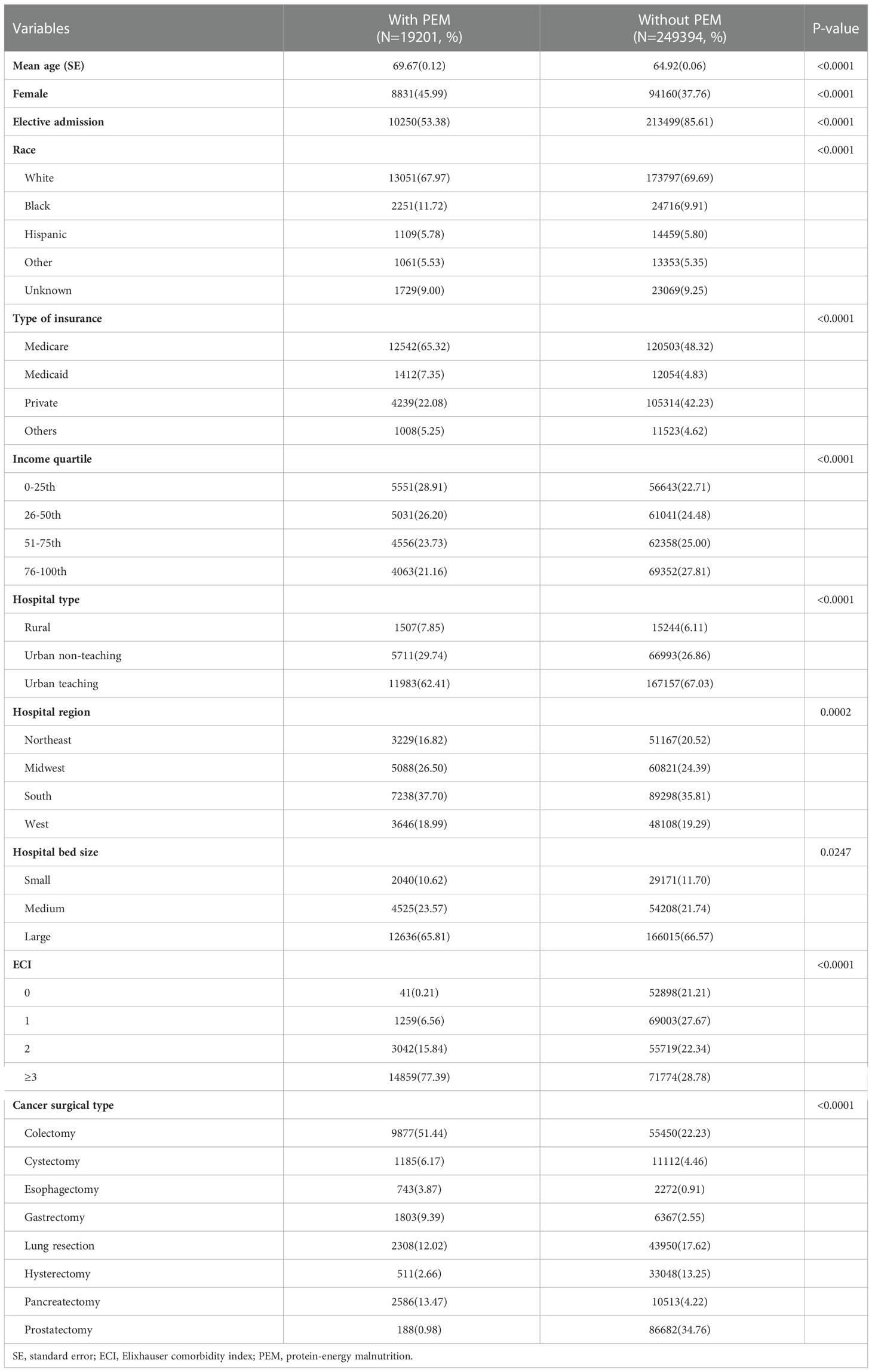
A total of 268,595 (weighted 1,335,681) patients who underwent MCS were selected from 2009 to 2015 of NIS database. Among them, 7.1% of patients had PEM. Patient with PEM were older, more likely to be female, higher percentage of black subjects, more likely to have Medicare as their primary health insurance and a lower income (Table 1). It was not surprising that patients with PEM had a higher comorbidity burden with a greater proportion of patients with ECI ≥ 3 (77.39% vs. 28.78%, P < 0.0001) (Table 1). As shown in Supplementary Table 3, almost all of the Elixhauser comorbid conditions were statistically significant between patients who underwent MCS with and without PEM (P < 0.05 for all).
Concerning the type and admission of surgery, patients with PEM had lower proportion of elective admission (53.38% vs. 85.61%, P < 0.0001) with highest proportion of colectomy (51.44%), followed by pancreatectomy (13.47%), lung resection (12.02%), gastrectomy (9.39%), cystectomy (6.17%), esophagectomy (3.87%), hysterectomy (2.66%) and prostatectomy (0.98%) (Table 1). Patients who underwent operations for gastrointestinal (GI) cancers had the highest prevalence of PEM. Esophageal cancer ranked first (24.6502%), gastric cancer ranked second (22.029%), followed by pancreatic cancer (19.7319%), and colon cancer (15.1097%). Patients treated surgically for lung cancer (4.9766%) and bladder cancer (9.6109%) had moderate rates of PEM. Patients who underwent operations for uterine cancer (1.5188%) and prostate cancer (0.2171%) had the lowest rates of PEM (Figure 1).
