According to the World Health Organization, around 1.6 million people worldwide died due to diabetes in 2016. It is estimated that 425 million people are living with diabetes all over the world. By 2045, projections show this number rising to some 629 million diabetics globally. Type 1 diabetes is an autoimmune condition where the pancreas cannot produce insulin, whereas type 2 diabetes is the body’s resistance to insulin.
Previous studies have linked viruses to diabetes, and recent studies have suggested that enteroviruses could potentially trigger diabetes, although its direct effect in vivo as well as its mechanism of action at the molecular level were unknown. A recent mouse study by researchers at the Spanish National Cancer Research Centre (CNIO) reveals how the enterovirus coxsackievirus type B4 (CVB4) could induce diabetes.
Their findings, “Coxsackievirus B Type 4 Infection in β Cells Downregulates the Chaperone Prefoldin URI to Induce a MODY4-like Diabetes via Pdx1 Silencing,” is published in Cell Reports Medicine and led by Nabil Djouder, PhD, group leader at CNIO.
The researchers suggest their findings could be of relevance for the COVID-19 pandemic, since clinical information indicates a possible relationship between SARS-CoV-2 viral infection and diabetes. The researchers suggest that since the receptor of SARS-CoV-2 is expressed in the endocrine pancreas, it could operate and lead to diabetes in a similar way that CVB4 does, independently of immune reactions.
“Enteroviruses are suspected to contribute to insulin-producing β cell loss and hyperglycemia-induced diabetes. However, mechanisms are not fully defined. Here, we show that coxsackievirus B type 4 (CVB4) infection in human islet-engrafted mice and in rat insulinoma cells displays loss of unconventional prefoldin RPB5 interactor (URI) and PDX1, affecting β cell function and identity. Genetic URI ablation in the mouse pancreas causes PDX1 depletion in β cells,” noted the researchers.
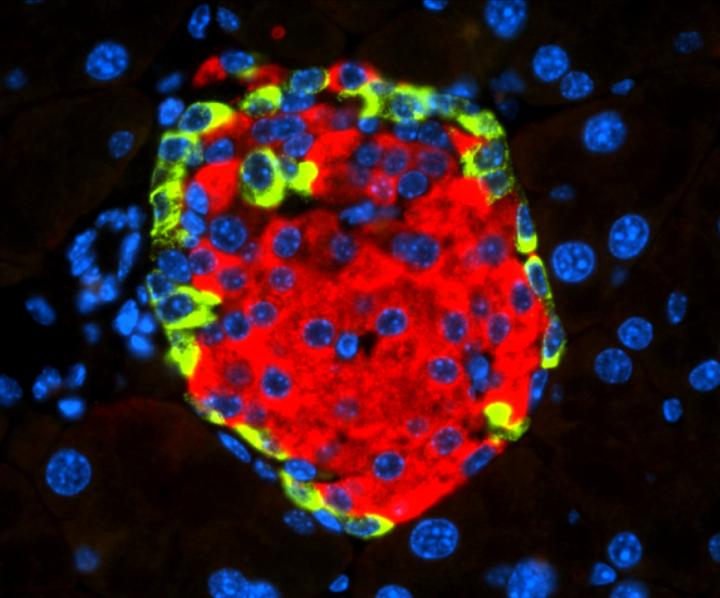
The researchers observed animal models engrafted with human pancreatic cells infected by CVB4, as well as with human and mouse insulin-producing cells, also infected with this virus. They discovered that CVB4 infection induces deregulation of URI, a protein that regulates the normal functions of numerous cellular activities.
“In this case, URI downregulation triggers a cascade of molecular events leading to modification of the genome via hypermethylation and silencing of Pdx1. This is a gene critical for the identity and the function of beta cells present in the endocrine pancreas, at the so-called Islets of Langerhans, and responsible for the production and secretion of insulin, a hormone that decreases blood glucose levels,” stated Djouder. “PDX1 silencing causes the loss of the identity and function of the beta cells, which become more like alpha cells, in charge of increasing blood glucose levels, and hence leading to hyperglycemia and subsequent diabetes, independently of any immune reactions.”
The researchers also observed that diabetic mice that overexpress URI in beta cells are more tolerant to glucose. They demonstrated that expression of URI, PDX1, and viral particles correlates in beta cells, highlighting a causal link between enterovirus infection and diabetes in humans.
“Similarly to our investigations on enteroviruses, some recent clinical observations have associated SARS-CoV-2, the virus responsible for COVID-19, to diabetes in infected patients,” explained Djouder. “Since the receptor of SARS-Co-V2 is present in beta cells, it would be interesting to study if this virus also alters URI function and silences the expression of PDX1 to affect beta-cell function, promoting diabetes.”
These findings could be a critical step to a new wave of therapeutic strategies and may aid in understanding the relationship between SARS-CoV-2 and diabetes.