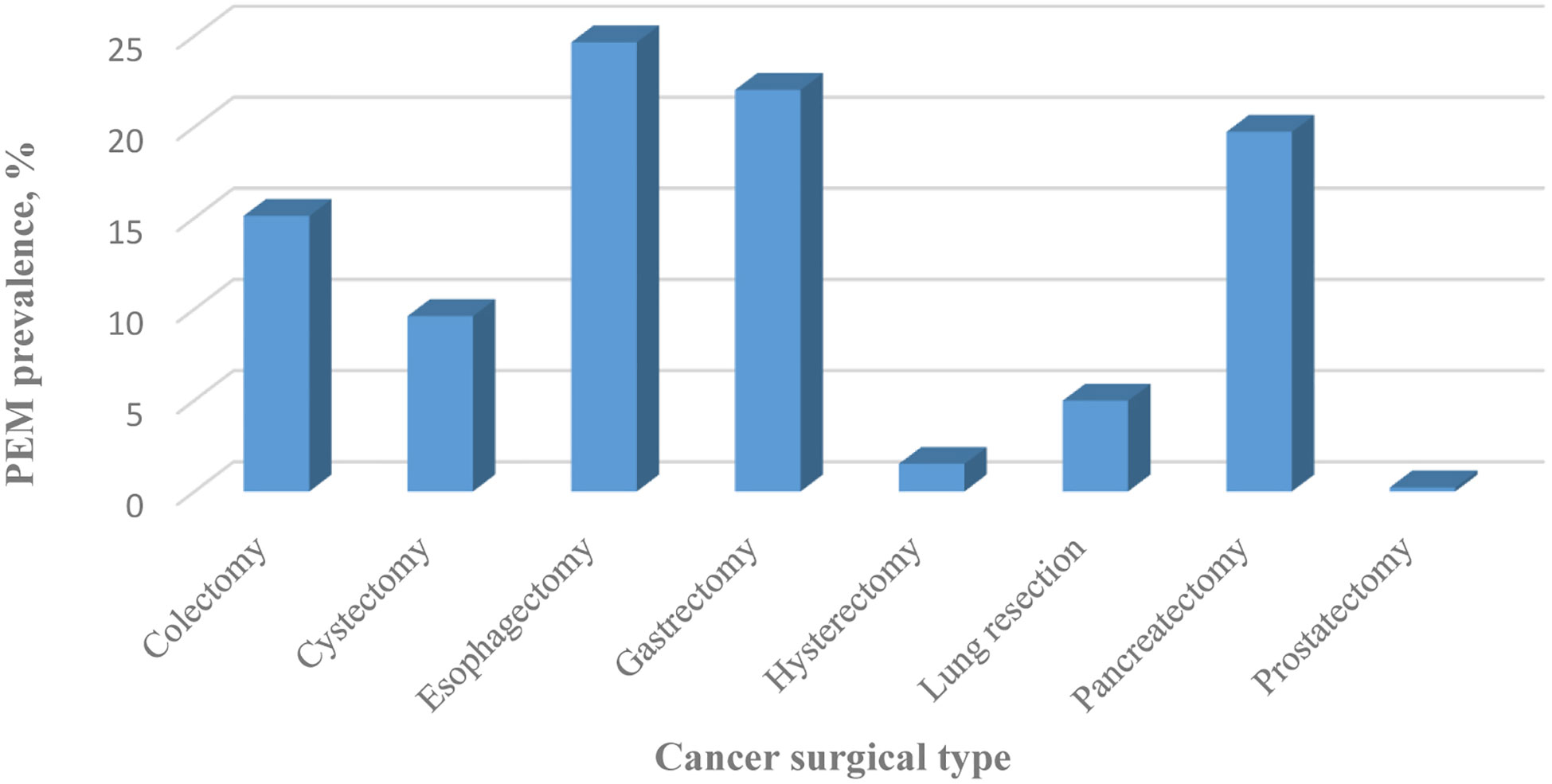
Figure 1 Prevalence of PEM classified by cancer surgery type between 2009 and 2015 in the United States.
3.2. Temporal trends of PEM, mortality and major complications
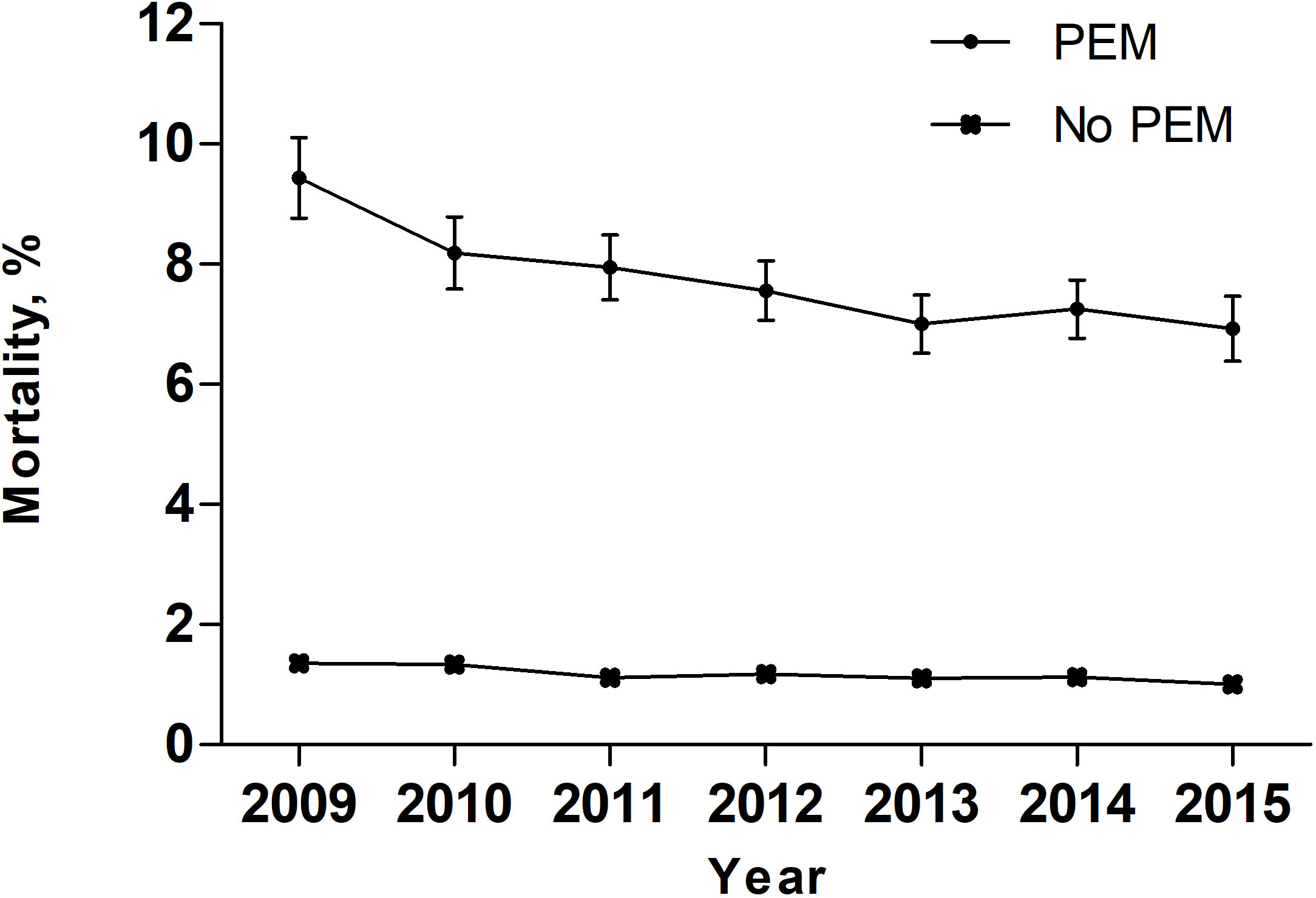
Over the entire study period, temporal trend analyses showed that the EAPC of PEM was +7.17% (95% confidence interval [CI]: 4-10.44; P = 0.0019) (Figure 2). During the same period, the EAPC of mortality in patients with PEM was -4.52% (95% CI: -6.58–2.41, P < 0.01) while the EAPC of mortality in patients without PEM was -4.21% (95% CI: -6.68–1.68, P < 0.01) (Figure 3). Meanwhile, the EAPC of major complications in patients with PEM was -1.21% (95% CI: -1.85–0.56, P = 0.0048), and the EAPC of major complications in patients without PEM showed no significant change (EAPC = 1.45, 95% CI: -0.43-3.36, P = 0.1046) (Figure 4).
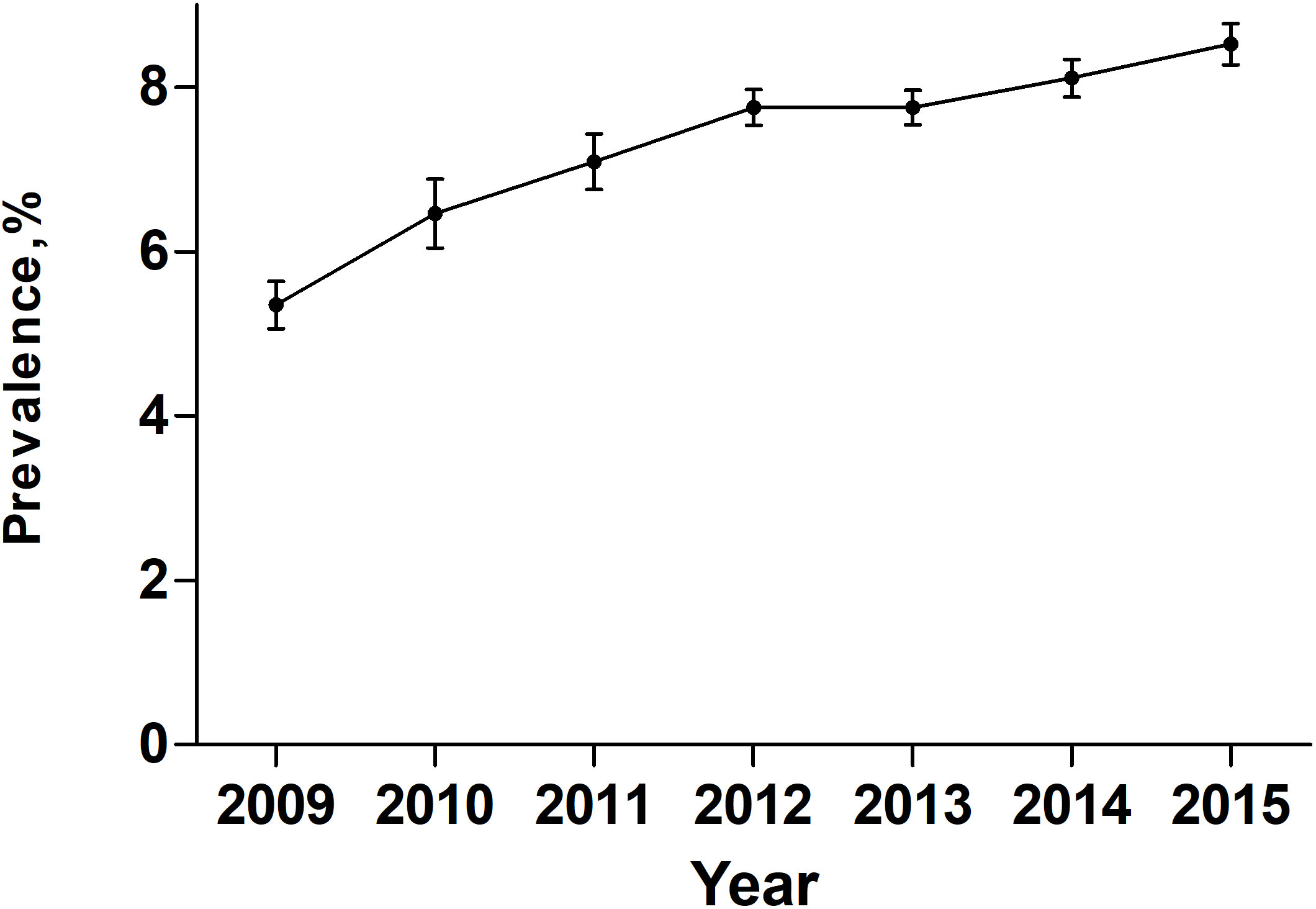
Figure 2 Prevalence of PEM in patients undergoing major cancer surgery patients between 2009 and 2015 in the United States.

Figure 4 Major complications in patients with and without PEM between 2009 and 2015 in the United States.
3.3. Perioperative outcomes after MCS in patient with PEM
Patients with PEM had poorer perioperative outcomes after MCS. The mortality rate was 7.77% in patients with PEM, which was 2.26-fold higher than those without PEM (1.19%) (odds ratio [OR]= 2.26, 95% CI: 2.08-2.44, P<0.0001) (Table 2). Moreover, PEM was associated with higher total cost ($35814 vs. $16825, P < 0.0001) and longer LOS (14 days vs. 4 days, P < 0.0001) (Table 2).
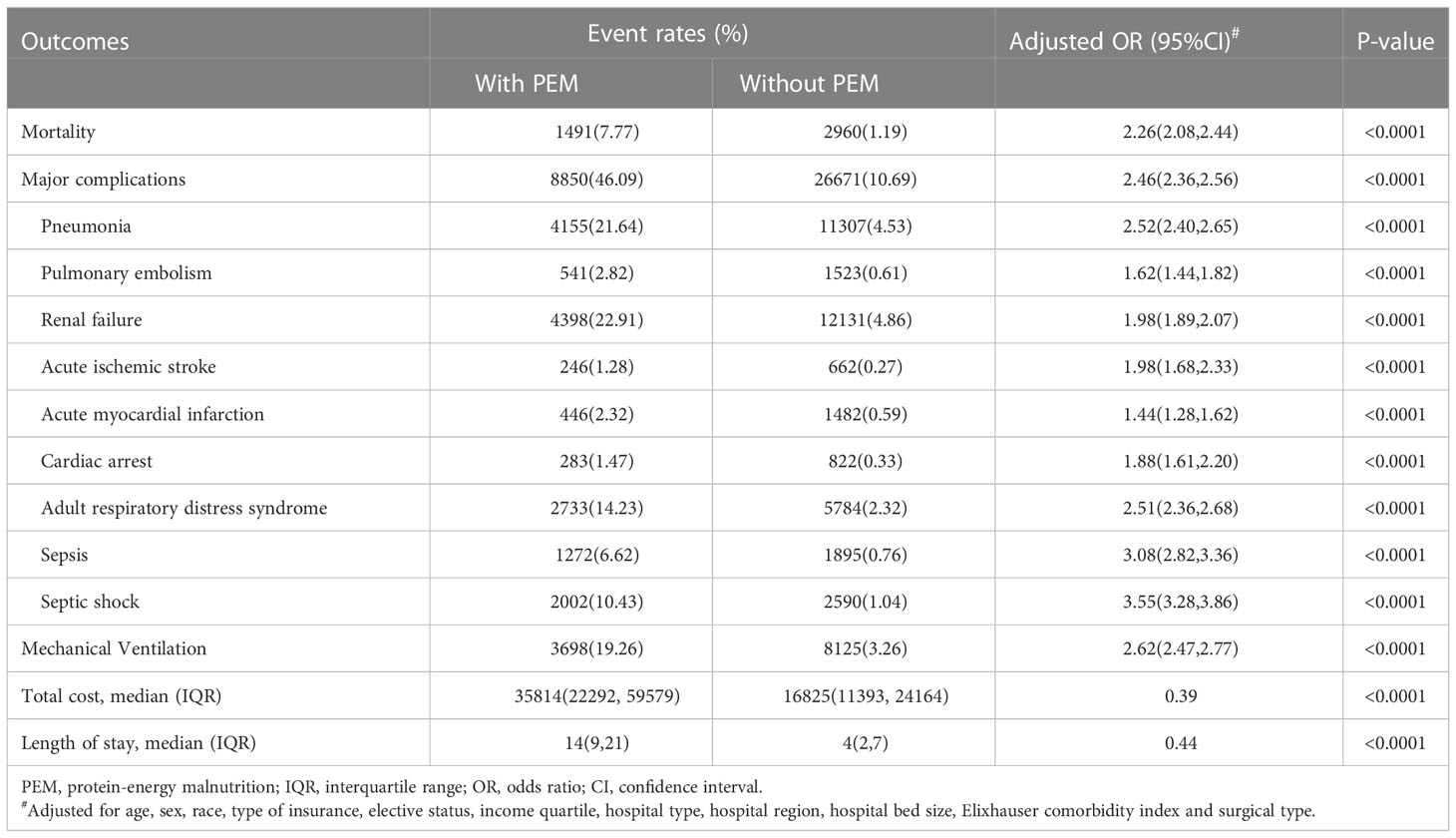
Table 2 Comparison of clinical outcomes following major cancer surgery in patients with and without PEM.
Considering major complications, PEM group showed a 2.46-fold increase of risk when compared with non-PEM group (OR=2.46, 95% CI: 2.36-2.56, P < 0.0001) (Table 2). More specifically, renal failure (22.91%), pneumonia (21.64%), adult respiratory distress syndrome (14.23%), and septic shock (10.43%) were most common in the PEM group. When compared with non-PEM group, patients with PEM had higher risk of septic shock (OR=3.55, 95%CI: 3.28-3.86) and sepsis (OR=3.08, 95%CI: 2.82-3.36), followed by pneumonia (OR=2.52, 95%CI: 2.40-2.65), adult respiratory distress syndrome (OR= 2.51, 95%CI: 2.36-2.68), renal failure (OR=1.98, 95%CI: 1.89-2.07), acute ischemic stroke (OR=1.98, 95%CI: 1.68-2.33), cardiac arrest (OR=1.88, 95%CI: 1.61-2.20), pulmonary embolism (OR=1.62, 95%CI: 1.44-1.82) and acute myocardial infarction (OR=1.44, 95%CI: 1.28-1.62). Moreover, patients with PEM showed a 2.62-fold increase in the need for mechanical ventilation after MCS compared with patients without PEM (OR=2.62, 95%CI: 2.47-2.77, P < 0.0001) (Table 2).
3.4. The influence of surgical type on perioperative outcomes
In order to investigate the influence of surgical type on the perioperative outcomes of PEM patients, subgroup analysis was conducted. The rate of mortality varied among surgical types (Supplementary Table 4). PEM patients underwent lung resection (10.27%) and colectomy (8.35%) had the highest mortality rate while those underwent prostatectomy had the lowest mortality (1.6%).
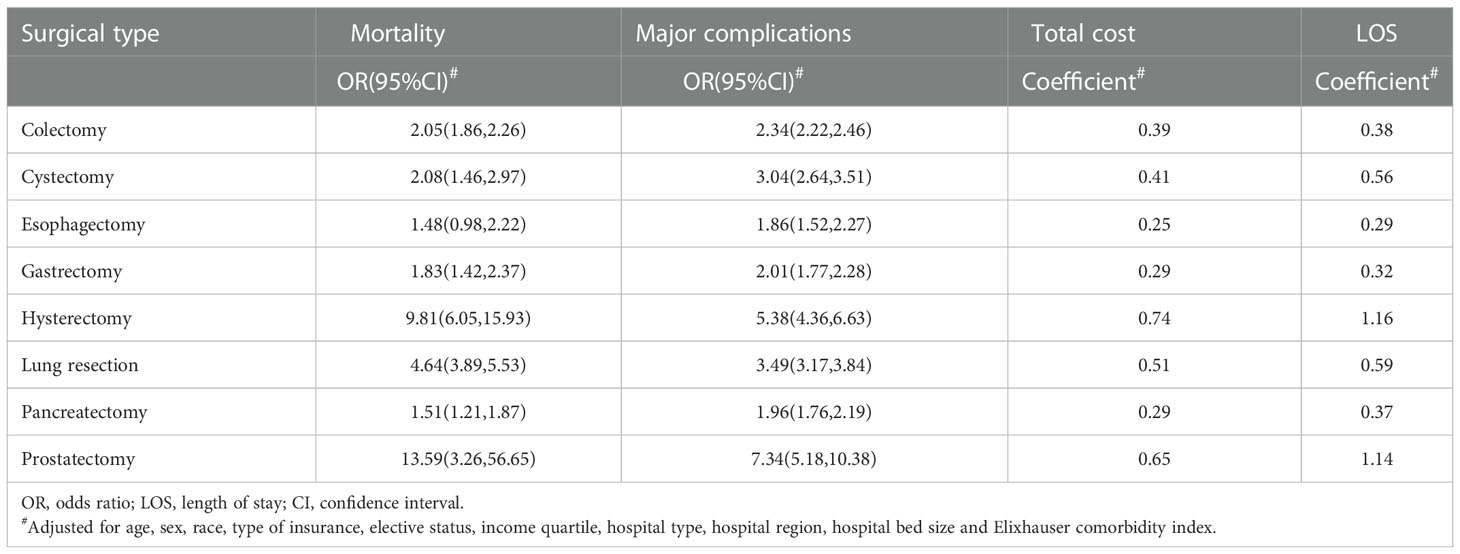
The risk of mortality and major complications also varied among surgical types. Patients with PEM underwent prostatectomy had the highest risk of mortality (OR=13.59, 95%CI: 3.26-56.65), and major complications (OR=7.34, 95%CI: 5.18-10.38), followed by patients underwent hysterectomy (mortality: OR=9.81; major complications, OR=5.38) and lung resection (mortality: OR=4.64; major complications, OR=3.49), which were all non-GI operations (Table 3). On the other hand, gastrointestinal operations, such as colectomy, esophagectomy, gastrectomy, pancreatectomy and cystectomy, had relatively lower risk (1-2 folds) of perioperative mortality in patients with PEM (Table 3).
3.5. Sensitivity analysis
In order to eliminate the influence of residual confounders on the robustness of the results, a sensitivity analysis was conducted. All the results, including mortality, major complications, total costs, and LOS remained statistically significant after the double robust inverse probability of treatment weighting method (Supplementary Table 5).
4. Discussion
In the present study, we found the rate of PEM in patients underwent MSC was 7.1% by analyzing data of more than 1 million patients from NIS database. Patients with PEM were older, more likely to be female, higher percentage of black subjects, a lower income, lower proportion of elective admission and higher proportion of operations for GI cancers. The EAPC of PEM was +7.17%. PEM patients had higher risk of mortality and major complications, as well as higher total cost and longer LOS when compared with non-PEM patients after MCS. PEM patients who underwent lung resection and colectomy had the highest mortality rate while PEM significantly increased the risk of mortality and major complications in those underwent prostatectomy, hysterectomy and lung resection.
PEM is a common problem in cancer patients and has been recognized as a poor prognostic factor of postoperative complications and death (23). In the past decade, early identification and prevention of PEM have attracted increasing attention, many screening tools for malnutrition and guidelines for clinical nutrition in cancer have been advanced (24). In the present study, we reported that the prevalence of PEM in patients undergoing MCS was 7.1% (Table 1), which is much lower than other reports to focus on the prevalence of malnutrition in patients with cancer (20-70%) (8). The inconsistency of PEM prevalence was contributing to difference of cancer stage, cancer type and patient age (25). It is reported that the prevalence of moderate and severe malnutrition in stage III and IV patients was 79%, which is significantly higher than in stage I and II patients (3%) (26). Since relatively early-stage cancers are indicated for surgery, the impact of cancer on nutrition for those who undergo MCS is less than those in the late stages of cancer. Our study also indicated that subjects with relative early-stage cancer and PEM were more likely to be older, female, black, have low incomes, receive the operation in rural, urban non-teaching hospitals and lower-volume centers, and have more comorbidities, and were less likely on private insurance (Table 1). The difference in PEM rates among patients with different races, income statuses, properties and regions of hospitals, and types of insurance may be attributable to socioeconomic factors. Concerning female PEM patients, accumulating evidence suggests that vitamin disbalance play an important role in women’s health and nutraceutical supplementation is an effective way to improve the situation (27, 28). Our results highlight the importance of targeting such groups who are susceptible to malnutrition and may lack nutrition support.
As cancer-related malnutrition is still largely unidentified, underestimated, and undertreated in clinical practice, many screening tools have been recommended. Groups including the European Society for Clinical Nutrition and Metabolism and the American Cancer Society have been developing guidelines regarding nutrition in cancer patients (29, 30). Our study revealed that the prevalence of PEM among patients for MCS was continuously risen. As the importance of assessing nutritional status before cancer surgery has gained more notice by surgeons, there is reason to believe that the increasing prevalence of PEM is owing to improvements in its detection rate. Meanwhile, the mortality rate in both the PEM and non-PEM groups decreased from 2009 to 2015, and the EAPC of mortality was -4.52 and -4.21%, respectively, which implies the rate of mortality decrease seen in the PEM group exceeds that of the non-PEM group. Notably, other studies have also shown a decreasing trend in mortality after MCS from 1999 to 2009, with a reported EAPC of -2.4%. During the same period, the overall mortality in all patients undergoing MCS was 2% (31). This study extends this knowledge. Meanwhile, a declining EAPC of major complications is only seen in the PEM group (-1.21%, 95% CI [-1.85–0.56], P < 0.01). This suggests that improved methods for the identification, prevention, and treatment of malnutrition in cancer patients have already made some difference.
Despite great advances in surgical techniques, postoperative recovery of cancer patients is tortuous, where malnutrition plays a major role (32). Our nationwide data analysis revealed that patients with PEM had a 2.26-fold risk of mortality compared to those without PEM after MCS, which was consistent with previous studies focusing on one specific cancer. Data analysis based on American College of Surgeons-National Surgical Quality Improvement Program from 2009 to 2013 indicated that patients with mild hypoalbuminemia, an indicator for malnutrition, had significantly higher postoperative mortality rates of colorectal cancer than those with normal albumin levels (OR=1.74; P < 0.001) (33). Furthermore, we made subgroup analysis to explore the influence of surgical type on mortality of PEM patients. Noteworthily, PEM patients had significantly high risk of mortality when undergoing non-GI surgery, including prostatectomy, hysterectomy and lung resection (Table 3). It is reasonable that malnutrition is more common in patients with GI cancers due to GI side effects of nausea, vomiting, anorexia, diarrhea, dysphagia, and malabsorption (34). However, once PEM occurs in patients with non-GI cancers, it always means that the patient’s physical condition is very poor; therefore, the impact of PEM may be more pronounced in such cases. Besides, it is reported that prostate cancers and cancers involving uterine corpus are generally diagnosed at lower stages and grades. In contrast, esophageal cancer and pancreatic cancer are generally diagnosed at later stages and are related to lower survival rates (4), which might also partially explain the strong effects of PEM on prostatectomy and hysterectomy as well as its relatively weak effects on esophagectomy and pancreatectomy. This suggests more attention should be paid to non-GI cancer patients with PEM whose nutritional statuses are always less noticed than GI cancer patients. Urgent and appropriate nutritional supplements should be administered to patients with PEM, thereby correcting PEM and improving their prognosis.
Apart from mortality, major complications play the key roles in perioperative recovery, hospital stay and total cost of cancer patients (35). Our study indicated that patients with PEM have a 2.46-fold increased risk of overall major complications compared to those without PEM after MCS (Table 2). It is worth noting that the highest OR related to PEM was sepsis (OR=3.08) and septic shock (OR=3.55), which was consistent with previous report (1). Cancer patients are considered to have baseline immunosuppression (36), and PEM worsens this condition, which inclines patients to immunologic deficiency due to protein deficiency and lack of immune mediators and consequently predisposes patients to susceptibility to infection (37). Sepsis always results in massive catabolism, characterized by the depletion of protein, fat, and glycogen energy reserves. It is common for patients with sepsis to experience muscle wasting and weight loss, which further causes or worsens malnutrition (38). Therefore, early screening of PEM and monitoring for infection symptoms, signs, and laboratory findings are crucial for cancer patients undergoing surgery. Furthermore, there was a higher risk of pneumonia (OR=2.52), adult respiratory distress syndrome (2.51), and mechanical ventilation (OR=2.62) in patients with PEM after MCS, which were resulted mainly from PEM-induced muscle weakness and PEM-related immunologic deficiency (39, 40). Also, higher risk of cardiac complications (acute myocardial infarction, cardiac arrest) were also observed from our study, which may result from high levels of inflammation and the progression of atherosclerosis (41) as well as cardiac structural alterations and the occurrence of heart failure (42).
There are several limitations of our study. First, the use of ICD-9-CM codes to identify these procedures and events relies largely on coding accuracy, which could be assigned erroneously. As PEM has not been rigorously validated in the NIS, if the misclassification occurs, it is impossible to access individual patient charts to confirm the diagnosis, which inevitably results in bias. Second, the NIS data set does not provide information for tumor stage and grade, laboratory values, or other cancer-related treatment received by the patients, which made it impossible to evaluating these parameters on outcomes. Third, the NIS data does not provide consistent surgeon identification, and there is a possible relationship between outcomes after MCS and the experience and practice patterns of surgeons or institutions. Fourth, as the information after discharge is not available from the NIS, the post-discharge outcomes could not be evaluated. Fifth, since the heterogenous patients and the restrictions of NIS database, it is not possible to extrapolate the information for each single cancer surgery. Despite these shortcomings, the NIS is a large and reliable database containing hospitalized patient data from over 4,000 hospitals in over 30 states in the United States, and temporal trend analyses are performed during a 6-year time span, which affords more power to the study. Moreover, the database has been widely applied in other retrospective studies. In addition, the impact of PEM on outcomes is independent of confounding variables in the multivariable and double robust inverse probability of treatment weighting method. Also, we investigate the influence of surgical type on perioperative outcomes, aiming to provide more comprehensive information concerning surgical type and relating outcome. Since the present study was observational, prospective studies are needed to verify the impact of PEM on worse outcomes after MCS.
In conclusion, we found PEM had severe impact on mortality, major complications, total cost and LOS of cancer patients underwent MCS by analyzing data of more than one million patients. PEM patients who underwent lung resection and colectomy had the highest mortality rate while PEM significantly increased the risk of mortality and major complications in those underwent prostatectomy, hysterectomy and lung resection. Also, we discovered consistently increasing PEM rates and the conversely decreasing EAPC of both mortality and major complications in the PEM group undergoing MCS from 2009 to 2015, which are likely the result of improved screening tools, evolving guidelines, and better management. Prompt recognition of PEM and the initiation of appropriate nutrition therapy is essential to achieve better outcomes after MCS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
JJ, XZ, ZD, YL, and HL designed research. JJ, XZ, PZ, and YX conducted research. JJ, ZD, and HH analyzed data. JJ, XZ, YL, and HL wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (2018YFC1314100), the Key-Area Research and Development Program of Guangdong Province (2019B020230001), the National Natural Science Foundation of China Youth Science Foundation (82201551), the Natural Science Foundation of Guangdong Province (2022A1515011306, 2020A1515010268, 2020A1515010049, 2019A1515010275), the Outstanding Young Talents Foundation of Guangdong Provincial People’s Hospital (KJ012019091), the Program of Science and Technology of Guangzhou (202103020166, 201904010424), NSFC Incubation Project of Guangdong Provincial People’s Hospital(KY0120220031), the Fundamental Research Funds for the Central Universities(2020ZYGXZR010).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.970187/full#supplementary-material
References
1. Adejumo AC, Akanbi O, Pani L. Protein energy malnutrition is associated with worse outcomes in sepsis-a nationwide analysis. J Acad Nutr Dietetics (2019) 119(12):2069–84. doi: 10.1016/j.jand.2019.04.019
2. Adejumo AC, Adejumo KL, Adegbala OM, Enwerem N, Ofosu A, Akanbi O, et al. Inferior outcomes of patients with acute myocardial infarction and comorbid protein-energy malnutrition. JPEN J parenteral enteral Nutr (2020) 44(3):454–62. doi: 10.1002/jpen.1680
3. Adejumo AC, Adejumo KL, Adegbala OM, Chinedozi I, Ndansi J, Akanbi O, et al. Protein-energy malnutrition and outcomes of hospitalizations for heart failure in the USA. Am J Cardiol (2019) 123(6):929–35. doi: 10.1016/j.amjcard.2018.12.014
4. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
5. Jiang D, Zhang L, Liu W, Ding Y, Yin J, Ren R, et al. Trends in cancer mortality in China from 2004 to 2018: A nationwide longitudinal study. Cancer Commun (London England). (2021) 41(10):1024–36. doi: 10.1002/cac2.12195
6. Laganà AS, Vitale SG, Nigro A, Sofo V, Salmeri FM, Rossetti P, et al. Pleiotropic actions of peroxisome proliferator-activated receptors (PPARs) in dysregulated metabolic homeostasis, inflammation and cancer: Current evidence and future perspectives. Int J Mol Sci (2016) 17(7):999. doi: 10.3390/ijms17070999
7. Vitale SG, Laganà AS, Nigro A, La Rosa VL, Rossetti P, Rapisarda AM, et al. Peroxisome proliferator-activated receptor modulation during metabolic diseases and cancers: Master and minions. PPAR Res (2016) 2016:6517313. doi: 10.1155/2016/6517313
8. Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr (Edinburgh Scotland). (2017) 36(5):1187–96. doi: 10.1016/j.clnu.2017.06.017
9. Lakananurak N, Gramlich L. The role of preoperative parenteral nutrition. Nutrients (2020) 12(5):1320. doi: 10.3390/nu12051320
10. Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Maeda S, Haraguchi N, et al. Prevalence of malnutrition among gastric cancer patients undergoing gastrectomy and optimal preoperative nutritional support for preventing surgical site infections. Ann Surg Oncol (2015) 22 Suppl 3:S778–85. doi: 10.1245/s10434-015-4820-9
11. Lee DU, Fan GH, Hastie DJ, Addonizio EA, Suh J, Prakasam VN, et al. The clinical impact of malnutrition on the postoperative outcomes of patients undergoing colorectal resection surgery for colon or rectal cancer: Propensity score matched analysis of 2011-2017 US hospitals. Surg Oncol (2021) 38:101587. doi: 10.1016/j.suronc.2021.101587
12. Fukami Y, Saito T, Arikawa T, Osawa T, Komatsu S, Kaneko K, et al. European Society for clinical nutrition and metabolism (ESPEN) malnutrition criteria for predicting major complications after hepatectomy and pancreatectomy. World J Surg (2021) 45(1):243–51. doi: 10.1007/s00268-020-05767-w
13. Bagan P, Berna P, De Dominicis F, Das Neves Pereira JC, Mordant P, de la Tour B, et al. Nutritional status and postoperative outcome after pneumonectomy for lung cancer. Ann Thorac Surg (2013) 95(2):392–6. doi: 10.1016/j.athoracsur.2012.06.023
14. Anandavadivelan P, Lagergren P. Cachexia in patients with oesophageal cancer. Nat Rev Clin Oncol (2016) 13(3):185–98. doi: 10.1038/nrclinonc.2015.200
15. Tobert CM, Hamilton-Reeves JM, Norian LA, Hung C, Brooks NA, Holzbeierlein JM, et al. Emerging impact of malnutrition on surgical patients: Literature review and potential implications for cystectomy in bladder cancer. J Urol (2017) 198(3):511–9. doi: 10.1016/j.juro.2017.01.087
16. Soloff MA, Vargas MV, Wei C, Ohnona A, Tyan P, Gu A, et al. Malnutrition is associated with poor postoperative outcomes following laparoscopic hysterectomy. JSLS (2021) 25(1):e2020.00084. doi: 10.4293/JSLS.2020.00084
17. Tan HJ, Saliba D, Kwan L, Moore AA, Litwin MS. Burden of geriatric events among older adults undergoing major cancer surgery. J Clin Oncol Off J Am Soc Clin Oncol (2016) 34(11):1231–8. doi: 10.1200/JCO.2015.63.4592
18. Sammon J, Trinh VQ, Ravi P, Sukumar S, Gervais MK, Shariat SF, et al. Health care-associated infections after major cancer surgery: temporal trends, patterns of care, and effect on mortality. Cancer (2013) 119(12):2317–24. doi: 10.1002/cncr.28027
19. Nguyen GC, Munsell M, Harris ML. Nationwide prevalence and prognostic significance of clinically diagnosable protein-calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflammatory bowel diseases. (2008) 14(8):1105–11. doi: 10.1002/ibd.20429
20. Burke LG, Frakt AB, Khullar D, Orav EJ, Jha AK. Association between teaching status and mortality in US hospitals. Jama (2017) 317(20):2105–13. doi: 10.1001/jama.2017.5702
21. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care (1998) 36(1):8–27. doi: 10.1097/00005650-199801000-00004
22. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med (2015) 34(28):3661–79. doi: 10.1002/sim.6607
23. McKenna NP, Bews KA, Al-Refaie WB, Colibaseanu DT, Pemberton JH, Cima RR, et al. Assessing malnutrition before major oncologic surgery: One size does not fit all. J Am Coll Surgeons. (2020) 230(4):451–60. doi: 10.1016/j.jamcollsurg.2019.12.034
24. Reber E, Schönenberger KA, Vasiloglou MF, Stanga Z. Nutritional risk screening in cancer patients: The first step toward better clinical outcome. Front Nutr (2021) 8:603936. doi: 10.3389/fnut.2021.603936
25. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J parenteral enteral Nutr (2014) 38(2):196–204. doi: 10.1177/0148607113502674
26. Ravasco P, Monteiro-Grillo I, Vidal PM, Camilo ME. Nutritional deterioration in cancer: the role of disease and diet. Clin Oncol (Royal Coll Radiologists (Great Britain)). (2003) 15(8):443–50. doi: 10.1016/S0936-6555(03)00155-9
27. Rizzo G, Laganà AS, Rapisarda AM, La Ferrera GM, Buscema M, Rossetti P, et al. Vitamin B12 among vegetarians: Status, assessment and supplementation. Nutrients (2016) 8(12):767. doi: 10.3390/nu8120767
28. Paul C, Laganà AS, Maniglio P, Triolo O, Brady DM. Inositol's and other nutraceuticals' synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: state-of-the-art and future perspectives. Gynecological Endocrinol Off J Int Soc Gynecological Endocrinol (2016) 32(6):431–8. doi: 10.3109/09513590.2016.1144741
29. Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, et al. American Cancer society guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA: Cancer J Clin (2012) 62(1):30–67. doi: 10.3322/caac.20140
30. Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, et al. ESPEN practical guideline: Clinical nutrition in cancer. Clin Nutr (Edinburgh Scotland). (2021) 40(5):2898–913. doi: 10.1016/j.clnu.2021.02.005
31. Trinh VQ, Karakiewicz PI, Sammon J, Sun M, Sukumar S, Gervais MK, et al. Venous thromboembolism after major cancer surgery: temporal trends and patterns of care. JAMA Surg (2014) 149(1):43–9. doi: 10.1001/jamasurg.2013.3172
32. Martínez-Ortega AJ, Piñar-Gutiérrez A, Serrano-Aguayo P, González-Navarro I, Remón-Ruíz PJ, Pereira-Cunill JL, et al. Perioperative nutritional support: A review of current literature. Nutrients (2022) 14(8):1601. doi: 10.3390/nu14081601
33. Hu WH, Eisenstein S, Parry L, Ramamoorthy S. Preoperative malnutrition with mild hypoalbuminemia associated with postoperative mortality and morbidity of colorectal cancer: a propensity score matching study. Nutr J (2019) 18(1):33. doi: 10.1186/s12937-019-0458-y
34. Garth AK, Newsome CM, Simmance N, Crowe TC. Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J Hum Nutr dietetics Off J Br Dietetic Assoc (2010) 23(4):393–401. doi: 10.1111/j.1365-277X.2010.01058.x
35. Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin Nutr (Edinburgh Scotland). (2021) 40(7):4745–61. doi: 10.1016/j.clnu.2021.03.031
36. Sammon JD, Klett DE, Sood A, Olugbade K Jr., Schmid M, Kim SP, et al. Sepsis after major cancer surgery. J Surg Res (2015) 193(2):788–94. doi: 10.1016/j.jss.2014.07.046
37. Batool R, Butt MS, Sultan MT, Saeed F, Naz R. Protein-energy malnutrition: a risk factor for various ailments. Crit Rev Food Sci Nutr (2015) 55(2):242–53. doi: 10.1080/10408398.2011.651543
38. Wischmeyer PE. Nutrition therapy in sepsis. Crit Care clinics. (2018) 34(1):107–25. doi: 10.1016/j.ccc.2017.08.008
39. Baba H, Tokai R, Hirano K, Watanabe T, Shibuya K, Hashimoto I, et al. Risk factors for postoperative pneumonia after general and digestive surgery: a retrospective single-center study. Surg Today (2020) 50(5):460–8. doi: 10.1007/s00595-019-01911-9
40. Howes N, Atkinson C, Thomas S, Lewis SJ. Immunonutrition for patients undergoing surgery for head and neck cancer. Cochrane Database systematic Rev (2018) 8(8):Cd010954. doi: 10.1002/14651858.CD010954.pub2
41. Raposeiras Roubín S, Abu Assi E, Cespón Fernandez M, Barreiro Pardal C, Lizancos Castro A, Parada JA, et al. Prevalence and prognostic significance of malnutrition in patients with acute coronary syndrome. J Am Coll Cardiol (2020) 76(7):828–40. doi: 10.1016/j.jacc.2020.06.058
Keywords: protein-energy malnutrition (PEM), major cancer surgery, mortality, postoperative complications, nationwide analysis
Citation: Jin J, Zhu X, Deng Z, Zhang P, Xiao Y, Han H, Li Y and Li H (2023) Protein-energy malnutrition and worse outcomes after major cancer surgery: A nationwide analysis. Front. Oncol. 13:970187. doi: 10.3389/fonc.2023.970187
Received: 15 June 2022; Accepted: 03 January 2023;
Published: 17 January 2023.
Edited by:
Lucia Moletta, University of Padua, ItalyReviewed by:
Lidia Santarpia, University of Naples Federico II, ItalyAntonio Simone Laganà, University of Palermo, Italy
Copyright © 2023 Jin, Zhu, Deng, Zhang, Xiao, Han, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai Li, lihai8@mail.sysu.edu.cn; Yanbing Li, liyb@mail.sysu.edu.cn
†These authors have contributed equally to this work
 Jiewen Jin
Jiewen Jin Xianying Zhu
Xianying Zhu Zhantao Deng
Zhantao Deng Pengyuan Zhang1
Pengyuan Zhang1 Hedong Han
Hedong Han Yanbing Li
Yanbing Li Hai Li
Hai Li